Abstract
Locus coeruleus (LC) is involved in the LHRH regulation by gonadal steroids. We investigated the expression of progesterone and estrogen receptors (PR; ER) in LC neurons of ERα (αERKO) or ERβ (βERKO) knockout mice, and their wild-type (αWT and βWT). Immunocytochemical studies showed that LC expresses PR and both ERs, although ERβ was more abundant. Estradiol benzoate (EB) decreased ERα-positive cells in WT and βERKO mice, and progesterone caused a further reduction, whereas none of the steroids influenced ERβ expression. ERβ deletion increased ERα while ERα deletion did not alter ERβ expression. In both WT mice, EB increased PR expression, which was diminished by progesterone. These steroid effects were also observed in αERKO animals but to a lesser extent, suggesting that ERα is partially responsible for the estrogenic induction of PR in LC. Steroid effects on PR in βERKO mice were similar to those in the αERKO but to a lesser extent, probably because PR expression was already high in the oil-treated group. This expression seems to be specific of LC neurons, since it was not observed in other areas studied, the preoptic area and ventromedial nucleus of hypothalamus. These findings show that LC in mice expresses αER, βER, and PR, and that a balance between them may be critical for the physiological control of reproductive function.
Keywords: Estradiol, Progesterone and estrogen receptors, Locus coeruleus, Knockout mice, LHRH
Introduction
It is well established that gonadal steroids, estrogen, and progesterone, control LHRH secretion and subsequent preovulatory LH surge in rodents. Although elevated levels of estradiol and progesterone are known to be essential for the synthesis and release of LHRH in the preoptic area (POA), it is not completely understood how they act on various parts of the brain to exert coordinately their regulatory action on LHRH and LH secretion.
Recent studies have shown that the locus coeruleus (LC) may be one of the key brain sites of estrogen action. Selective retrograde tracing showed that POA LHRH neurons receive direct projections from the LC, together with the brainstem A2 noradrenergic neurons [1]. LC, which is the main source of noradrenaline (NA) in the brain [2], has been demonstrated to play a critical role in facilitation of LHRH/LH surges. LC electrolytic lesion decreased NA content in the POA and ME and completely blocked the preovulatory LH surges as well as the surge induced by steroids in ovariectomized rats [3, 4]. It has also been shown that there is an increase in the number of FOS-immunoreactive (ir) neurons observed in the LC during the afternoon of proestrus [5] indicating an increase of neuronal activity in this area paralleled with LH release in response to LHRH surges.
LC neurons concentrate estradiol [6], express mRNA for estrogen receptor (ER) [7] as well as the two types of ERs, ERα and ERβ [8–11]. In addition, mRNA levels of NA synthesis enzyme, tyrosine hydroxylase, are increased by estrogen in the LC [12]. Therefore, it is hypothesized that noradrenergic LC neurons may be a target of estrogen actions, which are mediated by both types of ERs. The number of ERα-ir cells fluctuates during estrous cycle more profoundly in the LC than in the POA [13], however, it remains to be determined, how estrogen and progesterone regulate the levels of ERα and ERβ in the LC.
One of downstream effects of estrogen action is the induction of progesterone receptors (PR). Progesterone is known to work synergistically with estrogen to regulate LHRH/LH synthesis and release. For instance, progesterone administration to estradiol-primed ovariectomized rats results in the amplification and anticipation of LHRH [14] and LH [15] surges, and administration of PR antagonist RU486 to proestrus rats blocks LH surge [16]. Since a clear fluctuation of the number of PR immunoreactive cells has been described in the LC during the estrous cycle [13], it is possible that these effects of progesterone on the LHRH system are potentially mediated by its action on the LC neurons. Therefore, in this study, we examined estrogen-inducible PR in the LC in estrogen or estrogen plus progesterone treated ovariectomized mice. To determine the differential roles of two types of ERs in the induction of PR, we used ERα and ERβ knockout mice. Since surprisingly, a high number of LC neurons expressed PR in the oil-treated ovariectomized βERKO mice, we further examined the effects of ovarian steroids on PR expression in other brain regions related to reproduction, the POA and the ventromedial nucleus of hypothalamus.
Results
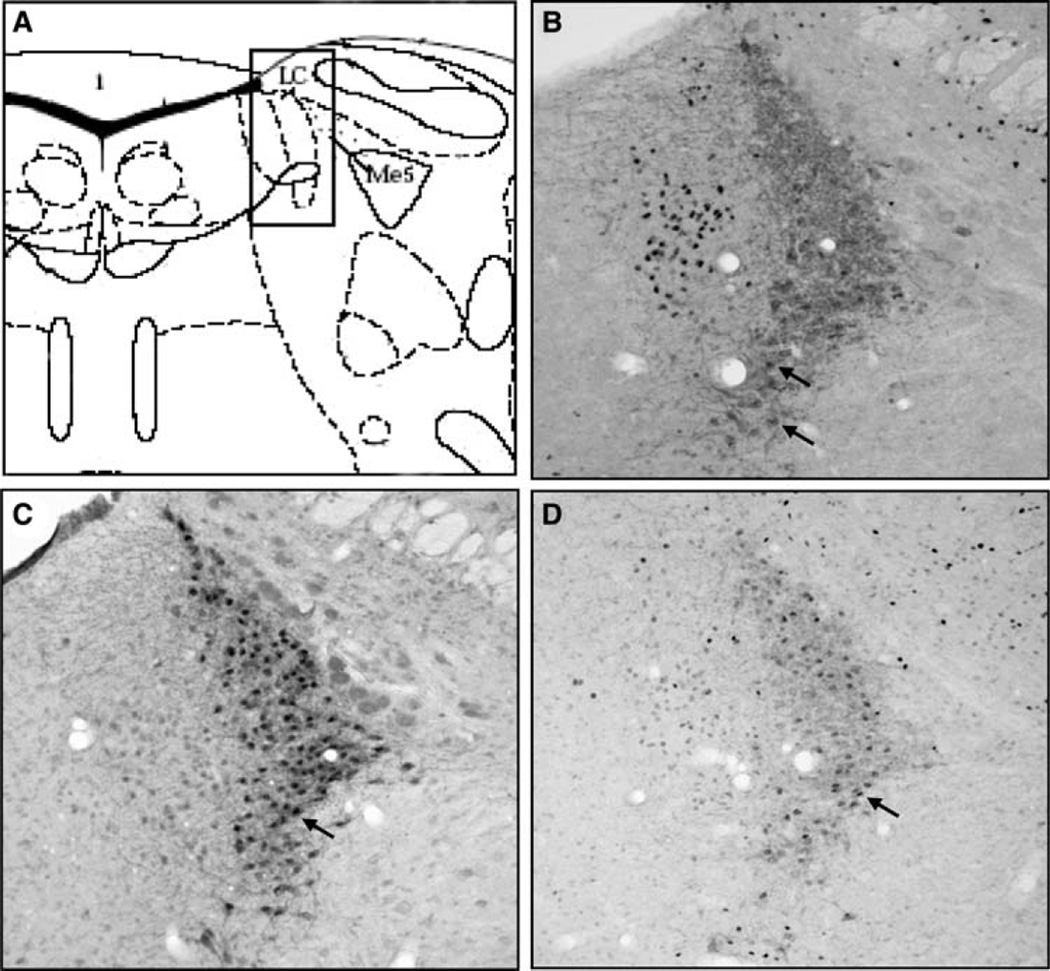
It was found that ERα, ERβ and PR-ir cells were all present in the LC. Figure 1 shows the LC localization (Fig. 1a) and a typical staining for each receptor in the middle portion of the LC of an oil-treated (Fig. 1b, c) and an estradiol-treated (Fig. 1d) αWT animal. All immunostaining was detected specifically within the cell nucleus. Overall, ERβ-ir neurons were more abundant than ERα-ir cells. In fact, nearly all TH-staining cells in LC express ERβ (Fig. 1c), while ERα expression was much more discreet and mainly localized ventrally (Fig. 1b). Interestingly, a strong staining for ERα was observed outside LC, in Barrington nucleus. Conversely, PR expression was scattered all over the LC nucleus (Fig. 1c).
Fig. 1.
Schematic view of the locus coeruleus (a) and representative photomicrographs (200×) showing nuclear immuno staining for ERα (b), ERβ (c) and PR (d) and cytoplasmatic staining for tyrosine hydroxylase in the locus coeruleus. The arrows indicate examples of double-labeled neurons. (Color figure online)
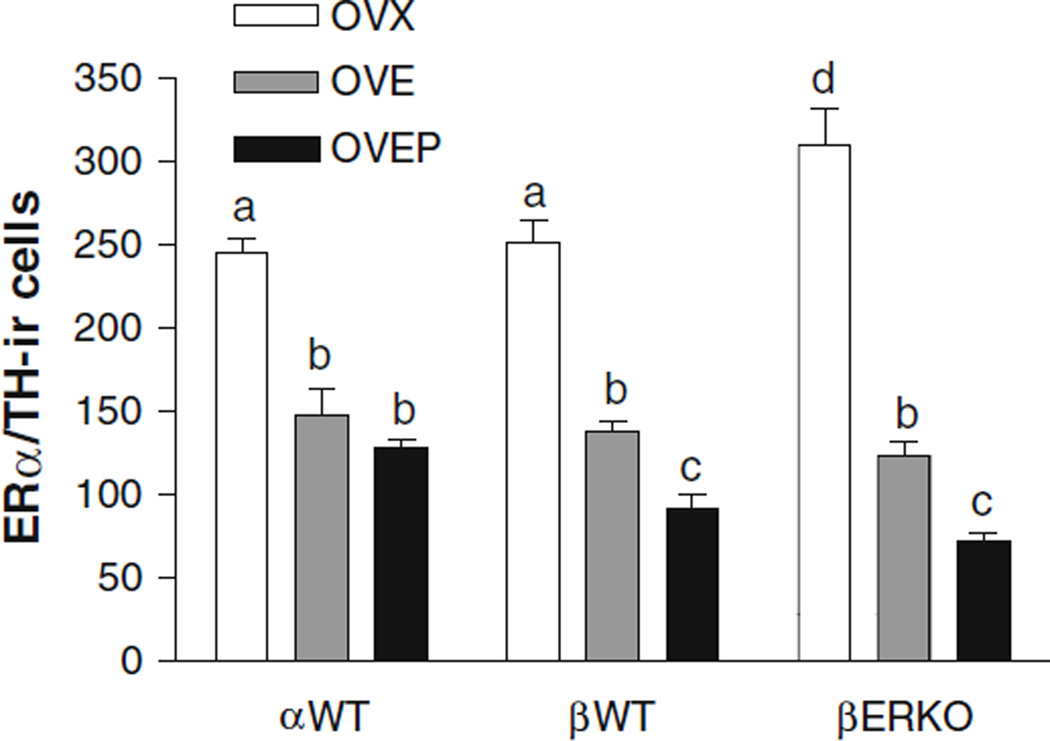
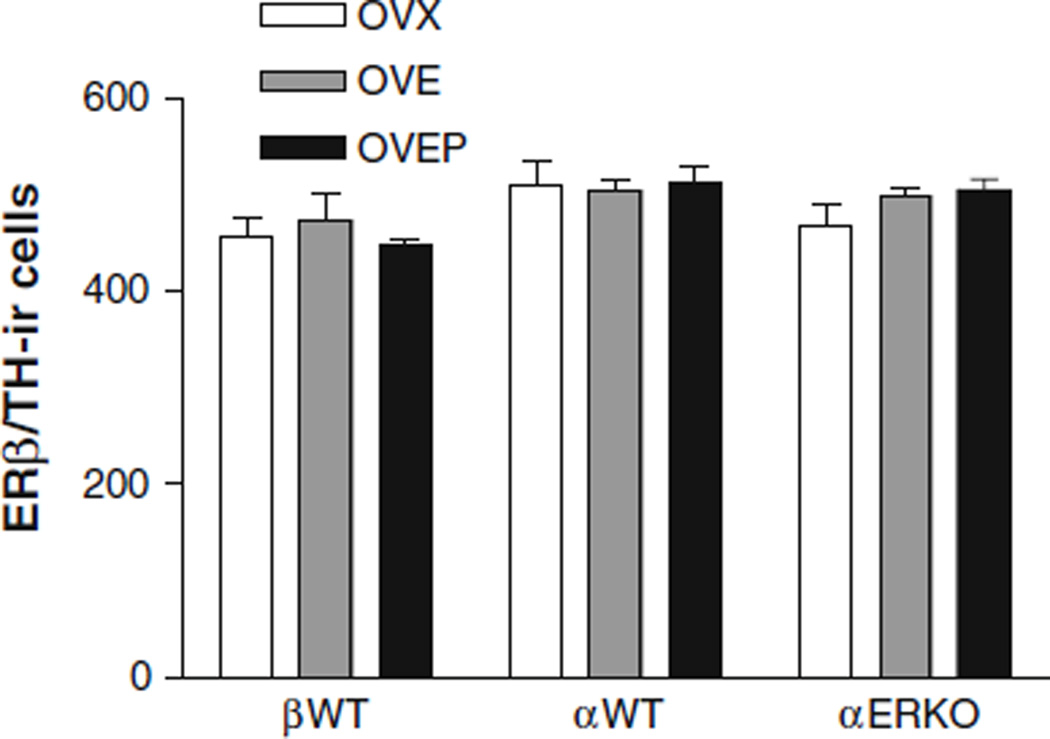
The number of ERα-ir cells was significantly higher (P < 0.05) in βERKO oil-treated group, when compared to the oil-treated βWT animals (Fig. 2). Two days of EB treatment significantly reduced the number of ERα-ir cells in αWT and βWT as well as in βERKO (P < 0.001 vs. OVX group) and progesterone treatment resulted in a further decrease in this number (P < 0.05 vs. OVE group). Unlike its effects on ERα-ir cells, EB did not down-regulate the number of ERβ-ir cells in αWT, βWT, or αERKO. Moreover, the number of ERβ-ir cells was similar in all groups studied, regardless the treatment or genotype (Fig. 3). It should also be noted that unlike an increase of ERα in βERKO mice, ERα gene disruption did not affect ERβ expression.
Fig. 2.
Histogram showing the effects of estradiol and progesterone treatment on the mean number (±SEM) of TH-immunoreactive cells expressing ERα immunoreactivity in the locus coeruleus of ovariectomized αWT, βWT, and βERKO mice treated with either oil (OVX), EB (OVE) or EB plus progesterone (OVEP). Different letters indicate statistical significance (n = 6–11/group)
Fig. 3.
Histogram showing the effects of estradiol and progesterone treatment on the mean number (±SEM) of TH-immunoreactive cells expressing ERβ immunoreactivity in the locus coeruleus of ovariectomized αWT, βWT and αERKO mice treated with either oil (OVX), EB (OVE) or EB plus progesterone (OVEP). (n = 5–7/group)
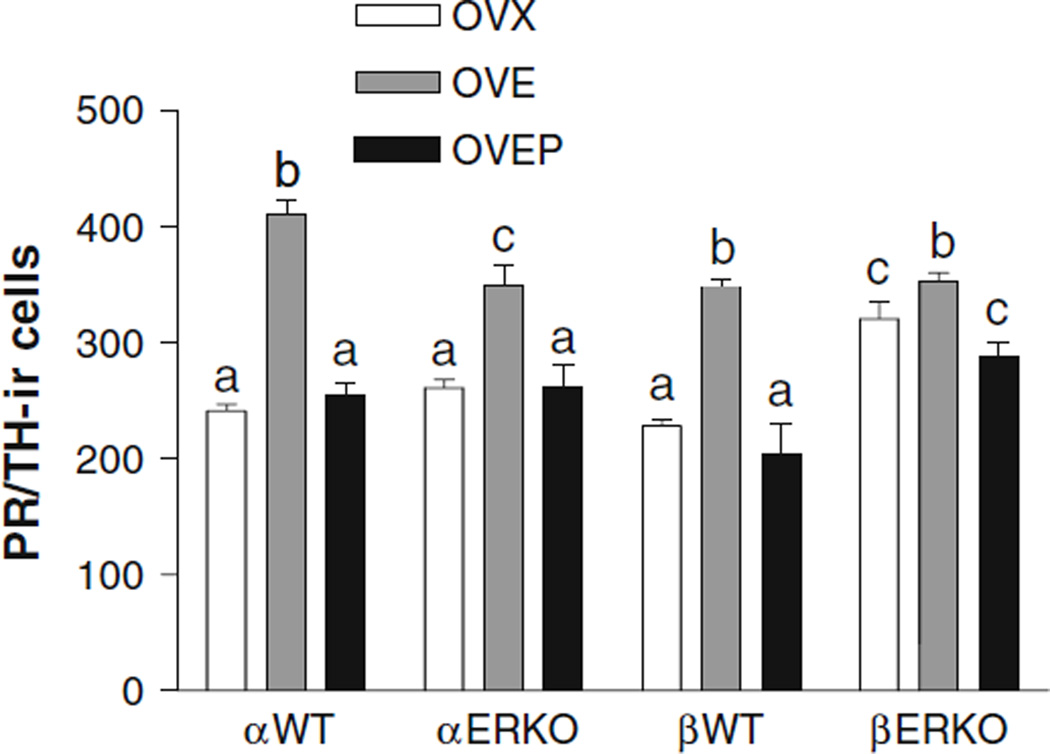
A substantial number of PR-ir cells was found in the LC of all oil-treated groups, though it was significantly higher in βERKO animals, when compared to the βWT mice (P < 0.001) as shown in Fig. 4. In both αWT and βWT mice, EB treatment significantly increased the number of PR-ir cells (P < 0.001 vs. OVX group) while progesterone treatment decreased it (P < 0.001 vs. OVE group) to the levels of the oil-treated groups. Although its effect was smaller than that seen in αWT, EB also increased the number of PR-ir cells (P < 0.01 vs. OVX group) in αERKO animals and progesterone treatment decreased it (P < 0.01 vs. OVE group) to the level of the oil-treated group. In βERKO mice, EB treatment induced a small, but still significant increase in the number of PR-ir cells (P < 0.05 vs. OVX group) and progesterone reversed the effect of EB (P < 0.05 vs. OVE group). It should be noted, however, that the number of PR-ir in βERKO mice in the OVEP group was also significantly higher than that in βWT mice (P < 0.05) as found in the oil-treated group.
Fig. 4.
Histogram showing the effects of estradiol and progesterone treatment on the mean number (±SEM) of TH-immunoreactive cells expressing PR immunoreactivity in the locus coeruleus of ovariectomized αWT, αERKO, βWT and βERKO mice treated with either oil (OVX), EB (OVE) or EB plus progesterone (OVEP). Different letters indicate statistical significance (n = 5–11/group)
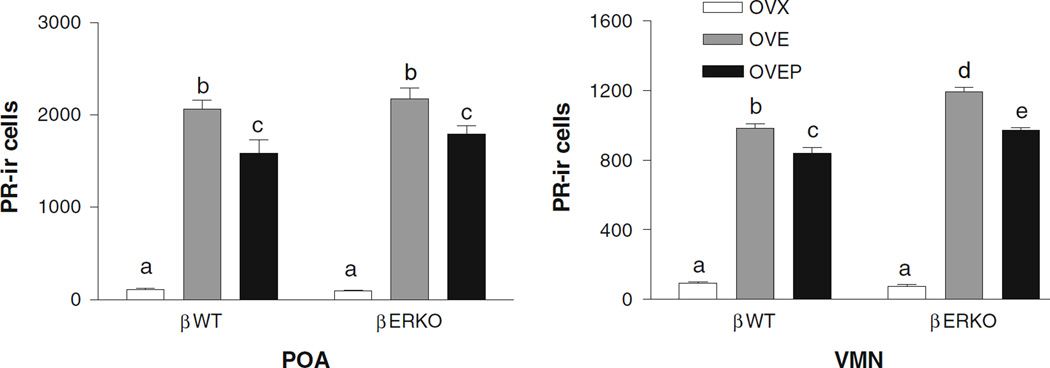
Since an elevated level of PR expression was observed in the LC neurons of oil-treated βERKO animals, we performed an additional experiment using separate groups of βERKO and βWT mice (n = 6/group for a total of six groups) to examine whether the profile observed in the LC might also be found in other brain areas. In the LC, we could replicate our original findings, i.e., oil-treated βERKO mice had significantly higher number of PR-ir than βWT mice. Unlike in the LC, PR expression in the POA and VMH of the oil-treated βERKO mice was minimal and not different from that in βWT (Fig. 5). The number of PR-ir cells dramatically increased after EB treatment (P < 0.001) and slightly decreased after progesterone injection (P < 0.05) regardless the genotype. The increase in PR expression in the OVE and OVEP groups was larger in the VMH of βERKO compared to βWT animals (P < 0.01 and P < 0.001, respectively), while the genotype did not influence the PR expression in the POA. Representative photomicrographs showing PR-ir staining in the POA and VMH for each treatment group are shown in Fig. 6. To further test the possibility that a higher number of PR-ir cells in the oil-treated βERKO mice may be due to an elevated level of residual estradiol in this group, we measured the plasma levels of estradiol and progesterone in these mice. We found that ovariectomy greatly decreased plasma levels of estradiol as well as progesterone almost equivalently in βWT and βERKO (Table 1). Furthermore, there were no genotype differences in plasma levels of steroids in any other treatment groups, OVE and OVEP.
Fig. 5.
Histogram showing the effects of estradiol treatment on the mean number (±SEM) of PR immunoreactive cells in the POA (a) and the VMN (b) of ovariectomized βWT and βERKO mice treated with either oil (OVX), EB (OVE) or EB plus progesterone (OVEP). Different letters indicate statistical significance (n = 6/group)
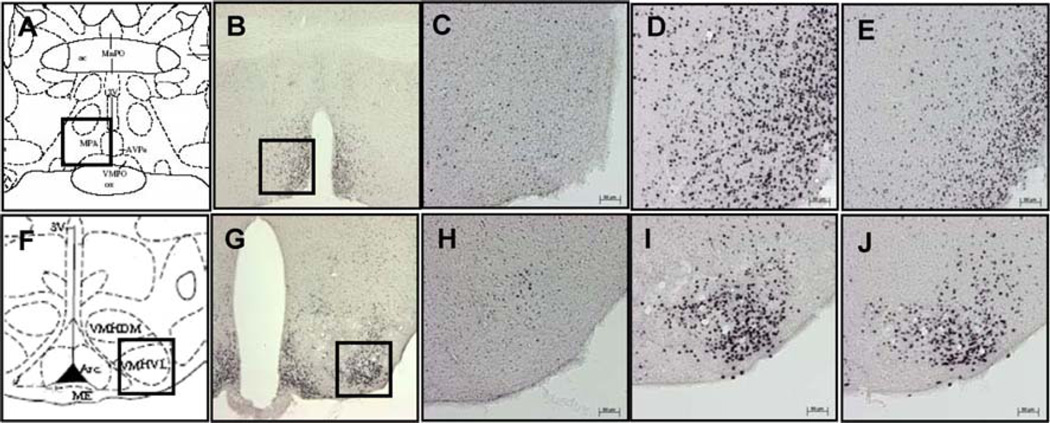
Fig. 6.
Schematic view and representative photomicrographs of POA (a–e) and VMN (f–j). Squares in the lower magnification (50×; photos b and g) indicate the same areas shown in the higher magnification (200×; photos c–e and h–j) where the PR immunoreactive cells were quantified in the POA (c–e) and VMN (h–j) of ovariectomized βWT mice treated with either oil (c and h), EB (d and i) or EB plus progesterone (e and j)
Table 1.
Mean (±SEM) plasma estradiol and progesterone concentrations of ovariectomized mice treated with oil (OVX), estradiol (OVE) or estradiol plus progesterone (OVEP)
| βWT | βERKO | |||||
|---|---|---|---|---|---|---|
| OVX | OVE | OVEP | OVX | OVE | OVEP | |
| Estradiol (pg/ml) | 11.0 ± 1.1 | 231.6 ± 59.9** | 274.9 ± 46.8** | 9.2 ± 0.9 | 241.7 ± 36.4** | 263.8 ± 73.1** |
| Progesterone (ng/ml) | 0.9 ± 0.2 | 1.1 ± 0.2 | 66.3 ± 11.4*** | 1.2 ± 0.2 | 1.1 ± 0.2 | 66.7 ± 16.3*** |
Different letters indicate statistical significance (n = 6/group)
P < 0.05;
P < 0.001
Discussion
It was found in this study that LC neurons express ERα, ERβ, and PR, and that both estradiol and progesterone treatment affects the number of ERα and PR-ir expressing cells, while it has no effect on the number of ERβ-ir cells. The expression of ERs and PRs in LC neurons as well as the control exerted by gonadal steroids may be related to the female reproductive function, since there is a well-defined relationship between noradrenaline from LC neurons and LHRH secretion [1, 3, 4].
Effects of estradiol and progesterone on ERα, ERβ, and PR expression
Although experimental evidence demonstrated the presence of low levels of ERβ mRNA [17] and protein [18] in LHRH neurons, there is no evidence of a role of ERβ in the regulation of GnRH neural physiology [19]. On the other hand, it is well known that ERα and PR are absolutely required for the generation of the preovulatory LH surge [20, 21]. As LHRH neurons do not express ERα [22] or PR [23] the positive steroid feedback upon LHRH neurons mainly occurs in an indirect manner, through neurotransmitter-producing neurons.
Similarly to the present results obtained in wild-type mice, previous studies using female rats demonstrated that LC neurons express ERα and PR and respond to ovarian steroid secretion [13], suggesting that these hormones could control LHRH secretion by acting on LC neurons. Although it is well known that estradiol increases TH mRNA synthesis [12] in the LC neurons, and presumably leads to increased capacity for catecholamine biosynthesis in this nucleus, it is not clear if there is a change in the number of TH-ir neurons. An attempt was made to calculate a percentage of TH neurons expressing each one of the steroid receptors studied, but as the LC is a very compact nucleus, it was not possible to distinguish clearly the shape of each TH-ir neuron in the area. Apparently, there was no change in the boundaries of TH staining in LC, but we clearly demonstrated here that estradiol treatment decreased the number of ERα-ir cells of LC in WT and βERKO animals, probably through down-regulatory mechanisms on its own receptor. The inhibitory effect of estrogen on ERα-expression was potentiated by progesterone treatment, which reduced the number of ERα-ir cells even more in both WT and βERKO animals. Indeed, progesterone has been demonstrated to be a suppressor of the ERα expression in the brain, diminishing ER hypothalamic and adenohypophysis concentrations in ovariectomized rats [24].
ERβ was clearly more abundant in LC neurons than ERα of all studied animals. Whereas a high ERβ/ERα ratio in LC was also observed by Mitra et al. [9], another study performed by Merchenthaler et al. [10] demonstrated a higher number of ERα-ir cells compared to ERβ-ir cells in the LC. A possible reason for this discrepancy seems to be differences in the antibodies used. Since we used the same antibody as the one used in Mitra’s study (80424), our results are in agreement with that study. Microscopic observation revealed that almost all TH immunostained cells express ERβ, whereas much smaller number of them express ERα. Interestingly, although ERβ expression is higher than ERα, neither estradiol nor progesterone affected ERβ expression, suggesting that ERβ has a minor contribution to the regulation of the HPG axis. It was previously shown that ovarian steroids replacement into ovariectomized monkeys also did not modify the expression of ERβ in several hypothalamic areas [25]. One possible role for the high expression of ERβ could be regulation of TH expression, since it has been demonstrated that ERβ plays a role in the transient sex difference in TH expression in the mouse LC [26]. However, ERβ has been shown to be required for several rapid, presumably nongenomic effects of estradiol on LHRH neurons such as CREB phosphorylation [27] and induction of galanin expression [28] that was related to the short-term negative feedback regulation of the LHRH levels. Recent studies reinforce that estradiol actions on LHRH control are exerted by ERα [29, 30] and that ERβ modulates these actions [31, 32].
As previously shown in other brain areas [33], estradiol treatment induced PR expression in the LC neurons in both WT mice. Although numbers of PR-ir cells were also increased by estrogen treatment in αERKO mice, this effect was smaller. This finding suggests that estradiol acts on LC neurons probably through ERα. Partial reduction of PR-induction by estradiol in αERKO mice was also found in the dorsal raphe nuclei (DRN) [34]. On the other hand, in hypothalamic regions, PR-induction by estradiol in αERKO mice was greatly reduced in comparison to WT control mice both at protein [35] and mRNA levels [36]. In this study, relative contribution of ERα and ERβ in the induction of PR by estrogen in the LC was not determined due to unexpectedly high levels of PR in oil-treated βERKO mice. It is possible that these high levels of PR are a result of increased ERα expression in these animals. Furthermore, PR can be induced by estradiol in hypothalamic regions of αβERKO female mice [37]. Therefore, it is likely that mechanisms other than those through ERβ and ERα may be involved in this estradiol-induced PR expression in the LC. Progesterone treatment diminished PR synthesis in the LC, POA and VMH neurons in WT animals, probably by this hormone down-regulating its own receptor. In all four genotypes studied, the number of PR-ir cells returned to its oil-treated levels after estrogen plus progesterone treatment in LC neurons, but not in the POA and VMH. Though increases in progesterone levels clearly decrease the number of PR-ir cells in peripheral tissues [38], progesterone action in the brain is variable in different brain areas [39].
Effects of ERα and ERβ gene disruption on estrogen and progesterone receptor expression
Interestingly, the number of cells expressing ERα in oil-treated βERKO animals was higher compared to that in oil-treated WT mice, suggesting that ERβ has an inhibitory role in ERα expression. Indeed, a previous in vitro study has demonstrated that ERβ sometimes acts as a negative regulator of ERα activity [40] On the other hand, ERα deletion did not change the number of ERβ containing cells in the LC neurons, consistent with the finding in several tissues that regulation of ERβ expression is not dependent on ERα [10, 41]. Conversely, a previous study demonstrated that ERβ protein levels were greatly reduced by ERα gene disruption site specifically in the POA [42]. These findings suggest that expression of one type of ER in certain brain region may be influenced by the lack of the other type of ER.
It has been shown that ERβ has biological roles that are distinct from those of ERα. Both ERα and ERβ activation is able to stimulate the transcription of the estrogen response element (ERE) through homo or heterodimeric complexes [43]. Thus, the activity of estradiol may depend on whether a cell contains ERα, ERβ or both. Indeed, there are differences in the rapid effects of estradiol on MAPK/CREB signaling pathways within the cells containing ERα or ERβ [44] that also suggests that maybe some of the biological functions of the ERβ may be dependent on the presence of ERα. Since we did not analyze ERα and ERβ expression in the same sections we cannot say whether they are colocalized in the same neurons. However, as most of the LC neurons express ERβ, it is likely that at least part of them express also ERα. Therefore, it is possible that interaction between ERα and ERβ plays a role in gonadal steroid regulation of various biological functions in the LC.
In this study, a considerable number of PR-ir cells were found in the LC of oil-treated mice from all four genotype groups. Actual numbers of PR-ir cells were not different among oil-treated animals except in the βERKO group, which showed a significantly higher number of PR-ir cells compared to oil-treated βWT group. These data suggest that ERβ may inhibit PR expression specifically in the LC neurons since in the other brain areas studied such as VMH and POA, the number of PR-ir cells was low in the oil-treated βERKO mice as seen as in other genotype groups. These results corroborate with previous findings that suggests that estradiol can regulate the expression of PR via multiple mechanisms, based upon brain region [34].
It is worth noting that the regulation of ERs and PR expression by ovarian steroids as well by other receptors on LC may also affect a wide variety of other functions. It is well known that LC is related to the autonomic and behavioral responses to stress challenges [45]; stress is also known to suppress HPG axis-activity [46] and estradiol decreases stress-induced Fos-expression in the LC neurons [47]. In addition, LC is involved with cognitive and behavioral functions, including arousal [48, 49]. For instance, arousal responses are greatly reduced by the ERα, but not ERβ disruption [50], but the mechanisms involved in such controls are still unknown.
Regarding reproductive function, findings in this study allow us to make advancements in the area of gonadal steroids action on LC neurons. Although the number of ERα expressing cells was much less than that of ERβ, only ERα seems to be regulated by ovarian steroids. As estradiol increases PR expression in the LC, ERα may play an important role on the positive feedback mechanisms in the LC for the LHRH release. The suppressive effect of ERβ on ERα expression could be relevant to the maintenance of low plasma LH levels during most of the cycle. However, during the LHRH and gonadotropin surges, this suppressive effect may be disrupted or superseded by other mechanisms, allowing an increase of ERα and PR expression, and consequently triggering the surges. Finally, since progesterone inhibits ERα expression, its release with the LH preovulatory surge could exert an important role in terminating the surge.
In summary, findings in this study demonstrate that LC neurons do express ERα, ERβ and PR and that the expression of these receptors is modulated not only by ovarian steroids but also by their own interdependence. Thus, a balance between these receptors in the LC may be critical for the physiological control of reproductive function.
Materials and methods
Mice
A total of 98 adult female αERKO, βERKO and their respective wild-type (WT) littermate mice were used. They were obtained from the αERKO and βERKO breeding colonies maintained at the Rockefeller University by mating heterozygous male and female mice. Original breeding pairs, which had been completely backcrossed to C57BL/6J, were obtained from the National Institute of Environmental Health Sciences. Throughout the study, mice were group housed under controlled temperature (22°C) on a 12-h light, 12-h dark cycle (lights off at noon) with food and water supplied ad libitum. All procedures were approved by the IACUC at the Rockefeller University.
Hormonal treatment
All mice were ovariectomized under methoxyflurane anesthesia at the age of 9–14 weeks and housed with the mice from the same genotype and treatment group (3 mice/cage). After 10 days (βERKO) or 14 days (αERKO), the animals received two subcutaneous injections of 0.1 ml of sesame oil (OVX) or 10 µg β-estradiol 3-benzoate (EB: Sigma Chemical Co., St. Louis, MO) in 0.1 ml oil at 48 and 24 h before perfusion (OVE). The third group of mice (OVEP) received two EB injections followed by a single injection of 0.5 µg progesterone (Sigma) in 0.1 ml oil at 5 h prior to perfusion, which was performed at 4 pm.
Preparation of brain samples
Mice were deeply anesthetized with Nembutal (75 mg/kg; i.p.). They were then perfused through the left ventricle, with 30 ml of 100 mM phosphate-buffered saline (PBS, pH 7.2) containing heparin, followed by 40 ml of 2% paraformaldehyde and 2.5% acrolein in 100 mM phosphate buffer (PB, pH 7.4). Blood samples were collected from the left ventricle prior to perfusion in some mice. Brain tissues were removed and post-fixed in the same fixative for 2 h at room temperature. After overnight washing in PB 100 mM at 4°C, the brains were incubated in a 30% sucrose solution in PB until it sank, frozen in cold 2-methylbutane (Fisher Scientific, Pittsburgh, PA) and sectioned using a sliding freezing microtome. The sections were stored in a cryoprotectant solution (30% glycerol, 30% ethylene glycol in 100 mM PB, pH 7.4) at −20°C until processed.
Single and dual label immunocytochemistry
Free-floating sections (30 µm) were washed several times with 50 mM Tris-buffered saline (TBS, pH 7.2) in order to thoroughly remove cryoprotectant. They were then treated with three kinds of blocking solutions. To remove residual aldehyde in acrolein fixed tissue and reduce non-specific staining, the sections were first incubated in a 0.5% sodium borohydrate (NaBH4) solution in TBS for 10 min followed by 0.1% hydrogen peroxide in TBS for 20 min to block endogenous peroxidase. They were rinsed three times (10 min each) in TBS between different blocking solutions. The sections were then incubated for 60 min in TBS containing 0.3% Triton X-100, 3% bovine serum albumin (BSA) and 3% normal goat serum (NGS). Without TBS rinse, the LC containing sections were incubated in one of three different primary antibodies, i. e., rabbit polyclonal anti ERα (C1355, Upstate Biotechnology, Lake Placid, NY, 1:2500), ERβ (80424, a gift of Merck Research Laboratories, 1:16000), and PR (SP2, LabVision Corporation, Fremont, CA, 1:600) for 72 h at 4°C. After washing with TBS (three times, 10 min each), the sections were incubated for 120 min at room temperature in a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Inc., Burlingame, CA, 1:400). All primary and secondary antibodies were diluted in the same buffer as used in the last blocking step. After TBS washes the tissues were incubated with avidin–biotin complex (ABC; Vectastain ABC Elite Kit, Vector Laboratories, 1:250) diluted in TBS for 60 min at room temperature, and washed again with TBS (three times, 10 min each). They were then developed using 0.02% diaminobenzidine (DAB; Sigma) and 0.01% hydrogen peroxide with 2.5% nickel sulfate in TBS. No staining was observed after incubation of sections obtained from αERKO animals with the αER antibody and from βERKO animals with the βER antibody (data not shown). Further ICC in POA and ventromedial nucleus of hypothalamus (VMH) sections from another group of βERKO and βWT animals were similarly stained only for PR. After stringent washing with TBS, only the LC-containing sections were incubated with a rabbit polyclonal anti-tyrosine hydroxylase antibody (CA-101, Protos Biotech Corp, New York, NY, 1:4000) overnight at 4°C. After washing with TBS, the sections were incubated for 60 min in a biotinylated goat anti-rabbit secondary antibody (1:400). After TBS washes, the tissues were incubated for 30 min with ABC, washed again with TBS and developed using DAB (0.02%) with 0.01% hydrogen peroxide. All sections were mounted on gelatin-coated slides, air-dried, dehydrated in ascending ethanol concentrations, cleared with xylene and coverslipped with DPX mounting medium (BDH, Poole, England).
Quantitative analysis
For quantification, three sections (120 µm apart) containing anterior, middle or posterior part of the LC were selected for each animal and the number of cells stained for each of ERα, ERβ, and PR was counted bilaterally at a magnification of 200× under a Nikon light microscope (Nikon, Japan). Since the majority of the LC cells express TH [51], we used TH immunostaining to identify the exact boundary of the LC regions. In the POA and VMH, PR-ir cells in an area of 500 µm2 in two sections for each brain region were quantified bilaterally. The sum of immunopositive cells in each brain area was calculated for each mouse and averaged for each genotype and treatment group.
Radioimunoassay
Blood samples collected from the further experiments with βERKO and βWT mice were used to measure plasma levels of estradiol and progesterone by double antibody radioimmunoassay (RIA) using the Estradiol and Progesterone Maia kits (Biochem Immunosystems, Serotec, Italy). The lower limit of detection was 6.71 pg/ml for estradiol and 0.43 ng/ml for progesterone. The intra-assay coefficient of variation was 2.1 and 3.2%, for estradiol and progesterone, respectively. In order to prevent inter-assay variation, all samples were measured together in a single assay.
Statistics
Data were analyzed first using a two-way analysis of variance (ANOVA) for main effects of genotype, treatment group and their interaction, followed by post-hoc one-way ANOVAs with Newman-Keuls for multiple comparisons or t-test. The level of significance was set at P < 0.05.
Acknowledgments
The authors gratefully acknowledge Dr. Celso R. Franci, for his generous support for the steroid radioimmunoassay and Ms. Sonia M. Zanon Batista for her technical assistance. This study was supported by CAPES and FAPESP (CVH) and NIMH 62147 (SO).
Contributor Information
Cleyde Helena, Department of Physiology, School of Medicine of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil; Laboratory of Neurobiology and Behavior, The Rockefeller University, New York, NY, USA.
Jan-Åke Gustafsson, Department of Biosciences and Nutrition, Karolinska Institute, Huddinge S-141 86, Sweden; Center for Nuclear Receptors and Cell Signaling, Department of Biology and Biochemistry, University of Houston, Houston, TX 7720, USA.
Kenneth Korach, Laboratory of Reproductive and Developmental Toxicology, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA.
Donald Pfaff, Laboratory of Neurobiology and Behavior, The Rockefeller University, New York, NY, USA.
Janete A. Anselmo-Franci, Email: jaafranc@usp.br, Laboratory of Neuroendocrinology of Reproduction, School of Dentistry of Ribeirão Preto, University of São Paulo, Av. Do Cafés/n, Ribeirão Preto, SP 14040-904, Brazil; Department of Physiology, School of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil.
Sonoko Ogawa, Email: ogawa@kansei.tsukuba.ac.jp, Laboratory of Neurobiology and Behavior, The Rockefeller University, New York, NY, USA; Laboratory of Behavioral Neuroendocrinology, Kansei, Behavioral and Brain Sciences Graduate School of Comprehensive Human Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba 305-8577, Ibaraki, Japan.
References
- 1.Campbell RE, Herbison AE. Endocrinology. 2007;148:5884–5890. doi: 10.1210/en.2007-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grzanna R, Molliver ME. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- 3.Anselmo-Franci JA, Franci CR, Krulich L, Antunes-Rodrigues J, McCann SM. Brain Res. 1997;767:289–296. doi: 10.1016/s0006-8993(97)00613-6. [DOI] [PubMed] [Google Scholar]
- 4.Helena CV, Franci CR, Anselmo-Franci JA. Brain Res. 2002;955:245–252. doi: 10.1016/s0006-8993(02)03471-6. [DOI] [PubMed] [Google Scholar]
- 5.Martins-Afferri MP, Ferreira-Silva IA, Franci CR, Anselmo-Franci JA. Brain Res. Bull. 2003;61:521–527. doi: 10.1016/s0361-9230(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 6.Heritage AS, Stumpf WE, Sar M, Grant LD. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- 7.Shughrue PJ, Lane MV, Merchenthaler I. J. Comp. Neurol. 1997;388(50):7–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Shughrue PJ, Merchenthaler I. J. Comp. Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 9.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 10.Merchenthaler I, Lane MV, Numan S, Dellovade TL. J. Comp. Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 11.Shughrue PJ, Scrimo PJ, Merchenthaler I. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 12.Serova L, Rivkin M, Nakashima A, Sabban EL. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- 13.Helena CV, de Oliveira PM, Sanvitto GL, Hayashi S, Franci CR, Anselmo-Franci JA. J. Endocrinol. 2006;188:155–165. doi: 10.1677/joe.1.06268. [DOI] [PubMed] [Google Scholar]
- 14.Levine JE, Ramirez VD. Endocrinology. 1980;107:1782–1790. doi: 10.1210/endo-107-6-1782. [DOI] [PubMed] [Google Scholar]
- 15.Krey LC, Tyrey L, Everett JW. Endocrinology. 1973;93:385–390. doi: 10.1210/endo-93-2-385. [DOI] [PubMed] [Google Scholar]
- 16.Bauer-Dantoin AC, Tabesh B, Norgle JR, Levine JE. Endocrinology. 1993;133:2418–2423. doi: 10.1210/endo.133.6.8243259. [DOI] [PubMed] [Google Scholar]
- 17.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- 18.Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- 19.Bodo C, Rissman EF. Front. Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Couse JF, Korach KS. Endocr. Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 21.Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O’Malley BW, Levine JE. Endocrinology. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
- 22.Herbison AE, Theodosis DT. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 23.Fox SR, Harlan RE, Shivers BD, Pfaff DW. Neuroendocrinology. 1990;51:276–283. doi: 10.1159/000125350. [DOI] [PubMed] [Google Scholar]
- 24.Blaustein JD, Brown TJ. Brain Res. 1984;304:225–236. doi: 10.1016/0006-8993(84)90325-1. [DOI] [PubMed] [Google Scholar]
- 25.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Brain Res. Mol. Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 26.Pendergast JS, Tuesta LM, Bethea JR. J. Neuroendocrinol. 2008;20:1155–1164. doi: 10.1111/j.1365-2826.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 27.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. J. Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merchenthaler I, Hoffman GE, Lane MV. Endocrinology. 2005;146:2760–2765. doi: 10.1210/en.2004-1562. [DOI] [PubMed] [Google Scholar]
- 29.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindzey J, Jayes FL, Yates MM, Couse JF, Korach KS. J. Endocrinol. 2006;191:309–317. doi: 10.1677/joe.1.06965. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Criado JE, de Las Mulas JM, Bellido C, Navarro VM, Aguilar R, Garrido-Gracia JC, Malagon MM, Tena-Sempere M, Blanco A. J. Endocrinol. 2006;188:167–177. doi: 10.1677/joe.1.06377. [DOI] [PubMed] [Google Scholar]
- 32.Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. Endocrinology. 2008;149:1627–1637. doi: 10.1210/en.2007-1540. [DOI] [PubMed] [Google Scholar]
- 33.MacLusky NJ, McEwen BS. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 34.Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. J. Comp. Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. J. Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shughrue PJ, Lubahn DB, Negro-Vilar A, Korach KS, Merchenthaler I. Proc. Natl. Acad. Sci. USA. 1997;94:11008–11012. doi: 10.1073/pnas.94.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudwa AE, Rissman EF. J. Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 38.Milgrom E, Thi L, Atger M, Baulieu EE. J. Biol. Chem. 1973;248:6366–6374. [PubMed] [Google Scholar]
- 39.Galeeva A, Tuohimaa P. Behav. Brain Res. 2001;119:41–47. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- 40.Pettersson K, Delaunay F, Gustafsson JA. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 41.Korach KS. J. Soc. Gynecol. Investig. 2000;7:S16–S17. doi: 10.1016/s1071-5576(99)00057-x. [DOI] [PubMed] [Google Scholar]
- 42.Nomura M, Korach KS, Pfaff DW, Ogawa S. Brain Res. Mol. Brain Res. 2003;110:7–14. doi: 10.1016/s0169-328x(02)00544-2. [DOI] [PubMed] [Google Scholar]
- 43.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mol. Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 44.Abraham IM, Todman MG, Korach KS, Herbison AE. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 45.Valentino RJ, Foote SL, Aston-Jones G. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- 46.Rivier C, Rivest S. Biol. Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 47.Ueyama T, Tanioku T, Nuta J, Kujira K, Ito T, Nakai S, Tsuruo Y. Brain Res. 2006;1084:67–79. doi: 10.1016/j.brainres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Harley CW. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1987;11:419–458. doi: 10.1016/0278-5846(87)90015-7. [DOI] [PubMed] [Google Scholar]
- 49.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Proc. Natl. Acad. Sci. USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, Korach KS, Ogawa S, Pfaff DW. Proc. Natl. Acad. Sci. USA. 2003;100:11019–11022. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pickel VM, Joh TH, Reis DJ. Proc. Natl. Acad. Sci. USA. 1975;72:659–663. doi: 10.1073/pnas.72.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]