Phosphatidylinositol 3-kinase promotes proton pump activation, resulting in vacuolar acidification and stomatal closing, thereby delaying methyl jasmonate-induced leaf senescence.
Abstract
PI3K and its product PI3P are both involved in plant development and stress responses. In this study, the down-regulation of PI3K activity accelerated leaf senescence induced by methyl jasmonate (MeJA) and suppressed the activation of vacuolar H+-ATPase (V-ATPase). Yeast two-hybrid analyses indicated that PI3K bound to the V-ATPase B subunit (VHA-B). Analysis of bimolecular fluorescence complementation in tobacco guard cells showed that PI3K interacted with VHA-B2 in the tonoplasts. Through the use of pharmacological and genetic tools, we found that PI3K and V-ATPase promoted vacuolar acidification and stomatal closure during leaf senescence. Vacuolar acidification was suppressed by the PIKfyve inhibitor in 35S:AtVPS34-YFP Arabidopsis during MeJA-induced leaf senescence, but the decrease was lower than that in YFP-labeled Arabidopsis. These results suggest that PI3K promotes V-ATPase activation and consequently induces vacuolar acidification and stomatal closure, thereby delaying MeJA-induced leaf senescence.
Leaf senescence is a complex, highly ordered process that occurs toward the end of leaf development (Lim et al., 2007). Although leaf senescence is an age-dependent process, it is also induced by diverse biotic and abiotic stresses (Wingler et al., 2006). Leaf senescence is regulated by several plant hormones, such as jasmonic acid, ethylene, abscisic acid (ABA), and cytokinins (Lim et al., 2007). During leaf senescence, metabolism is significantly altered; for example, there is a switch to carbon assimilation to catabolize energy resources and enhance chlorophyll degradation (Yue et al., 2012). In addition, the water loss of senescing leaves is more rapid than that of nonsenescing leaves (Zhang et al., 2012). Water control is primarily dependent on stomatal movement, which can be regulated by vacuolar acidification (Bak et al., 2013). In plants, three distinct proton pumps can generate pH gradients (Gaxiola et al., 2007). Vacuolar H+-ATPase (V-ATPase) is a key proton pump that is localized in tonoplasts and consists of 14 different subunits and two subcomplexes. The peripheral V1 domain is composed of eight distinct subunits (A3, B3, C1, D1, E2, F1, G2, and H1-2) and functions in ATP hydrolysis, whereas the integral V0 domain consists of six different subunits (a1, d1, en, c4-5, c′1, and c′′1) and is responsible for proton translocation (Cipriano et al., 2008). V-ATPase is involved in various physiological events and stress responses, including embryonic development, lateral root development, membrane trafficking, nutrient storage, and environmental stress tolerance (Dettmer et al., 2005; Marshansky and Futai, 2008; Krebs et al., 2010). The major mechanism of V-ATPase regulation is the reversible assembly/disassembly of the V0 and V1 sectors.
In renal epithelial cells induced with Glc, phosphoinositide 3-kinase type I (PI3K) promotes the assembly and translocation of V-ATPase (Sautin et al., 2005). PI3K activated by influenza A virus also interacts with the E subunit of the V1 domain of V-ATPase (Marjuki et al., 2011). In mammalian cells, there are three types of PI3K with distinct substrate specificities. The generation of phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) by PI3K type I activates a highly complex downstream signaling network that regulates different cellular responses. The most recognized downstream target of PtdIns(3,4,5)P3 is protein kinase B, which is involved in the regulation of cell growth, proliferation, survival, and metabolism, as well as in protein transcription and synthesis (Foster et al., 2003). Other well-characterized proteins can also function as downstream targets of PtdIns(3,4,5)P3 production. These proteins include Tyr kinase Tec, phospholipase PLCγ, most small GTPase activators, and V-ATPase (Hirsch et al., 2007; Sautin et al., 2005). PI3K type II uses phosphatidylinositol and phosphatidylinositol-4-phosphate as substrates and suppresses apoptotic cell death, cytoskeletal organization, exocytosis, and insulin signaling (Lee et al., 2010). PI3K type III, which is the only type of PI3K identified in plants, phosphorylates the d-3 position of phosphoinositides; nevertheless, the function of PI3K type III appears to be more comprehensive and multifaceted than previously reported (Bunney et al., 2000). In Arabidopsis, PI3K type III promotes protein trafficking and endocytosis as well as intracellular ROS production, as reflected in the enhanced stomatal closing, F-actin reorganization, and pollen development observed upon PI3K type III activation (Park et al., 2003; Choi et al., 2008; Lee et al., 2008). PI3K type III is also involved in nuclear functions (Bunney et al., 2000). Although various studies have been conducted on PI3K, information on the role of PI3K in leaf senescence is lacking.
Sugars accumulate in senescent Arabidopsis leaves (Wingler et al., 2012), and the accumulative signals of these compounds initiate the PI3K-V-ATPase pathway in senescent cells. The regulatory action of PI3K on V-ATPase has not been confirmed in plants, but recent reports have indicated a possible relationship between PI3K and V-ATPase (Aparicio-Fabre et al., 2006; daSilva et al., 2005; Ma et al., 2011; Schumacher and Krebs, 2010). V-ATPase is localized in various subcellular organelles in plants, such as the endoplasmic reticulum, Golgi apparatus, and vacuoles (Schumacher and Krebs, 2010). In each location, the targeting of V-ATPase to different membranes via vesicular trafficking is an important mechanism (Marshansky and Futai, 2008). PI3K type III plays a vital role in the trafficking of proteins to and from vacuoles. Treatment with PI3K inhibitors blocks the retrograde transport of vacuolar sorting receptors to the trans-Golgi (TGN), thereby resulting in vacuolation or swelling of the prevacuolar compartment (daSilva et al., 2005). PI3P is present in various compartments of plant cells, and it plays a role in the vacuolar trafficking of sporamin, which is normally localized in the large central vacuole (Kim et al., 2001). These studies have indicated that PI3K may regulate the transport of V-ATPase subunits from the TGN to the tonoplasts. V-ATPase activity is essential for endocytic and secretory trafficking in Arabidopsis (Dettmer et al., 2006), and V-ATPase may be regulated by PI3K in vacuolar trafficking. Structural biology studies have further demonstrated the possible relationship between V-ATPase and PI3K. The N-terminal domain of the V-ATPase subunit B in Arabidopsis contains a profilin-like motif (Ma et al., 2011). PI3K interacts with profilin in the poly-l-Pro-binding regions (Aparicio-Fabre et al., 2006) and with the V-ATPase subunit B. These interactions regulate the assembly of the V-ATPase complex and the activity of V-ATPase.
Mammalian PI3K type I promotes the assembly and translocation of V-ATPase (Sautin et al., 2005). Plant V-ATPase down-regulation results in an enhanced stomatal aperture (Allen et al., 2000; Zhang et al., 2013), which leads to rapid water loss and premature leaf senescence (Zhang et al., 2012). Given these findings, we speculated that leaf senescence proceeds in the following order: Glc-PI3K-activation of V-ATPase-vacuolar acidification-stomatal closure-reduction of water loss-alleviation of leaf senescence.
Wortmannin and LY294002 are two pharmacological PI3K inhibitors used to elucidate the function of PI3Ks. Wortmannin, a fungal metabolite, dose-dependently targets PI3K and PI4K (Takáč et al., 2012). At the subcellular level, wortmannin inhibits vacuolar trafficking and dilatation of the cell wall as well as the fusion, swelling, and vacuolization of the multivesicular body (Jaillais et al., 2008; Pimpl et al., 2006). LY294002, which is derived from the flavonoid quercetin, competes with ATP and binds to Lys residues in the ATP-binding pocket of PI3Ks (Walker et al., 2000). Generally, LY294002 suppresses PI3K-related kinases, such as DNA-dependent protein kinase and the target of rapamycin (Brunn et al., 1996). LY294002 is considered to be more selective than wortmannin (Leprince et al., 2014).
In this work, we found that the stomatal aperture increased in methyl jasmonate (MeJA)-induced senescence, and that inhibition of vacuolar acidification promoted stomatal opening and accelerated senescence. The activity of V-ATPase and gene expression of its subunits was also increased in MeJA-induced leaf senescence. Treatment with PI3K inhibitors or down-regulation of AtVPS34 gene expression further suppressed V-ATPase activation in MeJA-induced leaf senescence. The results of yeast two-hybrid and bimolecular fluorescence complementation (BiFC) assays demonstrated that PI3K bound to the V-ATPase subunit B2 (VHA-B2) in vitro and in vivo. Vacuolar pH and the stomatal aperture also increased in the pi3k mutant and in treatments with PI3K inhibitors. Our findings demonstrate that PI3K binds to VHA-B2 and promotes the activation of V-ATPase, resulting in vacuolar acidification and stomatal closure, thereby delaying MeJA-induced leaf senescence.
RESULTS
Enhanced Stomatal Aperture during MeJA-Induced Leaf Senescence
Detached wild-type Arabidopsis leaves were treated with 50 μM MeJA for 3 d and observed using standard microscopy and scanning electron microscopy (SEM) to assess the stomatal aperture during leaf senescence. The stomatal aperture increased with increasing treatment time (Fig. 1, A and B). A similar increase in the stomatal aperture was also observed via SEM (Supplemental Fig. S1). Given that vacuolar acidification is important in regulating stomatal movement, we determined the pH of the stomata by using acridine orange (OH), a pH-sensitive fluorescent dye. The pH was lower in the closing stomata than in the opening stomata (Fig. 1B). These results indicated a relationship between increased stomatal aperture and vacuolar alkalization during MeJA-induced leaf senescence.
Figure 1.
An increased stomatal aperture during leaf senescence. A, An increased stomatal aperture during leaf senescence. During the process of MeJA-induced senescence (1–3 d), the stomatal aperture was assayed by microscopy. Bars = 20 μm. Stomata are marked by arrowhead. Control means untreated with MeJA. B, Stomatal aperture were determined by microscopy and given as width to length ratio (n > 30). Asterisks (*) indicate significant difference by t test from the control treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test). C, The link between stomatal aperture and vacuolar acidification. Vacuolar acidification was observed using AO. After treatment with 50 μM MeJA for 3 d, the guard cells were stained with 50 μM AO for 100 min. A model no. LSM510 Meta microscope (Carl Zeiss) was used to detect the fluorescence ratio. The R/G ratio is displayed in pseudocolor. Regions with the lowest R/G ratio are in blue, while those with the highest R/G ratio (more acidic) are in red. The stomata in the square frame were enlarged in the right panel as shown in type 1 and type 2. Bars = 20 μm or 5 μm.
V-ATPase Inhibitor Accelerates Vacuolar Alkalization and Stomatal Opening, Thereby Promoting MeJA-Induced Leaf Senescence
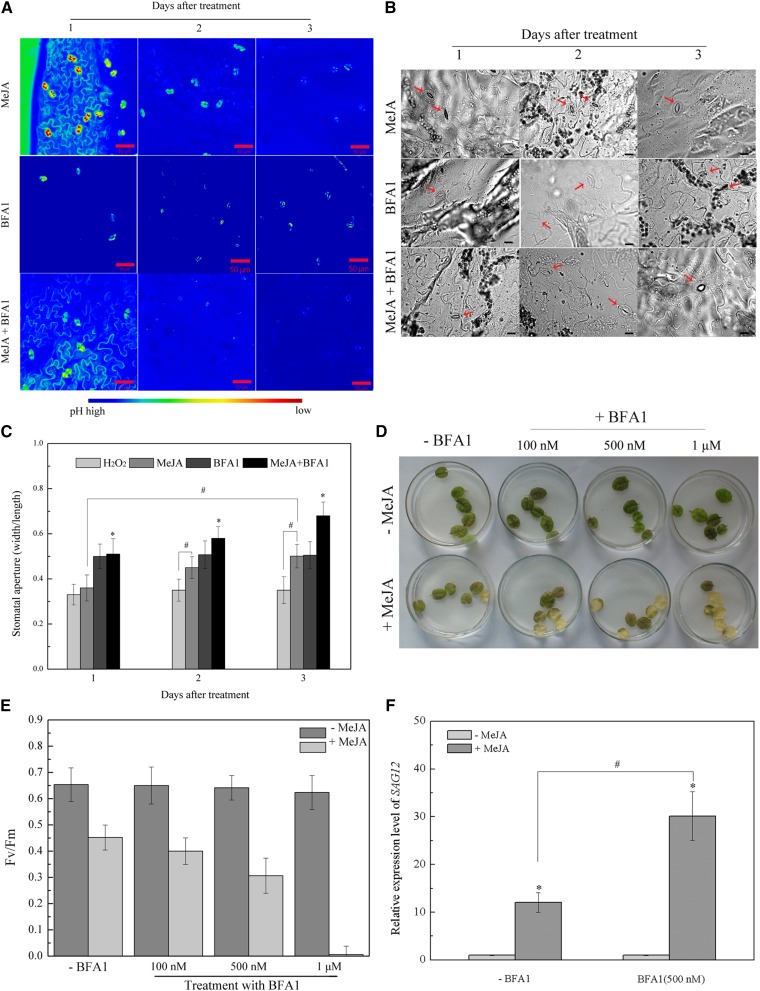
Bafilomycin A1 (BFA1), a V-ATPase-specific inhibitor, was used to elucidate the role of vacuolar alkalization in stomatal movement during leaf senescence. Treatment with 500 nm BFA1 accelerated vacuolar alkalization in leaf senescence induced by 50 μM MeJA in wild-type Arabidopsis (Fig. 2A). After 3 d of MeJA treatment, the stomatal aperture of leaves treated with 500 nm BFA1 increased by approximately 1.4-fold compared to leaves treated with only MeJA (Fig. 2, B and C). As concentrations of BFA1 increased (100 nM, 500 nM, and 1 μM), we observed more intense yellowing of leaves than that of controls treated with 50 μM MeJA for 3 d (Fig. 2D). The photochemical efficiency demonstrated a similar trend to that obtained in the photosystem II (PSII) assay (Fig. 2E). Conversely, leaf yellowing and photochemical efficiency were not significantly different in the series containing BFA1 without MeJA treatment (Fig. 2, D and E). Furthermore, treatment with BFA1 promoted gene expression of SAG12 in MeJA-induced leaf senescence (Fig. 2F). These results implied that vacuolar alkalization regulates stomatal opening and promotes MeJA-induced leaf senescence.
Figure 2.
Vacuolar alkalization induced by V-ATPase inhibitor promotes stomatal opening and accelerates MeJA-induced leaf senescence. A, Vacuolar acidification was suppressed by 500 nm Bafilomycins A1 (BFA1) during the process of 50 µM MeJA-induced leaf senescence. The vacuolar pH was determined by AO. B, C, Stomatal aperture were determined by microscopy (n > 30). The detached Arabidopsis leaves were treated with 50 μm MeJA supplemented with or without 500 nm BFA1. Asterisks (*) indicate significant difference by t test from the MeJA treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test). Stomata are marked by arrowhead. D, Photographs showed leaves with different concentrations of BFA1 (100 nM; 500 nM; 1 μM) treatment in 50 μM MeJA-induced leaf senescence in wild-type Arabidopsis for 3 d. E, Photochemical efficiency of detached leaves was shown. Each bar represents three replications. F, Determination of gene expression of SAG12. The detached Arabidopsis leaves were treated with 50 μm MeJA supplemented with or without 500 nm BFA1. Gene expression of SAG12 was determined by qRT-PCR. UBQ10 was used as an internal control. Asterisks (*) indicate significant difference by t test from no MeJA treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test). Fv/Fm = maximum quantum yield of PSII electron transport (maximum variable fluorescence/maximum yield of fluorescence).
Increased V-ATPase Activity and Gene Expression of V-ATPase Subunits during MeJA-Induced Leaf Senescence
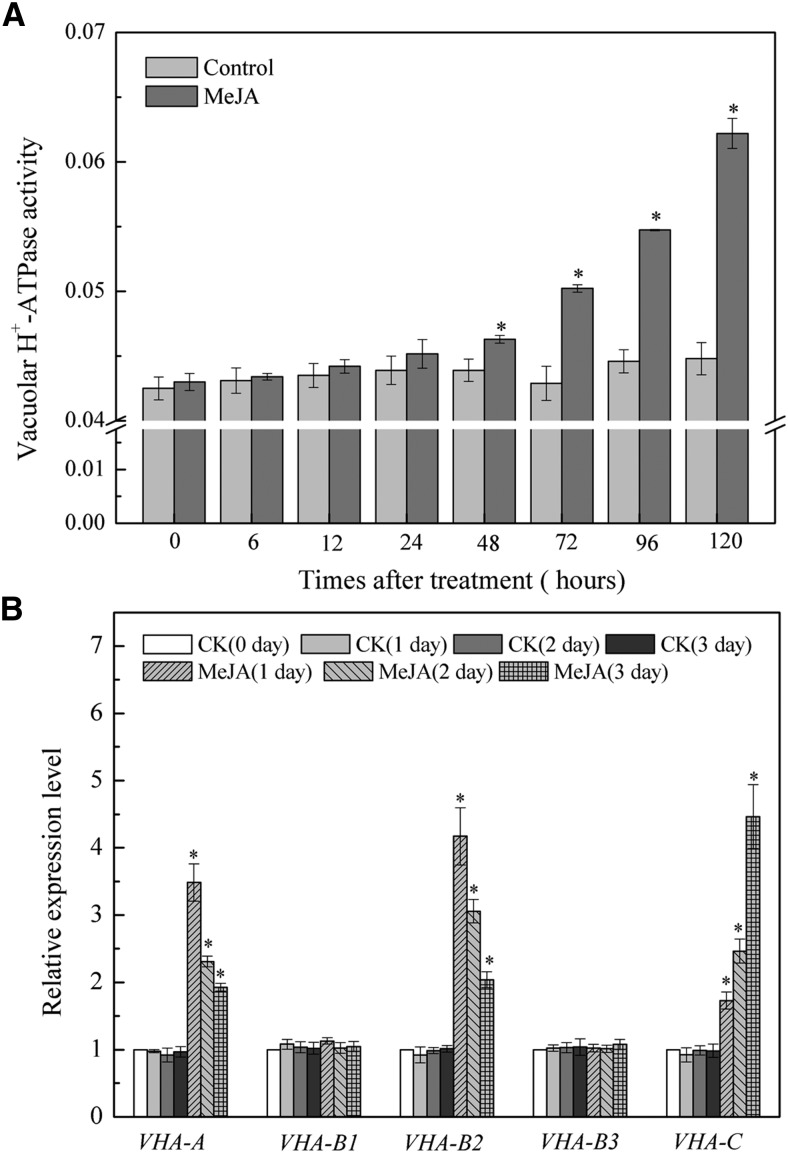
V-ATPase activity and the gene expression patterns of the V-ATPase subunits were examined to explore the mechanisms of MeJA-induced leaf senescence. Detached wild-type Arabidopsis leaves were treated with 50 μM MeJA for 5 d. V-ATPase activity was measured with concanamycin A (Con A), which is a sensitive indicator of ATP hydrolysis. As shown in Figure 3A, the activity of V-ATPase in tonoplasts increased continuously during MeJA-induced leaf senescence in wild-type Arabidopsis. We also examined V-ATPase activity in microsomal membrane fractions. A similar increase in the activity of V-ATPase was observed in leaves treated with MeJA compared to control leaves (Supplemental Fig. S2). Gene expression of the V-ATPase subunits was also determined upon treatment with 50 μM MeJA. The results showed that gene expression of V-ATPase subunit A (VHA-A), subunit C (VHA-C), and VHA-B2 were up-regulated in senescent leaves, but their expression patterns differed. The gene expression of VHA-A and VHA-B2 peaked on the first day after treatment and then decreased with increasing treatment time, whereas the gene expression of VHA-C continuously increased (Fig. 3B). Hence, MeJA-induced senescence in Arabidopsis correlates with elevated V-ATPase activity and vha gene expression.
Figure 3.
The activity of V-ATPase and genes expression of V-ATPase subunits during MeJA-induced leaf senescence. A, The activity of V-ATPase in leaf senescence. After treatment with 50 μM MeJA, the activity of V-ATPase was calculated as the difference measured in the absence or presence of 100 nm Con A in the required time. Each bar represents three replications. Asterisks (*) indicate significant difference by t test from the control in the indicated times (*, P < 0.05). Control means untreated with MeJA. B, Genes expression of VHA-A, VHA-B1, VHA-B2, VHA-B3, and VHA-C in leaf senescence. The detached Arabidopsis leaves were treated with 50 μM MeJA, and genes expression was determined in the indicated time points. Arabidopsis UBQ10 was used as an internal control. Expression levels for each gene were normalized to control treatment (0 d) of the wild-type plant. Asterisks (*) indicate significant difference by t test from the control in the indicated days (*, P < 0.05). CK, untreated with MeJA.
Given that sugars also accumulate in senescent Arabidopsis leaves, we treated 3-week-old detached leaves with Glc or mannitol at two different concentrations (0.5% or 1.8%) to detect the effect of Glc signaling on V-ATPase activation (Supplemental Fig. S3A). After 6 h, V-ATPase activity was up-regulated with an increasing concentration of Glc but was not significantly different from leaves treated with mannitol. Additionally, the activity of V-ATPase was measured at different times after 1.8% Glc or mannitol treatment (Supplemental Fig. S3B). The activity of V-ATPase increased with increasing treatment times.
Down-Regulation of PI3K Activity Inhibits the Activity of V-ATPase in MeJA-Induced Leaf Senescence
The role of PI3K type III in the regulation of V-ATPase activity was also determined in Arabidopsis. LY294002 (30 μM) was used in treatments with external 1.8% Glc. The results showed that LY294002 suppressed V-ATPase activation induced by Glc in Arabidopsis leaves (Supplemental Fig. S4). The gene expression of AtVPS34 encoding PI3K was also determined as senescence progressed. The gene expression of AtVPS34 increased in wild-type Arabidopsis after treatment with 50 μM MeJA for 1 d (Supplemental Fig. S5), which indicated that PI3K was expressed during MeJA-induced senescence in wild-type Arabidopsis.
Two pi3k mutants (pi3k-1: SALK_007281 and pi3k-2: GABI_418H02) and the PI3K inhibitors LY294002 (30 μM) and wortmannin (10 μM) were used to down-regulate the activity of PI3K type III in Arabidopsis. Gene expression of AtVPS34 was significantly down-regulated in the pi3k mutants compared to wild-type plants (Supplemental Fig. S6A). Consistently with previous results, these findings showed that MeJA treatment promotes V-ATPase activity (Fig. 4, A and B; Supplemental Fig. S7). Treatment with both PI3K inhibitors (30 μm LY294002 or 10 μm wortmannin) and mutation of AtVPS34 suppressed V-ATPase activity in detached and attached leaves during MeJA-induced senescence. In treatments without MeJA, V-ATPase activity was not significantly different in pi3k mutants or among treatments with the PI3K inhibitors (30 μm LY294002 or 10 μm wortmannin) compared with the activity in wild-type plants and under control conditions, respectively (Fig. 4; Supplemental Fig. S7). These results show that the PI3K gene is required for full activation of V-ATPase during MeJA-induced senescence.
Figure 4.
Down-regulation of PI3K activity suppresses the activity of V-ATPase in MeJA-induced leaf senescence. Determination of V-ATPase activity in treatment with PI3K inhibitors (A) or pi3k mutants (B) with or without 50 μM MeJA treatment. After treatment with 50 μM MeJA for 3 d or not, the activity of V-ATPase was assayed. Each bar represents three replications. Asterisks (*) indicate significant difference by t test from no MeJA treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test).
PI3K and V-ATPase Subunit B Interact in Vitro and in Vivo
Two different methods were used to demonstrate an interaction between PI3K and the V-ATPase subunits and to investigate the mechanism of PI3K in the regulation of V-ATPase activity. The physical interaction between PI3K and members of the V-ATPase subunits was examined in vitro. Analyses using yeast two-hybrid with full-length PI3K and full-length V-ATPase subunit proteins indicated that PI3K interacted with the VHA-B family, but no significant interactions between PI3K and VHA-A or VHA-C were detected (Fig. 5). This finding suggested that PI3K binds to the VHA-B family in vitro.
Figure 5.
The interaction of PI3K and V-ATPase subunit B examined by yeast two-hybrid analysis. The recombined bait plasmids, prey plasmids constructs, were cotransformed into the yeast strain EGY48 using LiAc-mediated yeast transformation. Assay of β-galactosidase activity on induction or no-induction plates was carried out 3 d after transformation. Quantification of β-galactosidase activity was measured by using ONPG as a substrate. The results shown represented the mean values of 10 independent β-galactosidase assays, and the error bars represent SE. Asterisks (*) indicate significant difference by t test from negative control (*, P < 0.05).
The V-ATPase subunit B2 was selected as the representative sample to examine the interaction between PI3K and V-ATPase subunits in vivo, through a BiFC assay. Strong fluorescence signals were observed in the tonoplasts of guard cells coexpressing PI3K-cYFP (C-terminal fragment of the yellow fluorescence protein) and VHA-B2-nYFP (N-terminal fragment of the yellow fluorescence protein) in tobacco leaf epidermis (Fig. 6).
Figure 6.
PI3K interacts with VHA-B2 in vivo. BiFC analysis of PI3K and VHA-B2 interacted in tobacco. Vectors containing the indicated constructs were cotransformed into Nicotiana benthamiana leaves. Both YFP fluorescence images merged with light view images (lower panel), and YFP-alone fluorescence images (upper panel) were shown. Bar = 5 μm. Pictures represent typical examples.
Down-Regulation of PI3K Activity Suppresses Vacuole Acidification and Stomatal Closing in MeJA-Induced Leaf Senescence
Given the key role of PI3K in V-ATPase activation and V-ATPase in vacuolar acidification, we examined the effect of PI3K on vacuolar acidification using AO. After incubation with 50 μM MeJA for 3 d, pi3k mutants and samples treated with PI3K inhibitors (30 μm LY294002 or 10 μm wortmannin) showed reduced vacuolar acidification, which differed from that under control conditions (Fig. 7, A and B). We also examined the stomatal aperture in detached leaves after MeJA treatment for 3 d pi3k mutants, and samples subjected to the PI3K inhibitor (30 μm LY294002 or 10 μm wortmannin) treatments showed increased stomatal apertures compared to wild-type Arabidopsis and samples under control conditions (Fig. 7; Supplemental Fig. S8). These data indicated that the down-regulation of PI3K activity inhibits vacuolar acidification and stomatal closing in MeJA-induced leaf senescence.
Figure 7.
Down-regulation of PI3K activity suppressed the vacuolar acidification and stomatal closing during MeJA-induced leaf senescence. A disturbance of pH value decrease in pi3k mutants (A) or PI3K inhibitor treatment (B) under leaf senescence, as determined by AO. Representative R/G ratio images of Arabidopsis guard cells were shown after treatment with 50 μM MeJA in pi3k mutants and wild type. Bar = 50 μm. C, D, The stomatal aperture was assayed in pi3k mutants or supplemented with PI3K inhibitors in 50 μM MeJA treatment for 3 d by microscopy. Bars = 20 μm. Stomata are marked by arrowhead. E, F, The stomatal aperture was assayed (n > 30). Asterisks (*) indicates significant difference by t test from no MeJA treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test).
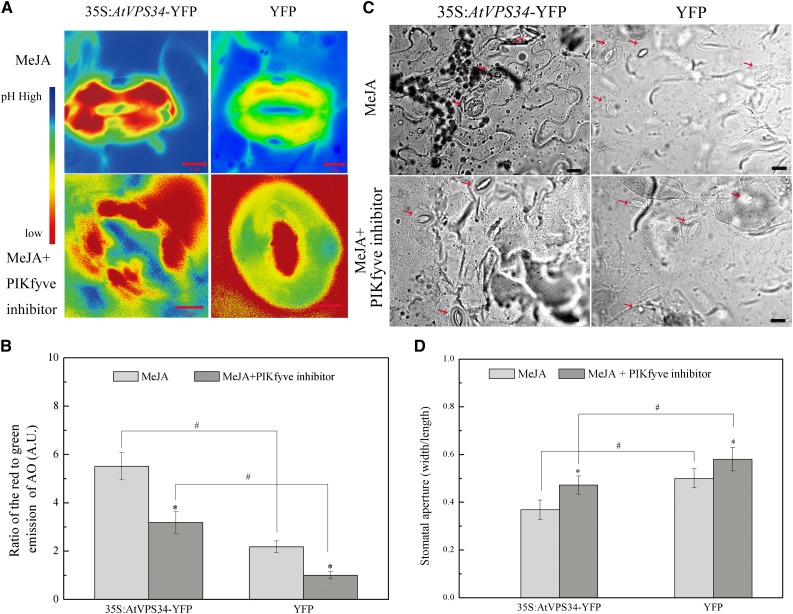
The PIKfyve inhibitor YM201636 was used to exclude the effect of phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2) on vacuolar acidification and stomatal movement. We stably expressed YFP-fused PI3K protein in Arabidopsis by Agrobacterium tumefaciens-mediated transformation, and named the resultant plants 35S:AtVPS34-YFP Arabidopsis. Increased protein levels of PI3K were found in 35S:AtVPS34-YFP Arabidopsis compared to YFP Arabidopsis (Supplemental Fig. S9). In Figure 8, vacuolar acidification was suppressed in leaves treated with the PIKfyve inhibitor in 35S:AtVPS34-YFP Arabidopsis during MeJA-induced senescence. Nevertheless, the decrease was lower than that in YFP Arabidopsis. Additionally, the stomatal aperture was examined. When leaves were treated with only MeJA, the stomatal aperture of 35S:AtVPS34-YFP Arabidopsis was 0.37, whereas the stomatal aperture of YFP Arabidopsis was 0.47. Under both MeJA and PIKfyve inhibitor treatments, the stomatal aperture of 35S:AtVPS34-YFP Arabidopsis was 0.50, whereas the stomatal aperture of YFP Arabidopsis was 0.58. These findings were consistent with the results for vacuolar acidification.
Figure 8.
The effect of PIKfyve inhibitor on vacuolar acidification and stomatal movement in 35S:AtVPS34-YFP Arabidopsis during MeJA-induced senescence. A, The detached Arabidopsis leaves from 35S:AtVPS34-YFP and YFP Arabidopsis leaves were treated with 1 μM PIKfyve inhibitor for 1 d supplemented with 50 μM MeJA. Vacuolar acidification was observed using AO. B, The extent of vacuolar acidification was quantified as the means of the R/G ratios. Each bar represents three replications (n ≥ 30). C, The stomatal aperture was assayed in YFP transgenic and 35S:AtVPS34-YFP transgenic Arabidopsis after 50 μM MeJA treatment for 3 d supplemented with 1 μm PIKfyve inhibitor or not by microscopy. Bars = 20 μm. Stomata are marked by arrowhead. D, The stomatal aperture was assayed (n > 30). Asterisks (*) indicate significant difference by t test from only MeJA treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test).
Down-Regulation of PI3K Activity Accelerates MeJA-Induced Leaf Senescence
The pi3k mutants and two PI3K inhibitors (30 μm LY294002 or 10 μm wortmannin) were used to further explore the effect of PI3K on leaf senescence. After detached and attached leaves were treated with 50 μM MeJA for 3 d, photochemical efficiency and chlorophyll content decreased, whereas SAG12 gene expression increased in pi3k mutants compared to wild-type Arabidopsis (Fig. 9; Supplemental Fig. S7). Similar results were also observed in leaves treated with PI3K inhibitors (30 μm LY294002 or 10 μm wortmannin; Fig. 9).
Figure 9.
PI3K promotes the MeJA-induced leaf senescence. Photographs show leaves after incubation with 50 μM MeJA in pi3k mutants (A) or treatment with PI3K inhibitors (B) for 3 d. Photochemical efficiency (C, F), chlorophyll content (D, G), and SAG12 gene expression (E, H) of detached leaves were shown. UBQ10 was used as internal control. Each bar represents three replications. Asterisks (*) indicates significant difference by t test from no MeJA treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test). Fv/Fm = maximum quantum yield of PSII electron transport (maximum variable fluorescence/maximum yield of fluorescence).
PI3K Is Involved in the Regulation of Dark-Induced Senescence but Does Not Function through the Regulation of V-ATPase Activity
The effect of PI3K on dark-induced senescence was also investigated. Mutation of the AtVPS34 gene (pi3k-1) resulted in more pronounced yellowing than in wild-type Arabidopsis in dark-induced senescence (Fig. 10A). Similar observations were made for chlorophyll content and the photochemical efficiency of PSII (Fig. 10, B and C). Interestingly, V-ATPase activity significantly increased in leaves in which senescence was induced with dark treatment compared to leaves under control conditions. However, V-ATPase activity minimally decreased in pi3k-1 mutants compared with wild-type plants (Fig. 10D). Additionally, vacuolar acidification was examined in detached leaves under dark treatment. Vacuolar acidification and stomatal closing were enhanced (Supplemental Fig. S10), but vacuolar acidification did not significantly differ between the pi3k-1 mutants and wild-type plants in dark-induced senescence (Fig. 10E). Similarly, the stomatal aperture did not differ between the pi3k-1 mutants and wild-type plants when senescence was induced with or without dark treatment (Fig. 10F; Supplemental Fig. S11).
Figure 10.
PI3K is involved in dark-induced leaf senescence but not through the regulation of V-ATPase activity. A, The detached Arabidopsis leaves of pi3k-1 mutant and wild-type Arabidopsis were put into dark for 3 d, and the phenotype of leaves was taken. Photochemical efficiency (B) and chlorophyll content (C) of the detached leaves were shown. D, Determination of V-ATPase activity in pi3k-1 mutant and wild-type Arabidopsis after dark treatment for 3 d. E, Vacuolar acidification was observed using AO in pi3k-1 mutant and wild-type Arabidopsis leaves for 3 d after dark treatment. F, The alteration of stomatal aperture was examined by microscopy. Each bar represents three replications. Asterisks (*) indicate significant difference by t test from no dark treatment (*, P < 0.05). Hash marks (#) indicate statistically significant differences between indicated samples (P < 0.05; Student’s t test). Fv/Fm = maximum quantum yield of PSII electron transport (maximum variable fluorescence/maximum yield of fluorescence).
DISCUSSION
In this study, we investigated the molecular mechanism underlying the function of PI3K in the delay of MeJA-induced leaf senescence. The down-regulation of AtVPS34 gene expression or treatment with PI3K inhibitors suppressed the activation of V-ATPase in MeJA-induced leaf senescence. In vitro and in vivo interaction analyses revealed that PI3K bound to VHA-B2. Treatment with PI3K or V-ATPase inhibitors also suppressed vacuolar acidification and stomatal closing. These results indicated that PI3K promotes vacuolar acidification and stomatal closing, thereby delaying leaf senescence.
PI3K Regulates the Activity of V-ATPase in MeJA-Induced Leaf Senescence but Not in Dark-Induced Leaf Senescence
PI3K type III, a hetero-oligomeric complex, consists of the catalytic subunit VPS34, putative protein kinase VPS15, and several accessory subunits, including Beclin 1 (Backer, 2008). As a catalytic subunit, VPS34 produces PI3P by phosphorylating the d-3 position of phosphoinositides. The localization and activity of VPS34 are regulated by other subunits. Although the principal function of PI3K type III is the production of PI3P on restricted membrane domains, its subunits may exhibit additional functions. In Phaseolus vulgaris, PI3K type III interacts with profilin; however, this interaction depends on the Tyr phosphorylation status within the Pro-rich binding domains (Aparicio-Fabre et al., 2006). The structural analysis of V-ATPase subunits indicated that the subunits B and C exhibited similar structural features to profilin (Ma et al., 2011). In renal epithelial cells, PI3K type I regulates V-ATPase assembly and translocation (Sautin et al., 2005). PI3K type III, which is the only type of PI3K identified in plants, phosphorylates the d-3 position of phosphoinositides; nevertheless, the function of PI3K type III appears to be more comprehensive and multifaceted than previously reported (Bunney et al., 2000). For instance, PI3K type I mediates the activation of MAPK via trimeric G protein-coupled receptors in animals (Lopez-Ilasaca et al., 1997). PI3K type III activity is also required for Cd2+-induced MAPK activation in rice roots (Yeh et al., 2007). Nevertheless, the relationship between PI3K type III and V-ATPase subunits in plants requires additional study.
The activity of V-ATPase was lower in the pi3k mutants and in samples treated with PI3K inhibitors in the presence of 50 μM MeJA than under control conditions. This finding indicated that PI3K regulates the activity of V-ATPase in senescing Arabidopsis leaves. Wortmannin suppressed the activation of V-ATPase and stomatal closing severely; this effect may have been due to the diverse inhibition on PIK activity (Fig. 4A). Moreover, no significant difference was detected between wild-type Arabidopsis and the pi3k mutants without MeJA treatment (Fig. 4B; Supplemental Fig. S7E).
In renal epithelial cells, Glc stimulation initiates PI3K type I-dependent signaling and consequently promotes V-ATPase-dependent acidification of endosomes as well as V-ATPase assembly and trafficking (Sautin et al., 2005). In addition, leaf senescence is induced by high light intensity, phytohormones, and long-day treatment duration, and these factors are associated with hexose accumulation (Diaz et al., 2005; Wingler et al., 2006; Rolland et al., 2006). A previous study has revealed that the V-ATPase subunit B is involved in Glc signaling in Arabidopsis (Cho et al., 2006). Considering these findings, we speculated that the accumulated sugar signaling might initiate PI3K signaling and promote the activation of V-ATPase during leaf senescence. To test this hypothesis, we used a PI3K inhibitor in V-ATPase activation stimulated by Glc in Arabidopsis leaves. The results showed that LY294002 treatment suppressed Glc-activated V-ATPase (Supplemental Fig. S4). Dark-induced leaf senescence was also achieved as indicated by the low levels of hexoses and disaccharides in the isolated Arabidopsis leaves stored under dark conditions (Quirino et al., 2000; van Doorn, 2008). Although vacuolar acidification is necessary for stomatal closing in dark-induced senescence (Supplemental Fig. S10), vacuolar acidification and stomatal aperture did not significantly differ between the pi3k-1 mutants and wild-type Arabidopsis (Fig. 10). A similar observation was made for the determination of V-ATPase activity in dark-induced senescence (Fig. 10). The blockage of sugar signaling might possibly have abolished initiation of the PI3K-V-ATPase pathway in leaf senescence. However, the upstream signaling mechanism linking Glc to PI3K remains unclear.
Because Glc and Glc-6-phosphate are well-known physiologically relevant allosteric regulators of a variety of enzymes (Bollen et al., 1998), they may be required for allosteric activation of the Vps34-VHA-B2 complex. Although PI3K is involved in the regulation of dark-induced senescence, Arabidopsis Vps34 may exist in different complexes and participate in different cellular functions. An autophagy-related function of PI3K class III may exist in dark-induced senescence. AMPK (homologous to Arabidopsis SnRK) is a key cellular energy sensor that maintains energy homeostasis upon Glc starvation (Hardie, 2007). In Glc-starved cells, AMPK inhibits the nonautophagy Vps34 complex by phosphorylating T163/S165 in Vps34, thereby inhibiting overall PI3P production and protecting cells from starvation. In addition, AMPK activates the proautophagy Vps34 complex by phosphorylating S91/S94 in Beclin1 to induce autophagy (Kim et al., 2013). The increased V-ATPase activity in pi3k-1 mutants during dark-induced senescence indicated that other regulators promote V-ATPase activation during leaf senescence. A previous study has revealed that dark treatment results in increased stability of V-ATPase (Schnitzer et al., 2011). Vacuolar acidification is accompanied by cytosolic alkalization of guard cells during ABA-induced stomatal closure (Bak et al., 2013). Ma et al. (2013) has revealed that cytosolic alkalization-mediated H2O2 and NO production are involved in darkness-induced stomatal closure in Vicia faba. However, the underlying mechanism that induces the alteration in vacuolar pH and the activation of V-ATPase in darkness remains unknown. Regulator of H+-ATPase of vacuolar and endosomal membranes (RAVE) is a V-ATPase exclusive assembly factor, but the formation of the RAVE-C and RAVE-V1 subcomplexes in the cytosol is not Glc-dependent (Parra et al., 2014). Interestingly, a recent study revealed that, unlike the change in assembly of the mammalian V-ATPase in response to other stimuli, the amino acid-dependent change in assembly does not appear to involve PI3K (Stransky and Forgac, 2015). Therefore, PI3K can be a significant regulator of V-ATPase activity but not an exclusive regulator of leaf senescence.
PI3K Binds to the V-ATPase Subunit B
Nakamura et al. (1997) has revealed that V-ATPase colocalizes in osteoclasts with p85 regulated by PI3K. Furthermore, Vps34p of the human pathogenic yeast Candida albicans interacts with the putative vacuolar H+-ATPase subunit Vma7p (Eck et al., 2005). The V1 subunit E protein of V-ATPase has recently been reported to be immunoprecipitated by an antibody specific for P-PI3K (p85α) during endosomal acidification induced by influenza A virus (Marjuki et al., 2011). Hence, we speculated that PI3K might interact with the V-ATPase subunits in plants, thereby promoting the activation of V-ATPase. In P. vulgaris, vesicular trafficking mediated by PI3K may rely on dynamic changes in the actin cytoskeleton because profilin, a regulator of actin dynamics, binds to PI3K in a phosphorylation-dependent manner (Aparicio-Fabre et al., 2006). Recent studies have additionally indicated that V-ATPase directly interacts with the actin cytoskeleton through actin-binding sites located in the N-terminal domain of subunit B. In this subunit, the N-terminal domains of plants contain a profilin-like motif (Ma et al., 2011). Thus, the V-ATPase subunit B may interact with PI3K type III in plants.
Because results of the yeast two-hybrid assay showed that PI3K bound to the three types of VHA-B (Fig. 5), we used the V-ATPase subunit B2 as the representative subunit B for the BiFC assay. PI3K interacted with the V-ATPase subunit B2 on the tonoplast of tobacco guard cells (Fig. 6). The interaction between VHA-B2 and PI3K may have promoted the assembly of V-ATPase on the tonoplast, but this finding should be confirmed in future studies. V-ATPase assembly in the presence of a full complement of subunits occurred via a concerted assembly pathway in which early associations between the V1 and V0 subunits preceded the full assembly of either complex rather than associations occurring with preassembled V1 and V0 sectors (Kane, 2006). Thus, the effect of PI3K on the colocalization efficiency of the V1 and V0 domains should be investigated in more detail. Additionally, the indirect role of PI3K on V-ATPase activation cannot be ruled out, especially a general trafficking role for PI3K in the translocation of VHA-B2 to the vacuoles. Additional experiments are necessary to confirm these possibilities; in particular, the effect of PI3K on the transport of VHA-B2 to the tonoplast should be examined. However, the domain of VHA-B that binds to PI3K is unknown. The profilin-like motif of VHA-B may be the domain that binds to PI3K, and this motif has also been demonstrated to bind to F-actin (Aparicio-Fabre et al., 2006). The alteration in subunit composition may also be attributed to changes in enzymatic properties (Ratajczak et al., 1998). The vacuoles isolated from the vps34∆ and vps15∆ have been reported to reduce the V-ATPase activity and decrease the levels of several V-ATPase subunits (Sambade et al., 2005). Given that PI3K is involved in nuclear function (Bunney et al., 2000), the PI3K-induced change in the abundance of the V-ATPase subunits may be another regulatory mechanism of V-ATPase activity.
Downstream of the PI3K-V-ATPase Pathway in MeJA-Induced Leaf Senescence
Water loss is accelerated in senescent leaves compared to young leaves (Jordan et al., 1975), and stomatal activity plays an important role in this process (Zhang et al., 2012). To control water transpiration, guard cells generally live longer than mesophyll cells in senescing leaves and remain functional until the late stages of senescence (Zeiger and Schwartz, 1982; Peñarrubia and Moreno, 2001). Zhang et al. (2012) have found that SAG113, which encodes a protein phosphatase that belongs to the PP2C family, antagonizes ABA signal transduction and accelerates water loss during leaf senescence. Nevertheless, the factors and mechanisms involved in the regulation of stomatal movement in senescent leaves remain unknown.
In this study, stomatal opening was enhanced with prolonged MeJA treatment (Fig. 1A and Fig. 2C) and an alkalized vacuolar lumen. Enhanced stomatal aperture was also found in the low-acidified vacuolar lumen in MeJA-induced leaf senescence (Fig. 1B). Bak et al. (2013) have further reported that vacuolar acidification is accompanied by ABA-induced stomatal closure. The alteration in vacuolar pH may be related to the stomatal opening in MeJA-induced leaf senescence. To further elucidate the possible relationships between vacuolar pH and stomatal activity, we treated senescent leaves induced by MeJA with the V-ATPase inhibitor BFA1 or the PIKfyve inhibitor to accelerate vacuolar alkalization (Figs. 2A and 8A). Stomatal openings were enhanced compared with those under control conditions (Figs. 2B and 8C), suggesting that the alteration of vacuolar pH is necessary for stomatal opening in MeJA-induced leaf senescence.
In plants, the pH homeostasis of the vacuole is regulated by antiporters and proton pumps (V-ATPase and V-PPase). V-ATPase, an important regulator of vacuolar acidification (Ferjani et al., 2011), is highly conserved. V-ATPase is a multisubunit complex containing 14 different subunits and two subcomplexes. Our data indicated that the gene expression of VHA-A, VHA-B2, and VHA-C was up-regulated and the activity of V-ATPase promoted in MeJA-induced leaf senescence (Fig. 3). A previous study has revealed that the vacuolar membrane probably plays an important role in the early stages of leaf senescence. Suzuki and Kasamo (1993) have found that ATPase activity and the rate of ATP-dependent proton-pumping increase until day 14, whereas they observed a rapid decrease in the rate of pumping on day 26, as indicated by the apparent loss of fresh weight in the cotyledons. We also found that the gene expression of VHA-A, VHA-B2, and VHA-C exhibited different expression patterns, which may have been due to the different roles of each subunit in V-ATPase activation and responses to environmental stress. The catalytic hexamer A3B3 is linked with the proton pathways by stalks (Marshansky and Futai, 2008). VHA-C has been reported to link VHA-E to the membrane-integral sector V0 and to act as a flexible stator subunit in regulating the coupling between ATP hydrolysis and proton transport (Li and Zhang, 2004; Schnitzer et al., 2011). ATP binding to VHA-C has also been demonstrated by Armbrüster et al. (2005). To further elucidate the role of V-ATPase in leaf senescence, we used the V-ATPase inhibitor BFA1. Treatment with the V-ATPase inhibitor significantly accelerated vacuolar alkalization (Fig. 2A), resulting in an enhanced stomatal aperture in MeJA-induced leaf senescence (Fig. 2C). Additional analysis of senescent characteristics revealed that BFA1 treatment hastened MeJA-induced senescence (Fig. 2, D, E, and F). Considering these findings, we speculated that V-ATPase activity might be enhanced in anti-MeJA-induced leaf senescence. This enhanced activity delayed vacuolar alkalization and suppressed stomatal opening, thereby maintaining water homeostasis in leaves and delaying leaf senescence.
V-ATPase may play another role in the regulation of leaf senescence. In the senescing petals of Japanese morning glory, treatment with Con A, a V-ATPase-specific inhibitor, accelerates petal senescence (Shibuya et al., 2009). The effect of Con A primarily results from the high pH conditions in the vacuole; under these conditions, vacuolar hydrolase is inactivated, and the accumulation of autophagic bodies is induced (Yoshimoto et al., 2004). These studies suggest that autophagy is associated with plant senescence and functions as the primary mechanism of degradation and remobilization of macromolecules. Autophagy also acts as an aid to cell survival rather than as a cause of cell death (Yamada et al., 2009). A recent report has revealed that chlorophyll breakdown is a detoxification process rather than a remobilization process (Schelbert et al., 2009). Thus, the duration of vacuolar acidification may minimize the risk of the accumulation of chlorophyll intermediates and potential phototoxicity, resulting in delayed leaf senescence.
PI3P is the product of PI3K and the precursor to another phosphoinositide, PtdIns(3,5)P2, which is produced by PI3P 5-kinase and is known as Fab1p in yeast and PIKfyve in mammals. PtdIns(3,5)P2 is a low-abundance phosphoinositide located in the endosomal, lysosomal, and vacuolar membranes of fungi and higher eukaryotes (Ho et al., 2012; Bak et al., 2013). Depending on the organism and cell type, PtdIns(3,5)P2 governs a diverse array of cellular functions, such as complete inhibition of PIKfyve, which induces vacuole formation from early endosomes. The vacuoles in fab1∆ yeast are less acidified than those in wild type (McCartney et al., 2014). PIKfyve is also required for the trafficking of protein from endosomes to the TGN (Zhang et al., 2007). Additionally, recent reports have revealed that polyphosphoinositides (including PtdIns(3,5)P2) are crucial for the trafficking, morphology, and functions of vacuoles in Arabidopsis (Nováková et al., 2014; Whitley et al., 2009; Vermeer et al., 2006).
PI3P and PtdIns(3,5)P2 have similar roles in plant development and vacuolar functions. Thus, to exclude the effect of PtdIns(3,5)P2 on stomatal movement, a PIKfyve inhibitor was used in 35S:AtVPS34-YFP Arabidopsis during MeJA-induced senescence. This approach distinguishes between both macromolecules because the upstream product of PtdIns(3,5)P2 is PI3P, which is the phosphorylated product of PI3K. Although vacuolar acidification was suppressed with treatment of the PIKfyve inhibitor in 35S:AtVPS34-YFP Arabidopsis during MeJA-induced senescence, the decrease was lower than that in YFP-labeled Arabidopsis (Fig. 8). PI3K may regulate vacuolar acidification in MeJA-induced senescence through the downstream product PtdIns(3,5)P2 as well as through other signaling pathways. These findings can be interpreted using the direct effect of PI3K on the activity of V-ATPase.
PI3K Is Widely Involved in Leaf Senescence
The inhibitory effects of PI3K activity on stomatal movement in Arabidopsis (Jung et al., 2002) and dayflower (Choi et al., 2008) have been established, and recent reports have revealed that PI3K may be involved in the regulation of leaf senescence. During petal senescence, the gene expression of AtVPS34 is up-regulated in the Japanese morning glory (Shibuya et al., 2009). In ethylene-accelerated petal senescence, treatment with the PI3K inhibitor 3-MA reduces the time to petal senescence (Yamada et al., 2009). In this study, PI3K inhibitors or down-regulation of AtVPS34 gene expression accelerated MeJA-induced leaf senescence (Fig. 9). Our data revealed that the down-regulation of PI3K activity suppresses vacuolar acidification and stomatal closing in MeJA-induced senescence (Fig. 7).
The down-regulation of PI3K activity also accelerated dark-induced leaf senescence (Fig. 10), but V-ATPase activity did not significantly differ between the pi3k mutants and wild-type Arabidopsis during this process (Fig. 10). This finding suggested that PI3K also regulates dark-induced senescence but does not do so through the PI3K-V-ATPase pathway. In plant development and stress responses, PI3K exhibits diverse functions, such as actin filament reorganization, plasma membrane endocytosis, and NADPH oxidase activation (Choi et al., 2008; Leshem et al., 2007; Liu et al., 2012). Thus, PI3K may be involved in the regulation of leaf senescence.
CONCLUSION
This work provides a comprehensive perspective of the PI3K-V-ATPase pathway in MeJA-induced leaf senescence. The binding of PI3K to the VHA-B subunits improved the assembly of V-ATPase and enhanced V-ATPase activity and vacuolar acidification, thereby suppressing stomatal opening and MeJA-induced leaf senescence.
MATERIALS AND METHODS
Chemicals
PI3K inhibitors LY294002 and wortmannin were obtained from Biyuntian (Shanghai, China). Bafilomycins A1 (BFA1), concanamycin A (Con A), 9-amino-6chloro-2-methoxyacridine (ACMA), and methyl jasmonate (MeJA) were purchased from Sigma-Aldrich (St. Louis, MO). Acridine orange (AO) was purchased from Invitrogen (Carlsbad, CA). And the PIKfyve inhibitor (YM201636) was offered by Calbiochem (Merck Millipore, Darmstadt, Germany).
Plant Materials and Growth Conditions
The plant materials containing the wild-type Arabidopsis (Col-0), pi3k-1 mutant Arabidopsis (SALK_007281), pi3k-2 mutant Arabidopsis (GABI_418H02), YFP Arabidopsis, and 35S:AtVPS34-YFP were grown in an environmentally controlled growth room with a 16-h light/8-h dark cycle at 22°C for 3 weeks. SALK_007281 contained T-DNA at position +2388 relative to the ATG of VPS34. Sequence-indexed T-DNA insertion line was generated by vacuum infiltration of Columbia (Col) plants with Agrobacterium tumefaciens vector pROK2; kanamycin was employed for selection of plants carrying a T-DNA. GABI_418H02 contained a T-DNA into the fifth exon at position +664; ampicillin was employed for selection of plants carrying a T-DNA. The pi3k mutant was germinated on MS agar medium containing kanamycin or ampicillin (50 mg l−1) to obtain Kan or Amp resistance heterozygous Arabidopsis seedlings since the homozygous seedlings are lethal. PCR-based genotyping analysis of these pi3k mutants were taken by the previous study (Lee et al., 2008). The Arabidopsis leaves at the approximate middle of the petioles were cut as the leaf sample. The determination of pi3k mutant, vha-B2 mutant, and 35S:AtVPS34-YFP was performed by quantitative reverse transcription (qRT)-PCR (Supplemental Fig. S6). One week after germination, the seedlings on MS agar medium (including wild type) were transplanted to soil.
Hormone Treatment and Dark-Induced Senescence
For in planta treatment, one week after seed germination, the seedlings were transplanted on fresh MS containing 50 μM MeJA for 1 week under a 16-h light/8-h dark cycle (He et al., 2002).
For senescence assay in detached leaves upon MeJA treatment, leaves numbers 4 and 5 were detached from rosettes from 3-week-old plants after sowing, and were immediately floated on 3 mm MES buffer (pH 5.8) solution with or without 50 μM MeJA under continuous lighting to incubate leaves for different times (Woo et al., 2001). Before the experiments, leaves were washed three times with distilled water. All the experiments were carried out at 22°C and repeated at least three times (Yue et al., 2012).
For senescence assay in detached leaves under dark treatment, leaves were cut from 21 d plants grown in soil and placed onto 3 mm MES buffer (pH 5.8) in the dark for 3 d (Shan et al., 2011).
Measurement of Chlorophyll Content and Photochemical Efficiency
Chlorophyll was extracted from two leaf discs by boiling the leaves in 95% ethanol at 80°C. And the chlorophyll content per fresh weight of leaf was calculated according to the methods described previously by Lichtenthaler (1987). The chlorophyll content was expressed as the percentage of the initial value of rosettes. The photochemical efficiency of photosystem II (PSII) was examined by using a PAM fluorometer (Walz, Eichenring, Germany) according to the protocol as described previously (Müller et al., 2001). The ratio of maximum variable fluorescence to maximum yield of fluorescence was represented as the photochemical efficiency of PSII (Oh et al., 1996).
Vacuolar Lumenal Acidification Determination
The vacuolar acidification in Arabidopsis guard cell was observed using the pH-dependent fluorescent dye AO (Bak et al., 2013). For vacuolar pH determination, Arabidopsis leaf epidermis with guard cells was stained with 50 mm AO for 100 min. AO fluorescence was captured by an LSM 510 META system (Carl Zeiss, Jena, Germany) following excitation at 488 nm and two band-pass (BP) filters (BP 615–660 nm and BP 530–540 nm) were used for the red and green channels, respectively. The quantitative analysis of fluorescence images was analyzed with an AxioVision 4.8.2 analysis program (Carl Zeiss) and ImageJ software (National Institutes of Health, Bethesda, MD).
Total RNA Extraction and Quantitative Reverse Transcript-PCR
Total RNA was extracted from Arabidopsis leaves according to manufacturer’s instruction using the TRIZOL reagent (Invitrogen). Concentration of RNA was determined by measuring OD at 260 nm. First-strand cDNA was synthesized with the SuperScript II First-Strand Synthesis System for quantitative reverse transcript-PCR (qRT-PCR; Invitrogen). The transgenic Arabidopsis was examined by qRT-PCR. qPCR amplification was carried out using UBQ10 as endogenous control. SYBR Green probes for each gene were used. The primers were listed in Supplemental Table S1. The primers in determination of pi3k mutant were as follows: 5′-GCTGTTCCCGCAAATGGTT-3′ and 5′-CCGCAGATGTCGCAGATGTA-3′. qPCR was carried out using 50 ng of cDNA and SYBR PCR master mix (TaKaRa, Clontech, Mountain View, CA), following the manufacturer’s protocol. Relative expression levels were calculated using the 2-ΔΔCT analysis method. Expression levels for each gene were normalized to control treatment of the wild-type plant (Livak and Schmittgen, 2001; Sun et al., 2012).
Plasmid Construction
DNA manipulations were carried out by using standard procedures (Zhang et al., 2013). The cDNA sequences of AtVPS34, AtVHA-A, AtVHA-B1, AtVHA-B2, AtVHA-B3, and AtVHA-C were amplified by reverse transcript-PCR for constructing different vectors as follows: For a yeast two-hybrid construct, AtVPS34 was cloned into the bait plasmid pEG202, and the AtVHA-A, AtVHA-B1, AtVHA-B2, AtVHA-B3, and AtVHA-C were cloned into the prey plasmid pGADT7 (Clontech; kindly provided by Dr. Yongsheng Liu, Hefei University of Technology, Hefei, China); for the bimolecular fluorescence complementation construct (BiFC), AtVPS34 and AtVHA-B2 were cloned into the pBiFC vectors, which contained either N-terminal 155 amino acids or C-terminal 84 amino acids of EYFP (Walter et al., 2004) to generate 35S-AtVPS34-cYFP and 35S-AtVHA-B2-nYFP, respectively. For 35S:AtVPS34-YFP vector construction, AtVPS34 was cloned into the pHB-YFP vector. The primers, GenBank accession number and restriction sites of them were listed in Supplemental Table S1.
Yeast Two-Hybrid Assays
Yeast two-hybrid assays were performed according to the Lex A two-hybrid system. Full-length open reading frame cDNA sequences of Arabidopsis PI3K, VHA-A, VHA-B1, VHA-B2, VHA-B3, and VHA-C were PCR-amplified using the primers in Supplemental Table S1. The PCR fragments were cloned into the prey plasmid pJG4-5 and the bait plasmid pEG202, respectively, and fused with DNA-binding protein (Lex A) and activation domain (B42) sequences of the yeast Lex A system. The recombined bait plasmids and prey plasmids constructs were cotransformed into the yeast strain EGY48 using LiAc-mediated yeast transformation. Photographs of resulting yeast colonies were taken 3 d after transfer and β-galactosidase assay (Invitrogen) was carried out according to the supplier’s protocol (Wang et al., 2008; Gao et al., 2013).
Bimolecular Fluorescence Complementation Assay
The 35S:AtVPS34-cYFP fusion protein construct together with 35S:AtVHA-B2-nYFP or 35S-nYFP, and the 35S:AtVHA-B2-nYFP fusion protein construct together with 35S:AtVPS34-cYFP or 35S-cYFP, were transiently transformed to Nicotiana benthamiana mediated by Agrobacterium tumefaciens GV3101. Cells with YFP fluorescence were monitored under a laser scanning confocal microscopy (YFP: excitation 514 nm, emission 525–550 nm), and the detailed protocol follows Walter et al. (2004) and Huang et al. (2009a).
Generation of Transgenic Plants
Transgenic Arabidopsis were generated by Agrobacterium tumefaciens-mediated transformation according to the described procedure previously (Clough and Bent, 1998). The resulting transgenic T1 seeds were selected by plating on 0.5× MS (pH 5.8) containing hygromycin (50 mg l−1) and confirmed by PCR using hpt (accession no. E00777.1)-specific primers (5′-TAGGAGGGCGTGGATATGTC-3′ and 5′-TACACAGCCATC GGTCCAGA-3′; Yue et al., 2012; Zhang et al., 2013). The overexpression of AtVPS34 gene in 35S:AtVPS34-YFP transgenic Arabidopsis was determined by qRT-PCR (Supplemental Fig. S6B).
Tonoplast Membrane Protein Extraction
Microsomal membrane fractions were extracted from leaves according to protocol described previously (Liu et al., 2012). The crude microsomal membrane protein was used to the extraction of tonoplast vesicles. The microsomal membrane pellet was resuspended in a buffer containing 2 mm BTP/MES, pH 7.0, 250 mm Suc, 1 mm DTT, 10% glycerol (v/v), 0.2% BSA (w/v) and covered with a 25/38% (w/w) discontinuous Suc density gradient, then centrifuged with 100,000g for 2 h. The fraction from the interface was the tonoplast protein. The concentrations of tonoplast membrane protein were determined by Lowry method (Zhang et al., 2013).
Determination of the Purity of the Tonoplast Fraction
To characterize the purity of the isolated tonoplast vesicles, hydrolytic and proton pumping activities of the vacuolar H+-ATPase (V-ATPase) were assayed in the presence and absence of appropriate inhibitors (Queirós et al., 2009; Snowden et al., 2015). Quantities of 50 mm KNO3, 0.1 mm Na3VO4, and 0.5 mm NaN3 were used to inhibit tonoplast V-ATPase, plasma membrane P-ATPase, and F-ATPase, respectively. In addition, the effect of the specific inhibitor of the tonoplast ATPase, Con A, on ATP-dependent proton pumping into tonoplast vesicles was also determined. ATP-dependent proton pumping was measured by the quenching of ACMA fluorescence (Shabala et al., 2011). The proton pump activity of V-ATPase was assayed in a reaction buffer containing 250 mm sorbitol, 50 mm KCl, 3 mm ATP, 50 μm NaVO4, 1 mm NaN3, 2 μm ACMA, and 10 mm MES-Tris (pH 7.5). A quantity of 3 mm MgSO4 was used to initiate the reaction. The fluorescence quenching was measured with an excitation wavelength 410 nm and an emission wavelength of 480 nm by an auto-microplate reader. The determination of purity of tonoplast fraction was shown in Supplemental Fig. S12.
V-ATPase Activity Assays
Ten micrograms of tonoplast membrane protein was applied to analyze the V-ATPase activity with 10 μg BSA as a negative control. The V-ATPase activity, which was calculated as the difference measured with or without 100 nm Con A, was examined in a reaction medium as described previously (Zhang et al., 2013).
Stomatal Aperture Measurements
Stomatal aperture was monitored by microscopy and scanning electron microscopy, respectively. After treatment, stomata were observed by microscopy and given as width to length ratio. At least 30 stomata were assayed (Zhou et al., 2013). To determine the stomatal aperture by scanning electron microscopy, leaves were fixed by 2.5% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) at 4°C for 24 h, washed twice with 0.1 m phosphate buffer for 10 min, then immersed in 50, 60, 70, 80, 90, and 100% ethanol sequentially for 10 min each. After drying at vacuum dryer, the samples were coated with gold for scanning electron microscopy analysis (Huang et al., 2009b).
Western Blot
Protein extracts were extracted from 35S:AtVPS34-YFP and YFP Arabidopsis leaf samples after washing with sterile water. Protein extracts were separated on a 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis minigel and then a western blot was taken.
To obtain the optimal antibody, amino acid sequences of VPS34 in different species was compared using DNAman (Supplemental Fig. S9). The VPS34 polyclonal antibody (Cat. no. AP1851b; Abgent, San Diego, CA) is generated from rabbits immunized with a KLH conjugated synthetic peptide between 770 and 801 amino acids from the C-terminal region of hVPS34. The antibody is purified through a protein G column, then eluted with high and low pH buffers and neutralized immediately, followed by dialysis against PBS. For detection of VPS34 protein, blots were probed with a 1:1000 dilution of VPS34 antibody. Subsequently, blots were washed and incubated with an HRP-labeled goat anti-rabbit secondary antibody (Wuhan Boster Biological Technology, Wuhan, China). Proteins were detected by using a Gbox Chemi XT4 system (Syngene, Cambridge, UK).
Statistical Analysis
All assays were repeated independently for a minimum of three times. Data are represented as mean ± sd. Statistical analysis was performed with the Student’s paired t test.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY139999 (UBQ10), NM_104735 (VPS34), NM_001036222 (VHA-A), NM_106251 (VHA-B1), NM_001036730 (VHA-B2), NM_101877 (VHA-B3); AY059872 (VHA-C), AF370131 (SAG12).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. The primers used for plasmid construction and functional analysis.
Supplemental Figure S1. Scanning electron microscopy analysis of stomatal aperture in leaf senescence.
Supplemental Figure S2. The V-ATPase activity of microsomal membrane fractions in MeJA-induced Arabidopsis leaf senescence.
Supplemental Figure S3. The V-ATPase activity under Glc treatment.
Supplemental Figure S4. The V-ATPase activity under Glc and LY294002 treatment.
Supplemental Figure S5. Genes expression of AtVPS34 and SAG12 in MeJA-induced senescence.
Supplemental Figure S6. Characterization of pi3k Arabidopsis mutants and 35S:AtVPS34-YFP transgenic Arabidopsis.
Supplemental Figure S7. The effect of PI3K on MeJA-induced senescence in attached Arabidopsis leaves.
Supplemental Figure S8. Scanning electron microscopy analysis of stomatal aperture during leaf senescence in pi3k mutant or supplemented with PI3K inhibitors.
Supplemental Figure S9. Determination of 35S:AtVPS34-YFP Arabidopsis seedling.
Supplemental Figure S10. The vacuolar acidification in dark-induced leaf senescence.
Supplemental Figure S11. Scanning electron microscopy analysis of stomatal aperture in dark-induced leaf senescence in pi3k-1 mutant.
Supplemental Figure S12. Assessment of purity of tonoplast membrane fractions.
Supplementary Material
Acknowledgments
We thank Prof. Yongsheng Liu for providing the yeast plasmids for a yeast two-hybrid assay and appreciate the conversation with the members of our group in developing some of the ideas presented in this study.
Glossary
- ABA
abscisic acid
- ACMA
9-amino-6chloro-2-methoxyacridine
- BFA1
bafilomycin A1
- BiFC
bimolecular fluorescence construct
- Con A
concanamycin A
- MeJA
methyl jasmonate
- MS
Murashige and Skoog agar medium
- PSII
photosystem II
- qRT
quantitative reverse transcript
- RAVE
regulator of H+-ATPase of vacuolar and endosomal membranes
- SEM
scanning electron microscopy
- TGN
trans-Golgi
- V-ATPase
vacuolar H+-ATPase
- VHA-A
V-ATPase A subunit
- VHA-B
V-ATPase B subunit
- VHA-C
V-ATPase C subunit
- VHA-E
V-ATPase E subunit
Footnotes
This research is supported by the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT0829), the Key Program of NSFC-Guangdong Joint Funds of China (no. U0931005), and the National High Technology Research and Development Program of China (863 Program; no. 2007AA10Z204).
References
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, Chory J, Schroeder JI (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Armbrüster A, Hohn C, Hermesdorf A, Schumacher K, Börsch M, Grüber G (2005) Evidence for major structural changes in subunit C of the vacuolar ATPase due to nucleotide binding. FEBS Lett 579: 1961–1967 [DOI] [PubMed] [Google Scholar]
- Aparicio-Fabre R, Guillén G, Estrada G, Olivares-Grajales J, Gurrola G, Sánchez F (2006) Profilin tyrosine phosphorylation in poly-L-proline-binding regions inhibits binding to phosphoinositide 3-kinase in Phaseolus vulgaris. Plant J 47: 491–500 [DOI] [PubMed] [Google Scholar]
- Backer JM. (2008) The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 410: 1–17 [DOI] [PubMed] [Google Scholar]
- Bak G, Lee EJ, Lee Y, Kato M, Segami S, Sze H, Maeshima M, Hwang JU, Lee Y (2013) Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5-bisphosphate. Plant Cell 25: 2202–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M, Keppens S, Stalmans W (1998) Specific features of glycogen metabolism in the liver. Biochem J 336: 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr., Abraham RT (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15: 5256–5267 [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, Watkins PA, Beven AF, Shaw PJ, Hernandez LE, Lomonossoff GP, Shanks M, Peart J, Drøbak BK (2000) Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell 12: 1679–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Yoo SD, Sheen J (2006) Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 127: 579–589 [DOI] [PubMed] [Google Scholar]
- Choi Y, Lee Y, Jeon BW, Staiger CJ, Lee Y (2008) Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant Cell Environ 31: 366–377 [DOI] [PubMed] [Google Scholar]
- Cipriano DJ, Wang Y, Bond S, Hinton A, Jefferies KC, Qi J, Forgac M (2008) Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta 1777: 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- daSilva LLP, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Schubert D, Calvo-Weimar O, Stierhof YD, Schmidt R, Schumacher K (2005) Essential role of the V-ATPase in male gametophyte development. Plant J 41: 117–124 [DOI] [PubMed] [Google Scholar]
- Diaz C, Purdy S, Christ A, Morot-Gaudry JF, Wingler A, Masclaux-Daubresse C (2005) Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis. A metabolic profiling approach. Plant Physiol 138: 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck R, Nguyen M, Günther J, Künkel W, Zipfel PF (2005) The phosphatidylinositol 3-kinase Vps34p of the human pathogenic yeast Candida albicans is a multifunctional protein that interacts with the putative vacuolar H+-ATPase subunit Vma7p. Int J Med Microbiol 295: 57–66 [DOI] [PubMed] [Google Scholar]
- Ferjani A, Segami S, Horiguchi G, Muto Y, Maeshima M, Tsukaya H (2011) Keep an eye on PPi: the vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 23: 2895–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster FM, Traer CJ, Abraham SM, Fry MJ (2003) The phosphoinositide (PI) 3-kinase family. J Cell Sci 116: 3037–3040 [DOI] [PubMed] [Google Scholar]
- Gao Y, Liu J, Zhang Z, Sun X, Zhang N, Fan J, Niu X, Xiao F, Liu Y (2013) Functional characterization of two alternatively spliced transcripts of tomato ABSCISIC ACID INSENSITIVE3 (ABI3) gene. Plant Mol Biol 82: 131–145 [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581: 2204–2214 [DOI] [PubMed] [Google Scholar]
- Hardie DG. (2007) AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47: 185–210 [DOI] [PubMed] [Google Scholar]
- He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128: 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Costa C, Ciraolo E (2007) Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol 194: 243–256 [DOI] [PubMed] [Google Scholar]
- Ho CY, Alghamdi TA, Botelho RJ (2012) Phosphatidylinositol-3,5-bisphosphate: no longer the poor PIP2. Traffic 13: 1–8 [DOI] [PubMed] [Google Scholar]
- Huang L, Yang S, Zhang S, Liu M, Lai J, Qi Y, Shi S, Wang J, Wang Y, Xie Q, Yang C (2009a) The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root. Plant J 60: 666–678 [DOI] [PubMed] [Google Scholar]
- Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX (2009b) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev 23: 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miège C, Gaude T (2008) Evidence for a sorting endosome in Arabidopsis root cells. Plant J 53: 237–247 [DOI] [PubMed] [Google Scholar]
- Jordan WR, Brown KW, Thomas JC (1975) Leaf age as a determinant in stomatal control of water loss from cotton during water stress. Plant Physiol 56: 595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JY, Kim YW, Kwak JM, Hwang JU, Young J, Schroeder JI, Hwang I, Lee Y (2002) Phosphatidylinositol 3- and 4-phosphate are required for normal stomatal movements. Plant Cell 14: 2399–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane PM. (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70: 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Eu YJ, Yoo CM, Kim YW, Pih KT, Jin JB, Kim SJ, Stenmark H, Hwang I (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL (2013) Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152: 290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M, Beyhl D, Görlich E, Al-Rasheid KAS, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010) Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA 107: 3251–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim ES, Choi Y, Hwang I, Staiger CJ, Chung YY, Lee Y (2008) The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development. Plant Physiol 147: 1886–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Munnik T, Lee Y (2010) Plant phyphatidylinositol 3-kinase. In Munnik T. ed, Lipid Signaling in Plants, 1st Ed, Vol 16 Springer-Verlag, Berlin, Germany, 95–106 [Google Scholar]
- Leprince AS, Magalhaes N, De Vos D, Bordenave M, Crilat E, Clément G, Meyer C, Munnik T, Savouré A (2014) Involvement of phosphatidylinositol 3-kinase in the regulation of proline catabolism in Arabidopsis thaliana. Front Plant Sci 5: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Seri L, Levine A (2007) Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J 51: 185–197 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang X (2004) Electron-microscopic structure of the V-ATPase from mung bean. Planta 219: 948–954 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. (1987) Chlorophylls a and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 18: 350–382 [Google Scholar]
- Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou J, Xing D (2012) Phosphatidylinositol 3-kinase plays a vital role in regulation of rice seed vigor via altering NADPH oxidase activity. PLoS One 7: e33817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−Δ Δ C(T) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R (1997) Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase γ. Science 275: 394–397 [DOI] [PubMed] [Google Scholar]
- Ma B, Xiang Y, An L (2011) Structural bases of physiological functions and roles of the vacuolar H+-ATPase. Cell Signal 23: 1244–1256 [DOI] [PubMed] [Google Scholar]
- Ma Y, She X, Yang S (2013) Cytosolic alkalization-mediated H2O2 and NO production are involved in darkness-induced stomatal closure in Vicia faba. Can J Plant Sci 93: 119–130 [Google Scholar]
- Marjuki H, Gornitzky A, Marathe BM, Ilyushina NA, Aldridge JR, Desai G, Webby RJ, Webster RG (2011) Influenza A virus-induced early activation of ERK and PI3K mediates V-ATPase-dependent intracellular pH change required for fusion. Cell Microbiol 13: 587–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V, Futai M (2008) The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20: 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney AJ, Zhang Y, Weisman LS (2014) Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. BioEssays 36: 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura I, Sasaki T, Tanaka S, Takahashi N, Jimi E, Kurokawa T, Kita Y, Ihara S, Suda T, Fukui Y (1997) Phosphatidylinositol-3 kinase is involved in ruffled border formation in osteoclasts. J Cell Physiol 172: 230–239 [DOI] [PubMed] [Google Scholar]
- Nováková P, Hirsch S, Feraru E, Tejos R, van Wijk R, Viaene T, Heilmann M, Lerche J, Rycke RD, Feraru MI, Grones P, van Montagu M, Heilmann I, Munnik T, Friml J (2014) SAC phosphoinositide phosphatases at the tonoplast mediate vacuolar function in Arabidopsis. Proc Natl Acad Sci USA 111: 2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SA, Lee SY, Chung IK, Lee CH, Nam HG (1996) A senescence-associated gene of Arabidopsis thaliana is distinctively regulated during natural and artificially induced leaf senescence. Plant Mol Biol 30: 739–754 [DOI] [PubMed] [Google Scholar]
- Park KY, Jung JY, Park J, Hwang JU, Kim YW, Hwang I, Lee Y (2003) A role for phosphatidylinositol 3-phosphate in abscisic acid-induced reactive oxygen species generation in guard cells. Plant Physiol 132: 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra KJ, Chan CY, Chen J (2014) Saccharomyces cerevisiae vacuolar H+-ATPase regulation by disassembly and reassembly: one structure and multiple signals. Eukaryot Cell 13: 706–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñarrubia L, Moreno J (2001) Senescence in plants and crops. In Pessarakli Mohammad. ed, Handbook of Plant and Crop Physiology, 2nd Ed, Vol 9 CRC Press, New York, 181–203 [Google Scholar]
- Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, Denecke J (2006) Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 18: 198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirós F, Fontes N, Silva P, Almeida D, Maeshima M, Gerós H, Fidalgo F (2009) Activity of tonoplast proton pumps and Na+/H+ exchange in potato cell cultures is modulated by salt. J Exp Bot 60: 1363–1374 [DOI] [PubMed] [Google Scholar]
- Quirino BF, Noh YS, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5: 278–282 [DOI] [PubMed] [Google Scholar]
- Ratajczak R, Feussner I, Hause B, Böhm A, Parthier B, Wasternack C (1998) Alteration of V-type H+-ATPase during methyljasmonate-induced senescence in barley (Hordeum vulgar L. cv. Salome). J Plant Physiol 152: 199–206 [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Sambade M, Alba M, Smardon AM, West RW, Kane PM (2005) A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 170: 1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL (2005) Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 25: 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbert S, Aubry S, Burla B, Agne B, Kessler F, Krupinska K, Hörtensteiner S (2009) Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 21: 767–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer D, Seidel T, Sander T, Golldack D, Dietz KJ (2011) The cellular energization state affects peripheral stalk stability of plant vacuolar H+-ATPase and impairs vacuolar acidification. Plant Cell Physiol 52: 946–956 [DOI] [PubMed] [Google Scholar]
- Schumacher K, Krebs M (2010) The V-ATPase: small cargo, large effects. Curr Opin Plant Biol 13: 724–730 [DOI] [PubMed] [Google Scholar]
- Shabala S, Baekgaard L, Shabala L, Fuglsang A, Babourina O, Palmgren MG, Cuin TA, Rengel Z, Nemchinov LG (2011) Plasma membrane Ca²+ transporters mediate virus-induced acquired resistance to oxidative stress. Plant Cell Environ 34: 406–417 [DOI] [PubMed] [Google Scholar]
- Shan X, Wang J, Chua L, Jiang D, Peng W, Xie D (2011) The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol 155: 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Yamada T, Suzuki T, Shimizu K, Ichimura K (2009) InPSR26, a putative membrane protein, regulates programmed cell death during petal senescence in Japanese morning glory. Plant Physiol 149: 816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden CJ, Thomas B, Baxter CJ, Smith JAC, Sweetlove LJ (2015) A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J 81: 651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky LA, Forgac M (2015) Amino acid availability modulates vacuolar H+-ATPase assembly. J Biol Chem 290: 27360–27369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Nie S, Xing D (2012) Nitric oxide-mediated maintenance of redox homeostasis contributes to NPR1-dependent plant innate immunity triggered by lipopolysaccharides. Plant Physiol 160: 1081–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kasamo K (1993) Effects of aging on the ATP-and pyrophosphate-dependent pumping of protons across the tonoplast isolated from pumpkin cotyledons. Plant Cell Physiol 34: 613–619 [Google Scholar]
- Takáč T, Pechan T, Samajová O, Ovečka M, Richter H, Eck C, Niehaus K, Šamaj J (2012) Wortmannin treatment induces changes in Arabidopsis root proteome and post-Golgi compartments. J Proteome Res 11: 3127–3142 [DOI] [PubMed] [Google Scholar]
- van Doorn WG. (2008) Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels? J Exp Bot 59: 1963–1972 [DOI] [PubMed] [Google Scholar]
- Vermeer JEM, van Leeuwen W, Tobeña-Santamaria R, Laxalt AM, Jones DR, Divecha N, Gadella TWJ Jr., Munnik T (2006) Visualization of PtdIns3P dynamics in living plant cells. Plant J 47: 687–700 [DOI] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6: 909–919 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, Harter K, Kudla J (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang S, Liu J, Feng Y, Niu X, Giovannoni J, Liu Y (2008) Altered plastid levels and potential for improved fruit nutrient content by downregulation of the tomato DDB1-interacting protein CUL4. Plant J 55: 89–103 [DOI] [PubMed] [Google Scholar]
- Whitley P, Hinz S, Doughty J (2009) Arabidopsis FAB1/PIKfyve proteins are essential for development of viable pollen. Plant Physiol 151: 1812–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Purdy S, MacLean JA, Pourtau N (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57: 391–399 [DOI] [PubMed] [Google Scholar]
- Wingler A, Stangberg EJ, Saxena T, Mistry R (2012) Interactions between temperature and sugars in the regulation of leaf senescence in the perennial herb Arabis alpina L. J Integr Plant Biol 54: 595–605 [DOI] [PubMed] [Google Scholar]
- Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG (2001) ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13: 1779–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ichimura K, Kanekatsu M, van Doorn WG (2009) Homologs of genes associated with programmed cell death in animal cells are differentially expressed during senescence of Ipomoea nil petals. Plant Cell Physiol 50: 610–625 [DOI] [PubMed] [Google Scholar]
- Yeh CM, Chien PS, Huang HJ (2007) Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J Exp Bot 58: 659–671 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, Ohsumi Y (2004) Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 16: 2967–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H, Nie S, Xing D (2012) Over-expression of Arabidopsis Bax inhibitor-1 delays methyl jasmonate-induced leaf senescence by suppressing the activation of MAP kinase 6. J Exp Bot 63: 4463–4474 [DOI] [PubMed] [Google Scholar]
- Zeiger E, Schwartz A (1982) Longevity of guard cell chloroplasts in falling leaves: implication for stomatal function and cellular aging. Science 218: 680–682 [DOI] [PubMed] [Google Scholar]
- Zhang H, Niu X, Liu J, Xiao F, Cao S, Liu Y (2013) RNAi-directed downregulation of vacuolar H+-ATPase subunit a results in enhanced stomatal aperture and density in rice. PLoS One 8: e69046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Xia X, Zhang Y, Gan SS (2012) An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J 69: 667–678 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS (2007) Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA 104: 17518–17523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Sun A, Xing D (2013) Modulation of cellular redox status by thiamine-activated NADPH oxidase confers Arabidopsis resistance to Sclerotinia sclerotiorum. J Exp Bot 64: 3261–3272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.