The heme oxygenase HY1 functions negatively and acts upstream in drought-induced ABA signaling, and this cascade depends on RbohD-derived ROS production in the regulation of stomatal closure.
Abstract
Heme oxygenase (HO; EC 1.14.99.3) has recently been proposed as a novel component in mediating wide ranges of the plant adaptive signaling processes. However, the physiological significance and molecular basis underlying Arabidopsis (Arabidopsis thaliana) HO1 (HY1) functioning in drought tolerance remained unclear. Here, we report that mutation of HY1 promoted, but overexpression of this gene impaired, Arabidopsis drought tolerance. This was attributed to the abscisic acid (ABA)-hypersensitive or -hyposensitive phenotypes, with the regulation of stomatal closure in particular. However, comparative transcriptomic profile analysis showed that the induction of numerous ABA/stress-dependent genes in dehydrated wild-type plants was differentially impaired in the hy1 mutant. In agreement, ABA-induced ABSCISIC ACID-INSENSITIVE4 (ABI4) transcript accumulation was strengthened in the hy1 mutant. Genetic analysis further identified that the hy1-associated ABA hypersensitivity and drought tolerance were arrested in the abi4 background. Moreover, the promotion of ABA-triggered up-regulation of RbohD abundance and reactive oxygen species (ROS) levels in the hy1 mutant was almost fully blocked by the mutation of ABI4, suggesting that the HY1-ABI4 signaling in the wild type involved in stomatal closure was dependent on the RbohD-derived ROS production. However, hy1-promoted stomatal closure was not affected by a nitric oxide scavenger. Correspondingly, ABA-insensitive behaviors in rbohD stomata were not affected by either the mutation of HY1 or its ectopic expression in the rbohD background, both of which responded significantly to exogenous ROS. These data indicate that HY1 functioned negatively and acted upstream of ABI4 in drought signaling, which was casually dependent on the RbohD-derived ROS in the regulation of stomatal closure.
Drought stress has been considered a central topic of plant stress physiology because it significantly reduces plant growth and crop production (Skirycz and Inzé, 2010; Tester and Langridge, 2010). In response to drought stress, terrestrial plants have developed a complex signaling network to limit water loss by regulating the stomatal aperture. The movement of the stomatal pore controls approximately 30% of the evaporation of total rainfall and is modulated precisely by the phytohormone abscisic acid (ABA) and several other well-known small molecules (reactive oxygen species [ROS] and nitric oxide [NO]; García-Mata and Lamattina, 2013). When water deficiency occurs, ABA is quickly synthesized, thereafter causing the closing of the stomatal pores by switching on a series of biochemical and physiological signaling cascades. This is an effective strategy to reduce transpirational water loss and critical for land plants to survive (Lee et al., 2009; Sirichandra et al., 2009). Drought-caused oxidative stress, which activates the plant’s defense response, is dependent on the functioning of complex gene networks (Pastori and Foyer, 2002; Munné-Bosch et al., 2013; Noctor et al., 2014). ROS, enzymatically generated via the NADPH oxidase isoforms AtrbohD and AtrbohF (Kwak et al., 2003), were identified as key bioregulators involved in ABA-induced stomatal signaling (Bright et al., 2006; Jammes et al., 2009; Xie et al., 2014). This deduction was confirmed by the observation that ABA-induced ROS generation and stomatal closure were largely impaired in the rbohD/F mutant (Hirayama and Shinozaki, 2007; Zhang et al., 2009).
ABSCISIC ACID-INSENSITIVE4 (ABI4), a member of the AP2-type transcription factor family, has been identified as a vital intermediate in regulating the ABA-dependent transcriptional profile that functions especially during seed dormancy, germination, and development (Finkelstein et al., 2002; Kerchev et al., 2011). Consistently, the ABI4 transcript level is restricted to seed maturation and within a few days following germination, and its steady-state mRNA levels drop sharply a few days thereafter (Wind et al., 2013). Some studies questioned the function of this protein and suggested its important roles in other aspects of plant development and metabolism. These roles were related to chloroplast-to-nucleus retrograde signal pathways (Sun et al., 2011), rosette growth and lateral root formation (Shkolnik-Inbar and Bar-Zvi, 2010), as well as cross talk between ABA and jasmonate (Giraud et al., 2009; Kerchev et al., 2011, 2013; Wind et al., 2013). Molecular evidence demonstrated that ABI4 could repress the expression of several downstream components of signaling cascades by directly binding to corresponding promoters of related genes, such as CYP707A1 and CYP707A2, as well as a subunit of the heme activator proteins (Zhang et al., 2013). As a consequence, the excellent studies cited above revealed that ABI4 was a versatile factor that functions in diverse pathways and was tightly regulated at the transcriptional and posttranscriptional levels (Finkelstein et al., 2011; Wind et al., 2013). Notwithstanding these insights, the physiological relevance of the regulatory mechanism of ABI4 in stomatal movement still requires further elucidation.
Heme oxygenase (HO; EC 1.14.99.3) is a ubiquitous, sensitive, and highly active enzyme that catalyzes the stereospecific cleavage of heme to biliverdin with the release of iron and carbon monoxide (Davis et al., 1999; Emborg et al., 2006). Initially identified as mediating phytochrome chromophore synthesis and retrograde signaling (Muramoto et al., 1999), plant HOs have gained increasing attention due to their indispensable and positive roles in a wide array of cellular adaptation and developmental processes (Shekhawat and Verma, 2010). Several casual links among HO activation, the phytohormone ABA, and the second messengers NO/ROS have been established (Cao et al., 2007; Xie et al., 2008, 2011, 2013; Han et al., 2014). HY1, the most highly expressed and inducible HO in Arabidopsis (Arabidopsis thaliana), was confirmed to be involved in the regulation of salt acclimation/tolerance in Arabidopsis (Xie et al., 2011, 2013). Shen et al. (2006) discovered that ABA normally induced stomatal closure in the hy1-1 mutant (CS67), and this mutant further showed open-stomata and partial ABA-insensitive phenotypes (Tomiyama et al., 2014). Therefore, it remains to be demonstrated conclusively whether HY1 is involved in plant drought tolerance.
In this investigation, we showed that disruption of HY1 (hy1-100 mutant; CS236) enhanced, but overexpression of this gene impaired, Arabidopsis drought tolerance, which could be attributed to the promoted or reduced sensitivity to ABA-induced stomatal closure particularly. However, RNA sequencing (RNA-Seq) experiments revealed that the transcriptional abundance of several clusters of ABA/stress-dependent genes induced by drought stress was lower in the hy1-100 mutant upon drought stress compared with the wild type, with the exception of a cluster of transporter genes. Further genetic analysis showed that abi4 almost fully blocked the ABA-hypersensitive and drought-tolerant phenotype of hy1-100. These results provided conclusive evidence of a strong linear interrelationship between HY1 and ABI4 involved in the regulation of a subset of ABA responses that determined plant resistance to water deficiency. By taking this genetic approach, we further clarified that the RbohD-dependent ROS production could serve as a downstream component of HY1-ABI4 signaling in controlling stomatal movement in wild-type plants. The biological role of the guard cell outward-rectifying K+ (GORK) channel was also preliminarily investigated. Taken together, this study established a signaling pathway leading to hy1-promoted stomatal closure and drought tolerance that involves ABI4 activated by RbohD-dependent ROS generation.
RESULTS
Disruption of HY1 Enhances, But Overexpression of This Gene Reduces, Arabidopsis Drought Tolerance
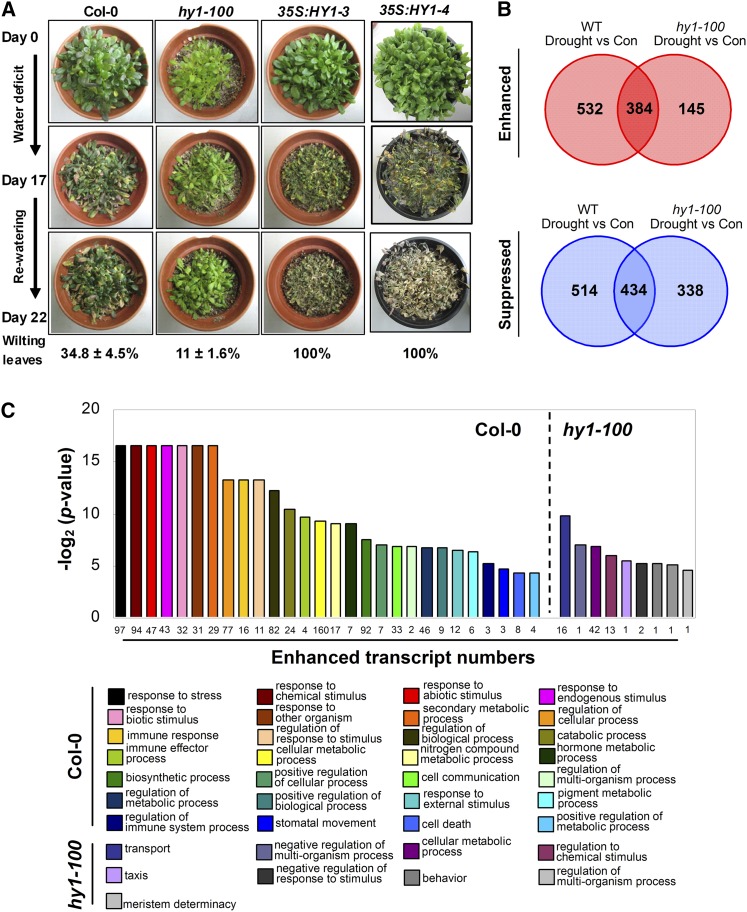
Our previous results showed that HY1 is an essential component of the salt acclimation signaling pathway. For example, the hy1-100 mutant displayed hypersensitivity to salinity, whereas overexpressing HY1 led to salt tolerance characteristics (Xie et al., 2011, 2013). Since salinity and drought signaling share a common cascade in the ABA-dependent pathway, we hypothesized that HY1 may also function as a key positive regulator of Arabidopsis drought tolerance. Surprisingly, after a 17-d water-withholding period, the rosette leaves of two HY1 gain-of-function mutants (35S:HY1-3 and 35S:HY1-4) wilted more severely compared with the wild type, with most leaves becoming darker and dying. By contrast, the HY1-loss mutant plants, hy1-100, which possessed much lower HO activity (measured as the production rate of biliverdin, one of the products of HO; Supplemental Table S1), almost remained turgid (Fig. 1A). The survival phenotypes after rewatering reconfirmed that the mutation of HY1 enhanced, but overexpression of this gene reduced, Arabidopsis drought tolerance. Similarly, compared with the ecotype Landsberg erecta as background, a second mutant allele of HY1 (hy1-1; CS67) also displayed a drought-tolerant phenotype (Supplemental Fig. S1). As such, the results above suggested that HY1 might function as an important negative regulator in the plant adaptation to drought stress.
Figure 1.
Phenotypes and transcriptional profiles of HY1 loss- and gain-of-function mutants upon drought stress. A, Four-week-old plants, including the wild type (Columbia-0 [Col-0]), hy1-100, 35S:HY1-3, and 35S:HY1-4 in the Col-0 ecotype background, were cultured in pots before stopping irrigation. The images illustrate plant phenotypes at day 0 and at day 17 after the application of drought stress. The pots were then rewatered, and the wilted leaves were analyzed after another 5 d. Values are means ± se from at least three independent experiments. B, Venn diagram for the proportions of genes showing significant changes in transcript levels in response to desiccation (3 h) in the detached leaves of 4-week-old hy1-100 relative to the wild type (WT) according to the set thresholds (drought stress relative to control [Con], q ≤ 0.05, fold change 2 or greater or 0.5 or less). C, Enriched Gene Ontology (GO) categories of the biological processes of genes specifically up-regulated in the wild type (532 genes) and the hy1-100 mutant (145 genes) upon drought stress (P < 0.05). Numbers below each bar indicate counts of each GO category that appeared in the total specific up-regulated genes in the wild type and the hy1-100 mutant.
Expression Profiling Analysis of the Wild Type and the hy1 Loss-of-Function Mutant under Well-Watered and Drought Stress Conditions
Sustained free heme status is critical for plant drought tolerance (Phung et al., 2011; Nagahatenna et al., 2015). Therefore, we quantified total noncovalently bound heme, a substrate of HO, in wild-type and hy1-100 seedlings. As shown in Supplemental Table S1, we found reduced levels of noncovalently bound heme in hy1-100 seedlings (3.17 ± 0.23 nmol g−1 fresh weight in the wild type versus 2.06 ± 0.24 nmol g−1 fresh weight in hy1-100), which would be expected to result from a feedback mechanism in the inhibition of the tetrapyrrole biosynthesis pathway (Terry and Kendrick, 1999).
To further elucidate the molecular mechanism of how and why HY1, a positive regulator of high-salinity signaling, negatively regulated Arabidopsis drought tolerance, a genome-wide transcriptional analysis was performed by determining the differentially expressed genes (DEGs) between wild-type and hy1-100 plants under both well-watered and desiccated conditions. The results of the RNA-Seq analyses are available in Supplemental Table S2. Upon drought treatment, 916 activated genes and 948 repressed genes were found in the wild type (fold change 2 or greater or 0.5 or less, respectively; Fig. 1B). In hy1-100, the number of genes activated or repressed as a consequence of drought stress was reduced to 529 or 772, respectively. A Venn diagram of the results also indicated that 384 up-regulated and 434 down-regulated DEGs overlapped between the wild type and hy1-100. In addition, cluster analysis revealed that the expression pattern for all genes upon drought stress in the wild type did not fully overlap with that of hy1-100 (Fig. 1C; Supplemental Fig. S2; Supplemental Table S2).
GO categories in biological processes consistently showed that genes in a broad range of pathways known to be associated with abiotic and biotic stress responses were enriched in the wild type upon drought stress but not in the hy1-100 mutant. These categories of genes included response to stress, response to chemical stimulus, response to abiotic stimulus, and response to endogenous stimulus (Fig. 1C). It was noteworthy that a large proportion of these enriched genes were ABA dependent, such as GLUTATHIONE S-TRANSFERASE11 (GST11; At1g02920) and DESICCATION-RESPONSIVE PROTEIN29A (RD29A; At5g52310; Supplemental Table S2). By contrast, the most strongly enriched GO category in hy1-100 was transport-related genes. To confirm these results, six stress/ABA-responsive genes, which belong to the response to stress and transport GO categories, were selected for real-time reverse transcription-PCR (Q-PCR) validation. The expression of these genes in the wild type and the hy1-100 mutant showed a similar pattern of expression when comparing RNA-Seq with Q-PCR results (Supplemental Fig. S3). Therefore, these results revealed that HY1 had a significant impact on the global gene expression profile of Arabidopsis upon drought stress and was indeed required for the modulation of the most stress/ABA-responsive gene expression. It was also indicated that other critical factor(s) might act as definitive and available strategies accounting for hy1-enhanced Arabidopsis drought tolerance.
HY1 Negatively Regulates ABA Responses in Germination and Stomatal Movement But Not in Primary Root Growth
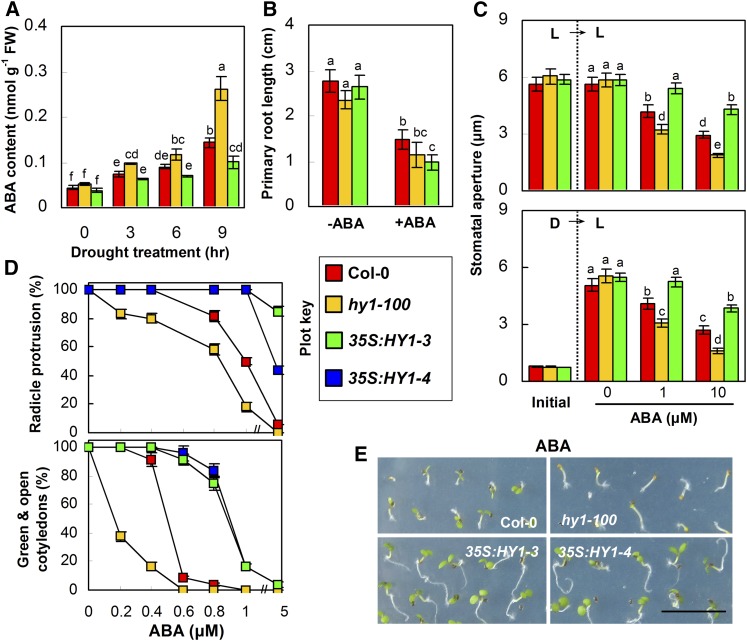
Plants can respond to drought stress in either an ABA-dependent or ABA-independent manner. Therefore, ABA contents in HY1-loss and -gain mutants under drought stress were analyzed. As expected, compared with the corresponding wild type, the drought-induced ABA contents were more pronounced in hy1-100 and hy1-1 mutants but attenuated in the HY1 transgenic overexpressing line 35S:HY1-3 (Fig. 2A; Supplemental Fig. S1). The altered expression of representative ABA-responsive genes (Supplemental Fig. S2; such as RD22 and RAB18, etc.) in the hy1-100 mutant upon drought stress strongly suggested that HY1 may play an unexpected role in ABA responses. Actually, ABA has been reported to inhibit primary root growth, germination, and postgermination processes as well as to regulate stomatal movement (Gimeno-Gilles et al., 2009; Cutler et al., 2010; Zhang et al., 2010). In the presence of ABA, primary root growth was markedly inhibited in the hy1-100 mutant (51% of the relative inhibition; Fig. 2B). Similar results were found in the hy1-1 mutant (Supplemental Fig. S1). However, this inhibited tendency was essentially parallel to that observed in wild-type plants (46.3% of the relative inhibition) while being strengthened in 35S:HY1-3 (63% of the relative inhibition). These results indicated that HY1 might not have much impact on ABA-inhibited primary root growth.
Figure 2.
Loss-of-function mutation in HY1 strengthens, but overexpression of HY1 blocks, drought-induced ABA content as well as ABA-induced stomatal closure and germination inhibition. A, ABA contents in 5-d-old seedlings of the wild type, the hy1-100 mutant, and HY1-overexpressing line 35S:HY1-3 in response to drought stress for the indicated times. FW, Fresh weight. B, Primary root growth for each genotype 7 d after transfer to Murashige and Skoog (MS) medium with or without 10 μm ABA (n = 15 from three independent experiments). C, ABA-induced stomatal closure (top) and inhibition of stomatal opening (bottom; n = 50 from three independent experiments). D, Darkness; L, cold light. D, Radicle protrusion and green and open cotyledon rate (%) of each genotype grown on MS medium containing the indicated ABA concentrations for 5 d (n = 50 from three independent experiments). The plot key illustrates the genotypes for each bar shown in A to D. Data are means ± se from at least three independent experiments. Differences among treatments were analyzed by one-way ANOVA, taking P < 0.05 as significant according to Tukey’s multiple range test. E, Photographs from the 0.4 μm ABA treatment. Bar = 1 cm.
Regarding ABA-promoted stomatal closure and ABA-inhibited stomatal opening, loss of function in HY1 caused ABA-hypersensitive phenotypes, while gain of function in HY1 resulted in hyposensitive phenotypes (Fig. 2C; Supplemental Fig. S1). Subsequently, the ABA response was examined by germination and postgermination assays. In medium supplemented with increasing concentrations of ABA, the germination rate of hy1-100 or hy1-1 seeds was more severely reduced than that of the wild type. In comparison, two HY1-overexpressing lines exhibited ABA-insensitive phenotypes (Fig. 2D; Supplemental Figs. S1 and S4). The parameters of seedling cotyledon opening and greening showed similar tendencies (Fig. 2, D and E). Interestingly, a hypersensitive or hyposensitive response to osmotic stress caused by the mutation or overexpression of HY1 was also observed, as evaluated by radicle protrusion, primary root growth, stomatal aperture, and green and open cotyledons of seedlings (Supplemental Fig. S5, A, C, and D). Furthermore, a functional redundancy was exhibited between HY1 and HO4 in terms of green and open cotyledons of seedlings (Supplemental Fig. S5B). Overall, the above results clearly indicated that HY1 negatively regulates the ABA responses during the germination and postgermination stages as well as stomatal movement.
ABI4 Acts Downstream of HY1 in Mediating a Subset of ABA Responses
ABI4 was a versatile activator or repressor in ABA signaling, particularly in the germination process and retrograde signaling (Penfield et al., 2006; Zhang et al., 2013). To verify whether ABI4 might functionally associate, at least in part, with HY1 for the regulation of a subset of ABA responses, we crossed hy1-100 with the abi4 mutant and identified plants with homozygous mutations in both HY1 and ABI4 genes by performing genotyping and phenotypic analysis. Since both ABI4 and HY1 were involved in Arabidopsis chloroplast-to-nucleus retrograde signaling, we further investigated whether retrograde signaling was altered in the hy1-100/abi4 mutant. In our experimental conditions, the addition of norflurazon (an inhibitor of carotenoid and ABA biosynthesis; Supplemental Fig. S6; Bartels and Watson, 1978; Feldman and Sun, 1986) and lincomycin (an inhibitor of plastid protein synthesis; Supplemental Fig. S7; Sullivan and Gray, 1999) to wide-type plants induced the retrograde signaling pathway by decreasing the accumulation of transcripts of nuclear genes encoding photosynthesis-related proteins, such as LHCB, CA, and CP (Supplemental Fig. S8). Therefore, the possibility that ABA was related to norflurazon-influenced HY1-ABI4 retrograde signaling could be partially excluded. By contrast, the repression of the above transcripts by these inhibitors was impaired in both hy1-100 and abi4 mutants to a certain extent. The additive impairment of repression for some of these marker genes (CP in particular) in hy1-100/abi4 plants suggested that there might exist a potent relationship between ABI4 and HY1 in the regulation of chloroplast-to-nucleus retrograde signaling.
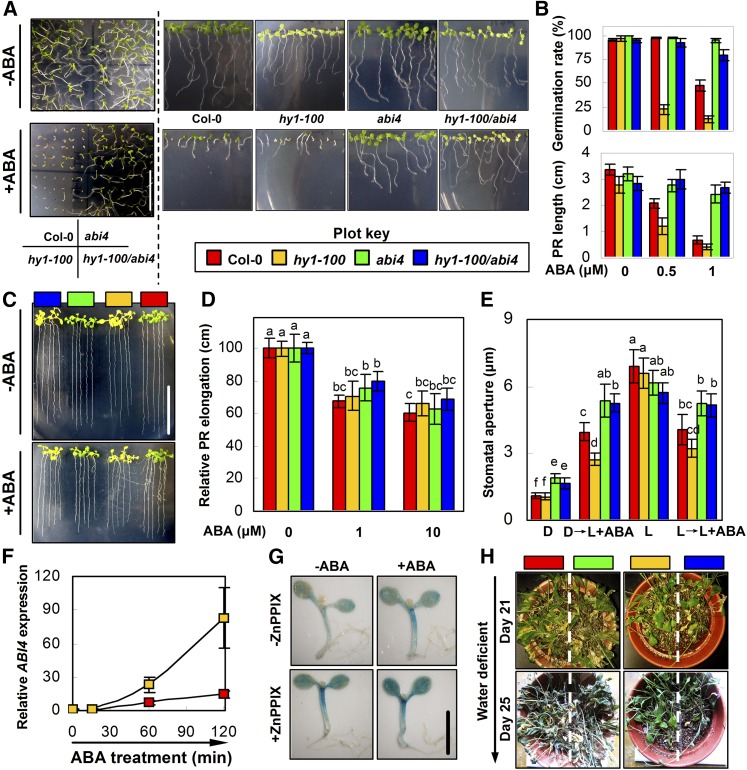
To further examine the interrelationship between ABI4 and HY1 in ABA responses, two approaches were adopted. First, seeds were sown directly in the ABA-containing medium to investigate the responses of germination and seedling growth to ABA. As expected, compared with the wild type, the hy1-100 or abi4 mutant showed hypersensitive or hyposensitive behaviors with regard to ABA-inhibited seed germination and seedling establishment (Fig. 3, A and B). These phenotypes in the hy1-100/abi4 mutant were much more comparable to its parent line abi4 than to hy1-100. Compared with the hy1-100 mutant, seedlings of hy1-100/abi4 exhibited an ABA-insensitive phenotype, since the inhibition of seedling germination and the development of hy1-100 were largely impaired by the mutation of ABI4. However, on the medium without ABA, no obvious difference was observed between hy1-100 and hy1-100/abi4. Second, 5-d-old seedlings were transferred to ABA-containing medium to investigate the responses of primary root growth (Fig. 3, C and D). However, no distinguishable differences were observed among all genotypes, indicating that HY1 and ABI4 did not directly affect the ABA-triggered inhibition of primary root growth.
Figure 3.
ABA and drought responsiveness of the wild type, hy1-100, abi4, and hy1-100/abi4. A and B, Germination and postgermination assays (n = 50 from three independent experiments). Seeds of each ecotype were sown on MS medium with or without the indicated concentrations of ABA (1 μm for A). Plates were placed horizontally (A, left) or vertically (A, right, and B) for 7 d. Germination rate and primary root (PR) length of seedlings grown vertically were then measured (B). Bar = 2 cm. C and D, Primary root growth (n = 15 from three independent experiments). Five-day-old seedlings of each ecotype were transferred to MS medium with or without the indicated concentrations of ABA (10 μm for C). Relative primary root elongation was measured 7 d after treatments, taking the elongation rate of each ecotype in ABA-free MS medium as 100%. Bar = 1 cm. E, ABA-induced (10 μm) stomatal closure and inhibition of stomatal opening of each genotype (n = 50 from three independent experiments). Leaves of each ecotype were floated on buffer containing 50 mm KCl and 10 mm MES-Tris (pH 6.15) in the dark (D) or cold light (L; 200 μmol m−2 s−1) alone for 2 h or followed by the cold light or dark condition for another 2 h in the presence of 10 μm ABA, and then apertures were recorded. F, ABA-induced (100 μm) ABI4 expression in wild-type and hy1-100 mutant leaves. G, GUS staining of 1-week-old ABI4:GUS plants. ABI4:GUS seedlings were treated with or without ABA (10 μm) for 2 h after 2 h of pretreatment with ZnPPIX (a specific inhibitor of HO1; 100 μm). Bar = 2 mm. H, Phenotypes of each ecotype upon drought stress. Four-week-old plants were cultured in pots before stopping irrigation. The photographs show plants at days 21 and 25 after the application of drought stress. Data are means ± se from at least three independent experiments. Differences among treatments were analyzed by one-way ANOVA, taking P < 0.05 as significant according to Tukey’s multiple range test.
Stomatal closure and density are two important factors in the regulation of leaf water loss. With regard to stomatal movement, Finkelstein (1994) reported that the abi4 mutants showed no difference from the wild type in stomatal closure (data not shown). However, in this study, ABA-promoted stomatal closure and the inhibition of stomatal opening were largely impaired in the abi4 mutant (Fig. 3E). Regardless of these points, most importantly, the mutation of ABI4 was able to abolish the ABA-hypersensitive phenotypes of hy1-100, manifested in the reduced sensitivity to ABA-promoted stomatal closure and the inhibition of stomatal opening (Fig. 3E). Thus, these data place ABI4 linearly downstream of HY1 in the regulation of a subset of ABA responses, but not including primary root growth. Interestingly, in the hy1-100 mutant, the stomatal density was reduced, and the stomatal size was increased at the vegetative growth stage (Supplemental Figs. S9 and S10). However, these parameters returned to an approximately similar level to that of the wild type by the mutation of ABI4, further indicating that the guard cell developmental process might be regulated by HY1 and ABI4. To verify this hypothesis, the ostiole length-stoma length ratio in all genotypes was measured. An ostiole length:stoma length ratio higher than 1:3 was regarded as mature stoma (Merlot et al., 2001). As expected, the mutation of HY1 had no significant impact on the percentage of mature stomata in Arabidopsis leaves (the values of both the wild type and hy1-100 were approximately above 90%; Supplemental Fig. S11). By contrast, this value was decreased by approximately 50% in the abi4 and hy1-100/abi4 mutants. Interestingly, the pore sizes of mature stoma of abi4 and hy1-100/abi4 mutants were smaller than that of the wild type or the hy1-100 mutant, further suggesting that ABI4 had an impact on stomata pore size (Supplemental Fig. S12). Taken together, these results indicate that the premature stoma observed under our experimental conditions resulted mainly from the ABI4 mutation.
The fact that abi4 almost fully arrested the ABA hypersensitivity of the hy1-100 mutant led us to investigate whether HY1 could regulate ABI4. The transcript level of ABI4 was time-dependently enhanced by ABA in wild-type plants, while this enhancement was more pronounced in the hy1-100 mutant, after 30 min of treatment (Fig. 3F). To address whether there was any tissue-specific regulation of ABI4 transcription by HY1, an Arabidopsis line containing an ABI4 promoter-driven GUS gene was applied. This line could clearly establish the sites of ABI4 transcription and help demonstrate its biological relevance in ABA responses. As reported previously (Finkelstein et al., 2011), GUS activity was detected in cotyledons and hypocotyls but was less evident in roots (Fig. 3G). The GUS activity was activated by ABA and was moderately increased in cotyledons and hypocotyls by the addition of ZnPPIX, a potent HY1 inhibitor (Xie et al., 2011). An additive effect for ABA and ZnPPIX was also observed.
To further clarify the contributions of HY1 and ABI4 in the stomatal movement in response to drought stress in Arabidopsis, water-deficient assays of the above-mentioned mutant plants were performed. Although hy1-100 showed enhanced resistance to drought, the susceptible phenotypes of both abi4 and hy1-100/abi4 were not discernibly different from that of the wild type (day 25; Fig. 3H).
Participation of RbohD-Dependent ROS in hy1- and ABI4-Regulated Stomatal Movement
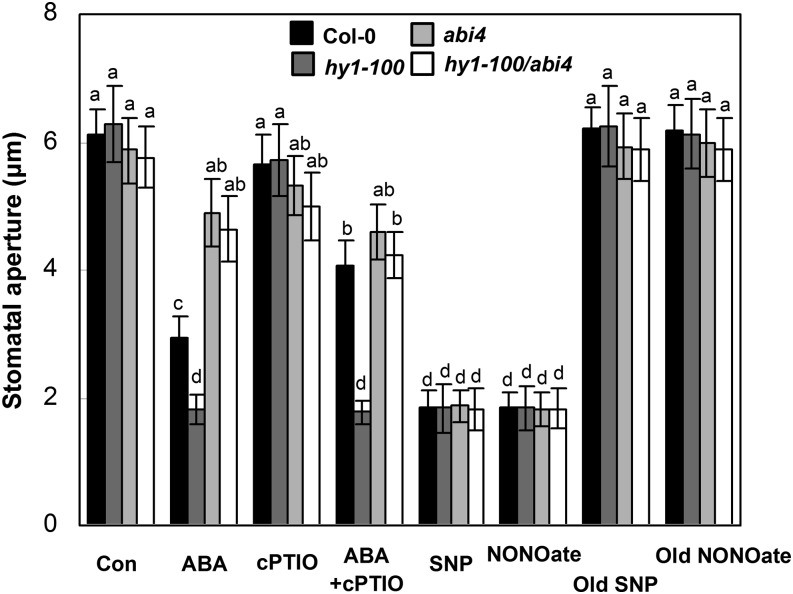
Since both NO and ROS are key signaling molecules involved in ABA-triggered stomatal closure (Bright et al., 2006), whether NO and ROS play any roles in the HY1- and ABI4-mediated signaling cascade was investigated. As expected, Figure 4 shows that cPTIO, a NO scavenger, could partially reverse ABA-induced stomatal closure in wild-type plants, while the stomatal aperture of hy1-100 (in particular), abi4, and hy1-100/abi4 was not much altered.
Figure 4.
Effects of ABA, sodium nitroprusside (SNP), NONOate, and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) treatments on the stomatal aperture of the wild type and hy1-100, abi4, and hy1-100/abi4 mutants. Arabidopsis leaves of each ecotype were treated with or without ABA (10 μm) or SNP (50 μm), NONOate (50 μm), Old SNP (50 μm), or Old NONOate (50 μm) in MES-KCl buffer for 2 h after 0.5 h of pretreatment with cPTIO (400 μm). Photographs were immediately taken with a microscope. Stomatal apertures were then measured (n = 50 from three independent experiments). Seedlings without chemical treatments were regarded as controls (Con). Data are means ± se from at least three independent experiments. Differences among treatments were analyzed by one-way ANOVA, taking P < 0.05 as significant according to Tukey’s multiple range test.
Meanwhile, the application of SNP or diethylamine NONOate sodium salt hydrate (NONOate), two well-known NO-releasing compounds as positive controls, was found to trigger a similar level of stomatal closure in all genotypes. By contrast, treatments with Old SNP and Old NONOate, which were used as negative controls of SNP and NONOate, respectively (Tossi et al., 2009; Xie et al., 2013), had no significant impact on the stomatal aperture of the wild type, abi4, hy1-100, and hy1-100/abi4. These results thus confirmed that NO, but not the degradation products of SNP and NONOate, contributed to the stomatal closure under our experimental conditions.
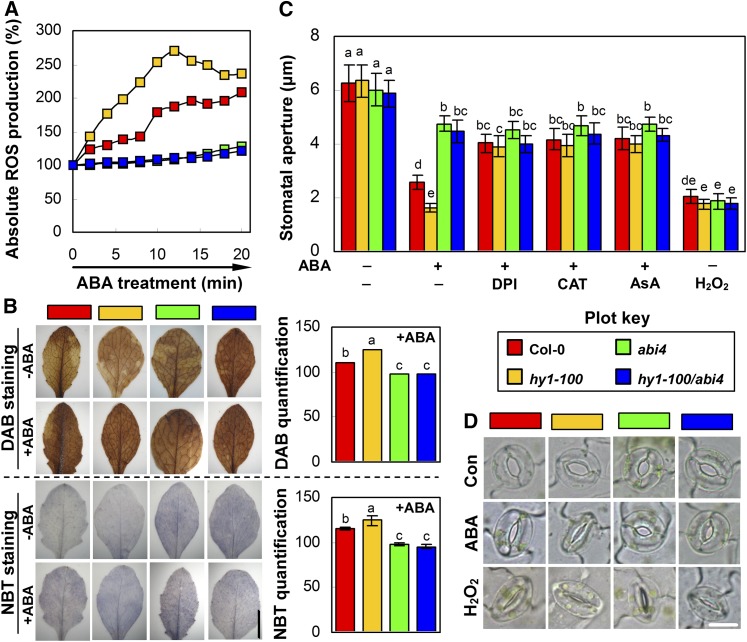
Laser scanning confocal microscopy was applied to measure ROS levels, taking relative ROS production of the wild type at 0 h as 100% (Kwak et al., 2003; Jannat et al., 2011). Subsequent data showed that, upon ABA treatment, the ROS levels in the stomata of hy1-100, abi4, and hy1-100/abi4 plants were generated differentially with respect to that of the wild type. As shown in Figure 5A, a basal level of ROS accumulation in all mutant stomata was close to the wild-type level (0 min; constitutive ROS production). In the wild type, ABA treatment boosted inducible ROS production, which was increased to a greater extent in ABA-treated hy1-100 stomata. By contrast, ROS production remained relatively constant in the guard cells of abi4 and hy1-100/abi4 stomata. Combined with the observed alternation of the stomatal aperture (Fig. 5D), these results indicated that ABA-inducible cytosolic ROS elevation, but not constitutive ROS production, functions in ABA-, HY1-, and ABI4-regulated stomatal closure (Jannat et al., 2011). However, these promoted or decreased inducible ROS levels were less pronounced as detected by nitroblue tetrazolium (NBT) or 3,3′-diaminobenzidine (DAB) staining. These results may be due to the tissue-specific modulation of endogenous ROS production in the guard cells or to the low specificity and resolution of histochemical staining (Fig. 5B).
Figure 5.
ROS generation is required for the hy1- and ABI4-mediated Arabidopsis stomatal closure. A, Confocal analysis of ROS production in the stomata of the wild type and hy1-100, abi4, and hy1-100/abi4 mutants upon 10 μm ABA for 20 min. ROS production of the wild type at 0 min was regarded as 100%. B, Histochemical detection of ABA-induced hydrogen peroxide (H2O2) and superoxide radical production. Four-week-old seedling leaves of each ecotype were treated with 10 μm ABA for 2 h and stained with DAB or NBT (left). Bar = 1 cm. Randomly selected leaves were used for quantification (right), separately taking values of corresponding untreated control lines (−ABA) as 100%. C, Stomatal aperture (n = 50 from three independent experiments). Arabidopsis leaves of each ecotype were treated with ABA (10 μm) or H2O2 (100 μm) in MES-KCl buffer for 2 h after 0.5 h of pretreatment with diphenyleneiodonium (DPI; 50 μm), catalase (CAT; 60 units mL−1), or ascorbic acid (AsA; 100 μm). Data are means ± se from at least three independent experiments. Differences among treatments were analyzed by one-way ANOVA, taking P < 0.05 as significant according to Tukey’s multiple range test. D, Representative images are shown. Bar = 10 μm.
The discrepancies of stomatal behaviors among these mutants were further compared. Stomatal bioassay experiments revealed that the mature stomatal aperture of the ABA-treated hy1-100 mutant decreased to a greater degree, with respect to the wild type, whereas it decreased to a much lesser extent in the abi4 and hy1-100/abi4 mutants upon ABA (Fig. 5, C and D). The parameter of pore size also displayed similar tendencies (Supplemental Fig. S12). The confirmation and potential sources of hy1-induced ROS generation were evaluated using CAT, AsA, or DPI, which either remove H2O2 or reduce ROS production by the inhibition of NADPH oxidase (Zhang et al., 2001). Pretreatment with all the above-mentioned compounds individually had no significant impact on ABA-induced stomatal closure in the abi4 and hy1-100/abi4 plants, whereas it greatly abolished that of hy1-100 to approximately the same level as the wild type. As a positive control, the stomata of all mutants were significantly closed by exogenous H2O2 to approximately the same degree as the wild type, as evaluated by the stomatal aperture and pore size (Fig. 5, C and D; Supplemental Fig. S12).
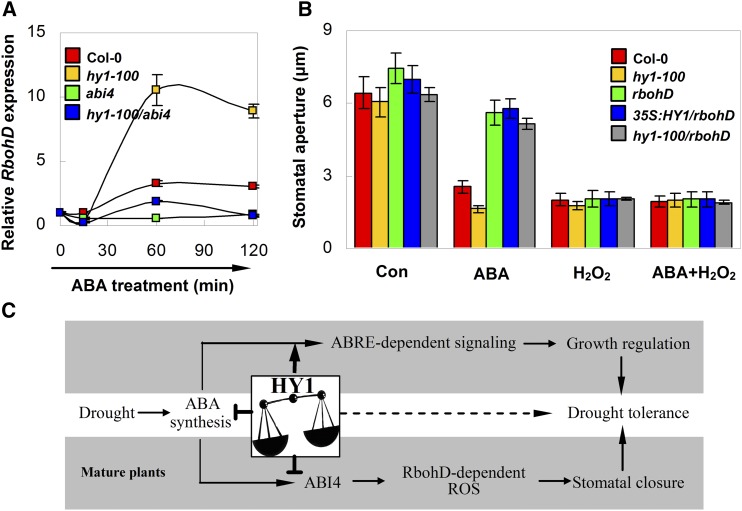
NADPH oxidase is mainly responsible for ABA-induced ROS generation and stomatal closure (Torres and Dangl, 2005), and the interrelationship between RbohD and HY1 involved in salt acclimation was reported previously (Xie et al., 2013). To characterize the involvement of RbohD in HY1/ABI4-mediated ABA signaling, we examined the time-course changes of RbohD transcripts in HY1-null and/or ABI4-null mutants upon ABA treatment for 120 min. The ABA-induced RbohD transcripts were strengthened in the hy1-100 mutant but largely attenuated in abi4 and hy1-100/abi4 (Fig. 6A). If RbohD is a downstream component of HY1/ABI4 signaling, we deduced that overexpression or mutation of HY1 may not change the reduced ABA sensitivity of the rbohD mutant in terms of stomatal movement. Therefore, the HY1 overexpression or HY1-null mutant in the rbohD mutant background was generated. In the presence of ABA, overexpression or mutation of HY1 in the rbohD background (35S:HY1/rbohD or hy1-100/rbohD) did not alter its ABA-insensitive phenotype with regard to stomatal closure (Fig. 6B). However, exogenously applied H2O2 could efficiently induce stomatal closure in rbohD, 35S:HY1/rbohD, and hy1-100/rbohD mutant lines as well as in the hy1-100 mutant.
Figure 6.
RbohD functions downstream of hy1 and ABI4 in ABA-induced stomatal closure. A, Time course of Q-PCR analysis of RbohD expression in 4-week-old leaves of each ecotype in response to ABA (10 μm) for 2 h. The expression of RbohD is presented relative to that of the corresponding samples at 0 h. B, Stomatal aperture (n = 50 from three independent experiments). Arabidopsis leaves of each ecotype were treated with ABA (10 μm) or H2O2 (100 μm) in MES-KCl buffer for 2 h. Values are means ± se of at least three independent experiments. C, Schematic model describing the hy1-mediated, drought-induced ABA responsiveness. The T-bar denotes inhibition. ABRE, ABA response element.
In concordance with the Q-PCR results obtained from the desiccation experiment (Supplemental Fig. S2), ABA-induced GORK activation was pronounced in the hy1-100 mutant compared with that of the wild type. In contrast, it was abolished by abi4 (Supplemental Fig. S13). Compared with the wild type, the stomatal closure of the gork mutant was greatly impaired when ABA or H2O2 was applied exogenously, individually or simultaneously (Supplemental Fig. S14). Taken together, the above results place RbohD-derived ROS as a downstream component of HY1/ABI4 signaling, subsequently activating GORK in ABA-induced stomatal closure and drought tolerance.
DISCUSSION
The increasing evidence of HO mediating a wide array of plant physiological processes contrasts with our limited knowledge about the identification of downstream components of its intrinsic signaling cascade (Shekhawat and Verma, 2010). In this study, by using genetic, pharmacological, and physiological approaches, we showed the biological functions of HY1 during drought stress responses, in which HY1 negatively regulated ABI4 in ABA-induced stomatal closure driven by the RbohD-derived ROS. By investigating the consequences of loss and gain of function of HY1 on the drought stress adaptation of Arabidopsis plants, we found that hy1 mutants were tolerant to drought stress (Fig. 1A). In agreement with the loss-of-function study, overexpression of HY1 consequently impaired the drought tolerance of transgenic plants. These results clearly demonstrated that HY1 can act as a negative regulator in drought stress signaling in Arabidopsis (Fig. 6C).
A subsequent question was, how did a positive regulator of salinity signaling (Xie et al., 2011) negatively regulate Arabidopsis drought tolerance? In an attempt to explain this phenomenon at the molecular level, we performed RNA-Seq analysis, which revealed an impaired expression of multiple stress- and/or ABA-inducible genes in the hy1-100 mutant upon water deficiency (Fig. 1, B and C). Some of these genes had functions in ABA-dependent pathways, including GST11 and RD29A, whose overexpression enhanced drought tolerance (Supplemental Table S1; Umezawa et al., 2006; Valliyodan and Nguyen, 2006; Seki et al., 2007). The transcriptomic results showed that HY1 might function upstream of these ABA-inducible genes and positively control the ABA signaling pathway by the induction of multiple stress-responsive genes. However, it was observed that the expression of these stress-inducible genes was enriched in the wild type, which seemed to contradict the drought-tolerant phenotype of hy1-100. Furthermore, it was noteworthy that a group of genes belonging to the GO transport category were strongly up-regulated in the hy1-100 mutant upon drought stress, in comparison with the wild type (Fig. 1C). These genes encode important transmembrane transporters that function to move ions or anions across the plasma membrane, such as AtHAK5, MATE efflux family proteins, as well as GORK (Supplemental Table S1). It is possible that the observed enhanced expression of the transporters could result in an increased ion flux across the membranes, with guard cell potassium efflux in particular, which may in turn regulate the cell osmolarity and degree of stomatal apertures, subsequently leading to drought tolerance. Accordingly, it was reported that Arabidopsis plants with impaired K+ efflux ability presented impaired ABA-mediated stomatal closing and reduced survival of drought stress (Osakabe et al., 2013). We will discuss and verify this point in detail in the following section.
It is well known that ABA regulates plant drought adaptation mainly through its functions in cellular dehydration tolerance and by conserving a higher water status (Lee and Luan, 2012). The former role has been attributed mainly to the induction of dehydration-responsive genes, and the latter trait was associated with the regulation of guard cell movement (Pierik and Testerink, 2014). Our examination of several physiological aspects demonstrated that the hy1-dependent Arabidopsis drought tolerance was ascribed to the function of HY1 in the regulation of ABA contents and responses, with guard cell movement in particular (Fig. 2; Supplemental Fig. S1). For example, compared with the wild type, seed germination, early seedling development and growth (except primary root growth), as well as stomatal closure were hypersensitive to ABA in the hy1-100 and hy1-1 mutant alleles but ABA insensitive in HY1-overexpressed transgenic plants. In particular, our stomata results illustrated that HY1 negatively affected ABA signaling in guard cells, thereafter modulating Arabidopsis drought tolerance. In comparison, Shen et al. (2006) and Tomiyama et al. (2014) showed normal or partial ABA-insensitive phenotypes in the genome uncoupled2 (gun2)/hy1 mutant, respectively. Combined with our findings, these results added more complex features to the control of guard cell signaling, and further investigation will be needed to fully clarify the direct target of HY1 involved in ABA signaling in guard cells. Meanwhile, one possible explanation of the above-mentioned impairment of the up-regulation of stress/ABA-inducible genes in drought-stressed hy1-100 plants might be its hypersensitivity in terms of stomatal closure. This, in turn, would conserve a higher water status, thereby resulting in down-regulation feedback on the induced genes. It was noteworthy that the ABA-induced responses, such as radicle protrusion (Fig. 2D) and stomatal movement (Fig. 2C), were greatly impaired but not fully eliminated when HY1 was overexpressed, indicating that some ABA signaling cascades remain. We deduced that additional factors may retain sufficient activity to trigger the observed residual response. Another possibility was that there was an HY1-independent, ABA-responsive pathway that controls these responses. It was also observed that disruption or overexpression of HY1 did not cause significant alternation of the ABA-inhibited primary root growth. These results implied the functional segregation of HY1 in the regulation of multiple ABA responses. Similar segregated behavior was found between Arabidopsis SnRK2.6 and SnRK2.2/2.3, suggesting that SnRK2.6 functions in guard cells, whereas SnRK2.2/2.3 specializes in seed germination and seedling growth (Fujii and Zhu, 2009). Taken together, the improved performance of the hy1-100 mutant plants under limiting water conditions was associated with its enhanced ABA content and increased ABA sensitivity, thus leading to the promotion of the ABA-induced stomatal closure.
The formation of biliverdin catalyzed by HY1 plays a fundamental role in phytochrome chromophore biosynthesis. Biliverdin is coordinated with phytochrome apoprotein biosynthesis pathways to synthesize photochemically active phytochrome, an essential step for proper photomorphogenesis in plants (Muramoto et al., 1999). The cytosol-synthesized PHYTOCHROME (PHY) apoproteins are encoded by small families of nuclear genes (PHYA–PHYE in Arabidopsis; Sharrock and Quail, 1989). It has been reported that PHYB increases drought tolerance by enhancing ABA sensitivity in Arabidopsis (González et al., 2012). It should be noted that hy1 is a phytochromobilin-deficient mutant (Muramoto et al., 1999). Therefore, the hypersensitive closed-stomata phenotype in hy1 might be unlikely due to the PHY-associated light signaling.
HY1 was identified through the characterization of gun2 mutants that block the chloroplast-to-nucleus retrograde signaling (Muramoto et al., 1999; Mochizuki et al., 2001). ABI4 has been proposed as the master switch that controls the expression of a large number of nuclear genes in response to plastid-derived signals (Wind et al., 2013). Ample evidence implies that plastid-to-nucleus signaling and ABA signaling may be interconnected (Voigt et al., 2010). For example, the chloroplast protein PTM connects the plastid GUN1 pathway with the nuclear ABI4 pathway (Sun et al., 2011), while downstream components of ABI4 are CYP707A1 and CYP707A2 as well as a cluster of the heme activator proteins (Zhang et al., 2013). Our results suggested a potent coordination between HY1 and ABI4 involved in chloroplast-to-nucleus retrograde signaling (Supplemental Fig. S8). It was further demonstrated that ABI4 appears to be a downstream component of HY1 in controlling a subset of ABA responses. First, ABA-induced ABI4 mRNA abundance was more pronounced in the hy1-100 mutant seedling, suggesting the processing role of hy1 in the activation of ABI4 (Fig. 3F). Second, ABI4 promoter-derived GUS activities were moderately higher when HY1 activity was blocked by ZnPPIX in the presence or absence of ABA (Fig. 3G). Therefore, the promotion of ABA-induced ABI4 is most likely caused by the loss of function of HY1 in the hy1-100 mutant. Third, the ABA-hypersensitive phenotype of hy1-100, in terms of ABA-inhibited seed germination and postgermination development, as well as the guard cell movement but not primary root growth, could be almost fully abolished by the mutation of ABI4 (Fig. 3, A–E). Finally, guard cell developmental processes were also regulated by HY1 and ABI4, as evaluated by stoma size and number (Supplemental Figs. S9 and S10). Importantly, the drought-tolerant phenotype of hy1-100 was markedly blocked by the mutation of ABI4, while a single mutation of ABI4 did not significantly alter the plant response to drought stress compared with that of the wild type (Fig. 3H). Similarly, the abi4 mutant showed weaker drought tolerance after norflurazon treatment (Zhang et al., 2013). These results all supported the view that tetrapyrrole and ABI4 signaling were coordinated and indicated the biological significance of retrograde signaling associated with plant drought tolerance. In contrast, Finkelstein (1994) reported that the abi4 mutants showed no difference from the wild type in stomatal closure (data not shown). These discrepancies indicated the complex signaling network of guard cells and plant drought tolerance.
Previous studies have reported the linearity existing among ABA, H2O2, and NO signaling in the control of stomatal closure and drought tolerance (Bright et al., 2006; Wilkinson and Davies, 2010). Previous investigations have also showed that ABI4 was activated to regulate redox signaling in mature plants (Kerchev et al., 2011, 2013). In this study, a genetic approach was adopted to characterize the downstream module of the HY1-ABI4-mediated ABA response with respect to stomatal closure and drought tolerance. Our assessment of stomatal aperture has clarified that the HY1-ABI4-controlled guard cell movement was associated with RbohD-dependent ROS production (Fig. 5). Remarkably, ROS production in guard cells or leaves in hy1-100 plants was promoted, as monitored using a ROS fluorescence probe. The concomitant stomatal closure was largely impaired by abi4 compared with the wild type (Fig. 5, A and C). Stomatal closure of the ABA-treated hy1-100 mutant could be attenuated by the removal of H2O2. In a number of physiological responses, especially guard movement, NO production was dependent on ROS generation (especially H2O2 synthesis; Bright et al., 2006). The data in this study demonstrated that ABA-induced stomatal closure in wild-type plants was greatly reduced in the presence of the NO scavenger cPTIO but was not so strongly affected in the hy1-100, abi4, and hy1-100/abi4 mutant lines (Fig. 4). These data implied that endogenous NO may not be involved in hy1-promoted ABA hypersensitivity in stomatal closure, at least under our experimental conditions. By contrast, all mutants responded significantly to SNP and NONOate, two NO-releasing compounds, as well as in the wild type. Our data thus provide evidence that the ABA-induced HY1-ABI4-ROS guard cell signaling propagation remains divergent from NO signaling at some points and may relate to a mechanism that is independent of de novo NO biosynthesis (Lozano-Juste and León, 2010).
It has been demonstrated that ROS mainly produced by NADPH oxidases are crucial for mediating ion homeostasis in Arabidopsis roots (Ma et al., 2012). In this study, we present evidence of a positive function for RbohD in HY1-ABI4 signal propagation and the activation of stomatal closure. The increasing tendencies of ABA-induced RbohD expression in the wild type were accelerated in hy1-100 but depressed in abi4 and hy1-100/abi4 mutants (Fig. 6A). These results implied a putative role of hy1 and ABI4 in the activation of RbohD expression, which would be important for controlling guard cell movement. Moreover, either overexpression or null mutation of HY1 could not alter the ABA hyposensitivity of rbohD in terms of stomatal closure (Fig. 6B), further placing RbohD as a downstream component of HY1-ABI4 signaling in the wild type. However, compared with wild-type plants, all mutant lines responded strongly to exogenous H2O2, which was similar to the behavior of SNP and NONOate (Fig. 4). Taken together, these results identified the requirement for RbohD-related ROS generation in the propagation of ABA-activated HY1-ABI4 signaling to control stomatal closure in wild-type plants. Our previous investigations reported the participation of RbohD-derived ROS in relaying HY1-mediated salt acclimation signaling (Xie et al., 2011). RbohD was also required for the pathogen response and systemic signaling (Marino et al., 2012). Therefore, in order to adapt to the continually exposed changes of living conditions, the differential organic localization (root or leaf, etc.) of RbohD appears to facilitate its broad-range functions in the spatiotemporal control of ROS production and delivering complex signaling processes.
Unequivocally, the GORK channel is highly expressed in guard cells and thought to provide the main barrier for K+ loss to drive stomatal closure, which is important for plant adaptation to environmental changes (Ache et al., 2000; Imes et al., 2013; Osakabe et al., 2013). Our transcriptomic and Q-PCR data showed that a subset of transporter transcripts were strongly up-regulated in the hy1-100 mutant upon drought stress, including GORK (Supplemental Table S2; Supplemental Fig. S1). Preliminary molecular, physiological, and genetic experiments illustrated that Arabidopsis lacking functional GORK displays impaired stomatal closure in response to ABA and H2O2 (Supplemental Fig. S14). These results implied the potential role of GORK activation (K+ homeostasis) for HY1-ABI4-RbohD-mediated ABA signaling in the induction of stomatal closure and drought stress responses. In addition, the differential cell part localization of HY1 (plastid; Muramoto et al., 1999), ABI4 (nucleus; Sun et al., 2011; Zhang et al., 2013), and GORK (guard cell; Ache et al., 2000; Osakabe et al., 2013) suggested that we still do not know what determines the relay of this observed cascade, directly or indirectly.
HY1 is involved in the tetrapyrrole biosynthetic pathway. Recently, the significance of tetrapyrrole-based drought stress signaling was addressed, and this signaling was favored toward free heme production owing to drought-induced secondary events, including chloroplast-localized oxidative stress (Nagahatenna et al., 2015). Therefore, it was reasonable to hypothesize that the hy1-promoted drought tolerance may result from the sustained free heme status (Phung et al., 2011). Free heme was proposed to activate a series of drought-responsive and ROS detoxification genes that could also be utilized to generate heme-derived antioxidant biomolecules for defense. However, noncovalently bound heme in hy1-100 seedlings was lower than that in the wild type (Supplemental Table S1). More importantly, the transcriptomic data showed that a broad range of genes related to abiotic/biotic stress responses appeared in the wild type upon drought stress, but not in the hy1-100 mutant (Fig. 1C). It was also observed that ROS production was more pronounced upon ABA treatment in hy1-100 leaves (Fig. 5, A and B). Therefore, these results indicated that free heme status does not account for the drought-tolerant phenotype of the hy1 mutant, indicating that other factors may be responsible for hy1-promoted Arabidopsis drought tolerance. Supporting this notion was the finding that Arabidopsis ferrochelatase1 mutants produced significantly less total heme upon oxidative stress when compared with wild-type plants (Nagai et al., 2007), whereas no expected hypersensitive mutant phenotype was observed (Scharfenberg et al., 2015). Thus, future investigations should unravel the interrelationship between the biological function of HY1 and free heme-associated ROS detoxification.
In conclusion, we have identified, to our knowledge, a novel pathway linking the mutation of HY1 with ABA biosynthesis, several ABA responses, and drought tolerance. Disruption of HY1 expression leads to the activation of ABI4, thereby increasing the RbohD enzyme with consequently elevated levels of ROS production (Fig. 6C). Thereafter, the increased ROS induce stomatal closure, as reflected in ABA-promoted drought tolerance. It is possible that ion channels such as GORK participate in this signal propagation and also function in the stomatal response, thus enhancing drought tolerance (Osakabe et al., 2013). These findings extend our knowledge of the gene expression regulation network to stress conditions. They also open the possibility to engineer transgenic plants with enhanced drought tolerance by reducing HY1 levels using a guard cell-specific approach.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) mutants hy1-100 (CS236; Col-0), ho2 (SALK_025840), ho3 (SALK_034321), ho4 (SALK_044934), and abi4 (CS8104; Col-0) were obtained from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/abrc), and the homogenous HY1 overexpression lines 35S:HY1-3 and 35S:HY1-4 (Col-0) were constructed previously and used (Xie et al., 2011, 2012; Jayakannan et al., 2013). The rbohD and hy1-1 (CS67; Landsberg erecta) homozygous mutants were a generous gift from W.H. Zhang (Department of Plant Science, Nanjing Agricultural University) and L.X. Zhu (College of Plant Science and Technology, Huazhong Agricultural University). The transgenic 35S:HY1/rbohD line was generated by overexpressing HY1 in the rbohD mutant background. The double mutant lines hy1-100/ho4, hy1-100/abi4, and hy1-100/rbohD were obtained by crossing ho4, abi4, and rbohD with hy1-100. Homozygous hy1-related mutants were identified by sequencing, combined with PCR-based genotyping and corresponding phenotypes (including yellow cotyledons and ABA hypersensitivity; Xie et al., 2013). Seeds were surface sterilized and washed three times with sterile water for 20 min, then cultured in petri dishes on solid MS medium (pH 5.8). Plants were grown in a growth chamber with a 16/8-h (23°C/18°C) day/night regime at 120 μmol m−2 s−1 irradiation.
Drought Tolerance and Phenotypic Analysis
For the drought stress survival assays, 4-week-old plants were grown in potting soil saturated with water. Drought stress was then conducted over the 17 d by withholding water, followed by a further 5 d of rewatering (21 and 25 d for hy1-100- and abi4-related plants). All experiments were repeated at least three times, and representative photographs were taken.
For phenotypic analysis, stratified seeds were germinated on the MS medium with or without ABA at the indicated concentrations for the indicated times. Alternatively, 5-d-old seedlings of each genotype were cultured in MS medium with or without the indicated concentrations of ABA for the indicated times. The phenotypes, including the primary root length, green and open cotyledons, germination rate, and primary root elongation, were then measured (Xie et al., 2011, 2013). Meanwhile, representative images were made.
For the stomatal bioassay, the experimental details are described in the figure legends. At the indicated times, stomatal apertures were captured using a light microscope equipped with an imaging camera (model Stemi 2000-C; Carl Zeiss) and analyzed with ImageJ software (supplied by the National Center for Biotechnology Information and available at http://rsb.info.nih.gov/ij) to measure apertures. Within each time point or treatment, 30 stomata were randomly selected and recorded in six independent replicates.
RNA-Seq Analysis
Detached leaves of wild-type and hy1-100 plants were sampled for RNA-Seq experiments. The process of RNA-Seq analysis was performed according to the standard procedure of the SOLEXA high-throughput sequencing service (Oebiotech). Data were extracted and normalized according to the manufacturer’s standard protocol. Log-fold changes of DEGs (up- or down-regulated) between the mutant and wild-type plants were selected with a significance threshold of P < 0.05. The biological process pathways annotation was conducted using the Database for Annotation, Visualization, and Integrated Discovery (Huang et al., 2009) and The Arabidopsis Information Resource database (http://www.Arabidopsis.org). The RNA-Seq results were then confirmed by Q-PCR.
Q-PCR Analysis
Q-PCR analysis was performed as described (Xie et al., 2014). The specific primers used for PCR are listed in Supplemental Table S3.
Analysis of ABA Content
The ABA concentration of leaves was measured by the Phytodetek competitive ELISA kit (Agdia). All plant leaves were detached and sampled at the indicated times, followed by an overnight extraction in 80% acetone (Artsaenko et al., 1995). ABA was quantified according to the manufacturer’s instructions.
Detection of GUS Activity
Arabidopsis plants containing the ABI4::GUS construct were grown on plates with one-half-strength MS medium at 22°C. To test the induction of ABI4 promoter activity, 1-week-old seedlings were subjected to different treatments. Afterward, the seedlings were immersed in staining buffer (50 mm sodium phosphate, 10 mm Na2EDTA, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, and 0.1% Triton X-100, pH 7), stained with 5-bromo-4-chloro-3-indolyl-β-glucuronidic acid at 37°C for 48 h, and cleared in 70% ethanol for 48 h at 37°C (Jefferson et al., 1987; Söderman et al., 2000). Photographs of the decolorized tissues were recorded using a stereomicroscope (model Stemi 2000-C; Carl Zeiss).
Measurement of Leaf Stomatal Density and Stomatal Dimension
For the measurements of stomatal density, dimension, and pore size, epidermal strips from 4-week-plant leaves were used. At the indicated times, all samples were captured using a light microscope (200×) equipped with a digital camera (model Stemi 2000-C; Carl Zeiss). The number of stomata was determined on both the adaxial and abaxial sides of five leaves and then converted into a stomatal density value (Kondo et al., 2010). Relative stomatal area, stomatal length, width, dimension, and pore size were analyzed with ImageJ software (supplied by the National Center for Biotechnology Information and available at http://rsb.info.nih.gov/ij) to measure apertures. Only mature stomata, whose ostiole length was greater than one-third of the length of stoma, were taken into account (Merlot et al., 2001).
Stomatal Bioassay
To study the effects of ABA and light on stomatal closure, Arabidopsis leaves of each ecotype were floated on MES-KCl buffer, which contained 50 mm KCl and 10 mm MES-Tris (pH 6.15), in the dark or cold light (200 μmol m−2 s−1) alone for 2 h or followed by the cold light or dark condition for another 2 h in the presence of ABA at the indicated concentrations. To investigate the effects of DPI, CAT, AsA, and H2O2 on ABA-induced stomatal closure, Arabidopsis leaves of each ecotype were treated with ABA (10 μm), H2O2 (100 μm), or their combination in MES-Tris buffer for 2 h after 0.5 h of pretreatment with DPI (50 μm), CAT (60 units mL−1), or AsA (100 μm; Zhang et al., 2001; Bright et al., 2006).
In our experimental conditions, the Old SNP/NONOate solutions were used as negative controls of SNP/NONOate by maintaining the SNP/NONOate solution (50 μm) for at least 10 d in the light in an open tube to eliminate NO (Tossi et al., 2009; Xie et al., 2013). Therefore, the Old SNP/NONOate solution contains only the degradation product of SNP/NONOate except NO. To investigate the effect of NO on ABA-induced stomatal closure, Arabidopsis leaves of each ecotype were treated with or without ABA (10 μm), SNP (50 μm), NONOate (50 μm), Old SNP (50 μm), or Old NONOate (50 μm) in MES-Tris buffer for 2 h after 0.5 h of pretreatment with 400 μm cPTIO. The chemical concentrations used in this study were determined from previous reports (Bright et al., 2006; Xie et al., 2013). Stomatal apertures were randomly selected for three independent repeats (n = 50) and measured with a microscope equipped with an imaging camera (model Stemi 2000-C; Carl Zeiss). Stomatal apertures were analyzed with ImageJ software.
Confocal Laser Scanning Microscopy
Endogenous ROS production was examined with 2′,7′-dichlorodihydrofluorescein diacetate (Sigma) as described previously (Bright et al., 2006; Xie et al., 2011, 2014). Epidermal fragments from 4-week-old plants were loaded with 50 μm 2′,7′-dichlorodihydrofluorescein diacetate for 15 min before washing in MES-KCl buffer three times for 5 min each. Fragments were then treated with various reagents as indicated in the figure legends. All manipulations were performed at 25°C ± 1°C. At each sampling time, at least 50 guard cells in three independent epidermal strips were observed using a TCS-SP2 confocal laser scanning microscope (excitation at 488 nm and emission at 500–530 nm). To measure the relative fluorescence intensity in guard cells, images acquired were analyzed via the Leica software (Sieberer et al., 2009; Liesche and Schulz, 2012). Data were calculated as means ± se of pixel intensities.
Histochemical Staining
The generation of H2O2 or superoxide radical was detected by DAB or NBT staining, respectively (Lv et al., 2011). Four-week-old plant leaves were immersed in freshly prepared 0.1% (w/v) DAB solution (pH 3.8), vacuum infiltrated, and then incubated overnight in darkness at 22°C. Alternatively, leaves were stained with 0.1% NBT solution in 10 mm potassium phosphate buffer (pH 7.8) containing 10 mm NaN3, vacuum infiltrated, and then incubated in darkness at 22°C for 1 h. Afterward, the stained leaves were placed in a solution containing acetic acid:glycerol:ethanol (1:1:3, v/v/v) at 95°C for 10 min and then stored in 95% ethanol until photographed (model Stemi 2000-C; Carl Zeiss).
Chloroplast-to-Nucleus Retrograde Signaling
Norflurazon and lincomycin treatments were performed to determine the retrograde signaling (Kerchev et al., 2011; Sun et al., 2011). Seedlings were treated as indicated in the figure legends, collected, and then frozen in liquid nitrogen, and total RNA was isolated. LHCB, CA, and CP transcript levels were determined with Q-PCR.
Statistical Analysis
Data are means ± se from at least three independent experiments. For statistical analysis, Tukey’s multiple range test (P < 0.05) was chosen.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ABI4(At2g40220), Actin2/7 (NM_121018), CA(At3g01500), CP(At3g62410), Gork (At5g37500), LHCB(At1g29910), RAB18(At5g66400), RbohD (At5g47910), RD22(At5g25610), RD29a(At5g52310), WRKY45(At3g01970).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phenotypes and ABA levels of the hy1-1 mutant in response to drought or ABA treatment.
Supplemental Figure S2. Hierarchical cluster of all DEGs in the RNA-Seq experiment.
Supplemental Figure S3. Q-PCR validation for the fold change of representative genes of wild-type and HY1-loss mutant detached leaves in response to drought stress desiccation for 3 h.
Supplemental Figure S4. Time-course analysis of the radicle protrusion of the wild type and HY1 loss- and gain-of-function mutants in response to 1 μm ABA.
Supplemental Figure S5. Osmotic phenotypic analyses of the wild type, hy1-100, ho2, ho3, and ho4 mutants, hy1-100/ho4, and the HY1 overexpression lines 35S:HY1-3/4.
Supplemental Figure S6. ABA contents in wild-type and hy1-100 mutant seedlings treated with or without norflurazon for 5 d.
Supplemental Figure S7. Impact of lincomycin treatment on gene expression in wild-type and hy1-100 seedlings.
Supplemental Figure S8. Impact of norflurazon and lincomycin treatments on LHCB, CA, and CP transcript levels in wild-type, hy1-100, abi4, and hy1-100/abi4 seedlings.
Supplemental Figure S9. Comparisons of stomatal and cell density and relative stomatal area in adaxial epidermis of the wild type and hy1-100, abi4, and hy1-100/abi4 mutants.
Supplemental Figure S10. Comparisons of stomatal length, stomatal width, and stomatal dimension in adaxial epidermis of the wild type and hy1-100, abi4, and hy1-100/abi4 mutants.
Supplemental Figure S11. Comparisons of the percentage of mature stoma in leaves of the wild type and hy1-100, abi4, and hy1-100/abi4 mutants.
Supplemental Figure S12. Comparisons of stomatal pore size in leaves of the wild type and hy1-100, abi4, and hy1-100/abi4 mutants.
Supplemental Figure S13. ABA-induced GORK gene expression in 4-week-old wild-type, hy1-100, abi4, and hy1-100/abi4 mutant leaves.
Supplemental Figure S14. Relative stomatal aperture of gork mutant plants in response to ABA or H2O2.
Supplemental Table S1. Quantification of noncovalently bound heme and HO activity in wild-type and hy1-100 seedlings.
Supplemental Table S2. DEGs in hy1-100 relative to the wild type under both well-watered and desiccated conditions.
Supplemental Table S3. Sequences of PCR primers for Q-PCR.
Supplementary Material
Glossary
- ABA
abscisic acid
- ROS
reactive oxygen species
- NO
nitric oxide
- RNA-Seq
RNA sequencing
- GORK
guard cell outward-rectifying K+
- DEGs
differentially expressed genes
- GO
Gene Ontology
- Q-PCR
real-time reverse transcription-PCR
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- SNP
sodium nitroprusside
- NBT
nitroblue tetrazolium
- DAB
3,3′-diaminobenzidine
- CAT
catalase
- AsA
ascorbic acid
- DPI
diphenyleneiodonium
- H2O2
hydrogen peroxide
- Col-0
Columbia-0
- MS
Murashige and Skoog
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31200195 to Y.X.), the Fundamental Research Funds for the Central Universities (grant no. KYTZ201529 to Y.X. and grant no. KYTZ201402 to W.S.), the Natural Science Foundation of Jiangsu Province (grant no. BK2012364 to Y.X.), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett 486: 93–98 [DOI] [PubMed] [Google Scholar]
- Artsaenko O, Peisker M, zur Nieden U, Fiedler U, Weiler EW, Müntz K, Conrad U (1995) Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J 8: 745–750 [DOI] [PubMed] [Google Scholar]
- Bartels P, Watson C (1978) Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci 26: 198–203 [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Cao Z, Huang B, Wang Q, Xuan W, Ling T, Zhang B, Chen X, Nie L, Shen W (2007) Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin Sci Bull 52: 2365–2373 [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Davis SJ, Kurepa J, Vierstra RD (1999) The Arabidopsis thaliana HY1 locus, required for phytochrome-chromophore biosynthesis, encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA 96: 6541–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg TJ, Walker JM, Noh B, Vierstra RD (2006) Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol 140: 856–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman LJ, Sun PS (1986) Effects of norflurazon, an inhibitor of carotenogenesis, on abscisic acid and xanthoxin in the caps of gravistimulated maize roots. Physiol Plant 67: 472–476 [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Lynch T, Reeves W, Petitfils M, Mostachetti M (2011) Accumulation of the transcription factor ABA-insensitive (ABI)4 is tightly regulated post-transcriptionally. J Exp Bot 62: 3971–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR. (1994) Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5: 765–771 [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA 106: 8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201-202: 66–73 [DOI] [PubMed] [Google Scholar]
- Gimeno-Gilles C, Lelièvre E, Viau L, Malik-Ghulam M, Ricoult C, Niebel A, Leduc N, Limami AM (2009) ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture: modifying enzymes and structural proteins in Medicago truncatula embryo axis. Mol Plant 2: 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E, Van Aken O, Ho LH, Whelan J (2009) The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol 150: 1286–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González CV, Ibarra SE, Piccoli PN, Botto JF, Boccalandro HE (2012) Phytochrome B increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana. Plant Cell Environ 35: 1958–1968 [DOI] [PubMed] [Google Scholar]
- Han B, Yang Z, Xie Y, Nie L, Cui J, Shen W (2014) Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol Plant 7: 388–403 [DOI] [PubMed] [Google Scholar]
- Hirayama T, Shinozaki K (2007) Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12: 343–351 [DOI] [PubMed] [Google Scholar]
- Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Imes D, Mumm P, Böhm J, Al-Rasheid KA, Marten I, Geiger D, Hedrich R (2013) Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J 74: 372–382 [DOI] [PubMed] [Google Scholar]
- Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, et al. (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA 106: 20520–20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannat R, Uraji M, Morofuji M, Islam MM, Bloom RE, Nakamura Y, McClung CR, Schroeder JI, Mori IC, Murata Y (2011) Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J Plant Physiol 168: 1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot 64: 2255–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchev PI, Karpińska B, Morris JA, Hussain A, Verrall SR, Hedley PE, Fenton B, Foyer CH, Hancock RD (2013) Vitamin C and the abscisic acid-insensitive 4 transcription factor are important determinants of aphid resistance in Arabidopsis. Antioxid Redox Signal 18: 2091–2105 [DOI] [PubMed] [Google Scholar]
- Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH (2011) The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23: 3319–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kajita R, Miyazaki A, Hokoyama M, Nakamura-Miura T, Mizuno S, Masuda Y, Irie K, Tanaka Y, Takada S, et al. (2010) Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol 51: 1–8 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35: 53–60 [DOI] [PubMed] [Google Scholar]
- Liesche J, Schulz A (2012) In vivo quantification of cell coupling in plants with different phloem-loading strategies. Plant Physiol 159: 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Juste J, León J (2010) Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol 152: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv WT, Lin B, Zhang M, Hua XJ (2011) Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156: 1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺ homeostasis in Arabidopsis under salt stress. J Exp Bot 63: 305–317 [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17: 9–15 [DOI] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25: 295–303 [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98: 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman HM (1999) The Arabidopsis photomorphogenic mutant hy1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid heme oxygenase. Plant Cell 11: 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahatenna DS, Langridge P, Whitford R (2015) Tetrapyrrole-based drought stress signalling. Plant Biotechnol J 13: 447–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S, Koide M, Takahashi S, Kikuta A, Aono M, Sasaki-Sekimoto Y, Ohta H, Takamiya K, Masuda T (2007) Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol 144: 1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164: 1636–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Ohiraki H, Yamada K, Seo SU, Abo M, et al. (2013) Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25: 609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH (2002) Common components, networks, and pathways of cross-tolerance to stress: the central role of “redox” and abscisic acid-mediated controls. Plant Physiol 129: 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Li Y, Gilday AD, Graham S, Graham IA (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung TH, Jung HI, Park JH, Kim JG, Back K, Jung S (2011) Porphyrin biosynthesis control under water stress: sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol 157: 1746–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Testerink C (2014) The art of being flexible: how to escape from shade, salt, and drought. Plant Physiol 166: 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfenberg M, Mittermayr L, von Roepenack-Lahaye E, Schlicke H, Grimm B, Leister D, Kleine T (2015) Functional characterization of the two ferrochelatases in Arabidopsis thaliana. Plant Cell Environ 38: 280–298 [DOI] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10: 296–302 [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH (1989) Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev 3: 1745–1757 [DOI] [PubMed] [Google Scholar]
- Shekhawat GS, Verma K (2010) Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot 61: 2255–2270 [DOI] [PubMed] [Google Scholar]
- Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Wang XL, Peng CC, Yu XC, Zhu SY, et al. (2006) The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443: 823–826 [DOI] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Bar-Zvi D (2010) ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22: 3560–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer BJ, Chabaud M, Timmers AC, Monin A, Fournier J, Barker DG (2009) A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol 151: 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Wasilewska A, Vlad F, Valon C, Leung J (2009) The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J Exp Bot 60: 1439–1463 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21: 197–203 [DOI] [PubMed] [Google Scholar]
- Söderman EM, Brocard IM, Lynch TJ, Finkelstein RR (2000) Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signaling networks. Plant Physiol 124: 1752–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JA, Gray JC (1999) Plastid translation is required for the expression of nuclear photosynthesis genes in the dark and in roots of the pea lip1 mutant. Plant Cell 11: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Feng P, Xu X, Guo H, Ma J, Chi W, Lin R, Lu C, Zhang L (2011) A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat Commun 2: 477. [DOI] [PubMed] [Google Scholar]
- Terry MJ, Kendrick RE (1999) Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol 119: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327: 818–822 [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Inoue S, Tsuzuki T, Soda M, Morimoto S, Okigaki Y, Ohishi T, Mochizuki N, Takahashi K, Kinoshita T (2014) Mg-chelatase I subunit 1 and Mg-protoporphyrin IX methyltransferase affect the stomatal aperture in Arabidopsis thaliana. J Plant Res 127: 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403 [DOI] [PubMed] [Google Scholar]
- Tossi V, Lamattina L, Cassia R (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181: 871–879 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17: 113–122 [DOI] [PubMed] [Google Scholar]
- Valliyodan B, Nguyen HT (2006) Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr Opin Plant Biol 9: 189–195 [DOI] [PubMed] [Google Scholar]
- Voigt C, Oster U, Börnke F, Jahns P, Dietz KJ, Leister D, Kleine T (2010) In-depth analysis of the distinctive effects of norflurazon implies that tetrapyrrole biosynthesis, organellar gene expression and ABA cooperate in the GUN-type of plastid signalling. Physiol Plant 138: 503–519 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ 33: 510–525 [DOI] [PubMed] [Google Scholar]
- Wind JJ, Peviani A, Snel B, Hanson J, Smeekens SC (2013) ABI4: versatile activator and repressor. Trends Plant Sci 18: 125–132 [DOI] [PubMed] [Google Scholar]
- Xie Y, Ling T, Han Y, Liu K, Zheng Q, Huang L, Yuan X, He Z, Hu B, Fang L, et al. (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ 31: 1864–1881 [DOI] [PubMed] [Google Scholar]
- Xie Y, Mao Y, Lai D, Zhang W, Zheng T, Shen W (2013) Roles of NIA/NR/NOA1-dependent nitric oxide production and HY1 expression in the modulation of Arabidopsis salt tolerance. J Exp Bot 64: 3045–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol 165: 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Xu D, Cui W, Shen W (2012) Mutation of Arabidopsis HY1 causes UV-C hypersensitivity by impairing carotenoid and flavonoid biosynthesis and the down-regulation of antioxidant defence. J Exp Bot 63: 3869–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DK, Yang Q, Shen WB (2011) Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J 66: 280–292 [DOI] [PubMed] [Google Scholar]
- Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, Rong H, Jürgens G, Knox JP, Wang MH (2010) ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J 64: 764–774 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009) Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21: 2357–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Feng LY, Cheng J, Tang H, Xu F, Zhu F, Zhao ZY, Yuan M, Chen YE, Wang JH, et al. (2013) The roles of two transcription factors, ABI4 and CBFA, in ABA and plastid signalling and stress responses. Plant Mol Biol 83: 445–458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.