Isolation and analysis of parasitic plant mutants with defects in haustorial hair development shows that these specialized root hairs are important for tight interactions with the host plant roots.
Abstract
A haustorium is the unique organ that invades host tissues and establishes vascular connections. Haustorium formation is a key event in parasitism, but its underlying molecular basis is largely unknown. Here, we use Phtheirospermum japonicum, a facultative root parasite in the Orobanchaceae, as a model parasitic plant. We performed a forward genetic screen to identify mutants with altered haustorial morphologies. The development of the haustorium in P. japonicum is induced by host-derived compounds such as 2,6-dimethoxy-p-benzoquinone. After receiving the signal, the parasite root starts to swell to develop a haustorium, and haustorial hairs proliferate to densely cover the haustorium surface. We isolated mutants that show defects in haustorial hair formation and named them haustorial hair defective (hhd) mutants. The hhd mutants are also defective in root hair formation, indicating that haustorial hair formation is controlled by the root hair development program. The internal structures of the haustoria in the hhd mutants are similar to those of the wild type, indicating that the haustorial hairs are not essential for host invasion. However, all the hhd mutants form fewer haustoria than the wild type upon infection of the host roots. The number of haustoria is restored when the host and parasite roots are forced to grow closely together, suggesting that the haustorial hairs play a role in stabilizing the host-parasite association. Thus, our study provides genetic evidence for the regulation and function of haustorial hairs in the parasitic plant.
Parasitic plant lineages are found in a wide range of angiosperm families and include approximately 4,000 species (Westwood et al., 2010). The family Orobanchaceae contains the largest variety of parasitic plants, including the most economically important parasites such as Striga spp. Striga spp. plants are major threats to world food security because they parasitize important crops, including maize (Zea mays), sorghum (Sorghum bicolor), rice (Oryza sativa), cowpea (Vigna unguiculata), and sugarcane (Saccharum officinarum), and cause severe yield reductions (Aly, 2007; Scholes and Press, 2008; Mutuku et al., 2015). In particular, in sub-Saharan Africa and Asia, Striga spp. cause yield losses valued at billions of U.S. dollars each year (Spallek et al., 2013).
A common feature of parasitic plants is the formation of a haustorium, a multicellular organ that establishes the host-parasite interaction (Yoshida and Shirasu, 2012; Ichihashi et al., 2015). Obligate Orobanchaceae parasites such as Striga and Orobanche spp. form terminal haustoria at the radicle tips and cease further development of the primary root. In contrast, facultative Orobanchaceae parasites such as Triphysaria and Phtheirospermum spp. form lateral haustoria at the host-interacting sites along the roots; thus, a single root can have multiple haustoria (Westwood et al., 2010). When a parasite root is in close proximity with the host root, root hair-like structures called haustorial hairs proliferate on the haustorium epidermis, and at the same time, cell division and swelling occur to make a globular haustorium structure (Baird and Riopel, 1983; Heide-Jorgensen and Kuijt, 1993). The haustorium penetrates the host tissues and connects its own vasculature with that of the host.
The initiation of haustorium development in Orobanchaceae plants is triggered by host root-derived chemicals called haustorium-inducing factors (HIFs). These include phenolic acids, flavonoids, and quinones (Estabrook and Yoder, 1998; Kim et al., 1998; Albrecht et al., 1999). The only HIF that has been identified directly so far from plant roots is 2,6-dimethoxy-p-benzoquinone (DMBQ; Chang and Lynn, 1986). The precise mechanisms by which these compounds are released and then trigger haustorium development are not well understood; however, some genes have been identified that may be involved in these processes. For instance, in the facultative parasite Triphysaria versicolor, a gene encoding a quinone oxidoreductase (TvQR1) is important for signal transduction in the haustorium. This enzyme converts quinones to semiquinones that function as intermediate products during redox cycling. Knockdown of TvQR1 resulted in reduced numbers of haustoria, suggesting that the semiquinone itself or redox cycling may be involved in the signal transduction pathway (Bandaranayake et al., 2010). This result, together with other investigations of redox compounds involved in haustorium induction, highlights the importance of reactive oxygen species (ROS) in haustorium formation (Kim et al., 1998). A current model proposes that phenolic acids derived from host cell wall degradation are oxidized by ROS and oxidative enzymes, generating HIF quinones (Keyes et al., 2001; Westwood et al., 2010).
The formation of haustorial hairs, which densely cover the haustorium, is one of the earliest structural events in haustorium development (Heide-Jorgensen and Kuijt, 1993). Haustorial hairs are present in different genera of the parasitic Orobanchaceae, including Agalinis, Triphysaria, Buchnera, Aureolaria, Striga, and Phtheirospermum (Baird and Riopel, 1983, 1985; Matvienko et al., 2001; Yoshida and Shirasu, 2009; Ishida et al., 2011). Species in the genera Orobanche and Phelipanche do not have haustorial hairs; however, small protuberances of epidermal cells on the haustoria of these species are thought to function similarly to haustorial hairs (Joel and Losnergoshen, 1994).
The morphology of haustorial hairs resembles that of root hairs. Root hairs are tubular outgrowths of epidermis cells. They absorb water and nutrients, interact with microorganisms, and anchor the plant to the soil (Ishida et al., 2008). Using Arabidopsis (Arabidopsis thaliana) as a model, the genetics and molecular mechanisms of root hair formation have been studied extensively. The root hairs develop in the differentiation zone of the root (Petricka et al., 2012). In Arabidopsis, the root epidermis layer consists of nonhair and hair cells, and their differentiation is tightly controlled by defined transcriptional complexes (Ishida et al., 2008). The polar growth of root hairs involves Ca2+ and ROS signals, which are required for the maintenance of cell wall integrity at the tip of growing root hairs (Datta et al., 2011).
Unlike root hairs, haustorial hairs secrete substances that act as glue to bind the haustorium to the host roots (Baird and Riopel, 1983; Heide-Jorgensen and Kuijt, 1995). However, the contribution of haustorial hairs to the establishment of parasitism is not clear, because previous reports were mostly based on histological observations rather than genetics. Phtheirospermum japonicum is a facultative root parasite that self-pollinates and has a short life cycle, and a root-transformation method has been established for this species (Ishida et al., 2011). P. japonicum is a suitable model for studying the molecular genetics of haustorium formation because it forms lateral haustoria similar to those of other facultative hemiparasites in the Orobanchaceae and its haustoria can be induced by DMBQ treatment. We performed a mutant screen on DMBQ-containing medium and isolated three mutants with recessive defects in their haustorial hairs. Characterization of these mutants suggested that haustorial hair development is controlled by the same loci that regulate root hair development and that the haustorial hairs play an important role in host attachment, which is needed to support parasitism.
RESULTS
Haustorium Formation in P. japonicum upon DMBQ Treatment
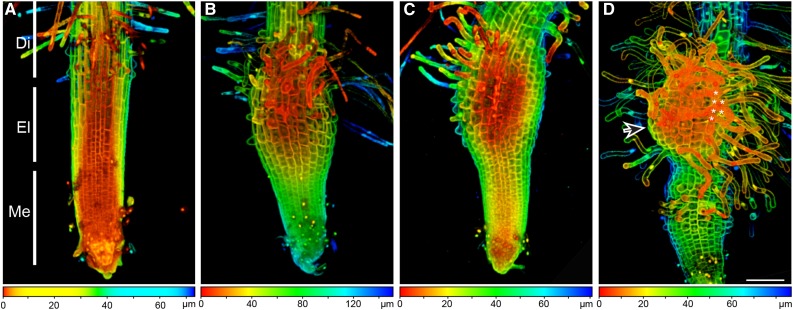
To understand the early events during haustorium formation in P. japonicum, we carefully examined 10-d-old seedlings in which haustorium development was induced by treatment with the HIF DMBQ. Without DMBQ treatment, the root structure was similar to that of Arabidopsis: the meristem zone included the root cap, the elongation zone contained small cells that were going to elongate, and the differentiation zone showed developing root hairs (Fig. 1A). About 8 h after beginning DMBQ treatment, the area around the elongation zone started to swell to form a haustorium (Fig. 1B). At this stage, the epidermal cells at the elongation zone became larger compared with those of untreated roots, suggesting that DMBQ treatment triggered rapid cell expansion at this very early stage of haustorium formation. Most haustoria were formed at the elongation zone, suggesting a high capacity of cells in this zone to differentiate into haustoria. Although some haustoria also formed later, in the differentiation zone, the frequency of haustoria in this zone was lower and haustorium initiation took much longer. In addition, haustorial hairs began to emerge from the epidermal cells of the swelling tissue at this stage (Fig. 1B). At 12 h after beginning DMBQ treatment, small cells were visible in the swelling tissue, indicating that cell division was very active at this early stage of haustorium formation (Fig. 1C). At 48 h, many fully elongated haustorial hairs had developed (Fig. 1D). In a previous report on Agalinis purpurea, where haustoria were induced by host root exudates or gum tragacanth (Baird and Riopel, 1983), there was an area at the haustorium tip where the epidermal cells did not produce haustorial hairs, and these cells were likely to intrude into the host roots. Similarly, there was an area of the haustorium in P. japonicum that did not form haustorial hairs (Fig. 1D, arrow). At this stage, a second haustorium often started to emerge at the elongation zone. These time-course observations indicated that haustorial development in P. japonicum is similar to that of other facultative parasites in the Orobanchaceae, including T. versicolor and A. purpurea (Baird and Riopel, 1983; Bandaranayake and Yoder, 2013). Importantly, DMBQ treatment did not induce xylem formation, which is normally seen upon host attachment. This suggests that other host-derived signal(s) are required for the establishment of a mature haustorium.
Figure 1.
Haustorium formation upon DMBQ treatment in P. japonicum. Ten-day-old seedlings of P. japonicum were transferred to agar plates supplemented without (A) or with (B–D) 10 μm DMBQ and incubated for 8 h (A and B), 12 h (C), or 48 h (D) before imaging. The root tips were stained with propidium iodide, and Z-stack images were taken using a confocal fluorescence microscope. Color-coded bars at bottom indicate image depths. The asterisks in D indicate adjacent epidermal cells of haustoria that formed haustorial hairs, and the arrow in D indicates a region of the haustorium that did not form haustorial hairs. Di, Differentiation zone; El, elongation zone; Me, meristem zone. Bar = 100 µm.
P. japonicum Mutant Screening
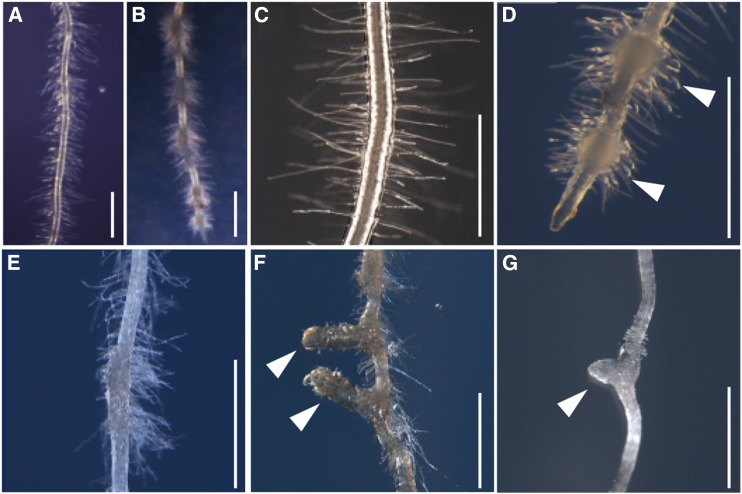
To understand the genetic programs underlying haustorium formation, we employed a forward genetic approach by establishing ethyl methyl sulfonate (EMS)-mutagenized lines of P. japonicum. Sterilized seeds of the second generation after mutagenesis (M2 seeds) were sown on 10 μm DMBQ agar medium and grown on vertically placed plates for 2 to 3 weeks. Then, the haustorium morphologies were observed using a stereomicroscope. The wild-type plants form several lateral haustoria, recognized as bump-like structures covered with haustorial hairs on the lateral part of a root, under these conditions (Fig. 2, A–D). After screening approximately 30,000 M2 seedlings, we identified one no-haustorium mutant (Fig. 2E), two mutants from independent pools with elongated haustoria (Fig. 2F), and five mutants from three independent pools that were defective in haustorial hair formation (Fig. 2G). In this study, we focused on the mutants that were defective in haustorial hair formation.
Figure 2.
Phenotypes of DMBQ-induced haustoria in the wild type and various mutant lines. A to D, Wild-type P. japonicum roots were grown without (A and C) or with (B and D) 10 μm DMBQ for 2 weeks. E to G, Haustorial morphologies of the mutants grown on 10 μm DMBQ medium for 2 weeks. E, No haustorium mutant. F, Elongated haustorium mutant. G, Haustorial hairless mutant. White arrowheads indicate haustoria. Bars = 1 mm.
Genetic Characterization of the haustorial hair defective Mutants
Three of the five haustorial hairless mutants were derived from the same seed pool, and when these plants were intercrossed, the F1 progeny showed the same mutant phenotype. Therefore, we defined these three mutants as one line that likely contains the same mutation. Thus, we identified a total of three independent lines as haustorial hairless mutants. To test the genetic behavior of the mutant lines, we backcrossed the mutants with the parental wild type. The F1 progeny from all three lines showed the wild-type phenotype, and the F2 progeny showed 3:1 segregation ratios of wild-type:mutant phenotypes, indicating that all three mutations are recessive (Table I). Reciprocal crossing among the three lines suggested that two of the three lines are allelic. Thus, we designated these mutant loci as haustorial hair defective1-1 (hhd1-1), hhd1-2, and hhd2 (Table II).
Table I. Segregation of root hair phenotypes in the F1 and F2 generations of the hhd mutants backcrossed with the wild type.
| Cross (♀ × ♂) | Total No. | Normal | Haustorial Hairless | Ratio | χ2 | Probability |
|---|---|---|---|---|---|---|
| F1 generation | ||||||
| hhd1-1 × wild type | 37 | 37 | 0 | 37:0 | 0 | 1 |
| hhd1-2 × wild type | 19 | 19 | 0 | 19:0 | 0 | 1 |
| hhd2 × wild type | 15 | 15 | 0 | 15:0 | 0 | 1 |
| F2 generation | ||||||
| hhd1-1 × wild type | 123 | 92 | 31 | 3:1 | 0.0 | 0.958 |
| hhd1-2 × wild type | 196 | 152 | 44 | 3:1 | 0.7 | 0.409 |
| hhd2 × wild type | 199 | 148 | 51 | 3:1 | 0.0 | 0.838 |
Table II. Root hair phenotypes of the F1 generation resulting from crosses among the hhd mutants.
| Cross (♀ × ♂) | Total No. | Normal | Haustorial Hairless |
|---|---|---|---|
| hhd1-1 × hhd1-2 | 10 | 0 | 10 |
| hhd1-2 × hhd1-1 | 10 | 0 | 10 |
| hhd2 × hhd1-2 | 10 | 10 | 0 |
| hhd1-2 × hhd2 | 10 | 10 | 0 |
The hhd Mutants Are Defective in Root Hair Formation
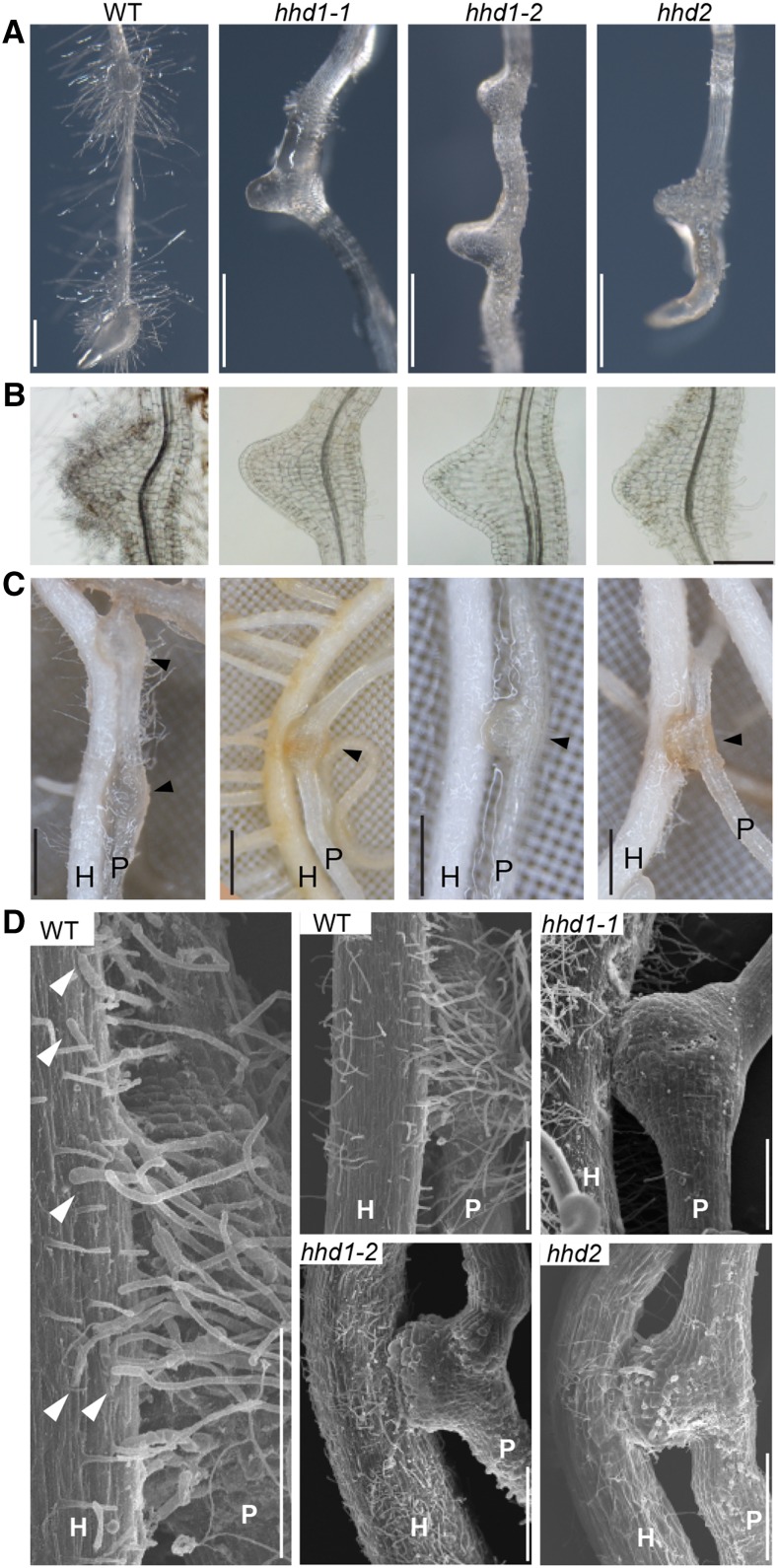
The hhd mutants lack haustorial hairs, which are usually induced by DMBQ (Fig. 3, A and B). However, the hhd mutants developed haustoria of similar size to those induced in wild-type plants exposed to DMBQ (Fig. 3, A and B). The haustoria of mutant and wild-type plants were also similar in size upon host rice infection (Fig. 3C). These results suggest that the hhd mutations affect haustorial hair development but not the development of the haustorium itself. For further characterization, we observed the haustoria that infected host rice roots by scanning electron microscopy (SEM; Fig. 3D). The wild-type plants had abundant haustorial hairs, whereas haustorial hairs were completely absent in the hhd1-1 and hhd1-2 mutants, and the hhd2 mutant produced a few very short haustorial hairs.
Figure 3.
Phenotypes of the hhd mutants. A, Lateral haustoria were induced on the roots of P. japonicum wild type (WT) and hhd mutants by incubating seedlings on agar medium containing DMBQ for 7 d. Bars = 500 µm. B, Cleared and magnified images of haustoria induced as described for A. Bar = 200 µm. C, Images of rice host roots (H) infected by P. japonicum wild type and hhd mutants (P) after growing them together in the rhizotron for 2 weeks. Arrowheads indicate the positions of haustoria. Bars = 0.5 mm. D, SEM images of P. japonicum haustoria after infection of rice host roots. Note that the haustorial hairs of the wild-type root grew toward the host root and formed physical interactions with the host root surface (arrowheads). At left is a magnified image from the wild type. Bars = 250 µm.
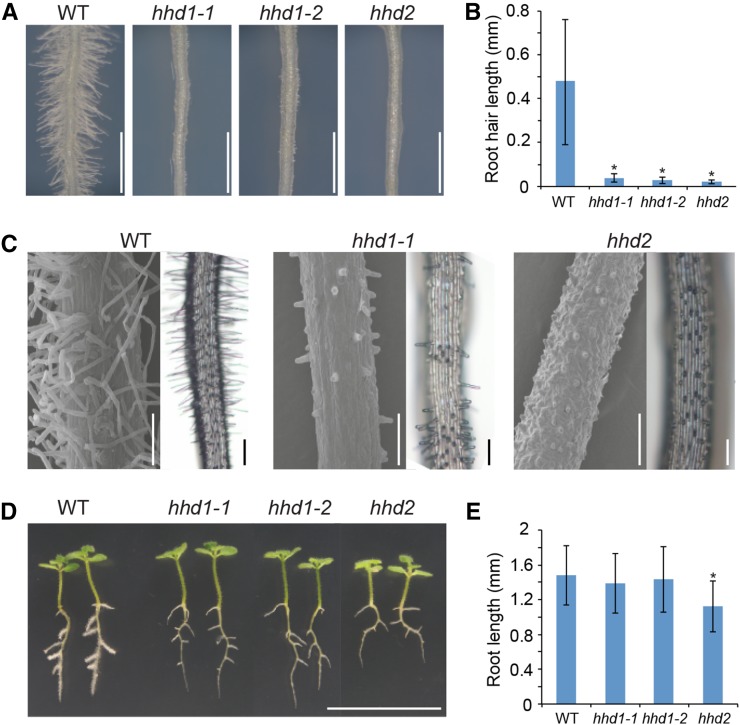
Another obvious phenotype in the hhd mutants is very short root hairs (Fig. 4, A–C). P. japonicum has type 1 root hairs that are randomly distributed along the root (Fig. 4, A and C). We found that all the mutants are impaired in root hair elongation both in the primary and lateral roots (Fig. 4, A–C). On wild-type plants, the root hairs are generally distributed evenly along the differentiation zone in the primary root, whereas hhd1-1 and hhd1-2 show patchy formation of very short root hairs. The regions having short root hairs are unevenly distributed among other regions that completely lack root hairs (Fig. 4C), indicating that these mutations affect both root hair initiation and elongation. On the other hand, the hhd2 mutant shows evenly distributed very short root hairs (Fig. 4C), suggesting that this mutation affects root hair elongation but not initiation. In addition, the hhd2 mutant has shorter roots compared with those of the wild type (Fig. 4, D and E), suggesting that the HHD2 locus might regulate general plant growth. This does not appear to be the case for HHD1, since both hhd1 alleles show similar root lengths to those of the wild type. Our results indicate that both root hair and haustorial hair development are impaired in the hhd mutants, providing genetic evidence that haustorial hair and root hairs share regulatory mechanisms controlling their development.
Figure 4.
Root hairs are also defective in the hhd mutants. A, Root hairs on the primary roots of 2-week-old wild-type (WT) and hhd mutant P. japonicum seedlings grown on a medium containing 1% Suc. Bar = 1 mm. B, Quantification of root hair length. Data represent means ± sd with 190 < n < 201 from 20 individual plants. Asterisks indicate statistically significant differences between the wild type and the mutants at P < 0.01 (Student’s t test). C, Scanning electron micrographs (left; bars = 100 µm) and light microscope images (right; bars = 200 µm) showing root hair distribution along primary roots. D, Two-week-old seedlings of the wild type and the hhd mutants grown on medium containing 1% Suc. Bar = 2 cm. E, Quantitative analysis of whole-root length per plant derived from D. Data represent means ± sd of three biological replicates with 80 < n < 104. The asterisk indicates a statistically significant difference between the wild type and the mutant at P < 0.01 (Student’s t test).
Expression of a Root Hair Marker Gene in the Haustorium
To further characterize the genetic identity of haustorial hairs, we aimed to identify a root hair marker in P. japonicum. We first investigated Arabidopsis EXPANSIN7 (AtEXP7) and AtEXP18, which are expressed exclusively in root hairs and have been used as root hair marker genes (Cho and Cosgrove, 2002). The promoter regions of these genes contain a root hair-specific cis-element called RHE (for root hair element), which is functionally conserved among root hair genes in a wide range of angiosperms with distinct root hair distribution patterns (Kim et al., 2006). A BLAST search using the full-length sequence of AtEXP7 against the draft assembly of the P. japonicum genome (Conn et al., 2015) identified nine P. japonicum contigs (Supplemental Fig. S1A), each of which encodes a protein with a conserved central expansin domain (Li et al., 2002). A phylogenetic tree using the conserved core domains showed that one of the contigs falls into the same clade as AtEXP7 and AtEXP18. We named this contig PjEXP7 because of its higher identity with AtEXP7 (69% amino acid sequences) than with AtEXP18 (64%). The root hair-specific cis-element RHE was found in the promoter region of PjEXP7 at approximately 200 bp upstream of the start codon (Supplemental Fig. S1B).
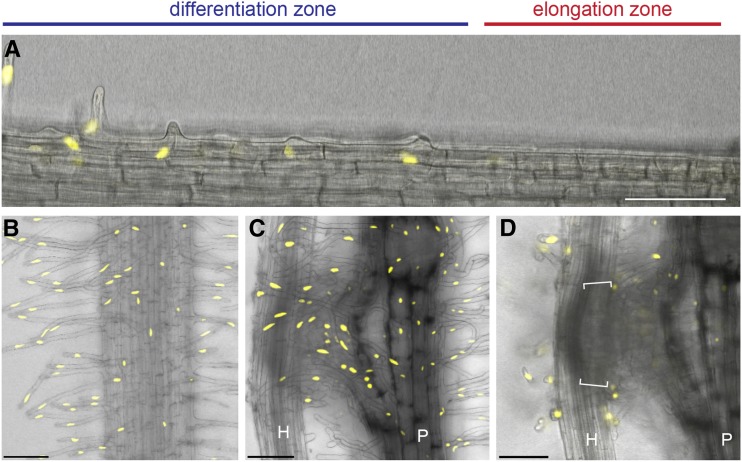
A 1,249-bp promoter region of PjEXP7 was amplified by PCR and cloned in front of three repeats of the VENUS gene, encoding a modified yellow fluorescent protein, and a nuclear localization signal (PjEXP7pro-VENUS-NLS). We transformed P. japonicum roots with the construct using the Agrobacterium rhizogenes transformation system (Ishida et al., 2011) and selected transformants with yellow fluorescence in their nuclei. Similar to the AtEXP7 expression pattern, the PjEXP7pro-VENUS-NLS construct was expressed exclusively in the root hairs of transformed plants (Fig. 5, A and B; Supplemental Fig. S2). The transformed roots were placed near host Arabidopsis roots, and VENUS protein fluorescence was observed in the nuclei of the haustorial hairs at 51 h after host infection (Fig. 5C). This result combined with the genetic evidence allowed us to conclude that the haustorial hairs are specialized root hairs that are induced upon host infection.
Figure 5.
Expression of the root hair-specific marker PjEXP7 in P. japonicum roots. Fluorescence images show P. japonicum roots expressing the nucleus-targeted VENUS marker under the control of the P. japonicum EXP7 promoter (PjEXP7pro-VENUS-NLS). The images show the expression of the marker in root hairs (A and B) and haustorial hairs (C and D) but not in intrusive cells, indicated by the square brackets in D. A host Arabidopsis root (H) was placed next to the P. japonicum root (P), and fluorescence was observed at 0 h (A and B), 51 h (C), and 66 h (D) after coincubation. Note that the yellow fluorescence signal was detected in the differentiation zone but not in the elongation zone. Images from multiple planes were taken and stacked into a single image with depths of 58.8 and 60.2 µm in B and C, respectively. D shows an image from a focal plane targeting the intrusive cells of a haustorium. Bars = 100 µm.
The Elongation of Parasite Intrusive Cells Is Not Altered by the hhd Mutations
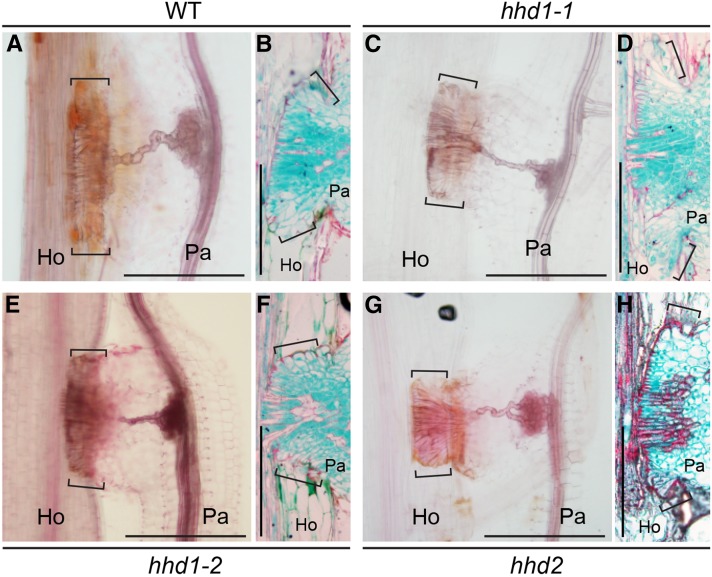
When Orobanchaceae plants invade host tissues, unique elongated cells, called intrusive cells, are formed in the haustorium at the interface between the host and the parasite (Heide-Jorgensen and Kuijt, 1993). Some of the intrusive cells later differentiate into xylem vessel cells after the haustorium reaches the host xylem. Due to the similarity between intrusive cells and root hair cells in terms of epidermal origin and polar outgrowth, we investigated whether the root hair development program is required for the formation of intrusive cells. We visualized the internal cell structures of the wild type and the hhd mutants using histochemical staining 7 d after contact with host roots (Fig. 6). At this stage, the xylem bridge connecting the haustorium to the host was fully developed (Fig. 6A). The wild-type P. japonicum haustorium, with Safranin O-stained (reddish) lignified cell walls, showed palisade-like elongated intrusive cells at the parasite-host interface (Fig. 6, A and B). Those cells were also strongly stained with Fast Green, indicating dense cytoplasm in these cells (Fig. 6B). In the parasite intrusive cells, the periclinal cell walls facing the host cells and parts of the anticlinal walls between parasite cells that were close to the host-parasite interface showed lignification. A few cells had lignified cell walls and no cell contents, indicating differentiation into xylem cells. The hhd mutants did not exhibit significant differences in shapes or numbers of intrusive cells compared with the wild type, nor did they show alterations in xylem bridge formation (Fig. 6, C–H). These results indicate that the hhd loci are not involved in the formation of intrusive cells or the xylem bridge. Furthermore, the intrusive cells did not show any fluorescence from expression of the root hair marker PjEXP7pro-VENUS-NLS (Fig. 5D). From these results, we concluded that the root hair development program mediated by HHD1 and/or HHD2 is not required for the formation of intrusive cells and that the genetic identity of the haustorial hairs is distinct from that of the intrusive cells.
Figure 6.
Xylem bridges and intrusive cells are formed in the hhd mutants upon host infection. The images show wild-type (WT; A), hhd1-1 (C), hhd1-2 (E), and hhd2 (G) xylem bridges and intrusive cells (indicated by square brackets) in the mature haustoria of P. japonicum after rice infection. The tissues were cleared with chloral hydrate and stained with Safranin O. In B, D, F, and H, the cross-sectioned images show closeup views of the intrusive cells. Cross sections of Technovit-fixed tissues from the wild type (B), hhd1-1 (D), hhd1-2 (F), and hhd2 (H) were stained with Safranin O and Fast Green. Ho and Pa indicate host and parasite roots, respectively. Bars = 200 µm.
The hhd2 Mutant Is More Sensitive to DMBQ
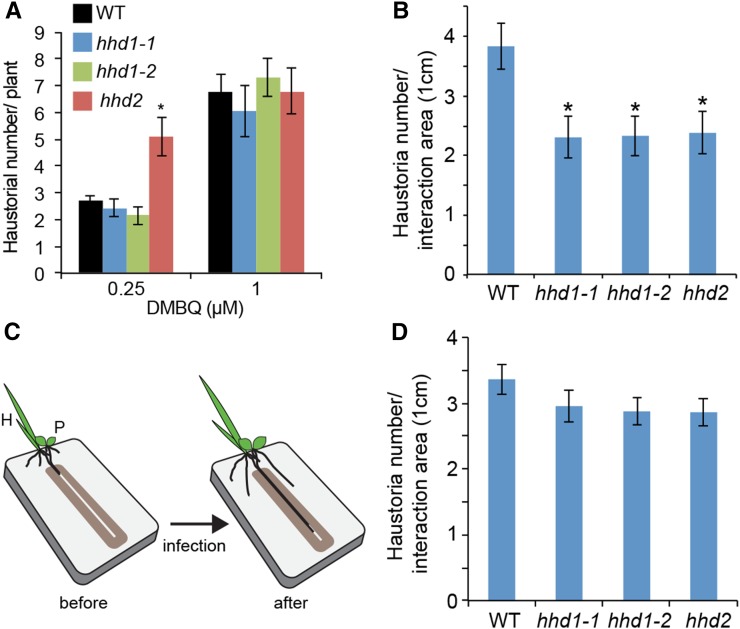
To understand the role of haustorial hairs in the formation of a haustorium, we quantified the number of haustoria on the roots of wild-type and hhd mutant plants upon DMBQ treatment (Fig. 7A). With 0.25 and 1 μm DMBQ, wild-type roots produced averages of about 2.5 and 6.5 haustoria, respectively. No significant differences were observed between hhd1 and the wild type, indicating that elongated haustorial hairs are not required for DMBQ-induced haustorium formation. Interestingly, however, the hhd2 mutant showed significantly higher numbers of haustoria after treatment with 0.25 μm but not 1 μm DMBQ. This indicated that hhd2 is more sensitive to DMBQ than wild-type or hhd1 plants. Taken together, the defects in haustorial hair formation do not desensitize DMBQ-derived haustorium-inducing signals, and the hhd2 mutation rather increases sensitivity to DMBQ.
Figure 7.
Haustorium formation by the wild type and hhd mutants after DMBQ treatment or host infection. A, Numbers of haustoria in the wild type (WT) and hhd mutants after exposure to DMBQ for 7 d. Data represent means ± se of three biological replicates (31 < n < 41). The asterisk indicates a statistically significant difference between the wild type and the mutant at P < 0.01 (Student’s t test). B, Quantification of haustorium numbers after rice host root infection for 2 weeks. The y axis indicates the number of haustoria formed per 1 cm of the root interaction area between P. japonicum and rice. Data represent means ± se of three biological replicates (29 < n < 37). Asterisks indicate statistically significant differences between the mutants and the wild type at P < 0.01. C, The rhizotron system with a 0.8-mm slit that directs the roots of P. japonicum (P) and rice (H) to grow together. D, Numbers of haustoria developed after 10 d in the rhizotron system described in C. Data represent means ± se of three biological replicates (32 < n < 48).
The hhd Mutants Form Fewer Haustoria upon Host Infection due to the Loss of Close Association with the Host
Next, we investigated whether the hhd mutations affect host infection. We counted the number of haustoria after host root infection using a rhizotron root growth system, in which the roots were grown on a two-dimensional surface for easy observation (Fig. 3C). We measured the number of haustoria per centimeter in the area where the host and parasite roots grew in close proximity. Wild-type P. japonicum averaged 3.8 haustoria per centimeter, whereas all three hhd lines produced about 2.3 haustoria per centimeter, on average (Fig. 7B). The reduced numbers of haustoria in the mutants indicate that haustorial hairs are required for efficient host interactions.
Haustorial hairs are believed to function by secreting adhesive compounds from their tips to ensure firm attachments with host roots (Baird and Riopel, 1983). Indeed, we observed that the tips of wild-type haustorial hairs attached to the surfaces of host roots, while such interactions were completely lacking in the hhd mutants (Fig. 3D). Therefore, we reasoned that the fewer haustoria in the hhd mutants may be due to the lack of firm attachments to the host. To test this hypothesis, we artificially forced the parasite and host roots to attach to one another during their growth. We used the modified rhizotron system and grew the host and parasite roots together in a narrow slit on the two-dimensional surface (Fig. 7C). In this system, the hhd mutants produced almost as many haustoria per centimeter as the wild-type plants, and the slight differences were not statistically significant (Fig. 7D; Supplemental Fig. S3). Our results indicate that the haustorial hairs play an important role in maintaining physical contact with the host roots to ensure efficient parasitism.
DISCUSSION
Haustorial hairs are commonly observed in root parasites in the Orobanchaceae family and have been described as adhesive trichomes resembling root hairs (Riopel and Musselman, 1979; Heide-Jorgensen and Kuijt, 1993). This study provides genetic evidence that at least two loci regulate the proliferation of haustorial hairs, and these loci are also required for root hair development. In addition, the specific expression of the root-hair identity marker PjEXP7 in haustorial hairs strongly suggests that haustorial hairs are specialized root hairs that are induced by signals from host roots. The timing and location of haustorium initiation are similar to those observed in the facultative parasites T. versicolor (Matvienko et al., 2001) and A. purpurea (Riopel and Musselman, 1979): the haustorium develops at the elongation zone of a root where root hairs are generally not developed. These results suggest that the ectopic activation of the root hair developmental program occurs upon DMBQ treatment or host interaction. However, the morphology and function of haustorial hairs are distinct from those of the root hairs. We observed that haustorial hairs are wavy, and the position of the nucleus tends to be close to the host root tissue (Fig. 5C). In addition, the haustorial hairs are known to produce adhesive compounds to promote attachment to host roots (Baird and Riopel, 1985). Therefore, additional unique genetic programs must be involved in haustorial hair function. In some plant species, trichomes, which often share a development program with root hairs, also secret adhesive materials to fight against insects and animals. For example, the glandular trichomes of tomato (Solanum lycopersicum) secret adhesive materials that are used for early defense against insects (Peiffer et al., 2009). The trichomes of a protocarnivorous plant, Roridula gorgonias, also produce a sticky glue to capture insects (Voigt et al., 2009). By analogy, the haustorial hairs of parasitic plants may have evolved similarly to trichomes for the secretion of adhesive chemicals.
The identification of haustorial hair mutants enabled us to test the importance of haustorial hairs in the host-parasite interaction. Our results indicate that the haustorial hairs are required for efficient host interactions but not for haustorium induction by chemical signals or for the development of haustorium structures. The reduced number of haustoria formed by hhd mutants after host root infection was reversed by forcing attachments between the host and parasite roots. This result suggests that haustorial hairs play a role in maintaining physical attachment to the host surface to ensure efficient parasitism. Without the firm attachment of the haustorial hairs to a host root, the parasite roots may lose their contact and fail to form mature haustoria. Indeed, we observed small bumps that had not penetrated host roots more frequently in the hhd mutants than in the wild type, suggesting that the haustoria were often initiated but then failed to develop further in hhd mutants. Firm attachment to hosts may also enhance the recognition of host signals for haustorium maturation. It was shown in Striga asiatica that a short exposure of the root tips to DMBQ induced immature and smaller haustoria than those formed by longer exposure (Smith et al., 1990), suggesting that the transduction of host signals must be maintained in order to continue haustorium expansion after the initiation of haustorium formation. As haustorial hairs are able to dramatically increase the surface areas of epidermal cells, it is also possible that haustorial hairs are important for an efficient response to host signals to maintain haustorium development.
Root hair development involves modifications of microtubules, actin filaments, ROS, the Ca2+ ion, auxin, and cell wall materials (Datta et al., 2011). The HHD1 and HHD2 loci may encode proteins associated with such molecular or cellular components. Although both the hhd1 and hhd2 mutants showed defects in root hair formation, the phenotypes are slightly different. HHD1 controls root hair initiation and elongation, while HHD2 regulates elongation but not initiation. In Arabidopsis, the GL2, AXR3, RHL1/2/3, RHD6, and CPC genes regulate root hair initiation (Masucci and Schiefelbein, 1994; Masucci et al., 1996; Schneider et al., 1997; Schellmann et al., 2002; Mishra et al., 2009). Most of the mutations in these genes produce additional defects in root hair elongation, resembling the root hair phenotypes of hhd1-1 and hhd1-2. Other genes, like HHD2, regulate root hair elongation through the regulation of various cellular components. Such genes include COW1, MYA2, RHD2, EXPA7, RHS2, RSL2, and ACTIN2 (Cho and Cosgrove, 2002; Ringli et al., 2002; Foreman et al., 2003; Böhme et al., 2004; Peremyslov et al., 2008; Won et al., 2009; Yi et al., 2010). For example, a widely characterized cellular component involved in root hair elongation is ROS, which is produced by NADPH activity. ROS facilitates the polar growth of root hair cells by mediating Ca2+ influx (Wymer et al., 1997; Carol et al., 2005). Apart from defects in root and haustorial hair elongation, the hhd2 mutant shows an overall reduction in the growth of primary roots (Fig. 4D), indicating that HHD2 is involved in other aspects of plant development in addition to root hair elongation.
A curious finding was that the hhd2 mutant is more sensitive to DMBQ than the hhd1 or wild-type plants with regard to the number of haustoria (Fig. 7A). This suggests that the HHD2 gene may play a role in DMBQ-mediated signal transduction, the molecular mechanisms of which are largely unknown. However, the numbers of haustoria produced by the hhd2 mutant were lower than, or similar to, those of wild-type plants in the host interaction and slit experiments (Fig. 7). The differences between the in vitro and host interaction experiments may be explained by the high probability that the host root exudate contains various HIFs in addition to DMBQ, such as phenolic acids and flavonoids (Lynn et al., 1981; Albrecht et al., 1999). The hhd2 mutant may react differently to these compounds. Alternatively, the physical distance between the host and parasite roots may be of primary importance in influencing the number of haustoria in the hhd2 mutant. The hhd2 mutant showed higher numbers of haustoria than the wild type when exposed to 0.25 μm DMBQ but similar numbers to the wild type after exposure to 1 μm DMBQ (Fig. 7A). As suggested by the slit experiment (Fig. 7D), the high concentration of HIFs near the host surface may mask the hhd2 phenotype with regard to DMBQ sensitivity. Identification of the HHD2 gene will provide answers to these questions in the near future.
The hhd mutant haustoria exhibit internal cell structures similar to those of the wild type. During penetration, the apices of the intruding organs contain elongated intrusive cells, which grow with polarity toward the host vasculature. Similar to Triphysaria spp., the intrusive cells of P. japonicum most likely have an epidermis origin (Heide-Jorgensen and Kuijt, 1993). Therefore, the origin, shape, and polar growth characteristics of the intrusive cells are similar to those of root hair cells. However, our results suggest that intrusive cell development is not regulated by the root hair development program that is controlled by the HHD1 and HHD2 loci. In this sense, the elongated intrusive cells may be unique to parasitic plants. In addition, xylem bridge formation is not influenced by the hhd mutations. Taken together, these observations indicate that the haustorial hairs are not crucial for haustorial development but are important for efficient parasitism.
CONCLUSION
This report provides, to our knowledge, the first example of a forward genetic study of a parasitic plant. Our results reveal convergent genetic regulation of root and haustorial hair development and provide insights into the specialized function of haustorial hairs in parasitism. The identification of the HHD loci, and a functional analysis of the encoded proteins, will provide further information about the mechanisms underlying haustorial hair development and haustorium signal transduction. The isolation of additional mutants with other haustorium-related phenotypes may further reveal the molecular program of haustorium development and its evolutionary origin in parasitic plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Phtheirospermum japonicum (ecotype Okayama) was used as the wild-type plant (Yoshida and Shirasu, 2009). The rice (Oryza sativa japonica) host was cv Koshihikari. Arabidopsis (Arabidopsis thaliana accession Col-0) was used as a host for the root hair gene expression experiment. P. japonicum and rice seeds were sterilized as described previously (Yoshida and Shirasu, 2009). P. japonicum seeds were germinated on 0.8% agar medium containing one-half-strength Murashige and Skoog (MS) medium and 1% Suc. The seedlings were incubated at 25°C for 3 d under dark conditions after 2 d of stratification at 4°C. The germinated seedlings were then grown at 25°C with 16 h of light and 8 h of dark. Rice seeds were germinated and grown at 26°C with 16 h of light and 8 h of dark.
Establishment of Mutagenized Lines and Mutant Screening
P. japonicum (ecotype Okayama) was self-crossed five times to establish an inbred line. The resulting seeds were used for mutagenesis by EMS (Sigma-Aldrich). Briefly, three groups of 2,000 seeds were each treated with 0.1%, 0.3%, or 0.5% EMS as described previously (Lightner and Caspar, 1998), and the chlorotic sectors in the M1 plants were monitored. In the 0.3% and 0.5% EMS-treated M1 populations, approximately 0.1% and 0.3% to 0.7% of plants showed chlorotic sectors, respectively, whereas chlorotic sectors were only rarely observed in the 0.1% EMS-treated population. Therefore, the 0.5% EMS-treated M2 population was used for the following screens. The M2 seeds derived from eight M1 plants were pooled, and 229 M2 pools were established from 1,832 M1 plants. For mutant screening, the M2 seeds were sterilized and grown on 1% agar with 10 μm DMBQ for 2 weeks, and the root and haustorium phenotypes were observed using a stereomicroscope (Zeiss Stemi 2000-C). The phenotypes were confirmed in the M3 populations by growth in the presence of 10 μm DMBQ. At the same time, the M3 plants were grown with rice host plants in the rhizotron system (see below) to investigate the host infection phenotype. The haustorium hairless mutants were isolated based on the absence of haustorial hairs in these screenings.
Quantifying Haustoria upon DMBQ Treatment
In preparation for DMBQ treatment, P. japonicum seedlings were grown for 6 d on one-half-strength MS medium with 1% Suc, transferred to 0.8% agar medium with no supplements, and grown for 4 d. The seedlings were then transferred to 0.8% agar medium with 0.25 or 1 μm DMBQ. The root parts on DMBQ-containing plates were covered with aluminum foil and were placed vertically for 10 d. To count the numbers of haustoria, whole roots were cleared overnight in a clearing solution (2.5 g mL−1 chloral hydrate and 50% [v/v] glycerol) and then analyzed with a bright-field microscope (Olympus BX51). The root lengths were measured using ImageJ (National Institutes of Health). For the negative controls (without DMBQ), the solvent dimethyl sulfoxide was added to the medium with the same concentration as that used on the 10 μm DMBQ plates.
Confocal Laser Scanning Microscopy
Time-lapse observations of haustorium formation were conducted using a confocal laser scanning microscope (Zeiss LSM 700). The root tips were stained by immersion in a 0.1 mg mL−1 propidium iodide solution for 10 min, then rinsed in water for a few minutes. The excitation and emission wavelengths were 555 nm and 559 to 600 nm, respectively. The pinhole size was 7.4 µm. A series of images was scanned with 3.72-µm intervals to acquire Z-stack confocal images. The 3D images were created using ZEN 2010 software with color-coded depths.
SEM
For SEM, P. japonicum roots were grown on MS medium for 2 weeks and rice roots were grown for 1 week after haustorium infection. The roots were dehydrated in a series of ethanol treatments (25%, 50%, 70%, 80%, 90%, 95%, and 100%; each step for 20 min at 4°C) and stored in 100% ethanol overnight at 4°C. The ethanol was replaced with isopentyl acetate, the samples were dried with a critical-point dryer (EM CPD030; Leica), and then the samples were coated with platinum using an ion sputter (JFC-1600; JEOL). The samples were observed with a scanning electron microscope (SU1510; Hitachi High-Technologies) at 15 kV.
Rice Infection in the Rhizotron System
The rhizotron system was set up as described previously (Yoshida and Shirasu, 2009). Briefly, a rhizotron consisted of a square petri dish filled with rockwool (Nichiasu) onto which nylon mesh was placed. The top and bottom of the petri dish were perforated to allow shoot growth and draining. One-week-old rice and P. japonicum seedlings were carefully arranged side by side in the rhizotrons and grown vertically for 2 weeks before the infections were analyzed.
P. japonicum and rice roots were also grown in narrow slits using the rhizotron system, to force the roots to grow in close proximity with one another. Ten-day-old P. japonicum and 7-d-old rice seedlings were grown separately in conventional rhizotrons for 10 and 3 d, respectively. Then, one rice and one P. japonicum seedling were carefully aligned side by side in a 0.8-mm slit on a 1- × 10-cm plastic plate, which had been placed between the nylon mesh and the petri dish lid. After the roots had been grown together for 10 d, the numbers of haustoria were counted with a stereomicroscope (Zeiss Stemi 2000-C). The interaction areas between the rice and P. japonicum roots were measured using ImageJ software, using images taken with a Nikon camera (D5100) attached to the stereomicroscope.
Sectioning and Staining
To observe haustorial structures, rice-infecting P. japonicum haustoria were fixed in 10% (v/v) formaldehyde, 5% acetic acid, and 50% ethanol for 5 min under a vacuum and then embedded in Technovit 7100 (Heraeus Kulzer) following the manufacturer’s instructions. Thin sections of 4 to 5 µm thickness were prepared using a Leica microtome (RM2135) equipped with a TC65 tungsten blade. The sections were fixed on APS (Amino Silane)-coated glass slides (Matsunami) and then stained with Safranin O (Wako Chemical) and Fast Green (Wako Chemical) as described previously (Yoshida and Shirasu, 2009). For the analysis of xylem bridge formation, rice roots infected by P. japonicum were heated at 90°C for 15 min in 10% KOH, washed three times with 1× phosphate-buffered saline (PBS) solution, and then transferred to a 0.1% Safranin O solution. After heating at 90°C for 5 min followed by three washes with 1× PBS, the root samples were soaked in a clearing solution overnight. The samples were washed with water and observed with an inverted light microscope.
Plasmid Construction and Hairy Root Transformation
The plasmid PjEXP7pro-VENUS-NLS was constructed using the golden gate cloning technique (Engler et al., 2014), with PCR primers listed in Supplemental Table S1. The PjEXP7 promoter was amplified from P. japonicum genomic DNA. The resulting 1,249-bp fragment (accession no. LC107785) upstream of the translational start was inserted into the level 0 vector pICH41295. Three VENUS coding sequences and the DNA fragment that encodes the N7 nuclear localization peptide were amplified from the plasmid pDR5rev::3×Venus-N7 (Heisler et al., 2005). The resulting fragments were inserted separately into the level 1 vector pAGM1311. Subsequently, these four level 1 fragments were assembled in frame into the level 0 vector pICH41308. Next, three level 0 fragments were excised from their plasmids as follows: the PjEXP7 promoter from pICH41295, the 3×VENUS-N7 sequence from pICH41308, and the 35S terminator sequence from pICH41414. The fragments were then assembled into the level 1 vector pICH47751. P. japonicum hairy root transformation was carried out according to Ishida et al. (2011) using the Agrobacterium rhizogenes strain AR1193. Confocal microscopy was performed using the laser scanning confocal microscope Leica SP5. The VENUS fluorescent protein was excited with a 488-nm laser, and its emission was detected in a 510- to 600-nm wavelength band.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers PjEXP7 promoter, LC10775.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phylogenetic analysis and promoter comparison of EXP genes in Arabidopsis and P. japonicum.
Supplemental Figure S2. Images of the root hairs and haustorial hairs expressing PjEXP7pro-VENUS-NLS in P. japonicum roots.
Supplemental Figure S3. Haustoria formed using the rhizotron system with a narrow slit.
Supplemental Table S1. Primer sequences.
Supplementary Material
Acknowledgments
We thank Ms. Mayumi Wakazaki and Dr. Mayuko Sato (RIKEN) for technical assistance with the SEM analysis, Shirasu laboratory members for their critical reading of the article, and Dr. Elliot Meyerowitz (California Institute of Technology) for providing the pDR5rev::3×Venus-N7 plasmid.
Glossary
- HIF
haustorium-inducing factor
- DMBQ
2,6-dimethoxy-p-benzoquinone
- ROS
reactive oxygen species
- EMS
ethyl methyl sulfonate
- SEM
scanning electron microscopy
- MS
Murashige and Skoog
Footnotes
This work was supported by MEXT KAKENHI (grant no. 25891029 to S.C., grant nos. 25128716, 25711019, and 15H01246 to S.Y., and grant nos. 24228008 and 15H05959 to K.S.) and by the Japan Society for the Promotion of Science (fellowship program grants to T.W. and S.B.S.).
Articles can be viewed without a subscription.
References
- Albrecht H, Yoder JI, Phillips DA (1999) Flavonoids promote haustoria formation in the root parasite Triphysaria versicolor. Plant Physiol 119: 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly R. (2007) Conventional and biotechnological approaches for control of parasitic weeds. In Vitro Cell Dev Biol Plant 43: 304–317 [Google Scholar]
- Baird WV, Riopel JL (1983) Experimental studies of the attachment of the parasitic angiosperm Agalinis purpurea to a host. Protoplasma 118: 206–218 [Google Scholar]
- Baird WV, Riopel JL (1985) Surface characteristics of root and haustorial hairs of parasitic Scrophulariaceae. Bot Gaz 146: 63–69 [Google Scholar]
- Bandaranayake PCG, Filappova T, Tomilov A, Tomilova NB, Jamison-McClung D, Ngo Q, Inoue K, Yoder JI (2010) A single-electron reducing quinone oxidoreductase is necessary to induce haustorium development in the root parasitic plant Triphysaria. Plant Cell 22: 1404–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaranayake PCG, Yoder JI (2013) Evolutionary origins. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies. Springer, Heidelberg, Germany, pp 69–70 [Google Scholar]
- Böhme K, Li Y, Charlot F, Grierson C, Marrocco K, Okada K, Laloue M, Nogué F (2004) The Arabidopsis COW1 gene encodes a phosphatidylinositol transfer protein essential for root hair tip growth. Plant J 40: 686–698 [DOI] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Chang M, Lynn DG (1986) The haustorium and the chemistry of host recognition in parasitic angiosperms. J Chem Ecol 12: 561–579 [DOI] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14: 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn CE, Bythell-Douglas R, Neumann D, Yoshida S, Whittington B, Westwood JH, Shirasu K, Bond CS, Dyer KA, Nelson DC (2015) Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 349: 540–543 [DOI] [PubMed] [Google Scholar]
- Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tam T, Vijayakumar P, Dolan L (2011) Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil 346: 1–14 [Google Scholar]
- Engler C, Youles M, Gruetzner R, Ehnert TM, Werner S, Jones JD, Patron NJ, Marillonnet S (2014) A golden gate modular cloning toolbox for plants. ACS Synth Biol 3: 839–843 [DOI] [PubMed] [Google Scholar]
- Estabrook EM, Yoder JI (1998) Plant-plant communications: rhizosphere signaling between parasitic angiosperms and their hosts. Plant Physiol 116: 1–7 [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Heide-Jorgensen HS, Kuijt J (1993) Epidermal derivatives as xylem elements and transfer cells: a study of the host-parasite interface in 2 species of Triphysaria (Scrophulariaceae). Protoplasma 174: 173–183 [Google Scholar]
- Heide-Jorgensen HS, Kuijt J (1995) The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. Am J Bot 82: 782–797 [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911 [DOI] [PubMed] [Google Scholar]
- Ichihashi Y, Mutuku JM, Yoshida S, Shirasu K (2015) Transcriptomics exposes the uniqueness of parasitic plants. Brief Funct Genomics 14: 275–282 [DOI] [PubMed] [Google Scholar]
- Ishida JK, Yoshida S, Ito M, Namba S, Shirasu K (2011) Agrobacterium rhizogenes-mediated transformation of the parasitic plant Phtheirospermum japonicum. PLoS ONE 6: e25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59: 365–386 [DOI] [PubMed] [Google Scholar]
- Joel DM, Losnergoshen D (1994) The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Can J Bot 72: 564–574 [Google Scholar]
- Keyes WJ, Taylor JV, Apkarian RP, Lynn DG (2001) Dancing together: social controls in parasitic plant development. Plant Physiol 127: 1508–1512 [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kocz R, Boone L, Keyes WJ, Lynn DG (1998) On becoming a parasite: evaluating the role of wall oxidases in parasitic plant development. Chem Biol 5: 103–117 [DOI] [PubMed] [Google Scholar]
- Kim DW, Lee SH, Choi SB, Won SK, Heo YK, Cho M, Park YI, Cho HT (2006) Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 18: 2958–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, Baldauf SL, McQueen-Mason SJ (2002) Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol 128: 854–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J, Caspar T (1998) Seed mutagenesis of Arabidopsis. Methods Mol Biol 82: 91–103 [PubMed] [Google Scholar]
- Lynn DG, Steffens JC, Kamut VS, Graden DW, Shabanowitz J, Riopel JL (1981) Isolation and characterization of the first host recognition substance for parasitic angiosperms. J Am Chem Soc 103: 1868–1870 [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW (1996) The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122: 1253–1260 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1994) The Rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106: 1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matvienko M, Torres MJ, Yoder JI (2001) Transcriptional responses in the hemiparasitic plant Triphysaria versicolor to host plant signals. Plant Physiol 127: 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BS, Singh M, Aggrawal P, Laxmi A (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 4: e4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku JM, Yoshida S, Shimizu T, Ichihashi Y, Wakatake T, Takahashi A, Seo M, Shirasu K (2015) The WRKY45-dependent signaling pathway is required for resistance against Striga hermonthica parasitism. Plant Physiol 168: 1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer M, Tooker JF, Luthe DS, Felton GW (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol 184: 644–656 [DOI] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV (2008) Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146: 1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol 63: 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C, Baumberger N, Diet A, Frey B, Keller B (2002) ACTIN2 is essential for bulge site selection and tip growth during root hair development of Arabidopsis. Plant Physiol 129: 1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riopel JL, Musselman LJ (1979) Experimental initiation of haustoria in Agalinis purpurea (Scrophulariaceae). Am J Bot 66: 570–575 [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jürgens G, Hülskamp M (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 21: 5036–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Wells B, Dolan L, Roberts K (1997) Structural and genetic analysis of epidermal cell differentiation in Arabidopsis primary roots. Development 124: 1789–1798 [DOI] [PubMed] [Google Scholar]
- Scholes JD, Press MC (2008) Striga infestation of cereal crops: an unsolved problem in resource limited agriculture. Curr Opin Plant Biol 11: 180–186 [DOI] [PubMed] [Google Scholar]
- Smith CE, Dudley MW, Lynn DG (1990) Vegetative/parasitic transition: control and plasticity in Striga development. Plant Physiol 93: 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallek T, Mutuku M, Shirasu K (2013) The genus Striga: a witch profile. Mol Plant Pathol 14: 861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt D, Gorb E, Gorb S (2009) Hierarchical organisation of the trap in the protocarnivorous plant Roridula gorgonias (Roridulaceae). J Exp Biol 212: 3184–3191 [DOI] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15: 227–235 [DOI] [PubMed] [Google Scholar]
- Won SK, Lee YJ, Lee HY, Heo YK, Cho M, Cho HT (2009) Cis-element- and transcriptome-based screening of root hair-specific genes and their functional characterization in Arabidopsis. Plant Physiol 150: 1459–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J 12: 427–439 [DOI] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42: 264–267 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shirasu K (2009) Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol 183: 180–189 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shirasu K (2012) Plants that attack plants: molecular elucidation of plant parasitism. Curr Opin Plant Biol 15: 708–713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.