A small subset of ethylene response factor genes emerge as main actors in controlling fruit ripening via both ethylene-dependent and RIN/NOR-mediated mechanisms.
Abstract
Our knowledge of the factors mediating ethylene-dependent ripening of climacteric fruit remains limited. The transcription of ethylene-regulated genes is mediated by ethylene response factors (ERFs), but mutants providing information on the specific role of the ERFs in fruit ripening are still lacking, likely due to functional redundancy among this large multigene family of transcription factors. We present here a comprehensive expression profiling of tomato (Solanum lycopersicum) ERFs in wild-type and tomato ripening-impaired tomato mutants (Never-ripe [Nr], ripening-inhibitor [rin], and non-ripening [nor]), indicating that out of the 77 ERFs present in the tomato genome, 27 show enhanced expression at the onset of ripening while 28 display a ripening-associated decrease in expression, suggesting that different ERFs may have contrasting roles in fruit ripening. Among the 19 ERFs exhibiting the most consistent up-regulation during ripening, the expression of 11 ERFs is strongly down-regulated in rin, nor, and Nr tomato ripening mutants, while only three are consistently up-regulated. Members of subclass E, SlERF.E1, SlERF.E2, and SlERF.E4, show dramatic down-regulation in the ripening mutants, suggesting that their expression might be instrumental in fruit ripening. This study illustrates the high complexity of the regulatory network connecting RIN and ERFs and identifies subclass E members as the most active ERFs in ethylene- and RIN/NOR-dependent ripening.
The plant hormone ethylene is involved in a wide range of developmental processes and physiological responses such as flowering, fruit ripening, organ senescence, abscission, root nodulation, seed germination, programmed cell death, cell expansion, and responses to abiotic stresses and pathogen attacks. In the last decades, tremendous progress has been made toward deciphering the mechanisms by which plants perceive and respond to ethylene (Benavente and Alonso, 2006; Ju et al., 2012). Studies on components of ethylene signaling have revealed a linear transduction pathway that ultimately leads to the activation of transcriptional regulators belonging to the ethylene response factor (ERF) family of transcription factors. While the upstream components of the ethylene transduction pathway are common to all ethylene responses, the apparent simplicity of the hormone signaling pathway cannot account for the wide diversity and specificity of biological responses. ERFs are one of the largest families of plant transcription factors, and in this regard, they represent a suitable step where the diversity and specificity of ethylene responses can be expressed. These downstream components of ethylene signaling are the main mediators of ethylene-dependent gene transcription.
Considering the importance of fleshy fruits for a healthy diet and the prominent role assigned to ethylene in the control of fruit ripening, substantial advances have been made to uncover the molecular mechanisms that control fruit development and especially ripening. Tomato (Solanum lycopersicum) has been collectively accepted as a reference species for Solanaceae genomics research and as a model system for studying fleshy fruit development, due to its advantages over other fleshy fruit species of agronomical interest (Gapper et al., 2013). Fruit ripening is a highly coordinated process culminating in dramatic physiological, metabolic, and textural changes that contribute to the buildup of a soft edible ripe fruit with desirable sensory qualities (Seymour et al., 2002; Klee and Giovannoni, 2011). As a climacteric fruit, tomato ripening is regulated by the phytohormone ethylene in conjunction with a set of developmental nonhormonal factors (for review, see Klee and Giovannoni, 2011; Karlova et al., 2014). Indeed, climacteric fruits have an absolute requirement for ethylene to ripen, and reduction in the hormone synthesis or interference with its perception inhibits this process. Inhibition or delay in fruit ripening by an antisense strategy targeting ACS2 or ACO1 in tomato provided direct evidence that ethylene biosynthesis is essential for climacteric fruit ripening (Hamilton et al., 1990; Oeller et al., 1991; Picton et al., 1993). Moreover, the tomato Never-ripe (Nr) mutant, a mutation in the ethylene receptor conferring ethylene insensitivity, produced nonripening fruits (Wilkinson et al., 1995). Another mutant, Green-ripe, a dominant ripening mutation that occurs in a gene encoding another component of ethylene signaling, failed to fully ripen as a consequence of reduced ethylene responsiveness (Barry et al., 2005). The identification of several key ripening regulators in tomato, such as RIN (RIPENING-INHIBITOR; Vrebalov et al., 2002), NOR (NON-RIPENING; Giovannoni, 2004), CNR (COLORLESS NON-RIPENING; Manning et al., 2006), TAGL1 (TOMATO AGAMOUS-LIKE1; Itkin et al., 2009; Vrebalov et al., 2009), AP2a (APETALA2a; Chung et al., 2010; Karlova et al., 2014), and SlHB1 (HD-Zip homeobox protein; Lin et al., 2008), provided novel insights into the understanding of the complex mechanisms underlying fruit ripening. These regulators act upstream of the ethylene-regulated biochemical events and control fruit ripening via the regulation of ethylene biosynthesis/perception genes.
The tomato rin mutant, which displays a nonripening phenotype, is affected in a gene encoding a MADS box transcription factor (Vrebalov et al., 2002). The RIN protein has been reported to interact directly with the promoter of genes involved in ethylene biosynthesis (ACS2 and ACS4), ethylene perception (NR and ETR4), and ethylene response (ERFs; Ito et al., 2008; Martel et al., 2011; Fujisawa et al., 2012, 2013; Zhong et al., 2013). The cnr mutation induces an epigenetic change that alters the promoter methylation of a SQUAMOSA promoter-binding protein, resulting in a pleiotropic ripening inhibition phenotype and inhibited expression of the ethylene-associated genes ACO1, E8, and NR (Thompson et al., 1999; Manning et al., 2006). The nor mutant causes retardation of tomato fruit ripening with a phenotype similar to the rin mutant (Giovannoni, 2004), and the NOR protein is a member of the NAC domain transcription factor family (Giovannoni, 2007). Recently, a systems biology approach indicated that NOR may have a more global regulation effect on ethylene synthesis/perception genes than RIN in controlling fruit ripening (Osorio et al., 2011). Another MADS box protein, TAGL1, controls fruit ripening by regulating ethylene synthesis via binding to the ACS2 promoter (Itkin et al., 2009; Vrebalov et al., 2009). Tomato plants down-regulated in TAGL1 produced yellow-orange fruit, whereas ectopic expression of TAGL1 in tomato resulted in sepal expansion and lycopene accumulation, supporting the active role of TAGL1 in ripening (Vrebalov et al., 2009). Down-regulation of SlAP2a results in early ripening and ethylene overproducer fruit, suggesting that this member of the AP2/ERF superfamily acts as a negative regulator of fruit ripening and ethylene production (Chung et al., 2010; Karlova et al., 2011). SlHB1 binds the promoter of SlACO1 (Lin et al., 2008), and its silencing via virus-induced gene silencing results in the down-regulation of SlACO1 expression associated with delayed fruit ripening. Overall, the characterization of these transcriptional regulators indicated that transcription factors play key roles in relaying ripening-inducing signals and regulating ethylene biosynthesis/perception and, hence, in controlling fruit ripening.
As described above, ethylene signaling is instrumental in climacteric fruit ripening, but the means by which ethylene selects the ripening-associated genes remains mostly unsolved. Because they are encoded by a large multigene family, ERFs are well suited to mediate the diversity and specificity of ethylene responses through recruiting the desired ethylene-responsive genes (Pirrello et al., 2012). Taking advantage of the recent release of the complete annotated tomato genome sequence (Tomato Genome Consortium, 2012), 77 tomato ERF genes were identified, but knowledge of their physiological significance has been hampered by the functional redundancy among members of this vast gene family. A number of the ERF genes identified in tomato are ethylene inducible and show a ripening-related expression pattern that highlights their putative role in fruit ripening (Sharma et al., 2010; Pirrello et al., 2012; Liu et al., 2014, 2015). Consistent with this hypothesis, tomato LeERF1 was reported to mediate the ethylene response, and its overexpression resulted in a constitutive ethylene response and accelerated fruit ripening and softening (Li et al., 2007). Likewise, SlERF6 plays an important role in fruit ripening by integrating ethylene and carotenoid synthesis pathways in tomato (Lee et al., 2012). More recently, the involvement of SlERF.B3 in controlling fruit ripening through the regulation of climacteric ethylene production and carotenoid accumulation was revealed using a dominant repression strategy (Liu et al., 2014). Although recent studies have shown that some ERF members are involved in fruit ripening, whether different ERF family members play specific roles in mediating ethylene-dependent ripening remains largely unknown. A better understanding of the regulatory mechanisms underlying ethylene action during climacteric fruit ripening requires the deciphering of the physiological function of ERFs and assigning specific roles to different members of this gene family. Building on the recently generated tools and genomics resources in tomato, this study aims at identifying the ERFs that are most active in fruit ripening. A large set of RNA sequencing (RNA-Seq) data available for multiple tomato cultivars was mined at the genome-wide scale using the newly developed bioinformatics platform TomExpress (http://gbf.toulouse.inra.fr/tomexpress), leading to the identification of a small subset of ERF genes displaying a consistent ripening-associated expression pattern. The connection of the selected ripening-related ERF genes to the mechanism underlying ethylene- and RIN/NOR-dependent ripening was investigated.
RESULTS
Consensus Nomenclature for Tomato ERF Genes
The important role attributed to ethylene in triggering and coordinating the ripening of climacteric fruits and the central role assigned to ERFs in mediating the hormone action prompted the search for the ERFs involved in ethylene responses during fleshy fruit ripening. Building on the achievement of the complete tomato genome sequence (SL2.40 genome sequence and ITAG2.30 whole protein sequences), we previously extended the total number of AP2/ERF genes from 112 (Sharma et al., 2010) to 146 (Pirrello et al., 2012). Among these, the nature of distinctive amino acid residues allowed us to assign 77 genes to the ERF subfamily and 48 to dehydration-responsive element-binding proteins, and the constructed phylogenetic tree clustered the 77 tomato ERF proteins into nine subclasses (A–J). To comply with the classification adopted for Arabidopsis (Arabidopsis thaliana), tomato ERF genes were given a letter (A–J) with reference to the subclass they belong to and a number to distinguish between members of the same subclass, according to their position in the neighborhood phylogenetic tree (Pirrello et al., 2012). Since a link between the physiological function of ERFs and their structural features has been suggested previously (Nakano et al., 2006; Pirrello et al., 2012), this study attempted to clarify the structure-based classification of all tomato ERFs in order to unify their nomenclature and make it compatible with that established for Arabidopsis. Table I provides the correspondence between the nomenclature proposed here and the Solyc chromosome identifier issued by ITAG 2.40 reference annotation (Tomato Genome Consortium, 2012) as well as the various names given in the literature to some tomato ERFs, and the reported putative functions of the few ERFs subjected to functional analysis are given as well.
Table I. Correspondence between the unified nomenclature of the ERF gene family and their Solyc identifiers.
Where relevant, others names proposed in the literature are also listed, as well as their reported putative functions.
| New Names | Solyc Identifier | Other Names | Reported Function | References |
|---|---|---|---|---|
| SlERF.A1 | Solyc08g078180 | |||
| SlERF.A2 | Solyc03g093610 | |||
| SlERF.A3 | Solyc05g052050 | pti4 | Disease resistance | Zhou et al. (1997); Gu et al. (2002) |
| SlERF.A4 | Solyc08g078170 | |||
| SlERF.A5 | Solyc08g007230 | |||
| SlERF.B1 | Solyc05g052040 | |||
| SlERF.B2 | Solyc03g093560 | ERF5 | Drought and salt tolerance | Pan et al. (2012) |
| SlERF.B3 | Solyc05g052030 | LeERF4 | Ethylene response and fruit ripening | Tournier et al. (2003); Liu et al. (2013, 2014) |
| SlERF.B4 | Solyc03g093540 | |||
| SlERF.B5 | Solyc03g093550 | |||
| SlERF.B6 | Solyc01g090300 | |||
| SlERF.B7 | Solyc01g090310 | |||
| SlERF.B8 | Solyc01g090320 | |||
| SlERF.B9 | Solyc01g090340 | |||
| SlERF.B10 | Solyc01g090370 | |||
| SlERF.B11 | Solyc05g050790 | |||
| SlERF.B12 | Solyc09g066350 | |||
| SlERF.B13 | Solyc08g078190 | |||
| SlERF.C1 | Solyc05g051200 | TERF1/JERF2 | Salt tolerance | Huang et al. (2004) |
| SlERF.C2 | Solyc04g014530 | |||
| SlERF.C3 | Solyc09g066360 | |||
| SlERF.C4 | Solyc09g089930 | TSRF1 | Pathogen resistance | Zhang et al. (2004b) |
| SlERF.C5 | Solyc02g077360 | |||
| SlERF.C6 | Solyc02g077370 | pti5 | Disease resistance | Gu et al. (2002) |
| SlERF.C7 | Solyc11g011740 | |||
| SlERF.C8 | Solyc11g011750 | |||
| SlERF.C9 | Solyc11g006050 | |||
| SlERF.C10 | Solyc03g005520 | |||
| SlERF.D1 | Solyc04g051360 | |||
| SlERF.D2 | Solyc12g056590 | |||
| SlERF.D3 | Solyc01g108240 | |||
| SlERF.D4 | Solyc10g050970 | |||
| SlERF.D5 | Solyc04g012050 | |||
| SlERF.D6 | Solyc04g071770 | |||
| SlERF.D7 | Solyc03g118190 | |||
| SlERF.D8 | Solyc12g042210 | |||
| SlERF.D9 | Solyc06g068830 | |||
| SlERF.E1 | Solyc09g075420 | LeERF2 | Ethylene response and seed germination | Pirrello et al. (2006) |
| SlERF.E2 | Solyc06g063070 | JERF1 | Salt tolerance | Zhang et al. (2004a) |
| SlERF.E3 | Solyc03g123500 | JERF3 | Salt tolerance | Wang et al. (2004) |
| SlERF.E4 | Solyc01g065980 | SlERF6 | Fruit ripening | Lee et al. (2012) |
| SlERF.E5 | Solyc12g049560 | |||
| SlERF.F1 | Solyc10g006130 | SlERF36 | Photosynthesis and growth regulation | Upadhyay et al. (2013) |
| SlERF.F2 | Solyc07g064890 | |||
| SlERF.F3 | Solyc07g049490 | |||
| SlERF.F4 | Solyc07g053740 | |||
| SlERF.F5 | Solyc10g009110 | SlERF3/LeERF3b | Stress response | Tournier et al. (2003); Chen et al. (2008) |
| SlERF.F6 | Solyc12g005960 | |||
| SlERF.F7 | Solyc03g006320 | |||
| SlERF.F8 | Solyc01g067540 | |||
| SlERF.F9 | Solyc05g013540 | |||
| SlERF.G1 | Solyc01g095500 | |||
| SlERF.G2 | Solyc06g082590 | pti6/SlCRF1 | Hormone and stress response | Zhou et al. (1997); Shi et al. (2014) |
| SlERF.G3 | Solyc03g007460 | |||
| SlERF.G4 | Solyc06g051840 | |||
| SlERF.H1 | Solyc06g065820 | LeERF1 | Fruit ripening | Li et al. (2007) |
| SlERF.H2 | Solyc06g068360 | |||
| SlERF.H3 | Solyc03g116610 | |||
| SlERF.H4 | Solyc01g090560 | |||
| SlERF.H5 | Solyc05g050830 | |||
| SlERF.H6 | Solyc03g120840 | |||
| SlERF.H7 | Solyc06g066540 | |||
| SlERF.H8 | Solyc08g066660 | |||
| SlERF.H9 | Solyc07g042230 | |||
| SlERF.H10 | Solyc04g054910 | |||
| SlERF.H11 | Solyc12g056980 | |||
| SlERF.H12 | Solyc04g072900 | |||
| SlERF.H13 | Solyc12g013660 | |||
| SlERF.H14 | Solyc05g052410 | |||
| SlERF.H15 | Solyc06g050520 | |||
| SlERF.H16 | Solyc01g008890 | |||
| SlERF.H17 | Solyc01g014720 | |||
| SlERF.H18 | Solyc01g091760 | |||
| SlERF.H19 | Solyc02g067020 | |||
| SlERF.J1 | Solyc02g090770 | |||
| SlERF.J2 | Solyc02g090790 | |||
| SlERF.J3 | Solyc05g009450 |
Identification of ERF Genes Exhibiting a Ripening-Associated Pattern of Expression
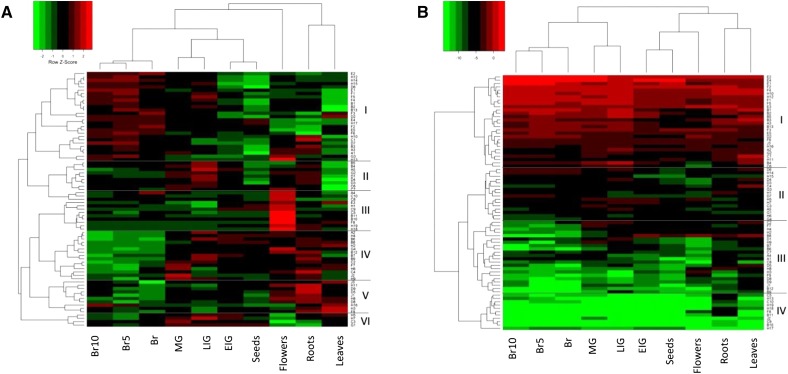
Comprehensive transcriptomic profiling of tomato ERF genes in vegetative and reproductive tissues was carried out using the online TomExpress platform and associated data-mining tools (http://gbf.toulouse.inra.fr/tomexpress). Heat map representations were constructed in two different ways, based on Spearman’s correlation or Euclidian distance, in order to cluster genes according to their expression pattern or level of expression, respectively. Heat map representation based on the expression pattern distributed the 77 tomato ERFs into six distinct clades (Fig. 1A). Clade I includes 27 genes (SlERF.A1, A3, B1–B3, B13, C1, D2, D6, D7, E1, E2, E4, E5, F1–F6, G3, H10, H12–H15, and H17) displaying an increase in their expression at the onset of ripening (breaker [Br] stage), peaking at 5 d postbreaker, and then declining at late ripening stages (10 d postbreaker). This pattern of expression suggests a potential role of these genes in regulating the ripening process. Clade II contains nine genes (SlERF.A5, B4, B5, C6, C7, D1, D3, D4, and G2) with preferential expression in young unripe fruits that declines at the onset of ripening. Genes from clade III (SlERF.A4, B10, B11, C5, C8–C10, E3, F8, H1, H18, and H19) show transcript accumulation mainly in roots, suggesting their specific involvement in the developmental process of this organ. Clade IV is made up of 15 genes (SlERF.A2, B6–B9, B12, C2, C4, F7, G4, H2, H4, H6, H9, and J2) strongly down-regulated during ripening and exhibiting high expression in roots, leaves, flowers, and immature fruits. Clade V genes (SlERF.D5, D8, D9, F9, H3, H8, H11, H16, J1, and J3) display the highest expression in roots, leaves, and flowers, whereas those of clade VI (SlERF.C3, G1, H5, and H7) are highly expressed at preripening stages, including early immature green, late immature green, and mature green (MG). Most ERFs (55 out of 77) exhibit a ripening-associated pattern of expression, with 27 genes up-regulated and 28 genes down-regulated during fruit ripening. A second heat map representation generated by applying the Euclidian distance method, to emphasize the expression level, classified the 77 ERFs into four separate clades (Fig. 1B). Clade I includes 28 genes corresponding to ERFs most highly expressed in both vegetative and reproductive tissues. By contrast, genes from clade IV display very weak expression in all tissues, while ERFs from clades II and III show intermediate expression levels.
Figure 1.
A, Heat map of the expression pattern of tomato ERF family genes in different tissues and developmental stages. The distance used for the clustering is based on the Spearman correlation, which allows clustering genes by expression patterns. For a given row of the heat map, green and red correspond, respectively, to low and high values of expression of the considered gene, which allows an easier comparison of similar patterns. B, Heat map of the expression levels of tomato ERF family genes in different tissues and developmental stages. The distance used for the clustering is based on the classical Euclidean distance, which allows clustering gene expression by expression levels. Green and red correspond, respectively, to low and high values of all expressions. For a given gene and tissue or stage, the expression value corresponds to the mean of normalized expressions of all cultivars contained in the TomExpress platform (from all available RNA-Seq data sets). Br5, Five days postbreaker; Br10, 10 d postbreaker; EIG, early immature green; LIG, late immature green.
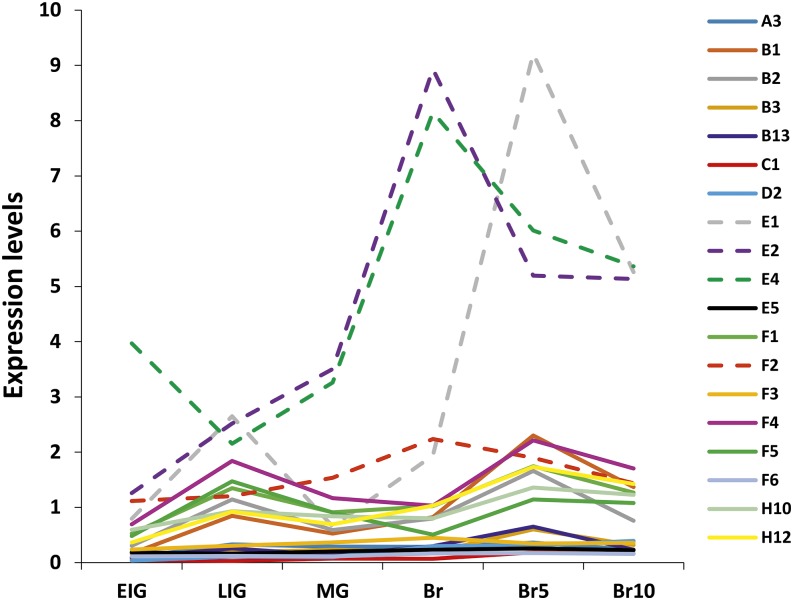
By comparing the output of the two clustering methods and taking into account both the level and pattern of expression, 19 genes (SlERF.A3, B1, B2, B3, B13, C1, D2, E1, E2, E4, E5, F1, F2, F3, F4, F5, F6, H10, and H12) were selected as the best candidates in actuating the ripening process based on their ripening-related pattern and high expression levels. Transcript accumulation patterns of the selected 19 ERF genes, assessed by quantitative real-time (qRT)-PCR, fully match those obtained using the online TomExpress pipeline and, hence, confirm the consistency and robustness of this platform (Supplemental Fig. S1). Of particular interest, members of subclass E (SlERF.E1, E2, and E4) are the most highly expressed during fruit ripening, displaying a net up-regulation at the onset of ripening starting after the MG stage (Fig. 2; Supplemental Fig. S1, A and B, top). Eight ERF genes (SlERF.B1, B2, F1, F2, F4, F5, H10, and H12) display up-regulation at the onset of ripening but have significantly lower levels of expression than subclass E members (Fig. 2; Supplemental Fig. S1, A and B, middle). Eight other ERFs (SlERF.A3, B3, B13, C1, D2, E5, F3, and F6) show the lowest levels of transcript abundance among the selected 19 ERF genes and display enhanced expression during ripening (Supplemental Fig. S1, A and B, bottom).
Figure 2.
Expression data of ERF genes obtained from the TomExpress platform. ERF E subclass genes show the highest expression levels during fruit ripening. The data were mined from the TomExpress online tool and represent the expression levels of ERFs during different developmental and ripening stages. Br5, Five days postbreaker; Br10, 10 d postbreaker; EIG, early immature green; LIG, late immature green.
Ripening-Related ERF Genes Show Altered Expression in the Tomato Ripening Mutants
To shed more light on the potential role of the selected ERFs in fruit ripening, we compared their expression in the wild type and rin, nor, and Nr ripening mutants at different ripening stages, including 20 DPA, MG, Br, 3 d postbreaker, and 10 d postbreaker. We took advantage of a newly generated tomato genetic resource where the rin, nor, and Nr mutant loci have been introgressed into the cv MicroTom genetic background. By minimizing the genotype effect, this plant material allows the more rigorous assignment of changes in the expression of ERF genes to the ripening mutation than to a variation in the genetic background. It is important to mention that the ripening phenotypes of Nr, rin, and nor in the cv MicroTom genetic background are strictly comparable to those described in other genotypes (Fig. 3).
Figure 3.
Ripening-impaired mutants in the cv MicroTom genetic background. Br3, Three days postbreaker; Br10, 10 d postbreaker; WT, wild type.
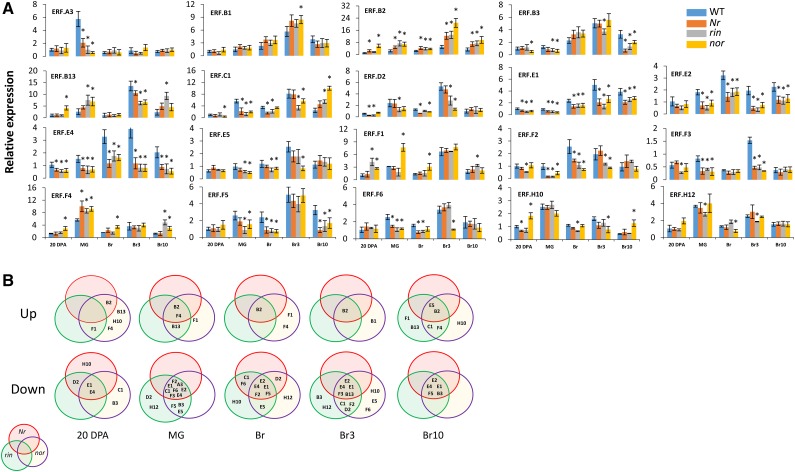
The qRT-PCR analyses indicated that among the 19 selected genes, the expression levels of 14 ERFs undergo alteration in the ripening mutants while, by contrast, the expression of five ERFs (SlERF.A3, B1, B13, H10, and H12) did not show consistent change (Fig. 4). Overall, 11 ERFs are consistently repressed (SlERF.B3, C1, D2, E1, E2, E4, E5, F2, F3, F5, and F6) during the maturation phases in the ripening mutants (Fig. 4B), whereas only three ERFs are consistently induced (SlERF.B2, F1, and F4). It is noteworthy that a higher number of ERFs are impacted in rin and nor mutants than in Nr throughout the ripening process, and all ERFs being down-regulated in the Nr mutant are also down-regulated in rin and nor (Fig. 4B). When considering specifically the onset of ripening (MG and Br stages), more ERFs are down-regulated in rin than in nor and Nr (Table II). Remarkably, four out of five members of ERF subclass E (SlERF.E1, E2, E4, and E5) display reduced expression in the ripening mutants, and the down-regulation of SlERF.E1, SlERF.E2, and SlERF.E4 is ubiquitous to all ripening mutants (Table II). On the other hand, SlERF.B2, SlERF.F1, and SlERF.F4, which exhibit down-regulation at the MG-to-Br transition in wild-type fruit (Supplemental Fig. S1, A and B, middle), are the only ERFs that undergo clear up-regulation in the ripening mutants, suggesting that reduced expression levels of these ERFs at the onset of ripening might be required for normal ripening. SlERF.B2 is the most consistently up-regulated ERF in all three rin, nor, and Nr mutants throughout ripening, indicating that high expression levels of this gene might restrain the ripening process. Likewise, the expression of SlERF.F1 and SlERF.F4, both encoding transcriptional repressors, shows constant up-regulation in the ripening mutants, suggesting that they might be involved in inhibiting some essential ripening genes. Of particular interest, the expression of SlERF.A3 displays strong down-regulation at the MG stage in all ripening mutants and then retrieves a normal expression level at later ripening stages.
Figure 4.
Modulated expression of ERF genes in Nr, rin, and nor ripening mutants. A, The levels of transcripts were assessed by qRT-PCR, and values represent means of three biological replicates. Error bars represent sd. *, P < 0.05 (Student’s t test). B, Regulation of ERF genes at different fruit developmental and ripening stages in ripening mutants shown by Venn diagram. Up, up-regulation in ripening mutants compared with the wild type; Down, down-regulation in mutants compared with the wild type. In each color circle, genes regulated in the corresponding mutant are indicated: Nr in red, rin in green, and nor in purple. Br3, Three days postbreaker; Br10, 10 d postbreaker.
Table II. ERF genes down-regulated at the onset of ripening in the tomato ripening mutants rin, nor, and Nr.
Numbers in parentheses correspond to the number of down-regulated ERFs in the corresponding ripening mutant(s).
| Mutants | ERFs Down-Regulated at MG and Br |
|---|---|
| rin | (8) SlERF.C1, SlERF.E1, SlERF.E2, SlERF.E4, SlERF.E5, SlERF.F2, SlERF.F5, SlERF.F6 |
| nor | (6) SlERF.E1, SlERF.E2, SlERF.E4, SlERF.E5, SlERF.F2, SlERF.F5 |
| Nr | (6) SlERF.C1, SlERF.E1, SlERF.E2, SlERF.E4, SlERF.F2, SlERF.F6 |
| rin-nor-Nr | (4) SlERF.E1, SlERF.E2, SlERF.E4, SlERF.F2 |
| rin-nor | (6) SlERF.E1, SlERF.E2, SlERF.E4, SlERF.E5, SlERF.F2, SlERF.F5 |
| rin-Nr | (6) SlERF.C1, SlERF.E1, SlERF.E2, SlERF.E4, SlERF.F2, SlERF.F6 |
| nor-Nr | (4) SlERF.E1, SlERF.E2, SlERF.E4, SlERF.F2 |
SlERF.E1, SlERF.E2, SlERF.E4, and SlERF.F2 Are the Main Ripening-Associated ERFs
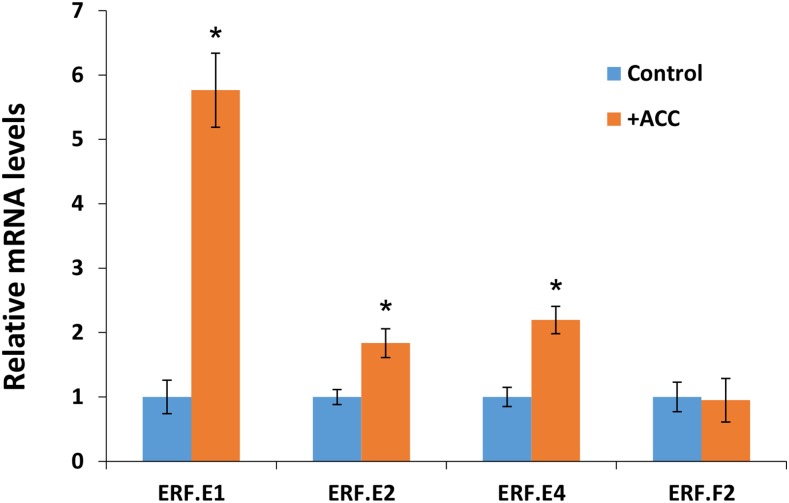
It is noteworthy that the expression pattern of SlERF.E2 and SlERF.E4 matches that of RIN, with the three genes displaying a parallel increase between MG and Br stages, while the increase in SlERF.E1 expression occurs later at postbreaker stages (Fig. 5). The search for conserved cis-regulatory elements indicated that the promoter regions of these four ERF genes (Fig. 6A) contain typical ethylene-response elements (ERE) and putative RIN-binding sites (known as CArG box). Accordingly, SlERF.E1, SlERF.E2, and SlERF.E4 display ethylene-induced expression in MG fruit (Fig. 7). Indeed, treatment with the ethylene precursor ACC results in an up to 6-fold increase in SlERF.E1 transcript accumulation and a 2-fold increase in the case of SlERF.E2 and SlERF.E4 but has no effect on SlERF.F2 expression. Correlation studies, using the TomExpress platform and associated coexpression tools, indicated that the expression of SlERF.E2, SlERF.E4, and SlERF.F2 is highly correlated (Supplemental Fig. S2). Furthermore, SlERF.E2 and SlERF.E4 expression shows a high coefficient of correlation with major ripening-associated and key ripening regulator genes, including NOR, NR, CTR1, ACS4, AP2a, E4, E8, ERF.E2, PG2a, PSY1, PDS, AAT1, AAT2, α-AMYLASE2, and α-AMYLASE1 (Supplemental Fig. S2).
Figure 5.
Expression data obtained from the TomExpress platform of RIN, NOR, SlERF.E1, SlERF.E2, SlERF.E4, and SlERF.F2. For each gene, the expression value represents the mean of normalized counts of all tomato cultivars contained in TomExpress. Br5, Five days postbreaker; Br10, 10 d postbreaker; LIG, late immature green.
Figure 6.
Transcriptional regulation of ERF.E1, ERF.E2, and ERF.E4. A, Presence of putative RIN-binding sites [CArG, C(C/T)(A/T)6(A/G)G; NAC-binding site (NBS), CATGTG; and ERE, A(A/T)TTCAAA] and a putative ERF-binding element (DRE) in the promoters of ERF genes. The cis-acting elements identified are represented by black bars and localized from ATG. B, Transactivation of ERF promoters by RIN. Protoplasts were cotransfected with the GFP reporter fused to the promoters of ERFs (ERF.E1, E2, E4, and F2) and an effector plasmid expressing RIN under the control of the 35S promoter. In the transactivation assay of the pGCC synthetic promoter (4× GCC box) by RIN and ERF.B3, protoplasts were cotransfected with the GFP reporter fused to the synthetic promoter and an effector plasmid expressing either RIN or ERF.B3 under the control of the 35S promoter. Gray bars correspond to the control for each GFP reporter. Values represent means of three biological replicates. Error bars represent sd. C, Transactivation of ERF.E1 promoters by ERF.E2 and ERF.E4. Protoplasts were cotransfected with the GFP reporter fused to the promoter of ERF.E1 and an effector plasmid expressing either ERF.E2 or ERF.E4 under the control of the 35S promoter. Values represent means of three biological replicates. Error bars represent sd. *, P < 0.01; **, P < 0.001; and ***, P < 0.0001.
Figure 7.
Ethylene regulation of ripening-associated ERFs. Wild-type fruits at MG stage were treated with 1-aminocyclopropane-1-carboxylic acid (ACC) solution by direct injection through the calyx end (Su et al., 2015), and then RNA was extracted 96 h after treatment. Control samples were injected only with buffer solution. The levels of transcripts were assessed by qRT-PCR, and values represent means of three biological replicates. Error bars represent sd. *, P < 0.05 (Student’s t test).
To investigate whether SlERF.E1, SlERF.E2, SlERF.E4, SlERF.F2, and RIN are involved in the same regulatory network, we first tested the ability of RIN protein to regulate the promoter activity of the four ERF genes. To this purpose, tobacco (Nicotiana tabacum) BY-2 protoplasts were cotransformed with the effector construct carrying the RIN coding sequence driven by the 35S constitutive promoter and with reporter constructs consisting of the GFP coding sequence driven by SlERF.E1, SlERF.E2, SlERF.E4, or SlERF.F2 promoters (Fig. 6A). Transactivation assays indicated that RIN is capable of acting as a positive regulator of the promoter activity of SlERF.E1, SlERF.E2, and SlERF.E4 but has no impact on that of the SlERF.F2 promoter (Fig. 6B). RIN has the strongest impact on SlERF.E4 (2.8-fold increase) compared with SlERF.E1 (1.8-fold increase) and SlERF.E2 (1.6-fold increase). The activation of SlERF.E1, SlERF.E2, and SlERF.E4 by RIN is consistent with the presence of a RIN-binding site in their promoter regions (Fig. 6A). To assess whether RIN activates the transcription of the target ERF.E genes via inducing the ethylene pathway, we tested the effect of RIN on the ethylene-inducible GCC box-containing promoter. As shown in Figure 6B, the activity of this highly ethylene-inducible promoter is not induced by RIN, thus ruling out the possibility that RIN activates ERF.E transcription through inducing ethylene production. In the same experiment (Fig. 6B), the synthetic ethylene-responsive promoter containing a GCC box was strongly induced by ERF.B3, shown previously to be an efficient activator of the GCC box (Pirrello et al., 2012). Further supporting the idea that these ERF.E genes undergo direct regulation by RIN, the use of chromatin immunoprecipitation (ChIP) sequencing and ChIP-chip approaches revealed the direct binding of RIN to ERF.E1 and ERF.E4 promoters (Fujisawa et al., 2013; Zhong et al., 2013). On the other hand, the delayed expression of SlERF.E1 compared with that of RIN (Fig. 5) and its strong ethylene-induced expression suggest the putative regulation of this ERF gene by SlERF.E2 or SlERF.E4, whose expression precedes that of SlERF.E1. Therefore, we tested the ability of SlERF.E2 and SlERF.E4 proteins to regulate the transcriptional activity of SlERF.E1 (Fig. 6C). The data indicate that SlERF.E4, but not SlERF.E2, is able to significantly enhance the transcriptional activity of the SlERF.E1 promoter (Fig. 6C).
DISCUSSION
Although ethylene has been known for a long time to be a key factor in initiating and orchestrating climacteric fruit ripening (Giovannoni, 2004), the molecular mechanisms by which this hormone recruits the ripening-associated genes remain poorly understood. The ERF transcription factors are downstream components of ethylene signaling, known to regulate the expression of ethylene-responsive genes (Solano et al., 1998; Pirrello et al., 2012). It is widely accepted that ethylene is instrumental in climacteric ripening, and ERFs have been assigned a central role in mediating ethylene responses. Nevertheless, so far, little is known about the role of the ERF family members in fruit ripening, and, strikingly, reports describing ripening mutants affected in ERF genes are lacking, likely due to functional redundancy among members of this large gene family. Up to 77 ERF genes are found in the tomato genome (Pirrello et al., 2012), but the functional significance of the overwhelming majority of these still awaits elucidation. The comprehensive expression profiling of tomato ERF genes performed in this study, combined with the use of the ripening-impaired mutants Nr, rin, and nor (Lanahan et al., 1994; Vrebalov et al., 2002; Giovannoni, 2004), allowed the identification of a small subset of ERF genes whose expression is highly linked to the ripening process. Overall, 19 ERFs exhibit ripening-associated patterns and elevated levels of expression in fruit. Among these, four ERFs (SlERF.E1, E2, E4, and F2), which display dramatic down-regulation in rin, nor, and Nr ripening mutants, emerge as strong candidates to play a key role in climacteric fruit ripening.
The cumulative RNA-Seq data processed by the TomExpress pipeline indicated that a high number of tomato ERF genes exhibit fruit development- and ripening-associated patterns of expression (Fig. 1). Indeed, among the 77 tomato ERFs, up to 55 members show high correlation with fruit ripening, which may explain why the majority of the ERF genes identified so far were reported to exhibit a ripening-related pattern of expression (Tournier et al., 2003; Chen et al., 2008; Sharma et al., 2010; Lee et al., 2012; Pirrello et al., 2012; Liu et al., 2014). Among the 77 tomato ERFs, 27 show enhanced expression at the onset of ripening while 28 others display a decreased expression during ripening, suggesting that different ERFs may have contrasting roles in fruit ripening. Interestingly, we show here that some genes belonging to the same clade exhibit similar expression profiles, suggesting a link between structural subclasses and physiological function. Of particular note, most ERFs from subclasses E (four out of five) and F (six out of nine) show a ripening-related pattern and high expression levels, suggesting their prominent role in fruit ripening. The potential role of members of subclasses E and F is consistent with the ripening-associated pattern described previously for SlERF.E1 (named LeERF2 in Tournier et al., 2003) and SlERF.F5 (named LeERF3b in Chen et al., 2008). In addition, SlERF.E1 was described as ethylene inducible and as a positive regulator of a feedback regulation loop via the control of the ethylene biosynthesis genes ACS and ACO (Zhang et al., 2009). Another subclass E member, SlERF.E4 (named SlERF6 in Lee et al., 2012), has been reported to play an important role in fruit ripening by integrating ethylene and carotenoid pathways. This is in agreement with a previous report on RAP2.2, a subclass E Arabidopsis ERF, shown to regulate the expression of carotenoid biosynthesis genes via binding to the ATCTA cis-element in the promoter regions of PSY and PDS (Welsch et al., 2007). Moreover, correlation analysis revealed that the expression of SlERF.F1 is positively correlated with α-carotene accumulation, suggesting the involvement of subclass F members in controlling fruit ripening through the regulation of carotenoid accumulation (Lee et al., 2012). More recently, a dominant repression strategy showed that SlERF.B3 controls fruit ripening through regulating climacteric ethylene production and carotenoid accumulation (Liu et al., 2014). On the other hand, SlERF.H1 (named LeERF1 in Li et al., 2007) was reported to affect some ripening aspects like fruit softening in tomato, even though it failed to show a typical ripening-related expression pattern (Li et al., 2007). Likewise, SlERF.C2 displays low expression in fruit but shows a negative correlation with trans-lycopene accumulation, suggesting its putative role in fruit ripening (Lee et al., 2012).
Interestingly, most ERF genes selected in this study, based on their consistent ripening-associated pattern of expression, also display altered expression in Nr, rin, and nor tomato ripening mutants, further suggesting their putative involvement in ethylene-mediated ripening regulatory networks. Among these, members of subclass E seem to be the most active, sustaining the hypothesis that this subclass might play a central role in controlling fruit ripening. The data corroborate previous studies showing that SlERF.E2 is down-regulated in the Nr mutant (Alba et al., 2005) and that SlERF.E1 and SlERF.E2 (previously named SlERF71 and SlERF72 in Kumar et al., 2012) are dramatically down-regulated at different ripening stages in the rin mutant. Likewise, the transcript level of SlERF.E4 was reported to undergo a significant decrease in all ripening-impaired mutants, including Nr, rin, and nor (Lee et al., 2012). In contrast to subclass E members, SlERF.B2, SlERF.F1, and SlERF.F4 display consistent up-regulation in the ripening mutants, suggesting a possible requirement for the down-regulation of these ERFs in normal ripening. The coordinated up-regulation of these ERFs in the rin mutant is consistent with the reported assumption that RIN plays a role in irreversibly promoting ripening via the negative regulation of some transcription factors (Fujisawa et al., 2013). Of particular interest, SlERF.B3, reported previously to have contrasting effects on tomato fruit ripening (Liu et al., 2013, 2014), exhibits a distinct expression pattern in the ripening mutants, with a significant down-regulation at 10 d postbreaker in all ripening mutants but up-regulation at the Br stage, suggesting that its role in controlling ripening is possibly stage dependent. On the other hand, SlERF.D1, which displays a typical down-regulation during fruit ripening (Fig. 1), was reported to be strongly down-regulated during ripening of the OrrDs/ORR heterozygous mutant (Nashilevitz et al., 2010), indicating that ERFs from different subclasses might contribute to the ripening process. Taken together, these data suggest that the coordinated expression of some ERFs is central to fruit ripening. Strikingly, ERFs from subclass F, encoding transcriptional repressors, also emerge as major regulators of fruit ripening in tomato. SlERF.F2, SlERF.F3, and SlERF.F5, which show ripening-associated expression, were also significantly down-regulated in all tomato ripening mutants (Fig. 4; Table II). This supports the hypothesis that these repressor ERFs may inhibit the expression of some negative regulators whose repression is instrumental to the output of the ripening program. Expression correlation analysis supports this last hypothesis, with SlERF.E2 and SlERF.E4 emerging as positive regulators of ripening, whereas SlERF.F2, whose expression is highly correlated to these two genes, might act through the down-regulation of a negative regulator of SlERF.E2 and SlERF.E4.
Of particular note, ERF genes that display clear down-regulation in the ripening mutant Nr are also down-regulated in rin and nor (Table II), supporting that NOR and RIN act upstream of NR. The down-regulation of SlERF.E1, SlERF.E2, and SlERF.E4 in the ripening mutants, together with the presence of conserved RIN-binding sites in their promoter regions (Fig. 6A), indicate that subclass E ERFs can be among the direct target genes regulated by the RIN protein. This is in agreement with the ChIP sequencing studies showing that SlERF.E1 and SlERF.E4 are potential targets of RIN (Zhong et al., 2013). In addition to SlERF.E1 and SlERF.E4, 21 other ERFs were reported to be potential targets of RIN (Fujisawa et al., 2013; Zhong et al., 2013). The transactivation assays performed in this study confirm that RIN is capable of inducing the transcriptional activity of SlERF.E1, SlERF.E2, and SlERF.E4 promoters (Fig. 6). Since RIN and NOR have been reported to play a crucial role in the attainment of competence to ripen (Osorio et al., 2011; Fujisawa et al., 2013; Zhong et al., 2013), it is conceivable that these master regulators affect fruit ripening through the direct regulation of a subset of ERF genes. Further supporting the possibility of a direct regulation of ERFs by RIN and NOR, the promoter regions of the ripening-associated ERF genes harbor well-conserved RIN-binding sites and a putative NAC protein-binding motif (Fig. 6A). Of particular interest, SlERF.F5 was reported to be a potential target of RIN, although it lacks a typical RIN-binding site (CArG box) in its promoter (Fujisawa et al., 2013). On the other hand, SlERF.F2 harbors a typical RIN-binding site in its promoter but failed to show RIN-mediated transcriptional activity (Fig. 6B). These data illustrate the high complexity of the network of regulation connecting RIN and ERFs. Moreover, the shift in the expression kinetics between SlERF.E4 and SlERF.E1, along with the ability of SlERF.E4 to activate the transcriptional activity of the SlERF.E1 promoter in a transactivation assay, support the hypothesis that the expression of some ERFs is connected. Taken together, these data suggest a complex RIN-dependent mechanism of regulation of ERFs where RIN initiates a cascade of events by turning on SlERF.E4 expression, which in turn activates the transcription of SlERF.E1.
Although an increasing number of studies addressing the functional significance of ERF genes are now becoming available, little is known about their position in the regulatory network triggering and orchestrating the ripening process. This study identifies a subset of ERF genes as being potentially important in controlling fruit ripening via both ethylene-dependent and RIN/NOR-mediated mechanisms. However, the involvement of other phytohormones such as auxin is also likely to be important in tuning the expression of ERFs during fruit ripening. This is supported by the recent study showing that SlARF2 is an important component of the regulatory mechanism controlling tomato fruit ripening (Hao et al., 2015). Interestingly, a high number of ERFs, including SlERF.E1 and SlERF.E4, are significantly down-regulated in the SlARF2 ripening-impaired mutant (Hao et al., 2015). Overall, the data designate ERFs from subclass E as priority targets for further functional characterization aiming to position these transcription factors in the gene regulatory networks underlying fruit ripening. It is worth mentioning here that a distinctive feature of subclass E ERFs is the presence of the N-terminal MCGGAII/L motif, conserved across all plant species (Tournier et al., 2003), which was shown recently to be responsible for posttranslational degradation through the N-end rule pathway under aerobic conditions (Licausi et al., 2011; Gibbs et al., 2015). Subclass E ERFs play an important role in the oxygen-sensing (hypoxic) response, and under low oxygen, they undergo relocalization into the nucleus, where they induce the transcription of their target genes (Licausi et al., 2011). Considering the marked rise in the expression of ERFs from subclass E at the onset of fruit ripening, which is associated with an increase in respiration, these ERFs might represent the missing link between the climacteric rise in respiration and autocatalytic ethylene production. In particular, it is important to further clarify whether members of this ERF subclass are involved in activating SYSTEM2 ethylene biosynthesis genes. The implementation of new approaches, such as in vivo ChIP coupled with high-throughput sequencing, is anticipated to yield essential information on the direct target genes of these ERFs and, hence, to provide clues concerning the control of the specific pathway(s) in which these transcriptional regulators are involved.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum ‘MicroTom’) wild type and Nr, rin, and nor fruit ripening-impaired mutants in the cv MicroTom background were grown under standard greenhouse conditions. Conditions in the culture chamber room were set as follows: 14-h-day/10-h-night cycle, 25°C/20°C day/night temperature, 80% hygrometry, and 250 µmol m−2 s−1 intense luminosity. Fruit samples were collected from different fruit development and ripening stages, including 20 DPA, MG stage, Br stage, 3 d postbreaker, and 10 d postbreaker. More than five fruits for each replicate were used for qRT-PCR analyses. Three independent biological repeats were performed for each experiment.
Expression Data Mining and Heat Map Generation
RNA-Seq data of the transcriptome in multiple tomato cultivars were obtained from the TomExpress bioinformatics platform (http://gbf.toulouse.inra.fr/tomexpress), focused on the ERF family genes during vegetative and reproductive development. TomExpress provides a unified approach to tomato gene expression from released RNA-Seq data sets. Expression data represent normalized counts per base and mean values of multiple cultivars for each tissue and stage and were used to generate heat map representations with R software (https://www.r-project.org). Both classical Euclidean distance and a correlation distance (Spearman) were used, respectively, to cluster together genes with similar expression levels and expression profiles.
RNA Isolation and qRT-PCR Analyses
Fruits from each developmental and ripening stage were harvested, frozen in liquid nitrogen, and stored at −80°C. Total RNA from pericarp of at least five individual fruits at each developmental stage analyzed here was extracted using the Plant RNA Purification Reagent (Invitrogen; catalog no. 12322-012) according to the manufacturer’s instructions. Total RNA was then DNase treated (Invitrogen; catalog no. AM1906) to remove any contaminating genomic DNA. First-strand complementary DNA was reverse transcribed from 2 μg of total RNA using the Omniscript Reverse Transcription kit (Qiagen; catalog no. 74904) following the manufacturer’s instructions. Gene-specific primers were designed by Primer Express software (PE-Applied Biosystems) and were further checked using BLAST against the tomato whole genome. qRT-PCR analyses were performed as described previously (Pirrello et al., 2006). The primer sequences used in this study are listed in Supplemental Table S1.
ACC Treatment
Tomato fruits were harvested at the MG stage and then injected with a buffer solution containing 10 mm MES, pH 5.6, 3% (w/v) sorbitol, or 100 μm ACC as described by Su et al. (2015). Three independent biological repeats were performed for each experiment.
Generation of the Correlation Network of ERFs and Ripening-Associated Genes
The network was generated from the analysis of the coexpression of ERFs and ripening-related genes in the TomExpress platform with a correlation threshold greater than 0.85. In the TomExpress platform, correlations are calculated with the Spearman correlation coefficient of gene expression during fruit development and ripening. Such a correlation coefficient allows the aggregation of genes that are coexpressed even if the coexpression is not linear.
Identification of cis-Acting Elements in the Promoter Sequences of SlERF.E1, SlERF.E2, SlERF.E4, and SlERF.F2
CArG box, ERE, and NAC-binding site cis-acting elements were identified manually in the 2-kb promoter of SlERF.E1, SlERF.E2, SlERF.E4, and SlERF.F2 based on the core sequences of the CArG box [C(C/T)(A/T)6(A/G)G], ERE box [A(A/T)TTCAAA], and NAC-binding site (CATGTG; Tran et al., 2004; Fujisawa et al., 2011).
Transient Expression Using a Single-Cell System
Protoplasts used for transfection were isolated from suspension-cultured tobacco (Nicotiana tabacum) BY-2 cells in accordance with Leclercq et al. (2005). The reporter construct was generated with native promoters, ERFs (SlERF.E1, SlERF.E2, SlERF.E4, and SlERF.F2), fused to GFP. Protoplast cotransfection assays were performed using the reporter plasmids and effector vectors carrying 35S:RIN or 35S:ERFs. GFP expression was analyzed and quantified by flow cytometry (FACS Cyflow space instrument; Partec; http://www.sysmex-partec.com/) 16 h following protoplast transfection. For each sample, 100 to 1,000 protoplasts were gated on forward light scatter; GFP fluorescence per population of cells corresponds to the average fluorescence intensity of the population of cells above the background. The data were analyzed using Flomax software (Sysmex Partec) and were normalized using an experiment with protoplasts transformed with the reporter vector in combination with the vector used as effector but lacking the RIN or ERF coding sequence.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. qRT-PCR results confirm the expression data of ERF genes obtained from the TomExpress platform.
Supplemental Figure S2. Correlation network of ERFs and ripening-associated genes.
Supplemental Table S1. Sequences of primers used in this study.
Supplementary Material
Acknowledgments
We thank D. Saint-Martin, O. Berseille, and L. Lemonnier for cultivating the tomato plants and M. Lauvernier for assistance in the setup of the TomExpress platform.
Glossary
- RNA-Seq
RNA sequencing
- Br
breaker
- MG
mature green
- qRT
quantitative real-time
- ChIP
chromatin immunoprecipitation
- ACC
1-aminocyclopropane-1-carboxylic acid
Footnotes
This work was supported by the Laboratoire d’Excellence entitled TULIP (grant no. ANR–10–LABX–41), by the European COST Action FA1106, and by the CAPES-COFECUB program (Ph.D. grant no. Sv 815–14 to B.L.G.).
Articles can be viewed without a subscription.
References
- Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17: 2954–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, McQuinn RP, Thompson AJ, Seymour GB, Grierson D, Giovannoni JJ (2005) Ethylene insensitivity conferred by the Green-ripe and Never-ripe 2 ripening mutants of tomato. Plant Physiol 138: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente LM, Alonso JM (2006) Molecular mechanisms of ethylene signaling in Arabidopsis. Mol Biosyst 2: 165–173 [DOI] [PubMed] [Google Scholar]
- Chen G, Hu Z, Grierson D (2008) Differential regulation of tomato ethylene responsive factor LeERF3b, a putative repressor, and the activator Pti4 in ripening mutants and in response to environmental stresses. J Plant Physiol 165: 662–670 [DOI] [PubMed] [Google Scholar]
- Chung MY, Vrebalov J, Alba R, Lee J, McQuinn R, Chung JD, Klein P, Giovannoni J (2010) A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J 64: 936–947 [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Ito Y (2011) Identification of potential target genes for the tomato fruit-ripening regulator RIN by chromatin immunoprecipitation. BMC Plant Biol 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25: 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Shima Y, Higuchi N, Nakano T, Koyama Y, Kasumi T, Ito Y (2012) Direct targets of the tomato-ripening regulator RIN identified by transcriptome and chromatin immunoprecipitation analyses. Planta 235: 1107–1122 [DOI] [PubMed] [Google Scholar]
- Gapper NE, McQuinn RP, Giovannoni JJ (2013) Molecular and genetic regulation of fruit ripening. Plant Mol Biol 82: 575–591 [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Conde JV, Berckhan S, Prasad G, Mendiondo GM, Holdsworth MJ (2015) Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress responses in plants. Plant Physiol 169: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2007) Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 10: 283–289 [DOI] [PubMed] [Google Scholar]
- Gu YQ, Wildermuth MC, Chakravarthy S, Loh YT, Yang C, He X, Han Y, Martin GB (2002) Tomato transcription factors pti4, pti5, and pti6 activate defense responses when expressed in Arabidopsis. Plant Cell 14: 817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Lycett GW, Grierson D (1990) Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346: 284–287 [Google Scholar]
- Hao Y, Hu G, Breitel D, Liu M, Mila I, Frasse P, Fu Y, Aharoni A, Bouzayen M, Zouine M (2015) Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato. PLoS Genet 11: e1005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang Z, Zhang X, Zhang H, Huang D, Huang R (2004) Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Lett 573: 110–116 [DOI] [PubMed] [Google Scholar]
- Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A (2009) TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J 60: 1081–1095 [DOI] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T (2008) DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J 55: 212–223 [DOI] [PubMed] [Google Scholar]
- Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Groth G, Tucker ML, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA 109: 19486–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, Chapman N, David K, Angenent GC, Seymour GB, de Maagd RA (2014) Transcriptional control of fleshy fruit development and ripening. J Exp Bot 65: 4527–4541 [DOI] [PubMed] [Google Scholar]
- Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23: 923–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma MK, Kapoor S, Tyagi AK, Sharma AK (2012) Transcriptome analysis of rin mutant fruit and in silico analysis of promoters of differentially regulated genes provides insight into LeMADS-RIN-regulated ethylene-dependent as well as ethylene-independent aspects of ripening in tomato. Mol Genet Genomics 287: 189–203 [DOI] [PubMed] [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1994) The never ripe mutation blocks ethylene perception in tomato. Plant Cell 6: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq J, Ranty B, Sanchez-Ballesta MT, Li Z, Jones B, Jauneau A, Pech JC, Latché A, Ranjeva R, Bouzayen M (2005) Molecular and biochemical characterization of LeCRK1, a ripening-associated tomato CDPK-related kinase. J Exp Bot 56: 25–35 [DOI] [PubMed] [Google Scholar]
- Lee JM, Joung JG, McQuinn R, Chung MY, Fei Z, Tieman D, Klee H, Giovannoni J (2012) Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J 70: 191–204 [DOI] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu B, Xu W, Zhu H, Chen A, Xie Y, Shao Y, Luo Y (2007) LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Rep 26: 1999–2008 [DOI] [PubMed] [Google Scholar]
- Lin Z, Hong Y, Yin M, Li C, Zhang K, Grierson D (2008) A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J 55: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Diretto G, Pirrello J, Roustan JP, Li Z, Giuliano G, Regad F, Bouzayen M (2014) The chimeric repressor version of an Ethylene Response Factor (ERF) family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytol 203: 206–218 [DOI] [PubMed] [Google Scholar]
- Liu M, Pirrello J, Chervin C, Roustan JP, Bouzayen M (2015) Ethylene control of fruit ripening: revisiting the complex network of transcriptional regulation. Plant Physiol 169: 2380–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pirrello J, Kesari R, Mila I, Roustan JP, Li Z, Latché A, Pech JC, Bouzayen M, Regad F (2013) A dominant repressor version of the tomato Sl-ERF.B3 gene confers ethylene hypersensitivity via feedback regulation of ethylene signaling and response components. Plant J 76: 406–419 [DOI] [PubMed] [Google Scholar]
- Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38: 948–952 [DOI] [PubMed] [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ (2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157: 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashilevitz S, Melamed-Bessudo C, Izkovich Y, Rogachev I, Osorio S, Itkin M, Adato A, Pankratov I, Hirschberg J, Fernie AR, et al. (2010) An orange ripening mutant links plastid NAD(P)H dehydrogenase complex activity to central and specialized metabolism during tomato fruit maturation. Plant Cell 22: 1977–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A (1991) Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254: 437–439 [DOI] [PubMed] [Google Scholar]
- Osorio S, Alba R, Damasceno CMB, Lopez-Casado G, Lohse M, Zanor MI, Tohge T, Usadel B, Rose JKC, Fei Z, et al. (2011) Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol 157: 405–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Seymour GB, Lu C, Hu Z, Chen X, Chen G (2012) An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep 31: 349–360 [DOI] [PubMed] [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D (1993) Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J 3: 469–481 [Google Scholar]
- Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, Tournier B, Khalil-Ahmad Q, Regad F, Latché A, Pech JC, Bouzayen M (2006) Sl-ERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant Cell Physiol 47: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Pirrello J, Prasad BCN, Zhang W, Chen K, Mila I, Zouine M, Latché A, Pech JC, Ohme-Takagi M, Regad F, et al. (2012) Functional analysis and binding affinity of tomato ethylene response factors provide insight on the molecular bases of plant differential responses to ethylene. BMC Plant Biol 12: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GB, Manning K, Eriksson EM, Popovich AH, King GJ (2002) Genetic identification and genomic organization of factors affecting fruit texture. J Exp Bot 53: 2065–2071 [DOI] [PubMed] [Google Scholar]
- Sharma MK, Kumar R, Solanke AU, Sharma R, Tyagi AK, Sharma AK (2010) Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Mol Genet Genomics 284: 455–475 [DOI] [PubMed] [Google Scholar]
- Shi X, Gupta S, Rashotte AM (2014) Characterization of two tomato AP2/ERF genes, SlCRF1 and SlCRF2 in hormone and stress responses. Plant Cell Rep 33: 35–45 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z, Roustan JP, Bouzayen M, Giuliano G, Chervin C (2015) Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol 15: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Tor M, Barry CS, Vrebalov J, Orfila C, Jarvis MC, Giovannoni JJ, Grierson D, Seymour GB (1999) Molecular and genetic characterization of a novel pleiotropic tomato-ripening mutant. Plant Physiol 120: 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F, Latché A, Pech JC, Bouzayen M (2003) New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett 550: 149–154 [DOI] [PubMed] [Google Scholar]
- Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16: 2481–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay RK, Soni DK, Singh R, Dwivedi UN, Pathre UV, Nath P, Sane AP (2013) SlERF36, an EAR-motif-containing ERF gene from tomato, alters stomatal density and modulates photosynthesis and growth. J Exp Bot 64: 3237–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Pan IL, Arroyo AJM, McQuinn R, Chung M, Poole M, Rose J, Seymour G, Grandillo S, Giovannoni J, et al. (2009) Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell 21: 3041–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Wang H, Huang Z, Chen Q, Zhang Z, Zhang H, Wu Y, Huang D, Huang R (2004) Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol Biol 55: 183–192 [DOI] [PubMed] [Google Scholar]
- Welsch R, Maass D, Voegel T, Dellapenna D, Beyer P (2007) Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol 145: 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang Z, Xie B, Chen Q, Tian X, Zhang X, Zhang H, Lu X, Huang D, Huang R (2004a) The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta 220: 262–270 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang D, Chen J, Yang Y, Huang Z, Huang D, Wang XC, Huang R (2004b) Tomato stress-responsive factor TSRF1 interacts with ethylene responsive element GCC box and regulates pathogen resistance to Ralstonia solanacearum. Plant Mol Biol 55: 825–834 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Quan R, Wang XC, Huang R (2009) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150: 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Fei Z, Chen YR, Zheng Y, Huang M, Vrebalov J, McQuinn R, Gapper N, Liu B, Xiang J, et al. (2013) Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat Biotechnol 31: 154–159 [DOI] [PubMed] [Google Scholar]
- Zhou J, Tang X, Martin GB (1997) The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO J 16: 3207–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.