Arabidopsis lignins, which are genetically p-coumaroylated up to the grass lignin level, display dramatic structural changes that make them more amenable to solubilization in alkali at room temperature.
Abstract
Grass lignins can contain up to 10% to 15% by weight of p-coumaric esters. This acylation is performed on monolignols under the catalysis of p-coumaroyl-coenzyme A monolignol transferase (PMT). To study the impact of p-coumaroylation on lignification, we first introduced the Brachypodium distachyon Bradi2g36910 (BdPMT1) gene into Arabidopsis (Arabidopsis thaliana) under the control of the constitutive maize (Zea mays) ubiquitin promoter. The resulting p-coumaroylation was far lower than that of lignins from mature grass stems and had no impact on stem lignin content. By contrast, introducing either the BdPMT1 or the Bradi1g36980 (BdPMT2) gene into Arabidopsis under the control of the Arabidopsis cinnamate-4-hydroxylase promoter boosted the p-coumaroylation of mature stems up to the grass lignin level (8% to 9% by weight), without any impact on plant development. The analysis of purified lignin fractions and the identification of diagnostic products confirmed that p-coumaric acid was associated with lignins. BdPMT1-driven p-coumaroylation was also obtained in the fah1 (deficient for ferulate 5-hydroxylase) and ccr1g (deficient for cinnamoyl-coenzyme A reductase) lines, albeit to a lower extent. Lignins from BdPMT1-expressing ccr1g lines were also found to be feruloylated. In Arabidopsis mature stems, substantial p-coumaroylation of lignins was achieved at the expense of lignin content and induced lignin structural alterations, with an unexpected increase of lignin units with free phenolic groups. This higher frequency of free phenolic groups in Arabidopsis lignins doubled their solubility in alkali at room temperature. These findings suggest that the formation of alkali-leachable lignin domains rich in free phenolic groups is favored when p-coumaroylated monolignols participate in lignification in a grass in a similar manner.
The cost-effective conversion of lignocellulosic feedstocks to the fuel ethanol is strongly dependent on the pretreatment step that makes cellulose accessible to enzymatic hydrolysis (Yang and Wyman, 2008; Kumar et al., 2009, Alvira et al., 2010; Hu and Ragauskas, 2012). Lignins are major targets of such pretreatments, as these phenolic polymers constitute resistant barriers between glycosidases and polysaccharides. Worldwide, research efforts are being carried out to decipher the relationship of lignin structure to lignin susceptibility to pretreatments. Mild alkali pretreatments are well suited to grass lignocelluloses, as native grass lignins can be substantially solubilized in alkali at room temperature (Beckman et al., 1923). The structural feature that governs this solubilization is the high frequency of free phenolic groups in grass lignins (Lapierre et al., 1989). Indeed, up to 50% of guaiacyl (G) grass lignin units are free phenolic, versus 25% or less in nongrass lignins (Lapierre, 2010). In addition, grass lignins have two other specific features: they are ether linked to ferulic acid (FA) units, which acylate arabinoxylans (Jacquet et al., 1995; Ralph et al., 1995), and they are acylated by p-coumaric acid (CA) on the γ-OH side chain of syringyl (S) and, to a lower extent, of G lignin units (Ralph et al., 1994; Grabber et al., 1996; Ralph, 2010). This p-coumaroylation, performed on monolignols prior to their polymerization (Lu and Ralph, 2002), is catalyzed by p-coumaroyl-coenzyme A monolignol transferase (PMT; Withers et al., 2012). Thanks to this PMT activity, lignins of grass mature stems can contain up to 10% to 15% (w/w) CA esters, as evidenced for purified maize (Zea mays) lignin fractions (Chazal et al., 2014). The highest lignin p-coumaroylation levels have been reported for grass species belonging to the C4 physiological type (Hatfield et al., 2009; Hatfield and Marita, 2010). It is now well established that CA esters linked to grass lignins keep free their phenolic group, by contrast to arabinoxylan-linked FA esters, which can be oxidatively etherified to lignin units (Ralph, 2010). However, it is not yet clearly established to what extent the substantial participation of CA conjugates to grass lignification can affect grass lignin structural traits other than the simple p-coumaroylation at Cγ.
In this study, we addressed the question of the possible relationship between the substantial p-coumaroylation level of grass lignins and their high frequency in free phenolic lignin units. The feasibility of introducing CA esters into dicot lignins was demonstrated recently by expressing the rice (Oryza sativa) OsPMT gene into two eudicots, Arabidopsis (Arabidopsis thaliana) and poplar (Populus spp.; Smith et al., 2015). The resulting trangenic PMT-Arabidopsis and PMT-poplar lines were found to display lignin p-coumaroylation, with CA levels ranging between 0.8% and 2% (w/w) of acetyl bromide lignin (Smith et al., 2015). In this study, we aimed to incorporate additional p-coumarate esters into Arabidopsis lignins, up to the level reported for the lignins of mature grass stems, and to investigate the impact of such a high incorporation on the structure of Arabidopsis lignins and their solubility in alkali at room temperature. This investigation was conducted on Arabidopsis transgenic lines expressing either the Brachypodium distachyon Bradi2g36910 (hereafter referred to as BdPMT1) or the B. distachyon Bradi1g36980 (hereafter referred to as BdPMT2) gene into the wild-type Columbia-0 (Col-0) genetic background under the control of the Arabidopsis cinnamate-4-hydroxylase (AtC4H) promoter, which has been shown to efficiently target gene expression to vascular tissues (Bell-Lelong et al., 1997; Weng et al., 2008). In addition, AtC4H promoter-driven BdPMT1 expression was also obtained in the Arabidopsis fah1 mutant devoid of S lignin units due to a mutation in the gene encoding ferulate 5-hydroxylase (Chapple et al., 1992) as well as in the ccr1g mutant deficient for CINNAMOYL-COENZYME A REDUCTASE1 (CCR1), which accumulate unusual levels of soluble ferulate derivatives and incorporate FA into lignins (Mir Derikvand et al., 2008; Ralph et al., 2008). The impacts of these transformations on the p-coumaroylation and structure of Arabidopsis lignins as well as on their solubility in alkali at room temperature are evaluated in this work.
RESULTS AND DISCUSSION
The Expression of Either BdPMT1 or BdPMT2 into Arabidopsis under the Control of the AtC4H Promoter Boosts Lignin p-Coumaroylation up to the Grass Lignin Level
The BdPMT1 gene was shown recently to encode for the monolignol-specific PMT1 enzyme, the activity of which results in the p-coumaroylation of B. distachyon lignins (Petrik et al., 2014). Lignin p-coumaroylation was decreased severely in the Bdpmt1 mutant, but not reduced to zero, suggesting that some other BdPMT proteins could act as BdPMT1 surrogates. Among the BdPMT gene family (Supplemental Fig. S1), we selected Bradi1g36980 (BdPMT2) as the best candidate to fulfill this role because of its substantial transcript level in B. distachyon lignified culm (PlaNET; http://aranet.mpimp-golm.mpg.de/). The full-length complementary DNA of BdMPT1 or BdPMT2 was cloned and introduced into wild-type and mutant Arabidopsis lines under the control of the AtC4H promoter. It is noteworthy that, throughout the production of transgenic lines (from T1 to T3 generation), transgenic and corresponding control plants displayed a similar phenotype (Supplemental Fig. S2).
The AtC4H promoter-driven expression of the BdPMT1 or BdPMT2 gene in Arabidopsis induced an impressive increase of alkali-releasable CA from the cell wall (CW) of wild-type mature stems (Table I). While minute, but measurable, amounts of CA were released systematically from wild-type samples, introducing one or the other BdPMT gene under the control of the AtC4H promoter boosted the amount of CW-linked CA esters up to the levels observed for mature grass stem samples (ranging between 5 and 18 mg g−1 CW; Table I). Such a high incorporation was obtained for two independent BdPMT1/wt or BdPMT2/wt homozygous lines (T3 generation plants). By contrast, when the BdPMT1 gene was expressed under the control of the maize constitutive ubiquitin (ZmUbi) promoter, CW p-coumaroylation was much lower, with values ranging between 0.8 and 1.6 mg g−1 CW (Supplemental Table S1). Such a modest p-coumaroylation level is similar to that obtained when the OsPMT gene was introduced in Arabidopsis under the control of the CELLULOSE SYNTHASE7 promoter (1–2 mg g−1 CW; Smith et al., 2015).
Table I. Amount of CA released by mild alkaline hydrolysis of the mature stems of Arabidopsis wild-type and BdPMT1- and BdPMT2-expressing lines under the control of the AtC4H promoter and of the corresponding purified DL fractions.
Data from grass samples are given for comparison purposes. The data for Arabidopsis CW represent means (and sd) from three different plant pools (about 20 plants per pool) for each line. Asterisks indicate significant differences (one-way ANOVA) compared with the value of the corresponding control at P < 0.001. For grass CWs, data are means (and sd) from analytical triplicates. For Arabidopsis samples, DL isolation was performed from the pool of biological triplicates, and each DL fraction was then subjected to analytical replicates (n = 2 or 3). For grass DL samples, each DL fraction was analyzed as analytical triplicates.
| Plant and Genotype |
CA from Whole CWs |
CA from Corresponding DL Fractions |
|---|---|---|
| mg g−1 | ||
| Arabidopsis extract-free mature stems | ||
| Wild-type Col-0 | 0.016 (0.002) | 0.93 (0.10) |
| BdPMT1/wt line1-3 | 7.84 (0.20)* | 48.0 (4.1) |
| BdPMT1/wt line12-8 | 12.57 (0.39)* | 66.3 (1.4) |
| Wild-type Col-0 | 0.010 (0.004) | 1.34 (0.04) |
| BdPMT2/wt line2-3 | 13.36 (0.81)* | 71.9 (4.3) |
| BdPMT2/wt line7-7 | 12.74 (0.23)* | 76.6 (2.1) |
| Grass extract-free mature stems | ||
| Maize stem (F2 line, silage stage) | 17.39 (0.66) | 119.8 (2.1) |
| B. distachyon Bd21-3 mature stems | 8.92 (0.63) | 35.4 (1.0) |
| Wheat straw (cv Champlein) | 4.76 (0.10) | 23.8 (1.4) |
Even though the specificity of BdPMT1 for monolignols is established (Petrik et al., 2014), it was necessary to ascertain that CW-linked CA units introduced in the BdPMT1/wt or BdPMT2/wt Arabidopsis line were linked to lignins. To this end, we isolated purified dioxane lignin (DL) fractions by mild acidolysis to check whether CA esters remained associated with lignins. The mild acidolysis procedure consists of refluxing lignified CWs in a dioxane:water mixture (9:1, v/v) containing 0.2 m HCl (30 min under N2) to recover a rough lignin extract, which was then purified to get a DL fraction. When applied to grass CWs, this rapid and mild procedure provides purified DLs with a high recovery yield (50%–70% of the whole lignin amount), a low sugar contamination (in the 3–10 weight percentage range), and with most lignin-linked CA esters preserved (Chazal et al., 2014). Purified DL fractions were obtained from transgenic and wild-type samples. When expressed as percentages of the sample Klason lignin (KL) content (discussed below), their recovery yield was higher for the transgenic lines (mean ± sd = 52% ± 4% for the four DL fractions isolated from BdPMT1- and BdPMT2-expressing lines) than for the control lines (25% ± 5% for the two DL fractions isolated from wild-type samples), which suggests that CA introduction in lignins has made these polymers more amenable to mild acidolysis extraction. Relative to the trace amounts observed for wild-type CWs (less than 0.02 mg g−1 CW; Table I), the corresponding DL fractions were found to be enriched in alkali-releasable CA (about 1 mg g−1 DL; Table I), which confirms that wild-type Arabidopsis lignins are p-coumaroylated, albeit to a very low extent. By contrast, significantly higher CA levels were released by mild alkaline hydrolysis of DL fractions isolated from BdPMT1/wt and BdPMT2/wt stems (Table I). These DL fractions contained 5- to 6-fold more alkali-releasable CA than the corresponding CWs, which conclusively establishes that CA esters are associated with lignins in the corresponding transgenic lines. The highest CA level reached 76 mg g−1 DL, a substantial value intermediate between the values from C3 grass species (wheat [Triticum aestivum] and B. distachyon DL in Table I) and the highly p-coumaroylated DL isolated from maize stems (Table I; Chazal et al., 2014). To evaluate whether some CA also may have acylated arabinose substituents that occur in Arabidopsis hemicelluloses, we subjected the BdPMT1/wt or BdPMT2/wt mature stems to analytical mild acidolysis, a method recently developed to estimate the amount of p-coumaroylated or feruloylated arabinose units in grass arabinoxylans (Petrik et al., 2014). Unlike grass CWs, no trace of p-coumaroylated arabinose could be detected in transgenic Arabidopsis lines.
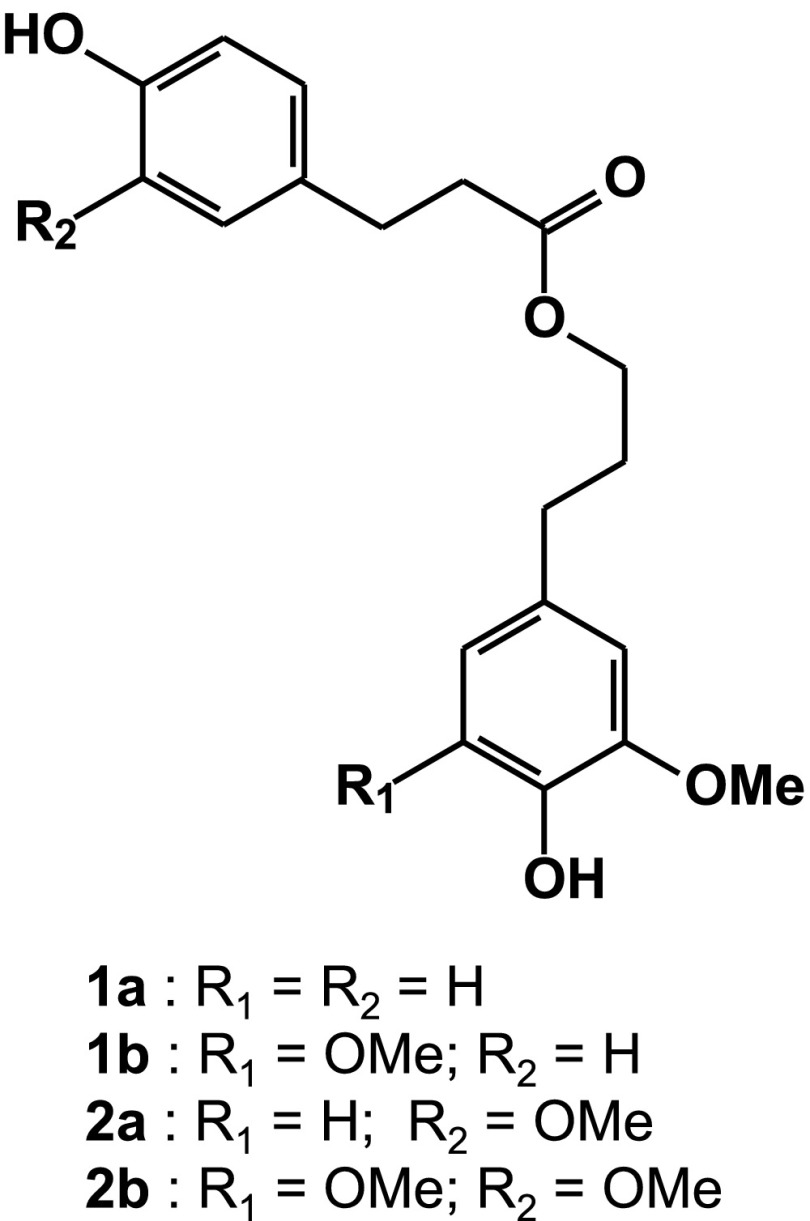
As a further confirmation that lignin units are p-coumaroylated in the BdPMT1/wt and BdPMT2/wt lines, we subjected the purified DL fractions to 1-h-long thioacidolysis experiments. The thioacidolysis lignin-derived mixtures were then desulfurated over Raney nickel, as described previously (Lapierre et al., 1995), and analyzed by gas chromatography-mass spectrometry (GC-MS) of their trimethylsilylated (TMS) derivatives. In contrast to the derivatization followed by reductive cleavage technique that leaves lignin γ-esters intact (Lu and Ralph, 1999), most p-coumaric esters present in grass lignins do not survive the standard 4-h-long thioacidolysis (Lapierre, 2010). However, some p-coumaroylated lignin units can give rise to diagnostic compounds identified previously among the thioacidolysis/Raney nickel products from maize lignins (Grabber et al., 1996). With 1-h-long thioacidolysis followed by desulfuration of BdPMT1/wt DLs, the diagnostic compounds 1a and 1b (Fig. 1) were easily observed on the GC-MS trace showing the separation of lignin-derived products (Supplemental Fig. S3). By comparing the mass spectra of their TMS derivatives (Supplemental Fig. S4; Supplemental Table S2) with published mass spectra (Grabber et al., 1996), compounds 1a and 1b were identified as dihydroconiferyl alcohol acylated by p-dihydrocoumaric acid and dihydrosinapyl alcohol acylated by p-dihydrocoumaric acid, respectively. Accordingly, p-coumaroylated G and S units only involved in β-O-4 bonds were the lignin parent structures of compounds 1a and 1b. With the standard 4-h-long thioacidolysis, these diagnostic compounds were minor or trace components relative to the main dimers, which are representatives of lignin-resistant interunit bonds (Supplemental Fig. S3). With short thioacidolysis, they displayed more substantial peaks (Supplemental Fig. S3), with a large predominance of compound 1b over 1a, suggesting that the main CA conjugate formed by PMT1 is the one with sinapyl alcohol.
Figure 1.
Acylated compounds diagnostic for p-coumaroylation of G and S lignin units (1a and 1b) and for feruloylation of G and S lignin units (2a and 2b). These compounds are recovered after short thioacidolysis (1 h) followed by Raney nickel desulfuration of Arabidopsis lignins isolated from BdPMT1-expressing lines. Compounds 2a and 2b are released only from the lignins of BdPMT1/ccr1g lines.
Type of Lignin Acylation Induced by Expressing the BdPMT1 Gene in the Arabidopsis fah1 or ccr1g Mutant
When AtC4H-driven BdPMT1 expression was performed in the fah1 mutant, which is impaired in the formation of sinapyl alcohol, a substantial CA incorporation also was obtained, up to about 7 mg g−1 CW, and for two independent homozygous lines (referred to as BdPMT1/fah1 lines), whereas alkali-releasable FA remained as a trace component (Table II). As done previously for the wild-type background, we could establish that CA was also closely associated with lignins in BdPMT1/fah1 lines, from the alkaline hydrolysis of the corresponding DL fractions (Table II) and by the identification of the diagnostic compound 1a obtained with 1-h-long thioacidolysis (Supplemental Fig. S3). This result means that the BdPMT1 enzyme is able to induce the appearance of p-coumaroylated G lignin units by catalyzing the formation of coniferyl p-coumarate conjugate. However, this p-coumaroylation was achieved to a lower extent in fah1 lignins than in wild-type ones, in agreement with the fact that sinapyl alcohol has been reported to be a better substrate of lignin-specific PMT than coniferyl alcohol (Withers et al., 2012).
Table II. Amounts of CA and FA released by mild alkaline hydrolysis of the mature stems of Arabidopsis BdPMT1-expressing lines in the fah1 and ccr1g backgrounds and of the corresponding purified DL fractions.
Data for Arabidopsis CW data represent means (and sd) from three different plant pools (about 20 plants per pool) for each line. Asterisks indicate significant differences (one-way ANOVA) compared with the value of the corresponding control at P < 0.001. DL isolation was performed from the pool of biological triplicates for three transgenic lines only, and each DL fraction was then subjected to analytical replicates (n = 2 or 3).
| Genotype | Whole CWs |
Corresponding DL Fractions |
||
|---|---|---|---|---|
| CA | FA | CA | FA | |
| mg g−1 | ||||
| fah1 | 0.021 (0.011) | 0.020 (0.002) | ||
| BdPMT1/fah1 line1-2 | 6.48 (0.17)* | 0.027 (0.10) | 27.3 (1.4) | 0.49 (0.05) |
| BdPMT1/fah1 line12-7 | 7.08 (0.09)* | 0.021 (0.005) | 29.6 (0.1) | 0.23 (0.02) |
| ccr1g | 0.025 (0.005) | 0.053 (0.001) | ||
| BdPMT1/ccr1g line2-7 | 3.53 (0.35)* | 1.58 (0.15)* | 20.0 (2.3) | 11.5 (1.3) |
| BdPMT1/ccr1g line6-5 | 5.28 (0.18)* | 2.49 (0.11)* | ||
When the AtC4H-driven PMT1 insertion was carried out in the Arabidopsis ccr1g background, CA incorporation was also successfully obtained for two independent lines (referred to as BdPMT1/ccr1g lines), albeit to a lower extent than in the BdPMT1/wt and BdPMT1/fah1 samples (Tables I and II). Such a lower incorporation might originate from the developmental problems of the CCR1-deficient Arabidopsis lines, echoed by their dwarf phenotype and lower lignin level (Mir Derikvand et al., 2008). Quite unexpectedly, and in parallel to the accretion of CA esters, a substantial amount of FA was released from the two BdPMT1/ccr1g lines. We previously established that CCR1-deficient Arabidopsis mutants accumulate unusual levels of soluble feruloyl malate (Mir Derikvand et al., 2008) and incorporate free FA into lignins by bis 8-O-4 cross coupling (Ralph et al., 2008). In addition, and as compared with the wild-type level, the amount of alkali-releasable FA is increased slightly by CCR1 deficiency (0.053 ± 0.001 mg g−1 CW for the ccr1g line [Table II] versus 0.020 ± 0.006 mg g−1 CW for the two wild-type samples). The results obtained for the two BdPMT1/ccr1g lines suggest that CCR1 deficiency induces the accumulation of feruloyl-CoA that might be used as a substrate by the PMT1 enzyme for the formation of monolignol ferulate conjugates. As done for the wild-type and fah1 backgrounds, we could ascertain that both CA and FA units introduced in BdPMT1/ccr1g CWs were associated with lignins by alkaline hydrolysis of the corresponding DL fraction (Table II) and by identification of the four diagnostic compounds 1a, 1b, 2a, and 2b (Fig. 1) among the desulfurated products released by 1-h-long thioacidolysis (Supplemental Fig. S5). The dihydroferuloylated conjugates 2a and 2b displayed mass spectra very similar to those of 1a and 1b, respectively, but with molecular ions at +30 D (Supplemental Fig. S4). In summary, the analyses of BdPMT1/ccr1g lines revealed that PMT1 is able to catalyze not only the formation of monolignol p-coumarate conjugates but also that of monolignol ferulate conjugates, provided that some alteration in the monolignol pathway induces a sufficient feruloyl-CoA buildup. In a recent study, lignin feruloylation mediated by the introduction of a gene encoding for an Angelica sinensis feruloyl-CoA monolignol transferase into poplar was reported as a novel strategy to introduce chemically labile linkages in lignins (Wilkerson et al., 2014). From our data here, lignin feruloylation also seems to be possible by introducing the BdPMT1 gene into the appropriate mutant or transgenic background.
Lignin p-Coumaroylation Induces Lower Lignin Content and Improved Saccharification of Arabidopsis Inflorescence Stems
Increasing the lignin p-coumaroylation level up to that of grass lignins induced a noticeable decrease of the KL content of Arabidopsis CWs (Table III). Relative to the corresponding control, the KL amount of BdPMT1- or BdPMT2- expressing lines was reduced by 10% to 30%. Such an impact was confirmed by acetyl bromide lignin (ABL) determination. All samples displayed an ABL level reduced by 10% to 20% relative to the corresponding control, except the two transgenic lines obtained in the ccr1g background. By contrast, no significant KL or ABL reduction could be observed for lower lignin p-coumaroylation degree, as reported recently (Smith et al., 2015) or as observed when the BdPMT1 gene was inserted under the control of the ZmUbi promoter (Supplemental Table S3).
Table III. Impact of expressing the BdPMT1 and BdPMT2 genes under the control of the AtC4H promoter in Arabidopsis lines on the KL content or ABL content of extract-free mature stems as well as on their saccharification efficiency.
Data represent means (and sd) from three different plant pools (about 20 plants per pool) per genotype. Asterisks indicate significant differences (one-way ANOVA) compared with the value of the corresponding control at P < 0.001.
| Genotype | KL | ABL | Saccharification Efficiency of Glc Release |
|---|---|---|---|
| % by weight | mg g−1 | ||
| Wild type | 18.51 (0.06) | 14.68 (0.47) | 131.0 (3.2) |
| BdPMT1/wt line1-3 | 15.84 (0.44)* | 13.17 (0.19)* | 146.3 (3.8)* |
| BdPMT1/wt line12-8 | 15.05 (0.21)* | 12.17 (0.52)* | 148.0 (2.3)* |
| fah1 | 21.12 (0.08) | 19.91 (0.59) | 115.2 (1.6) |
| BdPMT1/fah1 line1-2 | 18.39 (0.78)* | 16.37 (1.09)* | 150.4 (3.8)* |
| BdPMT1/fah1 line12-7 | 17.63 (0.17)* | 15.69 (0.49)* | 147.1 (6.5)* |
| ccr1g | 15.58 (0.41) | 12.45 (0.22) | 165.1 (1.8) |
| BdPMT1/ccr1g line2-7 | 13.29 (0.14)* | 12.12 (0.18) | 234.9 (12.9)* |
| BdPMT1/ccr1g line6-5 | 14.10 (1.05) | 12.13 (0.36) | 254.7 (8.4)* |
| Wild type | 19.84 (0.08) | 15.90 (0.22) | 122.8 (4.3) |
| BdPMT2/wt line2-3 | 15.01 (0.18)* | 13.34 (0.21)* | 147.6 (3.1)* |
| BdPMT2/wt line7-7 | 13.88 (0.21)* | 11.95 (0.26)* | 155.2 (1.8)* |
When calculated relative to the KL content of mature stems, the lignin p-coumaroylation was actually boosted up to 8% to 9% (w/w) for the transgenic lines obtained in the wild-type background, provided that the expression of one or the other PMT-encoding genes was driven by the AtC4H promoter, whereas the lignin CA level was only increased to 0.7% to 0.8% (w/w) with the constitutive ZmUbi promoter (Supplemental Fig. S6). The relative KL reduction observed in BdPMT1- or BdPMT2-expressing lines seemed to be somehow related to the lignin p-coumaroylation degree. This observation suggests that the lignification process might be indirectly or directly slowed down by the formation of CA-monolignol conjugates, provided that this formation induces a similar lignin p-coumaroylation extent to that in grass lignins.
The susceptibility of extract-free mature CWs to saccharification by a commercial cellulase preparation and without any pretreatment was evaluated by measuring the released Glc. As expected, the saccharification of Arabidopsis samples displaying a lower KL content yielded more Glc (Table III). However, the correlation between lignin content and Glc yield was low, which confirms that other factors than lignin content govern the CW susceptibility to saccharification. The most efficient saccharification was observed in the ccr1g background, with an unexpectedly high improvement afforded by introducing both CA and FA esters in lignins. In these samples, and relative to the control, a moderate KL reduction (about 10%) induced a large saccharification improvement (Glc release increased by about 50%).
The Substantial p-Coumaroylation of Arabidopsis Lignins Is Accompanied by Other Severe Structural Changes
The impact of p-coumaroylation on lignin structure was evaluated by thioacidolysis. This method specifically provides p-hydroxyphenyl (H), G, and S monomers from H, G, and S lignin units that are involved exclusively in labile β-O-4 interunit bonds. As discussed previously, compounds 1a, 1b, 2a, and 2b were obtained as minor or trace components with the standard 4-h-long thioacidolysis (Supplemental Figs. S3 and S5). This observation rules out the possibility that the yield of lignin-derived S or G monomers might be noticeably reduced by the survival of CA or FA ester linked at the Cγ group of lignin units. With this consideration in mind, and when expressed relative to the lignin content, thioacidolysis yield closely reflects the frequency of the parent lignin structure. As a corollary, thioacidolysis yield is reduced when the frequency of resistant interunit bonds is increased in lignins.
As shown in Table IV, the molar frequencies of lignin-derived H, G, or S monomers released from mature extract-free stems were found to be similar in the BdPMT1- or BdPMT2-expressing lines and in the corresponding controls. By contrast, when expressed on a KL basis (in µmol g−1 KL), the total yield of thioacidolysis monomers was reduced noticeably for the Arabidopsis samples displaying substantial lignin p-coumaroylation. Such a reduction is very likely diagnostic for a higher frequency of resistant interunit bonds, which counteracts the recovery of thioacidolysis monomers. This impact on lignin structure, reflected by reduced thioacidolysis yield, was not observed when lower lignin p-coumaroylation was achieved by ZmUbi-driven BdPMT1 expression (Supplemental Table S3).
Table IV. Thioacidolysis of extract-free mature stems from Arabidopsis control lines and transgenic lines expressing the BdPMT1 or BdPMT2 gene under the control of the AtC4H promoter.
Data represent means (and sd) from three different plant pools per genotype. Asterisks indicate significant differences (one-way ANOVA) compared with the value of the corresponding control at P < 0.001. ND, Not detected.
| Line | Total Yield | H | G | S |
|---|---|---|---|---|
| µmol g−1 KL | % | |||
| Wild type | 1,064 (34) | 1.0 (0.1) | 69.7 (0.2) | 29.3 (0.1) |
| BdPMT1/wt line1-3 | 891 (53) | 1.0 (0.1) | 68.5 (0.2) | 30.5 (0.3) |
| BdPMT1/wt line12-8 | 713 (7)* | 1.3 (0.2) | 68.7 (0.1) | 30.0 (0.1) |
| fah1 | 962 (40) | 0.9 (0.2) | 99.1 (0.2) | ND |
| BdPMT1/fah1 line1-2 | 675 (23)* | 1.3 (0.1) | 98.7 (0.1) | ND |
| BdPMT1/fah1 line12-7 | 673 (5)* | 1.1 (0.0) | 98.9 (0.0) | ND |
| ccr1g | 808 (60) | 0.9 (0.1) | 63.1 (0.6) | 36.1 (0.7) |
| BdPMT1/ccr1g line2-7 | 602 (78) | 0.7 (0.1) | 65.9 (1.0) | 33.4 (0.9) |
| BdPMT1/ccr1g line6-5 | 523 (18)* | 0.7 (0.1) | 64.9 (0.2) | 34.5 (0.2) |
| Wild type | 853 (17) | 0.9 (0.1) | 69.7 (0.2) | 29.4 (0.3) |
| BdPMT2/wt line2-3 | 532 (15)* | 1.3 (0.2) | 70.8 (0.7) | 27.9 (0.7) |
| BdPMT2/wt line7-7 | 503 (42)* | 1.3 (0.2) | 71.5 (0.5) | 27.3 (0.5) |
The percentage of terminal units with free phenolic groups in native lignins is a major structural feature that governs their solubility in alkaline medium. This feature can be evaluated by the thioacidolysis of permethylated samples (Lapierre, 2010). Whatever the sample and in agreement with past results, about 85% to 90% of H units giving rise to H thioacidolysis monomers were found to be terminal units. In control samples (i.e. in wild-type, fah1, and ccr1g lines), this percentage was weak for S units (less than 1.5% of β-O-4-linked S units were free phenolic; Table V) and low for G units (14%–16%; Table V), in agreement with previous data reported for Arabidopsis stem lignins (Ralph et al., 2008; Lapierre, 2010). As another major structural change accompanying the p-coumaroylation of Arabidopsis lignins, the thioacidolysis of permethylated samples revealed that lignins from BdPMT1- or BdPMT2-expressing lines were enriched in G or S terminal units with free phenolic groups (Table V). A higher frequency of terminal lignin units relative to internal ones may originate from a higher branching degree and/or a lower average Mr of native lignins. This structural alteration suggests that the participation of p-coumaroylated monolignols to lignification has somehow affected the lignin polymerization mode.
Table V. Relative percentages of free phenolic groups in G or S lignin units only involved in β-O-4 bonds, as revealed by thioacidolysis of permethylated extract-free stems from an Arabidopsis control line and from BdPMT1- or BdPMT2-expressing lines under the control of the AtC4H promoter.
Calculations were done as described previously (Lapierre, 2010). Data represent means (and sd) from duplicate analyses of different plant pools per genotype. Asterisks indicate significant differences (one-way ANOVA) compared with the value of the corresponding control at P < 0.001.
| Line | G | S |
|---|---|---|
| Wild-type Col-0 | 13.7 (0.1) | 1.3 (0.1) |
| BdPMT1/wt line1-3 | 21.8 (0.3)* | 2.2 (0.1)* |
| BdPMT1/wt line12-8 | 26.0 (0.0)* | 2.7 (0.2)* |
| fah1 | 16.7 (0.1) | – |
| BdPMT1/fah1 line1-2 | 19.1 (0.1)* | – |
| BdPMT1/fah1 line12-7 | 20.2 (0.6)* | – |
| ccr1g | 16.0 (0.1) | 1.1 (0.1) |
| BdPMT1/ccr1g line2-7 | 41.6 (3.9)* | 4.3 (0.5)* |
| BdPMT1/ccr1g line6-5 | 55.4 (0.3)* | 6.0 (0.5)* |
| Wild-type Col-0 | 14.0 (0.1) | 1.3 (0.1) |
| BdPMT2/wt line7-7 | 26.3 (0.7)* | 2.6 (0.2)* |
Arabidopsis p-Coumaroylated Lignins Are Twice More Soluble in Alkali and at Room Temperature
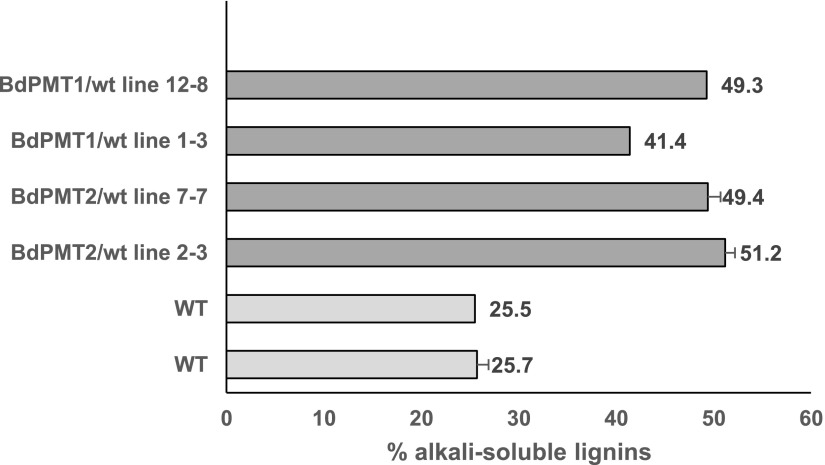
A higher frequency of terminal units with a free phenolic group in lignins may have a significant consequence on the solubilization of lignins in alkaline medium and at room temperature, as exemplified by the case study of grass lignins (Lapierre, 2010). Based on this rationale, and to evaluate the extent to which the lignins of BdPMT1/wt and BdPMT2/wt lines mirrored the solubility properties of p-coumaroylated grass lignins, we performed simple solubilization tests. The extract-free stem samples from wild-type and transgenic lines were subjected to lignin solubilization assays in 1 m NaOH at 20°C overnight. The resulting saponified CWs were carefully recovered, washed, and weighed before the determination of their KL content. Thus, the percentage of alkali-solubilized lignins could be evaluated from the alkali-induced weight loss together with the KL levels before and after this mild alkaline treatment (Fig. 2). This simple experiment revealed that the solubility of Arabidopsis lignins was increased from 25% in the wild-type lines to about 50% in the transgenic lines displaying lignins as p-coumaroylated as in grass lignins.
Figure 2.
The p-coumaroylation of Arabidopsis lignins up to the grass lignin level doubles their solubility in alkali at room temperature. Values show percentages of Arabidopsis lignins solubilized in 1 m NaOH at 20°C (overnight) from extract-free mature stems of wild-type (WT) and BdPMT1- and BdPMT2-expressing lines under the control of the AtC4H promoter. Data were obtained from duplicate solubilization experiments when error bars are given.
CONCLUSION
It is now well established that grass lignin p-coumaroylation results from the PMT-mediated p-coumaroylation of monolignols (Hatfield and Marita, 2010; Withers et al., 2012; Petrik et al., 2014). In a recent study, the introduction of a rice PMT gene into Arabidopsis and poplar induced the formation of p-coumaroylated lignins, with lignin p-coumaroylation degree ranging between 0.8% and 2% (w/w) of ABL lignins, without any impact on lignin content (Smith et al., 2015). Compared with this recent study, and most likely thanks to the use of the AtC4H promoter to drive BdPMT expression, we obtained 5- to 10-fold higher p-coumaroylation of lignins from Arabidopsis stems (up to 8%–9% [w/w] KL; Supplemental Fig. S6). Such a high p-coumaroylation level of Arabidopsis lignins, actually approaching those of lignins from mature maize stems, was the prerequisite for deciphering herein the tremendous impact of p-coumaroylated monolignols on lignification.
In this work, we report the very effective p-coumaroylation of Arabidopsis lignins, which was achieved not only with the introduction of the Bradi2g36910 gene (BdPMT1) encoding the bona fide lignin-specific PMT enzyme but also with the Bradi1g36980 gene (BdPMT2), another PMT-encoding gene with a still ill-defined role. Results from the introduction of the BdPMT1 gene into the fah1 background confirmed that PMT1 is able to efficiently drive the p-coumaroylation of G lignin units, which paves the way for the p-coumaroylation of gymnosperm lignins. In addition, the introduction of BdPMT1 into the ccr1g background revealed that PMT1 is also able to catalyze in vivo the formation of monolignol-ferulate conjugates if feruloyl-CoA is readily available.
When reaching the grass lignin level, the p-coumaroylation of Arabidopsis lignins was associated with severe structural alterations, namely a higher frequency of resistant interunit bonds and a higher frequency of terminal units with free phenolic groups. As could be anticipated from the higher frequency of G units with free phenolic groups, the solubilization of Arabidopsis lignins in alkali and at room temperature was about doubled for the transgenic lines provided with a high lignin p-coumaroylation degree. With these structural and solubility modifications, the p-coumaroylated Arabidopsis lignins are similar to grass lignins. Taken together, these results suggest genetically induced p-coumaroylation as a new and efficient strategy to make nongrass lignins as amenable to mild alkali pretreatment as grass lignins.
MATERIALS AND METHODS
Production of Plant Materials
The full-length complementary DNA sequences of Brachypodium distachyon BdPMT1 andBdPMT2 genes were amplified by reverse transcription-PCR from Bd21-3 stems using the BdPMT1 forward primer 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGAGAAGAAGTTCACGGTG-3′ and reverse primer 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCTCACTTCCCGGCGGTGAAGGCG-3′ and the BdPMT2 forward primer 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGCGGAGCGCGGGCGCGGTG-3′ and reverse primer 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCTCAGTCGAGGCGCATCATGTCC-3′, respectively.
The PCR products were ligated into the pDONR207 plasmid by BP Gateway (Invitrogen) reaction. The resulting pEntry clone was used to transfer the BdPMT1 or BdPMT2 gene into the pCC0996 vector, allowing expression under the control of the AtC4H promoter (Weng et al., 2008) by LR Gateway (Invitrogen) reaction. In addition, the BdPMT1 gene was transferred into the pIPKb2 vector containing the maize (Zea mays) ZmUbi promoter (Himmelbach et al., 2007). These various constructs were introduced into the wild-type, fah-1, or ccr1g background. Two lines for each construct and genetic background were selected and used for chemical analysis. Plants were grown together in a greenhouse for 12 weeks at 22°C in a 16-h-light/8-h-dark photoperiod and with 55% relative humidity.
The mature stems of control and transgenic plants were collected, ground to 0.5 mm, and subjected to exhaustive water and ethanol extraction in a Soxhlet apparatus. The extract-free samples were dried at 45°C for 2 d and then used for the analyses of CW-linked phenolics (lignins and CA or FA esters). In this study, the dried extract-free samples are referred to as CW samples.
Lignin Determination
All measurements were performed on biological triplicates. The determination of KL content was carried out from approximately 300 mg (weighed to the nearest 0.1 mg) of extract-free sample according to a protocol adapted from Dence (1992).
The determination of ABL was performed according to a procedure adapted from Fukushima and Hatfield (2001) using 6 to 8 mg of extract-free samples (weighed to the nearest 0.1 mg) mixed with 1.5 mL of freshly prepared 25% (v/v) acetyl bromide in acetic acid in a 2-mL glass vial provided with a Teflon-lined screw cap. The mixture was then digested at 55°C and at 650 rpm in a mixing block for 2.5 h. After cooling, 0.2 mL of the solution was diluted with 3 mL of acetic:acid:2 M NaOH mixture (50:9, v/v) and 0.5 mL of 0.5 m hydroxylamine chlorhydrate. The absorbance of the diluted solution was read at 280 nm against a reagent blank. ABL lignin content was calculated from this absorbance using the extinction coefficient 20 g L−1 cm−1.
Analytical Mild Acidolysis
We looked for the putative occurrence of p-coumaroylated arabinose units in the CWs of wild-type, BdPMT1/wt, and BdPMT2/wt stems by analytical mild acidolysis according to a recently reported procedure (Petrik et al., 2014).
Isolation of DL Fractions
About 2 g of extract-free sample was suspended in 50 mL of a dioxane:water mixture (9:1, v/v) containing 0.2 m HCl. The suspension was refluxed for 30 min under N2. The cooled reaction mixture was filtrated over a Büchner funnel, and the residue was washed three times with 10 mL of a dioxane:water mixture (9:1, v/v). All the filtrates containing the dissolved lignins were pooled, and the pH of the resulting solution was adjusted to 3 to 4 (saturated NaHCO3 aqueous solution). The solution was concentrated to about 10 mL and by rotoevaporation at 45°C. The concentrated DL solution was then injected into about 100 mL of cold water under magnetic stirring. The lignin precipitate was recovered by centrifugation (30 min at 2,000g and 10°C), washed with pure water, centrifuged again, and freeze-dried to recover a purified DL fraction.
Analysis of CA and FA Released by Mild Alkaline Hydrolysis of CW or DL Samples
About 5 to 10 mg of CW or DL samples (weighed to the nearest 0.1 mg) was put into a 2-mL Eppendorf plastic tube together with 1 mL of 1 m NaOH aqueous solution with 0.1 mL of o-coumaric acid internal standard methanolic solution. The internal standard amount was adapted to the analyzed samples (0.01 mg for control samples and 0.1 mg for transgenic samples). The mild alkaline hydrolysis was carried out overnight at room temperature on a carousel. After acidification with 0.2 mL of 6 m HCl, the tubes were centrifuged for 15 min at 2,000g and the supernatant was subjected to solid-phase extraction, then HPLC-diode-array detection analysis of the recovered methanolic extracts was performed as described previously (Ho-Yue-Kuang et al., 2016). To further confirm the occurrence of alkali-released CA or FA from control or transgenic samples, the methanolic extracts were diluted with water and the low-Mr phenolics were reextracted with ethyl acetate before GC-MS analyses of their TMS derivatives.
Lignin Analysis by Thioacidolysis
The simplified thioacidolysis was applied to extract-free CW or DL samples as described previously (Méchin et al., 2014). In addition, thioacidolysis assays were also performed after comprehensive permethylation of extract-free sample as follows. About 50 mg of extract-free CW sample was suspended in 1 mL of methanol and 1 mL of 2 m trimethylsilyldiazomethane solution in hexane (Acros Organics). The permethylation was then allowed to proceed for 24 h in a 4-mL glass vial provided with a Teflon-lined screw cap under agitation in a mixing block. After 24 h, the agitation was stopped, the vial was carefully opened (to release the nitrogen formed from the permethylation reagent), and the insoluble sample was allowed to decant before removal of the supernatant and the addition of fresh methylating mixture. This permethylation step was repeated twice with fresh reagent before washing of the permethylated sample with methanol and freeze-drying.
The desulfuration of the thioacidolysis mixture over Raney nickel was performed at 45°C in dioxane as described previously (Lapierre et al., 1995).
Saccharification Assays
About 30 mg of extract-free CWs was put into a 5-mL plastic tube together with 4 mL of 5 mm acetate buffer (pH 4.7) containing 4 mg mL−1 commercial cellulase preparation (Cellulase Onozuka R10 from Trichoderma viride; Serva) and 0.1 mg mL−1 sodium azide. The reaction tubes together with a blank tube (containing only the enzyme solution) were placed at 45°C for 72 h on a carousel. After centrifugation (1,500g for 20 min), the supernatant was subjected to Glc determination using the K-Gluc Megazyme Kit (http://www.megazyme.com) adapted to a 96-well format. In each well of the microplate, 200 µL of the Glc reagent was put together with 10 µL of supernatant. The microplate was then covered and the reaction was allowed to proceed for 20 min at 45°C before reading the A510 in a Spectramax microplate reader. The Glc amount released from the CW samples was calculated based on a Glc standard curve after subtraction of the Glc measured in the blank sample.
Alkali Solubilization Assays
About 600 mg (weighed to the nearest 0.1 mg) of extract-free samples was put into a 50-mL plastic tube together with 20 mL of 1 m NaOH. The suspension was agitated on a carousel overnight at room temperature, then carefully filtrated over a Büchner porcelain funnel protected with a Whatman paper filter number 4. The residue was then carefully washed with water, then with 1 m HCl, and finally with water again, before freeze-drying. The final dry saponified residue was carefully weighed to calculate its recovery yield and then subjected to KL determination.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phylogeny of the PMT-related subset of the BAHD superfamily in angiosperms.
Supplemental Figure S2. Representative images of Arabidopsis plants expressing the BdPMT1 or BdPMT2 gene under AtC4H promoter control in the wild-type, fah1, and ccr1g backgrounds.
Supplemental Figure S3. GC-MS separation of the main lignin-derived dimers obtained by thioacidolysis (1 h or 4 h long) and Raney nickel desulfuration of DL fractions purified from Arabidopsis mature stems of BdPMT1/wt and BdPMT1/fah1 lines.
Supplemental Figure S4. Mass spectra (electronic impact, 70 eV) of the four lignin-derived compounds diagnostic for the incorporation of CA or FA into Arabidopsis lignins.
Supplemental Figure S5. GC-MS separation of the main lignin-derived dimers obtained by thioacidolysis (1 h or 4 h long) and Raney nickel desulfuration of a DL fraction purified from Arabidopsis mature stems of the BdPMT1/ccr1g line.
Supplemental Figure S6. Calculated weight percentage of CA linked to the lignins of Arabidopsis mature stems of BdPMT1- and BdPMT2-expressing lines.
Supplemental Table S1. Amount of CA released by mild alkaline hydrolysis of extract-free mature stems from Arabidopsis wild-type and transgenic lines expressing BdPMT1 under ZmUbi promoter control.
Supplemental Table S2. Abbreviated mass spectra (electronic impact, 70 eV) of the main TMS derivatives of lignin-derived dimers recovered after thioacidolysis and Raney nickel desulfuration of Arabidopsis extract-free stems.
Supplemental Table S3. Analysis of extract-free mature stems from Arabidopsis wild-type and transgenic lines expressing the BdPMT1 gene under the control of the ZmUbi promoter.
Supplementary Material
Acknowledgments
We thank Clint Chapple for kindly providing the vector pCC0996 allowing the expression of PMT under the control of the AtC4H promoter and Sébastien Antelme for growing B. distachyon 21-3 plants.
Glossary
- G
guaiacyl
- FA
ferulic acid
- CA
p-coumaric acid
- S
syringyl
- Col-0
Columbia-0
- CW
cell wall
- DL
dioxane lignin
- KL
Klason lignin
- GC-MS
gas chromatography-mass spectrometry
- TMS
trimethylsilylated
- ABL
acetyl bromide lignin
- H
p-hydroxyphenyl
Footnotes
This work was supported by LabEx Saclay Plant Sciences-SPS (grant no. ANR–10–LABX–0040–SPS to the Institut Jean-Pierre Bourgin).
References
- Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101: 4851–4861 [DOI] [PubMed] [Google Scholar]
- Beckman E, Liesche O, Lehman F (1923) Qualitative und quantitative Unterschiede der Lignine einiger Holz-und Stroharten. Biochem Z 139: 491 [Google Scholar]
- Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C (1997) Cinnamate-4-hydroxylase expression in Arabidopsis: regulation in response to development and the environment. Plant Physiol 113: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple CC, Vogt T, Ellis BE, Somerville CR (1992) An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell 4: 1413–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal R, Robert P, Durand S, Devaux MF, Saulnier L, Lapierre C, Guillon F (2014) Investigating lignin key features in maize lignocelluloses using infrared spectroscopy. Appl Spectrosc 68: 1342–1347 [DOI] [PubMed] [Google Scholar]
- Dence CW. (1992) The determination of lignin. In Lin SY, Dence CW, eds, Methods in Lignin Chemistry. Springer-Verlag, New York, pp 33–61 [Google Scholar]
- Fukushima RS, Hatfield RD (2001) Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J Agric Food Chem 49: 3133–3139 [DOI] [PubMed] [Google Scholar]
- Grabber JH, Quideau S, Ralph J (1996) p-Coumaroylated syringyl units in maize lignin: implications for beta-ether cleavage by thioacidolysis. Phytochemistry 43: 1189–1194 [Google Scholar]
- Hatfield RD, Marita JM (2010) Enzymatic processes involved in the incorporation of hydroxycinnamates into grass cell walls. Phytochem Rev 9: 35–45 [Google Scholar]
- Hatfield RD, Marita JM, Frost K, Grabber J, Ralph J, Lu F, Kim H (2009) Grass lignin acylation: p-coumaroyl transferase activity and cell wall characteristics of C3 and C4 grasses. Planta 229: 1253–1267 [DOI] [PubMed] [Google Scholar]
- Himmelbach A, Zierold U, Hensel G, Riechen J, Douchkov D, Schweizer P, Kumlehn J (2007) A set of modular binary vectors for transformation of cereals. Plant Physiol 145: 1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Yue-Kuang S, Alvarado C, Antelme S, Bouchet B, Cézard L, Le Bris P, Legée F, Maia-Grondard A, Yoshinaga A, Saulnier L, et al. (2016) Mutation in Brachypodium caffeic acid O-methyltransferase 6 alters stem and grain lignins and improves straw saccharification without deteriorating grain quality. J Exp Bot 67: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Ragauskas A (2012) Pretreatment and lignocellulosic chemistry. BioEnergy Res 5: 1043–1066 [Google Scholar]
- Jacquet G, Pollet B, Lapierre C (1995) New ether-linked ferulic acid-coniferyl alcohol dimers identified in grass straws. J Agric Food Chem 43: 2746–2751 [Google Scholar]
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48: 3713–3729 [Google Scholar]
- Lapierre C. (2010) Determining lignin structure by chemical degradations. In Heitner C, Dimmel DR, Schmidt JA, eds, Lignin and Lignans. CRC Press, Boca Raton, FL, pp 11–48 [Google Scholar]
- Lapierre C, Jouin D, Monties B (1989) On the molecular origin of the alkali solubility of Gramineae lignins. Phytochemistry 28: 1401–1403 [Google Scholar]
- Lapierre C, Pollet B, Rolando C (1995) New insights into the molecular architecture of hardwood lignins by chemical degradative methods. Res Chem Intermed 21: 397–412 [Google Scholar]
- Lu F, Ralph J (1999) Detection and determination of p-coumaroylated units in lignins. J Agric Food Chem 47: 1988–1992 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (2002) Preliminary evidence for sinapyl acetate as a lignin monomer in kenaf. Chem Commun (Camb) 90–91 [DOI] [PubMed] [Google Scholar]
- Méchin V, Laluc A, Legée F, Cézard L, Denoue D, Barrière Y, Lapierre C (2014) Impact of the brown-midrib bm5 mutation on maize lignins. J Agric Food Chem 62: 5102–5107 [DOI] [PubMed] [Google Scholar]
- Mir Derikvand M, Sierra JB, Ruel K, Pollet B, Do CT, Thévenin J, Buffard D, Jouanin L, Lapierre C (2008) Redirection of the phenylpropanoid pathway to feruloyl malate in Arabidopsis mutants deficient for cinnamoyl-CoA reductase 1. Planta 227: 943–956 [DOI] [PubMed] [Google Scholar]
- Petrik DL, Karlen SD, Cass CL, Padmakshan D, Lu F, Liu S, Le Bris P, Antelme S, Santoro N, Wilkerson CG, et al. (2014) p-Coumaroyl-CoA:monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J 77: 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J. (2010) Hydroxycinnamates in lignification. Phytochem Rev 9: 65–83 [Google Scholar]
- Ralph J, Grabber JH, Hatfield RD (1995) Lignin-ferulate cross-links in grasses: active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr Res 275: 167–178 [Google Scholar]
- Ralph J, Hatfield RD, Quideau S, Helm RF, Grabber JH, Jung HJG (1994) Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J Am Chem Soc 116: 9448–9456 [Google Scholar]
- Ralph J, Kim H, Lu F, Grabber JH, Leplé JC, Berrio-Sierra J, Derikvand MM, Jouanin L, Boerjan W, Lapierre C (2008) Identification of the structure and origin of a thioacidolysis marker compound for ferulic acid incorporation into angiosperm lignins (and an indicator for cinnamoyl CoA reductase deficiency). Plant J 53: 368–379 [DOI] [PubMed] [Google Scholar]
- Smith RA, Gonzales-Vigil E, Karlen SD, Park JY, Lu F, Wilkerson CG, Samuels L, Ralph J, Mansfield SD (2015) Engineering monolignol p-coumarate conjugates into poplar and Arabidopsis lignins. Plant Physiol 169: 2992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JK, Li X, Stout J, Chapple C (2008) Independent origins of syringyl lignin in vascular plants. Proc Natl Acad Sci USA 105: 7887–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson CG, Mansfield SD, Lu F, Withers S, Park JY, Karlen SD, Gonzales-Vigil E, Padmakshan D, Unda F, Rencoret J, et al. (2014) Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344: 90–93 [DOI] [PubMed] [Google Scholar]
- Withers S, Lu F, Kim H, Zhu Y, Ralph J, Wilkerson CG (2012) Identification of grass-specific enzyme that acylates monolignols with p-coumarate. J Biol Chem 287: 8347–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin 2: 26–40 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.