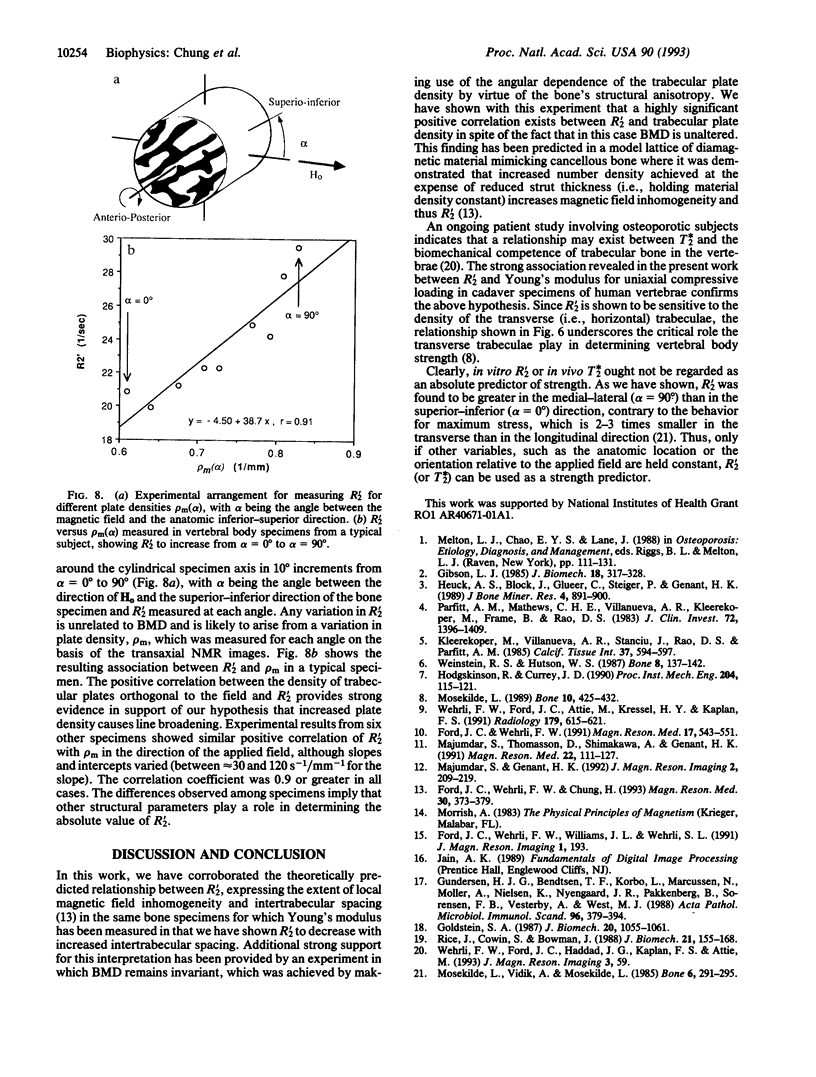
Abstract
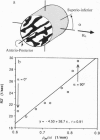
Structure, biomechanical competence, and incremental NMR line broadening (R'2) of water in the intertrabecular spaces of cancellous bone were examined on 22 cylindrical specimens from the lumbar vertebral bodies of 16 human subjects 24-86 years old (mean, 60 years old). A strong association (r = 0.91; P < 0.0001) was found between Young's modulus of elasticity and R'2 for a wide range of values corresponding to cancellous bone of very different morphologic composition. NMR line broadening is caused by the inhomogeneity of the magnetic field induced as a consequence of the coexistence of two adjacent phases of different diamagnetic susceptibility--i.e., mineralized bone and water in the marrow spaces. Structural analyses performed by means of NMR microscopy and digital image processing indicated that the variation in R'2 is closely related to the trabecular microstructure. Mean trabecular plate density measured along the direction of the magnetic field was found to play a major role in predicting R'2 (r = 0.74; P < 0.0001). This behavior was confirmed when the plate density was varied in individual specimens, which was achieved by rotating the specimen, making use of the bone's structural anisotropy. It is concluded that the NMR transverse relaxation rate in human cancellous bone of the spine is significantly determined by trabecular structural parameters relevant to biomechanical strength. The results further underscore the important role played by the transverse trabeculae in contributing to cancellous bone strength. The work has implications on possible in vivo use of quantitative magnetic resonance for the assessment of fracture risk in osteoporotic patients.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ford J. C., Wehrli F. W., Chung H. W. Magnetic field distribution in models of trabecular bone. Magn Reson Med. 1993 Sep;30(3):373–379. doi: 10.1002/mrm.1910300316. [DOI] [PubMed] [Google Scholar]
- Ford J. C., Wehrli F. W. In vivo quantitative characterization of trabecular bone by NMR interferometry and localized proton spectroscopy. Magn Reson Med. 1991 Feb;17(2):543–551. doi: 10.1002/mrm.1910170225. [DOI] [PubMed] [Google Scholar]
- Gibson L. J. The mechanical behaviour of cancellous bone. J Biomech. 1985;18(5):317–328. doi: 10.1016/0021-9290(85)90287-8. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A. The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech. 1987;20(11-12):1055–1061. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Heuck A. F., Block J., Glueer C. C., Steiger P., Genant H. K. Mild versus definite osteoporosis: comparison of bone densitometry techniques using different statistical models. J Bone Miner Res. 1989 Dec;4(6):891–900. doi: 10.1002/jbmr.5650040614. [DOI] [PubMed] [Google Scholar]
- Hindré F., Le Plouzennec M., de Certaines J. D., Foultier M. T., Patrice T., Simonneaux G. Tetra-p-aminophenylporphyrin conjugated with Gd-DTPA: tumor-specific contrast agent for MR imaging. J Magn Reson Imaging. 1993 Jan-Feb;3(1):59–65. doi: 10.1002/jmri.1880030111. [DOI] [PubMed] [Google Scholar]
- Hodgskinson R., Currey J. D. The effect of variation in structure on the Young's modulus of cancellous bone: a comparison of human and non-human material. Proc Inst Mech Eng H. 1990;204(2):115–121. doi: 10.1243/PIME_PROC_1990_204_240_02. [DOI] [PubMed] [Google Scholar]
- Kleerekoper M., Villanueva A. R., Stanciu J., Rao D. S., Parfitt A. M. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985 Dec;37(6):594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- Majumdar S., Genant H. K. In vivo relationship between marrow T2* and trabecular bone density determined with a chemical shift-selective asymmetric spin-echo sequence. J Magn Reson Imaging. 1992 Mar-Apr;2(2):209–219. doi: 10.1002/jmri.1880020215. [DOI] [PubMed] [Google Scholar]
- Majumdar S., Thomasson D., Shimakawa A., Genant H. K. Quantitation of the susceptibility difference between trabecular bone and bone marrow: experimental studies. Magn Reson Med. 1991 Nov;22(1):111–127. doi: 10.1002/mrm.1910220112. [DOI] [PubMed] [Google Scholar]
- Mosekilde L. Sex differences in age-related loss of vertebral trabecular bone mass and structure--biomechanical consequences. Bone. 1989;10(6):425–432. doi: 10.1016/8756-3282(89)90074-4. [DOI] [PubMed] [Google Scholar]
- Mosekilde L., Viidik A., Mosekilde L. Correlation between the compressive strength of iliac and vertebral trabecular bone in normal individuals. Bone. 1985;6(5):291–295. doi: 10.1016/8756-3282(85)90317-5. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Mathews C. H., Villanueva A. R., Kleerekoper M., Frame B., Rao D. S. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983 Oct;72(4):1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J. C., Cowin S. C., Bowman J. A. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988;21(2):155–168. doi: 10.1016/0021-9290(88)90008-5. [DOI] [PubMed] [Google Scholar]
- Wehrli F. W., Ford J. C., Attie M., Kressel H. Y., Kaplan F. S. Trabecular structure: preliminary application of MR interferometry. Radiology. 1991 Jun;179(3):615–621. doi: 10.1148/radiology.179.3.2027962. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Hutson M. S. Decreased trabecular width and increased trabecular spacing contribute to bone loss with aging. Bone. 1987;8(3):137–142. doi: 10.1016/8756-3282(87)90012-3. [DOI] [PubMed] [Google Scholar]