Abstract
Despite a growing number of studies showing relationships between behavior and resting-state functional MRI measures of large-scale brain network connectivity, no study to our knowledge has sought to investigate whether intrinsic connectivity-behavioral relationships are stable over time. In this study, we investigated the stability of such brain-behavior relationships at two timepoints, approximately 1 week apart. We focused on the relationship between the strength of hippocampal connectivity to posterior cingulate cortex and episodic memory performance. Our results showed that this relationship is stable across samples of a different age and reliable over two points in time. These findings provide the first evidence that the relationship between large-scale intrinsic network connectivity and episodic memory performance is a stable characteristic that varies between individuals.
Keywords: resting-state fMRI, hippocampus, individual differences, reliability, memory
Introduction
The brain is intrinsically organized into large-scale distributed functional networks, measureable in living individuals using resting-state functional connectivity MRI (fcMRI) (Biswal et al., 1995; Greicius et al., 2003; Smith et al., 2009; Yeo et al., 2011), among other imaging methods. A growing body of literature focuses on how individual differences in the strength of intrinsic connectivity of these networks relate to individual differences in a variety of experiences and behaviors such as in motor function (Fox et al., 2007), learning (Supekar et al., 2013; Ventura-Campos et al., 2013), working memory (Hampson et al., 2006), episodic memory (Wang et al., 2010a; Wang et al., 2010b), intelligence (van den Heuvel et al., 2009), executive function (Gordon et al., 2013; Seeley et al., 2007; Touroutoglou et al., 2012), social behaviors (Bickart et al., 2012; Di Martino et al., 2009) and affective experiences (Seeley et al., 2007; Touroutoglou et al., 2012; van Marle et al., 2010) as well as personality traits (Fulwiler et al., 2012; LaBar et al., 2001). Intrinsic connectivity differences have also been proposed as biomarkers for various neuropsychiatric disorders (Fox and Greicius, 2010; Greicius, 2008; Menon, 2011; Zhang and Raichle, 2010).
The growing evidence that fcMRI measures of large-scale neural network connectivity predict a range of normal human behavior raises a critically important question: To what extent are these brain-behavior relationships stable over time (i.e., can they be considered trait-like) vs. how much do they fluctuate from one scanning/testing session to the next (i.e., should they be considered state-like)? This raises further questions about the reliability of intrinsic connectivity network measures, studies of which have typically shown moderately high reliability (Braun et al., 2012; Guo et al., 2012; Shehzad et al., 2009; Song et al., 2012; Van Dijk et al., 2010; Zuo et al., 2010) on the network level. Other data show, however, that intrinsic connectivity networks can be dynamic (Hutchison et al., 2013), with recent task-evoked cognitive (Albert et al., 2009; Waites et al., 2005) and affective states (Harrison et al., 2008) or even task-free states of previous runs within the same resting-state scan session (Van Dijk et al., 2010) influencing connectivity strength, particularly in individual pairwise correlations between brain regions. Thus, fMRI-derived intrinsic connectivity measures are thought to reflect a combination of stable and transient influences (Buckner, 2010; Buckner et al., 2013; Deco et al., 2010).
Despite the optimistic results of initial resting-state reliability studies, no study to our knowledge has sought to investigate whether the relation between intrinsic connectivity and behavioral measures are stable over time. In this study, we used a strong a priori hypothesis-driven approach and investigated the stability of the relationship between the strength of hippocampal connectivity to posterior/precuneus cortex (PCC) and episodic memory performance, a finding previously reported by our laboratory in Wang et al. (2010a). We analyzed behavioral and scan data collected at two timepoints approximately 1 week apart. We hypothesized that the same relationship observed in Wang et al. (2010a)’s study would generalize to a sample of new participants of a different age, and further that the relationship within our sample would be reliable over two points in time. Support for this hypothesis would provide evidence that the relationship between hippocampal connectivity and episodic memory performance is a stable trait that varies between individuals.
Materials and Methods
Participants
Forty adults ranging in age from 18 to 54 (mean age = 27.97, SD= 9.60; 23 females) participated in this study, which involved the collection of resting-state blood oxygenation-level dependent (BOLD) data as well as task-evoked BOLD data. All participants were right-handed, native English speakers and had normal or corrected-to-normal vision. No participant reported a history of neurological or psychiatric disorders.
Behavioral Data Acquisition: Encoding and Retrieval Tasks
Participants completed in the scanner an associative memory encoding task followed by a retrieval memory task for two timepoints, approximately 1 week apart. During the first scan session, following resting state scans, participants were engaged in an affective task unrelated to the analyses of this study and then completed the associative memory encoding task. The affective task was part of a larger study examining the aging effects on affect and memory and included 9 high arousal, negative valence IAPS slides (arousal – Mean = 5.73, SD = 0.81, valence – Mean = 2.718, SD = 0.7) presented for six seconds each in order to induce negative affect. After the first round of induction, participants then began an associative memory encoding task. On each of 80 trials, participants were presented with a neutral image or scene as well as a common word selected from the Medical Research Council Psycholinguistic Database (Coltheart, 1981). Each image/word pair was presented for six seconds, and a total of 20 pairs were encoded in a single run. To ensure depth of encoding, participants were asked to judge whether the word ‘matched’ the picture. As picture/word pairs were created randomly, and pairs with an obvious semantic connection were excluded, this judgment was subjective. Next, participants completed a second affect induction and a second run of associative encoding, followed by two more induction and encoding runs, for a total of four runs each.
A retention delay of approximately 10 minutes followed, during which other scans unrelated to these analyses were performed. After this delay, recognition testing began. Participants were presented with all 80 pairs learned during encoding, as well as 40 pairs made up of new words and pictures, and 40 rearranged pairs made of words and pictures seen previously, but not previously associated. Each picture was presented for six seconds, during which time the participant responded by button press whether the pair had appeared during encoding, or whether it was a new or rearranged pair (yes/no).
Approximately one week later, the second scan session was performed. Participants returned and underwent an identical procedure with two differences: 1) neutral, rather than negative, affect was induced during induction runs through the presentation of low arousal, neutral valence IAPS images (arousal – Mean = 3.3, SD = 0.9, valence – Mean = 5.39, SD = .76), and 2) a new set of words and images were used during encoding and retrieval.
Each recognition trial was coded as a hit, miss, false alarm or correct rejection, and recognition accuracy was computed in terms of d′, a measure which controls for individual response bias (d′ = z(FA)-z(hits)). Thus, for each subject, the memory measure were d′ Timepoint 1 (d′ under negative induction condition) and d′ Timepoint 2 (d′ under neutral induction condition). d′ was then used in the subsequent analyses described below.
MRI data acquisition and preprocessing
Imaging data were collected on a 3T Magnetom Tim Trio system (Siemens Medical Systems, Iselin, NJ) at Massachusetts General Hospital, equipped for echo planar imaging (EPI) with a 12-channel phased-array head coil. Head motion was minimized using head restraints, including a pillow and foam padding. Noise was attenuated with ear plugs. Structural MRI data were acquired using a T1-weighted 3D MPRAGE sequence [TR/TE/flip angle = 2530ms/3.48ms/7°, resolution = 1.0 mm isotropic).
Whole-brain resting state fMRI data were acquired with echo-planar sequence [TR=5000ms; TE=30ms; FA=90°). These parameters allowed us to obtain 55 slices and have a spatial resolution of 2.0 mm isotropic voxels. The resting state scan was 6.40 minutes long and the data involved one run of 76.8 time points. During all resting-state fMRI runs, participants were directed to keep their eyes open without fixating and to remain as still as possible. Resting state fMRI runs preceded the task-based fMRI runs.
Preprocessing of the resting state fMRI data involved a series of previously established resting state functional connectivity MRI (rs-fcMRI) procedures (Biswal et al., 1995; Van Dijk et al., 2010; Vincent et al., 2007). After removing the first four functional volumes, the following steps were completed: correction for slice-dependent time shifts (SPM2, Wellcome Department of Cognitive Neurology, London, United Kingdom), correction for head motion with rigid-body transformation in three translation and three rotations (FMRIB, Oxford, UK), spatial normalization to Montreal Neurological Institute (MNI) atlas space, resampling to 2mm isotropic voxels, spatial smoothing using a 6mm full width at half-maximum (FWHM) Gaussian kernel, and temporal band-pass filtering to remove frequencies > 0.08Hz. We then removed sources of spurious variance and their temporal derivatives from the data through linear regression (six parameters derived from the rigid-body head motion correction, the signal averaged over a region within the deep white matter, and the signal averaged over the ventricles) and the residual BOLD time course was retained for functional connectivity analysis. In addition to these factors, we regressed out the signal averaged over the whole brain, as it has been suggested (Fox et al., 2009; Weissenbacher et al., 2009) that the use of global signal regression in brain systems expected to show positive correlations [such as the hippocampal connectivity to PCC (Van Dijk et al., 2010; Vincent et al., 2008; Wang et al., 2010a)] reduces certain elements of noise related to false positives.
Resting state fMRI analysis
To examine the intrinsic functional connectivity strength between the hippocampus and PCC, we used seed-based rs-fcMRI analysis. A hypothesis-driven approach was taken for this analysis, creating spherical regions of interest (ROIs) (4-mm radius) around hippocapampal formation (right hippocampus coordinates: + 21, −6, −18, MNI) and PCC bilaterally (right PCC coordinates: + 3, −51, 39 and left PCC coordinates: −9, −54, 39). The coordinates of these seeds were determined from the results of a previous study by our laboratory (Wang et al., 2010) that examined the relationship between hippocampal intrinsic connectivity and episodic memory performance (see Figure 1). In that study, peak activations in a contrast comparing encoding activity for subsequently remembered items vs. fixation were found in the right and left hippocampi. Additionally, a contrast comparing fixation vs. subsequently remembered items showed peak activations in PCC bilaterally. The strength of connectivity between each pair of these task-defined ROIs was then tested for correlation with each participants’ overall memory performance. The authors showed significant correlations between episodic memory performance and connectivity in the right hippocampus and bilateral PCC ROIs (see Figure 1). These ROIs were used as seeds in the functional connectivity analysis of the current study.
Figure 1.

Hippo-PCC connectivity significantly predicted episodic memory performance in an independent sample previously published by our laboratory (Wang et al. 2010a). In that study, a contrast comparing high confidence hits vs. fixation identified clusters containing hippocampi activation peaks (left, top) and a contrast comparing fixation vs. high confidence hits identified clusters containing PPC deactivations (left, bottom). Wang et al. (2010a) then used these task-based ROIs as seed ROIs in a subsequent resting state functional connectivity analysis. The strength of intrinsic connectivity between the right hippocampal ROI and right PPC ROI (scatter plot on the right) predicted overall memory performance in their sample of older adults. Figure adapted from Wang et al. 2010a.
To examine the intrinsic functional connectivity of the right hippocampus and bilateral PCC seeds we computed Pearson’s product moment correlations, r, between the mean signal time course of the hippocampus and bilateral PCC. Fisher’s r-to-z correlation coefficients were then calculated between each pair of hippocampus-PCC ROIs. The averaged pairwise connectivity measure of z(r) values of the right hippocampus to left PCC and the right hippocampus to right PCC was used for the stability analyses of the brain-behavioral relationships.
Stability of memory performance and hippocampal connectivity
To assess the stability of memory performance, we computed intraclass correlation coefficients (ICC) (two way random effects with absolute agreement) between memory performance at Timepoint 1 and memory performance at Timepoint 2. The Pearson’s product moment correlations, r, of memory performance at Timepoint 1 (d′) with memory performance at Timepoint 2 (d′) were also calculated and the relationship between memory performance at these timepoints was displayed in scatterplots.
Correspondingly, to assess the reliability of the strength of connectivity of the hippocampal connectivity, we computed intraclass correlation coefficients (ICC) (two way random effects with absolute agreement) between the z(r) values of the right hippocampus to PCC at Timepoint 1 and the z(r) values of the right hippocampus to PCC at Timepoint 2. The Pearson’s product moment correlations, r, of hippocampal connectivity to PCC at Timepoint 1 with hippocampal connectivity to PCC at Timepoint 2 were also computed and the relationship between the hippocampal connectivity at these timepoints was displayed in scatterplots. All analyses were conducted using PASW Statistics 21, Release Version 21.0.0 (SPSS, Inc., 2009, Chicago, IL, www.spss.com). Results were considered statistically significant at p < 0.05.
Stability of the brain-behavior relationship
We examined the relationship between hippocampal connectivity to PCC and memory performance, using a series of linear regression analyses. We conducted a linear regression analysis using hippocampal connectivity at Timepoint 1 (averaged pairwise connectivity measure of z(r) values of the right hippocampus with left and right PCC) as independent variable and memory performance at Timepoint 1 (d′) as the dependent variable. The same analysis was repeated for Timepoint 2.
To assess whether hippocampal connectivity at either timepoint predicts memory performance during the other timepoint, we conducted a linear regression analysis using hippocampal connectivity at one timepoint (e.g., Timepoint1) as independent variable and memory performance at the other timepoint (e.g., Timepoint 2) as the dependent variable. Brain-behavior analyses were conducted using PASW Statistics 21, Release Version 21.0.0 (SPSS, Inc., 2009, Chicago, IL, www.spss.com). Results were considered statistically significant at p < 0.05.
Results
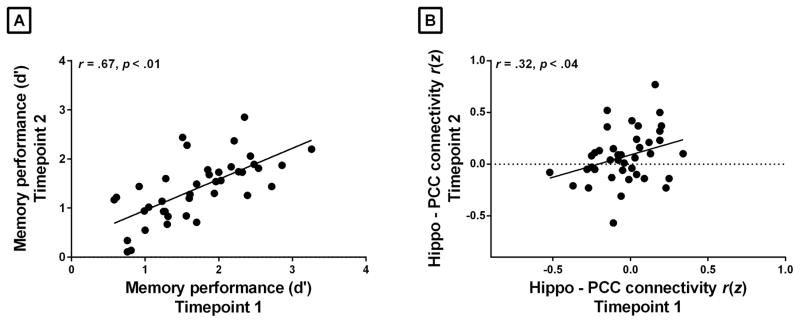
Consistent with prior evidence (Braun et al., 2012; Zuo et al., 2010), our reliability analyses revealed that the hippocampal intrinsic connectivity between the two timepoints (approximately one week apart) was moderately reliable with ICC = 0.47. A scatterplot displaying the relationship of the hippocampal intrinsic connectivity across the two sessions (r = 0.32, p < 0.04) is shown in Figure 2 (for comparison of reliability of the connectivity across the entire default mode network, to which the hippocampus-PCC connection belongs, and within the PCC and medial prefrontal nodes of the network, see Supplementary Material). Contrary to the moderate stability of the hippocampal intrinsic connectivity, episodic memory performance was highly stable across the two timepoints with ICC = 0.80. Figure 2 shows the relationship between memory performance at two timepoints (r = 0.67, p < 0.01).
Figure 2.
Stability of memory performance and hippocampal connectivity to PCC. (A) Recognition discriminability memory performance (d′) was highly stable across Timepoint 1 and 2 (r = 0.67, p <0.01). (B) The relationship between the hippocampal connectivity to PCC between Timepoint 1 and Timepoint 2 (z-transformed correlation coefficients computed by correlating the spontaneous activity between the right hippocampus and bilateral PPC) was relatively weak across the two sessions (r = 0.32, p = 0.04).
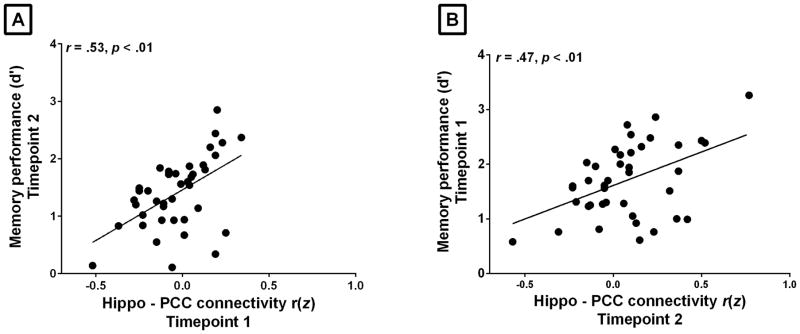
Using linear regression analysis, we found that hippocampal connectivity to PCC was reliably associated with memory performance (d′) at both Timepoint 1 and Timepoint 2 (r = 0.34, p < 0.03 and r = 0.38, p < 0.02, respectively) (see Figure 3 and Table 1) (for comparison of the relationship between hippocampal connectivity to PCC and item and associative memory d′ sub-scores, see Supplementary Material Table 1S). The regression analysis also revealed that hippocampal connectivity to PCC at one point in time is associated with memory performance at another point in time. The strength of connectivity between hippocampus and PCC at Timepoint 1 predicted future memory performance at Timepoint 2 (r = 0.53, p < 0.01). The strength of connectivity between hippocampus and PCC at Timepoint 2 was associated with past performance in memory at Timepoint 1 (r = 0.47, p < 0.01) (See Figure 4 and Table 1) (for comparison of reliability of the relationship between memory and connectivity across the entire default mode network, and within the PCC and medial prefrontal nodes of the network, see Supplementary Material Table 2S and 3S).
Figure 3.
Intrinsic connectivity between the hippocampus and PCC predicted memory performance within sessions. The z-transformed correlation coefficients (x axis) computed by correlating the spontaneous activity between the right hippocampus and bilateral PPC ROIs reliably predicted individual recognition discriminability memory performance (d′, x axis) within a session. This relationship was consistent at two separate time points, demonstrating the reliability of this brain-behavior relationship.
Table 1.
Increased hippocampal connectivity to PCC predicts better memory performance within and across sessions (N = 40)
| Memory performance (d′) | Memory performance (d′) | |||
|---|---|---|---|---|
| Timepoint 1 | Timepoint 2 | |||
| B | Total R2 | B | Total R2 | |
| Strength of the hippocampal intrinsic connectivity to PCC at Timepoint 1 | 0.34† | 0.12† | 0.53* | 0.28* |
| CI: 0.12 – 2.3 | CI: 0.83 – 2.70 | |||
|
| ||||
| Strength of the hippocampal intrinsic connectivity to PCC at Timepoint 2 | 0.47* | 0.22* | 0.38† | 0.14† |
| CI: 0.47 – 1.98 | CI: 0.18 – 1.67 | |||
Note. This table displays standardized regression coefficients (B) as well as the total variance (Total R2) in memory performance at two points in time predicted by the independent variables, each entered into a single linear regression model. Confidence Interval (CI),
p < 0.01;
p < 0.05
Figure 4.
Intrinsic connectivity between the hippocampus and PCC predicts memory performance across sessions separated by approximately one week. The z-transformed correlation coefficients (x axis) computed by correlating the spontaneous activity between the right hippocampus and bilateral PPC ROIs, are plotted against individual memory performance (d′, x axis) at two points in time. The strength of connectivity (A) between hippocampus and PCC at one point (Timepoint 1) predicts memory performance in the future (Timepoint 2) and (B) between hippocampus and PCC at one point in time (Timepoint 2) is associated with memory performance in the past (Timepoint 1).
Discussion
The present findings demonstrate a relationship between intrinsic functional connectivity and behavior that is stable over time and across samples of a different age. While previous studies have related intrinsic functional connectivity measures to individual differences in performance on various cognitive tasks, it has not been clear whether brain-behavior relationships identified with intrinsic connectivity fMRI reflect stable traits, transient states, or a combination thereof. The present data provide clear evidence that such a brain-behavior relationship persists across multiple sessions. That is, intrinsic connectivity between the hippocampus and PCC predicted performance on a memory task one week later. Our results thus suggest that the connectivity between the hippocampus and PCC –key nodes of the large-scale default mode network, known to be involved in episodic memory (Buckner et al., 2008; Dickerson and Eichenbaum, 2010; Raichle et al., 2001) –exhibits characteristics consistent with a trait-level neurobiological measure which predicts associative memory ability, rather than a transient state.
Intrinsic functional connectivity as measured by rs-fcMRI is a heterogeneous measure, which includes persistent sources such as anatomic connectivity and long-term changes in synaptic efficiency, temporary sources such as synaptic changes due to recent experience and state of arousal (Vaisvaser et al., 2013; Veer et al., 2011), and confounding sources such as motion, cardiac and respiratory activity (Buckner, 2010; Buckner et al., 2013; Hutchison et al., 2013). The reliability of intrinsic connectivity observed here is comparable to the moderate levels of reliability reported in other studies. Intra-class correlation between the two timepoints was 0.40, roughly in the center of the range of reliability of networks reported in studies that have examined this question on the whole-brain level (Braun et al., 2012; Zuo et al., 2010). Previous research on the default mode network, to which the hippocampus-PCC system examined in this study belongs, has reported somewhat higher levels of reliability than we observed here (Guo et al., 2012). Our analysis also showed higher reliability of the average connectivity across the entire network, compared to the connectivity between hippocampus and PCC. It thus appears that the cortical-cortical connections between other nodes of the default mode network are comparatively more stable, than the cortical-hippocampal connections. Consistent with this view, our analysis showed that the reliability of the connection between PCC and medial prefrontal cortex – two key cortical nodes of the default mode network –was greater than the reliability of the hippocampus to PCC connection.
Yet despite the many potential sources of noise present in intrinsic functional connectivity measures, a stable within-subject signal is evident through its relationship with behavior one week later. Thus, although functional connectivity MRI measures obtained using the present analytic techniques appear to be only modestly reliable, the connectivity-behavior relationship described here is stable enough to be detected in the context of the variety of sources of session-level variance in the measures.
Our findings provide further support for the use of resting state connectivity in the study of individual differences in brain-behavior relationships. Consistent with our findings, two recent studies have shown that variability in intrinsic functional connectivity predicts differences in personality traits such as agreeableness (LaBar et al., 2001; Vaisvaser et al., 2013), neuroticism, extraversion, and openness (LaBar et al., 2001). Similarly, intrinsic connectivity has been associated with variability in a wide number of behavioral domains where performance is believed to be stable, including visual and motor performance, language, learning, episodic memory, executive function, and emotional functioning [see (Veer et al., 2011), for review]. Evidence that resting state connectivity can predict stable differences in personality and behavior points to its potential value as a biomarker for vulnerability to various neuropsychiatric disorders (Buckner, 2013; Fox and Greicius, 2010; Greicius, 2008; Menon, 2011; Zhang and Raichle, 2010), particularly because of the low task demands on participants. However, the utility of this method depends critically on whether intrinsic connectivity measures are stable within individuals, and are not overly influenced by state-level factors. Intrinsic connectivity has also been shown to reflect transient affective states, such as stressor-evoked anticipatory anxiety (Seeley et al., 2007) and cardiovascular reactivity (Vaisvaser et al., 2013). However, the present findings demonstrate that individual differences in resting connectivity can meaningfully predict trait-level differences in behavior despite multiple potential sources of transient variability.
One limitation of this study is that the present analytic approach employed only two timepoints, approximately 1 week apart. As such, our data cannot speak to questions of reliability of resting state fMRI data over longer time frames. Additionally, as this study focused on the relationship between episodic memory performance and hippocampal connectivity to PCC, further research will be needed to assess whether these findings generalize to relationships between more diverse behaviors and nodes of other networks. Finally, further research is needed on the sources of variance in resting state fMRI data if we ultimately want to use measures derived from these data as markers for vulnerability to or the presence of neuropsychiatric illness.
Supplementary Material
Acknowledgments
We thank Jiahe Zhang, Michael Stepanovic, Morenikeji Adebayo, and Christina Caso, for their assistance in data acquisition and preprocessing.
Footnotes
Author Contributions
AT., J.A., L.F.B., and B.C.D. designed the study, analyzed the data and wrote the manuscript. L.F.B. and B.C.D. contributed to grant funding. This work was supported by the National Institutes of Health Director’s Pioneer Award (DP1OD003312) to Lisa Feldman Barrett, and a National Institute on Aging grant (R01 AG030311-06A1) to Lisa Feldman Barrett and Brad Dickerson. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or the National Institute on Aging.
References
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19(12):1023–7. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 2012;32(42):14729–41. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Braun U, Plichta MM, Esslinger C, Sauer C, Haddad L, Grimm O, Mier D, Mohnke S, Heinz A, Erk S, et al. Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage. 2012;59(2):1404–12. doi: 10.1016/j.neuroimage.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci U S A. 2010;107(24):10769–70. doi: 10.1073/pnas.1005987107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci. 2013;15(3):351–8. doi: 10.31887/DCNS.2013.15.3/rbuckner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–7. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature Reviews Neuroscience. 2010;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166(8):891–9. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35(1):86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56(1):171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The Global Signal and Observed Anticorrelated Resting State Brain Networks. J Neurophysiol. 2009 doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, King JA, Zhang N. Amygdala-orbitofrontal resting-state functional connectivity is associated with trait anger. Neuroreport. 2012;23(10):606–10. doi: 10.1097/WNR.0b013e3283551cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Devaney JM, Bean S, Vaidya CJ. Resting-State Striato-Frontal Functional Connectivity is Sensitive to DAT1 Genotype and Predicts Executive Function. Cereb Cortex. 2013 doi: 10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH, Seeley WW. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61(4):1471–83. doi: 10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(51):13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3(3):e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–78. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2349. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19(10):2209–29. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Desphande AS, Meier TB, Tudorascu DL, Vergun S, Nair VA, Biswal BB, Meyerand ME, Birn RM, Bellec P, et al. Age-related differences in test-retest reliability in resting-state brain functional connectivity. PLoS One. 2012;7(12):e49847. doi: 10.1371/journal.pone.0049847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L, Menon V. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci U S A. 2013;110(20):8230–5. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF. Dissociable large-scale networks anchored in the anterior insula subserve affective experience and attention/executive function. NeuroImage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, Fruchter E, Wald I, Pine DS, Tarrasch R, et al. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29(23):7619–24. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage. 2010;53(1):348–54. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57(4):1534–41. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Ventura-Campos N, Sanjuan A, Gonzalez J, Palomar-Garcia MA, Rodriguez-Pujadas A, Sebastian-Galles N, Deco G, Avila C. Spontaneous brain activity predicts learning ability of foreign sounds. J Neurosci. 2013;33(22):9295–305. doi: 10.1523/JNEUROSCI.4655-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of neurophysiology. 2008;100(6):3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010a;51(2):910–7. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010b;20(3):345–51. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–16. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–77. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.