Abstract
The ability of tumor cells to adapt to therapeutic regimens by activating alternative survival and growth pathways remains a major challenge in cancer therapy. Therefore, the most effective treatments will involve interactive strategies that target multiple non-overlapping pathways while eliciting synergistic outcomes and minimizing systemic toxicities. Nanoliposomal irinotecan is FDA-approved for gemcitabine-refractory metastatic pancreatic cancer (PanCa). However, the full potential of irinotecan treatment is hindered by several cancer cell survival mechanisms, including ATP-binding cassette G2 (ABCG2) transporter-mediated irinotecan efflux from cells. Here we demonstrate that benzoporphyrin derivative (BPD)-based photodynamic therapy (PDT), a photochemical cytotoxic modality that activates the apoptotic pathway, reduced ABCG2 expression to increase intracellular irinotecan levels in PanCa. Moreover, we show that PDT inhibited survivin expression. While PDT potentiated irinotecan treatment, we also demonstrate that irinotecan reduced the tumoral expression of monocarboxylate transporter 4 (MCT-4), which was upregulated by PDT. Notably, using orthotopic xenograft models, we demonstrate that combination of single low-dose PDT and a subclinical dose of nanoliposomal irinotecan synergistically inhibited tumor growth by 70% for 3-weeks compared to 25% reduction after either monotherapies. Our findings offer new opportunities for the clinical translation of PDT and irinotecan combination therapy for effective PanCa treatment.
Keywords: Photodynamic therapy, irinotecan, nanoliposomes, pancreatic cancer, combination treatment
Introduction
Cancer cells can ingeniously adapt themselves to therapeutic insults by adjusting their growth and survival signaling circuitry through crosstalk loops. (1) Thus, there is an increasing focus on combination therapies that offer enhanced effects by imparting damage to different cellular compartments and molecular pathways. (2) Besides their therapeutic benefits, these combinations are more attractive and viable for translation if the agents are FDA-approved and have non-overlapping side effects. (2)
Irinotecan, a camptothecin derivative, is approved for treatment of pancreatic and other cancers. This S phase-specific chemo-agent inhibits topoisomerase I action by binding to topoisomerase-DNA complexes, preventing DNA religation, causing DNA-strand-breaks and leading to cell death. (3) In a recent Phase III trial for patients with gemcitabine-refractory metastatic pancreatic cancer (PanCa), a nanoliposomal irinotecan formulation (MM-398) combined with fluorouracil and leucovorin extended the median overall survival (OS, primary endpoint) to 6.1 months, compared to 4.2 months for the fluorouracil and leucovorin control. (4,5) This marginal improvement in OS was viewed as a significant breakthrough, highlighting the dismal nature of PanCa, and reaffirming the need for alternative, intelligently-designed interventions.
Although efforts have been made to develop chemotherapeutic and biological cocktails to address challenges, such as multidrug resistance and upregulation of survival pathways, these attempts have largely been hindered by high toxicities associated with these agents adding to the already toxic regimens. It is, therefore, important to devise combinations that complement each other mechanistically to abrogate resistance pathways, while minimizing systemic toxicities.
Photodynamic therapy (PDT) is a well-established photochemistry-based approach where a non-cytotoxic agent (i.e. photosensitizer) is excited by appropriate-wavelength light to generate cytotoxic molecular species, killing or modulating cells. (6) PDT uniquely stimulates cell death by directly activating apoptosis, and therefore bypasses many cell-death signaling pathways required for chemoradiation to be effective. Kessel et al. first report that PDT-induced mitochondrial photodamage results in loss of mitochondrial membrane potential, destruction of mitochondria-associated Bcl-2, release of cytochrome c and subsequent apoptosis initiation, (7) a finding confirmed by Oleinick and colleagues. (8) This direct induction of apoptosis makes PDT effective even against chemo/radio-resistant cancers with defective signaling pathways. (9) Several studies have also shown that the unique mechanisms of cell death activated by PDT can re-sensitize drug resistant cells (10) and synergize with both chemo and biological therapies, e.g., receptor tyrosine kinase inhibitors. (11,12) Busch et al. showed that priming PDT with EGFR inhibitor erlotinib improved treatment efficacy in non-small cell lung carcinoma xenografts, even in erlotinib-resistant tumors. (11) Our group has demonstrated that PDT cooperates mechanistically with anti-EGFR antibody, Erbitux, to synergistically increase survival in disseminated ovarian cancer models. (12) In the context of chemotherapeutic combinations, Duska et al. showed that PDT via photoimmunoconjugates enhanced the cytotoxicity of cisplatin in ovarian cancer, and such enhancement is also synergistic on platinum-resistant cells. (10) PDT, which has received regulatory approval worldwide, is already a successful adjuvant therapy in clinical trials for several malignancies where most therapies have failed. For pancreatic cancer (PanCa), Bown et al. showed that chlorin-based PDT improved the median survival from 6–10 to 12.5 months in locally advanced PanCa patients. (13) Our Phase I/II trial reaffirms that benzoporphyrin derivative (BPD)-based PDT consistently induced tumor necrosis at 40J/cm in patients with localized PanCa. (14)
Here, we demonstrate multiple cooperative mechanistic interactions between PDT and irinotecan, showing for the first time that PDT reduces ATP-binding cassette G2 (ABCG2) efflux transporter expression to increase intracellular irinotecan concentrations and that PDT inhibits survivin expression to enhance apoptosis. We also show that irinotecan reduces the tumoral expression of the monocarboxylate transporter 4 (MCT-4), a biomarker that was upregulated by PDT. The combination of PDT and irinotecan is also attractive for cancer treatment due to their non-overlapping side effects. The systemic toxicities associated with irinotecan include grade 3–4 diarrhea and neutropenia, (4) and patients often require dose reduction or preemptive management. In contrast, PDT is well-tolerated in PanCa treatment and the only major adverse event of ‘mild’ abdominal pain can be alleviated using analgesics. (14) Therefore, we hypothesize that a low-dose PDT and irinotecan combination (12–20 fold lower than equivalent clinical effective doses; Supplementary Table S1) would be more tolerable and synergistic due to the unique counterbalancing mechanisms.
Advances in nanoliposomes have provided the means to preferentially deliver chemo-agents or photosensitizers to tumors, reducing systemic toxicities and improving outcomes. (15) Clinically, nanoliposomes improved the pharmacokinetics and biodistribution of irinotecan, minimizing side effects. (4) Non-pegylated nanoliposomal BPD (Visudyne®) is FDA-approved for treatment of age-related macular degeneration, and used in PanCa clinical studies. (14) Encouraged by these clinical advances, and motivated by the need for innovative, easily translatable treatments, we hypothesized that the distinct mechanisms of PDT and irinotecan, combined with mutually reinforcing molecular responses, would provide synergistic outcomes. Using nanoliposomal formulations of BPD and irinotecan, we investigated the anti-tumor efficacy of low-dose combination therapy in orthotopic MIA PaCa-2 and AsPC-1 tumor models.
Materials and Methods
Nanoliposome preparation and characterization
Nanoliposomal BPD (L-BPD) and nanoliposomal irinotecan (L-IRI) were prepared via freeze-thaw extrusion (Supplementary Methods).(15) Zetasizer NanoZS (Malvern) measured particle size and zeta potential. Concentrations of BPD and irinotecan were determined based on their absorbance spectra in dimethyl sulfoxide (DMSO) using established molar extinction coefficients (BPD: ε=34,895 M−1cm−1 at 687nm; Irinotecan: ε=21,835 M−1cm−1 at 384nm). Entrapment efficacy is defined as the molar ratio of drug entrapped into nanoliposomes to total drug added initially. Loading capacity is determined based on the ratio of final drug weight to overall weight of the nanoliposomes. BPD quenching, photobleaching and singlet oxygen (1O2) generation were studied in 96-well plates. L-BPD and singlet oxygen sensor green (SOSG) at 5μM were irradiated with 690nm light at different fluences (0–75J/cm2, 50mW/cm2, Intense-High Power Devices, Series-7401). A microplate reader (Molecular Devices) acquired fluorescence signals of BPD (Ex/Em:422/650–750nm) or SOSG (Ex/Em:504/525nm) before and after irradiation. Drug release was studied in 10% human serum at 37°C using dialysis.
In vitro molecular characterization post-PDT
MIA PaCa-2 and AsPC-1 cells were obtained from ATCC between 2012–2015, cultured per vendor’s instructions and tested for mycoplasma contamination. 150,000 cells grown overnight were incubated with L-BPD (250nM) for 1-hour. Before PDT, L-BPD-containing medium was replaced with fresh medium. Cells were irradiated with 690nm light at different fluences (0.5–5J/cm2, 50mW/cm2). At 1, 6 or 24-hours post-PDT, detached cells were removed via phosphate buffered saline (PBS) washes and remaining cells assessed for viability (MTT assay, Invitrogen) or biomarkers (ABCG2, survivin) by immunofluorescence and immunoblotting (Supplementary Methods).
Evaluation of intracellular irinotecan accumulation post-PDT
250,000 cells grown overnight were incubated in medium containing L-BPD (250nM) and L-IRI (0.24mg/mL) for 1-hour. Before PDT (690nm, 0.5J/cm2), the medium was replaced with either L-IRI-containing medium or ‘drug-free’ medium. At 1 or 6-hours post-PDT, cells were washed and lysed with Solvable™. A microplate reader (Molecular Devices) measured fluorescence signal from irinotecan (Ex/Em:355/460nm) and its metabolite SN-38 (Ex/Em:355/538nm). Readouts were normalized to protein concentration (Pierce BCA Protein Assay).
Orthotopic mouse model
Animal protocols were approved by MGH Institutional Animal Care and Use Committee (IACUC). Orthotopic MIA PaCa-2 or AsPC-1 tumors were established in male athymic (nu/nu) Swiss mice (4–6-weeks old). A 1cm incision on the left abdominal flank was made to exteriorize the pancreas of ketamine/xylazine-anesthetized mouse. Cancer cells (1×106) suspended in 50μL medium-Matrigel® mixture, were injected into the pancreas, and the incision was sutured aseptically.
Pharmacokinetics and biodistribution of L-BPD and L-IRI
Tumor-bearing mice received intravenous (IV) injection of L-IRI (20mg/kg) and L-BPD (0.25mg/kg). Blood, tumor and tissues were collected 10 minutes, 1, 4, 12, and 24-hours post-injections. BPD and irinotecan were extracted from tissue samples (Supplementary Methods), and then quantified via liquid chromatography-tandem mass spectrometry (LC-MS/MS, Agilent) for determination of %ID/g (percentage injected dose per gram tissue).
In vivo combination treatment
Treatments were initiated 9 days post-implantation when tumors reached ~25mm3 (Supplementary Fig. S1A). One-hour post-IV injection of low-dose L-BPD (0.25mg/kg) and L-IRI (20mg/kg), an incision was made on the left abdominal flank to exteriorize the tumor of anesthetized mice. A vertical light beam was focused on the tumor, and cloth was used to protect animal skin from light exposure. PDT was performed with a 690nm laser (75J/cm2, 100mW/cm2). Light irradiation coincides with the BPD Q-band absorbance (Fig. 1A, arrow) without overlapping with irinotecan absorbance. Post-treatment, incisions were sutured. Tumor growth in every animal was longitudinally monitored every 3–5 days using non-invasive ultrasound imaging (Supplementary Methods). At each time point, tumor volume was calculated using ellipsoid estimation (width×length×height×π/6), which was validated against the three-dimensional volume reconstruction algorithm of the Vevo2100 software (Supplementary Fig. S1B,C). Animals were euthanized on day 30. Collected tissues were processed for (i) histological (hematoxylin and eosin stain, H&E) analysis of necrosis and (ii) immunofluorescence analysis of microvessel density (MVD, CD31 vascular endothelial marker) and proliferation (Ki-67) (Supplementary Methods). In a small group of animals, tumors were excised at 24-hours post-treatment to analyze hypoxia (pimonidazole) and biomarker (MCT-4) (Supplementary Methods and Table S2). H&E and immunofluorescence slides were imaged using a whole slide scanning fluorescence imaging system (Hamamatsu) or a confocal microscope (Olympus) (Supplementary Methods). In a separate study, mice were treated with two cycles of combination therapy on days 9 and 27 post-implantation.
Fig. 1.
Physical characterization of L-BPD (red) and L-IRI (blue). (A) Absorption spectra of L-BPD and L-IRI in DMSO. Arrow indicates light excitation wavelength. (B, C) Long-term stability of L-BPD and L-IRI stored in dark conditions (4°C) determined by size and polydispersity. (D) L-BPD and L-IRI exhibit differential drug release profiles under biologically relevant conditions (37°C, 10% serum) (E) Plasma pharmacokinetics of L-BPD (0.25mg/kg) and L-IRI (20mg/kg) in athymic nu/nu mice. (F) Tissue biodistribution of L-BPD (0.25mg/kg) and L-IRI (20mg/kg) in tumor-bearing mice at 1-hour post-IV injection. Extracts from tissue homogenates were analyzed by LC-MS/MS. (N≥3)
Statistical analysis
To compare treatment responses, one-way ANOVA statistical tests were carried out to avoid type-I error (GraphPad Prism). Synergy between PDT and L-IRI is determined to be significant via random slopes model (Supplementary Methods and Table S3). Results are presented in mean ± standard error mean (SEM).
Results
Synthesis and characterization of nanoliposomes
For L-BPD and L-IRI syntheses, lipid compositions of dipalmitoylphosphatidylcholine, cholesterol, and distearoylphosphatidylethanolamine-methoxy polyethylene glycol were used based on clinically approved formulations. (15) Both L-BPD and L-IRI were grafted with 3-mol% of polyethylene glycol and are ~120–135nm with a narrow size distribution (polydispersity index, PdI<0.1) (Supplementary Table S4). The surface charge of nanoliposomes was engineered to be neutral-to-slightly cationic (+12mV)–by introducing dioleoyltrimethylammoniumpropane (8-mol%) to the lipid composition–to balance toxicity, circulation and tumor accumulation. (15) Concentrations of BPD or irinotecan in nanoliposomes were determined based on their respective absorbance spectra in DMSO (Fig. 1A). BPD was loaded in the lipid-bilayer via hydrophobic and ionic interactions (16) at entrapment efficiency of 70.7±8%. Hydrophilic irinotecan was encapsulated in the aqueous core through passive equilibration (17) at a lower entrapment efficiency of 56.6±4%. L-IRI loading capacity (10.2±1%) was higher than that of L-BPD (0.42±0.05%) due to the high aqueous-to-lipid compartment volume ratio of nanoliposomes. (18) This corresponded to approximately 14,000 irinotecan molecules per L-IRI, and 600 BPD molecules per L-BPD. Our long-term stability study suggested that four months dark storage at 4°C did not affect overall size or monodispersity of nanoliposomes (Fig. 1B,C).
Drug release profiles of L-BPD and L-IRI were evaluated under biologically relevant conditions (37°C, 10% serum). At 1-hour post-incubation, ~10% and ~30% of BPD and irinotecan were released from nanoliposomes, respectively (Fig. 1D). Relatively fast drug release from L-IRI (t1/2=2h) compared to L-BPD (t1/2=48h) suggests that irinotecan will be readily available to tumors when PDT is in action.
Our in vivo pharmacokinetic study (Fig. 1E) shows that plasma clearance of L-IRI (Clearance rate, pCL: 0.61L/kg×h; Area under curve, AUC: 32.52 μg×h/mL) is faster than that of L-BPD (pCL: 0.06L/kg×h; AUC: 4.267μg×h/mL). It is important to note that plasma clearance of L-IRI is still significantly slower than that of free irinotecan (pCL: 2.61L/kg×h; AUC: 7.65μg×h/mL).(19) This suggests that nanoliposomes improve the circulation profile of irinotecan, an important factor in the cytotoxicity of irinotecan. At 1-hour post-IV injection, accumulation of irinotecan was higher in tumor (15.3±2.2%ID/g) than in normal pancreas (3.4±0.3 %ID/g). Liver and spleen were organs of major L-BPD and L-IRI accumulation, respectively (Fig. 1F).
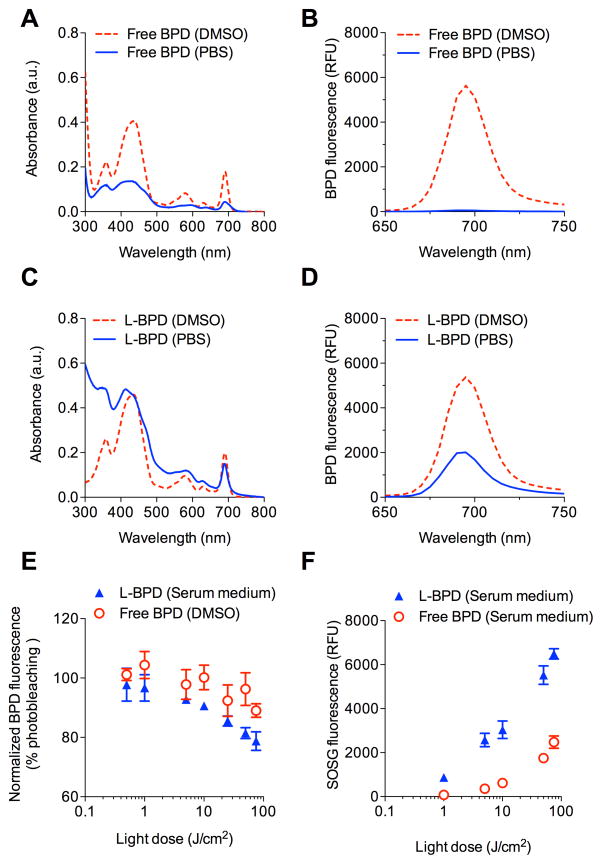
Photostability and photoactivity of L-BPD
Nanoliposomes have been shown to maintain the stability and photoactivity of photosensitizers in biologically relevant environments for effective PDT.(20) However, photosensitizers could also be prone to self-quenching when packed in lipid bilayers at high concentrations. (16) Here, we evaluated the photostability and quenching of free BPD and L-BPD by monitoring changes in BPD absorbance and fluorescence before and after dissolution in different solvents. In our BPD stability studies, the absorbance value at 690nm for free BPD in PBS was reduced by 81.3±3% compared to free BPD fully-dissolved in DMSO, indicating a rapid aggregation of free BPD molecules in PBS (Fig. 2A). Aggregation of free BPD in PBS led to a nearly complete (~95%) fluorescence quenching compared to free BPD in DMSO (Fig. 2B). In contrast, nanoliposomes facilitated monomerization of photosensitizers and restored BPD’s fluorescence emission by 40% in PBS (Fig. 2C). Up to 75% ‘recovery’ of BPD’s 690nm peak was restored by the nanoliposomes (Fig. 2D).
Fig. 2.
L-BPD photophysical characterization. (A) Reduced absorbance intensity of free BPD in PBS (blue solid line) compared to free BPD in DMSO (red dotted line), due to aggregation (self-quenching) of hydrophobic BPD in PBS. (B) Fluorescence of free BPD is quenched in PBS. (C) Formulating BPD in nanoliposomes as L-BPD restores absorbance of BPD in PBS (blue solid line). (D) Nanoliposomes de-quench and recover BPD fluorescence in PBS. Values are in relative fluorescence units (RFU). (E) BPD photobleaching post-PDT as a function of light dose (0.5–75J/cm2) determined by BPD fluorescence. (F) SOSG reports 1O2 production from photoactivated L-BPD and free BPD in medium. (N≥3)
We further tested if L-BPD can be photoactivated to generate cytotoxic 1O2 in serum-containing medium. Upon light-activation of L-BPD, we observed a light-dose-dependent decrease of BPD fluorescence due to photobleaching of BPD (Fig. 2E). Approximately 20% of BPD molecules in nanoliposomes were photobleached at 75J/cm2, and this was found similar to the photobleaching of free BPD molecules that are well-dispersed in DMSO. Subsequently, the formation of 1O2 upon light-activation of L-BPD was monitored using SOSG sensors. During light irradiation of L-BPD, we observed a dose-dependent increase of SOSG fluorescence intensity, which was markedly higher than that generated by free BPD under the same conditions (Fig. 2F). These data suggested that nanoliposomes retain a significant portion of BPD in their monomeric photoactive form. Therefore, L-BPD could effectively produce cytotoxic 1O2 upon photoactivation.
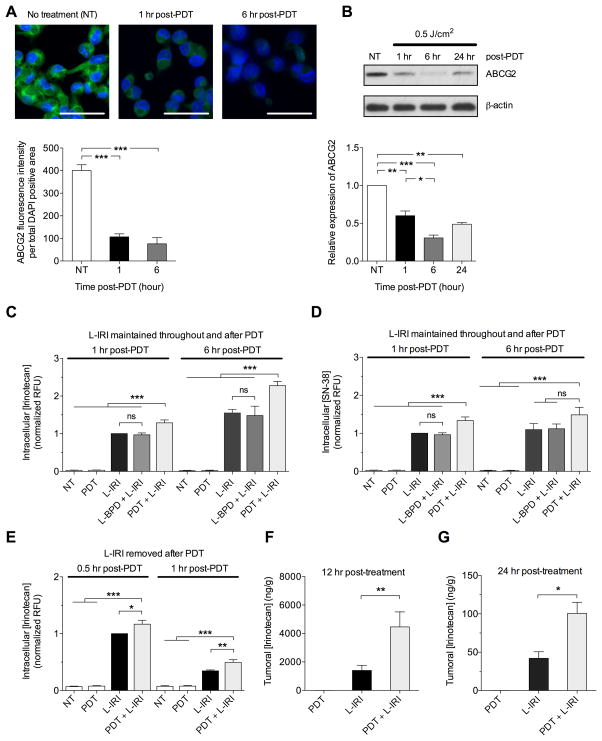
Photodestruction of ABCG2 increases intracellular irinotecan
A major obstacle encountered in cancer chemotherapy is multidrug resistance, a process mediated by ATP-binding cassette (ABC) transmembrane transporters that utilize energy from ATP-binding/hydrolysis to actively pump chemo-agents out of cells. (21) Overexpression of ABCG2 confers chemoresistance to PanCa cells and limits irinotecan efficacy. (22) Several ABC transporter inhibitors have been investigated, but their drug-drug interactions with chemo-agents and toxicities limited their clinical usage. (23) It is known that BPD is a substrate for ABCG2,(24) and others have suggested the possibility of photochemical destruction of ‘ABCG2-rich’ extracellular vesicles. (25) Here, we demonstrated that non-cytotoxic low-dose PDT at 0.5J/cm2 (Supplementary Fig. S2A, B) significantly reduced the ABCG2 immunofluorescence signal from MIA PaCa-2 by ~70% at 1 and 6-hours post-PDT (Fig. 3A). Western blots confirmed decreased proteins levels of ABCG2 at these time points in MIA PaCa-2 cells following the same PDT dose (Fig. 3B). Similar changes were observed in AsPC-1 cells (Supplementary Fig. S2C–E).
Fig. 3.
Sub-cytotoxic PDT (L-BPD: 0.25μM; hv: 690nm, 0.5J/cm2) reduces ABCG2 expression and increases intracellular irinotecan in MIA PaCa-2. (A) Immunofluorescence imaging of MIA PaCa-2 cells post-PDT. ABCG2 signal (green) is decreased at 1 and 6-hours post-PDT. Nuclei stained blue with DAPI. Scale bar=50μm. ABCG2 fluorescence intensity quantified per DAPI area. (B) Immunoblotting of ABCG2 in cell lysates collected 1, 6, and 24-hours post-PDT shows reduced ABCG2 expression. ABCG2 expressions relative to no-treatment were normalized to β-actin. (C,D) Intracellular levels of irinotecan and SN-38 increased post-PDT. MIA PaCa-2 cells were incubated with L-IRI (0.24mg/mL) and L-BPD (0.25μM) for 1-hour before replacing with L-IRI-containing media (0.24mg/mL) immediately before irradiation. Intracellular irinotecan levels from cell lysates at 1 and 6-hours post-PDT were determined by fluorescence signals of irinotecan (Ex/Em:355/460nm) and SN-38 (Ex/Em:355/538nm). (E) Following incubation with media containing L-IRI (0.24mg/mL) and L-BPD (0.25μM) for 1-hour, fresh media (no L-IRI) was added immediately before irradiation such that any increase in irinotecan levels is only due to retention. Readouts in RFU were normalized to protein concentration. (F,G) In vivo, irinotecan per gram of tumor (ng/g) increased at 12 and 24-hours post-PDT. (*P<0.05,**P<0.01,***P<0.001; One-way ANOVA Tukey’s range test) (N=3–6)
Given this PDT-based modulation of ABCG2, for which irinotecan is a known substrate, we subsequently tested if PDT-mediated ABCG2 reduction could affect intracellular irinotecan accumulation. We observed that MIA PaCa-2 cells treated with combination of PDT and L-IRI showed 28±7% and 49±2% increase in intracellular irinotecan concentrations at 1 and 6-hours post-PDT, compared to cells treated with L-IRI alone (Fig. 3C). MIA PaCa-2 treated with both PDT and L-IRI also showed statistically significant enhancement in intracellular SN-38 (an active metabolite of irinotecan) over cells treated with L-IRI at 1-hour post-PDT. Increase in intracellular SN-38 was not significant at 6-hours post-PDT (Fig. 3D). This saturation of SN-38 is presumably due to a limited intracellular concentration of the carboxylesterase enzyme, (26) which is responsible for conversion of irinotecan to SN-38. L-BPD alone (without light) did not increase intracellular concentrations of irinotecan and SN-38. In a separate study, L-IRI was removed from the media immediately before PDT, such that there is no contribution from uptake of L-IRI due to PDT. Higher intracellular levels of irinotecan were observed 1-hour post-PDT, suggesting that higher amounts of irinotecan were ‘retained’ in cells (Fig. 3E). Similar enhancements of intracellular irinotecan and SN-38 concentrations post-PDT were observed in AsPC-1 (Supplementary Fig. S2F–G). These promising results prompted us to determine whether the combination would improve irinotecan accumulation in vivo. Indeed, the combination significantly (P<0.05) increased intratumoral irinotecan levels by 3.2-fold and 2.4-fold at 12 and 24-hours post-PDT, respectively, as compared to L-IRI alone (Fig. 3F–G).
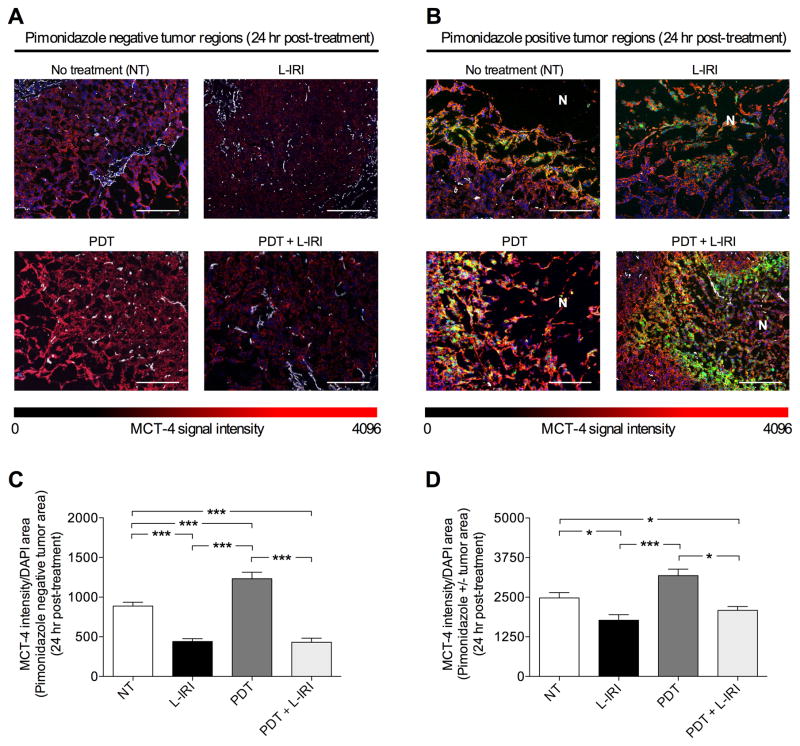
Irinotecan downregulates tumoral MCT-4 expression upregulated by PDT
Sections from MIA PaCa-2 tumors, excised 24-hours post-treatment, were stained with (i) CD31 (white) to identify vasculature; (ii) pimonidazole (green), a bioreductive chemical probe that forms protein adducts in viable hypoxic cells with partial oxygen pressure (pO2) less than 10-mmHg in tumors; (iii) MCT-4 (red), a lactate efflux pump that allows cancer cells to utilize aberrant glycolytic metabolism and can be influenced by abnormal tumor micro-environment such as that caused by hypoxia;(27) and, (iv) DAPI (blue) to mark nuclei. Figure 4 shows representative immunofluorescence images for these biomarkers in MIA PaCa-2 tumors at 24-hours post-treatment, as well as the corresponding quantification of MCT-4 expression, a biomarker that has been linked with poor prognosis in pancreatic cancer. (28) In determining the treatment-induced changes in MCT-4 expression, we separately analyzed pimonidazole positive and pimonidazole negative regions, since pimonidazole only stains distinct regions with pO2 below 10-mmHg. These areas are typically adjacent to necrotic zones that are chronically and severely depleted of oxygen and, thus, could potentially exhibit a differential response profile from other regions. The MCT-4 intensity was normalized to DAPI intensity in either pimonidazole negative regions only (Fig. 4A, C) or the whole cross tumor section irrespective of pimonidazole status (Fig. 4D).
Fig. 4.
L-IRI reduces MCT-4 expression, which is upregulated by PDT. (A, B) Immunofluorescence images of regions with and without pimonidazole (green), respectively, in MIA PaCa-2 tumors 24-hours post-treatment showing CD31 for endothelial cells (white) and MCT-4 (red). Scale bar=500μm. N=necrosis. (C) MCT-4 (red) intensity increases with oxygen-consuming PDT, but reduces with L-IRI in both L-IRI alone and combination groups. (D) Similar changes in MCT-4 expression observed in both pimonidazole positive and negative regions. (*P<0.05,***P<0.001; One-way ANOVA Tukey’s range test) (N≥15)
We demonstrated in vivo that L-IRI reduced the immunofluorescence signal of MCT-4 by ~52% at 24-hours post-injection compared to no-treatment controls in pimonidazole negative regions, while PDT increased MCT-4 staining compared to no-treatment (Fig. 4A, C). In the combination group, MCT-4 significantly decreased (P<0.001) in non-pimonidazole stained areas compared to no-treatment controls. Similarly, when both pimonidazole positive and negative regions were considered, a decrease (P<0.05) in MCT-4 signal was also observed in L-IRI and combination groups (Fig. 4B, D). However, this magnitude of difference is less pronounced, suggesting that MCT-4 expression may be harder to affect in regions with low pO2 (≤10-mmHg). Overall these results indicate that in combination, L-IRI acts to reduce the expression of MCT-4, which we observe to be upregulated by oxygen-consuming BPD-PDT.
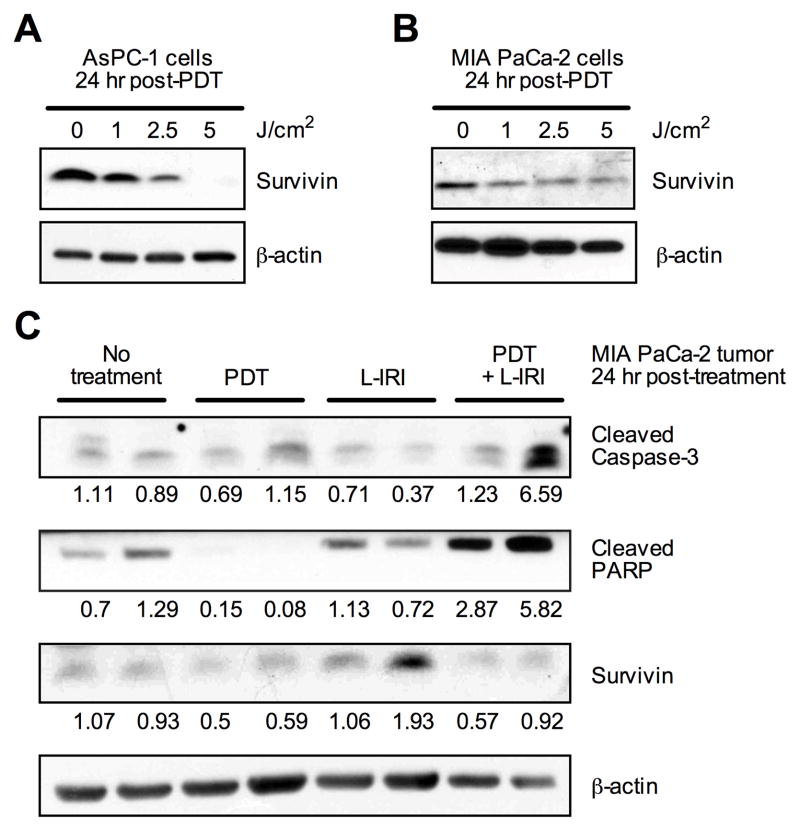
Survivin-downregulating PDT in combination with L-IRI enhances apoptosis in vivo
Survivin, an inhibitor of apoptosis, plays an essential role in regulating apoptosis by inhibiting caspases. (29) Decreased survivin expression has been shown to enhance the efficacy of camptothecin analogues for several cancers. (30) Gomer et al. and others demonstrated that PDT can regulate survivin expression in cancers, and such apoptotic modulation is dependent on photosensitizer and cell type. (31) At 24-hours post-PDT, we observe that PDT decreased survivin expression in MIA PaCa-2 and AsPC-1 cells, both in vivo and in vitro (Fig. 5A-C). Additionally, immunoblots of MIA PaCa-2 tumors show that survivin expression remained low in vivo at 24-hours after the combination treatment (Fig. 5C). In these same tumors, we observe a concomitant increase in tumoral expression of cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) overall. Together, the low expression of survivin and increased levels of cleaved PARP and cleaved caspase-3 suggest that the combination enhances apoptosis in vivo, relative to the monotherapies.
Fig. 5.
PDT decreases survivin in vitro and in vivo. (A, B) AsPC-1 and MIA PaCa-2 cells collected 24-hours post-PDT (L-BPD: 0.25 μM) shows dose-dependent decrease in survivin by immunoblotting. (C) Tissue lysates from two individual MIA PaCa-2 tumors collected 24-hours post-PDT suggests that survivin expression is not upregulated in the combination treatment. Corresponding increases in pro-apoptotic markers, cleaved caspase-3, and cleaved PARP are observed. Densitometry values are normalized to β-actin.
Combination treatment enhances tumor growth inhibition
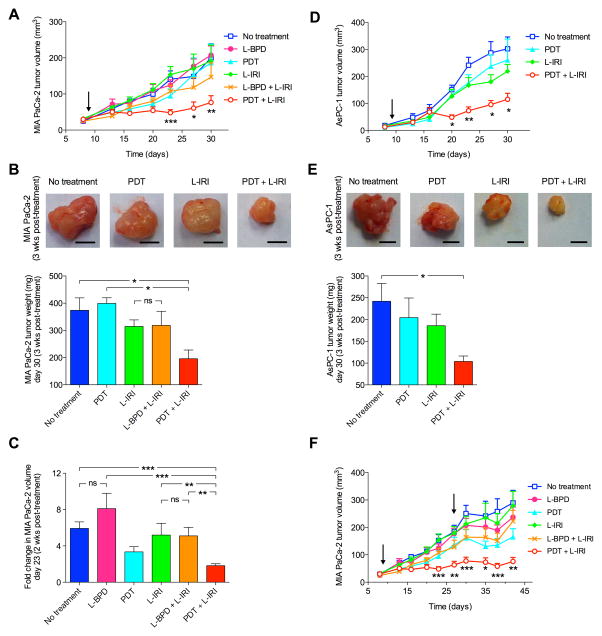
The above mechanistic insights provided us a compelling rationale to assess the combination PDT and L-IRI in orthotopic mouse models of MIA PaCa-2 and AsPC-1. Metastesis-derived AsPC-1 tumors exhibited faster growth rates than MIA PaCa-2 (Supplementary Fig. S3), in accordance with previous reports citing differences in tumorigenicity, immunocytochemistry and histology for these cell lines. (32) A single low-dose combination of PDT (L-BPD: 0.25mg/kg) and L-IRI (20mg/kg) demonstrated superior MIA PaCa-2 tumor volume growth inhibition by ~70% for at least 3-weeks, compared to <25% reduction after monotherapies (Fig. 6A). The slope of linearized growth curves for the combination group (PDT+L-IRI) was significantly lower (P=0.0191) than the ‘sum’ of the slopes for both monotherapies (PDT alone and L-IRI alone), suggesting the combination is synergistic (Supplementary Table S3).
Fig. 6.
Combination therapy inhibits tumor growth. (A) Combination PDT and L-IRI enhanced MIA PaCa-2 tumor volume reduction, determined by longitudinal ultrasound imaging. (B) Representative snapshots of MIA PaCa-2 tumors 3-weeks post-treatment. Scale bar=2cm. Tumor weights at this time showed the combination inhibited tumor growth. (C) Fold change in tumor volume 2-weeks post-treatment shows significantly lower volume in the combination group. (D) Combination PDT and L-IRI also enhanced AsPC-1 tumor volume reduction. (E) Representative snapshots of AsPC-1 tumors 3-weeks post-treatment. Scale bar, 2cm. Tumor weights at this time showed the combination slowed tumor growth. (F) A sustained and prolonged MIA PaCa-2 tumor growth inhibition was observed after two cycles of combination administered on 9 and 27 days post-implantation. Arrow indicates day of treatment. (*P<0.05,**P<0.01,***P<0.001, Kruskal–Wallis One-way ANOVA) (N=8–18)
At 3-weeks post-treatment (30 days post-implantation), tumor weights in the combination group (195±30mg) were significantly lower (P<0.05) than that of no-treatment (373±45mg) and L-BPD groups (399±30mg) (Fig. 6B). There was no statistically significant difference in tumor weight between the L-IRI (314±24mg) and L-BPD+L-IRI groups (318±57mg), suggesting that L-BPD alone without light is not tumoricidal. Although tumor weights in the combination group (195±30 mg) are smaller than that in the L-IRI group (314±24mg), this is not statistically significant. Given this observation, we also compared changes in tumor volume at 2-weeks post-treatment (i.e. right before tumor regrowth was observed in the combination group) (Fig. 6C). Interestingly, at 2-weeks post-treatment, the fold-increase in tumor volume for the combination group (~1.8-fold) was found to be significantly lower than the L-IRI group (~5.2-fold), L-BPD+L-IRI group (~5.1-fold), and no-treatment group (~5.9-fold), but not significantly different than the PDT group (~3.3-fold). These data suggest that this combination balances the initial PDT actions with the sustained irinotecan therapeutic effects. In vivo efficacy of the combination regimen was further evaluated in AsPC-1 tumors. At 3-weeks post-treatment, a single combination treatment cycle reduced AsPC-1 tumor volume (Fig. 6D) and weight (Fig. 6E) by an average of 66.6% and 58.1%, respectively, compared to no-treatment. In contrary, tumor weights and volumes in the PDT (e.g. 260±86mm2) and L-IRI groups (e.g. 219±28mm2) were not significantly different from no-treatment (e.g. 302±48mm2).
For both MIA PaCa-2 and AsPC-1, tumor regrowth was observed 10–14 days after a single cycle of low-dose combination treatment. A second cycle of low-dose combination therapy prolonged the inhibition of MIA PaCa-2 tumor growth up to 42 days post-implantation (Fig. 6F). No animal death or changes in mouse weight were observed (Supplementary Fig. S4). General behavior and physiological activities of mice were normal per IACUC guidelines.
Effect of PDT and L-IRI on tumor-related biomarkers
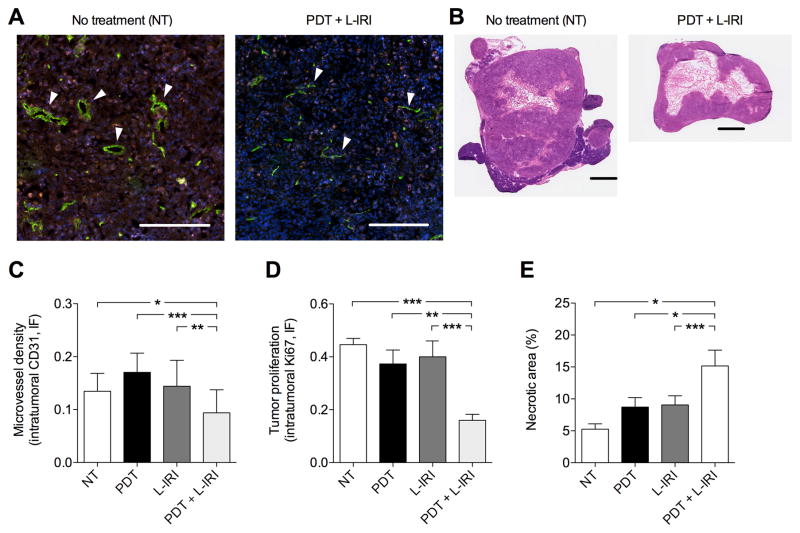
Studies show that microvessel density (MVD) estimation is a promising treatment response indicator. (33) Here we evaluate MVD via CD31-staining of vascular endothelial cells to determine tumor vascularization. Confocal images of MIA PaCa-2 cross-sections at 3-weeks post-treatment show immunostaining of both endothelial cells (green) and Ki-67 (proliferation marker, red) are significantly lower in the combination group compared to no-treatment (Fig. 7A). White arrows indicate microvessels, which are clearly smaller post-combination therapy. Quantitative analyses revealed that the combination resulted in a significant decrease (P<0.05) in MVD by ~30%, while sub-optimal PDT alone or L-IRI alone did not reduce tumoral MVD (Fig. 7C). Consistent with this anti-vascular effect, quantitative analyses revealed a dramatic (P<0.05) reduction of Ki-67-staining by >60% in tumors receiving the combination treatment, compared to no-treatment (Fig. 7D). Supporting the synergistic impact of the treatments on proliferation and vasculature destruction, we observed enhanced necrotic volumes in combination-treated tumors compared to all other groups (Fig. 7B, E). A low-dose combination treatment provided sustained tumor growth inhibition but did not eradicate the tumor completely. Indeed, H&E images of tumors in combination treatment group (Fig. 7B) shows a viable rim of tumor 3-weeks post-treatment indicating the necessity of multiple treatments for potentially complete treatment.
Fig. 7.
Anti-vascular and anti-proliferation effects 3-weeks post-treatment (30 days post-implantation). (A) Representative fluorescence images of MIA PaCa-2 tumor cross-section with immunostaining of endothelial cells (green), Ki-67 (red), and nuclei (blue) without and with combination therapy. Arrows indicate microvessels. Scale bar=250μm. (B) H&E images of MIA PaCa-2 cross-sections 3-weeks post-treatment. Scale bar=1cm. (C,D,E) Quantitative analyses revealed that a single combination decreased intratumoral MVD as determined by CD31 intensity, inhibited PanCa proliferation as determined by Ki-67, and enhanced necrosis. (*P<0.05,**P<0.01,***P<0.001; One-way ANOVA Tukey’s range test) (N≥11 cross-sections from N≥3 mice/group)
Discussion
The uniqueness of the PDT and L-IRI combination is that they mechanistically cooperate with each other, beyond their individual tumor destruction pathways, to enhance reduction in tumor burden with non-overlapping toxicities. Here, three cooperative pathways are demonstrated to elucidate the synergism: (i) PDT reduces ABCG2 expression, thereby increasing intracellular irinotecan and SN-38 levels. (ii) Irinotecan reduces tumoral expression of MCT-4, which is upregulated by PDT. (iii) PDT downregulates survivin expression, and amplification of the apoptotic and anti-proliferative effects was observed in the combination. The dramatic enhancement in tumor growth inhibition by the combination treatment confirms the strength of developing mechanistically-cooperative combinations, where each monotherapy enhances and benefits from the other, for difficult-to-treat diseases, e.g., PanCa.
Late diagnosis and limited effectiveness of standard treatments have resulted in a five-year survival rate of ≤5% in PanCa patients. (34) PanCa is notoriously resistant to chemoradiotherapies, partly attributable to inherent genetic complexities and abnormal microenvironment. (35) Several combination therapies have been clinically evaluated for PanCa, but most were abandoned due to ineffectiveness and high toxicities. (36) Recently, Abraxane® plus gemcitabine showed modest 2-month improvements in median OS compared to gemcitabine alone. (37) FOLFIRINOX, a combination of four chemo-agents (leucovorin, fluorouracil, irinotecan, oxaliplatin), generated much excitement after extending the median OS from 6.8 to 11.1 months. (38) However, FOLFIRINOX is limited to a small proportion of healthy patients due to high toxicities. Against this background, PDT is emerging as a clinically promising locoregional therapy for PanCa, (13,14) given its efficacy against gemcitabine-insensitive cells, (39) synergism with chemo/biological agents, and low incidence of mild adverse events clinically. (14) PDT has also been shown to induce antitumor immunity in various immunocompetent cancer models. (40)
Driven by this concept of developing rapidly translatable combinations where each component reinforces the other, our study is motivated by advances in nanoliposomal BPD-based PDT (Phase I/II) and nanoliposomal irinotecan (MM-398) for PanCa. (4,14) In this study, nanoliposomes improved the photochemical stability of BPD and the circulation profile of irinotecan, supporting the reduced systemic toxicity and increased efficacy of nanoliposomal BPD and irinotecan that have been established. (20,41) In our in vivo combination, L-BPD and L-IRI were administered IV simultaneously and PDT was performed 1-hour post-injection, when both agents were readily available at the tumor for their cooperative therapeutic actions.
Here, sub-lethal PDT reduced ABCG2 expression and increased intracellular concentrations of irinotecan and SN-38 by up to ~50% 6-hours post-treatment (Fig. 3C, D), while L-BPD alone does not interfere with intracellular irinotecan accumulation. Moreover, PDT-mediated ABCG2 reduction was sustainable for up to 24-hours (Fig. 3B). Studies have shown that durable maintenance of high intratumoral irinotecan and SN-38 levels is a critical determinant of antitumor activity. (42) Therefore, a sustained window of ABCG2-reduction could be important for enhanced irinotecan efficacy. It should be noted that at 24-hours, expression of ABCG2 appears to recover (Fig. 3B), although remaining lower than pre-PDT levels. This observation suggests that subsequent treatments of PDT and future optimization of PDT dose may be warranted for prolonged ABCG2 reduction.
In vivo, PDT (one-hour L-BPD-light interval) enhanced intratumoral irinotecan accumulation by more than 3-fold at 12-hours post-treatment, compared to L-IRI alone. Our previous work demonstrated that PDT with 1-hour BPD-light interval targets both tumor cells and vasculature. (43) Therefore, it is important to note that, besides the reduction of cellular ABCG2 expression, other mechanisms could also contribute to PDT-mediated enhancement of intratumoral drug accumulation. Previous studies have shown that BPD-PDT at a short photosensitizer-light interval, i.e., 15 minutes mainly causes vascular permeabilization, which leads to a modest enhancement (≤2-fold) of macromolecule delivery to tumors. (44) Future studies varying the photosensitizer-light interval to balance cellular damage (including ABCG2 photodestruction) and vascular permeabilization for maximal drug accumulation are merited. Although many attempts have been made to enhance the chemotherapy effectiveness by overcoming ABC transporter-mediated drug efflux with small molecular inhibitors, most studies have failed due to drug interactions and systemic toxicities. (23) PDT, an FDA-approved regimen with non-overlapping toxicities, provides a unique opportunity for inhibition of irinotecan efflux transporters, while avoiding those toxicity-related pitfalls.
Several studies have shown that oncogenic Ras-driven signals in PaCa cells can propel abnormal metabolic alterations, such as dependence on glycolysis. MCT-4 is a critical component in the glycolytic metabolism of PanCa cells, and can be further upregulated via several tumor microenvironmental changes such as hypoxia. (45) Studies have shown that high MCT-4 expression is a poor prognostic indicator in PanCa and that inhibition of MCT-4 leads to tumor growth suppression and increased cellular apoptosis. (28) PDT can create tumor hypoxia when oxygen is depleted by photochemical consumption or when oxygen supply is compromised by microvascular damage. (46) These hypoxic conditions can stimulate metabolic alterations, harbor treatment resistant cells and drive the cells towards metastatic invasion through activation of pathways, such as HIF-1α.(47) A unique feature of our combination is that irinotecan alleviates a compensatory response elicited by PDT. We show that while PDT elevates MCT-4 expression, the irinotecan component of the combination mitigates this increase and reduces MCT-4 to levels comparable to irinotecan treatment alone (Fig. 4). This observed effect, in accordance with the ability of irinotecan to downregulate HIF-1α as reported by others, (48) may also reduce metastatic dissemination of the disease in the combination treatment, and is currently being investigated. A sustained decrease in MVD is observed with the combination treatment compared to other groups at 3-weeks post-treatment (Fig. 7). While chronic vascular damage likely contributes to enhanced growth arrest, nutrient depletion and reduced blood supply could potentially lead to further hypoxia and corresponding survival signals. These conditions suggest that multiple irinotecan cycles may further enhance the two therapies synergism, through its effect on hypoxia-induced markers. Although reduction of blood supply may have been a concern for delivery of subsequent treatments, a second dose of the combination at 27 days post-implantation continues to exhibit synergistic efficacy and prolongs significant tumor growth inhibition (Fig. 6F).
Reduction in systemic toxicity is another attractive feature of PDT and L-IRI combination. Clinically, irinotecan can be prescribed for up to 12-cycles. However, due to its significant systemic toxicities (grade 3–4 diarrhea and neutropenia), patients receive an average of 6-cycles and often require dose reductions, compromising the chemo-effectiveness. PDT is associated with low incidence of mild abdominal pain, which can be relieved with analgesia. Normal pancreatic tissue healing has been shown after interstitial PDT without significant impact to structure or function. (14) In addition to non-overlapping side effects, the combination of PDT and L-IRI is significant in that enhanced cytotoxic efficacy can be achieved using drug doses at 12–20 fold lower than equivalent clinical doses (Supplementary Table S1). In this study we demonstrated that a single cycle, low-dose combination dramatically inhibited tumor volume growth by 70%, while others demonstrated that five doses of standard gemcitabine (250mg/kg) is ineffective against orthotopic MIA PaCa-2 tumors. (49) A second cycle of combination therapy further prolonged MIA PaCa-2 tumor growth inhibition, and was well-tolerated by mice. This compares favorably with the modest (~30%) tumor weight reduction of orthotopic PanCa tumors after 6 cycles of 25mg/kg irinotecan. (50)
In summary, this study shows that low-dose combination of PDT and L-IRI synergistically enhanced tumor growth inhibition compared to either treatment alone and was more effective than reported outcomes with a standard chemotherapeutics for PanCa. (49,50) Given the clinical promise of the individual therapies, this combination is easily translatable for clinical management of PanCa and other cancers. Further studies employing multi-cycle dosing for durable response, and evaluating metastasis control and survival enhancement are merited.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by NIH grants P01CA084203, R01CA156177, R01CA160998, S10ODO1232601 (T. Hasan), MGH-Tosteson-FMD-Fellowship 224889 (H.C. Huang), F32CA165881 (S. Mallidi) and K99CA175292 (I. Rizvi)
The authors thank Dr. Zhao and Mrs. Wu (WCP Photopathology) for help with histology, Dr. Schoenfeld (MGH Biostatistics Center) for support in statistical analyses, Drs. Spring and Palanisami for discussions.
Footnotes
All authors claim no conflicts of interest.
References
- 1.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nature biotechnology. 2012;30(7):679–92. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 3.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chemistry & biology. 2010;17(5):421–33. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko AH, Tempero MA, Shan YS, Su WC, Lin YL, Dito E, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. British journal of cancer. 2013;109(4):920–5. doi: 10.1038/bjc.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saif MW. MM-398 achieves primary endpoint of overall survival in phase III study in patients with gemcitabine refractory metastatic pancreatic cancer. JOP : Journal of the pancreas. 2014;15(3):278–9. doi: 10.6092/1590-8577/2507. [DOI] [PubMed] [Google Scholar]
- 6.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chemical reviews. 2010;110(5):2795–838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessel D, Castelli M. Evidence that bcl-2 is the target of three photosensitizers that induce a rapid apoptotic response. Photochemistry and photobiology. 2001;74(2):318–22. doi: 10.1562/0031-8655(2001)074<0318:etbitt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Xue LY, Chiu SM, Oleinick NL. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2001;20(26):3420–7. doi: 10.1038/sj.onc.1204441. [DOI] [PubMed] [Google Scholar]
- 9.Spring BQ, Rizvi I, Xu N, Hasan T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochemical & Photobiological Sciences. 2015;14(8):1476–91. doi: 10.1039/c4pp00495g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duska LR, Hamblin MR, Miller JL, Hasan T. Combination photoimmunotherapy and cisplatin: effects on human ovarian cancer ex vivo. Journal of the National Cancer Institute. 1999;91(18):1557–63. doi: 10.1093/jnci/91.18.1557. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher-Colombo SM, Miller J, Cengel KA, Putt ME, Vinogradov SA, Busch TM. Erlotinib Pretreatment Improves Photodynamic Therapy of Non-Small Cell Lung Carcinoma Xenografts via Multiple Mechanisms. Cancer research. 2015;75(15):3118–26. doi: 10.1158/0008-5472.CAN-14-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.del Carmen MG, Rizvi I, Chang Y, Moor AC, Oliva E, Sherwood M, et al. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. Journal of the National Cancer Institute. 2005;97(20):1516–24. doi: 10.1093/jnci/dji314. [DOI] [PubMed] [Google Scholar]
- 13.Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, et al. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50(4):549–57. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. British journal of cancer. 2014;110(7):1698–704. doi: 10.1038/bjc.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature reviews Drug discovery. 2005;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhary RK, Shariff I, Dolphin D. Drug release characteristics of lipid based benzoporphyrin derivative. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2003;6(1):13–9. [PubMed] [Google Scholar]
- 17.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale research letters. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szoka F, Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(9):4194–8. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneda N, Nagata H, Furuta T, Yokokura T. Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer research. 1990;50(6):1715–20. [PubMed] [Google Scholar]
- 20.Chen B, Pogue BW, Hasan T. Liposomal delivery of photosensitising agents. Expert opinion on drug delivery. 2005;2(3):477–87. doi: 10.1517/17425247.2.3.477. [DOI] [PubMed] [Google Scholar]
- 21.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nature reviews Molecular cell biology. 2009;10(3):218–27. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konig J, Hartel M, Nies AT, Martignoni ME, Guo J, Buchler MW, et al. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. International journal of cancer Journal international du cancer. 2005;115(3):359–67. doi: 10.1002/ijc.20831. [DOI] [PubMed] [Google Scholar]
- 23.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature reviews Drug discovery. 2006;5(3):219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, et al. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(8):2463–70. doi: 10.1158/1078-0432.CCR-06-1599. [DOI] [PubMed] [Google Scholar]
- 25.Goler-Baron V, Assaraf YG. Overcoming multidrug resistance via photodestruction of ABCG2-rich extracellular vesicles sequestering photosensitive chemotherapeutics. PLoS One. 2012;7(4):e35487. doi: 10.1371/journal.pone.0035487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobin P, Clarke S, Seale JP, Lee S, Solomon M, Aulds S, et al. The in vitro metabolism of irinotecan (CPT-11) by carboxylesterase and beta-glucuronidase in human colorectal tumours. British journal of clinical pharmacology. 2006;62(1):122–9. doi: 10.1111/j.1365-2125.2005.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. Journal of Biological Chemistry. 2006;281(14):9030–37. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 28.Baek G, Tse YF, Hu Z, Cox D, Buboltz N, McCue P, et al. MCT4 defines a glycolytic subtype of pancreatic cancer with poor prognosis and unique metabolic dependencies. Cell reports. 2014;9(6):2233–49. doi: 10.1016/j.celrep.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, et al. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nature cell biology. 1999;1(8):461–6. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 30.Bevins RL, Zimmer SG. It’s about time: scheduling alters effect of histone deacetylase inhibitors on camptothecin-treated cells. Cancer research. 2005;65(15):6957–66. doi: 10.1158/0008-5472.CAN-05-0836. [DOI] [PubMed] [Google Scholar]
- 31.Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ. Survivin, a member of the inhibitor of apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer research. 2007;67(10):4989–95. doi: 10.1158/0008-5472.CAN-06-4785. [DOI] [PubMed] [Google Scholar]
- 32.Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39(4):425–35. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nature reviews Clinical oncology. 2009;6(6):327–38. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrystoja CC, Diamandis EP, Brand R, Ruckert F, Haun R, Molina R. Pancreatic cancer. Clin Chem. 2013;59(1):41–6. doi: 10.1373/clinchem.2012.196642. [DOI] [PubMed] [Google Scholar]
- 35.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56(8):1134–52. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: are we there yet? Therapeutic advances in medical oncology. 2013;5(1):81–9. doi: 10.1177/1758834012462463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 39.Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers in surgery and medicine. 2011;43(7):565–74. doi: 10.1002/lsm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nature reviews Cancer. 2006;6(7):535–45. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer research. 2006;66(6):3271–7. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 42.Kalra AV, Kim J, Klinz SG, Paz N, Cain J, Drummond DC, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer research. 2014;74(23):7003–13. doi: 10.1158/0008-5472.CAN-14-0572. [DOI] [PubMed] [Google Scholar]
- 43.Chen B, Pogue BW, Hoopes PJ, Hasan T. Combining vascular and cellular targeting regimens enhances the efficacy of photodynamic therapy. International journal of radiation oncology, biology, physics. 2005;61(4):1216–26. doi: 10.1016/j.ijrobp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(3 Pt 1):917–23. doi: 10.1158/1078-0432.CCR-05-1673. [DOI] [PubMed] [Google Scholar]
- 45.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. The Journal of biological chemistry. 2006;281(14):9030–7. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 46.Henderson BW, Fingar VH. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer research. 1987;47(12):3110–4. [PubMed] [Google Scholar]
- 47.Merrimack Pharmaceuticals Announces MM-398 Achieves Primary Endpoint of Overall Survival in Phase 3 Trial in Post-Gemcitabine Metastatic Pancreatic Cancer. merrimackpharmacom. 2014 Press Release. [Google Scholar]
- 48.Guerin E, Raffelsberger W, Pencreach E, Maier A, Neuville A, Schneider A, et al. In vivo topoisomerase I inhibition attenuates the expression of hypoxia-inducible factor 1α target genes and decreases tumor angiogenesis. Mol Med. 2012;18:83–94. doi: 10.2119/molmed.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bornmann C, Graeser R, Esser N, Ziroli V, Jantscheff P, Keck T, et al. A new liposomal formulation of Gemcitabine is active in an orthotopic mouse model of pancreatic cancer accessible to bioluminescence imaging. Cancer Chemother Pharmacol. 2008;61(3):395–405. doi: 10.1007/s00280-007-0482-z. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Yue GG, Fung KP, Leung PC, Lau CB, Leung PS. Establishment of an orthotopic model of pancreatic cancer to evaluate the antitumor effects of irinotecan through the biomarker carbohydrate antigen 19–9 in mice. Pancreas. 2014;43(7):1126–8. doi: 10.1097/MPA.0000000000000183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.