Abstract
The use of xylazine, a veterinary sedative, with ketamine for rat anesthesia has been shown to enhance the pulmonary capillary hemorrhage (PCH) effect of diagnostic ultrasound. This study was undertaken to assess whether the sedative/analgesic dexmedetomidine, commonly used in the ICU, can also enhance ultrasound induced PCH. Female Sprague Dawley rats were anesthetized with various combinations of ketamine plus xylazine or dexmedetomidine. The dosage of dexmedetomidine was reduced for some groups to doses relevant to human clinical usage. The right thorax of all rats was shaved and depilated for ultrasound transmission and the rats were scanned with diagnostic ultrasound using a 7.6 MHz linear array in a 38 °C degassed water bath. There was no significant difference in PCH results for the recommended anesthetic dosages of ketamine plus xylazine and ketamine plus 500 μg/kg dexmedetomidine. The varied doses of dexmedetomidine enhanced the PCH, even for the lowest dose of 4 μg/kg, equivalent to a low human dose of 0.64 μg/kg. There was no significant difference in PCH for 500 μg/kg dexmedetomidine with or without ketamine. Further research is needed to identify and characterize other factors which may modify the patient risk from ultrasound induced PCH.
Keywords: Pulmonary ultrasound, Mechanical index, Comet-tail artifact, dexmedetomidine anesthesia
Introduction
Diagnostic ultrasound can induce capillary hemorrhage in lung, presenting a potential risk of injury for patients receiving trans-thoracic pulmonary ultrasound examination. Pulmonary capillary hemorrhage (PCH) has been studied for more than 25 y, since its discovery by Child et al. (1990), but the risk to patients remains uncertain. All the while, the use of diagnostic ultrasound for thoracic examination has grown. Indeed, direct pulmonary examination has become routine for diagnosis in the point of care settings of emergency and intensive care (Volpicelli, 2013), as well as in traditional radiology studies. The ultrasound does not image the interior of healthy lungs, but shows the surface as a bright line with multiple echoes and other artifacts displayed beyond the surface (Lichtenstein et al. 2009). For example, comet-tail artifacts (CTAs, also known as B-lines), which are bright lines of multiple echoes extending deep into the image, are indicative of interstitial syndrome, pulmonary edema and pulmonary effusion, among other problems. More information is needed to fully understand the mechanisms and dosimetry of the PCH bioeffect and the potential risks of PCH for the broad spectrum of patients receiving thoracic ultrasound.
Research has indicated that the PCH phenomenon occurs in mice, rats and pigs and can be characterized by a threshold for a specific set of ultrasound exposure conditions (American Institute of Ultrasound in Medicine, 2000; Church et al. 2008a). A study involving human transesophageal echocardiography at 3.5 MHz and up to 2.4 MPa (on screen Mechanical Index 1.3), which was believed to be near or slightly above the PCH threshold, was negative for PCH (Meltzer et al. 1998). However, the appearance of the lung surface, the incident ultrasound parameters, and the range of medications used for these examinations during cardiac surgery are all uncertain in relation to present pulmonary examinations. Recently, we found that relatively high resolution diagnostic ultrasound with a 7.6 MHz linear array displayed growing CTAs during pulmonary scanning in rats, that corresponded with visible PCH on the lung surface (Miller, 2012). Further study revealed that the PCH was strongly influenced by anesthesia methods in the animal tests, and was particularly enhanced by the use of xylazine in the anesthesia technique for sedation and analgesia (Miller et al. 2014; Miller et. al. 2015a). This finding that the exact anesthetics were nearly as important as physical exposure parameters in the etiology of PCH induction by ultrasound greatly complicates the estimation of possible patient risk of PCH.
More information is needed on human drugs (xylazine is a veterinary sedative and is typically not used in humans) or other physiological situations which may be critical for any assessment of patient susceptibility to this bioeffect. A common sedative/analgesic is Dexmedetomidine, which, like xylazine, is an alpha-2 receptor agonist but with higher specificity. This class of drugs is used in addition for treating hypertension, attention-deficit disorder, panic disorders, and opioid, benzodiazepine and alcohol withdrawal (Giovannitti et al. 2015). The human formulation in the USA is Precedex (0.1 mg/ml, dexmedetomidine HCL injection, Hospira Inc. Lake Forest IL,USA), while a veterinary formulation is Dexdomitor® (0.5 mg/ml, dexmedetomidine HCL injection, Orion Corp, Espoo, FD). Dexmedetomidine , commonly used for ICU sedation and non-intubated procedural sedation, is best known for causing minimal or no respiratory depression. It is under evaluation for many other applications (Pichot et al. 2013; Bajwa and Kulshrestha, 2013; Giovannitti et al. 2015). This study was undertaken to assess whether or not dexmedetomidine could enhance the PCH effect of diagnostic ultrasound.
Materials and Methods
All in vivo animal procedures were conducted with the approval and guidance of the University Committee on Use and Care of Animals (UCUCA). In this study, a total of 86 female rats (Sprague Dawley, Charles River, Wilmington, MA, USA) were tested at 8-12 weeks of age. The rat’s weight averaged of 228±21 gm. Three rats were lost from the study due anesthetic death and 5 were excluded due to other problems. The remaining 78 rats were randomly separated into 14 test groups of 5-7 rats each. For most rats, physiological data was collected on heart rate and percentage of oxygen saturation (%SpO2) using a pulse oximeter (SurgiVet V3395 TPR, Smiths Medical Inc. St Paul, MN USA).
Anesthetic techniques
Anesthesia was induced by intraperitoneal injection (IP) of 1.75 ml/kg of mixed anesthesia components and saline, as listed in Table 1. Xylazine was given at the recommended dosage (9 mg kg−1) plus ketamine (91 mg kg−1). The recommended dosage of dexmedetomidine (500 μg/kg) normally was given with ketamine (75 mg/kg). Both of these drug dosages are well tolerated by rats when given IP, and produce general anesthesia sufficient for surgical procedures (Wellington et al. 2013). Ketamine only was given to some rats to show the effect of omitting the xylazine or dexmedetomidine, and dexmedetomidine only was given to show the effect of omitting the ketamine. Both of these single drug doses produced anesthesia sufficient to perform the ultrasound scanning. The dosage of dexmedetomidine also was reduced for some groups to explore the possible dose-response changes of PCH induction for doses relevant to human usage. For humans, initial doses of 0.5 to 2.5 μg/kg are given for sedation by various routes, including IV infusion, intramuscular injection, intranasal and oral administration (Mason et al. 2011; Peng et al. 2014; Bajwa and Kulshrestha 2015). Interestingly, one advantage of using dexmedetomidine is its minimal respiratory depression (Giovannitti et al. 2015), and there is some evidence that it can reduce lung capillary permeability in rats (Kumagai et al 2008).
Table 1.
The components of anesthetic mixtures (ml) to make up 1.75 ml, all of which were given at an intraperitoneal dose of 1.75 ml/kg.
| Mixture | Ketamine 100 mg/ml |
Xylazine 100 mg/ml |
Dexmedetomidine 500 μg/ml |
Saline |
|---|---|---|---|---|
| KO | 0.91 | 0 | 0 | 0.84 |
| KX | 0.91 | 0.9 | 0 | 0.75 |
| KD500 | 0.75 | 0 | 1.0 | 0 |
| KD100 | 0.75 | 0 | 0.2 | 0.8 |
| KD20 | 0.75 | 0 | 0.04 | 0.96 |
| KD4 | 0.75 | 0 | 0.008 | 0.992 |
| DO | 0 | 0 | 1.0 | 0.75 |
The dosage adjustment for different sizes of animals is based on surface area (rather than only weight) (Dexdomitor® package insert, 2013). The formula for translating dosages by weight from the animal dose (AD) in μg/kg to the human equivalent dose (HED) in μg/kg is
| (1) |
in which AK and HK are factors based on body surface area (Reagan-Shaw et al. 2007). For adults and children, HK is equal to 37 and 25, respectively, while AK for rats is equal to 6. The recommended rat dose of dexmedetomidine of 500 μg/kg for surgical anesthesia therefore has a HED of 81 μg/kg and 120 μg/kg for adults and children, respectively. For the reduced dexmedetomidine doses in this study (see Table 1) of 100, 20 and 4 μg/kg, the HEDs are 16.2, 3.2 and 0.64 μg/kg for adults or 24, 4.8 and 0.96 μg/kg for children, which encompass the normal human dose range.
The right thorax of all rats was shaved and depilated for ultrasound transmission. The rats were mounted on a plastic board and immersed in a 38 °C degassed water bath together with the ultrasound probe. This exposure method has reliable ultrasound coupling, and maintains the body temperature of the rats.
Ultrasound scanning
A Phillips HDI 5000 (Philips Healthcare, Andover MA USA) diagnostic ultrasound machine with CL15-7 linear array was used for ultrasound aiming and exposure, as described previously (Miller 2012). This probe was mounted in the water bath on a three dimensional positioning apparatus and aimed horizontally at the rat thorax, at the level of the right cranial or middle lobe. The imaging was in B mode with 2 cm image depth, 1 cm focal depth, and 39 frames per second at 7.6 MHz. To avoid PCH incidence during setup and aiming, the image of the lung was acquired using a sub-threshold Mechanical Index setting of MI=0.21. The probe was partially in contact with the skin, and the pleural surface was at a depth of about 5-6 mm. The image was adjusted for a bright pulmonary surface echo and avoiding ribs as much as possible. To perform an exposure, the MI setting was rapidly elevated using the output toggle switch to the desired value of 0.27, 0.37, 0.52, 0.7 or 0.9 for 5 min. The exposure parameters at the lung surface were previously estimated by calibrated hydrophone measurements of the ultrasound attenuated by chest wall samples (Miller et al. 2015b), and are listed in Table 2. The pulse repetition frequency was 10 kHz for the 250 ns duration pulses.
Table 2.
In situ exposure parameters(mean ± standard deviation) for the Mechanical Index (MI) setting: PCPA, peak compressional pressure amplitude, PRPA, peak rarefactional pressure amplitude, PMPA, peak mean pressure amplitude, and ISPPA, spatial peak, pulse peak intensity.
| Setting MI |
PCPA MPa |
PRPA MPa |
PMPA MPa |
Isppa
W cm−2 |
|---|---|---|---|---|
| 0.9 | 3.41 ± 0.29 | 2.13 ± 0.21 | 2.77 ± 0.18 | 170 ± 20 |
| 0.7 | 2.73 ± 0.22 | 1.71 ± 0.19 | 2.22 ± .016 | 114 ± 15 |
| 0.52 | 2.02 ± 0.20 | 1.34 ± 0.13 | 1.68 ± 0.14 | 75 ± 13 |
| 0.37 | 1.42 ± 0.12 | 1.02 ± 0.11 | 1.22 ± 0.11 | 40.5 ± 7.4 |
| 0.27 | 0.98 ± 0.09 | 0.76 ± 0.08 | 0.87 ± 0.09 | 20.8 ± 4.0 |
| 0.21 | 0.70 ± 0.06 | 0.55 ± 0.06 | 0.62 ± 0.06 | 10.4 ± 2.0 |
Measured endpoints and experimental plan
The study was designed to assess the PCH resulting from varied exposure MI values with ketamine and 500 μg/kg dexmedetomidine in order to determine the exposure-response and the threshold for PCH. In addition, PCH variation was explored for MI = 0.9 due to anesthesia with normal ketamine and xylazine doses, with ketamine or dexmedetomidine only and with ketamine plus varied dexmedetomidine doses. The length of the CTA region (indicative of PCH) was observed in the final image, and was measured as a percentage of the length of the bright line image of the pleural surface. Five minutes after scanning or sham scanning, the rats were sacrificed by exsanguination under anesthesia for evaluation of the lungs. The trachea was occluded and the intact lung was removed for photography. The length, width and area of the hemorrhage region were measured on the lungs. Statistical comparisons between groups were performed using SigmaPlot for Windows V. 11.0 (Systat Software Inc., San Jose CA, USA), with statistical significance assumed at P<0.05. The statistical comparison of the groups versus the respective shams was made using the Mann-Whitney rank sum test for the area data, rather than the t-test, due to the non-normal distributions of the data. In addition, linear regression was used to characterize the exposure response function.
Results
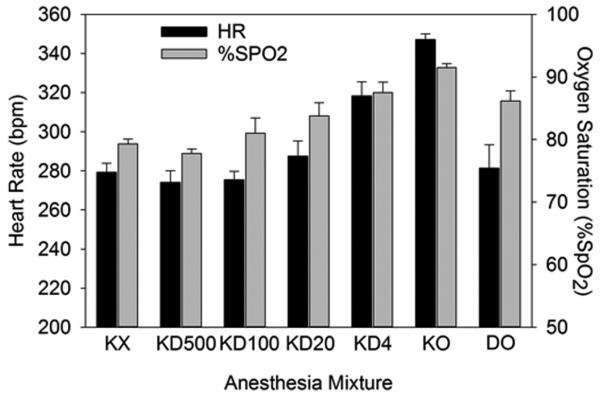
The results of the heart rate and %SpO2 measurements are shown in Figure 1. The sedatives produced essentially the same values of heart rate and %SpO2 (mean ± standard deviations): 279±20 and 79±3 (n=17) with ketamine plus xylazine, and 274±36 and 78±4 (n=35) with ketamine and dexmedetomidine (500 μg/kg). Ketamine plus dexmedetomidine (KD500) essentially duplicated the results for ketamine plus xylazine (KX). The ketamine only (KO) condition produced significantly higher values for both parameters relative to the standard anesthetics, and poor sedation alone relative to anesthesia including dexmedetomidine, or dexmedetomidine only (DO). The reduced dexmedetomidine doses had significantly higher %SpO2 for 4 μg/kg and 20 μg/kg than the higher doses. As noted above, these doses are roughly equivalent to doses used for human sedation (but without ketamine) and the results indicated minimal respiratory suppression.
Figure 1.
The mean results for heart rate and % SpO2 for the different anesthetic mixtures. The values for ketamine and xylazine (KX) include all three such groups, and the ketamine plus 500 μg/kg dexmedetomidine (KD500) value includes all six such groups. Values for DO (dexmedetomidine only), and for KO, KD4, KD20 and KD100 (ketamine plus zero, 4 μg/kg, 20 μg/kg and 100 μg/kg, respectively) include only the specific group.
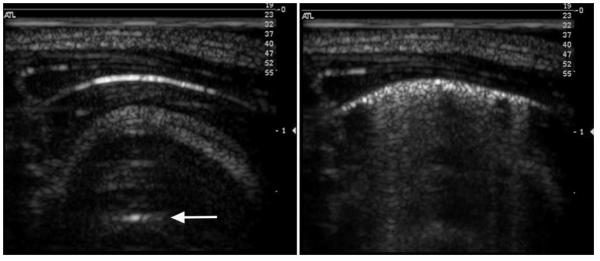
The ultrasound images revealed CTAs for the higher MIs which corresponded to PCH on the surface of the lungs. For example, the beginning and ending images for a rat scanned at MI = 0.9 for ketamine plus 4 μg/kg dexmedetomidine are shown in Fig. 2. The start image has the bright curved line extending horizontally in the image at a depth of about 5 mm. This bright line is the lung surface image, and the image features at greater depths are artefacts resulting from multiple reflections from the lung surface and the probe. These include a distorted image of the chest wall and short horizontal reflections of the lung surface, called A lines (Lichtenstein et al. 2009). The after-scanning image shows CTAs across 80% of the 14 mm bright line image, some of which extend more than a cm in depth to the bottom of the image. The CTAs have been termed “B lines”, and are predictive of some patient conditions (Lichtenstein et al. 2009). Figure 3 shows the representative lung sample from the ultrasound exposure in Fig. 2, which crossed the cranial and medial lobes. The positions of the lobes have shifted after removal from the thorax, such that the PCH line on the two lobes do not line up as they were when induced by the scan plane in vivo. The total length of the PCH was 13 mm, with an average width of about 1 mm, and a total area of 15.4 mm2.
Figure 2.
Representative images of the lung before (left) and after (right) MI=0.9 scanning with ketamine plus 4 μg/kg dexmedetomidine anesthesia. The bright curved line is the lung surface reflection and multiple reflection gives an “A line” (arrow). During scanning, the lung surface image breaks up with “comet tail” artefacts apparently extending vertically into the interior of the lung during scanning.
Figure 3.
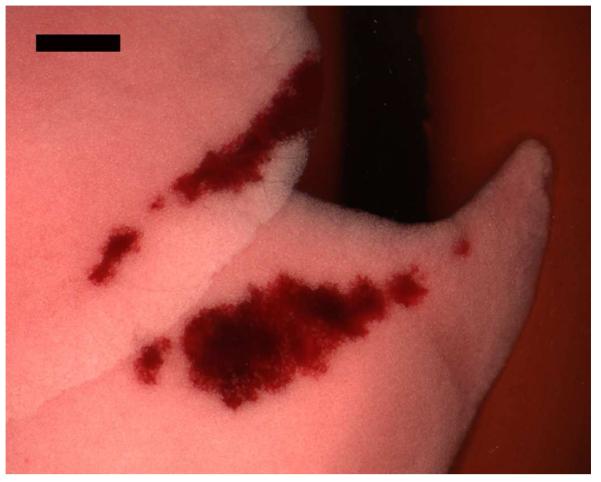
A photomicrograph of the cranial and medial lobes of the right lung showing PCH induced by ultrasound scanning (Fig. 2). In situ, the two parts of the PCH area would line up, as they were created along the line of incidence of the scan plane. Scale Bar: 2 mm.
Data on the proportion of PCH occurrence, percentage width of CTAs, and the PCH for all the groups is presented in Table 3. To enhance statistical sensitivity for the shams and the lowest exposures and doses of dexmedetomidine, extra rats were added to increase these groups to 6 or 7 rats (see Table 3). The two recommended anesthetics gave essentially the same results, with no significant difference between results for MI=0.9 or for 0.52. The lowest dexmedetomidine dose KD4 gave somewhat reduced surface area and length of the PCH relative to the other doses, with a statistically significant reduction from the KD100 PCH area (P<0.05). The KD4 result was statistically significantly greater than the sham result.
Table 3.
Data for measured parameters for all the groups presented as the means plus/minus one standard deviation. The number of positive results is listed as a fraction of the number (n) in the group. Comet tail artefacts (CTA) are given as a percentage of the bright lung surface image. In addition, the statistical P value of Mann-Whitney rank sum tests for PCH area relative to shams are listed with P>0.05 shown as not significant (NS).
| MI Setting |
Anesthesia Mixture |
Proportion + PCH /n |
CTA percent |
PCH Length mm |
PCH Area mm2 |
Test re Sham P |
|---|---|---|---|---|---|---|
| 0.9 | KX | 5/5 | 87±16 | 8.5±1.7 | 14.5±9.7 | 0.004 |
| 0.52 | KX | 5/6 | 37±21 | 2.2±2.4 | 2.1±2.7 | 0.015 |
| 0 | KX | 0/6 | 0 | 0 | 0 | - |
| 0.9 | KO | 3/5 | 4.7±6.5 | 1.3±1.5 | 0.7±1.0 | NS |
| 0.9 | DO | 5/5 | 88±10 | 8.9±3.2 | 16.0±9.0 | 0.008 |
| 0.9 | KD500 | 5/5 | 88±13 | 8.6±1.6 | 13.1±5.8 | 0.003 |
| 0.9 | KD100 | 5/5 | 85±9 | 10.9±3.5 | 19.3±7.6 | 0.003 |
| 0.9 | KD20 | 5/5 | 70±36 | 10.1±5.7 | 21.0±16.5 | 0.003 |
| 0.9 | KD4 | 5/7 | 33±40 | 5.5±5.6 | 6.7±7.2 | 0.026 |
| 0.7 | KD500 | 5/5 | 72±14 | 6.7±2.5 | 8.2±2.5 | 0.003 |
| 0.52 | KD500 | 5/7 | 44±27 | 3.4±3.8 | 3.9±5.0 | 0.026 |
| 0.37 | KD500 | 2/5 | 22±31 | 0.5±0.8 | 0.14±0.2 | NS |
| 0.27 | KD500 | 0/5 | 4±6 | 0 | 0 | - |
| 0 | KD500 | 0/7 | 0 | 0 | 0 | - |
The exposure response results are plotted in Fig. 4. A linear regression on the four dexmedetomidine data points with non-zero effect gave a zero-crossing threshold of 0.97 MPa PRPA (r2=0.60). The estimated value of the MI corresponding to this in situ threshold is 0.35. If the threshold is defined as midway between the highest PRPA group without significant PCH and the lowest PRPA group with a significant effect, then the threshold would be slightly higher at 1.15 MPa. The xylazine results were slightly different (a linear regression is not given, because there were only 2 points), but both the KX and KD500 results were statistically significantly higher than the shams at MI=0.52, indicating both drugs would yield nearly identical PCH thresholds.
Figure 4.
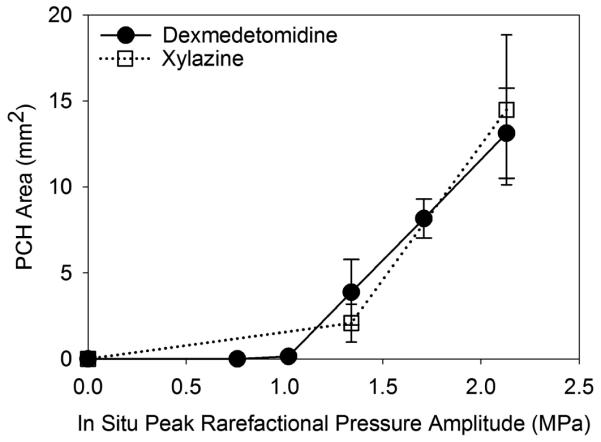
A plot of the PCH area results for the exposure response tests (Table 3) showing very similar trends for both dexmedetomidine and xylazine and thresholds at about 1 MPa.
Results for the varying dexmedetomidine dose groups are plotted in Fig. 5. A bar graph was used for this presentation, because a plot against a linear scale would greatly compress the spacing of the lower values. All the PCH results with dexmedetomidine, including the lowest 4 μg/kg dose, were statistically significantly greater than the sham. All the ketamine plus dexmedetomidine results were significantly greater than the results for ketamine only, except for the lowest dose result. As the dose of dexmedetomidine added to the ketamine was increased, there appeared to be a rise in effect to a roughly constant plateau. Interestingly, when the ketamine was omitted, the result for dexmedetomidine alone was not significantly different from the results with the combination.
Figure 5.
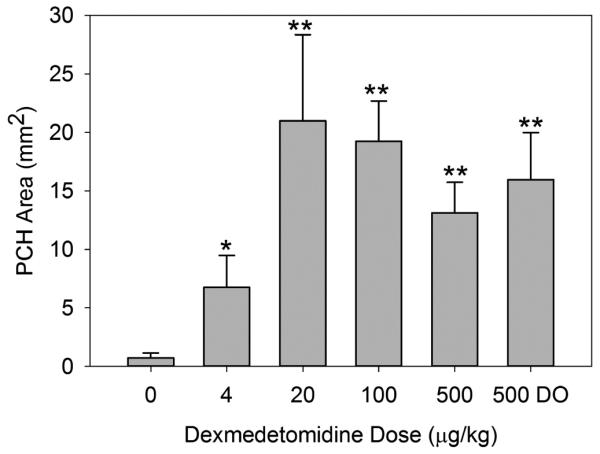
A graph of the PCH area results for the different dexmedetomidine doses (Table 3). The PCH is not significantly greater than shams for the ketamine with zero dexmedetomidine, but is statistically significant for the lowest dose of 4 μg/kg (P<0.05, *), and the other doses (P<0.01, **). The result for dexmedetomidine only (DO) is not significantly different from the same dose with added ketamine.
4. Discussion
Pulmonary capillary hemorrhage induced by diagnostic ultrasound in rats is strongly influenced by anesthetic techniques (Miller et al. 2015a). This finding indicates that physiological conditions, in addition to physical exposure parameters, can be critical factors in determining PCH risks. In the previous research, the recommended use of xylazine, a veterinary sedative, with ketamine for anesthesia in rats was found to lower the threshold and increase the magnitude of PCH. In this present study, the implication for human risk was explored by comparing the influence of dexmedetomidine, a common clinical sedative, to xylazine on the susceptibility of rats to PCH. Dexmedetomidine is used in human patients for radiological procedures, sedation in the ICU and other applications (Giovannitti et al. 2015).
Ketamine plus xylazine and ketamine plus dexmedetomidine at the recommended doses for rats produced similar reductions of heart rate and %SpO2 measurements relative to ketamine only (Figure 1). However the recommended dose of dexmedetomidine (500 μg/kg) for surgical anesthesia in rats is high relative to human doses for sedation (0.5-2.5 μg/kg). To test more clinically relevant conditions, dexmedetomidine doses were adjusted by the formula for equivalent doses in rats and humans (Eq. 1). Reduction of the dexmedetomidine dose to 4 or 20 μg/kg, equivalent to human doses in the clinical range, led to significantly higher %SpO2 than the higher doses, indicating reduced respiratory suppression.
There was no significant difference in the PCH results for the two recommended anesthetics (KX and KD500). The exposure response test for ketamine plus 500 μg/kg dexmedetomidine showed steadily increasing PCH areas above a threshold of 0.97 MPa, see Fig. 4. This was essentially identical to previously determined minimum thresholds (Miller et al. 2015a).
All the PCH results at MI=0.9 with dexmedetomidine, including the lowest 4 μg/kg dose, were significantly greater than sham. The ketamine plus dexmedetomidine results were uniformly greater than with ketamine alone, except for the lowest dose result. As the dose of dexmedetomidine dose was increased, PCH susceptibility reached a plateau over a wide range (more than 100x), a dose response characteristic of receptor-mediated drugs. The PCH result for sedation with dexmedetomidine only was not significantly different from the results with ketamine plus dexmedetomidine. This finding suggests that the ketamine may not influence on the PCH effect.
The dependence of PCH susceptibility with diagnostic ultrasound on detailed use of sedatives and anesthetics greatly complicates the prediction of patient risk. These results show that dexmedetomidine increases susceptibility to PCH even for low, human-equivalent doses. Ultrasound-induced PCH are likely to be influenced by sedatives which cause very little respiratory depression. Other similar medications, like clonidine, would likely have a similar influence on this bioeffect. Further study is needed to identify other drugs, patient conditions or other physiological factors which may modify the occurrence of ultrasound induced PCH.
Acknowledgments
This study was supported by the National Institutes of Health, National Heart Lung and Blood Institute, via grant number HL116434.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Institute of Ultrasound in Medicine Section 4--bioeffects in tissues with gas bodies. J Ultrasound Med. 2000;19:97–108. doi: 10.7863/jum.2000.19.2.97. 154-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa S, Kulshrestha A. Dexmedetomidine: an adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–483. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child SZ, Hartman CL, Schery LA, Carstensen EL. Lung damage from exposure to pulsed ultrasound. Ultrasound Med Biol. 1990;16:817–825. doi: 10.1016/0301-5629(90)90046-f. [DOI] [PubMed] [Google Scholar]
- Church CC, Carstensen EL, Nyborg WL, Carson PL, Frizzell LA, Bailey MR. The risk of exposure to diagnostic ultrasound in postnatal subjects: nonthermal mechanisms. J Ultrasound Med. 2008;27:565–592. doi: 10.7863/jum.2008.27.4.565. [DOI] [PubMed] [Google Scholar]
- Dexdomitor® Package Insert, Orion Corp, Espoo FD, NADA 141-267.
- Giovannitti JA, Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62:31–39. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai M, Horiguchi T, Nishikawa T, Masaki Y, Tobe Y. Intravenous dexmedetomidine decreases lung permeability induced by intracranial hypertension in rats. Anesth Analg. 2008;107:643–647. doi: 10.1213/ane.0b013e3181770e6f. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DA, Meziére GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136:1014–1020. doi: 10.1378/chest.09-0001. [DOI] [PubMed] [Google Scholar]
- Mason KP, Lubisch NB, Robinson F, Roskos R. Intramuscular dexmedetomidine sedation for pediatric MRI and CT. AJR Am J Roentgenol. 2011;197:720–725. doi: 10.2214/AJR.10.6134. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Adsumelli R, Risher WH, Hicks GL, Jr, Stern DH, Shah PM, Wojtczak JA, Lustik SJ, Gayeski TE, Shapiro JR, Carstensen EL. Lack of lung hemorrhage in humans after intraoperative transesophageal echocardiography with ultrasound exposure conditions similar to those causing lung hemorrhage in laboratory animals. J Am Soc Echocardiogr. 1998;11:57. doi: 10.1016/s0894-7317(98)70120-8. 60. [DOI] [PubMed] [Google Scholar]
- Miller DL. Induction of pulmonary hemorrhage in rats during diagnostic ultrasound. Ultrasound Med Biol. 2012;38:1476–1482. doi: 10.1016/j.ultrasmedbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Miller DL, Suresh MV, Dou C, Yu B, Raghavendran K. Characterization of ultrasound-induced pulmonary capillary hemorrhage in rats. Microvasc Res. 2014;93:42–5. doi: 10.1016/j.mvr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Dou C, Raghavendran K. Anesthetic techniques influence the induction of pulmonary capillary hemorrhage during diagnostic ultrasound scanning in rats. J Ultrasound Med. 2015a;34:289–97. doi: 10.7863/ultra.34.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Dou C, Raghavendran K. Dependence of thresholds for pulmonary capillary hemorrhage on diagnostic ultrasound frequency. Ultrasound Med Biol. 2015b;41:1640–50. doi: 10.1016/j.ultrasmedbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: a systematic review and meta-analysis. Clinics (Sao Paulo) 2014;69:777–86. doi: 10.6061/clinics/2014(11)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: from second- to first-line sedative agents in the critical care setting? J Intensive Care Med. 2012;27:219–237. doi: 10.1177/0885066610396815. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:6596–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Volpicelli G. Lung sonography. J Ultrasound Med. 2013;32:165–171. doi: 10.7863/jum.2013.32.1.165. [DOI] [PubMed] [Google Scholar]
- Wellington D, Mikaelian I, Singer L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci. 2013;52:481–487. [PMC free article] [PubMed] [Google Scholar]