Abstract
Background
Obsessive-compulsive disorder (OCD) is characterized by five major dimensions, including contamination/washing, harm/checking, symmetry/ordering, hoarding, and forbidden thoughts. How these dimensions may relate etiologically to the symptoms of other obsessive-compulsive related disorders (OCRDs) and anxiety disorders (ADs) is not well known. The aim of this study was to examine the genetic and environmental overlap between each major obsessive-compulsive dimension with the symptoms of other OCRDs and ADs.
Methods
Two thousand four hundred ninety-five twins of both sexes, aged between 18 and 45 years, were recruited from the Australian Twin Registry. Measures used scores on four dimensions (obsessing (forbidden thoughts), washing, checking, and ordering) of the Obsessive-Compulsive Inventory-Revised, Dysmorphic Concerns Questionnaire, Hoarding Rating Scale, Anxiety Sensitivity Index, Social Phobia Inventory, and Stress subscale of the Depression, Anxiety, and Stress Scale. Multivariate twin modeling methods using continuous and categorized variables were performed, also controlling for age and gender.
Results
Our findings suggested that forbidden thoughts and washing demonstrated the strongest genetic overlap with other AD symptoms, while ordering was genetically related to OCRD symptoms. Common genetic influences on checking symptoms were best estimated when modeling OCRDs together with AD symptoms. Common environmental factors of ordering and checking were shared with AD symptoms.
Conclusions
Important shared genetic and environmental risk factors exist between OCD, OCRDs, and ADs, but which vary alongside the expression of its major dimensions.
Keywords: OCD, anxiety/anxiety disorders, BDD, hoarding, twin studies
INTRODUCTION
There is consistent evidence to suggest that obsessive– compulsive disorder (OCD) encompasses a few consistent and temporally stable symptom dimensions, which may coexist within an individual patient.[1] These major dimensions typically include contamination/washing, harm/checking, symmetry/ordering, hoarding, and forbidden (sexual/religious) thoughts.[1,2] Each has been associated with distinct patterns of genetic and environmental influence[3,4]; comorbidity with other psychiatric disorders[5,6]; and treatment responsiveness.[7,8] Neurobiological studies also suggest that these symptom dimensions may, in part, reflect distinct underlying pathophysiological processes.[9–11] For example, elevated amygdala responsiveness to threat—a common finding in other anxiety disorders (ADs)—is most evident in OCD patients with prominent harm/checking and/or forbidden thoughts.[12] Thus, there is accumulating evidence to suggest that dimension-specific etiological influences contribute to the overall presentation of OCD, although precisely how such influences manifest remains a topic for ongoing research. Gaining further clarity on this question may ultimately have important implications for the continued refinement of diagnostic and etiological models of OCD.
In a recent population-based twin study of OC-related disorders (OCRDs) and ADs symptoms, we demonstrated that the proportion of common genetic variance in OCD symptoms was higher when modeling with both groups of disorders, compared to when modeling OCRDs alone.[13] In other words, we did not observe a stronger genetic commonality between OCD symptoms and other OCRDs (hoarding disorder (HD), body dysmorphic disorder (BDD)) versus OCRDs and ADs (social phobia (SP), panic disorder (PD), and generalized AD (GAD))—a distinction that might be expected based on recent conceptualizations of OCRDs and ADs.[14] Instead, these results were more consistent with evidence from past multivariate twin studies, which have indicated OCD is influenced by moderately heritable genetic factors that are mostly shared with other OCRDs[15] and ADs.[16]
Considering our recent twin study findings, together with accumulating support for the “multidimensional model of OCD,” the aim of the current study was to investigate the structure of genetic and environmental influences between OC symptom dimensions and the symptoms of these five aforementioned OCRDs and ADs. These relationships have yet to be investigated in a multivariate twin study. Nevertheless, on the basis of existing evidence, we anticipated that harm/checking and sexual/religious symptoms, in particular, might demonstrate greater genetic overlap with the symptoms of ADs. This prediction is based on the neurobiological evidence linking these dimensions more closely to ADs,[9,11,12] as well as the generally higher rate of comorbidity between these dimensions and other ADs.[17]
MATERIAL AND METHODS
PARTICIPANTS AND MEASURES
Participants (aged 18–45) were recruited from the Australian Twin Registry (ATR) to complete an online survey. The final sample available for the study included 2,495 twins, 1,281 MZ, and 1,214 DZ twins (1,027 males and 1,468 females). Briefly, the sample contained 503 MZ pairs, 445 DZ pairs, and 599 twins without their cotwins (275 MZ; 324 DZ). All participants provided informed consent. The study was approved by the ATR and the Melbourne Health Human Research Ethics Committee (Victoria, Australia). Full recruitment details are provided in López-Solà` et al.[19]
OC symptom dimensions were assessed with the Obsessive– Compulsive Inventory–Revised (OCI-R)[18]: a widely validated self-report measure of OCD symptoms for use in general and clinical populations. The OCI-R is an 18-item questionnaire comprising six sub-scales to assess OC symptom dimensions, which are conventionally labeled as (1) “checking”—corresponding to harm-related obsessions and associated checking compulsions; (2) “obsessing”—corresponding to sexual/religious (forbidden/taboo) thoughts; (3) “washing”— corresponding to contaminations fears and associated cleaning compulsions; (4) “ordering”—corresponding to symmetry/order-related obsessions and compulsions; (5) “neutralizing”—corresponding to mental (i.e., counting/numeric) compulsions; (6) “hoarding”— corresponding to excessive acquisition/inability to discard. The OCI-R total score and subscales scores have demonstrated excellent psychometric properties, with the exception of the neutralizing subscale.[18] Because, neutralizing does not correspond to the most well-replicated symptom dimensions (in factor analytic studies), it was excluded from our analysis. Because HD symptoms were assessed with a specific scale (see below), the OCI-R hoarding dimension was excluded here. With respect to OCI-R cut-off scores, clinical levels of symptoms are suggested to correspond to scores higher than 3 on the washing subscale, scores higher than 5 on the obsessing and checking subscales, and scores higher than 7 on the ordering subscale.[18]
Five validated self-report measures were also used to assess other OCRD and AD symptoms (also detailed in López-Solà et al.[19]). For OCRDs, hoarding symptoms were assessed with the Hoarding Rating Scale-Self Report (HRS-SR)[20] and BDD symptoms with the Dysmorphic Concern Questionnaire (DCQ).[21] For ADs, SP symptoms were assessed with the Social Phobia Inventory (SPIN)[22]; PD symptoms with the Anxiety Sensitivity Index (ASI)[23]; and GAD symptoms with the “Stress” subscale of the Depression, Anxiety, and Stress Scale-21 (DASS-21).[24]
STATISTICAL ANALYSIS
To ensure data normality and to retain the maximum number of variables in their original continuous form, all questionnaire responses underwent Box–Cox transformations .[25] However, four OCI-R subscales could not be normalized using this method and were instead categorized using aforementioned cut-offs scores. Each was transformed into a three-category variable with two thresholds: for example, washing scores from 0 to 2 (category 0) represented non-clinical levels (i.e., no reported distress); scores from 2 to 3 (category 1) represented sub clinical levels, and scores above 3 (category 2) were indicative of clinical levels of OCD for this dimension. Because univariate twin modeling of this data indicated the presence of genetic sex differences in some of the scales,[19] and because standardized residuals could not be applied to the analysis of OCI-R subscale scores, all multivariate models were performed including age and sex as covariates. To address the study aims, we conducted four multivariate twin models, one for each of the OC dimensions. Each model therefore contained one ordinal and five continuous variables.
A series of structural equation models were fitted by maximum likelihood. Firstly, we tested a baseline saturated model in which all possible correlations were freely estimated. Next, genetic and environmental variance component models were estimated using classical multivariate twin models.[26] Model 1 is a fully saturated Cholesky decomposition that estimated one additive genetic (1A), one shared environment (1C), and one nonshared environment (1E) factor for each phenotype making no assumptions about the nature of their underlying covariance. Model 2 is an “independent pathway” (IP) model, which estimates a set of common Ac, Cc, and Ec factors to directly influence all phenotypes versus specific As, Cs, and Es factors that may explain remaining phenotypic variance. Model 3 corresponds to a “common pathway” (CP) model, which estimates whether the covariance among phenotypes was influenced via one latent factor taking into account the shared contribution of common A, C, and E factors. The Akaike information criterion (AIC) value was used to measure the relatively goodness of fit of these models, whereby the model with the lowest AIC was taken to be the most parsimonious. Reduced submodels were systematically tested to derive the most parsimonious model fitting results. For the most parsimonious model, confidence intervals (CIs) for the factor loadings at the path diagram were calculated to provide the best estimate for each parameter of the model. Extra analyses (Cholesky and IP models) were carried out using identical procedures to that explained above, but instead contrasting each of the main OC symptom dimensions with the OCRD and AD groups, separately (e.g., checking and OCRDs symptoms in one model versus checking and ADs symptoms in another model). It should be noted that each model estimates the parameters depending on the variables included, implying for instance, that the Ac factor for checking in the OCRD model will not be directly comparable to the Ac factor of checking in the ADs model. All analyses were carried out in R (http://www.R-project.org/) using the OpenMx 2.0 package.[27]
RESULTS
Cross-twin–cross-trait correlations in both groups (MZ and DZ) are presented in Table 1.
TABLE 1.
Cross-twin-cross-trait correlations in both groups of twins (MZ and DZ) for each OC dimension
| OC dimensions | Zygosity | SP | PD | GAD | BDD | HD |
|---|---|---|---|---|---|---|
| Checking | MZ | 0.23 | 0.21 | 0.29 | 0.33 | 0.15 |
| DZ | 0.16 | 0.21 | 0.15 | 0.07 | −0.001 | |
| Obsessing | MZ | 0.24 | 0.31 | 0.34 | 0.21 | 0.19 |
| DZ | 0.12 | 0.09 | 0.01 | 0.07 | 0.01 | |
| Washing | MZ | 0.23 | 0.27 | 0.19 | 0.23 | 0.14 |
| DZ | 0.04 | 0.10 | 0.10 | 0.08 | 0.04 | |
| Ordering | MZ | 0.16 | 0.13 | 0.20 | 0.19 | 0.09 |
| DZ | 0.13 | 0.15 | 0.18 | 0.05 | 0.01 |
MZ, monozygotic twins; DZ, dizygotic twins; SP, social phobia symptoms, PD, panic disorder symptoms; GAD, generalized anxiety disorder symptoms; BDD, body dysmorphic disorder symptoms; HD, hoarding disorder symptoms.
BEST-FITTING MODELS AND ESTIMATED FACTOR LOADINGS
Table 2 and Fig. 1 present results for the most parsimonious model for each of the OC symptom dimensions with the symptoms of ADs and OCRDs together. Figure 1 also displays the factor loadings (with CIs) for common and specific genetic and environmental influences estimated for each dimension. With reference to Table 2, the four symptom dimensions demonstrated best fit with the same single factor structure; namely, the IP model with ACE as common factors and AE as specific factors. Estimates for the best-fitting IP model are emphasized in bold text (Table 2). Figure 1 presents the values of the factor loadings for each dimension, indicating a unique pattern of genetic and environmental overlap with ADs and other OCRDs.
TABLE 2.
Model-fitting results for checking, obsessing, washing, and ordering controlled by age and gender
| Models | Estimated parameter |
Fit statistic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Common factors |
Specific factors |
−2LL | df | AIC | Δχ2 | Δdf | P value | Compared with model |
|
| Checking | |||||||||
| 1 Cholesky saturated | ACE | ACE | 26,369.5 | 14,889 | −3,408.49 | - | - | - | - |
| 2 IP | ACE | ACE | 26,424.7 | 14,916 | −3,407.28 | 55.2 | 27 | .001 | 1 |
| 3CP | ACE | ACE | 26,550.9 | 14,928 | −3,305.08 | 181.4 | 39 | <.0001 | 1 |
| 4 IP | ACE | AE | 26,431.1 | 14,922 | −3,412.87 | 6.42 | 6 | .38 | 2 |
| 5 IP | ACE | CE | 26,457.1 | 14,922 | −3,386.86 | 32.42 | 6 | <.0001 | 2 |
| 6 IP | ACE | E | 26,546.05 | 14,928 | −3,309.95 | 121.33 | 12 | <.0001 | 2 |
| 7 IP | AE | ACE | 26,443.16 | 14,922 | −3,400.84 | 18.45 | 6 | .005 | 2 |
| 8 IP | CE | ACE | 26,462.7 | 14,922 | −3,381.28 | 38.01 | 6 | <.0001 | 2 |
| 9 IP | E | ACE | 26,550.9 | 14,928 | −3,305.08 | 126.21 | 12 | <.0001 | 2 |
| Obsessing | |||||||||
| 1 Cholesky saturated | ACE | ACE | 26,470.77 | 14,889 | −3,307.23 | - | - | - | - |
| 2 IP | ACE | ACE | 26,501.49 | 14,916 | −3,330.51 | 30.72 | 27 | .283 | 1 |
| 3CP | ACE | ACE | 26,624.40 | 14,928 | −3,231.60 | 153.6 | 39 | <.0001 | 1 |
| 4 IP | ACE | AE | 26,501.96 | 14,922 | −3,342.04 | 0.475 | 6 | .998 | 2 |
| 5 IP | ACE | CE | 26,528.07 | 14,922 | −3,315.93 | 26.58 | 6 | .0002 | 2 |
| 6 IP | ACE | E | 26,612.21 | 14,928 | −3,243.79 | 110.72 | 12 | <.0001 | 2 |
| 7 IP | AE | ACE | 26,526.10 | 14,922 | −3,317.89 | 24.62 | 6 | .0004 | 2 |
| 8 IP | CE | ACE | 26,542.30 | 14,922 | −3,301.71 | 40.81 | 6 | <.0001 | 2 |
| 9 IP | E | ACE | 26,624.40 | 14,928 | −3,231.60 | 122.92 | 12 | <.0001 | 2 |
| Washing | |||||||||
| 1 Cholesky saturated | ACE | ACE | 26,625.44 | 14,889 | −3,152.56 | - | - | - | - |
| 2 IP | ACE | ACE | 26,646.16 | 14,916 | −3,185.84 | 20.72 | 27 | .799 | 1 |
| 3CP | ACE | ACE | 26,759.26 | 14,928 | −3,096.74 | 133.81 | 39 | <.0001 | 1 |
| 4 IP | ACE | AE | 26,646.16 | 14,922 | −3,197.84 | 0.0002 | 6 | 1 | 2 |
| 5 IP | ACE | CE | 26,679.85 | 14,922 | −3,164.15 | 33.7 | 6 | <.0001 | 2 |
| 6 IP | ACE | E | 26,761.82 | 14,928 | −3,094.18 | 115.6 | 12 | <.0001 | 2 |
| 7 IP | AE | ACE | 26,661.07 | 14,922 | −3,182.93 | 14.9 | 6 | .02 | 2 |
| 8 IP | CE | ACE | 26,677.20 | 14,922 | −3,166.80 | 31.04 | 6 | <.0001 | 2 |
| 9 IP | E | ACE | 26,759.26 | 14,928 | −3,096.74 | 113.1 | 12 | <.0001 | 2 |
| Ordering | |||||||||
| 1 Cholesky saturated | ACE | ACE | 26,289.1 | 14,889 | −3,488.91 | - | - | - | - |
| 2 IP | ACE | ACE | 26,321.4 | 14,916 | −3,510.63 | 32.28 | 27 | .22 | 1 |
| 3CP | ACE | ACE | 26,439.5 | 14,928 | 3,416.51 | 150.39 | 39 | <.0001 | 1 |
| 4 IP | ACE | AE | 26,321.4 | 14,922 | −3,522.57 | 0.06 | 6 | .999 | 2 |
| 5 IP | ACE | CE | 26,348.5 | 14,922 | −3,495.49 | 27.14 | 6 | .0001 | 2 |
| 6 IP | ACE | E | 26,437.7 | 14,928 | −3,418.32 | 116.31 | 12 | <.0001 | 2 |
| 7 IP | AE | ACE | 26,343.9 | 14,922 | −3,500.10 | 22.53 | 6 | .0007 | 2 |
| 8 IP | CE | ACE | 26,358.9 | 14,922 | −3,485.12 | 37.5 | 6 | <.0001 | 2 |
| 9 IP | E | ACE | 26,439.5 | 14,928 | −3,416.51 | 118.12 | 12 | <.0001 | 2 |
−2LL, minus twice the log-likelihood; df, degrees of freedom; AIC, Akaike information criterion; Δχ2, difference in goodness-of-fit statistic between the submodel and the full model; Δdf, change in degrees of freedom between the submodel and the full model; A, additive genetic factor; C, shared environmental factor; E, nonshared environmental factor; IP, independent pathway; CP, common pathway.
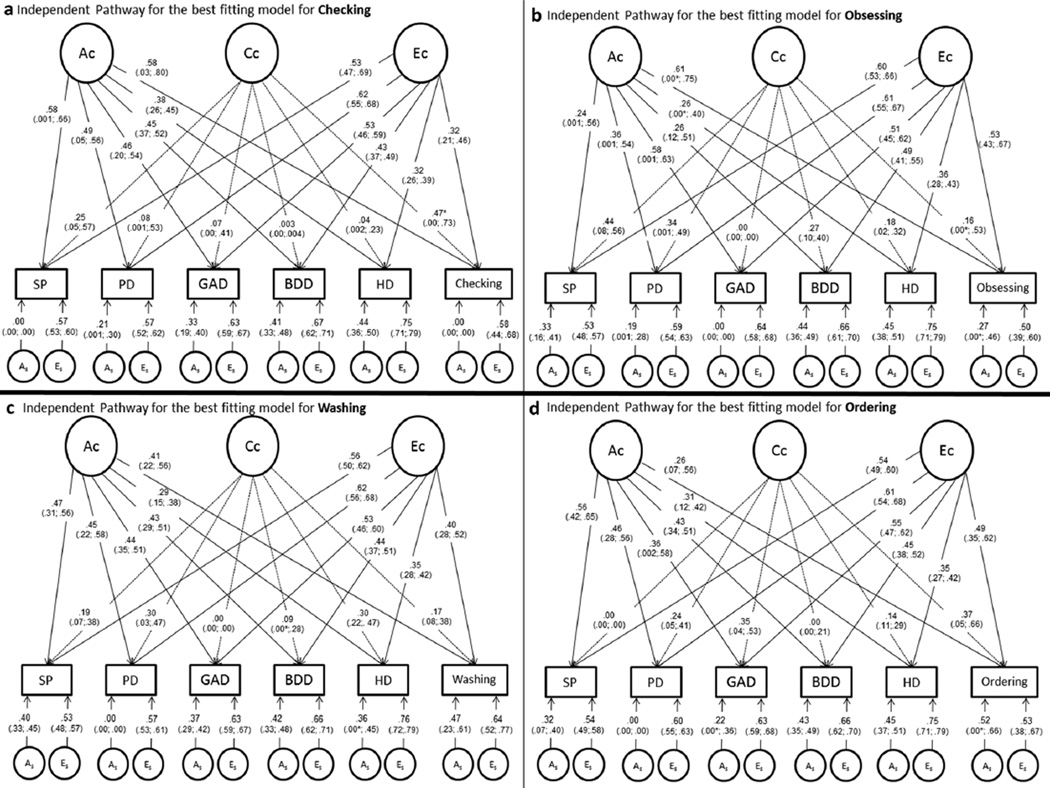
Figure 1.
Path diagrams (standardized factor loadings and confidence intervals) for the best-fitting independent pathway model for each obsessive–compulsive dimension.
Ac, common additive genetic factor; Cc, common shared environmental factor; Ec, common nonshared environmental factor; As, specific additive genetic factor; Es, specific nonshared environmental factor; HD, hoarding disorder symptoms; BDD, body dysmorphic disorder symptoms; PD, panic disorder symptoms; GAD, generalized anxiety disorder symptoms; SP, social phobia disorder symptoms
*The lower CI could not be reliably estimated.
Figure 1a presents results for checking symptoms and indicates that these symptoms share all genetic factor influences (λgc = 0.58) with ADs and OCRDs, while specific genetic factors were zero. This result implies that 100% of the genetic variance in checking symptoms is accounted for by the common genetic factor. Shared environmental influences also emerged as relatively important (λcc = 0.47) in the expression of checking symptoms.
Figure 1b presents the best-fitting model for obsessing symptoms and indicates that these symptoms share higher common genetic factor influences (Ac) (λgc = 0.61) with ADs and OCRDs compared to specific genetic influences (As) (λgs = 0.27). In other words, 84% of the genetic variance in obsessing symptoms is accounted for by the common genetic factor. Shared environmental influences (Cc) were very low (λcc = 0.16) between obsessing symptoms and the other domains, and were not significant.
Figure 1c presents results for washing symptoms, which demonstrated a similar proportion of common (λgc = 0.41) and specific genetic influences (λgs = 0.47). Accordingly, 43% of the genetic variance in washing symptoms was accounted for by the common genetic factor. Shared environmental influences between washing symptoms and the other domains were very low (λcc = 0.17).
Figure 1d presents results for ordering symptoms and indicates that these symptoms have weaker common genetic factor influences (λgc =0.26)with ADs and OCRDs compared to specific genetic influences (λgs = 0.52). For ordering symptoms, only 20% of the genetic variance is accounted for by the common genetic factor. Shared environmental influences also emerged as relatively important in the expression of ordering symptoms (λcc = 0.37).
ESTIMATED GENETIC INFLUENCES
In relation to the total genetic variance, standardized parameters for each OC dimension confirmed that obsessing (84%) and checking (100%) showed the highest percentage of common genetic variance with ADs and other OCRDs. Washing (43%) shared almost half of its genetic variance with these domains, while ordering had the lowest percentage of common genetic variance (20%).
Because DSM-5 endorses the idea that OCD and its dimensions are more etiologically aligned with the OCRDs, we conducted separate multivariate analyses (one for each OC dimension) with two Ac latent factors: one loading on all symptom domains and another loading only on each OC symptom dimension, HD and BDD symptoms. However, the standardized parameters of these models do not add significant information to the simplified model with only one Ac factor and therefore will not be reported further (available upon request).
In order to estimate more precisely the genetic covariance between each OC dimension and the other symptom domains (ADs and OCRDs, respectively), additional multivariate analyses were performed, which compared each OC dimension with the ADs and OCRDs alone. The following tables present the results of the most parsimonious model in a different but informative way compare to the results presented above.
Table 3 presents equivalent model estimates results for checking symptoms. Checking demonstrated around 8% of the total variance due to common genetic factors shared with ADs alone. When estimating its overlap with OCRDs alone, the percentage of shared genetic influence was higher (26%), with the strongest association being observed with BDD symptoms. These results can be compared to an estimated shared genetic influence of 34% (λgc = 0.58, squared is approximately 0.34) when the ADs and OCRDs were modeled together. These results suggest that checking shares stronger common genetic influence with ADs and OCRDs, although a relatively strong common influence was seen with BDD symptoms. Interestingly, BDD shared 100% of its genetic variance only with checking and not with any other OC symptom dimension.
TABLE 3.
Checking dimension with anxiety disorders and checking dimension with obsessive-compulsive related disorders: best-fitting models and standardized parameters
| Estimated parameter |
Fit statistic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Common factors | Specific factors | −2LL | df | AIC | χ2 | Δdf | P value | |
| Checking, social phobia, panic, and generalized anxiety disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 22,792.9 | 9,938 | 2,916.95 | - | - | - | |
| 2 IP | ACE | AE | 22,800.4 | 9,948 | 2,904.43 | 7.48 | 10 | .68 | |
| Checking, body dysmorphic, and hoarding disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 6,568.7 | 7,458 | −8,347.24 | - | - | - | |
| 2 IP | ACE | AE | 6,569.5 | 7,461 | −8,352.51 | 0.727 | 3 | .87 | |
| Standardized parameters for each best-fitting model number 2 (95% CI) | |||||||||
| Additive genetic (A) |
Shared environment (C) |
Nonshared environment (E) |
|||||||
| Common | Specific | Total A | Common | Specific | Total C | Common | Specific | Total E | |
| SP | 0.26(0.10–0.38) | 0.14(0.003–0.21) | 0.40(0.25–0.47) | 0.01 (0.00–0.14) | - | 0.01(0.00–0.14) | 0.29 (0.23–0.36) | 0.29 (0.24–0.34) | 0.59(0.52–0.65) |
| PD | 0.25(0.06–0.36) | 0.02 (0.00–0.08) | 0.27(0.10–0.36) | 0.03 (0.00–0.17) | - | 0.03(0.00–0.17) | 0.39(0.31–0.48) | 0.31 (0.25–0.36) | 0.70 (0.63–0.78) |
| GAD | 0.14(0.02–0.26) | 0.13 (0.00–0.18) | 0.27 (0.12–0.38) | 0.05(0.00–0.17) | - | 0.05(0.00–0.17) | 0.28(0.21–0.36) | 0.39(0.34–0.45) | 0.68 (0.61–0.75) |
| Checking | 0.08(0.00–0.56) | 0.03 (0.00–0.36) | 0.11 (0.00–0.64) | 0.41 (0.00–0.59) | - | 0.41 (0.00–0.59) | 0.14(0.06–0.24) | 0.34(0.23–0.47) | 0.48 (0.33–0.63) |
| BDD | 0.37(0.12–0.44) | 0.00 (0.00–0.24) | 0.37(0.29–0.44) | 0.001 (0.00–0.05) | - | 0.001 (0.0–0.05) | 0.06(0.02–0.12) | 0.57 (0.50–0.65) | 0.63 (0.56–0.70) |
| HD | 0.06(0.03–0.25) | 0.25(0.005–0.32) | 0.31 (0.18–0.38) | 0.0005(0.0–0.11) | - | 0.0005(0.00–0.11) | 0.49(0.25–0.75) | 0.19(0.00–0.43) | 0.68 (0.62–0.75) |
| Checking | 0.26(0.07–0.56) | 0.00(0.00–0.18) | 0.26(0.07–0.56) | 0.31 (0.06–0.51) | - | 0.31(0.06–0.51) | 0.15(0.06–0.32) | 0.27(0.13–0.41) | 0.42 (0.30–0.56) |
−2LL, minus twice the log-likelihood; df, degrees of freedom; AIC, Akaike information criterion; χ2, difference in goodness-of-fit statistic between the submodel and the full model; Δdf, change in degrees of freedom between the submodel and the full model; A, additive genetic factor; C, shared environmental factor; E, nonshared environmental factor; IP, independent pathway; CI, confidence intervals; HD, hoarding disorder symptoms; BDD, body dysmorphic disorder symptoms; PD, panic disorder symptoms; GAD, generalized anxiety disorder symptoms; SP, social phobia disorder symptoms.
At the bottom of the table is presented the standardized parameters for each best-fitting model (checking + ADs and checking + OCRDs). Total A, C, and E do not sum to 1 due to rounding. Each parameter can be multiplied by 100 to obtain the percentage (e.g., SP shows 40% of total A, 1% of total C, and 59% of total E).
Results presented at the bottom of Table 4 detail the percentages of common and specific genetic and environmental influence for obsessing symptoms and ADs and, separately, for obsessing symptoms and OCRDs. For obsessing, 46% of the total variance was due to common genetic influences with ADs alone (100% of its genetic variance), while the specific additive genetic component emerged as nonsignificant (Table 4). PD and GAD symptoms shared 100% and 66%, respectively, of their genetic variance with obsessing symptoms. When estimating its covariance with OCRDs alone, the percentage of variance due to shared genetic influence decreased to 21%. These results can be compared to an estimated shared genetic influence of 37% when the ADs and OCRDs were modeled together. These results suggest that obsessing symptoms have a stronger common genetic correlation with ADs than with OCRDs symptoms.
TABLE 4.
Obsessing dimension with anxiety disorders and obsessing dimension with obsessive-compulsive-related disorders: best-fitting models and standardized parameters
| Estimated parameter |
Fit statistic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Common factors | Specific factors | −2LL | df | AIC | χ2 | Δdf | P value | |
| Obsessing, social phobia, panic, and generalized anxiety disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 22,852.2 | 9,938 | 2,976.2 | - | - | - | |
| 2 IP | ACE | AE | 22,862.6 | 9,948 | 2,966.6 | 10.41 | 10 | .41 | |
| Obsessing, body dysmorphic, and hoarding disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 6,878.7 | 7,458 | −8,037.3 | - | - | - | |
| 2 IP | ACE | AE | 6,878.7 | 7,461 | −,043.3 | 0.00003 | 3 | 1 | |
| Standardized parameters for each best-fitting model number 2 (95% CI) | |||||||||
| Additive genetic (A) |
Shared environment (C) |
Nonshared environment (E) |
|||||||
| Common | Specific | Total A | Common | Specific | Total C | Common | Specific | Total E | |
| SP | 0.08 (0.00–0.24) | 0.15(0.08–0.21) | 0.24(0.10–0.42) | 0.14(0.008–0.26) | - | 0.14(0.008–0.26) | - | 0.28 (0.23–0.34) | 0.62 (0.55–0.68) |
| PD | 0.13 (0.02–0.30) | 0.007 (0.00–0.06) | 0.13 (0.01–0.30) | 0.14(0.01–0.25) | - | 0.14(0.01–0.25) | 0.40(0.32–0.48) | 0.33 (0.27–0.38) | 0.72 (0.65–0.79) |
| GAD | 0.20 (0.07–0.40) | 0.10(0.00–0.15) | 0.30(0.17–0.40) | 0.03 (0.00–0.13) | - | 0.03 (0.00–0.13) | 0.28(0.22–.35) | 0.39 (0.34–0.44) | 0.67 (0.60–0.74) |
| Obsessing | 0.46(0.15–0.60) | 0.00 (0.00–0.00) | 0.46 (0.27–0.60) | 0.01 (0.00–0.15) | - | 0.01 (0.00–0.15) | 0.27(0.18–0.38) | 0.25(0.16–0.37) | 0.52 (0.40–0.67) |
| BDD | 0.18(0.07–0.41) | 0.18(0.00–0.30) | 0.36 (0.26–0.43) | 0.00 (0.00–0.07) | - | 0.00 (0.00–0.07) | 0.22(0.13–0.36) | 0.41 (0.28–0.51) | 0.63 (0.56–0.71) |
| HD | 0.13 (0.03–0.34) | 0.16(0.00–0.28) | 0.29(0.09–0.38) | 0.03 (0.00–0.19) | - | 0.03 (0.00–0.19) | 0.13 (0.07–0.21) | 0.56(0.47–0.64) | 0.69 (0.61–0.76) |
| Obsessing | 0.21 (0.06–0.53) | 0.25 (0.00–0.41) | 0.46 (0.23–0.61) | 0.01 (0.00–0.19) | - | 0.01 (0.00–0.19) | 0.34(0.19–0.56) | 0.18(0.00–0.36) | 0.52 (0.39–0.68) |
−2LL, minus twice the log-likelihood; df, degrees of freedom; AIC, Akaike information criterion; χ2, difference in goodness-of-fit statistic between the submodel and the full model; Δdf, change in degrees of freedom between the submodel and the full model; A, additive genetic factor; C, shared environmental factor; E, nonshared environmental factor; IP, independent pathway; CI, confidence intervals; HD, hoarding disorder symptoms; BDD, body dysmorphic disorder symptoms; PD, panic disorder symptoms; GAD, generalized anxiety disorder symptoms; SP, social phobia disorder symptoms.
At the bottom of the table is presented the standardized parameters for each best-fitting model (obsessing + ADs and obsessing + OCRDs). Total A, C, and E do not sum to 1 due to rounding. Each parameter can be multiplied by 100 to obtain the percentage (e.g., SP shows 24% of total A, 14% of total C, and 62% of total E).
Table 5 presents results for the washing symptoms. Washing demonstrated 32% of the total variance due to common genetic factors shared with ADs alone (around 76% of its total genetic variance), while when estimating its covariance with OCRDs alone, the percentage of shared genetic influence was 20%. These results can be compared to an estimated shared genetic influence of 17% of the total variance when the ADs and OCRDs were modeled together. These results suggest that washing symptoms have a stronger common genetic correlation with ADs versus OCRDs symptoms.
TABLE 5.
Washing dimension with anxiety disorders and washing dimension with obsessive-compulsive related disorders: best-fitting models and standardized parameters
| Estimated parameter |
Fit statistic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Common factors | Specific factors | −2LL | df | AIC | χ2 | Δdf | P value | |
| Washing, social phobia, panic, and generalized anxiety disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 23,004.4 | 9,938 | 3,128.4 | - | - | - | |
| 2 IP | ACE | AE | 23,005.6 | 9,948 | 3,109.6 | 1.23 | 10 | .999 | |
| Washing, body dysmorphic, and hoarding disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 6,841.1 | 7,458 | −8,074.9 | - | - | - | |
| 2 IP | ACE | AE | 6,841.1 | 7,461 | −8,080.9 | −0.0001 | 3 | 1 | |
| Standardized parameters for each best-fitting model number 2 (95% CI) | |||||||||
| Additive genetic (A) |
Shared environment (C) |
Nonshared environment (E) |
|||||||
| Common | Specific | Total A | Common | Specific | Total C | Common | Specific | Total E | |
| SP | 0.19(0.04–0.33) | 0.16(0.09–0.21) | 0.35(0.19–0.46) | 0.06(0.00–0.19) | - | 0.06(0.00–0.19) | 0.31 (0.25–0.38) | 0.28 (0.24–0.34) | 0.59(0.53–0.66) |
| PD | 0.27 (0.09–0.37) | 0.00 (0.00–.05) | 0.27 (0.09–0.37) | 0.03 (0.00–0.18) | - | 0.03 (0.00–0.18) | 0.38(0.31–0.46) | 0.32 (0.27–0.36) | 0.70 (0.63–0.77) |
| GAD | 0.16(0.05–0.24) | 0.15(0.10–0.20) | 0.30(0.18–0.39) | 0.02(0.00–0.12) | - | 0.02 (0.00–0.12) | 0.28(0.22–0.35) | 0.39 (0.34–0.45) | 0.67 (0.61–0.75) |
| Washing | 0.32 (0.05–0.58) | 0.10(0.00–0.34) | 0.42(0.11–0.60) | 0.02 (0.00–0.25) | - | 0.02 (0.00–0.25) | 0.14(0.06–0.24) | 0.43 (0.28–0.59) | 0.56 (0.40–0.75) |
| BDD | 0.26(0.13–0.43) | 0.10(0.00–0.24) | 0.36 (0.27–0.43) | - | 0.00 (0.00–0.06) | 0.00 (0.00–0.06) | 0.15(0.06–0.31) | 0.49(0.33–0.59) | 0.63 (0.57–0.71) |
| HD | 0.08 (0.03–0.17) | 0.24(0.04–0.31) | 0.32 (0.12–0.39) | - | 0.00(0.00–0.15) | 0.00(0.00–0.15) | 0.20 (0.09–0.47) | 0.48 (0.22–0.61) | 0.68 (0.61–0.76) |
| Washing | 0.20 (0.06–0.42) | 0.26 (0.00–0.45) | 0.46(0.16–0.62) | - | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.15(0.05–0.32) | 0.39(0.22–0.59) | 0.54(0.38–0.73) |
−2LL, minus twice the log-likelihood; df, degrees of freedom; AIC, Akaike information criterion; χ2, difference in goodness-of-fit statistic between the submodel and the full model; Δdf, change in degrees of freedom between the submodel and the full model; A, additive genetic factor; C, shared environmental factor; E, nonshared environmental factor; IP, independent pathway; CI, confidence intervals; HD, hoarding disorder symptoms; BDD, body dysmorphic disorder symptoms; PD, panic disorder symptoms; GAD, generalized anxiety disorder symptoms; SP, social phobia disorder symptoms.
At the bottom of the table is presented the standardized parameters for each best-fitting model (washing + ADs and washing + OCRDs). Total A, C, and E do not sum to 1 due to rounding. Each parameter can be multiplied by 100 to obtain the percentage (e.g., SP shows 35% of total A, 6% of total C, and 59% of total E).
Table 6 presents results for ordering symptoms. Ordering demonstrated less than 1% of the total variance due to common genetic factors shared with ADs alone. When estimating its covariance with OCRDs alone, the percentage of shared genetic influence was higher (18%), but did not surpass the estimate of specific genetic variance (As = 24%). These results can be compared to an estimated shared genetic influence of 7% when the ADs and OCRDs were modeled together. These results suggest that ordering has stronger genetic correlation with OCRDs versus ADs symptoms, although it also displays more prominent specific genetic influences.
TABLE 6.
Ordering dimension with anxiety disorders and ordering dimension with obsessive-compulsive related disorders: best-fitting models and standardized parameters
| Estimated parameter |
Fit statistic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Common fectors | Specific fectors | −2LL | df | AIC | χ2 | Δdf | P value | |
| Ordering, social phobia, panic, and generalized anxiety disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 22,681.7 | 9,938 | 2,805.7 | - | - | - | |
| 2 IP | ACE | AE | 22,685.3 | 9,948 | 2,789.3 | 3.66 | 10 | .96 | |
| Ordering, body dysmorphic, and hoarding disorder | |||||||||
| 1 Cholesky saturated | ACE | ACE | 7,954.6 | 7,458 | −6,961.4 | - | - | - | |
| 2 IP | ACE | AE | 7,954.6 | 7,461 | −6,967.4 | 0.00 | 3 | 1 | |
| Standardized parameters for each best-fitting model number 2 (95% CI) | |||||||||
| Additive genetic (A) |
Shared environment (C) |
Nonshared environment (E) |
|||||||
| Common | Specific | Total A | Common | Specific | Total C | Common | Specific | Total E | |
| SP | 0.21 (0.05–0.33) | 0.16(0.09–0.21) | 0.37(0.21–0.47) | 0.04(0.00–0.17) | - | 0.04(0.00–0.17) | 0.30(0.24–0.37) | 0.29 (0.24–0.34) | 0.59(0.53–0.66) |
| PD | 0.26 (0.08–0.36) | 0.00 (0.00–0.06) | 0.26 (0.08–0.36) | 0.04(0.00–0.18) | - | 0.04(0.00–0.18) | 0.38(0.31–0.46) | 0.32(0.27–0.37) | 0.70 (0.63–0.77) |
| GAD | 0.10(0.002–0.30) | 0.11 (0.00–0.17) | 0.21 (0.06–0.36) | 0.10(0.00–0.22) | - | 0.10(0.00–0.22) | 0.30(0.23–0.37) | 0.39(0.33–0.45) | 0.69 (0.62–0.76) |
| Ordering | 0.009 (0.00–0.30) | 0.02(0.00–0.35) | 0.02 (0.00–0.50) | 0.39(0.02–0.53) | - | 0.39(0.02–0.53) | 0.26(0.13–0.40) | 0.33 (0.18–0.49) | 0.59(0.41–0.75) |
| BDD | 0.23 (0.07–0.42) | 0.13 (0.00–0.27) | 0.36(0.25–0.43) | 0.003 (0.00–0.08) | - | 0.003(0.00–0.08) | 0.15(0.08–0.24) | 0.49(0.39–0.57) | 0.64(0.56–0.71) |
| HD | 0.11 (0.02–0.34) | 0.18(0.00–0.30) | 0.29(0.11–0.38) | 0.02(0.00–0.17) | - | 0.02(0.00–0.17) | 0.20(0.11–0.34) | 0.49(0.35–0.59) | 0.69 (0.62–0.76) |
| Ordering | 0.18(0.03–0.46) | 0.24 (0.00–0.40) | 0.42(0.12–0.56) | 0.02 (0.00–0.25) | - | 0.02(0.00–0.25) | 0.21(0.10–0.36) | 0.35(0.21–0.49) | 0.56 (0.44–0.69) |
−2LL, minus twice the log-likelihood; df, degrees of freedom; AIC, Akaike information criterion; χ2 , difference in goodness-of-fit statistic between the submodel and the full model; Adf, change in degrees of freedom between the submodel and the full model; A, additive genetic factor; C, shared environmental factor; E, nonshared environmental factor; IP, independent pathway; CI, confidence intervals; HD, hoarding disorder symptoms; BDD, body dysmorphic disorder symptoms; PD, panic disorder symptoms; GAD, generalized anxiety disorder symptoms; SP, social phobia disorder symptoms.
At the bottom of the table is presented the standardized parameters for each best-fitting model (ordering + ADs and ordering + OCRDs). Total A, C, and E do not sum to 1 due to rounding. Each parameter can be multiplied by 100 to obtain the percentage (e.g., SP shows 37% of total A, 4% of total C, and 59% of total E).
In summary, with regards to genetic influences, (1) checking was found to be genetically associated with BDD and AD symptoms; (2) obsessing and washing demonstrated the highest genetic association with AD symptoms; while (3) symmetry demonstrated the highest degree of genetic specificity.
ESTIMATED ENVIRONMENTAL INFLUENCES
As shown in Tables 3 and 6, only checking and ordering demonstrated relevant findings regarding common environmental influences (zero or close to zero Cc factor loadings were obtained for obsessing and washing; Tables 4 and 5, respectively). Checking had an increased percentage of common environmental influence when assessed with ADs alone (41%) and OCRDs alone (31%), versus the full model with ADs and OCRDs together (22% of the total variance). With respect to ordering, the common environmental factor increased to 39% when assessed in relation to ADs alone, whereas the additive genetic factor (either common or specific) decreased almost to zero. In summary, these results indicate that checking shares common environmental influences with OCRDs and ADs, whereas ordering shares common environmental influences with ADs alone.
DISCUSSION
The current study supports the idea that OCD is both clinically and etiologically heterogeneous. Three main conclusions can be drawn from its findings. First, obsessing and washing symptoms had the highest genetic correlations with the symptoms of ADs. Second, ordering was the highest genetic correlation with HD and BDD symptoms, but shared common environmental influences with Ads .Third, common genetic influences on checking symptoms were best estimated when modeling OCRDs (in particular BDD symptoms) together with ADs, rather than when modeling either group alone. In summary, important shared genetic and environmental risk factors exist between OCD, OCRDs, and ADs, but which vary alongside the expression of its major symptom dimensions.
GENETIC INFLUENCES
Checking
Checking symptoms were found to share genetic factors with the symptoms of both ADs and OCRDs, but in particular with BDD. This result did not support the original study prediction that checking would be the OC symptom dimension most closely associated with AD symptoms only. Considering that checking, compared to other symptom dimensions, is predictive of OCD diagnosis as a whole,[28] this general pattern of findings is consistent with our previous study where the common genetic liability to OCD symptoms was higher when modeling both ADs and OCRDs compare to either group alone.[13] Checking symptoms have been previously linked to comorbid ADs,[17] as well as BDD.[29] It has also been demonstrated that OCD patients with comorbid BDD have increased aggressive/checking, symmetry, and reassurance-seeking severity.[29] BDD patients also demonstrate compulsively checking behaviors,[29] which supports the genetic correlation between BDD and checking symptoms observed here.
Obsessing
Obsessing symptom demonstrated the strongest estimated genetic association with ADs. Although we anticipated this relationship as a broad study prediction on the basis of other work by our group,[12] it nonetheless appears to be a novel finding. One previous twin study provides indirect support for this finding, having demonstrated genetic overlap between obsessing symptoms (i.e., forbidden thoughts) and neuroticism[30]—the latter being strongly linked to mood and ADs.[31] Obsessing, aggressive, and somatic symptoms have also been reported to demonstrate higher rates of comorbidity with ADs (GAD, panic/agoraphobia, and SP),[17] which fits with the pattern of findings here. One potential explanation is that obsessive thoughts represent a general cognitive bias toward the anticipation of possible threating events, including the self-censorship of one’s own behavior.
Washing
Washing was more genetically associated with the symptoms of ADs compared to OCRDs. Clinical and epidemiological studies have reported a consistent association between washing symptoms and comorbid depression and ADs,[32] which is consistent with our results. One potential linking factor between washing symptoms and ADs is disgust sensitivity—an emotional state associated with avoidance behavior of disgusting-threating stimuli.[33, 34] However, such associations needs to be examined further, particularly as other studies have reported a presence of comorbid OC spectrum disorders in association with washing symptoms.[33]
Ordering
The genetic correlation of ordering was greater with OCRDs than with ADs. This result is consistent with one recent study of female twin pairs, which reported that ordering and obsessing were the OC symptom dimensions most strongly genetically associated with BDD symptoms.[35] Clinical studies have also documented that OCD patients with ordering symptoms display higher comorbidity with OCRDs, such as HD,[36] and that a substantial proportion of patients with BDD exhibit marked appearance-related symmetry concerns.[37]
Ordering also demonstrated a relatively high proportion of specific genetic influences, which is interesting in view of molecular genetic studies that have reported distinct relationships candidate polymorphisms of monoaminergic system genes in OCD patients and in the severity of symmetry/ordering symptoms.[38] Thus, it is possible that OCD patients with prominent ordering symptoms, together with these other features, may represent a distinct phenotype of OCD.[39]
ENVIRONMENTAL INFLUENCES
Our results indicated that checking shares common environmental influences with OCRDs and ADs, whereas ordering was more strongly linked with ADs. Although the influence of stressful life events is widely recognized as a general etiological factor in the development of psychiatric disorders,[40] few studies have identified which life events may consistently contribute to the manifestation of OCD, ADs, and other OCRDs. In one study, perinatal insults were identified as a risk factor to ADs and OCD with prominent ordering symptoms[41]— such factors have not been explored in relation to HD and BDD. Thus, while perinatal events, psychosocial stressors, trauma, and inflammatory processes have been linked generally to the development of OCD,[42,43] little remains known about their specific contribution to OC symptom dimensions, or other OCRDs.
LIMITATIONS
Certain limitations should be acknowledged. First, all symptoms were assessed by self-report measures, which imperfectly align with the diagnostic criteria for ADs and OCRDs. For example, the DASS-Stress subscale is not a direct measure of “worry”—a principal construct thought to underlie GAD. However, it has been successfully used to predict the presence of GAD akin to other commonly used measures such as the Penn State Worry Questionnaire (PSWQ).[44] These measures are also mixed in terms of their emphasis on current (OCI-R, DASS, SPIN) versus lifetime symptoms (ASI, DCQ, HRS), which may impact on the generalizability of findings. Second, despite the good psychometric properties of the OCI-R, it provides only a brief assessment of OC symptom dimensions (three items per domain) compared to other measures available. It will be important to replicate the current findings in future studies that include broader assessments of OC symptom dimensions. Third, we were unable to reliably estimate some CIs for all the parameters in the full model (Fig. 1) due to the complex nature of the multivariate models, which may reflect limited sample size despite the large number of participants included in our study. Finally, the OCRD group was not fully assessed due to low response rates for the skin-picking and hair-pulling self-report questionnaires. These questionnaires were only completed if participants first endorsed some screening questions.[19] Nevertheless, the inclusion of BDD and HD was illuminating, particularly the observed associations between OC symptom dimensions and BDD symptoms. Our results also suggest that HD is genetically quite specific, despite some etiological overlap with obsessing symptoms.
CONCLUSIONS
The results from this study may have (1) nosological, (2) clinical, and (3) biological implications for understanding of OCD and its symptoms dimensions. In nosological terms, although DSM-5 has endorsed a separation between OCD and ADs, our results are more consistent with previous proposals that OCRDs and ADs should be merged as an overarching diagnostic concept.[45,46] The current study adds another layer of support to this idea by demonstrating that OC symptom dimensions comprise etiological factors that clearly overlap with AD symptoms.
One clinical implication of the current work is that OCD patients with prominent ordering symptoms should also be evaluated for the presence of other OCRDs, particularly hoarding, considering the genetic commonalities observed here. This suggestion also takes into account previous research linking ordering symptoms and tic-related disorders. Similarly, in the case of OCD patients with prominent checking, obsessing, and/or washing symptoms, the presence of other ADs should be carefully evaluated, and additionally, with respect to checking symptoms, the presence of BDD should be considered. In other words, a more holistic clinical approach may facilitate earlier detection and treatment, and potentially help to minimize the risk factors associated with overlapping conditions.
Finally, regarding biological implications, the current results appear to endorse a view that OCRDs and ADs are perhaps best understood as the manifestation of developmentally mediated neural processes whereby innate and learned responses to common threat and safety/reward cues (or signals) become dysregulated and expressed as excessive forms of avoidance and/or approach behaviors. This hypothesis partially aligns with existing neurobiological models of OCD and ADs—which intersect anatomically, particularly in their emphasis on the role of ventral prefrontal cortical brain systems, encompassing both ventromedial and orbitofrontal cortices.[10,47] Indeed, there is little or no evidence from neurobiological studies to suggest that OCRDs and ADs can be reliably distinguished at the level of brain systems. Experimentally, cross-diagnostic studies of fear and safety/reward learning and their contextual modulation (e.g., social/nonsocial) would now be particularly interesting and potentially lead to the identification of core neurobiological domains of function (or dysfunction) with greater explanatory power on the common comorbidity of these disorders and their major symptom dimensions.
Acknowledgments
Contract grant sponsor: The University of Melbourne; Contract grant sponsor: National Health and Medical Research Council of Australia (NHMRC) Clinical Career Development Fellowship; Contract grant number: 628509; Contract grant sponsor: Spanish Ministry of Education, Culture and Sport; Contract grant number: FPU12/01636; Contract grant sponsor: NIDA; Contract grant number: DA-026119; Contract grant sponsor: Instituto de Salud Carlos III cofunded by FEDER funds/European Regional Development Fund (ERDF) - a way to build Europe. Contract numbers: ISCCIII-PI13/01958 and PI14/00413; Contract grant sponsor: National Health and Medical Research Council; Contract grant number: 628911.
This research was facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911)from the National Health and Medical Research Council. We extend considerable thanks to staff at the Australian Twin Registry and all participating twins for their valuable contribution to this study.
Footnotes
Conflict of interest. All of the co-authors have no conflicts of interest to declare.
REFERENCES
- 1.Mataix-Cols D, Rosario-Campos MC do, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- 2.Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. 2008;165:1532–1542. doi: 10.1176/appi.ajp.2008.08020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasler G, Pinto A, Greenberg BD, et al. Familiality of factor analysis-derived YBOCS dimensions in OCD-affected sibling pairs from the OCD Collaborative Genetics Study. Biol Psychiatry. 2007;61:617–625. doi: 10.1016/j.biopsych.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 4.Katerberg H, Delucchi KL, Stewart SE, et al. Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behav Genet. 2010;40:505–517. doi: 10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leckman JF, Denys D, Simpson HB, et al. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety. 2010;27:507–527. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuels J, Bienvenu OJ, Riddle MA, et al. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther. 2002;40:517–528. doi: 10.1016/s0005-7967(01)00026-2. [DOI] [PubMed] [Google Scholar]
- 7.Mataix-Cols D, Rauch SL, Baer L, et al. Symptom stability in adult obsessive-compulsive disorder: data from a naturalistic two-year follow-up study. Am J Psychiatry. 2002;159:263–268. doi: 10.1176/appi.ajp.159.2.263. [DOI] [PubMed] [Google Scholar]
- 8.Rufer M, Fricke S, Moritz S, Kloss M, Hand I. Symptom dimensions in obsessive-compulsive disorder: prediction of cognitive-behavior therapy outcome. Acta Psychiatr Scand. 2006;113:440–446. doi: 10.1111/j.1600-0447.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 9.Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 10.Harrison BJ, Pujol J, Cardoner N, et al. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry. 2013;73:321–328. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel OA, Remijnse PL, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 12.Via E, Cardoner N, Pujol J, et al. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br J Psychiatry. 2014;204:61–68. doi: 10.1192/bjp.bp.112.123364. [DOI] [PubMed] [Google Scholar]
- 13.López-Solà C, Fontenelle LF, Bui M, et al. Etiological overlap between obsessive-compulsive related and anxiety disorder symptoms: a multivariate twin study. Br J Psychiatry. 2015 doi: 10.1192/bjp.bp.114.156281. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatry Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 15.Monzani B, Rijsdijk F, Harris J, Mataix-Cols D. The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry. 2014;71:182–189. doi: 10.1001/jamapsychiatry.2013.3524. [DOI] [PubMed] [Google Scholar]
- 16.Tambs K, Czajkowsky N, Røysamb E, et al. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasler G, LaSalle-Ricci VH, Ronquillo JG, et al. Obsessive-compulsive disorder symptom dimensions show specific relationships to psychiatric comorbidity. Psychiatry Res. 2005;135:121–132. doi: 10.1016/j.psychres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess. 2002;14:485–495. [PubMed] [Google Scholar]
- 19.López-Solà C, Fontenelle LF, Alonso P, et al. Prevalence and heritability of obsessive-compulsive spectrum and anxiety disorder symptoms: a survey of the Australian Twin Registry. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:314–325. doi: 10.1002/ajmg.b.32233. [DOI] [PubMed] [Google Scholar]
- 20.Tolin DF, Frost RO, Steketee G. A brief interview for assessing compulsive hoarding: the Hoarding Rating Scale-Interview. Psychiatry Res. 2010;178:147–152. doi: 10.1016/j.psychres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oosthuizen P, Lambert T, Castle DJ. Dysmorphic concern: prevalence and associations with clinical variables. Aust N Z J Psychiatry. 1998;32:129–132. doi: 10.3109/00048679809062719. [DOI] [PubMed] [Google Scholar]
- 22.Connor KM, Davidson JR, Churchill LE, Sherwood A, Foa E, Weisler RH. Psychometric properties of the Social Phobia Inventory (SPIN) New self-rating scale. Br J Psychiatry. 2000;176:379–386. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- 23.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 24.Antony M, Bieling P, Cox B, Enns M, Swinson R. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. 1998;10:176–181. [Google Scholar]
- 25.Box G, Cox D. An analysis of transformations. J R Stat Soc Ser B. 1964;26:211–252. [Google Scholar]
- 26.Neale MC, Maes HM. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers B.V; 2001. [Google Scholar]
- 27.Neale MC, Hunter MD, Pritikin JN, et al. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika. 2015 doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stasik SM, Naragon-Gainey K, Chmielewski M, Watson D. Core OCD symptoms: exploration of specificity and relations with psychopathology. J Anxiety Disord. 2012;26:859–870. doi: 10.1016/j.janxdis.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart S, Stack D, Wilhelm S. Severe obsessive-compulsive disorder with and without body dysmorphic disorder: clinical correlates and implications. Ann Clin Psychiatry. 2008;20:33–38. doi: 10.1080/10401230701844463. [DOI] [PubMed] [Google Scholar]
- 30.Bergin J, Verhulst B, Aggen SH, et al. Obsessive compulsive symptom dimensions and neuroticism :an examination of shared genetic and environmental risk. Am J Med Genet B Neuropsychiatr Genet. 2014;165:647–653. doi: 10.1002/ajmg.b.32269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldinger M, Stopsack M, Ulrich I, et al. Neuroticism developmental courses—implications for depression, anxiety and everyday emotional experience; a prospective study from adolescence to young adulthood. BMC Psychiatry. 2014;14:210. doi: 10.1186/s12888-014-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masi G, Millepiedi S, Perugi G, et al. A naturalistic exploratory study of the impact of demographic, phenotypic and comorbid features in pediatric obsessive-compulsive disorder. Psychopathology. 2010;43:69–78. doi: 10.1159/000274175. [DOI] [PubMed] [Google Scholar]
- 33.Torresan RC, Ramos-Cerqueira ATA, Shavitt RG, et al. Symptom dimensions, clinical course and comorbidity in men and women with obsessive-compulsive disorder. Psychiatry Res. 2013;209:186–195. doi: 10.1016/j.psychres.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Olatunji BO, Cisler J, McKay D, Phillips ML. Is disgust associated with psychopathology? Emerging research in the anxiety disorders. Psychiatry Res. 2010;175:1–10. doi: 10.1016/j.psychres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Monzani B, Rijsdijk F, Iervolino AC, Anson M, Cherkas L, Mataix-Cols D. Evidence for a genetic overlap between body dysmorphic concerns and obsessive-compulsive symptoms in an adult female community twin sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:376–382. doi: 10.1002/ajmg.b.32040. [DOI] [PubMed] [Google Scholar]
- 36.Wheaton M, Timpano KR, Lasalle-Ricci VH, Murphy D. Characterizing the hoarding phenotype in individuals with OCD: associations with comorbidity, severity and gender. J Anxiety Disord. 2008;22:243–252. doi: 10.1016/j.janxdis.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart AS, Phillips KA. Symmetry concerns as a symptom of body dysmorphic disorder. J Obsessive Compuls Relat Disord. 2013;2:292–298. doi: 10.1016/j.jocrd.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taj MJRJ, Viswanath B, Purushottam M, Kandavel T, Janardhan Reddy YC, Jain S. DRD4 gene and obsessive compulsive disorder: do symptom dimensions have specific genetic correlates? Prog Neuropsychopharmacol Biol Psychiatry. 2013;41:18–23. doi: 10.1016/j.pnpbp.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Miguel EC, Leckman JF, Rauch S, et al. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2005;10:258–275. doi: 10.1038/sj.mp.4001617. [DOI] [PubMed] [Google Scholar]
- 40.Faravelli C, Lo Sauro C, Godini L, et al. Childhood stressful events, HPA axis and anxiety disorders. World J Psychiatry. 2012;2:13–25. doi: 10.5498/wjp.v2.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grisham JR, Fullana MA, Mataix-Cols D, Moffitt TE, Caspi A, Poulton R. Risk factors prospectively associated with adult obsessive-compulsive symptom dimensions and obsessive-compulsive disorder. Psychol Med. 2011;41:2495–2506. doi: 10.1017/S0033291711000894. [DOI] [PubMed] [Google Scholar]
- 42.Vasconcelos MS, Sampaio AS, Hounie AG, et al. Prenatal, perinatal, and postnatal risk factors in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:301–307. doi: 10.1016/j.biopsych.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Lafleur DL, Petty C, Mancuso E, et al. Traumatic events and obsessive compulsive disorder in children and adolescents: is there a link? J Anxiety Disord. 2011;25:513–519. doi: 10.1016/j.janxdis.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gloster AT, Rhoades HM, Novy D, et al. Psychometric properties of the Depression Anxiety and Stress Scale-21 in older primary care patients. J Affect Disord. 2008;110:248–259. doi: 10.1016/j.jad.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein DJ, Fineberg NA, Bienvenu OJ, et al. Should OCD be classified as an anxiety disorder in DSM-V? Depress Anxiety. 2010;27:495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- 46.Bienvenu OJ, Samuels JF, Wuyek LA, et al. Is obsessive-compulsive disorder an anxiety disorder, and what, if any, are spectrum conditions? A family study perspective. Psychol Med. 2012;42:1–13. doi: 10.1017/S0033291711000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]