Abstract
It is well known that gender differences exist in experimental or clinical stroke with respect to brain damage and loss of functional outcome. We have previously reported neuroprotective properties of Ginkgo biloba/EGb 761® (EGb 761) in transient and permanent mouse models of brain ischemia using male mice, and the mechanism of action was attributed to the upregulation of the hemeoxygenase 1 (HO1)/Wnt pathway. Here, we sought to investigate whether EGb 761’s protective effect in ovariectomized female mice following stroke is also mediated by the HO1/Wnt pathway. Female mice were ovariectomized (OVX) to remove the protective effect of estrogen and were treated with EGb 761 for 7 days prior to inducing permanent middle cerebral artery occlusion (pMCAO) and allowed to survive for additional 7 days. At day 8, animals were sacrificed, and brains were harvested for infarct volume analysis, western blots, and immunohistochemistry. The OVX female mice treated with EGb 761 showed significantly lower infarct size as compared to that of Veh/OVX animals. EGb 761 treatment in female mice inhibited apoptosis by preventing caspase-3 cleavage and blocking the extrinsic apoptotic pathway. EGb 761 pretreatment significantly enhanced neurogenesis in OVX mice as compared to the Veh/OVX group and significantly upregulated androgen receptor expression with no changes in HO1/Wnt signaling. These results suggest that EGb 761 prevented brain damage in OVX female mice by improving grip strength and neurological deficits, and the mechanism of action is not through HO1/Wnt but via blocking the extrinsic apoptotic pathway.
Keywords: Ginkgo biloba, gender differences, hemeoxygenase 1, Wnt signaling, stroke
Introduction
Stroke affects approximately 795,000 people across the U.S., killing more than 137,000 people, with the majority of patients dying within the first month after the onset of stroke; and a third of stroke survivors experience permanent disability [1]. Men experience a higher incidence of stroke than women throughout their lifespan; however, at older ages, more women than men suffer from stroke [2]. The action of neuroprotection is attributed to estrogen, due to the beneficial effect of the hormone in preventing coronary heart disease [3]. A deficiency of estrogen after menopause is associated with a greater risk of memory decline, cognitive impairment, Alzheimer’s disease (AD) and stroke [4, 5]. Recent studies suggest that ovariectomy exacerbates brain damage in middle cerebral artery (MCA) occlusion, and the infarct size is comparable to that of male rodents. Estrogen pretreatment reduced the ischemic lesion in ovariectomized (OVX) female rats and showed a similar reduction in male rats, an effect that was due to changes in the regional cerebral blood flow [6]. However, Dubal et al. found no changes in regional cerebral blood flow between estrogen-pretreated and oil-treated animals. Post-stroke estrogen administration has been shown to play a beneficial role in reducing ischemic lesion in postmenopausal women [7–9]. However, long-term exposure to estrogen replacement therapy in menopausal women showed poor compliance and side effects. Also, a women’s health initiative study demonstrated that estrogen or hormone replacement therapy increases stroke and heart attack in healthy post-menopausal females as compared to placebo [10]. In spite of advances in understanding the pathophysiology of underlying cerebral ischemia, current treatments are limited concerning efficacy (e.g., recombinant tissue plasminogen activator) and utility [11]. Thus, there is a need for basic research to investigate potential neuroprotective candidates to determine whether their clinical investigation should be warranted in future.
There is a wealth of experimental evidence suggesting neuroprotective properties of EGb 761 in various neurological conditions such as AD, Parkinson's and stroke [12, 13]. A decade of research on EGb 761 and stroke provides valid data explaining its neuroprotective role in male mice [14, 15]. Recently, our lab showed neurogenesis-enhancing properties of EGb 761 following a stroke, and the neuroprotective mechanism has been attributed to the HO1/Wnt canonical pathway [16] However, all the studies were conducted in male animals, so EGb 761’s protective effects and mechanism of action in females is yet to be determined.
In the current study, we investigated whether pretreatment with EGb 761 in OVX mice is neuroprotective after permanent ischemia. To determine whether EGb 761 is neuroprotective, we combined the measures of infarct volume, grip strength, neurological outcome, protein expression, neurogenesis, and androgen receptor (AR) activation-enhancing properties. This study allowed us to understand the molecular mechanism of EGb 761 neuroprotection in females, which might lead to safer and alternative treatments for stroke.
Materials and Methods
Animals and treatment
All animal protocols were approved by The University of Toledo Health Science Campus Institutional Animal Care and Utilization Committee, and the guidelines of the National Institutes of Health were followed throughout the study. Female C57BL/6 mice, 8–10 weeks old and 20–25 grams in weight, were procured from Charles River Laboratories and were housed at 22 ± 1 °C with a 12h:12h light/dark cycles. All the animals were randomized and distributed into different groups. EGb 761 was kindly provided by Dr. Willmar Schwabe Pharmaceuticals (Karlsruhe, Germany).
Ovariectomy and drug treatment
All groups (sham, vehicle and EGb 761 treatment) underwent OVX. Mice were anesthetized under 5% isoflurane and then stabilized at 1.5% throughout the surgical procedure [17]. A single incision of 5.5–10 mm long is made into the muscle wall on both the right and left sides, approximately 1/3 of the distance between the spinal cord and the ventral midline. The ovary and the oviduct are exteriorized through the muscle wall. A hemostat is clamped around the uterine vasculature between the oviduct and uterus. Each ovary and part of the oviduct are removed with single cuts through the oviducts near the ovary. The hemostat is removed, and the remaining tissue is replaced into the peritoneal cavity. The ovary on the other side is removed in a similar manner. All the animals are allowed to recover for 7 days following OVX and prior to experimental ischemia. In the drug treatment group, EGb 761 was orally administered 24h after OVX and continued for 7 days. For neurogenesis study BrdU was administered intraperitoneally 4h after pMCAO and continued until the day of sacrifice.
Permanent middle cerebral artery occlusion (pMCAO)
Our previously optimized protocol for pMCAO was used in this study [16, 18]. An intracranial approach was used to occlude the distal part of the MCA permanently. Sterile conditions were maintained throughout the surgical procedure by using sterile drapes, surgical gloves and autoclaved equipment. Animals were briefly anesthetized using 5% isoflurane and later stabilized at 1–2% under a surgical microscope. A rectal thermometer was used to monitor the body temperature, and a heating blanket was used to maintain the body temperature at 37° C ± 0.5. An approximately 1 cm vertical skin incision was made between the right eye and ear; using forceps, the temporal muscle was separated, and the underlying skull was exposed. A dental drill was used to drill a 2.0mm hole above the visible MCA region, and the dura was carefully separated. The distal part of the MCA was carefully occluded and severed using a bipolar coagulator. Animals were moved to a temperature-controlled incubator to recover from surgery and later moved to home cages.
Grip Strength
Grip strength was evaluated by holding the mice by the tail and placing their forelimbs on a specially designed pull bar assembly (Grip strength meter, Columbus Instruments, OH). The peak amount of force an animal exerts is shown on the digital display and is noted. Each animal is tested three times per trial, 4h before and 24h and 7 days after pMCAO surgery.
Neurological deficit scoring (NDS)
NDS is evaluated by a 28-point score pattern optimized by our group [18]. A person blinded to the treatment plan but expert in the NDS evaluation performed NDS 7 days after pMCAO; the evaluation includes both sensory and motor deficits, such as body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response. Each of the seven tests included in the 28-point NDS is graded from 0 to 4, with higher scores indicating severe deficits.
Western blotting
To perform Western blotting for protein expression levels, a separate cohort of mice (n=5/group) were sacrificed 7 days after ischemia using carbon dioxide overdose and brain cortices of the ischemic and non-ischemic sides of the mice were dissected, weighed, and homogenized. The brain tissues were homogenized to extract cytoplasmic protein using a buffer (10mM HEPES-KOH pH 7.9, 1.5mM MgCl2, 10mM KCl and 1mM EDTA). Prior to the extraction process, freshly prepared 0.5mM DTT and 0.2mM PMSF, along with phosphatase and protease inhibitors, were added to the buffer. Bradford reagent (Bio-Rad laboratories) was used to determine protein concentration, and samples were analyzed by loading equivalent amounts of proteins (20µg) on 12%-15% SDS polyacrylamide gel. Proteins were transferred to the PVDF membrane and blocked by 5% dry nonfat milk for 1h at room temperature, followed by overnight incubation at 4 °C with the antibodies for: mouse anti-actin (1:2000; Sigma); mouse anti-HO1 (1:1000); rabbit anti vascular endothelial growth factor (VEGF) (1:1000, Santa Cruz, TX); rabbit anti tumor necrosis factor-α (TNF-α) (1:1000, Santa Cruz, TX); anti rabbit Wnt (1:1000, Abcam, MA); anti rabbit endothelial nitric oxide synthase (eNOS) (1:1000, Abcam, MA); anti rabbit caspase-3 (1:1000, cell signaling, MA); and anti-rabbit caspase-8 (1:1000, Bioss, MA). After washing, membranes were incubated with the buffer containing secondary antibody (goat anti-mouse or anti-rabbit, 1:2000; Jackson ImmunoResearch Laboratories) at room temperature (RT) for 1h. The densitometric values were normalized against the values of actin immunoreactivity to correct for any loading and transfer differences between samples.
Brain sectioning and immunohistochemistry
In a separate cohort (n=3/group), mice were anesthetized with sodium pentobarbital and transcardially perfused with saline followed by 4% paraformaldehyde. Mice brains were dissected out and stored for 24h in 4% paraformaldehyde at 4 °C before cutting into 6µm sections, and four to five brain sections were mounted on each slide and stored at − 80 °C until further use. Brain sections were incubated in 4% paraformaldehyde for 20 min, washed with 1XPBS buffer, and antigen-retrieved with 10mM citrate buffer (pH=6.0) using a pressure cooker for 4 min; sections were allowed to cool for 10 min at RT. Permeability was performed by incubating the sections with 1% Triton- X100 in 1X PBS buffer for 30 min, then washing again with 1X PBS buffer. Denaturation was accomplished with 2N HCl for 30 min at RT, and the slides were neutralized with 0.1M sodium borate for 20 min at RT and washed again. For studies involving neurogenesis, the slides were blocked with 3% BSA fraction IV (RPI Corp., Mount Prospect, IL) for 1h at RT, followed by incubation with primary antibodies overnight at 4°C for: anti-BrdU mouse (Thermo Scientific, West Palm Beach, FL), chicken anti-Netrin (Thermo Scientific), rabbit-anti UNC5B (Abcam, Cambridge, MA), rabbit anti-AR (Thermo scientific) and rabbit anti-DCC (Abcam) using the following dilutions: 1:300, 1:300, 1:250 and 1:250, respectively. The slides were again washed with 1X PBS for 10 min, followed by incubation with fluorescent anti-mouse, anti-rabbit and/or anti-chicken secondary antibody (Jackson Immuno Research, West Grove, PA) at the dilution of 1:500 for 2h at RT. After another set of washings, the slides were mounted with DAPI (Santa Curz, Dallas, TX) and then sealed with nail polish. For BrdU analysis, 12 – 15 captures were taken from the side of the injury (ipsilateral side) for each section with four to five brain sections per mouse, with three mice in each group. The sections were cut approximately between the coordinates of −0.94 to −2.92 from bregma. The Netrin-BrdU positive cells are counted and averaged, and the ratio was expressed by dividing Netrin-BrdU positive cells by the total DAPI cell counts.
Assay for caspase-3 activity
Aliquots of cytosolic extracts were mixed with equal volumes of 40µm Ac-DEVd-AMC in the buffer provided by Promega, Madison, WI. Free AMC accumulation, which resulted from cleavage of the aspartate-AMC bond, was monitored continuously in each sample over 30 min in 96-well microtiter plates using ELISA plate reader at 360nm excitation and 460nm emission wavelengths. The emission from each well was plotted against time.
Transfection and reporter assays
Expression constructs containing AR or empty vector were transiently transfected into COS-7 cells (African green monkey kidney cells lacking an endogenous AR). AR activity was measured by luciferase assay using the AR-responsive minimal reporter pGRE2EIB-Luc[19] and pRL-CMV Renilla reporter for normalization to transfection efficiency. Transient transfection was achieved using GeneFect (Alkali Scientific, Inc., Pompano Beach, FL), according to the manufacturers protocol. Twenty-four hour post-transfection cells were treated with vehicle or 10nM R1881 or 0.1, 1.0, 10, or 100 mg/ml EGb 761 for an additional 24h until harvest. Cell lysates were obtained, and assay was performed using the Promega dual luciferase assay system.
Statistical Analysis
All behavioral parameters and infarct volume analyses among different groups were analyzed by using one-way ANOVA and Newman-Keuls post hoc test. For the WB protein expression analysis and reporter assay, the differences between groups were determined by student's t test. A value of p< 0.05 was considered to be significant for all parameters.
Results
Effect of EGb 761 pretreatment on OVX female mice following permanent ischemia
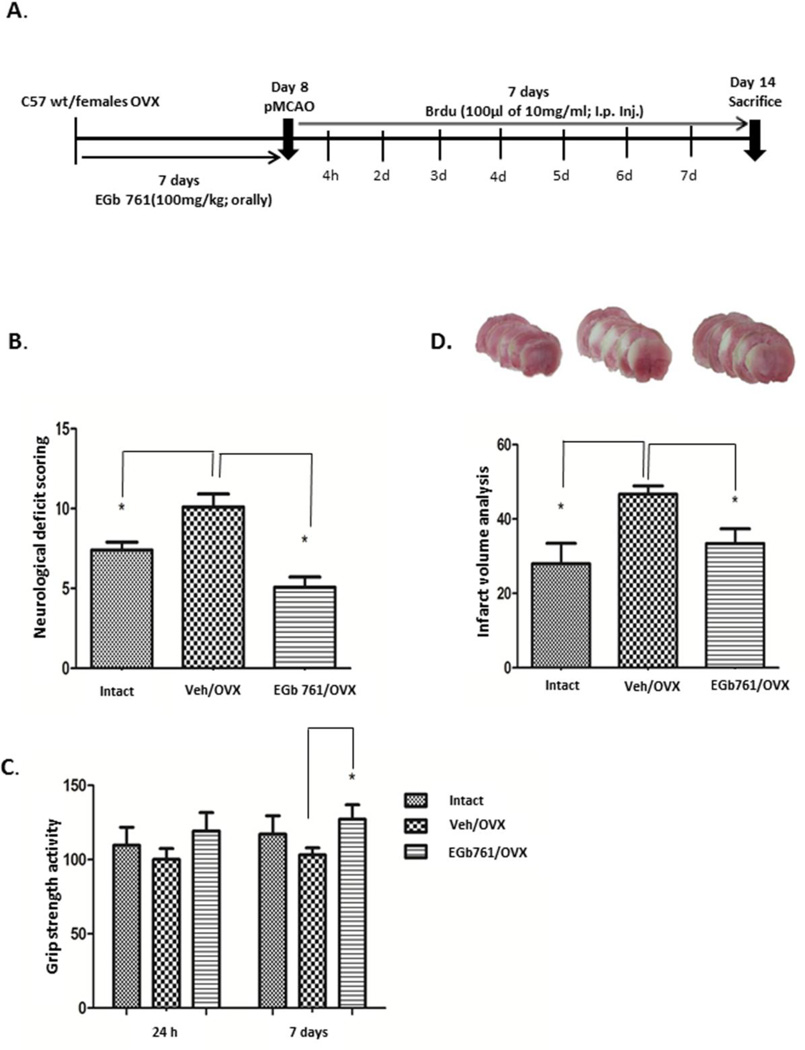
Previously, we demonstrated that EGb 761 pretreatment protected male mice from brain injury. Here, we wanted to investigate whether EGb 761 pretreatment also protects OVX female mice from ischemic brain injury. Randomly cycling female mice underwent ovariectomy and were allowed to recover for 7 days. EGb 761 was orally administered 24 h after ovariectomy and daily for 7 days followed by pMCAO at day 8, and mice were sacrificed after 8 days. OVX female mice pretreated with EGb 761 showed lower NDS and improved grip strength on day 8 after pMCAO (Fig. 1A and B). Similarly, infarct volume was significantly reduced in the EGb 761/OVX pretreated group as compared to the Veh/OVX group following pMCAO (Fig. 1C). Together, these results suggest that EGb 761 pretreatment improved stroke recovery in OVX female mice by enhancing neurobehavioral parameters and reducing stroke lesions.
Figure 1.
Pretreatment with EGb 761 attenuates the brain damage following pMCAO. A) Schematic diagram showing experimental protocol. Mice were randomly selected and ovariectomized and allowed to recover for 7 days. Female mice were divided into vehicle and treatment groups and administered either vehicle or EGb 761 for 7 days prior to pMCAO and survived additional 7 days, followed by sacrifice on day 8. B) NDS in EGb 761 pretreated OVX mice were significantly lower as compared to Veh/OVX. C) Grip strength showed significant improvement on day 7 in the EGb 761/OVX pretreated group as compared to Veh/OVX. D) Representative coronal brain sections show higher infarction in Veh/OVX female mice, which was significantly reduced in EGb 761 treated OVX mice. E) Infarct volume was significantly lower in the EGb 761 pretreated group as compared to Veh/OVX. Data are expressed as mean ± SEM; *p<0.05, vs. vehicle-treated control or Intact (n=7); Veh/OVX (n=8) and EGb 761/OVX (n=8)
Neuroprotection of EGb 761 is independent of HO1/Wnt pathway in OVX female mice
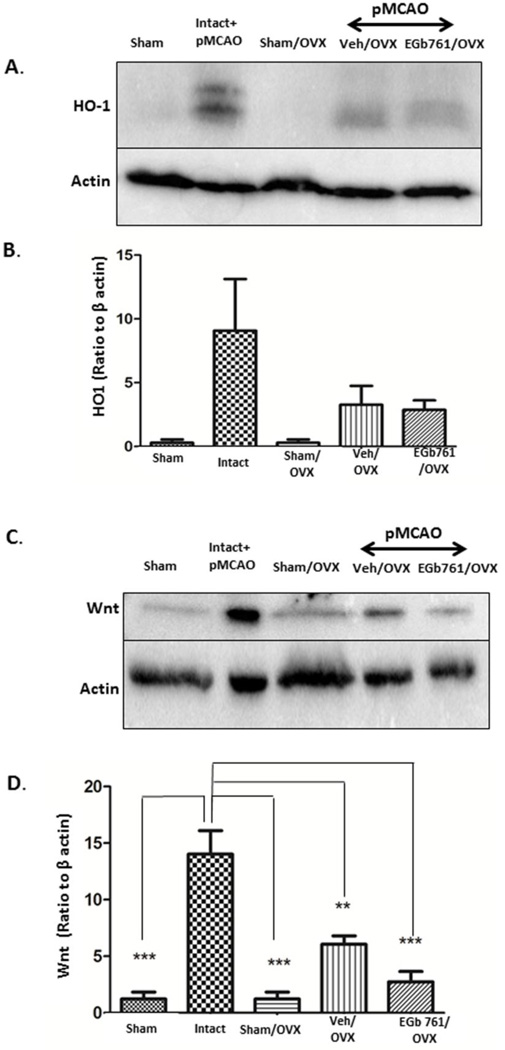
Several studies on male mice, including ours, showed that EGb 761 mediated neuroprotection is dependent on the HO1/Wnt canonical pathway [16, 20]. Here, we examined whether neuroprotective properties of EGb 761 in female mice were attributed to overexpression of HO1 and Wnt following permanent ischemia. HO1 and Wnt expression levels were observed to be unchanged in the EGb 761/OVX pretreated group when compared to the Veh/OVX group after ischemia (Fig. 2A-B). Intact female mice were used as positive control. Together, these results suggest that neuroprotection mediated by HO1/Wnt expression after EGb 761 treatment is beneficial only in males[16], and this neuroprotective pathway is ineffective in OVX female mouse.
Figure 2.
Non-involvement of HO1/Wnt pathway in EGb 761 neuroprotection. A) Protein expression levels showed that EGb 761 pretreatment did not upregulate HO1 expression after pMCAO as a protective mechanism. Intact female mice were used as positive control. C) Wnt expression also showed a similar pattern as that of HO1. (B and D) Corresponding graph shows the densitometric analysis normalized to actin. Data are expressed as mean ± SEM; *p<0.05, vs. vehicle-treated control or Intact (n=5), Veh/OVX (n=5) and EGb 761/OVX (n=5)
EGb 761 inhibits extrinsic apoptotic pathway and angiogenesis in OVX female mice
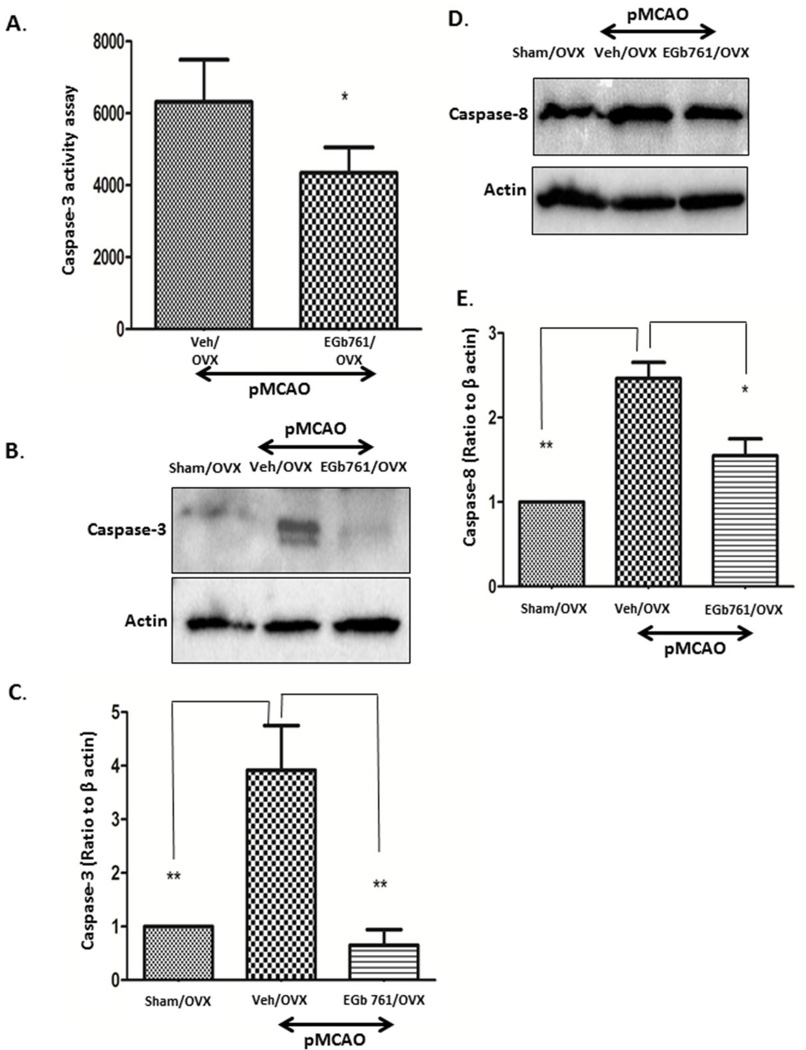
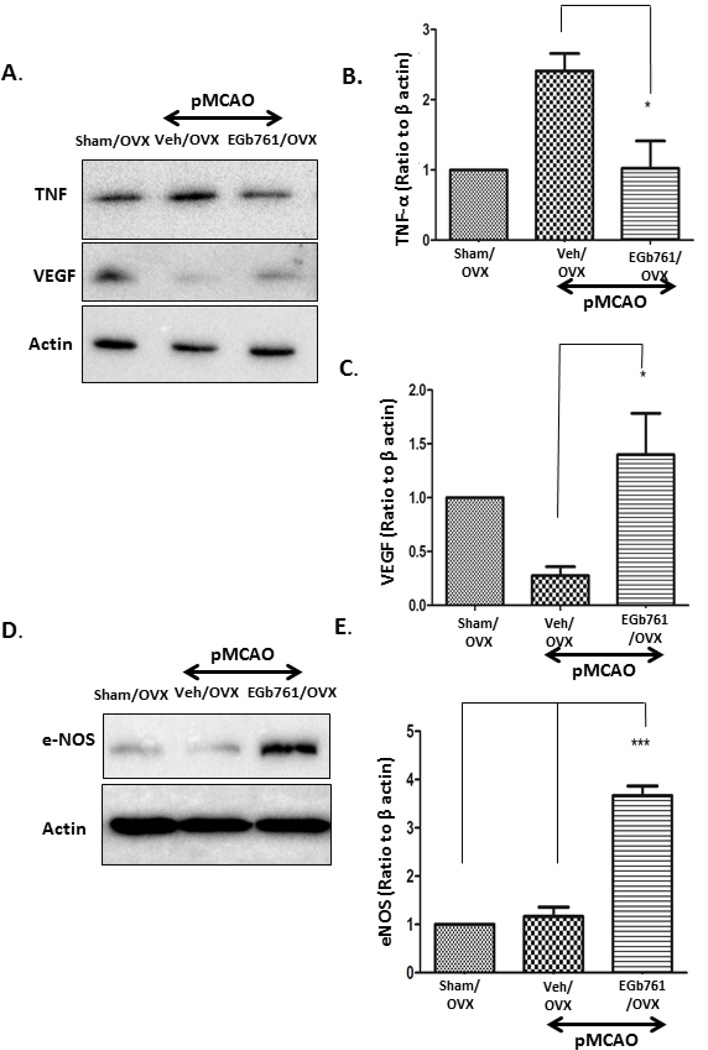
To assess the apoptotic mechanism in OVX female mice subjected to permanent ischemia, we measured the caspase-3 activity in both Veh/OVX and EGb 761/OVX treated groups and further confirmed the results by Western blot analysis. Caspase-3 activity assay showed significantly lower caspase-3 activity in the EGb 761/OVX group as compared to the Veh/OVX group (Fig. 3A). To confirm our results, we performed Western blot analysis, and expression of cleaved caspase-3 was significantly down-regulated in the EGb 761/OVX treated group as compared to the Veh/OVX group (Fig 3B and C). Our results are in conjunction with the previous studies suggesting a primary role of caspase-3 activation in apoptotic cell death in female mice [21, 22]. The expression levels of cytochrome C in the EGb 761/OVX treated group subjected to pMCAO showed no changes (data not shown); however, caspase-8 expression in the EGb 761/OVX group was significantly reduced when compared to the Veh/OVX group (Fig 3D and E). To further illustrate the neuroprotective properties of EGb 761 in female mice, we investigated the role of the inflammatory marker, TNF-α, which was observed to have significantly reduced expression in the EGb 761/OVX treated group as compared to Veh/OVX group (Fig. 4A). In addition, angiogenic markers, VEGF, and eNOS were significantly elevated in the EGb 761/OVX treated group as compared to the Veh/OVX group (Fig 4A, B, C and D). Our results from this study are in parallel with our previous data suggesting angiogenic properties of the EGb 761 in male mice [15]. Together, these findings suggest that in female mice, EGb 761 mediates neuroprotection by inhibiting extrinsic apoptotic pathway mediated cell death, reducing TNF-α mediated inflammation and upregulating the angiogenic proteins, VEGF, and eNOS.
Figure 3.
EGb 761 blocks caspase-3 activity assay and expression. A) Caspase-3 activity assay showed that EGb 761 significantly inhibits caspase-3 activity as compared to Veh/OVX. B) Protein expression analysis showed that OVX animals have higher expression of cleaved caspase-3, and EGb 761/OVX group showed significantly reduced levels of caspase-3 as compared to Veh/OVX. D) Significant reduction in expression levels of caspase-8 in EGB 761 treated OVX group as compared to OVX vehicles following pMCAO. C and E) Corresponding graph shows the densitometric analysis normalized to actin. Data are expressed as mean ± SEM; *p<0.05, vs. sham OVX (n=4); Veh/OVX (n=5) and EGb 761/OVX (n=5)
Figure 4.
EGb 761 reduces inflammation and induces angiogenesis. A) EGb 761 pretreatment reduced TNF-α expression significantly as compared to Veh/OVX. On the other hand, expression of VEGF was significantly higher in EGb 761 treated OVX group as compared to that of Veh/OVX. D) eNOS expression was significantly higher in EGb 761/OVX group as compared to that of Veh/OVX group following pMCAO. (B, C and E) Corresponding graph shows the densitometric analysis normalized to actin. Data are expressed as mean ± SEM; *p<0.05, vs. sham OVX (n=4); Veh/OVX (n=5) and EGb 761/OVX 761 (n=5)
EGb 761 pretreatment enhances neurogenesis independent of the HO1/Wnt pathway in female mice
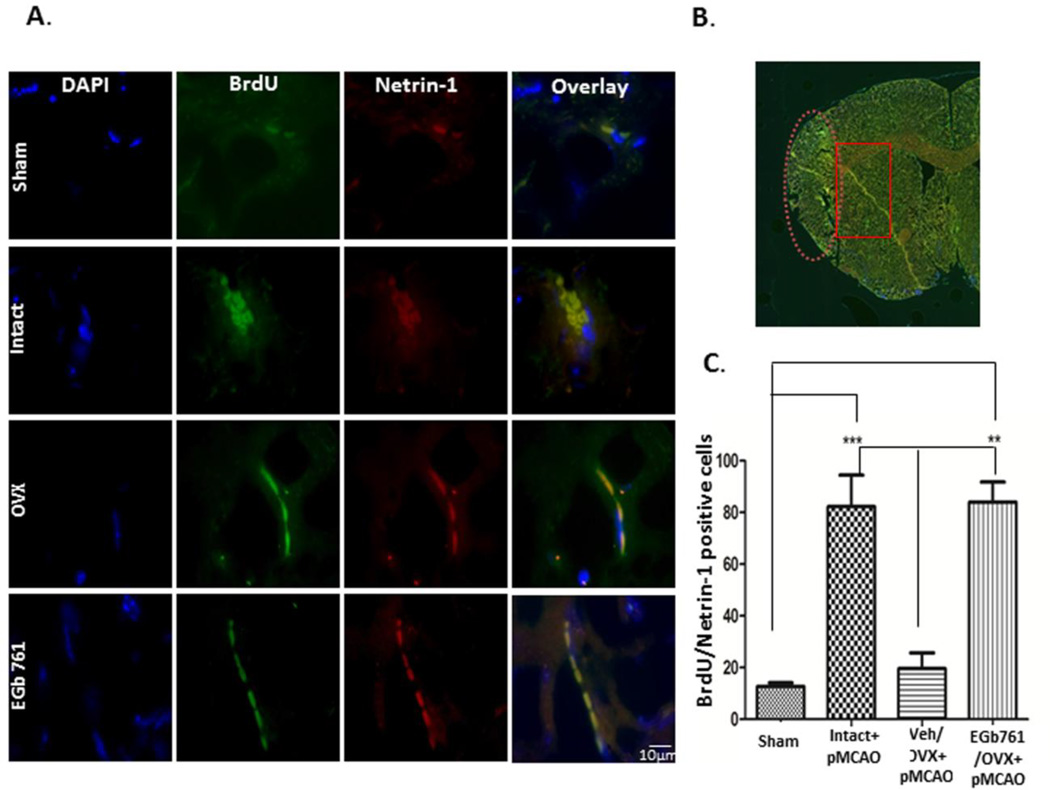
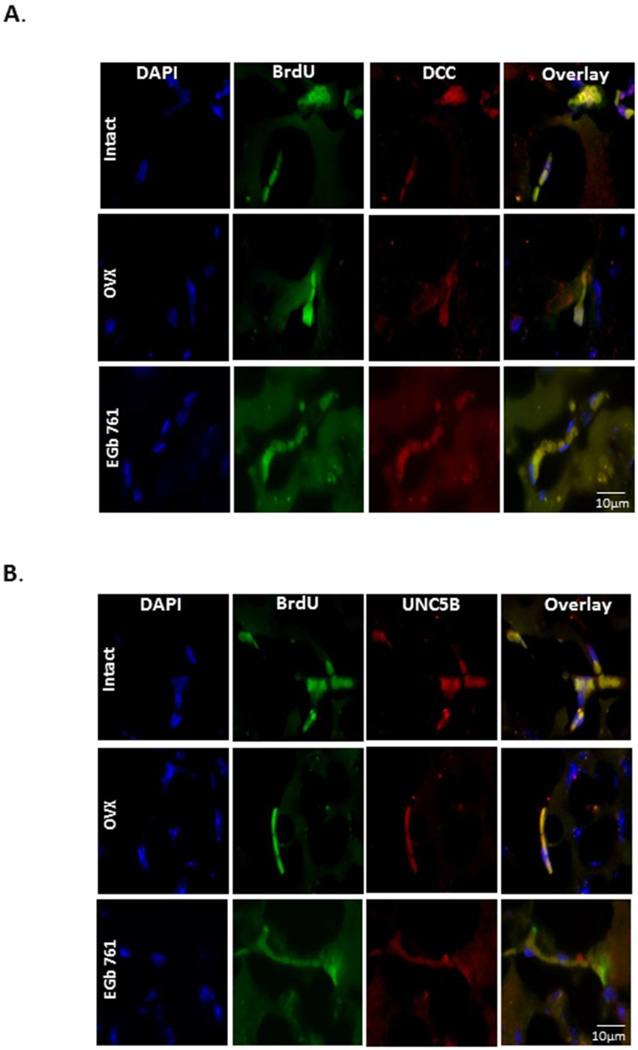
Our group previously reported the neurogenesis-enhancing properties of EGb 761 after ischemia in male mice, but this phenomenon has not been elucidated in female mice. The neurogenesis-enhancing properties of estrogen have been widely studied, so female mice with intact ovaries were used as positive control. OVX female mice treated with EGb 761 showed the same level of neurogenesis as observed in intact females. Surgical removal of ovaries showed a markedly reduced number of BrdU/Netrin-1 positive cells (Fig. 5A and B). As shown in Fig. 5A, stem cells were distinguished from non-stem cells by using a stem cell marker called netrin-1. Studies from our lab and various others have already shown that ischemia itself upregulates neurogenesis after 24h; however, in this study, we showed higher levels of netrin-1 expression in NSPCs after 7 days of ischemia. NSPCs were counted manually in the region near the peri-infarct area, only cells expressing BrdU and Netrin-1 were counted, and the graph was plotted (Fig. 5B). We also demonstrated that netrin-1 and its receptors, deleted colorectal cancer (DCC) and uncoordinated gene 5B (UNC5B), were expressed only in NSPCs. Expression of DCC and UNC5B was elevated after 7 days of ischemia in the EGb 761/OVX treated group (Fig.6A-B). For all the immunohistochemistry experiments, control slide was stained with only secondary antibody was used as a negative control (data not shown). Together, these results suggest that netrin-1 and its receptors, DCC, and UNC5B, are required for migration of NSPCs from SVZ to the injury site.
Figure 5.
EGb 761 pretreatment enhances neurogenesis. Mice pretreated with EGb 761 after OVX for 7 days prior to pMCAO and allowed to survive additional 7 days without drug treatment were sacrificed at day 8. BrdU was injected 4 h after pMCAO and then daily for 7 days (schematic diagram of Fig. 1A). A) Triple immunofluorescence staining of DAPI (blue), BrdU (green), and netrin-1 (red) and overlay of brain sections (6 µm) of female mice brains. B) The image represents the area from which the netrin+BrdU cells were counted; the image was taken from Cytation 5 immunofluorescence microscope (Biotek, Winooski, VT); the ischemic region is in the oval shaped (dotted) area, whereas as the NSPCs are counted from the rectangular area. C) The number of netrin+BrdU positive cells were counted near the peri infarct area and the graph was plotted. Data are expressed as mean±SEM; p < 0.05 vs. sham; ***p <0.05 vs. Intact, p< 0.05 vs. Veh/OVX; **p <0.05 vs. EGb 761/OVX (n=3 each group). The pictures were taken by Nikon Eclipse Ti microscope using × 100 objectives.
Figure 6.
Expression of netrin-1 and its receptors, UNC5B and DCC, in NSPCs. A) Triple immunofluorescence staining of DAPI (blue), BrdU (green), and DCC (red). B) Staining of UNC5B (red) and overlay of brain sections (6 µm) of intact, Veh/OVX and EGb 761/OVX mice sacrificed at day 8 after pMCAO. Netrin-1 receptors, DCC and UNC5B, were only observed to be expressed in the NSPCs. The pictures were taken by Nikon Eclipse Ti microscope using × 100 objectives, (n=3 each group).
EGb 761 induces neuroprotection via androgen receptors in ovariectomized female mice
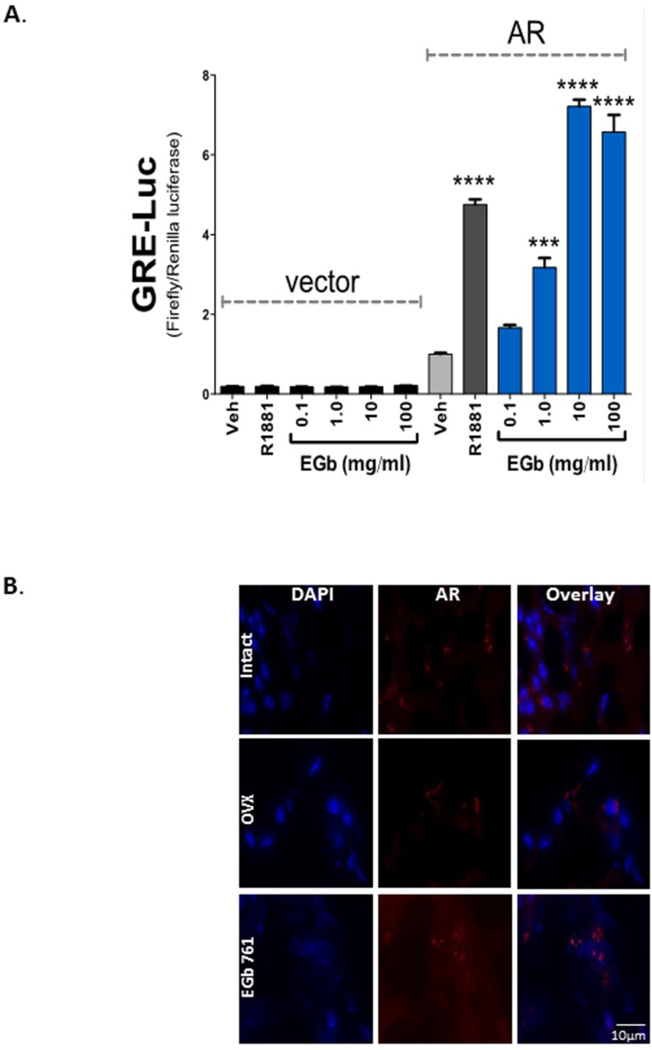
Androgens function by binding and activating the AR, and are typically considered male hormones [23]. Females, however, secrete androgens from adrenal, ovarian and adipose tissues, with circulating levels much lower than in men. On the other hand, androgen regulation of brain activity is essential for the initiation of sexual activity, libido and mating behavior in both male and females [23, 24]. Eliminating the female estrogen hormone by removal of the ovaries, and combining pretreatment with EGb 761, protected the mice from ischemic brain injury. To determine whether the neuroprotective mechanism of EGb 761 is through activation of AR in the brains of these mice we used Cos7 cells, which do not express AR. The Cos7 cells were transfected with AR or empty vector plasmid that constitutively expresses the receptor and were further co-transfected with a minimal luciferase response element, ARE-luc, and a pRL-CMV plasmid for renilla luciferase as a control for transfection efficiency (Fig. 7A). As a positive control, we treated Cos7 cells with the synthetic androgen, R1881, which did not activate the ARE-luc in the vector with no AR present. With AR expressed, R1881 significantly (p<0.0001) increased the ARE-luc, 4.75-fold. Interestingly, AR transcriptional activity was significantly (p<0.001) increased by 1 mg/ml EGb 761 treatment by 3.17-fold, and further showed a dose-dependent increase. At 10 mg/ml EGb 761 the AR transcriptional activity level was comparable to 10 nM R1881. Immunohistochemical studies also showed enhanced expression of AR in EGb 761/OVX female mice after ischemia (Fig. 7B).
Figure 7.
EGb 761 treatment increased AR expression level in OVX female brain after ischemia. A) A dose-dependent increase of EGb 761 showed significantly (p<0.001) increased AR activity (3.17-fold) at 1.0 mg/ml. The highest increase in AR activity with EGb 761 treatment was with 10 mg/ml (7.21-fold) and 100 mg/ml (6.57-fold). B) Immunohistochemical studies showed that DAPI (blue) and AR receptors (red) were expressed highly in the EGb 761/OVX group as compared to Veh/OVX group. The pictures were taken by Nikon Eclipse Ti microscope using × 100 objectives, (n=3 each group).
Discussion
In this study, we observed that EGb 761 pretreatment in OVX female mice reduced infarct volume and improved functional recovery after permanent ischemia. The surgical removal of ovaries reduced the expression of HO1 and Wnt, and the EGb 761 treated OVX mice did not show any upregulation of this pathway, suggesting the neuroprotective mechanism is independent of the HO1/Wnt pathway. Furthermore, EGb 761 treatment inhibited apoptotic cell death pathway by preventing the cleavage and activation of caspase-3 and downregulating caspase-8 and TNFα expression and also resulted in increased VEGF and endothelial NOS production. The pretreatment with EGb 761 enhanced proliferation, differentiation of NSPCs. It was also observed that EGb 761 treatment was able to bind to and activate the AR. Together these results suggest that EGb 761 neuroprotection is independent of HO1/Wnt expression, and sexual differences may exist in HO1/Wnt mediated neuroprotection. The mechanism of EGb 761 neuroprotection is mainly attributed to inhibition of caspase-dependent apoptotic cell death pathway and overexpression of angiogenic proteins and activation of AR.
Epidemiological studies in females showed that estrogen is considered to be protective against cardiovascular disease, and incidence of coronary heart disease is lower among women as compared to men before menopause; however, these differences are abolished after menopause [25]. Some studies have reported beneficial effects of estrogen in various neurological disorders including stroke, but recent clinical studies have shown that estrogen replacement after menopause does not enhance cognitive behavior and has serious side-effects, including risk of breast and uterine cancer [5, 26–28]. Moreover, studies also report the adverse indication of hormone replacement therapy in prevention of stroke due to increased occurrence of death in the estrogen-treated group as compared to placebo [10]. The role of EGb 761 has been widely studied in male rodents and to our knowledge, only one study has investigated OVX female animals and its protective mechanism in chronic stress. EGb 761 treatment attenuated cognitive impairments and prevented CA3 hippocampal neuronal loss in OVX/stress subjected female rats, as was observed with estrogen replacement [29, 30]. EGb 761 is an effective antioxidant that has been shown to provide protection against cardiovascular and neurological disorders [31, 32]. Many groups, including ours, have previously shown that EGb 761 attenuates brain damage in male rodents after permanent, transient and global models of ischemia [15, 16, 33]. The neuroprotective properties of EGb 761 are mainly attributed to HO1 induction, and we have shown the synergistic effect of EGb 761 in attenuating brain damage by upregulating the HO1/Wnt pathway and inducing neurogenesis in male mice [15, 16]. The mechanism of ischemic protection against ischemic injury by EGb 761 in females has not yet been determined. Therefore, we tested whether pretreatment with EGb 761 attenuates brain injury in OVX mice and whether the mechanism of action is mediated through the HO1/Wnt pathway and enhanced neurogenesis.
Previous studies on male mice and estrogen replacement studies in females as well as in males have shown that the Wnt canonical pathway plays a crucial role in central nervous system development. It was demonstrated that the estrogen replacement therapy regulates diverse processes including proliferation, differentiation and migration [34, 35]; and plays a role in differentiation of NSPCs to neurons [36]. Recent studies from our lab and by Vanella et al. showed that HO1 acts upstream of the canonical Wnt signaling cascade [20]. We also observed that HO1 and Wnt expression are down-regulated after ovariectomy. Surprisingly, EGb 761 treatment showed no upregulation in HO1 expression in OVX females, as was noted in males. These results suggest that the neuroprotective mechanism of EGb 761 is sexually dimorphic and that the HO1/Wnt canonical pathway is beneficial in males but not in female mice. While a considerable part of the observed gender differences in vascular reactivity has been attributed to genomic modulation by sex hormones, non-genomic effects of those hormones also exist [25]. Choudhary et al. [37] showed that estrogen administration upregulates HO activity during traumatic brain injury and hemorrhage. Posa et al. showed that inhibiting HO1 increases contraction in the aorta in females and males, but at the basal level, female rats with intact ovaries had lower blood pressure and lowered aortic contraction as compared to male rats. Several possible reasons can be postulated for the difference between Posa et al.’s study and our investigation, such as the type of disease, organ system and non-hormonal treatment [25]. In fact, very little is known about the HO activity in the female brain after ischemia.
The involvement of inflammation has been widely documented in the exaggeration of brain injury [38, 39]. One of the known inflammatory cytokines expressed during cerebral ischemia is TNF-α. However, the function of TNF-α is elusive in brain ischemia. At the pathophysiological level, TNF-α is shown to be involved in not only necrosis but also in the regulation of caspases and other apoptotic factors followed by disruption of the blood-brain barrier. Our data suggest that EGb 761 treatment in OVX females significantly attenuates TNF-α expression as compared to OVX female mice, and the caspase-3 activity a showed significant reduction in EGb 761 treated OVX females as compared to Veh/OVX treated females. To further determine caspase activation, we began by assessing cytochrome C expression for the intrinsic apoptotic pathway and caspase-8 expression for the extrinsic apoptotic pathway. We did not observe any changes in cytochrome c expression (data not shown); however, caspase-8 expression was significantly lower in the EGb 761/OVX treated group as compared to the Veh/OVX group. In addition, EGb 761 treatment in the OVX female mice significantly upregulated VEGF and eNOS expression that might have resulted in angiogenesis. Together, these findings suggest that EGb 761 promotes cell survival after ischemic injury by preventing activation of the extrinsic apoptotic pathway of cell death, reducing inflammation via TNF-α inhibition, and augmenting new blood vessel formation via expression of VEGF and eNOS. The possible reason for differences in signaling pathways between male and female are the presence of testosterone in males and estrogen in females. In our previous studies involving male animals, presence of testosterone might have partly mediated the neuroprotective mechanism of EGb 761 whereas in ovariectomized females the level of circulating estrogen was reduced which resulted in the increase in stroke volume. However, EGb 761 treated ovariectomized females demonstrated inhibition of caspase cleavage and its activation as an apoptotic pathway in females is shown to be caspase-dependent whereas in male mice it is PARP-AIF mediated. Our results agree with previous findings related to the dimorphic role of male and female ischemic cascade [26].
To address the recovery and repair properties of EGb 761, we further investigated its neurogenesis-enhancing properties that were previously observed in male mice. Axon attraction and repulsion are mediated by netrin-1 and its receptors, DCC and UNC5B, which are expressed highly during embryonic development as well as in adulthood, albeit at lower levels [40, 41]. Studies suggest that netrin-1 [42] and its overexpression result in angiogenesis, thus inducing neovascularization and vessel remodeling after stroke [43]. Our previous study illustrated that ischemia acts as an inducer of neurogenesis [16]. In the present study, we demonstrate that netrin-1 and its receptors, DCC, and UNC5B, are only expressed in NSPCs in all treatment groups, which suggests the role of netrin-1 in the migration of NSPCs towards the injury site. The number of netrin-1/NSPCs positive cells was higher in animals with intact ovaries, suggesting the role of estrogen in neurogenesis, whereas Veh/OVX showed lower numbers. However, EGb 761 treatment enhanced netrin-1/NSPCs positive cells, signifying enhanced endogenous neurogenesis after permanent ischemia. Taken together, our findings suggest that EGb 761 enhances neurogenesis during stroke in female mice, independent of the ovarian hormone, estrogen.
At the cellular and molecular levels, gonadal steroids have a profound effect on target neurons, including the sexual development of neural structure and organization [44]. Gonadal steroids have been shown to exert a neuroprotective effect by rescuing neurons from cell death in cases of various neurological insults [45, 46]. Until the late 1990s, estrogen was thought to be neuroprotective in the central nervous system; [47] however, recent studies have shown that cerebellar granule neurons can be protected from oxidative stress by androgens [48, 49]. Several laboratories have described nerve regeneration activity of androgens in motor neuron injury, where androgen treatment showed enhanced axon regeneration in both male and female rats [50–52]. In cerebral ischemia, androgen receptor over-expression in AR-Tg male mice showed neuroprotection following MCAO and reperfusion [53]. The role of androgen receptor activation is still poorly understood in OVX female mice. Our in vitro studies using a vector plasmid expressing the androgen receptor showed that EGb 761 dose-dependently increased AR activation, and immunohistochemical studies on brain sections also suggest increased expression of the AR in OVX females mice treated with EGb 761.
Conclusion
In summary, our results suggest that EGb 761 pretreatment prevents cell death and protects the brain from further damage, specifically in the cortex region, which further aids in the improvement of neurological and grip strength deficits induced by the permanent brain ischemia. The molecular studies on OVX female mice treated with EGb 761 show its beneficial neuroprotective actions are independent of HO1/Wnt expression. Furthermore, EGb 761 prevented cellular apoptosis by inhibiting the extrinsic pathway of apoptosis and augmenting the angiogenic response. EGb 761 pretreatment in OVX females showed increased neurogenesis and AR activation in the brain, in the absence of ovarian estrogen.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institutes of Health by the National Center for Complementary and Integrative Medicine (NCCIM) under award number R00AT004197 (Z.A.S.), the National Heart, Lung, and Blood Institute (NHLBI) under award Number K01HL125445 (T.D.H.), the NIH Division of Loan Repayment L32MD009154 (T.D.H.), and start-up funds from the University of Toledo (Z.A.S.) (T.D.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: Authors declare no conflict of interest.
Ethical approval: The University of Toledo Health Science Campus Institutional Animal Care and Utilization Committee, and the guidelines of the National Institutes of Health were followed throughout the study.
References
- 1.Turtzo LC, et al. Deletion of macrophage migration inhibitory factor worsens stroke outcome in female mice. Neurobiol Dis. 2013;54:421–431. doi: 10.1016/j.nbd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkayed NJ, et al. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 4.McCullough LD, et al. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32(3):796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 5.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14(5):228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 6.Hurn PD, et al. Postischemic cerebral blood flow recovery in the female: effect of 17 beta-estradiol. J Cereb Blood Flow Metab. 1995;15(4):666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- 7.Sampei K, et al. Stroke in estrogen receptor-alpha-deficient mice. Stroke. 2000;31(3):738–43. doi: 10.1161/01.str.31.3.738. discussion 744. [DOI] [PubMed] [Google Scholar]
- 8.Dubal DB, et al. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Murphy S, et al. Estrogen and selective estrogen receptor modulators: neuroprotection in the Women’s Health Initiative era. Endocrine. 2003;21(1):17–26. doi: 10.1385/endo:21:1:17. [DOI] [PubMed] [Google Scholar]
- 10.Billeci AM, et al. Hormone replacement therapy and stroke. Curr Vasc Pharmacol. 2008;6(2):112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- 11.Gibson CL, Coomber B, Murphy S. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134(Pt 7):2125–2133. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, et al. Ginkgo biloba extract in an animal model of Parkinson's disease: a systematic review. Curr Neuropharmacol. 2013;11(4):430–435. doi: 10.2174/1570159X11311040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen IM, et al. Ginkgo biloba in Alzheimer’s disease: a systematic review. Wien Med Wochenschr. 2010;160(21–22):539–546. doi: 10.1007/s10354-010-0844-8. [DOI] [PubMed] [Google Scholar]
- 14.Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tulsulkar J, Shah ZA. Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int. 2013;62(2):189–197. doi: 10.1016/j.neuint.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nada SE, Tulsulkar J, Shah ZA. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761(R)) after permanent ischemic stroke in mice. Mol Neurobiol. 2014;49(2):945–956. doi: 10.1007/s12035-013-8572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idris AI. Ovariectomy/orchidectomy in rodents. Methods Mol Biol. 2012;816:545–551. doi: 10.1007/978-1-61779-415-5_34. [DOI] [PubMed] [Google Scholar]
- 18.Nada SE, et al. A derivative of the CRMP2 binding compound lanthionine ketimine provides neuroprotection in a mouse model of cerebral ischemia. Neurochem Int. 2012;61(8):1357–1363. doi: 10.1016/j.neuint.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allgood VE, Oakley RH, Cidlowski JA. Modulation by vitamin B6 of glucocorticoid receptor-mediated gene expression requires transcription factors in addition to the glucocorticoid receptor. J Biol Chem. 1993;268(28):20870–20876. [PubMed] [Google Scholar]
- 20.Vanella L, et al. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther. 2013;4(2):28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, et al. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel C, et al. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A. 2011;108(28):11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dart DA, et al. Visualising androgen receptor activity in male and female mice. PLoS One. 2013;8(8):e71694. doi: 10.1371/journal.pone.0071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa S, et al. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96(22):12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posa A, et al. Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid Med Cell Longev. 2013;2013:521563. doi: 10.1155/2013/521563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough LD, et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25(4):502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 27.Takuma K, et al. Ginkgo biloba extract EGb 761 attenuates hippocampal neuronal loss and cognitive dysfunction resulting from chronic restraint stress in ovariectomized rats. Neuroscience. 2007;149(2):256–262. doi: 10.1016/j.neuroscience.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 28.Genazzani AR, et al. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13(2):175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- 29.Walesiuk A, Trofimiuk E, Braszko JJ. Gingko biloba extract diminishes stress-induced memory deficits in rats. Pharmacol Rep. 2005;57(2):176–187. [PubMed] [Google Scholar]
- 30.Walesiuk A, Trofimiuk E, Braszko JJ. Ginkgo biloba normalizes stress-and corticosterone-induced impairment of recall in rats. Pharmacol Res. 2006;53(2):123–128. doi: 10.1016/j.phrs.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekaran K, et al. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;(36 Suppl 1):S89–S94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- 32.Nada SE, Shah ZA. Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis. 2012;46(1):180–189. doi: 10.1016/j.nbd.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung JH, et al. Ginkgo biloba extract (EGb 761) prevents the ischemic brain injury-induced decrease in parvalbumin expression. Lab Anim Res. 2012;28(2):77–82. doi: 10.5625/lar.2012.28.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valvezan AJ, Klein PS. GSK-3 and Wnt Signaling in Neurogenesis and Bipolar Disorder. Front Mol Neurosci. 2012;5:1. doi: 10.3389/fnmol.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H, et al. PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell. 2010;17(5):497–509. doi: 10.1016/j.ccr.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response--effect of gender differences. Injury. 2007;38(12):1382–1391. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvin B, et al. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev. 1996;20(3):445–452. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 39.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manitt C, Thompson KM, Kennedy TE. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J Neurosci Res. 2004;77(5):690–700. doi: 10.1002/jnr.20199. [DOI] [PubMed] [Google Scholar]
- 41.Bradford D, Cole SJ, Cooper HM. Netrin-1: diversity in development. Int J Biochem Cell Biol. 2009;41(3):487–493. doi: 10.1016/j.biocel.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Masuda T, et al. Netrin-1 acts as a repulsive guidance cue for sensory axonal projections toward the spinal cord. J Neurosci. 2008;28(41):10380–10385. doi: 10.1523/JNEUROSCI.1926-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu H, et al. Netrin-1 hyperexpression in mouse brain promotes angiogenesis and long-term neurological recovery after transient focal ischemia. Stroke. 2012;43(3):838–843. doi: 10.1161/STROKEAHA.111.635235. [DOI] [PubMed] [Google Scholar]
- 44.Phoenix CH, et al. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 45.Bialek M, et al. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004;56(5):509–518. [PubMed] [Google Scholar]
- 46.Pike CJ, et al. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53(5):693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 48.Ahlbom E, et al. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11(4):1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 49.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 50.Yu WH. Sex difference in the regeneration of the hypoglossal nerve in rats. Brain Res. 1982;238(2):404–406. doi: 10.1016/0006-8993(82)90114-7. [DOI] [PubMed] [Google Scholar]
- 51.Yu WH. Effect of testosterone on the regeneration of the hypoglossal nerve in rats. Exp Neurol. 1982;77(1):129–141. doi: 10.1016/0014-4886(82)90149-2. [DOI] [PubMed] [Google Scholar]
- 52.Yu WH, Yu MC. Acceleration of the regeneration of the crushed hypoglossal nerve by testosterone. Exp Neurol. 1983;80(2):349–360. doi: 10.1016/0014-4886(83)90288-1. [DOI] [PubMed] [Google Scholar]
- 53.Ayala P, et al. Androgen receptor overexpression is neuroprotective in experimental stroke. Transl Stroke Res. 2011;2(3):346–357. doi: 10.1007/s12975-011-0079-z. [DOI] [PubMed] [Google Scholar]