Abstract
Background
Several peroxisome proliferator-activated receptor (PPAR) agonists reduce voluntary alcohol consumption in rodent models, and evidence suggests that PPARα and γ subunits play an important role in this effect. To define the subunit dependence of this action, we tested selective PPARα and α/γ agonists and antagonists in addition to null mutant mice lacking PPARα.
Methods
The effects of fenofibrate (PPARα agonist) and tesaglitazar (PPARα/γ agonist) on continuous and intermittent two-bottle choice drinking tests were examined in male and female wild-type mice and in male mice lacking PPARα. We compared the ability of MK886 (PPARα antagonist) and GW9662 (PPARγ antagonist) to inhibit the effects of fenofibrate and tesaglitazar in wild-type mice. The estrogen receptor antagonist, tamoxifen, can inhibit PPARγ-dependent transcription and was also studied in male and female mice.
Results
Fenofibrate and tesaglitazar reduced ethanol consumption and preference in wild-type mice, but these effects were not observed in mice lacking PPARα. MK886 inhibited the action of fenofibrate, but not tesaglitazer, while GW9662 did not inhibit either agonist. The PPAR agonists were more effective in male mice compared to females, and drinking in the continuous two-bottle choice test was more sensitive to fenofibrate and tesaglitazar compared to drinking in the intermittent access test. Tamoxifen also reduced ethanol consumption in male mice and this action was inhibited by GW9662, but not MK886, suggesting that it acts by activation of PPARγ.
Conclusions
Our study using selective PPAR agonists, antagonists, and null mutant mice indicates a key role for PPARα in mediating reduced ethanol consumption by fenofibrate and tesaglitazar.
Keywords: fenofibrate, tesaglitazar, MK886, GW9662, PPARα
Introduction
Peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors that act as ligand-activated transcription factors. Although prescribed for dyslipidemia and type 2 diabetes (Chigurupati et al., 2015, Grygiel-Gorniak, 2014), PPAR agonists also demonstrate anti-addictive properties in preclinical models (Le Foll et al., 2013).
Activation of two isoforms of PPARs, α and/or γ (by fenofibrate, pioglitazone, and tesaglitazar), reduced ethanol intake and preference in male C57BL/6J mice; however, a selective PPARδ agonist or an agonist for all three PPAR isoforms did not decrease ethanol consumption (Blednov et al., 2015). The PPARα agonist, fenofibrate, reduced ethanol intake in UChB rats (Karahanian et al., 2014). Another PPARα agonist, gemfibrozil, also decreased voluntary ethanol consumption in male Sprague Dawley rats (Barson et al., 2009). The PPARγ agonists, pioglitazone and rosiglitazone, decreased ethanol consumption, stress-induced relapse, and withdrawal in alcohol-preferring rats (Stopponi et al., 2013, Stopponi et al., 2011). Furthermore, data from a human genome-wide association study completed in the Collaborative Study on the Genetics of Alcoholism supported an association of single nucleotide polymorphisms in PPARA and PPARG with alcohol withdrawal and PPARGC1A with alcohol dependence but found no association for PPARD with either phenotype (Blednov et al., 2015). Overall, these findings support the involvement of PPARα and γ subunits in alcohol drinking in animals and humans.
In light of these findings, several key considerations remain to be addressed: i) determining the subunit dependence and the dose-response relationship for reducing ethanol intake, ii) defining potential "off-target" actions of PPAR agonists (Wright et al., 2014), and iii) studying potential sex differences of PPAR agonists on ethanol drinking. In this study, we defined the selective role of PPAR agonists in alcohol drinking, and in the companion study (Blednov et al., in press), we explored their actions in other alcohol-dependent and -independent behaviors.
Materials and Methods
Mice
Male and female C57BL/6J (B6) mice were taken from a colony maintained at The University of Texas at Austin (original breeders were purchased from Jackson Laboratories, Bar Harbor, ME). 129S4/SvJae-Pparatm1Gonz/J (hereafter referred to as 129S4) (stock #003580) mice were purchased from Jackson Laboratories, and the colony was maintained by heterozygous breeding. In pilot experiments we found that these mice would not consume high concentrations of ethanol. Therefore, mutant mice were backcrossed 4 times on a B6 background, and this new colony was maintained by heterozygous breeding and wild-type littermates were used as controls. Before the drinking studies, mice were group-housed 4–5 to a cage. Food and water were available ad libitum. The vivarium was maintained on a 12:12 hour light:dark cycle with lights on at 7:00 a.m.; however, the experimental rooms used for all drinking experiments were maintained on a reversed 12:12 hour light:dark cycle with lights off at 10:00 a.m. Before beginning experiments, mice were moved to their experimental room and remained there for at least 2 weeks to adapt to the new light cycle. The temperature and humidity of the rooms were kept constant. Baseline drinking began when the mice were 2–3 months old, and mice were individually housed. Throughout the study, males and females were housed in separate rooms. Experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin.
Baseline Drinking
Before beginning the drinking tests (described below), mice consumed 10 or 15% (v/v) ethanol for at least 3 weeks before the continuous (every day) two-bottle choice test and for about 5 weeks before the intermittent (every other day) two-bottle choice test. After these periods, 24-hour ethanol consumption was measured for at least 4 days of the continuous test and for 4 drinking days of the intermittent test to ensure stable consumption. Consumption was considered stable if the average 2-day intake measured over 4 drinking days was similar. For the continuous two-bottle choice test, ethanol intake was then measured after saline administration for 2 drinking days, and mice were grouped to provide similar mean levels of ethanol intake. From drinking day 3 in both tests, mice were administered saline or drugs once daily (in the intermittent test, injections were given daily even though ethanol drinking was every other day) and results are presented as the average from 2-day periods of consecutive drinking using different bottle positions. Overall, mice were exposed to ethanol for 3–5 weeks followed by 4 days of measured drinking before beginning the drug studies, which lasted up to 14 days. We consider this chronic drinking because it is at least 5 weeks of ethanol exposure. A 10% ethanol solution was used in the two-bottle choice experiment with wild-type and PPARα knockout mice on a 129S4 background (Supplemental Fig. 1), whereas all other experiments were performed using 15% ethanol.
Drug Administration
For the 24-hour continuous access test, fenofibrate (10, 50, 100 and 150 mg/kg) and tesaglitazar (1.5 mg/kg) were administered orally by gavage (p.o.). The PPARγ inhibitor (GW9662) (Leesnitzer et al., 2002) and PPARα inhibitor (MK886) (Kehrer et al., 2001) were injected i.p. at 5 mg/kg 30 minutes before injection of PPAR agonists. Tamoxifen (40 mg/kg, i.p.) was also tested with PPAR antagonists (which were administered p.o. 15 minutes after tamoxifen). Tamoxifen was then injected at 5, 25, and 75 mg/kg (i.p.) 1 hour before drinking sessions. Drugs were purchased from Sigma-Aldrich (St. Louis, MO), Tocris Bioscience (Minneapolis, MN), or Santa Cruz Biotechnology, Inc. (Dallas, TX). All drugs were freshly prepared as suspensions in saline with 4–5 drops of Tween-80 and administered in a volume of 0.05 ml/10 g of body weight (for fenofibrate and tesaglitazar), 0.1 ml/10 g of body weight (for PPAR antagonists), or 0.3 ml/10 g of body weight (for tamoxifen). Saline containing 4–5 drops of Tween-80 was administered to control groups. Single use, sterile Becton Dickinson gavage needles (27.5 gauge; model #305109) were used.
Ethanol Drinking - 24-hour two-bottle choice
Two drinking bottles were continuously available to individually housed mice. One contained water and the other 10 or 15% ethanol (v/v). A 10% ethanol solution was used in the two-bottle choice experiment with wild-type and PPARα knockout mice on a 129S4 background (Supplemental Fig. 1), whereas all other experiments were performed using 15% ethanol. Bottle positions were changed daily to control for position preferences. Measurements were carried out by weighing bottles containing ethanol and water before and after each drinking day. The amount of ethanol (in g) was based on the weight of ethanol solution consumed and the percentage (v/v) and density of ethanol and was then expressed as g ethanol/kg of body weight. Total amount of fluid consumed was the total amount of water and ethanol solution (in g) expressed as g total fluid/kg of body weight.
Once stable ethanol consumption was reached (see Baseline Drinking), we measured ethanol intake after 2 days of saline injections and grouped mice to provide similar mean levels of ethanol intake and preference (day 2 in graphs). Slightly lower ethanol intake was observed following injections, perhaps related to the stress of the injection procedure. However, all drugs were compared to the respective control (saline) level of intake and thus represent specific drug-induced effects. We measured consumption (g/kg body weight/time) and calculated preference as the amount of ethanol consumed divided by the total amount of fluids consumed per day (a value >50% indicates a preference for ethanol). Food was available ad libitum, and mice were weighed every 4 days beginning on day 1. Adult mouse weights are stable, and measuring weight every 4 days is adequate to ensure accuracy; furthermore, no differences in weight between groups were observed during the course of this study. Measurements of ethanol intake, preference, and total fluid intake were calculated after 24 hours and averaged over 2-day periods with different bottle positions. Each point in the graphs (days 2, 4, 6, etc.) represents the average of 2 days of measurement. For example, day 2 is the average of days 1–2 after saline for both control and drug groups; day 4 is the average of days 3–4 after saline or drug, and day 6 is the average of days 5–6 after saline or drug.
Ethanol Drinking - 24-hour access every other day (intermittent drinking)
Intermittent access to ethanol increases voluntary ethanol consumption in rats (Simms et al., 2008) and mice (Melendez, 2011; Osterndorff-Kahanek et al., 2013). We used an intermittent method to assess ethanol consumption in mice with access to one bottle of 15% ethanol and one bottle of water during 24-hour sessions every other day. The placement of the ethanol bottle was alternated with each ethanol drinking session to control for side preferences. Ethanol intake, preference, and total fluid intake were calculated after 24 hours.
Statistical Analyses
The number of mice in each group is shown in the Supplemental Tables and Figure Legends. Data are reported as the mean ± S.E.M. The statistics software program GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) was used to perform one-way or two-way repeated measures ANOVA and Bonferroni post hoc tests. Data from males and females were analyzed separately. Complete statistical analyses are found in the Supplemental Tables.
Results
Effects of PPAR Agonists on Ethanol Consumption
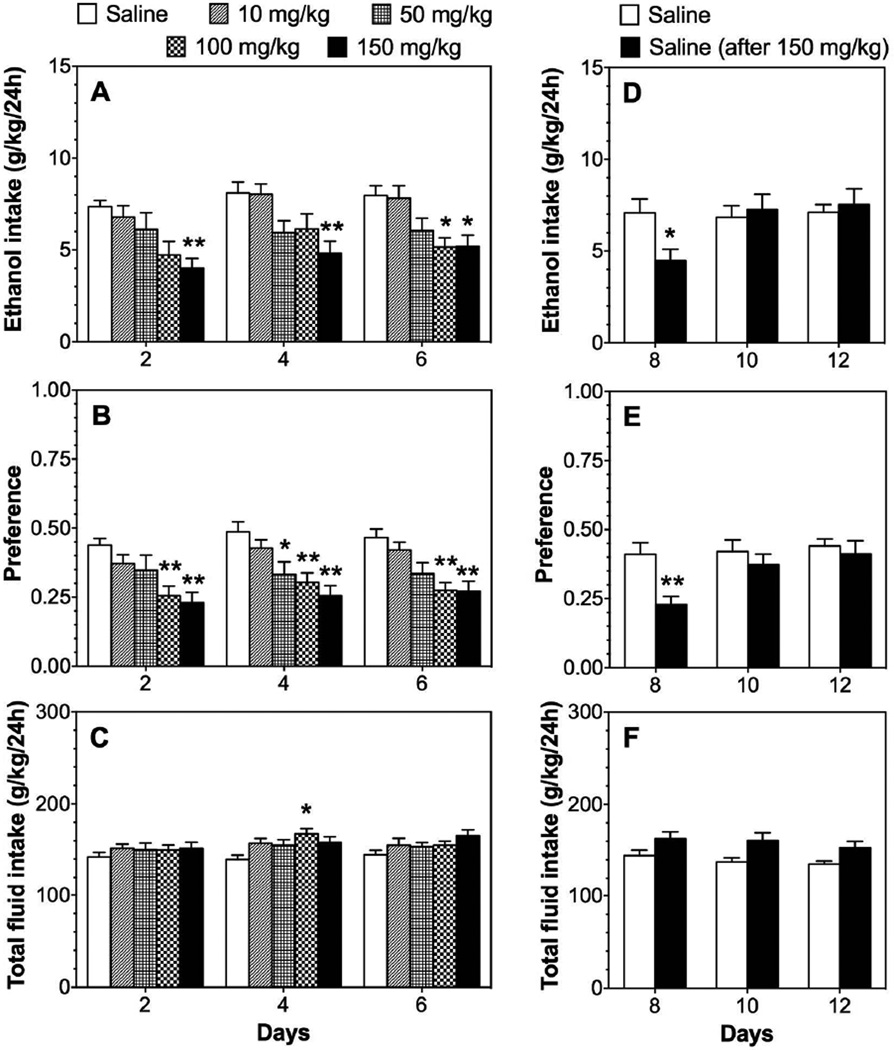
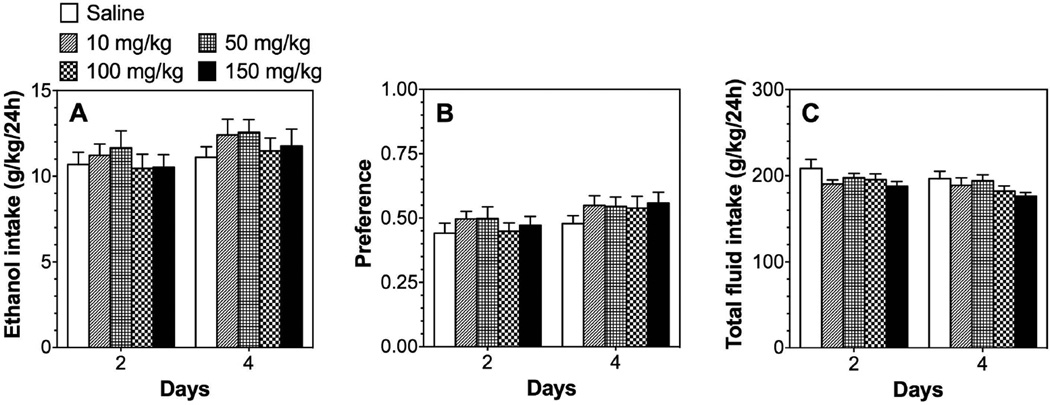
In the two-bottle choice test (continuous 24-hour access to ethanol and water), treatment with the PPARα agonist, fenofibrate, dose dependently reduced ethanol intake [F(4,59)=5.9, p<0.001] and preference [F(4,59)=7.7, p<0.001] without changing total fluid intake in B6 male mice (Fig. 1 A–C; Supplemental Table 1). Post hoc analyses showed significant reduction at doses of 100–150 mg/kg, whereas lower doses (10–50 mg/kg) did not alter ethanol intake. During the washout phase, when injection of fenofibrate was replaced by saline, mice previously treated with 150 mg/kg of fenofibrate showed reduced ethanol consumption [F(2,46)=7.9, p<0.01 treatment × time interaction] and preference [F(2,46)=7.2, p<0.01 treatment × time] only during the first 2 days of saline administration (Fig. 1 D–F; Supplemental Table 1). In contrast to males, fenofibrate did not affect ethanol consumption in females (Fig. 2; Supplemental Table 2).
Figure 1. Fenofibrate dose-dependently reduces 15% ethanol intake in the 24-hour two-bottle choice test in C57BL/6J (B6) male mice.
A–C. Effects of 10–150 mg/kg fenofibrate on ethanol intake, preference, and total fluid intake in male mice; n=12–13 per group. D–F. Extinction of fenofibrate effects in mice previously treated with 150 mg/kg of fenofibrate; n=12–13. Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test for multiple comparisons (*p<0.05, **p<0.01 compared to saline control).
Figure 2. Fenofibrate does not reduce ethanol intake in the 24-hour two-bottle choice test in B6 female mice.
A–C. Effects of 10–150 mg/kg fenofibrate on 15% ethanol intake, preference, and total fluid intake in female mice; n=10 per group. Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test.
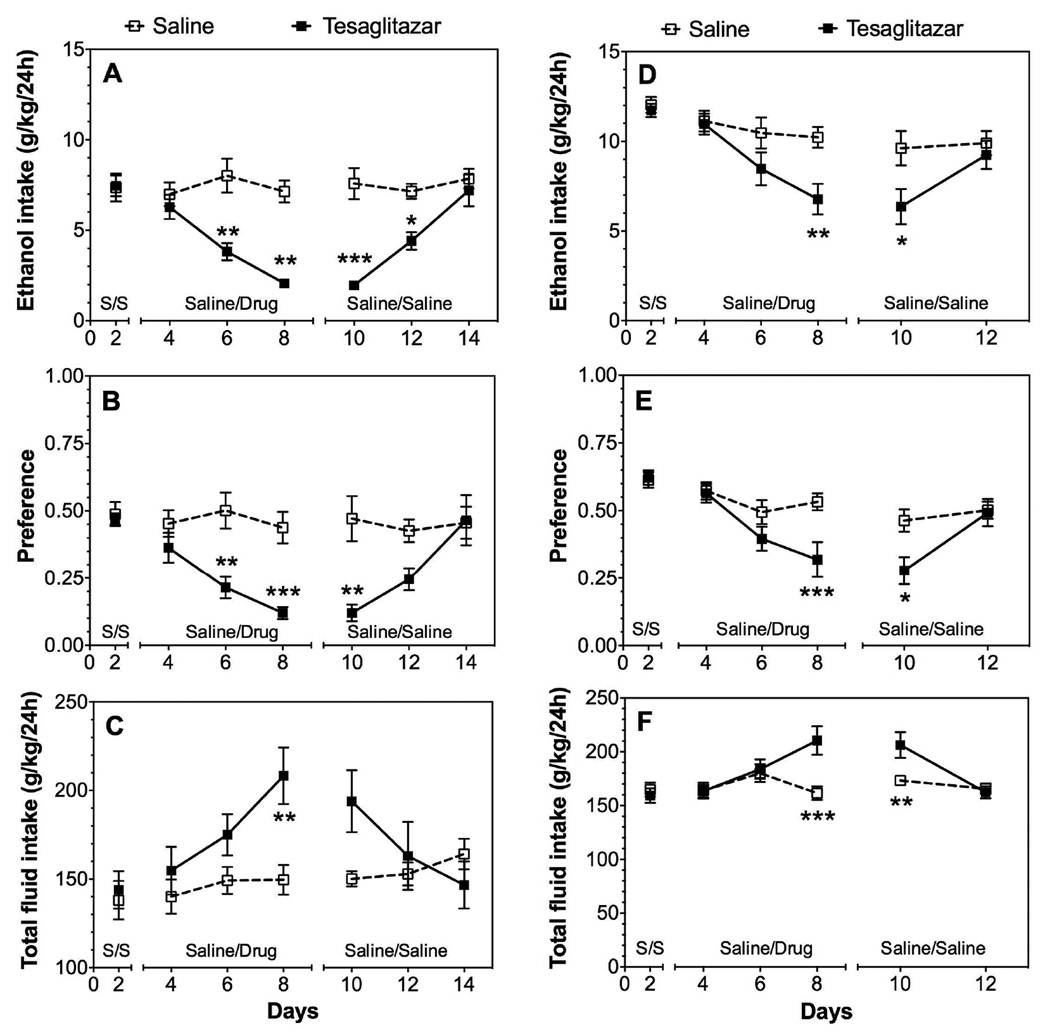
Treatment with tesaglitazar, a dual PPARα and γ agonist, produced a strong reduction of ethanol intake [F(1,10)=19.4, p<0.01] and preference [F(1,10)=13.8, p<0.01] in B6 male mice (Fig. 3 A, B; Supplemental Table 1). Total fluid intake also increased [F(2,20)=9.9, p<0.01 treatment × time] (Fig. 3 C; Supplemental Table 1). In females, much smaller reductions in ethanol intake [F(1,45)=8.9, p<0.01 for fenofibrate; F(2,40)=24.5, p<0.001 for tesaglitazar] and preference [F(1,45)=25.5, p<0.001 for fenofibrate; F(2,40)=23.8, p<0.001 for tesaglitazar] were observed over time (Fig. 3 D–F; Supplemental Table 2). As shown in Fig. 3, the effect of tesaglitazar was long lasting in male mice and was observed for at least 2 days after switching from drug to saline injection. There were significant treatment and time-dependent effects on the amount of ethanol consumed [F(1,10)=20.8, p<0.01 treatment; F(2,20)=13.2, p<0.001 treatment × time], preference for ethanol [F(1,10)=5.4, p<0.05 treatment; F(2,20)=9.3, p<0.01 treatment × time], and total fluid intake [F(2,20)=21.1, p<0.001 treatment × time] (Supplemental Table 1).
Figure 3. Tesaglitazar reduces 15% ethanol intake in the 24-hour two-bottle choice test in male and female B6 mice.
A–C. Effects of 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male mice and extinction of these effects in mice previously treated with tesaglitazar; n=10 per group. D–F. Effects of 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in female mice and extinction of these effects in mice previously treated with tesaglitazar; n=11 per group. On days 3–8, mice received saline or tesaglitazar daily (treatment period). On days 9 and after, mice from both treatment groups received saline daily (extinction period). Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test (*p<0.05, **p<0.01, ***p<0.001 compared to saline control). S represents treatment with saline.
In the two-bottle choice test with intermittent access to ethanol, treatment with 150 mg/kg fenofibrate reduced ethanol intake [F(2,22)=5.3, p<0.05 in males; F(2,24)=6.1, p<0.01 in females] and preference [F(2,22)=3.8, p<0.05 in males; F(2,24)=5.7, p<0.01 in females] in both sexes (Fig. 4, Supplemental Table 3).
Figure 4. Effects of fenofibrate on 15% ethanol intake in the 24-hour intermittent two-bottle choice test in male and female B6 mice.
A–C. Effects of 100 and 150 mg/kg fenofibrate on ethanol intake, preference, and total fluid intake in male mice; n=8–9 per group. D–F. Effects of 100 and 150 mg/kg fenofibrate on ethanol intake, preference, and total fluid intake in female mice; n=9 per group. Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test (*p<0.05 compared to saline control).
As in our previous study (Osterndorrff-Kahanek et al., 2013), we did not observe large differences in ethanol intake between continuous and intermittent access tests. Indeed, intermittent access to alcohol is not always accompanied by large increases in intake compared with continuous drinking (Crabbe et al., 2012). Nevertheless, we did observe increased alcohol preference during intermittent access. Preference for 15% alcohol in male control mice treated with saline was approximately 0.5 in the continuous test (Fig. 1B) and increased to approximately 0.75 in the intermittent test (Fig. 4B). Similar effects on preference for 15% alcohol were reported in B6 male mice in these two drinking tests (Melendez, 2011).
Effects of PPARα and PPARγ Antagonists on Ethanol Consumption
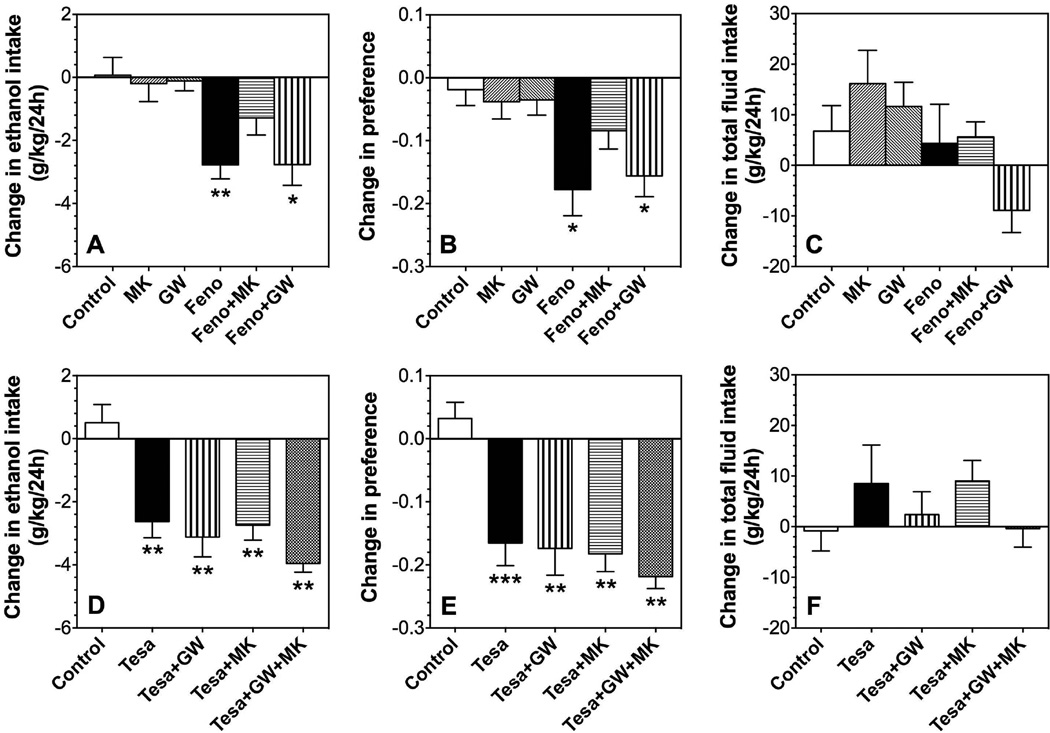
We next tested specific antagonists to determine if the effects of fenofibrate and tesaglitazer on continuous access two-bottle choice drinking in male mice were mediated by PPARα or PPARγ. As shown above, 150 mg/kg fenofibrate reduced ethanol intake (p<0.01) and preference (p<0.05) compared to control but did not change total fluid intake (Fig. 5 A–C). In the absence of fenofibrate, the PPARγ antagonist (GW9662) or the PPARα antagonist (MK886) did not alter ethanol consumption or preference. Pretreatment with MK886 reduced the effect of fenofibrate on ethanol intake and preference. However, pre-treatment with GW9662 did not change the effectiveness of fenofibrate.
Figure 5. Effects of fenofibrate, tesaglitazar, MK886, and GW9662 on 15% ethanol intake in the 24-hour two-bottle choice test in B6 male mice.
A–C. Effects of 150 mg/kg fenofibrate (Feno) in the absence and presence of 5 mg/kg MK886 (MK) or 5 mg/kg GW9662 (GW) on ethanol intake, preference, and total fluid intake in male mice; n=9–10 per group. D–F. Effects of 1.5 mg/kg tesaglitazar (Tesa) in the absence and presence of 5 mg/kg MK886 or 5 mg/kg GW9662 on ethanol intake, preference, and total fluid intake in male mice; n=10 per group. Data are presented as the differences between 2-day averages after drug injection (days 3, 4 after fenofibrate or days 5,6 after tesaglitazar) and the first 2 days of saline (control) injections (days 1, 2). Data were analyzed by one-way ANOVA followed by Bonferroni’s test (*p<0.05, **p<0.01, ***p<0.001 compared to control).
As previously observed, 1.5 mg/kg tesaglitazar reduced ethanol intake (p<0.01) and preference (p<0.001) compared to control but did not change total fluid intake (Fig. 5 D–F). Pre-treatment with GW9662 or MK886 did not block the effects of tesaglitazar on ethanol intake or preference (p<0.01 compared to control). Co-administration of GW9662 and MK886 also failed to prevent the effects of tesaglitazar (p<0.01 compared to control).
Effects of Fenofibrate and Tesaglitazar on Ethanol Consumption in Mice Lacking PPARα
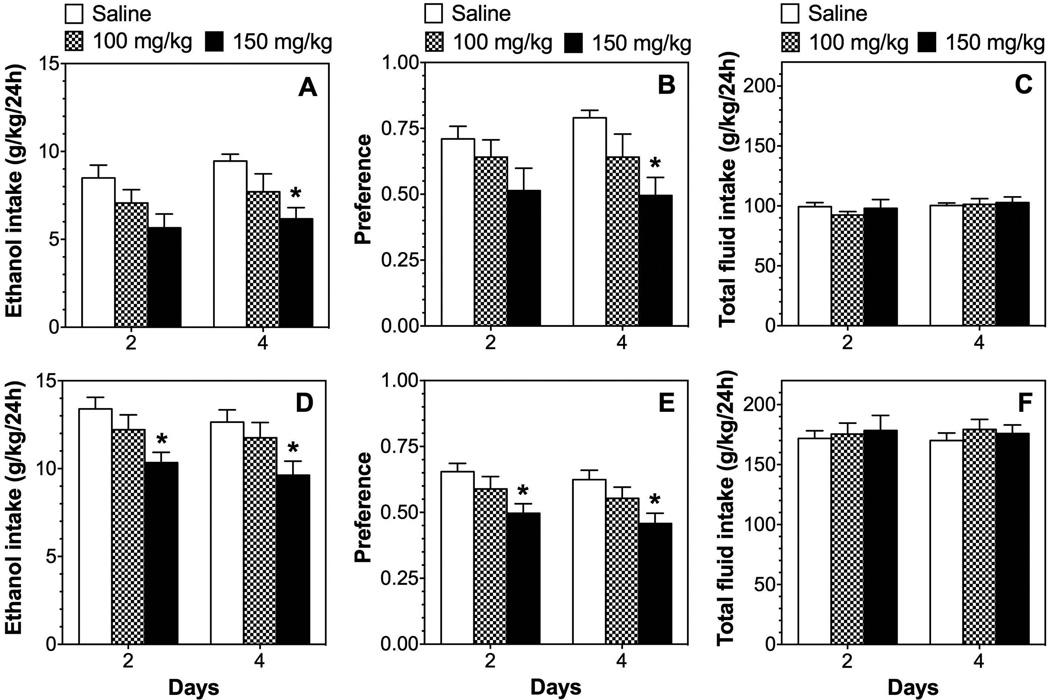
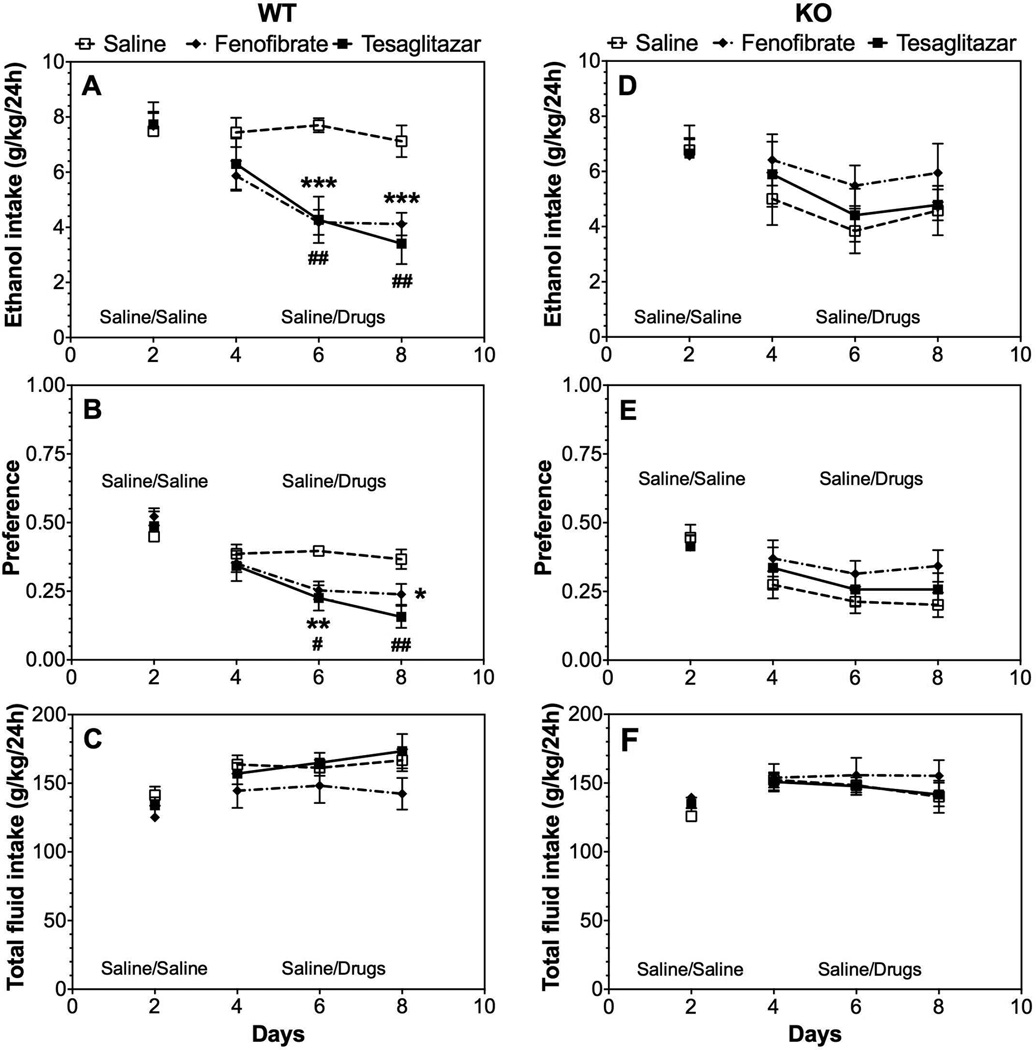
We extended our studies of the role of PPARα by testing the effects of fenofibrate and tesaglitazar in wild-type and null mutant mice lacking this receptor. In wild-type mice produced by heterozygous breeding (129S4/SvJae-Pparatm1Gonz/J), both drugs reduced ethanol intake and preference after 6 injections (Supplemental Fig. 1 A, B), but did not affect total fluid intake (Supplemental Fig. 1 C). Fenofibrate and tesaglitazar, respectively, produced significant treatment × time interaction effects on ethanol intake [F(2,44)=6.5, p<0.01; F(2,44)=8.4, p<0.001] and preference for ethanol [F(2,44)=6.1, p<0.01; F(2,44)=3.7, p<0.05] (Supplemental Table 4). In mice lacking PPARα, the drugs were ineffective in all tests (Supplemental Fig. 1 D–F). However, these mice have an inbred 129 genetic background and ethanol consumption was low compared to B6 mice. Furthermore, the 129S4 mice would not consume the 15% ethanol solution used in the other experiments, so 10% ethanol was used instead. Because of these potentially confounding factors, mice were backcrossed 4 times on a B6 genetic background, and wild-type and knockout mice were then tested using 15% ethanol (after backcrossing, mice consumed the 15% ethanol solution). In wild-type mice from this colony, both drugs reduced ethanol intake and preference without changing total fluid intake (Fig. 6 A–C). Treatment with fenofibrate and tesaglitazar, respectively, produced significant effects on ethanol intake [F(1,16)=25.6, p<0.001; F(1,16)=10.3, p<0.01] and preference for ethanol [F(1,16)=7.5, p<0.05; F(1,16)=7.9, p<0.05] (Supplemental Table 5). However, fenofibrate and tesaglitazar had no effect in mice lacking PPARα (Fig. 6 D–F).
Figure 6. Effects of fenofibrate and tesaglitazar on 15% ethanol intake in the 24-hour two-bottle choice test in male wild-type and PPARα knockout mice on a 129S4 × B6(N4) genetic background.
A–C. Effects of 150 mg/kg fenofibrate or 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male wild-type (WT) mice; n=9 per group. D–F. Effects of 150 mg/kg fenofibrate or 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male PPARα knockout (KO) mice; n=7 per group. Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test (*p<0.05, **p<0.01, ***p<0.001 fenofibrate compared to saline control; # p<0.05, ## p<0.01 tesaglitazar compared to saline control).
Effect of Tamoxifen on Ethanol Consumption
The estrogen receptor (ER) can bind to PPARγ response elements and inhibit PPAR-dependent transcription (Bonofiglio et al., 2005). We explored the possibility that ERs may exert a tonic inhibition of PPARγ function and administration of the ER antagonist, tamoxifen, would lead to activation of PPARγ signaling. If this hypothesis is correct, then tamoxifen should reduce alcohol consumption in a PPARγ-dependent manner and the effect should be greater in male compared to female mice.
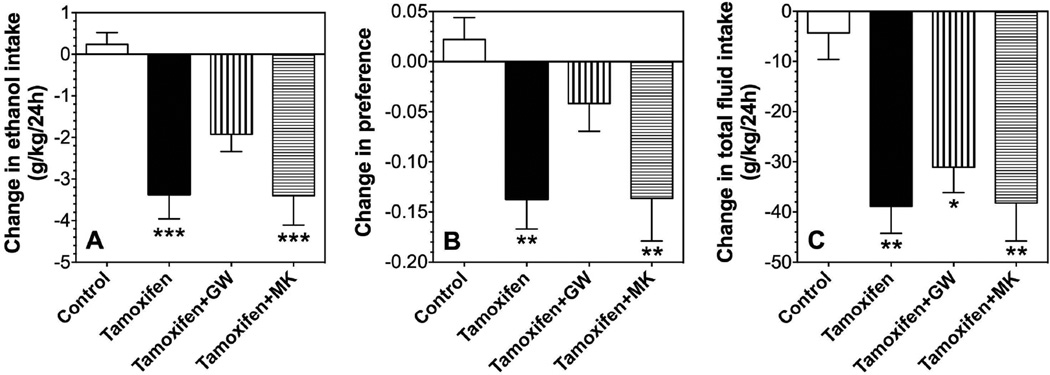
Tamoxifen (40 mg/kg) reduced ethanol intake (p<0.001), preference (p<0.01), as well as total fluid intake (p<0.01) in male mice in the continuous access test (Fig. 7 A–C). Pre-treatment with the PPARγ antagonist, GW9662 (5 mg/kg), blocked the reduction of ethanol intake and preference but not the reduction of total fluid intake (Fig. 7 A–C). Pre-treatment with the PPARα antagonist, MK886 (5 mg/kg), did not alter the effect of tamoxifen on ethanol intake, preference, or total fluid intake (Fig. 7 A–C).
Figure 7. Effects of tamoxifen, MK886, and GW9662 on ethanol intake in the 24-hour two-bottle choice test in B6 male mice.
A–C. Effects of 40 mg/kg tamoxifen in the absence and presence of 5 mg/kg MK886 (MK) or 5 mg/kg GW9662 (GW) on 15% ethanol intake, preference, and total fluid intake in male mice; n=10–12 per group. Data are presented as the differences between 2-day averages after drug injection (days 3, 4) and the first 2 days of saline (control) injections (days 1, 2). Data were analyzed by one-way ANOVA followed by Bonferroni’s test (*p<0.05, **p<0.01, ***p<0.001 compared to control).
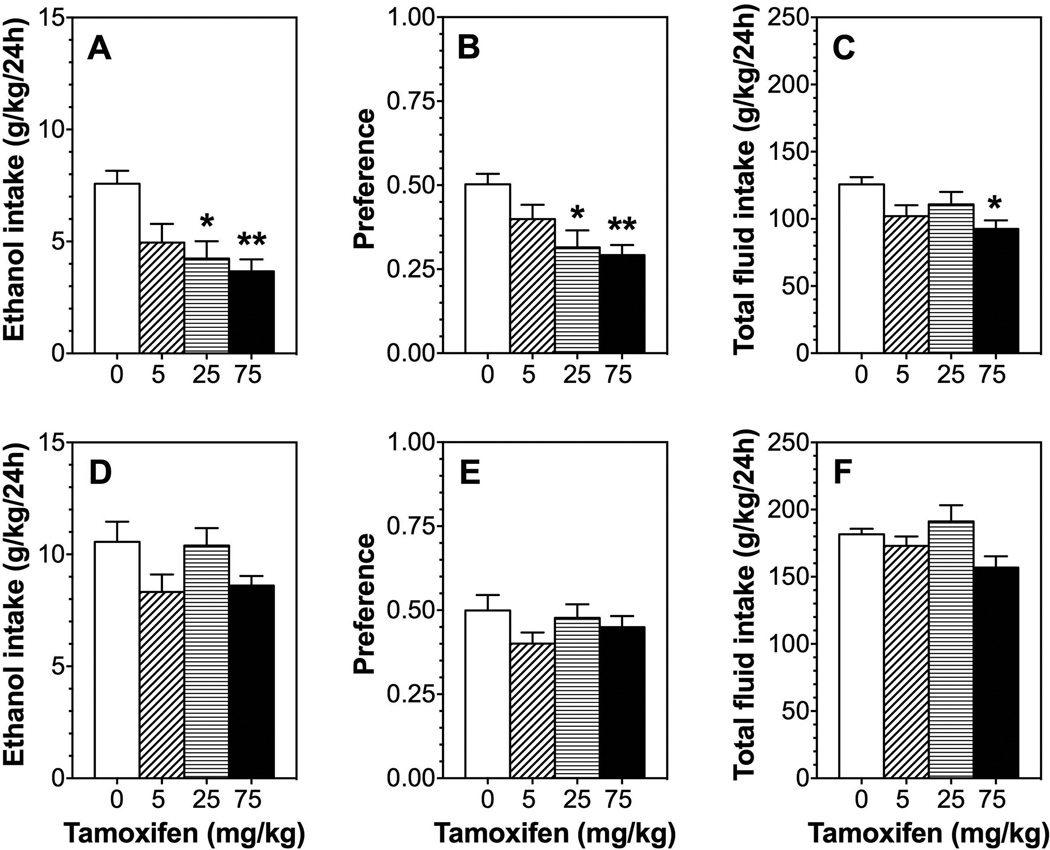
Tamoxifen dose-dependently reduced ethanol consumption [F(3,28)= 6.3; p<0.01] and preference [F(3,28)= 5.9; p<0.01] in male mice but did not significantly affect total fluid intake (Fig. 8 D–F). In female mice, tamoxifen did not alter ethanol consumption, preference, or total fluid intake (Fig. 8 A–C).
Figure 8. Tamoxifen dose-dependently reduces ethanol intake in the 24-hour two-bottle choice test in male but not female B6 mice.
A–C. Effects of tamoxifen (0–75 mg/kg) on 15% ethanol intake, preference, and total fluid intake in male mice; n=8 per group. D–F. Effects of tamoxifen (0–75 mg/kg) on ethanol intake, preference, and total fluid intake in female mice; n=8–9 per group. Data were analyzed by one-way ANOVA followed by Bonferroni’s test (*p<0.05, **p<0.01 compared to control).
Discussion
Both fenofibrate and tesaglitazar reduced ethanol intake in B6 mice, confirming other studies showing that PPAR agonists reduce ethanol consumption in rats and mice (Barson et al., 2009, Blednov et al., 2015, Ferguson et al., 2014, Karahanian et al., 2014, Stopponi et al., 2013, Stopponi et al., 2011). Reduced intake was observed for a few days after the last PPAR agonist injection, suggesting a long-acting transcriptional mechanism consistent with the function of PPARs as nuclear receptors (Ferguson et al., 2014). The ability of PPAR agonists to decrease ethanol consumption was dependent on the drinking test used and the sex of the mice, with the effects on continuous drinking being more sensitive than intermittent drinking. In the continuous access test, the drugs reduced drinking only in males. Similar sex-dependent effects were reported in a study of pain hypersensitivity, where fenofibrate reduced allodynia in male but not female mice (Sorge et al., 2015).
We did not observe large increases in ethanol intake during intermittent compared to continuous access, although intermittent drinking does not always produce higher levels of intake (Crabbe et al., 2012; Osterndorff-Kahanek et al., 2013). We did, however, observe increased preference for alcohol in the intermittent test. It is also important to consider that although B6 mice may consume similar amounts of total ethanol in the continuous and intermittent access tests, different effects on brain and liver transcriptomes have been observed (Osterndorff-Kahanek et al., 2013). Distinct genomic effects induced by the alcohol exposure protocol may explain the specific effects of PPAR agonists in the two drinking tests. Differing drug effects on continuous vs. intermittent drinking have been observed previously (Hopf et al., 2011), providing further support for unique neurobiological mechanisms underlying these two methods of alcohol administration.
We provide clear evidence that the reduction of ethanol intake by fenofibrate is mediated by PPARα. This was demonstrated by i) a reduced fenofibrate effect in the presence of a selective PPARα antagonist (MK886) but not a PPARγ antagonist (GW9662), and ii) mice lacking PPARα did not show any changes in ethanol intake after treatment with fenofibrate. However, it is more difficult to explain the mechanism of tesaglitazar, which activates PAPRα and γ simultaneously (Ljung et al., 2002). A role for PPARγ in ethanol drinking was suggested because PPARγ agonists (pioglitazone and rosiglitazone) decreased ethanol consumption in a two-bottle choice paradigm (Stopponi et al., 2013, Stopponi et al., 2011). These effects were prevented by injection of GW9662 into the lateral cerebroventricle, showing the importance of central PPARγ subtypes in reducing alcohol drinking (Stopponi et al., 2011). We previously found that, pioglitazone reduced ethanol intake in B6 mice, but only for 6 hours after injection (Blednov et al., 2015). In the current study, we used tamoxifen because it can activate PPARγ signaling by blocking the inhibitory effect of the ER on the subunit (Bonofiglio et al., 2005). Tamoxifen has other effects due to its action on all ERs, but we reasoned that if its effects on ethanol consumption were blocked by GW9662 (and not by MK886), then this was due to activation of PPARγ. This was confirmed in our study, providing additional evidence that activation of PPARγ reduces alcohol consumption. Because female mice drink more than males, one might expect tamoxifen to reduce drinking in females; however, this was not observed, implying that the increased drinking in females is not likely to be driven by ERs.
In view of the accumulating evidence for a role of both PPARα and PPARγ in reducing alcohol intake, it is surprising that neither MK886 nor GW9662 (nor both injected together) prevented the reduction of alcohol intake by tesaglitazar. This suggests that not all forms of PPARα or PPARγ are sensitive to MK886 or GW9662 and that genetic deletion is required to completely block PPARα signaling. Also, α/β/γ isoforms may function as a triad, and the expression level and activity of PPARα and γ may be regulated by β/δ (Aleshin et al., 2013), raising the possibility that binding of an antagonist to this complex has different effects from global subunit deletion. We should also note that the therapeutic applications of dual agonists like tesaglitazar are limited by side effects. Tesaglitazar and other dual PPAR agonists (muraglitazar, aleglitazer) have been investigated in late-phase clinical trials, but side effects, such as congestive heart failure and renal toxicity, caused termination of the clinical development of these agents (Lincoff et al., 2014, Rosenson et al., 2012).
Overall, there is extensive and compelling evidence that PPAR agonists reduce alcohol consumption and also mitigate behavioral and addictive actions of other drugs, including nicotine and opioids (de Guglielmo et al., 2015, Le Foll et al., 2013). Our present work shows that the PPARα subunit is critical for the reduction of alcohol consumption by fenofibrate and tesaglitazar. We also demonstrate that some effects on drinking depend on sex, emphasizing the importance of testing both male and female animals. An ongoing clinical trial of fenofibrate for use in the treatment of alcohol use disorder further highlights the potential role of PPARα subunits in addictive behaviors (https://clinicaltrials.gov/ct2/show/NCT02158273).
Supplementary Material
A–C. Effects of 150 mg/kg fenofibrate or 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male wild-type (WT) mice; n=11–12 per group. D–F. Effects of 150 mg/kg fenofibrate or 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male PPARα knockout (KO) mice; n=11–12 per group. A 10% ethanol solution was used for these experiments because the 129S4 mice would not consume 15% ethanol. Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test (*p<0.05 fenofibrate compared to saline control; # p<0.05 tesaglitazar compared to saline control).
Acknowledgments
The authors thank Jody Mayfield for help writing and editing the manuscript and Geyu Liu for technical assistance. This research was supported by the National Institutes of Health National Institute of Alcohol Abuse and Alcoholism (grants AA013520/INIA-West and AA006399).
Footnotes
Author Contributions. Y.A.B. designed and performed experiments, analyzed data, prepared graphs/tables, and wrote the manuscript; M.B., J.M.B., and E.E.S performed experiments; R.A.H. designed experiments and wrote the manuscript.
The authors declare no conflicts of interest.
REFERENCES
- Aleshin S, Strokin M, Sergeeva M, Reiser G. Peroxisome proliferator-activated receptor (PPAR)beta/delta, a possible nexus of PPARalpha- and PPARgamma-dependent molecular pathways in neurodegenerative diseases: Review and novel hypotheses. Neurochem Int. 2013;63:322–330. doi: 10.1016/j.neuint.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, Leibowitz SF. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol. 2009;43:433–441. doi: 10.1016/j.alcohol.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, Edenberg HJ, Wetherill L, Hesselbrock V, Foroud T, Harris RA. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res. 2015;39:136–145. doi: 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA. PPAR agonists: II. Fenofibrate and tesaglitazar alter behaviors related to voluntary alcohol consumption. Alcohol Clin Exp Res. doi: 10.1111/acer.12972. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Ando S. Estrogen receptor alpha binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor gamma signaling in breast cancer cells. Clin Cancer Res. 2005;11:6139–6147. doi: 10.1158/1078-0432.CCR-04-2453. [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Dhanaraj SA, Balakumar P. A step ahead of PPARgamma full agonists to PPARgamma partial agonists: therapeutic perspectives in the management of diabetic insulin resistance. Eur J Pharmacol. 2015;755:50–57. doi: 10.1016/j.ejphar.2015.02.043. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P. Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol Alcohol. 2012;47:509–517. doi: 10.1093/alcalc/ags067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, Giordano A, Senzacqua M, Somaini L, Cippitelli A, Gaitanaris G, Demopulos G, Damadzic R, Tapocik J, Heilig M, Ciccocioppo R. PPARgamma activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology. 2015;40:927–937. doi: 10.1038/npp.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson LB, Most D, Blednov YA, Harris RA. PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology. 2014;86:397–407. doi: 10.1016/j.neuropharm.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry. 2011;69:618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Fernandez K, Israel Y. Fenofibrate--a lipid-lowering drug--reduces voluntary alcohol drinking in rats. Alcohol. 2014;48:665–670. doi: 10.1016/j.alcohol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS, La E, Thuillier P, Datta K, Fischer SM, Vanden Heuvel JP. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochemical J. 2001;356:899–906. doi: 10.1042/0264-6021:3560899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr Drug Targets. 2013;14:768–776. doi: 10.2174/1389450111314070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Tardif JC, Schwartz GG, Nicholls SJ, Ryden L, Neal B, Malmberg K, Wedel H, Buse JB, Henry RR, Weichert A, Cannata R, Svensson A, Volz D, Grobbee DE, AleCardio I. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311:1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- Ljung B, Bamberg K, Dahllof B, Kjellstedt A, Oakes ND, Ostling J, Svensson L, Camejo G. AZ 242, a novel PPARalpha/gamma agonist with beneficial effects on insulin resistance and carbohydrate and lipid metabolism in ob/ob mice and obese Zucker rats. J Lipid Res. 2002;43:1855–1863. doi: 10.1194/jlr.m200127-jlr200. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA. Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PloS One. 2013;8:e59870. doi: 10.1371/journal.pone.0059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Wright RS, Farkouh M, Plutzky J. Modulating peroxisome proliferator-activated receptors for therapeutic benefit? Biology, clinical experience, and future prospects. Am Heart J. 2012;164:672–680. doi: 10.1016/j.ahj.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, Ubaldi M, Heilig M, Demopulos G, Gaitanaris G, Ciccocioppo R. Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res. 2013;37:1351–1360. doi: 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Wright MB, Bortolini M, Tadayyon M, Bopst M. Minireview: Challenges and opportunities in development of PPAR agonists. Mol Endocrinol. 2014;28:1756–1768. doi: 10.1210/me.2013-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–C. Effects of 150 mg/kg fenofibrate or 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male wild-type (WT) mice; n=11–12 per group. D–F. Effects of 150 mg/kg fenofibrate or 1.5 mg/kg tesaglitazar on ethanol intake, preference, and total fluid intake in male PPARα knockout (KO) mice; n=11–12 per group. A 10% ethanol solution was used for these experiments because the 129S4 mice would not consume 15% ethanol. Data were analyzed by two-way repeated measures ANOVA followed by Bonferroni’s test (*p<0.05 fenofibrate compared to saline control; # p<0.05 tesaglitazar compared to saline control).