Abstract
BACKGROUND
The relative roles of the mother and fetus in signaling for labor remain poorly understood.
OBJECTIVE
We previously demonstrated using gene-knockout (KO) mice that E. coli-induced preterm delivery is completely dependent upon MyD88, a toll-like receptor adaptor protein. Here, we leveraged this finding to conduct a genetic experiment testing whether the mother, the fetus, or both signal for parturition in bacterially induced labor.
STUDY DESIGN
Six different maternal/fetal genotype combinations for MyD88 were studied: Wild-type (WT) dams carrying either (1) WT or (2) MyD88 heterozygous (het) fetuses (generated by mating WT females with WT or MyD88-KO males, respectively); (3) WT dams carrying MyD88-KO fetuses (generated by replacing the ovaries of WT females with MyD88-KO ovaries, followed by mating with MyD88-KO males). A similar strategy was used to generate MyD88-KO dams carrying (4) MyD88-KO, (5) MyD88 het or (6) WT fetuses. On day 14.5 of gestation, mice received intrauterine injections of either 1 × 109 killed E. coli or sterile medium. Delivery of ≥1 fetus within 48h was considered preterm. A separate group of similarly treated pregnant mice was euthanized 5 hours after surgery for gene expression and tissue analysis.
RESULTS
E. coli-induced preterm delivery is dependent upon maternal and not fetal genotype: >95% of WT and < 5% of MyD88-KO dams deliver prematurely regardless of fetal genotype (p=0.0001). In contrast, fetal survival in utero is influenced by fetal genotype: in MyD88-KO dams, in which premature birth rarely occurs, only 81% of WT and 86% of MyD88-heterozygous fetuses were alive 48 hours after surgery compared to 100% of MyD88-KO fetuses (p < 0.01). mRNAs for the inflammatory mediators IL-1β, TNF, IL-6 and COX-2 were elevated in uterine tissues only in WT mothers treated with E. coli, and were low or undetectable in the uteri of KO mothers or in animals treated with saline. Serum progesterone levels were lower in KO mothers with WT ovaries than in WT mothers with KO ovaries, but bacterial exposure did not impact these levels.
CONCLUSIONS
In the murine E. coli-induced labor model, preterm delivery and uterine expression of inflammatory mediators is determined by the mother and not the fetus, and is not attributable to a decline in serum progesterone.
Keywords: inflammation, mouse model, parturition, preterm labor
Introduction
Despite decades of research, the mechanisms underlying the initiation and maintenance of parturition are not well understood. In particular, the relative roles of the mother and fetus in initiating labor processes are not known. In simplistic terms: does the fetus signal that it is time to be delivered? Does the mother? Is there collaboration (cross-talk) between mother and fetus to generate labor signals? What is the nature of these signals? Recent studies conducted in the mouse provide evidence that the fetus initiates spontaneous labor at term through fetal expression of steroid receptor coactivators (SRC) -1 and -2, which regulate fetal production of surfactant protein A (SP-A), which in turn induces inflammatory pathways in maternal tissues [1–3]. Whether a similar phenomenon occurs in humans remains to be determined, as does the answer to an additional question: when these processes occur at abnormal times (i.e. prematurely), do they recapitulate normal term labor, or are there alternative pathways to parturition? Correct understanding of the mechanisms of parturition has obvious implications for developing interventions to treat preterm labor, a disorder that remains the major cause of neonatal morbidity and mortality world-wide and for which treatment options are limited [4].
Among the various factors that are associated with preterm labor, infection and inflammation represent significant primary causes. As many as 50% of preterm labor cases are associated with infection and/or inflammation within the gestational compartment (with a higher proportion at earlier gestational ages) [5–7]. Infection induces a cascade of molecular events, including activation of the innate immune system, with subsequent expression of inflammatory cytokines, prostaglandins, contraction-associated proteins (CAPs) and ultimately end-organ phenomena characteristic of labor, including uterine contractions, cervical dilation and rupture of fetal membranes.
We took advantage of an experimental model in which an absolute dependence of bacterially induced preterm delivery was previously demonstrated for the toll-like receptor (TLR) adaptor protein MyD88 (myeloid differentiation primary response gene 88) [8]. MyD88 is a protein necessary for transduction of intracellular signals following engagement of most TLRs, which are membrane-bound receptors responsible for activation of the innate immune response to molecular constituents of microbes (known as pathogen-associated molecular patterns, or PAMPs). Such PAMPs include lipopolysaccharide (LPS, which is derived from Gram negative bacteria and engages TLR4) and peptidoglycan (PGN, which is derived from Gram positive bacteria and engages TLR2). We showed previously that following administration of an intrauterine inoculum of killed E. coli sufficient to cause preterm delivery in 100% of wild-type pregnant mice, mice deficient in MyD88 (KO) and carrying MyD88-KO fetuses were completely protected from preterm delivery [8]. This absolute requirement for MyD88 for the dichotomous outcome of preterm delivery allowed us to design an experiment to examine the relative roles of mother and fetuses in parturition by manipulation of maternal-fetal MyD88 genotype combinations.
Materials and Methods
Mice
All procedures involving animals were approved by the NorthShore University HealthSystem Animal Care and Use Committee and conform to the Guide for Care and Use of Laboratory Animals (1996, National Academy of Sciences). MyD88-deficient (KO) mice used in the present studies were from two sources: a) a strain developed and contributed by Professor Shizuo Akira, which was on a mixed C57BL/6J-129 background [9, 10]; and b) a commercially obtained strain (B6.129P2(SJL)-Myd88tm1.1Defr/J) back-crossed onto the inbred C57BL/6J line (The Jackson Laboratory, Bar Harbor, Maine). These two lines were used to account for possible effects due to background strain. Given that there were no differences between the two strains, the results for both were combined. Controls were B6129F2/J and C57BL/6J (Jackson Lab), respectively, and were also combined, with no differences between the two strains. WT dams carrying WT and MyD88-heterozygous fetuses were created by mating WT females with either WT or MyD88-KO studs, respectively. In a similar fashion, MyD88-KO dams carrying MyD88-KO and MyD88-heterozygous fetuses were creating by mating MyD88-KO females with either MyD88-KO or WT studs. To generate maternal-fetal genotype combinations not achievable in nature, ovarian transplants were performed, exchanging ovaries between wild-type and MyD88-KO mice. Doing so allows, through subsequent mating to appropriate male studs, for WT dams to carry MyD88-KO fetuses and for MyD88-KO dams to carry WT fetuses (see procedures below).
Ovarian transplant procedure
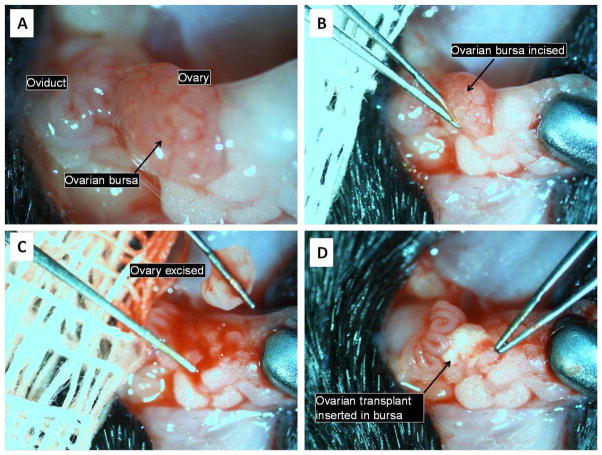
To perform the ovarian exchange, one 3–4 week-old WT female and one MyD88-KO female of similar age were anesthetized with ketamine (80–100mg/kg) and xylazine (5–10mg/kg). In each of these mice, a low dorsal midline skin incision was made and one ovary/oviduct/fat pad complex exposed (Figure 1). An incision was made in the ovarian bursal membrane, the edges of which were teased away laterally to expose the ovary. A curved forceps was then inserted under the ovary, which was lifted bluntly out of the bursa and placed in room-temperature PBS. A drop of 1% lidocaine-HCl mixed with epinephrine 1:100,000 was placed into the empty bursal sac to minimize blood loss. Once one ovary from each of the WT and MyD88-KO mice had been excised, the transplant procedure was performed: the donated ovary was gently placed within the empty ovarian bursa, and the edges of the sac were pulled over its surface to maintain it in position. The ovarian complex was then returned to the abdominal cavity. After both animals had donated and received one ovary apiece, the identical procedures were done on the contralateral side. The skin incision was closed with a single metal wound clip and the animals were allowed to awaken from anesthesia. Post-operative analgesia was provided with buprenorphine 0.1μg/g (immediately after surgery) and meloxicam 2 μg/g (immediately after surgery and again 24 hours later). Animals were allowed to recover for at least 2 weeks prior to being mated for the pregnancy experiment.
Figure 1.
Ovarian transplantation procedure.
See text for details.
Similar published protocols using fresh ovaries have produced high levels of fertility, with live offspring resulting from the transplanted ovary in 77–100% of cases [11, 12]. The offspring of such pregnancies themselves exhibited normal reproductive capacity [12].
Bacterially induced preterm labor model
After recovery from ovarian transplantation, female mice in estrus were selected by the gross appearance of the vaginal epithelium and were mated. Mating was confirmed by the presence of a vaginal plug, and the morning of plug formation was counted as Day 0.5 of pregnancy. The preterm labor experiment was performed on day 14.5 as previously described [13, 14]. Briefly, animals were anesthetized with 0.015 ml/g body weight of Avertin (2.5% tribromoethyl alcohol and 2.5% tert-amyl alcohol in PBS). A 1.5 cm midline incision was made in the lower abdomen. In the mouse, the uterus is a bicornuate structure in which the fetuses are arranged in a ‘beads-on-a-string’ pattern. Intrauterine injections of a 100 μl solution containing killed bacteria suspended in PBS (see below) or PBS only (controls) were performed in the midsection of the right uterine horn at a site between two adjacent fetuses, taking care not to inject individual fetal sacs. The abdomen was closed in two layers, with 4-0 polyglactin sutures at the peritoneum and wound clips at the skin. Surgical procedures lasted approximately 10 minutes. Pregnant animals were excluded from the preterm labor experiment if there were fewer than three conceptuses in the injected uterine horn (n= 4). Animals recovered in individual, clean cages in the animal facility, and occurrence of preterm delivery and number of delivered conceptuses was determined. Preterm delivery was defined as delivery of one or more fetuses within 48 hours.
For determination of delivery phenotype, animals were followed through the earlier of delivery or 48 hours after surgery, at which time they were euthanized by CO2 inhalation followed by necropsy for observation of retention and survival status of fetuses. Fetal remains in the cage and fetuses retained in utero were counted and correlated with the number of implantation sites (which remain easily visible in the mouse even after delivery). Because cannibalization of pups by mothers is common, our usual practice is to consider a sudden drop in maternal girth without finding fetal remains in the cage presumptive evidence of delivery, to be confirmed at autopsy. This did not occur in the present study. Samples were obtained for determination of fetal genotype (see below) from all conceptuses, whether they were retained in utero or delivered. Tissue harvests were conducted using a separate group of mice treated identically, with necropsies performed 5 hours after surgery. The inoculated/right horn was incised longitudinally along the anti-mesenteric border. Uteri (from regions inclusive of the decidual caps underlying placental attachment sites) and placentas were harvested, washed in ice-cold PBS, flash-frozen in liquid nitrogen and stored at −85°C for mRNA and protein extraction.
Bacterial preparation
Bacteria were prepared as previously described [8]. A fresh culture of previously frozen E. coli (American Type Culture Collection No. 12014) was grown overnight in 4,000 ml Luria-Bertani (LB) broth. The overnight culture was concentrated by centrifugation and suspension in 10 ml of PBS. The concentration of this suspension was subsequently determined by plating serial dilutions in duplicate. The bacteria within the suspension were killed by immersion in a boiling water bath for 5 minutes and then frozen at −20°C. Killing was verified by lack of overnight growth on plates and in broth culture. Once the concentration of the frozen stock was known, it was thawed and diluted to 2 × 109 organisms per ml. This latter suspension was frozen at −80°C in aliquots and thawed and diluted as needed prior to each experiment.
MyD88 genotyping
In order to confirm that each pregnancy was conceived with gametes from the transplanted ovary and not from the native ovary, the genotypes of all fetuses conceived after ovarian transplantation were determined by PCR of DNA extracted from the fetal rump. These specimens were obtained postpartum from delivered offspring or at necropsy from fetuses retained in utero. Primer sequences were: wild-type forward primer (GTTGTGTGTGTCCGACCGT); mutant forward primer (CCACCCTTGATGACCCCCTA); common reverse primer (GTCAGAAACAACCACCACCATGC). Using these primers, the MyD88 WT amplicon was 266 bp in length and the MyD88 mutant amplicon was 520 bp.
Real-Time PCR
Total RNA was extracted from homogenized tissues with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Five μg of total RNA were used as a template for cDNA synthesis. cDNA was prepared using random primers and the M-MLV reverse transcriptase system (Invitrogen, Carlsbad, CA). All PCR primers and probes were purchased from Applied Biosystems (Foster City, CA) (IL-1β Mm00434228; IL-6 Mm00446190; CCL5 (RANTES) Mm01302428; TNF Mm00443258; cyclooxygenase-2 (COX-2) Mm00478374; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (20X) 4452339E). Use of TaqMan PCR Reagent Kits was in accordance with the manufacturer’s manual. Reactions were performed in a 10μL mixture containing 1 μL cDNA. Duplex RT-PCR was performed with one primer pair amplifying the gene of interest and the other an internal reference (GAPDH) in the same tube. Thermocycler parameters were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Semi-quantitative analysis of gene expression was done using the comparative CT (ΔΔCT) method, normalizing expression of the gene of interest to GAPDH. PCR assays were performed in triplicate.
Serum progesterone determination
Blood was collected by trans-thoracic cardiac puncture at necropsy. Serum was separated and stored at −80°. ELISA assays for progesterone were performed using a Progesterone ELISA Kit (Enzo Life Sciences, Catalog number ADI-900-011, Farmingdale, NY USA) according to the directions of the manufacturer, and read at 405 nm with a microtiter plate reader.
Statistical methods
Categorical values were tested for statistical significance using Chi-squared test or Fisher’s exact test. The normality assumption for continuous variables (expressed as means or medians) was assessed using the Shapiro-Wilk test. The Kruskal-Wallis test was used as global test (overall test for heterogeneity) for overall differences among groups. If the global test was significant, post-hoc pair-wise comparisons of medians among groups were performed as appropriate by multiple comparisons for non-normally distributed data. The adjusted p-values were computed using the permutation resampling rank test method. Statistical analysis was performed using the SAS 9.3 platform (Cary, NC). P value < 0.05 indicates statistically significant differences.
Results
Confirmation of expected offspring genotypes after ovarian transplantation
The genotypes of all fetuses conceived after ovarian transplantation were determined by PCR in order to verify that each pregnancy was conceived with gametes from the transplanted ovary and not from the native ovary. In 86% (19 of 22) pregnancies in MyD88-KO dams following ovarian transplantation with WT ovaries and subsequent mating with WT males, all fetuses in each litter had the expected genotype (i.e. WT). In 9% (2 of 22) pregnancies, one fetal sample out of the litter amplified both WT and KO sequences, and in 4.5% (1 of 22) all fetal samples amplified both WT and KO sequences. Among pregnancies in WT dams following ovarian transplantation with KO ovaries and subsequent mating with KO males, the proportions of unexpected genotypes was higher: in 60% (15 of 25) pregnancies, all fetuses had the expected genotype (i.e. MyD88-KO). In 12% (3 of 25) one fetal sample in the litter amplified both WT and KO sequences, in 16% (4 of 25) two fetal samples in the litter amplified both WT and KO sequences, and in 12% (3 of 25) all fetal samples in the litter amplified both WT and KO sequences. The above occurrences of unexpected fetal genotypes might have resulted from either retained endogenous maternal ovarian tissue or from contamination of the fetal specimens with maternal DNA. We believe the latter explanation is more likely because of the following considerations: a) our confidence in the complete surgical excision of native ovaries; b) the sensitivity of PCR, which would tend to artifactually amplify even minute amounts of contaminant; and c) the fact that such discrepancies were more likely to occur in WT dams. In WT but not KO dams preterm labor occurred (see below), leading to either preterm delivery or significant resorption of fetal tissues, both of which increase the likelihood of maternal contamination. Nonetheless, in order to minimize concerns regarding the completeness of ovarian excision, animals were excluded if more than one fetus per litter had an unexpected genotype (i.e. 7 of 47 litters), except in one case, in which a litter of 7 fetuses had two fetal samples with both WT and KO DNA.
E. coli induced preterm delivery is mediated by the mother and not the fetus
Table 1 shows the delivery outcomes of pregnancies for the various maternal-fetal genotype combinations following intrauterine inoculation with 109 killed E. coli organisms. Preterm delivery was dictated by maternal genotype regardless of fetal genotype (86 – 100% preterm delivery (pooled 96%) in wild-type mothers, whether fetuses were WT, heterozygous or MyD88-KO, and 0 – 14% preterm delivery (<5%) in MyD88-KO mothers, whether fetuses were KO, heterozygous or WT).
Table 1.
Delivery outcomes stratified by maternal and fetal genotype following intrauterine inoculation with 109 killed E. coli organisms on day 14.5 of gestation. Preterm delivery was defined as delivery of ≥ 1 fetus within 48 hours. P values calculated by contingency tables.
| Maternal MyD88 genotype | Fetal MyD88 genotype | N | PTD rate (%) | P value |
|---|---|---|---|---|
| WT | WT | 8 | 100 | |
| WT | Het | 7 | 86 | 0.3 |
| WT | KO | 8 | 100 | |
| KO | KO | 7 | 0 | |
| KO | Het | 7 | 14 | 0.3 |
| KO | WT | 8 | 0 | |
| P value | 0.0001 |
Fetal survival in utero is influenced by fetal genotype
Table 2 shows effects of intrauterine inoculation on in utero fetal survival. In cases where preterm delivery occurred (i.e. in WT dams), approximately one third to two fifths of fetuses remained in utero regardless of fetal genotype. None of these fetuses were alive at the time of necropsy. This result suggests that regardless of fetal genotype, either bacterial exposure in a WT intrauterine environment or killed bacteria-induced preterm labor in and of itself leads to intrauterine fetal demise. In contrast, with bacterial exposure in the absence of labor (i.e. in MyD88-KO dams) there was a significant effect of fetal genotype on survival (median survival 100% in MyD88-KO fetuses versus 86% and 81% in MyD88-heterozygous and WT fetuses, respectively p < 0.01).
Table 2.
Effects of intrauterine heat-killed E. coli inoculation on fetal retention and survival. Evaluations were performed at necropsy immediately after preterm delivery, or 48 hours after intrauterine injection in animals who had not delivered. P values were calculated by Kruskall-Wallis test.
| Maternal MyD88 genotype | Fetal MyD88 genotype | N | Median % of fetuses retained in utero per litter | P value | Median % of fetuses alive in utero per litter | P value |
|---|---|---|---|---|---|---|
| WT | WT | 8 | 65 | 0 | ||
| WT | Het | 7 | 29 | 0.6 | 0 | 0.2 |
| WT | KO | 8 | 24 | 0 | ||
| KO | KO | 7 | 100 | 100 | ||
| KO | Het | 7 | 100 | 0.3 | 86 | .01 |
| KO | WT | 8 | 100 | 81 | ||
| P value | <0.0001 | <0.0001 |
Inflammatory cytokines in gestational tissues
To assess the effects of maternal and fetal genotype on the expression of genes related to inflammation and labor in gestational tissues, RT-PCR was performed. Genes chosen for assay had the following characteristics: relevance to the MyD88-dependent signal transduction pathway of toll-like receptors (i.e. interleukin (IL-) 1β and IL-6); relevance to the MyD88-independent pathway (i.e. CCL5, also known as chemokine (C-C motif) ligand 5, or RANTES); or relevance to both pathways (tumor necrosis factor alpha (TNF)). Prostaglandin synthase (cyclooxygenase 2 (COX-2)), the rate-limiting enzyme in the synthesis of prostaglandins, was also assayed because of its importance for the genesis and maintenance of labor [15, 16]. Close correlation between mRNA and protein expression [17, 18] was an additional factor in gene selection.
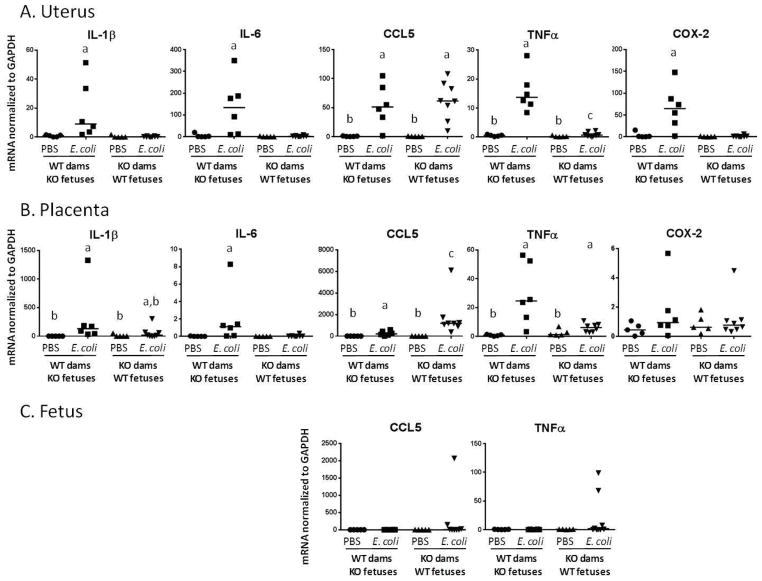
We find that the expression of these inflammatory mediators is correlated with both their known patterns of dependence upon MyD88 signaling and with the preterm labor phenotype. Thus, in uterine tissue (Figure 2A) IL-1β and IL-6 are robustly induced by E. coli in WT dams only, whereas CCL5 is induced in both WT and MyD88-KO dams. TNF is induced in both genotypes, but to a significantly lesser extent in MyD88 knockouts than in WT dams. Similar to IL-1β and IL-6, COX-2 is up-regulated by bacterial exposure in WT dams only.
Figure 2. Cytokine expression in gestational tissues.
mRNA expression was determined by RT-PCR in gestational tissues harvested 5 hours after treatment with intrauterine PBS (control) or killed E. coli. Values are normalized to the housekeeping gene GAPDH. Statistically significant differences (p < 0.05) were determined by Kruskal-Wallis test with post-hoc permutation resampling rank test to identify differences among treatment groups. Statistically significant differences between groups are indicated by different letter labels (i.e. groups not different from each other share the same letter label; groups different from each other have different letter labels). When there are no differences between treatment groups, no labels are used. Fetal expression of IL-1β, IL-6 and COX-2 was undetectable.
The placenta is a fetal organ, however in the mouse to a far greater extent than the human, the intact placenta after separation from the uterus has attached to it a significant amount of maternally derived decidual tissue [19]. In keeping with this mixed origin of tissue layers in the postnatal placenta, we find that placental expression of the above markers parallels that of the uterus (Figure 2B) with the exception of COX2, which is no different between the various treatment groups. Placental responses are blunted in comparison to those of the uterus, but like the uterus are determined primarily by maternal genotype and type of exposure (i.e. bacteria vs. control).
Fetal expression of the inflammatory markers tested (Figure 2C) was undetectable or low, with no differences between treatment groups.
Serum progesterone correlates with ovarian genotype and is not altered by exposure to intrauterine heat-killed E. coli
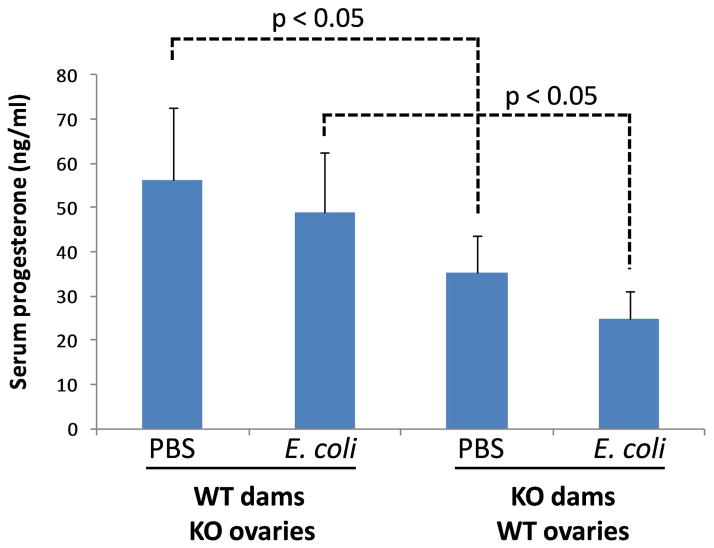
In the mouse, the ovaries (specifically corpora lutea) are the source of progesterone during pregnancy [20, 21], unlike the human, where the majority of circulating progesterone in the second and third trimesters derives from the placenta. We previously showed that there is a 30% drop in serum progesterone 5 hours after bacterial exposure in WT dams carrying WT fetuses, but there is no decline in MyD88-KO dams carrying MyD88-KO fetuses [8]. Here we show that following ovarian transfer there is no significant change in serum progesterone levels due to bacterial treatment in either mouse strain (Figure 3). There is an unexplained higher level of circulating progesterone in pregnant WT dams carrying MyD88-KO ovaries than in MyD88-KO dams carrying WT ovaries, both in controls and in animals exposed to E. coli. Despite this higher progesterone level in WT dams, these animals delivered prematurely after exposure to E. coli, while KO mice did not despite significantly lower circulating progesterone levels. This, in combination with prior results using pregnant MyD88-KO dams with their native ovaries, suggests that neither ovarian function nor serum progesterone have a role in labor initiation or maintenance in this bacterially induced preterm birth model.
Figure 3.
Serum progesterone by ELISA 5 hours after the indicated treatments.
Comment
We have shown using a genetic experiment that preterm delivery induced by heat-killed E. coli in the mouse depends on maternal and not fetal MyD88 genotype. We have further shown that survival in utero (among fetuses from litters that do not deliver prematurely) is influenced by fetal genotype. Thus, it appears that responses generated by the bacterial stimulus are important on the maternal side for labor signals – most likely a cascade of cytokines, chemokines and other inflammation-related effectors –leading to labor, while on the fetal side similar signals – most likely a fetal inflammatory response syndrome (FIRS) [22] manifesting as shock – can lead to fetal demise.
There is other evidence to support the interpretation that an inflammatory cascade is the upstream cause of both preterm labor and fetal demise. For example, administration of a broad-spectrum chemokine inhibitor resulted in the suppression of preterm delivery and fetal demise in mice given LPS intraperitoneally [23]. Further, an anti-inflammatory treatment regimen (ampicillin + betamethasone + indomethacin compared to ampicillin alone) led to prolongation of gestation and suppression of inflammatory markers in rhesus macaques in whom labor was induced using group B streptococcus [24]. Other lines of evidence suggest the existence of substantial redundancy in inflammatory response networks [25–28], possibly explaining the failure of narrowly targeted interventions to prevent preterm delivery. Because MyD88 is an essential linchpin for myriad effects associated with the innate immune response, its targeting results in inhibition of a broad range of downstream events that contribute to labor and fetal demise. There is also evidence that MyD88 is essential for the function of macrophages [29, 30] and NK cells [31, 32]. These two types of leukocyte (but not neutrophils) are critical for mediating LPS-induced preterm delivery in mouse models [33–36].
In the mouse, spontaneous labor at term results from regression of the corpus luteum and thus depends upon ovarian function [20]. The ovarian transplantation strategy used in the current experiments, along with previously published data showing that deletion of MyD88 simultaneously from both the maternal (including the ovaries) and fetal compartments prevents E. coli-induced labor [8], leads to the conclusion that the delivery phenotype in this inflammatory model is not mediated through effects on ovarian function. This conclusion would not have been as definitive were maternal/fetal genotype combinations not achievable in nature created using an embryo transfer strategy instead of ovarian transplantation.
Our results cannot be extrapolated to spontaneous labor occurring at the natural end of pregnancy, but it is notable that MyD88-KO females carrying MyD88-KO fetuses deliver normally at term. Thus, unlike inflammation-induced preterm labor, normal labor in the mouse is not dependent upon MyD88. A recent mouse study suggests that spontaneous parturition at term results from fetal signals through the actions of steroid receptor coactivators (SRC) -1 and -2 [2]. Thus, recent data add to previously existing observations suggesting that the molecular signals leading to initiation of pathologic preterm labor and physiologic term labor may differ in important ways, despite their similar clinical endpoints (uterine contractions, cervical change, and rupture of the membranes, among other phenomena). The mouse model used in these experiments was developed in our lab [14] and is widely used, with various substances employed to induce labor, including live and killed bacteria, various toll-like receptor ligands (such as LPS and peptidoglycan), and other materials [16, 37–41]. Infection- and inflammation-induced preterm labor in the mouse has many similarities to preterm labor in humans, including a lack of dependence upon a decline in circulating progesterone ([42] and the present study); induction of labor by infections either in the gestational compartment or in remote locations [43]; and similar expression patterns of oxytocin receptors, gap junction proteins, prostaglandin synthase enzymes and inflammatory cytokines [14, 27, 44–47]. However, several potential limitations of this model should be noted. When a large bolus of killed bacteria (as was used in the present study) or bacterial extracts are used, the model is best thought of not as infectious but rather inflammatory. This bolus-type inflammatory stimulus does not well model the gradual ascending colonization that is presumed to occur in human intrauterine infection in pregnancy. Further limiting extrapolation to human gestation are differences in uterine anatomy; multifetal gestation in the rodent; placental and fetal membrane structure; and regulatory functions of fetal hypothalamus, adrenals and placenta. Also, the complete deletion (‘knockout’) of a gene, though suggestive of its function, might not reflect the role of that same gene in an animal in which the gene is functional in other tissues and times. Finally, though E. coli is a common organism in intrauterine infections, many such infections are caused by other organisms, and other infections are polymicrobial. The dependence of these infections on MyD88 signaling may be variable, limiting the generalizability of our findings, although it is notable that all toll-like receptors with the exception of TLR3 signal through MyD88.
It cannot be assumed that interventions designed to interfere with MyD88 signaling or indeed the function of any other inflammatory mediator will benefit either mother or fetus in clinical situations. While such interventions may limit the damage caused by inflammatory responses, they might also impair the host’s ability to eliminate infection. The degree to which MyD88 inhibition will prove beneficial in the setting of ascending, gradual infection with live organisms in humans remains to be determined.
Acknowledgments
This research is supported by MOD #21-FY06-573, 1R01 HD056118 and the Satter Fund in Perinatal Research. The funding sources had no role in any aspect of the design, execution or publication of the study.
Footnotes
The authors report no conflict of interest.
Presented in part at the 34th Annual Meeting of the Society for Maternal-Fetal Medicine, February 3 – 8, 2014, New Orleans LA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA, Chambon P, Xu J, O’Malley BW, Mendelson CR. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. 2015;125:2808–2824. doi: 10.1172/JCI78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154:483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17:12–19. doi: 10.1016/j.siny.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamont RF. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. BJOG. 2003;110(Suppl 20):71–75. doi: 10.1016/s1470-0328(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 7.Klein LL, Gibbs RS. Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am J Obstet Gynecol. 2004;190:1493–1502. doi: 10.1016/j.ajog.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice. Am J Obstet Gynecol. 2009;200:e1–8. doi: 10.1016/j.ajog.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 11.Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–1304. doi: 10.1093/humrep/15.6.1300. [DOI] [PubMed] [Google Scholar]
- 12.Gunasena KT, Villines PM, Critser ES, Critser JK. Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997;12:101–106. doi: 10.1093/humrep/12.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal V, Smart K, Jilling T, Hirsch E. Surfactant Protein (SP)-A Suppresses Preterm Delivery and Inflammation via TLR2. PLoS One. 2013;8:e63990. doi: 10.1371/journal.pone.0063990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 15.Chang EY, Zhang J, Sullivan S, Newman R, Singh I. N-acetylcysteine prevents preterm birth by attenuating the LPS-induced expression of contractile associated proteins in an animal model. J Matern Fetal Neonatal Med. 2012;25:2395–2400. doi: 10.3109/14767058.2012.697942. [DOI] [PubMed] [Google Scholar]
- 16.Ilievski V, Hirsch E. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod. 2010;83:767–773. doi: 10.1095/biolreprod.110.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels I, Cavill D, Murray IA, Long RG. Elevated expression of iNOS mRNA and protein in coeliac disease. Clin Chim Acta. 2005;356:134–142. doi: 10.1016/j.cccn.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Zhao TZ, Xia YZ, Li L, Li J, Zhu G, Chen S, Feng H, Lin JK. Bovine serum albumin promotes IL-1beta and TNF-alpha secretion by N9 microglial cells. Neurol Sci. 2009;30:379–383. doi: 10.1007/s10072-009-0123-x. [DOI] [PubMed] [Google Scholar]
- 19.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 20.McCormack J, Greenwald G. Progesterone and oestradiol-17beta concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol. 1974;62:101–107. doi: 10.1677/joe.0.0620101. [DOI] [PubMed] [Google Scholar]
- 21.Skarnes RC, Harper MJ. Relationship between endotoxin-induced abortion and the synthesis of prostaglandin F. Prostaglandins. 1972;1:191–203. doi: 10.1016/0090-6980(72)90004-4. [DOI] [PubMed] [Google Scholar]
- 22.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 23.Shynlova O, Dorogin A, Li Y, Lye S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J Cell Mol Med. 2014 doi: 10.1111/jcmm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, Novy MJ. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197:518e511–518. doi: 10.1016/j.ajog.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194:1334–1340. doi: 10.1016/j.ajog.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin 1 signaling. Am J Obstet Gynecol. 2002;186:523–530. doi: 10.1067/mob.2002.120278. [DOI] [PubMed] [Google Scholar]
- 27.Mussalli GM, Blanchard R, Brunnert SR, Hirsch E. Inflammatory cytokines in a murine model of infection-induced preterm labor: cause or effect? J Soc Gynecol Invest. 1999;6:188–195. doi: 10.1016/s1071-5576(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura K, Hirsch E. Interleukin-6 is neither necessary nor sufficient for preterm labor in a murine infection model. J Soc Gynecol Investig. 2003;10:423–427. doi: 10.1016/s1071-5576(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa S, Miyake M, Fujii R, Konishi Y. MyD88 associated ROS generation is crucial for Lactobacillus induced IL-12 production in macrophage. PLoS One. 2012;7:e35880. doi: 10.1371/journal.pone.0035880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J Immunol. 2007;178:3731–3739. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 31.Baratin M, Roetynck S, Lepolard C, Falk C, Sawadogo S, Uematsu S, Akira S, Ryffel B, Tiraby JG, Alexopoulou L, Kirschning CJ, Gysin J, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Natl Acad Sci U S A. 2005;102:14747–14752. doi: 10.1073/pnas.0507355102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarajan UM, Sikes J, Prantner D, Andrews CW, Jr, Frazer L, Goodwin A, Snowden JN, Darville T. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect Immun. 2011;79:486–498. doi: 10.1128/IAI.00843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179:838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200:308e301–309. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinaldi SF, Catalano RD, Wade J, Rossi AG, Norman JE. Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor. J Immunol. 2014;192:2315–2325. doi: 10.4049/jimmunol.1302891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filipovich Y, Agrawal V, Crawford SE, Fitchev P, Qu X, Klein JH, Emmet Depletion of polymorphonuclear leukocytes has no effect on preterm delivery in a mouse model of E. coli-induced labor. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2015.07.025. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudley DJ, Chen CL, Branch DW, Hammond E, Mitchell MD. A murine model of preterm labor: inflammatory mediators regulate the production of prostaglandin E2 and interleukin-6 by murine decidua. Biol Reprod. 1993;48:33–39. doi: 10.1095/biolreprod48.1.33. [DOI] [PubMed] [Google Scholar]
- 38.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods. 1998;39:147–154. doi: 10.1016/s1056-8719(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 41.Yang Q, El-Sayed Y, Rosenberg-Hasson Y, Hirschberg DL, Nayak NR, Schilling J, Madan A. Multiple cytokine profile in plasma and amniotic fluid in a mouse model of pre-term labor. Am J Reprod Immunol. 2009;62:339–347. doi: 10.1111/j.1600-0897.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod. 2002;67:1337–1341. doi: 10.1095/biolreprod67.4.1337. [DOI] [PubMed] [Google Scholar]
- 43.Mussalli GM, Brunnert SR, Hirsch E. Preterm delivery in mice with renal abscess. Obstet Gynecol. 2000;95:453–456. doi: 10.1016/s0029-7844(99)00571-2. [DOI] [PubMed] [Google Scholar]
- 44.Cook JL, Zaragoza DB, Sung DH, Olson DM. Expression of myometrial activation and stimulation genes in a mouse model of preterm labor: myometrial activation, stimulation, and preterm labor. Endocrinology. 2000;141:1718–1728. doi: 10.1210/endo.141.5.7474. [DOI] [PubMed] [Google Scholar]
- 45.Gross G, Imamura T, Muglia LJ. Gene knockout mice in the study of parturition. J Soc Gynecol Investig. 2000;7:88–95. [PubMed] [Google Scholar]
- 46.Kimura T, Ogita K, Kusui C, Ohashi K, Azuma C, Murata Y. What knockout mice can tell us about parturition. Rev Reprod. 1999;4:73–80. doi: 10.1530/ror.0.0040073. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch E, Blanchard R, Mehta S. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1999;180(2 Pt 1):429–434. doi: 10.1016/s0002-9378(99)70227-9. [DOI] [PubMed] [Google Scholar]