Abstract
Inducing sustained, robust CD8+ T cell responses is necessary for therapeutic intervention in chronic infectious diseases and cancer. Unfortunately, most adjuvant formulations fail to induce substantial cellular immunity in humans. Attenuated acute infectious agents induce strong CD8+ T cell immunity, and are thought to therefore represent a good road map for guiding the development of subunit vaccines capable of inducing the same. However, recent evidence suggests that this assumption may need reconsideration. Here we provide an overview of subunit vaccine history as it pertains to instigating T cell responses. We argue that in light of evidence demonstrating that T cell responses to vaccination differ from those induced by infectious challenge, research in pursuit of cellular immunity-inducing vaccine adjuvants should no longer follow only the infection paradigm.
Introduction
The vast majority of information over the last 25 years regarding the molecular and cellular requirements of robust cellular immunity have come from the study of the host response to infectious challenge. An underlying assumption has been that information gained from these infectious models will be directly applicable to the design, development and formulation of subunit vaccines. That is, the immunological rules guiding infection-elicited T cell responses will be the same as those guiding subunit vaccine elicited T cell responses. Recent findings, however, begin to question this assumption. While infectious models have shown central roles for type I IFN and IL-12 for mediating T cell differentiation and memory formation, these cytokines are often dispensable in the T cell response to subunit vaccination [1]. In contrast, IL-27 signaling appears to be required for the T cell responses to a host of subunit adjuvants [2], while the response to infectious challenge is either unaffected or even elevated in the absence of this cytokine [3, 4]. TNF receptor superfamily members expressed by T cells largely enhance various qualitative aspects of the T cell responses during infection [5–8], but instead dictate the quantitative magnitude of the response in subunit vaccine settings [9–15]. In short, the success or failure to produce a cellular response by subunit vaccination may be guided by different underlying mechanisms than those that govern infectious challenge.
In evaluating the relationship between infection-elicited and subunit vaccine-elicited cellular responses, one is reminded of the Chinese folklore of the Fauna of Mirrors. As the ancient legend has it, mirrors not only reflect objects in the present world, but also contain entirely new worlds behind their surfaces, possessing completely different flora and fauna. Inhabitants of both worlds were, for a time, allowed to roam freely between the two. Applying the metaphor, vaccine-elicited T cell responses could either match the reflection from the world of infectious biology, or alternatively could more closely resemble a world on the other side of the mirror, possessing familiar creatures but with unique traits and functions. In this version of reality, understanding comes not from increasingly better analysis of the reflection, but from exploring the new world behind the mirror, interrogating its rules, quirks and subtleties and in so doing, gaining a comprehension of its inhabitants. Here we provide a discussion of findings that suggest divergent underlying mechanisms between infection and subunit vaccination leading to robust antigen specific cytotoxic T cell responses.
B cell vaccinology… a better reflection of infection
Some of the earliest vaccines (circa Jenner to Pasteur) focused on the use of live attenuated infectious agents, capable of generating robust cellular and humoral immunity. Being a live infection, there are inherent problems with vaccine production and storage, adverse reactions and even reversion to virulence that plagued their use as vaccines. These issues inspired early vaccinologists to explore the use of vaccines that instead contained either whole, killed microbes or components of microbes against which effective lasting immunity could be established. In the 1920s and 30s, Alexander Glenny demonstrated that the precipitation of Diphtheria toxoid on an aluminum salt dramatically enhanced the efficacy of the subunit vaccine to elicit anti sera [16–18]. This milestone not only marked the dawn of vaccine adjuvants, it also helped inextricably link neutralizing antibody formation as the gold standard metric for evaluating vaccine efficacy.
Alum was the adjuvant of the 20th century, contributing to the near eradication of dangerous and prevalent infections like diphtheria, tetanus, pertussis, and polio from the developed world. Alum has its limits however, one of them being that it is largely incapable of inducing any significant degree of cytotoxic T cell immunity [19]. While generally perceived to be less critical for mediating prophylactic immunity against infectious challenges, robust cellular immunity is almost certainly required for effective therapeutic vaccination against chronic viral infections or cancer [20]. Unfortunately, the majority of new vaccine adjuvants developed thus far, likewise, do not generate clinically significant cell-mediated immunity [19]. Consequently, the field turned back to the study of infectious agents and the robust cellular immune responses they instigate.
Ironically, utilizing these natural infections have facilitated the design and implementation of vaccines that induce better humoral, not cellular, immunity. For example, the study of bacterial infections ultimately lead to the identification of the receptor for LPS [21], Toll Like receptor (TLR) 4, and ultimately all the other TLRs and numerous other families of innate receptors. Given the inflammation they induce, the molecularly defined agonists for these receptors were seen as ideal vaccine adjuvants. Indeed, the addition of a single innate receptor agonist usually improves the magnitude and/or affinity of antigen specific antibody induced by a vaccine [22]. In contrast, only rarely does the addition of single, or even multiple, innate receptor agonists induce any substantial protective cellular immunity even after multiple boosts [23].
T cell vs. B cell fauna… all about the numbers
This disparity in how protective T and B cell responses are generated by vaccination may be largely attributed to a game of numbers. Though optimal antibody generation is restricted by a series of T cell mediated checks and balances, the multimeric nature of microbial and virally associated antigens allows not only T-independent Ig production but it can also permit mechanisms of extra-follicular class switching that do not require the participation of antigen specific T cells [24]. Even for T dependent responses, the organized architecture of secondary lymphoid organs makes it possible for a relatively small number of antigen specific T cells to support the maturation of the B cell response. B cells already begin at a substantially larger antigen specific precursor frequency than the antigen specific precursor frequency of CD8+ T cells [25–28]. Furthermore, once activated, the functional effect of even a small number of activated B cells is further amplified by differentiation into plasma cells, producing antibody in great abundance over a long period of time. With minimal instigation (ie antigen+LPS), a robust antibody response can be achieved with the mobilization of perhaps 10–20,000 plasmablasts [25, 26].
For cytotoxic T cells on the other hand, the biology of protection is based upon T cell contact with target cells. Therefore, the T cells themselves must be in sufficient number so as to track down, contact and destroy infected/oncogenic cells before they propagate the malady. This reality was exquisitely highlighted by mathematical predictions of sterilizing immunity to cancer and viral infections based upon relative abundance of antigen specific cytotoxic T cells to either viral titer or tumor burden [20]. Reasonably similar estimates were empirically derived by assessing the required number of T cells for sterilizing immunity against malaria in a mouse model [29]. Suffice it to say, the numbers of antigen specific T cells identified in these studies are orders of magnitude larger than 10–20,000; roughly 106–107 T cells per cm of tissue. Until recently, even the best subunit vaccines produced antigen specific T cells several orders of magnitude lower than model natural infections, such as Listeria, Vaccinia or LCMV.
Good evidence for the primacy of sustainable T cell numbers in protective cellular responses comes from the infusion of Adoptive Cell Therapy (ACT) of in vitro activated T cells into late stage cancer patients. T cell ACT, bearing either tumor specific TCRs or engineered Chimeric Antigen Receptors (CARs), has been performed in the clinic for the better part of the last 2 decades with only limited success [30, 31]. Indeed, very few durable responses were ever observed despite the infusion into patients of exceedingly high numbers (>109) of tumor reactive T cells. These poor overall responses rates were better understood when examination of the patients blood revealed precipitous losses of the transferred cells, culminating in only small numbers of tumor reactive T cells that could persist in the host [32–34]. ACT began seeing its first consistent successes only after researchers and clinicians elaborated methods by which the high numbers of transferred T cells could be maintained through time points long after initial transfer [35–37]. Indeed, the cases where this treatment successfully controls the tumor are often the patients in which the transferred T cells expand to become 40–80% of the total T cell pool [37]. These frequencies are within a few fold of what is observed in response to model infections such as LCMV, but are multiple orders of magnitude larger than what is induced by most adjuvanted subunit vaccines. While it is certainly true that qualitative aspects of T cell behavior can enhance or detract from the efficiency of the overall response [38, 39], a good argument can be made that the primary deficiency in subunit immunization is a simple failure to evoke sufficient T cell numbers requisite for therapeutic cytotoxic responses.
Through the looking glass
If the key to making effective T cell-eliciting vaccines is getting the vaccine to make more T cells, it stood to reason that the place to look for the right clues would be infectious models. In response to an infection such as LCMV, upwards of 50% of the activated CD8+ T cell pool is composed of virus specific T cells (targeting 3–4 dominant antigens) only 7 days after initial viral challenge [40]. Other infectious challenges such as vaccinia, influenza or Listeria monocytogenes (LM) are little different, typically producing CD8+ T cell responses that dedicate anywhere from 15–30% of the T cell pool toward the infectious challenge[41–43]. When one considers that the pre-challenge precursor frequency of CD8+T cells in a mouse specific for any one of these epitopes is in the range of 200–2000 cells [27, 28], this represents an antigen specific T cell expansion that is somewhere around 3–4 orders of magnitude. To date, there are only a few subunit vaccine platforms capable of producing this level of T cell expansion, one of which is a combination adjuvant stimulating both an innate receptor (such as a TLR) and CD40 (combined innate/CD40). Much like the B cell response, the use of either of these single adjuvants typically produces around 10–50,000 total antigen specific CD8+ T cells [2], a number that is non-protective with respect to T cell responses [13]. In contrast, after a single exposure, the combined innate/CD40 adjuvant can produce a similar number of antigen specific T cells (~1–3 million antigen specific T cells), and in the same time frame (7 days), as the infectious challenge [2, 10, 13, 44, 45]. As a result of the potency of this vaccine adjuvant, we and others [46–48] have spent years studying its underlying mechanisms in mice and have recently shown its capacity to produce robust CD8 and CD4 responses in non-human primates [49].
We initially believed that this adjuvant represented an opportunity to not only make better vaccines, but could also be used to better understand the infectious process. We reasoned that a reductionist adjuvant system that reproduced both the magnitude and character of the infectious T cell response through the targeting of only 2 molecules would “obviously” be better at cutting through the inflammatory noise of the infection to identify the critical adaptive signals buried beneath. In hindsight, we should not have been surprised to find that rather than revealing secrets of the hidden infectious process, we identified signals central only to the efficacy of subunit vaccine adjuvants. Thus far, two signaling pathways have stood out as critical to vaccine-elicited CD8+ T cell responses; CD27 [10, 12, 13, 45] and (coincidently) IL-27 [2]. While the “27s” also influence and modulate the infectious response, their role in the infection is far more qualitative and nuanced than in vaccination where they dictate (in a necessary-but-not-sufficient manner) the magnitude of the primary response and the function of the memory response.
CD27
The canonical TNFR1 and TNFR2 family members are known for their roles in determining cell fate through either cell survival or apoptosis in a host of cells from epithelial/tumor cells (as the name suggests) to T cells, B cells and myeloid cells of the immune system [50]. As such, many of the members of the TNF receptor superfamily have been explored deeply for the machinery responsible for T cell life and death decisions [51]. Infectious biology has highlighted the role of these family members to influence overall T cell number but usually in later stages of a developing primary T cell response . For example, whereas the CD27, OX40 and 41BB influence the CD8+ T cell response to challenge with a range of infections, their effects are typically seen in specific tissue sites or at later stages of the developing T cell response [52]. These effects are more often qualitative, influencing the overall numbers of antigen specific T cells a few fold but having a more substantial impact on their function and/or phenotype [5–8, 53, 54]. One unique illustration of this principal is seen in studies of CD27 where high affinity T cells to influenza had a dispensable relationship with CD27 whereas low affinity T cells required CD27 ligation by CD70 in order to survive long enough to contribute to the memory pool [7]. Similarly, OX40 deficiency has little effect on the magnitude of the primary T cell response to LM challenge, rather influencing the cell fate decision between short lived effectors and longer term, self-renewing memory T cells [55]. Even more qualitative are the role of TNFR effects in response to VV infection where neither CD27 or OX40 play much of a role in the response to more virulent VV and only influence the development of protective memory to less virulent strains [56].
These results stand in reasonably sharp contrast to the role of these receptors in a vaccine setting, where the blockade of CD27 produces dramatic (>10 fold) inhibition of the primary expansion of CD8 T cells [9–15, 45, 57–59]. Curiously, OX40 can serve as a reasonable substitute to support CD8+ T cell expansion to vaccination, but only in the developmental absence (KO animals) of CD27[12]. This likely has to do with the similarities in CD27 and OX40 signaling coupled with the fact that OX40 is poorly expressed on CD8 T cells unless CD27 is absent. OX40 has a less obscure role in CD4 cell response to vaccination where both CD27 and OX40 signaling are required for peak responses in the WT host [45]. While we have studied this extensively in response to the combined innate/CD40 vaccine adjuvant, others, using a diversity of immunization platforms (adjuvants, in vitro derived DCs, etc) have observed a similar importance for CD27 in a vaccine-elicited T cell response [9–15, 45, 57–59]. In the majority of these systems, this CD27 dependency influences expansion, survival and programming of effectors and memory.
Indeed, strong evidence suggests that potent CD27 signaling in the CD8+ T cell can render both its primary and secondary response CD4 T cell-independent [60, 61]. Because of the obvious benefits of this in a setting like a HIV infection or cancer where a patient’s degree of immune competence is questionable at best, CD27 is now an active target in the rapidly expanding field of immuno-oncology. Given this central role for these TNF receptors in the vaccine elicited response, it is certainly not a coincidence that the inclusion of 41BB signaling components into the cytoplasmic tail of CARs has dramatically augmented the long term survival of CAR T cells and their therapeutic efficacy [36].
IL-27
IL-27 is a pleiotropic cytokine that is closely related to IL-6/IL-12/IL-23 and has been linked to both the promotion and the inhibition of cell-mediated immunity in the context of autoimmunity and infectious disease [3, 62]. Through STAT1 signaling, IL-27 can induce early Tbet activation and predispose a developing CD4 response toward Th1 and away from Th17 responses [63, 64]. Somewhat paradoxically however, IL-27 also signals through STAT3 and its activity is critical for suppressing rampant inflammatory responses. Indeed, the existing infectious literature is far more consistent with a suppressive function for IL-27 in T cell biology [4, 62, 65–67]. For example, in the response to influenza, IL-27 induces IL-10 production in CD4+ T cells simultaneous to their IFNγ production [68]. Though initially counter intuitive, it ultimately allows a degree of inflammatory control while maintaining sufficient inflammatory momentum to clear the virus. Similarly, in T gondii infection, a loss of IL-27 leads to a lethal level of lymphproliferation and IFNγ driven inflammation [69]. In our hands [2], as in others [70], CD8+ T cell responses to vaccinia or Listeria challenge are relatively unconcerned with IL-27 deficiency, the response being either similar or slightly elevated relative to WT responses. Notably, an exception to the suppressive function of IL-27 has been observed in cancer immunity where the addition of IL-27 in vivo augments the CTL response against a variety of tumors (reviewed in [71]). Further, IL-27Rα−/− hosts are more sensitive to tumor growth [72], suggesting some role for endogenous IL-27 signaling in this response. Even in this case, however, substantial tumor specific immunity could still be induced in the IL-27Rα−/− hosts. Collectively, the preponderance of data is consistent with the interpretation that while IL-27 may not be mandatory for tumor specific CTL activity, its presence or absence can influence the induction/maintenance of tumor specific immunity.
Regardless of its specific role in cancer immunity, it is fair to say that at least in area of infectious biology, the established functions of IL-27 would not have predicted any central and/or necessary role in subunit vaccine-elicited immunity. Indeed, the majority of data implicated cytokines such as IL-12 or type I IFN as the most likely “signal 3” mediators for CD8+ T cell responses to vaccination. As described by Mescher and colleagues more than 15 years ago, only when specific “signal 3” cytokines are included during the early phases of T cell activation (IL-12 and IFN for CD8s, IL-1 for CD4s) would T cells develop effector functions, survive long-term and proliferate in response to secondary challenge [73]. In these years since these initial reports, IL12 and IFN have been validated as critical for full CD8+ T cell differentiation and memory formation in a variety of model systems [73–77]. Thus, once again, the available data from various infectious model systems might have more likely pointed one toward IL-12/IFN and away from IL-27 as primary mediators of vaccine-elicited cellular immunity.
In part because the vaccine-elicited CD8 response was independent of both IL-12 and IFN signaling in T cells, we began investigating possible contributions of IL-27 to the T cell response to the innate/CD40 vaccine. We discovered that a loss of IL-27R on T cells, much like CD27, resulted in a >10 fold reduction in both CD4 and CD8 responses [2]. Perhaps even more surprisingly, this dependence on IL-27 was maintained for a spectrum of single adjuvants as well, indicating a dependence on IL-27 that was more broad than for just our combined adjuvant. Effective IL-27 signaling in this context required STAT1/STAT3 and, again similar to CD27, affected not only the peak expansion of the primary response, but also affected appropriate programming of protective memory. Subunit vaccine-elicited memory IL-27 deficient T cells fail to expand upon rechallenge with LM, despite the fact that the primary response to LM is IL-27 independent. Even more surprising, memory IL-27 deficient T cells which had responded normally to priming with LM also failed to expand when boosted by subunit vaccination. Thus, IL-27 plays a unique role in programming the expansion, effector function, and memory formation of T cells in response to both primary and secondary encounters with subunit vaccination. Again, these functions for IL-27 are largely un-mirrored during the T cell response to infectious challenge.
Fauna yet to be discovered
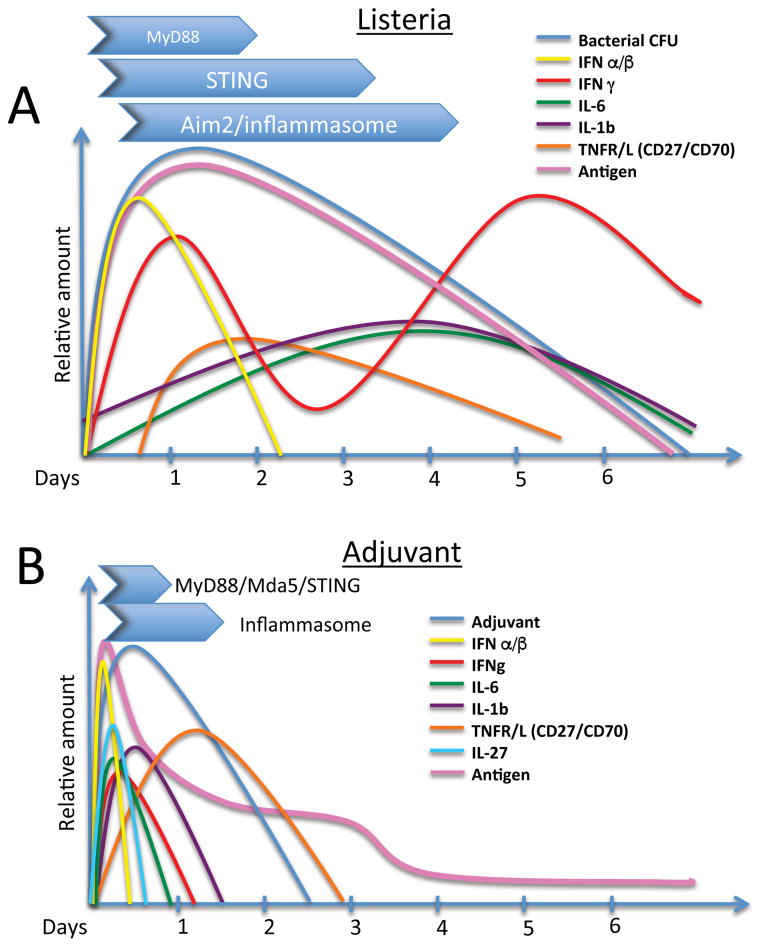
Collectively the data indicate that the “27s” play quantitatively and qualitatively different roles in the infectious vs. subunit vaccine-elicited T cell response. To revisit our analogy, deeply staring into the mirror reflecting infectious biology would not have likely revealed the necessity for these molecules in the vaccine elicited response. It is worth nothing that each signal is necessary but not sufficient, indicating that they are likely critical steps along a linear path of stimuli, the loss of any one of which leads to the dissolution of the path entirely. This may be informative in regards to understanding why infectious and vaccine responses are so disparate in their dependence on the 27s. During the infectious process, numerous innate receptors will be triggered during the many days over which the pathogen replicates (Figure 1A). These innate pathways induce the production of a host of inflammatory mediators, some in series and some in parallel, which over time not only reduce pathogenic burden and dissemination, but also serve as stimuli for professional antigen presenting cells (APCs) to induce the levels of antigen processing and presentation, co-stimulatory molecule expression and cytokine production necessary for T cell activation, proliferation, survival, effector function, and resolution to memory [78]. Conceptually, an antigen specific T cell has an extended opportunity to integrate various stimuli as they arise under the broad arc of the infectious process, sampling the inflammatory milieu for the signals it needs to sustain its expansion, function and survival. In sharp contrast to this, the inflammation induced by the adjuvant in a subunit vaccination is limited in magnitude, diversity and geography, producing a regionally isolated innate inflammatory burst which at best is likely operative over only the first 36 hours post-delivery (Figure 1B). For example, adjuvant-elicited IL-27 production rises and falls within the first ~8–12 hours after vaccination (NP and RMK, unpublished results). CD70 induction on antigen bearing APCs (the ligand for CD27) peaks between 12–18 hours after vaccination [9, 13], and its stimulatory activity is necessary within the first 24 hours of vaccination in order to achieve maximal effect on T cell expansion. Contrast this to the many days of cytokine production and rounds of APC activation, usually occurring in multiple anatomical and immunologically distinct niches, in response to infectious challenge. Logically, one might anticipate that the temporal sequence, limited diversity and overall magnitude of the inflammatory mediators induced by the adjuvant would have the very effects on the T cell response that we do indeed observe; i) T cells are more demanding of both the level and sequence of each inflammatory factor induced, and thus, far more sensitive to their loss if removed, and ii) the factors the vaccine depends upon are different from those induced, and therefore depended upon, by an infection.
Figure 1.
Schematic diagram comparing and contrasting the innate response to a model infectious organism (L. monocytogenes) with the response to a vaccine adjuvant. A. Major innate pathways induced and an estimate of the time frame over which they are active is shown in the blue arrows above. In general, most cytokines induced by these pathways extend over multiple days as bacterial growth expands and wanes. Various inflammatory cytokines are induced over time which facilitate stages of both innate and adaptive control of bacterial growth. Antigen load expands and contracts with bacterial load which is eventually eliminated 5–6 days after challenge. B. In contrast to the infectious process, the inflammation induced by the adjuvant spikes early and begins to wane a few days after injection, tracking with the level of antigen. The limited inflammation induced creates fewer and less inflammatory cytokines which are also produced within a highly compressed time frame as compared to the infectious process. By days 3–4 after injection, while the antigenic load and inflammatory factors are still increasing to the infection, factors important to the adjuvant-elicited response such as IL-27 and CD27 are likely already becoming highly limited in the inflammatory milieu of the adjuvant.
This fundamental difference in the inflammation induced by infections and subunit vaccination suggest that there are other factors that could use greater scrutiny and exploration in a subunit vaccine setting. T cell signaling strength is a matter of both TCR affinity for peptide MHC as well as the overall avidity of APC-associated multimeric peptide:MHC. Because the strength of T cell signaling can dictate a T cell’s dependency of other factors within the milieu [7, 79, 80], the antigenic load is a critical component in directing T cell fates. As an infectious burden increases over the first dew days of its replication, so increases the antigenic load. While the precise levels of antigen generated during the course of infection is difficult to calculate the efficiency by which it stimulates T cells is augmented by the spectrum of inflammatory factors induced [79, 80]. In addition, APCs are often directly infected, enhancing classical presentation of endogenous antigen into class I MHC. Contrast this with the fact that the amount of target antigen in a subunit vaccination will never be greater than the amount first injected, waning rapidly (at least one hopes so… see below) after vaccination. Curiously, the amount of antigen typically utilized in clinical subunit vaccines is usually about the same absolute amount we use in mice; eg. the Hepatitis B vaccine contains only 20ug of protein in its formulation. While clearly sufficient for eventually inducing protective antibody responses, this relative dose (for a 60kg person, 0.0003 mg/kg or ~0.01mg/m2) is probably 10–100 fold lower than what can be reasonably expected to induce T cell responses even in mice where we use optimized antigens and adjuvants. Compounding the impact of this scarcity of antigen is the relative inefficiency of processing and presentation of exogenous antigens by APCs. Along side of the fore-mentioned limited inflammatory environment, it perhaps becomes more understandable why our subunit vaccinations do not produce clinically relevant T cell responses.
One solution that early vaccinologists utilized to slow the loss of antigen after injection was the use of precipitates and emulsions, capable of maintaining antigen for many weeks after immunization [17, 81]. Though shown more than 60 years ago to be unnecessary for effective antibody production [82], the formation of such an “antigen depot” is still assumed to be useful in vaccine formulations for the generation of immunity. While it is true that B cell responses proceed relatively unaffected by the presence or absence of such antigen depots in an injection site, there is very strong evidence that antigen depots are actively damaging to the formation of enduring T cell responses [46, 83, 84]. Work by Overwijk and colleagues convincingly showed that the it is precisely the duration of antigen within the depot that proves the downfall for T cells, drawing nascent responders into the antigen-rich injection site, distracting them from target sites of interest and inducing their apoptosis [83]. Adjuvants that facilitate the maintenance of peripheral tissue depots of antigen for any extended length of time beyond the rise of the induced T cell response (10–15 days?) may be compromising the efficacy of the vaccine by just such a mechanism. As such, other methods need to be explored which can increase the amount of antigen presented to T cells without forming destructive antigen reservoirs. One area that is proving productive in this regard is the targeting of antigen to APCs [57, 85–89]. By conjugating antigens to TLR agonists or to antibodies specific for molecules expressed by DC subsets, the efficiency of antigen uptake is greatly magnified, allowing far lower amounts of antigen to be presented effectively. Targeted antigen in this fashion can dramatically increase the efficiency of cross-presentation [87, 88], and in some cases even prolonging the presence of the antigen for extended periods of time [89], though apparently not long enough to compromise the induction of robust T cell immunity. The success of these methods supports the notion that antigen dose/load is a critical determinant of a successful T cell response and provides a reasonable path forward for feasible augmentation of antigen dose in the clinic.
Lastly, despite the essential and fate-determining effect of cellular metabolism on antigen specific T cells, there is very little information on the in vivo metabolic programming directed by subunit immunizations. How a T cell meets it metabolic demands dictates whether the cell can survive during both the expansion and contraction of the response. Energetic deficiency leads to cells that are either unable to divide or unable to carry out effector functions requiring protein synthesis and motility. While cytokine signaling, such as IL-12 and IL-2, drives the expression of fate-determining transcriptions factors (Eomes/Bcl6 for memory and Tbet/Blimp-1 for effectors) [90, 91], these cell fates are also affected by the metabolic pathways active within the cell [92]. Evidence for this is the growing data that T cells can be skewed toward different cell fates by nothing more than manipulation of their metabolic pathways during initial priming [92–94]. Many of the inflammatory cytokines produced during an infectious response can influence genes important in glycolysis or oxidative phosphorylation. It therefore remains to be determined how the metabolic demands of a T cell are met in a subunit vaccine setting and whether or not the T cell meets these demands in a fashion similar to or divergent from a T cell in an infectious setting.
Concluding remarks
The past 20 years has seen the rise, fall and rise again of Immunotherapy. In the present era we celebrate the “Year of Immunotherapy” and enjoy the popularization of immune-centric terms such as “Immuno-oncology” (or just “IO” for those in Pharma) and “ immune checkpoint inhibitors”. The capacity of the immunotherapeutics in the clinic to so powerfully influence T cell function in vivo, in conjunction with the increasing success in ACT and CAR T cell therapies, might tempt one to speculate whether the pursuit of T cell-inducing vaccines is at best past its prime or at worst clinically irrelevant. As we consider a range of outstanding questions in the field (see Outstanding Questions box), three points are noteworthy in regards to the need for pursuing T cell-eliciting vaccine approaches. First, aseptic T cell priming and expansion probably follows rules much more akin to subunit vaccination than infection. For this reason, pursuits in understanding the laws in either of these realms may reinforce understanding of the other. Second, targeting T cell checkpoint inhibitors appears to achieve success through activating a number of T cells in vivo that is orders of magnitude less than the number transferred during ACT [95]. Much work has been done to increase the efficiency of adoptive transfers by identifying the characteristics of in vitro generated T cells which permit them make them to survive and function in the recipient [96]. It is almost certainly not coincidental that their phenotype is similar to the phenotype of T cells generated by successful non-infectious vaccine methods [61]. Thus, ACT would likely benefit from the development and use of a robust subunit vaccine method that could be employed post adoptive transfer. Indeed, animal models of ACT predict as much [97]. Lastly, as with the animal data on ACT, most of the checkpoint regulators currently in the clinic did not successfully control tumor growth in early animal models unless some form of vaccination was used in combination [98–100]. Indeed, it is arguably a surprise that the use of the checkpoint regulators alone, or even in combination with one another, has been as successful as they have without some form of vaccination on board. Given this, the development of vaccine platforms that can generate even modest T cell responses is almost certain to increase the already impressive response rates for these powerful therapeutics and promote substantially higher rates of stable disease and disease free survivals.
OUTSTANDING QUESTIONS BOX.
What is the sequence and duration of innate factors induced by vaccine adjuvants that induce robust cellular immunity? The inflammatory response to adjuvant injection is substantially different than that in response to infection. The quantity and quality of the T cell response depends on the right sequence of the right factors. A careful time course monitoring inflammatory factors and stimulatory receptors within the vicinity of the developing T cell response, will inform the development of better adjuvants.
What other factors regulate the subunit vaccine-elicited response and not the infectious response? Continuing to compare the infectious response and vaccine-elicited response will reveal what other factors are specifically required for the latter and not the former.
What aspects of vaccine-elicited cellular immunity are conserved between mice and primates? Many adjuvants produce some degree of cellular immunity in mice but fail to do so when used in non-human primates. Some adjuvants (combined innate/CD40, TLR7 agonist-antigen conjugates) induce potent immunity in both species. These types of adjuvants require further study in primates to inform adjuvant development suitable for clinical usage.
What antigen doses are best suited for inducing clinically relevant vaccine induced cellular immunity? Current doses of antigen used in clinical vaccines are barely suitable for producing CD8+ T cell responses in mice let alone humans. More effective dose responses need to be explored in humans to insure that our best adjuvants are not (literally) starving for success.
What are the best methods for delivering the proper antigen dose? A quick fix for the antigen dose issue is to simply use more antigen. However, numerous antigen-targeting methods increase the efficiency of antigen uptake and presentation and show substantial promise in animal models. More data in higher primates is needed to understand what targeting modalities are best suited for the clinic.
What is the metabolic profile of T cells responding to subunit vaccination? The response to infectious challenge relies heavily on aerobic glycolysis to support both the energetic and biosynthetic demands of the massive T cell expansion that occurs. The balance between glycolysis and oxidative phosphorylation is can be heavily influenced by the inflammatory milieu. Given the differences in this milieu between infections and adjuvants, its is currently unclear whether a T cell responding to subunit vaccination uses similar or divergent means to meets its metabolic demands and balance the formation of effectors and memory phenotype T cells.
In returning to our metaphor, the legend tells us that occupants of both sides of the mirror were once able to transit between the worlds. Eventually conflict and confusion arose between the earthly realm and that which lay beyond the mirror, compelling the Emperor to close the portal between the two. Though we contend that subunit vaccinology lies in the world beyond the mirror, this by no means suggests that its understanding is unreachable. Indeed, arming ourselves with all of the available “omics”, and other formerly incomprehensible technological tools, should allow for immunologists to once again make informed transit from one side of the mirror to the other and influence the development of subunit vaccines for better clinical outcomes.
Trends Box.
Evidence is growing for divergent underlying mechanisms guiding the magnitude and duration of vaccine-elicited and infection-elicited CD8+ T cell responses.
B cell responses can be successful after producing 10–20,000 “effectors” (antibody secreting cells) whereas successful T cell responses require the mobilization of exponentially larger numbers of effectors.
Most modern adjuvants and formulations are able to produce the required number of B cell effectors but generally cannot produce the required number of CD8 T cells.
The few adjuvants which can induce potent cellular immunity in mice and primates rely heavily on TNF receptors (CD27/OX40) and IL-27 to promote sufficient T cell expansion and survival.
These specific pathways are utilized very differently in response to infection, challenging whether the study of infectious responses can be expected to reveal a path forward for the development of T cell-inducing vaccine adjuvants.
Acknowledgments
Thanks to Laurel Lenz for his help in estimating the type and relative time frame of inflammatory cytokines induced by LM for Figure 1A. The authors apologize to all colleagues whose work could not be cited in this article due to length restrictions. This work was supported by grants AI066121 and AI101205 from NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Starbeck-Miller GR, Harty JT. The Role of Il-12 and Type I Interferon in Governing the Magnitude of CD8 T Cell Responses. Adv Exp Med Biol. 2015;850:31–41. doi: 10.1007/978-3-319-15774-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Pennock ND, Gapin L, Kedl RM. IL-27 is required for shaping the magnitude, affinity distribution, and memory of T cells responding to subunit immunization. Proc Natl Acad Sci U S A. 2014;111:16472–16477. doi: 10.1073/pnas.1407393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183:4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 6.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 7.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten Brinke A, Tak PP, Eldering E, Nolte MA, et al. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Ballesteros-Tato A, Leon B, Lee BO, Lund FE, Randall TD. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity. 2014;41:127–140. doi: 10.1016/j.immuni.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams JA, Sanchez PJ, Haluszczak C, Gapin L, Kedl RM. Multiple innate signaling pathways cooperate with CD40 to induce potent, CD70-dependent cellular immunity. Vaccine. 2010;28:1468–1476. doi: 10.1016/j.vaccine.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowley TF, Al-Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J Immunol. 2004;172:6039–6046. doi: 10.4049/jimmunol.172.10.6039. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez PJ, Kedl RM. An alternative signal 3: CD8 T cell memory independent of IL-12 and type I IFN is dependent on CD27/OX40 signaling. Vaccine. 2012;30:1154–1161. doi: 10.1016/j.vaccine.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181:1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 15.Taraban VY, Rowley TF, Kerr JP, Willoughby JE, Johnson PM, Al-Shamkhani A, Buchan SL. CD27 costimulation contributes substantially to the expansion of functional memory CD8(+) T cells after peptide immunization. Eur J Immunol. 2013;43:3314–3323. doi: 10.1002/eji.201343579. [DOI] [PubMed] [Google Scholar]
- 16.Glenny AT. The Principles of Immunity applied to Protective Inoculation against Diphtheria. The Journal of hygiene. 1925;24:301–320. doi: 10.1017/s0022172400008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenny AT. Insoluble Precipitates in Diphtheria and Tetanus Immunization. British medical journal. 1930;2:244–245. doi: 10.1136/bmj.2.3632.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenny AT, Barr M. The precipitation of diphtheria toxoid by potash alum. The Journal of Pathology and Bacteriology. 1931;34:131–138. [Google Scholar]
- 19.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budhu S, Loike JD, Pandolfi A, Han S, Catalano G, Constantinescu A, Clynes R, Silverstein SC. CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues. The Journal of experimental medicine. 2010;207:223–235. doi: 10.1084/jem.20091279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 22.Vasilakos JP, Smith RM, Gibson SJ, Lindh JM, Pederson LK, Reiter MJ, Smith MH, Tomai MA. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 2000;204:64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- 23.Oh JZ, Kedl RM. The capacity to induce cross-presentation dictates the success of a TLR7 agonist-conjugate vaccine for eliciting cellular immunity. J Immunol. 2010;185:4602–4608. doi: 10.4049/jimmunol.1001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Yang JC, Robbins PF, Wunderlich JR, Hwu P, Sherry RM, Schwartzentruber DJ, Topalian SL, Restifo NP, Filie A, et al. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother. 2003;26:385–393. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 41.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 42.Harty JT, Lenz LL, Bevan MJ. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurche JS, Burchill MA, Sanchez PJ, Haluszczak C, Kedl RM. Comparison of OX40 ligand and CD70 in the promotion of CD4+ T cell responses. J Immunol. 2010;185:2106–2115. doi: 10.4049/jimmunol.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68:9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina Ma, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8 T cell sequestraon, dysfuncon and deleon. Nature medicine. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson EA, Liang F, Lindgren G, Sandgren KJ, Quinn KM, Darrah PA, Koup RA, Seder RA, Kedl RM, Lore K. Human Anti-CD40 Antibody and Poly IC:LC Adjuvant Combination Induces Potent T Cell Responses in the Lung of Nonhuman Primates. J Immunol. 2015 doi: 10.4049/jimmunol.1500078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 52.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 53.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, Schoenberger SP, Croft M. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 2010;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 59.Taraban VY, Rowley TF, Tough DF, Al-Shamkhani A. Requirement for CD70 in CD4+ Th cell-dependent and innate receptor-mediated CD8+ T cell priming. J Immunol. 2006;177:2969–2975. doi: 10.4049/jimmunol.177.5.2969. [DOI] [PubMed] [Google Scholar]
- 60.Dong H, Franklin NA, Roberts DJ, Yagita H, Glennie MJ, Bullock TNJ. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. Journal of immunology (Baltimore, Md : 1950) 2012;188:3829–3838. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards LE, Haluszczak C, Kedl RM. Phenotype and function of protective, CD4-independent CD8 T cell memory. Immunol Res. 2013;55:135–145. doi: 10.1007/s12026-012-8356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 63.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 64.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 65.Findlay EG, Greig R, Stumhofer JS, Hafalla JC, de Souza JB, Saris CJ, Hunter CA, Riley EM, Couper KN. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J Immunol. 2010;185:2482–2492. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- 66.Freitas do Rosário AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O'Garra A, Langhorne J. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. Journal of immunology (Baltimore, Md : 1950) 2012;188:1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 68.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nature immunology. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–114. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 70.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 71.Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, Matsui M, Mizuguchi J, Yoshimoto T. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. Journal of biomedicine & biotechnology. 2010;2010:605483–605483. doi: 10.1155/2010/605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, Hara H, Yoshida H. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. International journal of cancer Journal international du cancer. 2009;124:1372–1378. doi: 10.1002/ijc.24107. [DOI] [PubMed] [Google Scholar]
- 73.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 75.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 76.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 77.Xiao Z, Casey Ka, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. Journal of immunology (Baltimore, Md : 1950) 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 79.Bullock TN, Mullins DW, Engelhard VH. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. J Immunol. 2003;170:1822–1829. doi: 10.4049/jimmunol.170.4.1822. [DOI] [PubMed] [Google Scholar]
- 80.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity. 2013;38:140–152. doi: 10.1016/j.immuni.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White RG, Coons AH, Connolly JM. Studies on antibody production. III. The alum granuloma. J Exp Med. 1955;102:73–82. doi: 10.1084/jem.102.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holt LB. Quantitative studies in diphtheria prophylaxis; the primary response. British journal of experimental pathology. 1949;30:289–297. pl. [PMC free article] [PubMed] [Google Scholar]
- 83.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matos I, Mizenina O, Lubkin A, Steinman RM, Idoyaga J. Targeting Antigens to Dendritic Cells In Vivo Induces Protective Immunity. PloS one. 2013;8:e67453. doi: 10.1371/journal.pone.0067453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2012;118:3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kastenmuller K, Wille-Reece U, Lindsay RW, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest. 2011;121:1782–1796. doi: 10.1172/JCI45416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 90.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 91.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol Rev. 2014;257:264–276. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, Overwijk WW, Restifo NP, Melief CJ, Offringa R, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]