Abstract
B lymphopoiesis is necessary to generate a diverse pool of naïve B cells that are able to respond to a broad spectrum of antigens during immune responses to pathogens and to vaccination. Rabbits have been utilized for many years to generate high affinity monoclonal and polyclonal antibodies. Specific antibodies generated in rabbits have greatly advanced scientific discoveries, but the unique qualities of rabbit B cell development have been underappreciated. Unlike in humans and mice, where B lymphopoiesis declines in mid to late life, B lymphopoiesis in rabbits arrests early in life, between 2–4 months of age. This review focuses on the early loss of B cell development in rabbits and the contribution of the bone marrow microenvironment to this process. We also propose directions for future research in this area, and discuss how the rabbit can be used as a model to understand the decline of B lymphopoiesis that occurs in humans late in life. Such studies will be important for developing therapeutics targeted to prevent and/or reverse declining B lymphopoiesis in the elderly, as well as boosting immunity and antibody responses after infection or vaccination.
1. Introduction
Numerous seminal findings in the area of immunoglobulin (Ig) structure and B cell biology were discovered through the study of rabbits. In addition to the Nobel Prize awarded to Rodney Porter in 1972 for his studies of rabbit Ig structure (Fleischman et al., 1963), the concepts of allotypes (Oudin, 1956) and allelic exclusion (Cebra et al., 1966, Pernis et al., 1965), the genetics of antibody formation (Feinstein, 1963, Gilman-Sachs et al., 1969, Todd, 1963), and recognition of the use of gene conversion for somatic diversification of Ig genes (Becker and Knight, 1990) were of crucial importance. Another discovery, now considered a pillar in B cell biology, showed that rabbit B lymphocytes express surface Ig receptors (antibody) (Pernis et al., 1970, Sell and Gell, 1965). This finding led the way to understanding the mechanism by which B cells participate in immune responses and in the production of high affinity antibody. While the number of immunological studies performed in rabbits has waned over the years, recent work reviewed here, continues to advance our knowledge of hematopoiesis and the microenvironment in which B cells develop.
Establishment of a diverse antibody repertoire is imperative to protect a host from pathogens, as well as to generate effective immune responses after vaccination. Generation of an antibody repertoire is dependent on the production of naïve B lymphocytes during the process of B lymphopoiesis. Rabbit B lymphopoiesis, similar to humans and mice, initially occurs in the fetal liver (Hayward et al., 1978, McElroy et al., 1981) before moving to the bone marrow (BM) after birth. Pre-B cells are first found in the fetal BM during gestation d25 and increase in number after birth. Between birth and two weeks of age pre-B cells make up 9–19% of rabbit BM hematopoietic cells, but this acutely declines to negligible levels at about 2 months of age. By 4 months of age, almost no pro-B or pre-B cells are found in the BM (Jasper et al., 2003), in contrast to humans and mice where B-lymphopoiesis continues at a high level in young adults and its loss is protracted from mid to late life (McKenna et al., 2001, Scholz et al., 2013).
Short-lived B lymphopoiesis in the BM does not appear to impair the rabbit’s ability to mount antibody responses after immunization, as rabbits are commonly used to generate antigen-specific high affinity antibodies. The development of rabbit monoclonal antibody technology by Knight and colleagues (Spieker-Polet et al., 1995) has proven to be a valuable tool both because rabbits make high affinity antibody, and because they readily produce antibodies to antigens that are poorly immunogenic in mice, e.g., carbohydrates (Bystryn et al., 1982). For the production of antibody, rabbits are typically immunized as adults, when B lymphopoiesis is no longer found in the BM. We will review the studies that provide the basis for our current understanding of factors that contribute to the loss of B cell development in rabbit BM. Additionally, we propose mechanisms that may help maintain immune competency even in the absence of ongoing B lymphopoiesis.
2. Resolution of rabbit B cell progenitor stages
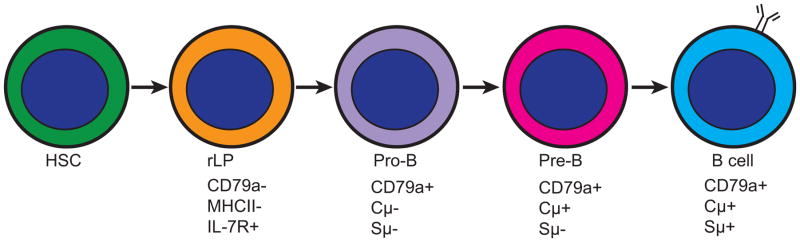
Similar to humans and mice, B cell development in rabbit presumably begins with the hematopoietic stem cell (HSC) and progresses through several developmental progenitor stages before becoming immature B cells. Many progenitor stages have been identified in humans and mice based on phenotypic markers and functions, but this process is less defined in rabbit. While the phenotype of rabbit HSCs is undefined, several B lineage progenitors have been described. The earliest B lineage progenitor population termed rabbit lymphoid progenitor (rLP) was described by Kalis et al. (2007) and defined as cells that bind IL-7 and do not express MHC Class II molecules (MHCII−IL-7R+). This population expresses Tdt, EBF, and Pax5, and is thought to contain a population of cells equivalent to the Common Lymphoid Progenitor (CLP). Downstream of the rLP in the B cell maturation scheme is the pro-B cell described by Jasper et al. (2003) as CD79a+ cytoplasmic μ− surface μ− (CD79a+Cμ− Sμ−), followed by the pre-B cell CD79a+Cμ+ Sμ− (Hayward et al., 1978, Jasper et al., 2003, McElroy et al., 1981), and finally, the B cell CD79a+Cμ+ Sμ+ (Jasper et al., 2003, Pernis et al., 1965, Sell and Gell, 1965) (Figure 2).
Figure 2. B cell development stages in rabbit BM.
Several B lineage progenitors are identified in rabbit BM. The earliest B lineage progenitor identified in the B cell development scheme is the rLP, which expresses Tdt, EBF, and Pax5. Next is the pro-B cell with successful DJ gene rearrangements in the IgH locus, and the pre-B cell with successful VDJ gene rearrangements. Finally, IgM+ B cells develop. HSC- hematopoietic stem cell, Tdt- terminal deoxynucleotidyl transferase, EBF- early B cell factor, Pax5- paired box protein 5, Cμ - cytoplasmic IgM, Sμ - surface IgM
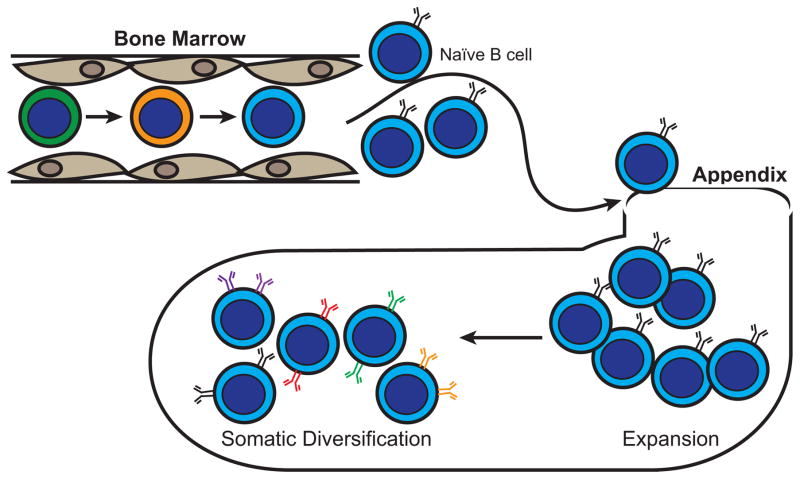
Similar to B lymphopoiesis in other vertebrates, the maturation of progenitor B cells is accompanied by VDJ gene recombination. This process is important for generating a diverse pre-immune antibody repertoire that can recognize a broad spectrum of antigens. Jasper et al. (2003) detected DJ gene rearrangements in pro-B cells and productive VDJ gene rearrangements in purified pre-B cells. Although V(D)J gene recombination occurs in rabbits, combinatorial joining of multiple V, D and J gene segments is not the primary mechanism by which rabbits expand and generate a large heavy chain antibody repertoire. In fact, rabbits primarily utilize only one VH, VH1, the 3′-most VH gene segment, during this process (Becker et al., 1990, Friedman et al., 1994, Knight and Becker, 1990, Raman et al., 1994, Tunyaplin and Knight, 1995). In contrast, the κ light chain repertoire is derived from combinatorial joining of numerous Vκ and Jκ, gene segments (Sehgal et al., 1999), although the extent to which this increases the functional repertoire has not been studied. The diversity of Ig and B cell receptors (BCR) is increased when naïve B cells leave the BM and traffic to gut associated lymphoid tissue (GALT) where the Ig genes undergo somatic diversification (Figure 1). The major site of somatic diversification is the appendix where naïve B cells receive microbe-dependent signals to proliferate and undergo somatic diversification by gene conversion and somatic hypermutation to modify their BCRs. Other species that use GALT to expand the B cell repertoire by gene conversion or somatic hypermutation, include chickens and sheep (Alitheen et al., 2010, Ratcliffe, 2006, Reynaud et al., 1987, Reynaud et al., 1995). In chickens, this diverse antibody repertoire develops in the bursa of Fabricius prior to birth, while in rabbits, it develops shortly after birth, and requires signals from gut bacteria (Rhee et al., 2004). While the expansion and diversification of rabbit B cells in the appendix is important for B cell maturation and the generation antibody diversity, this process has been reviewed recently (Lanning et al., 2000, Mage et al., 2006) and is not the focus of this review. Instead, we focus on B lymphopoiesis in the BM, which acts as a source of naïve B cells that seed the appendix for further maturation.
Figure 1. Rabbit B cell development and affinity maturation.
B cell development and generation of antibody diversity in rabbits occurs primarily in the BM and appendix. Hematopoietic progenitors in the BM differentiate through a series of maturation stages resulting in the development of naïve B cells. The BM acts as a source of naïve B cells which then travel to appendix in GALT. Here, naïve B cells receive microbe-mediated signals resulting in proliferation and somatic diversification of Ig genes by gene conversion and somatic hypermutation to generate a diverse pre-immune antibody repertoire.
3. Early Decline of Rabbit B lymphopoiesis in Bone Marrow
The earliest evidence suggesting that B lymphopoiesis ceases in the BM of young rabbits came in the 1960’s, from studies now known as allotype suppression experiments. Neonatal rabbits, heterozygous for IgH (heavy chain) allotypes, were injected with anti-paternal IgH allotype antibody resulting in the depletion of a specific Ig allotype (Dray, 1962). Recovery of the Ig with the suppressed allotype was not found even after two years of age (Eskinazi et al., 1979), suggesting either that active allotype-specific suppression was ongoing throughout life or that B cell development was arrested. Although low numbers of progenitors with the suppressed Ig allotype were reportedly found in rabbit BM (Simons et al., 1979), the paternal allotype never recovered, suggesting a block in B cell development must exist. In contrast when performing similar allotype suppression experiments, in mice, Lalor et al. (1989) found that expression of the suppressed allotype recovered 6 wk after suppression, consistent with the idea that B lymphopoiesis does not wane early in life in mice.
Rabbit BM was found to support B lymphopoiesis early in life (Hayward et al., 1978), and several studies report that the numbers of pro-B cells (Jasper et al., 2003) and pre-B cells (Gathings et al., 1981, Gathings et al., 1982, Hayward et al., 1978, McElroy et al., 1981) increase soon after birth and decline shortly thereafter. Jasper et al. (2003) used flow cytometry to follow the kinetics of the appearance and disappearance of pro-B and pre-B cells in BM and found that while pre-B and pro-B cells were at their highest percentage (each representing ~7% of BM hematopoietic cells) between birth and 4 weeks of life, they declined to only 1% of the BM cells by 2 months of age, and were undetectable by 4 months of age. The authors also found that as the number of B lineage progenitors decreased with age, the number of B cells in rabbit BM increased. The increase in mature B cells detected in adult rabbit BM is likely due to trafficking of these cells back to the BM from the periphery. The absence of B lineage progenitors indicated that B lymphopoiesis is markedly reduced in rabbit BM by 2–4 months of age, and no other anatomic site with pro-B or pre-B cells has been reported.
Additional data showing that B lymphopoiesis in rabbit BM declines early in life was obtained by Crane et al. (1996) who searched for IgH B cell excision circles (BRECs) that form during VD and DJ gene rearrangements in developing B lineage progenitors. Consistent with decreased numbers of B lineage progenitors in adult BM, the authors found that BRECs, which were abundant in newborn BM, were greatly decreased in adult BM. Similarly, quantitative studies showed that the highest levels of BRECS were found in BM in the first 3 weeks of life; by 2 months of age, they were greatly reduced, and were almost undetectable in BM from rabbits more than 4 months of age. These studies show on a molecular level, that B lymphopoiesis declines soon after birth in rabbits.
4. What mechanisms contribute to the loss of B cell development?
Decreases in B lymphopoiesis can be due to both intrinsic changes in hematopoietic progenitors and/or to extrinsic changes in the BM microenvironment. This topic has been widely studied and declining B lymphopoiesis in old mice is now attributed to changes in both hematopoietic progenitors and the BM microenvironment (Cho et al., 2008, Kondo et al., 1997, Labrie et al., 2004a, Miller and Allman, 2003, Miller and Allman, 2005, Montecino-Rodriguez et al., 2013). To determine if there are intrinsic defects in the rabbit hematopoietic progenitors, Kalis et al. (2007) enriched the rLP (MHCII−IL-7R+) early B lineage progenitor stage of cells from adults and tested if they have an intrinsic defect. When cultured in vitro on OP9 BM stromal cells (Holmes and Zuniga-Pflucker, 2009, Kalis et al., 2007), these cells differentiated into B lineage cells, suggesting that B lineage progenitors remained in adults, and were not intrinsically defective. The authors concluded that the decline in B lymphopoiesis was likely due to changes in the microenvironment.
BM HSCs from aged mice have increased expression of myeloid lineage genes and decreased expression of lymphoid genes compared to HSCs from young BM (Cho et al., 2008, Guerrettaz et al., 2008, Muller-Sieburg et al., 2004, Rossi et al., 2005). Additionally, pro-B cells from BM of aged mice were found to have impaired responsiveness to IL-7 stimulation (Stephan et al., 1997). Multiple studies have further addressed this issue in vivo through transfers of hematopoietic progenitors from aged mouse BM into young irradiated recipients, and then assessing the development of B lineage cells. One study found that the transferred progenitors only differentiated into myeloid lineage cells (Sudo et al., 2000), suggesting intrinsic changes in BM progenitors with age, while other studies found the transferred progenitors developed normally into B lineage cells (Chen et al., 1999, Miller and Allman, 2005, Morrison et al., 1996). In rabbits, transfer of GFP+ adult rabbit BM cells into GFP− young rabbits resulted in the production of GFP+ pre-B cells in the young recipients (Kalis et al., 2007), indicating that changes in the BM microenvironment, rather than in the early hematopoietic precursor cells are the major contributor to the decline of B lymphopoiesis in adult rabbits. A confounding feature of the in vivo adoptive cell transfer studies is that host irradiation is used, which likely changes factors secreted in the microenvironment. Therefore, other strategies have been used to search for differences between BM from young and adult rabbits.
4.1 Age-related changes in bone marrow
Interactions with BM stromal cells are critical for B lineage progenitors to differentiate into immature B cells. BM stromal cells consist of osteoblasts, endothelial cells, and adventitial reticular cells, which form niches to support B lymphopoiesis (Calvi et al., 2003, Jacobsen and Osmond, 1990, Kiel et al., 2005, Lichtman, 1981, Taichman et al., 1996, Tokoyoda et al., 2004, Weiss, 1976). These cells produce important factors for B cell development, such as, Interleukin 7 (IL-7) (Hardy et al., 1991, von Freeden-Jeffry et al., 1995), C-X-C motif chemokine 12 (CXCL12) (Egawa et al., 2001, Nagasawa et al., 1994), stem cell factor (SCF) (Driessen et al., 2003, Waskow et al., 2002), insulin-like growth factor 1 (IGF-1) (Gibson et al., 1993), and Flt3 Ligand (Kiel et al., 2005, Tokoyoda et al., 2004). Loss of these factors due to genetic deletion, aging, or pathology has a negative impact on B cell development. For example, IL-7−/− mice (Tsapogas et al., 2011, Wei et al., 2000), Flt3-L−/− mice (McKenna et al., 2000), and IL7Rα−/− Flt3-L−/− mice (Jensen et al., 2008, Sitnicka et al., 2003) have impaired B lymphopoiesis. While rabbits with genetic deletions of these factors are not available, IL-7 was found to be required for rabbit B cell development in vitro (Kalis et al., 2007) and therefore, if decreased in adult rabbit BM, may contribute to arrested B lymphopoiesis.
4.2 Changes in bone marrow supportive factors
IL-7 isoforms
BM stromal cells from aged mice secrete less IL-7 than do stromal cells from younger mice, and have an impaired capacity to support B lymphopoiesis in vitro (Stephan et al., 1998). The decline in B lymphopoiesis in rabbit does not appear to be due to decreased levels of IL-7 in BM (Kalis et al., 2007), because by northern blot analysis the level of IL-7 transcripts in total BM actually increases with age, rather than decreases. Further, analysis of adult rabbit BM revealed an isoform of IL-7, IL-7II, expression of which also increases with age (Siewe et al., 2010). IL-7II was expressed in vitro and it was found not only to bind the classical IL-7R, but it also showed no evidence of inhibiting B lymphopoiesis in vitro, suggesting that this isoform does not contribute to the decline of rabbit B cell development. Together, these data suggest that the early loss of rabbit B lymphopoiesis is not due to a reduction of the supportive cytokine IL-7.
Periostin
To identify BM stroma-derived supportive factors that decrease with age, Siewe et al. (2011) took a global approach to identify microenvironmental changes by performing a cDNA representational difference analysis (RDA). This analysis compared BM stromal cells, presumably mesenchymal stem cells (MSC), isolated and expanded in vitro, from a newborn rabbit to those from a two year old rabbit. The authors found that the extracellular matrix protein periostin was the most highly down-regulated protein in stromal cells from adult rabbits. To ask if periostin is required for rabbit B lymphopoiesis, Siewe et al. (2011) used a small interfering RNA (siRNA) knock down of periostin in OP9 stromal cells and found that in vitro, B lymphopoiesis was inhibited, suggesting that periostin is required in vitro. Further microarray analysis of OP9 cells with decreased production of periostin showed decreased expression of CXCL12 and IL-7, molecules needed for B cell development. While periostin−/− mice have normal B cell development, this study identified periostin as an important factor in vitro, that when decreased in vivo, may be compensated by other factors. Other molecules found by the cDNA RDA to be down-regulated in stromal cells from a 2 year old rabbit included the extracellular matrix components fibronectin, collagen type I, and thrombospondin, a ubiquitin Ct hydrolase, the tumor suppressor FAT, intersectin (component for endocytosis), and frizzled 4 (WNT signaling), although the impact of these factors on B lymphopoiesis, if any, remains to be determined.
5. Age-related changes to the BM microenvironment
While the transfer study (Kalis et al., 2007) suggested that changes in the BM microenvironment that occur at two to four months of age are responsible for the decline in rabbit B lymphopoiesis, altered expression of a factor that supports B cell development has not been identified. Bilwani and Knight (2012) examined BM CFU-Fibroblasts (CFU-F), presumably mesenchymal stem cells (MSC) (Mareschi et al., 2012), progenitors of both osteoblasts and adipocytes, and found that the number of MSCs in BM declined dramatically soon after birth. Further, the MSCs isolated from 9 week to 11-month-old rabbits appeared to have a greater propensity to differentiate into adipocytes, rather than osteoblasts, than did MSCs from rabbits less than 9 weeks-of-age. These data show that conditions in adult rabbit BM favor the generation of adipocytes instead of osteoblasts, suggesting that adult rabbit BM contains fewer osteoblasts (which support B lymphopoiesis) and a greater number of adipocytes. Consistent with this observation Tavasolli and colleagues found a substantial amount of adipose tissue in adult rabbit BM (Bigelow and Tavassoli, 1984). The accumulation of adipocytes in BM (femur and tibia) appears to be conserved among higher vertebrates, because adipocytes also increase in human and mouse BM with age (Chinn et al., 2012, Justesen et al., 2001, Lecka-Czernik et al., 2010, Rosen et al., 2009, Tuljapurkar et al., 2011). In these cases, the accumulation of adipocytes occurs later in life, coincident with the decline in B lymphopoiesis.
5.1. Adipocytes and myeloid-derived suppressor cells Inhibit B lymphopoiesis
The accumulation of fat occurs in rabbit BM at the time B lymphopoiesis declines, led Bilwani and Knight (2012) to test if adipocytes produce molecules that negatively regulate B lymphopoiesis. B lymphopoiesis cultures of rabbit BM cells and OP9 stromal cells were performed in the presence of adipocyte-conditioned medium (ACM), and they found that the production of CD79a+ B lineage cells was greatly inhibited, suggesting that adipocytes produce factors that negatively regulate B lymphopoiesis. In similar cultures of progenitors from human and mouse BM, ACM also inhibited B lymphopoiesis (Bilwani and Knight, 2012, Kennedy and Knight, 2015). In fact, the inhibitory activity of ACM appears to be conserved between mammals, as we found human, mouse, and rabbit B lymphopoiesis were all inhibited by ACM generated from adipocytes of these species interchangeably. Further examination of the mouse cultures revealed that ACM not only inhibited B lymphopoiesis, but also promoted the production of CD11b+Gr1+ myeloid-derived suppressor cells (MDSC). These MDSCs directly inhibited B lymphopoiesis in cultures without adipocyte factors, suggesting that adipocyte-derived factors promote the accumulation of MDSCs which then inhibit B lymphopoiesis.
MDSCs are most known for their capacity to inhibit T cell responses (Gabrilovich and Nagaraj, 2009), while only few studies have explored their interactions with other cell types (Green et al., 2013, Green et al., 2015, Kennedy and Knight, 2015, O’Connor et al., 2015, Zhu et al., 2007). Because the identification of MDSCs as suppressors of B lymphopoiesis is novel, we became intrigued by how this might be occurring. By cytokine array analysis, we found that these MDSCs produce inflammatory factors, most notably IL-1, which was identified as the key factor by which MDSCs inhibit B lymphopoiesis (Kennedy and Knight, 2015). While IL-1 can affect many cell types, we found that the hematopoietic target of IL-1-mediated inhibition in our system was the multipotent progenitor (MPP) cell. IL-1 treatment inhibited B lymphopoiesis by skewing MPP differentiation toward myelopoiesis at the expense of B lymphopoiesis. We hypothesize that if this mechanism also occurs in vivo in adult rabbits, large amounts of IL-1 and high numbers of myeloid cells will be found in the BM by two months of age, the time at which fat accumulates in the BM. Studies are currently under investigation in rabbits to determine if MDSCs accumulate in BM by a few months of age. It is of interest that CD11b+Gr1+ MDSCs accumulate in BM of 22 month old mice (Enioutina et al., 2011), a time at which B lymphopoiesis has declined (Labrie et al., 2004b, Miller and Allman, 2003, Stephan et al., 1996, Stephan et al., 1997, Stephan et al., 1998).
Several years ago, Soderberg and colleagues (1984a, 1984b) identified two types of suppressor cells in rabbit BM, one of which may be similar to the recently-identified MDSCs. The first suppressive cell, an adherent macrophage-like population inhibited proliferation and activation of BM cells after immune complex stimulation. While it is not known if these cells produce IL-1, this phenotype resembles the monocytic MDSCs induced by ACM (Kennedy and Knight, 2015). The other suppressor population found in rabbit BM, described as non-adherent FcRγ+ complement receptor negative cells, suppressed baseline proliferation of rabbit BM cells. While the lineage of the FcRγ+ suppressor cells was not identified, they are not likely MDSCs as they appear similar to natural suppressor lymphocytes (T, B, or NK lineage) characterized in mice at the time (Maes et al., 1988). In addition to suppressing BM cell proliferation, FcRγ+ suppressor cells were also found to inhibit T cell responses and mediate suppressive activity by blocking IL-2 (Maes et al., 1988, Soderberg, 1985). While this inhibition is due to a soluble factor, the identity of this molecule remains unknown. These FcRγ+ cells could be B10(reg) cells (Tedder, 2015) or aged B cells (ABC) found to negatively regulate B lymphopoiesis (Ratliff et al., 2013). Advancements in cell purification/separation techniques and the availability of additional cell-lineage-specific antibodies will allow one to establish the identity of these cells and determine if either is similar to the recently identified MDSCs (Kennedy and Knight, 2015).
Adipocyte-derived molecules promote MDSCs, but the adipocyte molecule(s) responsible for this has not been identified. We do however, know that MDSCs function in vitro by producing IL-1, suggesting that adipocytes trigger IL-1 production in MDSCs. Typically, IL-1β is expressed as a full length, inactive precursor, and inflammasome activation is required for cleavage of pro-IL1β into its active form by caspase-1 (Garlanda et al., 2013). Adipose tissue is known to produce many inflammasome activators, such as lipid crystals, fatty acids, and S100 proteins (Nagareddy et al., 2014, Wen et al., 2011, Youm et al., 2012), and we suggest one of these molecules is responsible for the generation or expansion of MDSCs, as well as for activation of the inflammasome, although this is yet to be determined.
6. Nature of BM adipocytes with increasing age
The accumulation of adipose tissue in 2–4 month old (adolescent) rabbits mirrors the pattern of accumulation in humans in which approximately 40–50% of the proximal femur and 70% of the tibia are filled with adipose tissue in the elderly (Li et al., 2013). In addition, the accumulation of adipocytes occurs coincident with the time when both species exhibit declining B lymphopoiesis, making rabbits a unique model for understanding the mechanism responsible for the decline in B lymphopoiesis and also for developing treatments aimed at restoring B lymphopoiesis in humans. Important issues to be addressed, are: What causes adipocyte accumulation in BM; what is the nature of these adipocytes; and would a decline in these cells from BM increase B lymphopoiesis?
As discussed above, MSCs from adult rabbit BM cells appear to be skewed toward adipocyte differentiation (Bilwani and Knight, 2012), although we cannot be certain if this is an intrinsic change in MSCs or an effect of the microenvironment on MSCs. Diascro et al. (1998) showed that differentiation of human osteosarcoma, mouse osteoblastic, and rat osteosarcoma cell lines to adipocytes was induced by rabbit serum, suggesting that it contains pro-adipogenic factors. Biochemical analysis of the active molecules suggested they are lipid in nature, specifically, palmitic, oleic, and linoleic free fatty acids. Whether serum fatty acids induce epigenetic changes in adult rabbit BM MSCs remains to be determined. Another curiosity is the possible connection between WNT-signaling and differentiation of adipocytes. Expression of frizzled 4, a receptor for WNT ligands is reportedly decreased in rabbit MSC after B lymphopoiesis is arrested (Kalis et al., 2007). Decreased WNT signaling in MSCs, which would lead to low levels of active β-catenin, could result in differentiation of adipocytes (Sen et al., 2008). Further study will be needed to confirm if frizzled 4 expression is decreased in BM stromal cells, and to identify the cause of free fatty acid accumulation in serum. The accumulation of fatty acids in serum and adipocytes in the BM could be related to life style characteristics such as diet and exercise. Therefore, as will be discussed later, perhaps diet and exercise could be modulated to reduce BM adipocyte accumulation and the loss of B lymphopoiesis in rabbits and humans.
Understanding BM adipose tissue in humans and mice is of growing interest today, but some of the earliest insight into this topic was provided by Tavasoli and colleagues, who identified two types of adipocytes in rabbit BM based on staining with performic acid Schiff reagent (PFAS) that stains unsaturated fatty acids (Tavassoli, 1976). These adipocytes were identified as PFAS+ and PFAS−. PFAS+ adipocytes, now termed regulated marrow adipose tissue (rMAT) (Scheller and Rosen, 2014), are found in red marrow where active hematopoiesis occurs. The adipocytes of rMAT may act as an energy source for hematopoiesis as rMAT vanishes when rabbit hematopoiesis is stimulated with hemolysis agents (Tavassoli, 1976). The second class of adipocyte, PFAS− was identified in yellow marrow, and is termed constitutive MAT (cMAT) (Scheller and Rosen, 2014), because the volume of cMAT is not responsive when hematopoiesis is induced and is resistant to changes in diet. In fact, Tavasoli found that after 10 days of starvation, cMAT in the distal tibia was not depleted (Tavassoli, 1974). This finding in rabbits is similar to instances of anorexia in humans and calorie restriction in mice where MAT increases, even when total peripheral body fat is reduced (Bredella et al., 2009, Devlin et al., 2010). Overall, there is a correlation between high MAT volume and low incidence of hematopoiesis (Bigelow and Tavassoli, 1984), suggesting BM adipocytes have a negative impact on hematopoiesis. Because active hematopoiesis depletes rMAT, and cMAT accumulates with increasing age, we suggest that the adipocytes responsible for the loss of B lymphopoiesis are from cMAT.
BM adipocytes in young mice have a brown phenotype, but with aging (24 months), these cells lose characteristics of brown adipocytes and retain properties of white adipocytes (Krings et al., 2012). This is consistent with the observation in rabbits that yellow marrow stromal cells resemble 3t3.L1 white adipocytes (Bainton et al., 1986). Recent studies of aging and obesity have identified adipose tissue as a source of inflammatory adipokines and danger-associated molecular patterns (DAMPS) (Lago et al., 2007, Wen et al., 2011, Youm et al., 2012, Youm et al., 2013), which we suggest may contribute to the loss of B cell development, as inflammatory factors are known to negatively regulate B lymphopoiesis (Dorshkind, 1988, Hirayama et al., 1994, Kennedy and Knight, 2015, Maeda et al., 2005, Maeda et al., 2009, Ratliff et al., 2013).
7. Strategies for restoring B lymphopoiesis
7.1 Calorie restriction and exercise
Growing evidence suggests that pathologies occurring in aging and obesity share similarities. Some of these include accumulation of adipocytes in the BM (Adler et al., 2014, Chinn et al., 2012, Justesen et al., 2001, Rosen et al., 2009) and thymus (Yang et al., 2009a, 2009b), increased systemic inflammation (Baylis et al., 2013, Nagareddy et al., 2014, Osborn and Olefsky, 2012, Vasto et al., 2007), and reduced B and T lymphopoiesis. Elegant studies by Dixit and colleagues linked these phenotypes in the context of thymic involution and decreased T lymphopoiesis in mice (Yang et al., 2009a, 2009b, Youm et al., 2012). They found that thymic atrophy with aging is accompanied by adipocyte infiltration and increased inflammation. Thymic decline is accelerated by a high fat diet (Yang et al., 2009b), and decelerated by calorie restriction (Yang et al., 2009a), suggesting that adipocyte products and diet affect this process. In the context of B lymphopoiesis, Adler et al. (2014) found that a high fat diet increased BM adiposity in as little as 6weeks, and resulted in decreased B lymphopoiesis. These studies suggest that calorie restriction may lead to increased B lymphopoiesis. However, calorie restriction also increases MAT (Cawthorn et al., 2014, Devlin et al., 2010), as observed in anorexic patients who have significant amounts of BM fat (Bredella et al., 2009). Although calorie restriction has a positive effect on thymic health, the increase in MAT leads to an increase in adiponectin (Cawthorn et al., 2014) which is known to inhibit B lymphopoiesis (Yokota et al., 2003). Consequently, calorie restriction may not improve B lymphopoiesis in fatty BM. Additional studies are needed to characterize how calorie restriction changes MAT and determine how these changes overall affect B lymphopoiesis.
While calorie restriction does not appear to reduce BM adipose tissue, exercise can decrease or prevent adipocyte accumulation in the BM in both healthy and obese mice (Styner et al., 2014). This effect could be due to mechanical stimulus, because mechanical stimulation of MSCs in vitro, lowers PPAR-y signaling which is required for adipogenesis (Case et al., 2013), and mechanical stimulation of MSCs in vivo, skews MSC differentiation from adipocytes to osteoblasts (Rubin et al., 2007). Because osteoblasts support B lymphopoiesis and adipocytes inhibit this process (Bilwani and Knight, 2012, Calvi et al., 2003, Kennedy and Knight, 2015, Naveiras et al., 2009, Visnjic et al., 2004, Zhang et al., 2003), exercise may be beneficial. Because MSCs isolated from older rabbits may undergo decreased WNT signaling (Kalis et al., 2007), and because mechanical stretching of MSCs inhibits adipocyte differentiation by increasing β-catenin levels (downstream of WNT receptor signaling) (Case et al., 2010, Sen et al., 2008), increased exercise may provide a beneficial means to reduce BM adipose tissue.
7.2 Inflammation and MDSC accumulation
Adipocyte products lead to both MDSC accumulation (Kennedy and Knight, 2015) and inflammation (Coppack, 2001, Lago et al., 2007, Vandanmagsar et al., 2011, Wen et al., 2011). Therefore, strategies to prevent MDSC accumulation or block inflammation downstream of adipocytes may be effective strategies to reverse the loss of B cell development. Adipocytes produce danger-associated molecular patterns (DAMPS) e.g., lipid crystals (Youm et al., 2012), which trigger inflammasome activation in surrounding cells leading to production of inflammatory molecules such as IL-1 (Duewell et al., 2010, Youm et al., 2012). In the context of thymic atrophy, mice lacking nod-like receptor NLRP3, which acts as a sensor of DAMPs, exhibit delayed atrophy (Youm et al., 2012), suggesting that blocking 16 inflammation through NLRP3 is sufficient to delay thymic aging. While NLRP3−/− rabbits are currently unavailable to test the requirement of this pathway in the decline of B lymphopoiesis, several inhibitors are available. Alternatively, because adipocyte-induced MDSCs produce IL-1 downstream of activation by adipocyte-derived molecules, blocking IL-1 with IL-1 receptor antagonist (IL-1RA) or depleting MDSCs with treatments such as all-trans retinoic acid may be sufficient to restore B lymphopoiesis in rabbits and aged humans.
8. Remaining questions and Conclusion
While recent studies reviewed here have advanced our understanding of the mechanisms that negatively regulate rabbit B lymphopoiesis (Figure 3), many questions remain. For instance, what factors do adipocytes produce to negatively regulate B cell development, and how do they function? While adiponectin is known to negatively regulate B lymphopoiesis (Yokota et al., 2003) and rabbit BM fat produces adiponectin (Cawthorn et al., 2014), adipocytes produce at least one additional factor that can inhibit rabbit B lymphopoiesis (Bilwani and Knight, 2012). One such, as-yet-undescribed factor induces the accumulation of IL-1-secreting MDSCs, which inhibits B lymphopoiesis (Kennedy and Knight, 2015). It will be interesting to determine if these MDSCs are similar to the macrophage-like suppressor cells found in rabbit BM by Soderberg (1984a), or whether another inhibitory cell contributes to the decline of B lymphopoiesis. Other important issues include understanding what initiates changes in the BM microenvironment and increased fat deposition; are MSCs affected by changes in the microenvironment, and to what extent do they contribute to the changes; can the microenvironment, especially the large amount of fat in adult BM be reversed, and if so, how will this affect hematopoiesis.
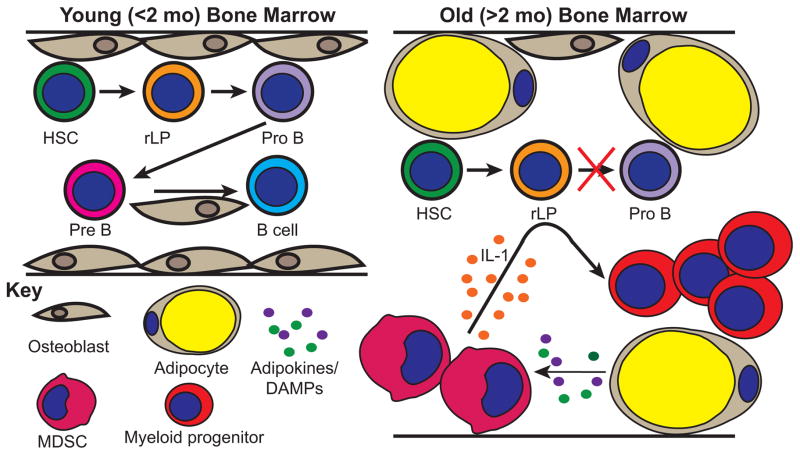
Figure 3. Proposed mechanisms that contribute to the arrest of B cell development.
BM from young rabbits is populated by osteoblasts which support B cell development. At this age, osteoblasts, pro-B cells, and pre-B cells are found at their highest numbers in rabbit BM. By 2 months of age these populations are greatly decreased. The BM microenvironment is the major contributor to the loss of B lymphopoiesis, which appears to be blocked at the rLP→pro-B cell stage. In >2 month old rabbits, the BM is filled with adipocytes. We hypothesize that adipocytes produce adipokines and DAMPs which lead to the accumulation of MDSCs which produce IL-1. We further hypothesize that IL-1 acts on rLPs, or yet to-be-defined MPPs, promoting myelopoiesis at the expense of B lymphopoiesis.
Adult rabbits have been used for decades to generate polyclonal and monoclonal antibodies. In the absence of significant on-going B lymphopoiesis, how do they maintain the capacity to resist infections and produce high affinity antibody following immunization? As mentioned earlier, naïve B cells generated in the BM leave and traffic to GALT for further diversification and maturation. Does rabbit GALT maintain the antibody repertoire throughout life in the absence of ongoing B lymphopoiesis? Are rabbit B cells long lived? A study by Raman and Knight (1992) suggests that rabbit B cells may be maintained through self-renewal. Rabbit B lymphocytes express CD5, a common marker for mouse B1a cells which are maintained by self-renewal throughout life (Forster and Rajewsky, 1987, Hayakawa et al., 1985, 1986, Herzenberg et al., 1986, Herzenberg and Kantor, 1993). While rabbit B cells resemble mouse B1a cells phenotypically, the ability to self-renew remains to be elucidated.
In conclusion, B lymphopoiesis in rabbit BM is a complex process that is regulated by the state of the BM microenvironment. Accumulation of adipocytes and suppressor cells in the BM likely contribute to a block in B cell development in vivo at the rLP stage. Strategies to modulate BM adipocyte phenotype and volume, and to deplete suppressor populations in rabbit BM are needed as a possible means for restoring or maintaining B cell development. The rabbit model has an advantage, because the decline of B lymphopoiesis occurs in a short time interval compared to humans and mice. Knowledge discovered in rabbit can be applied to humans to improve B cell immunity and response to vaccines in the elderly.
Table 1.
Antibodies reactive with rabbit immune-related antigens.
| Antibody reactivity | Clone | Specificity |
|---|---|---|
| CD1b | LAT3 | Rabbit |
| CD3 | PC3/188A | Rabbit |
| CD4 | Ken4 | Rabbit |
| CD9 | MM2 | Rabbit |
| CD10 | CD-CALLA | Human, cross reacts with rabbit |
| CD11b | 198 | Rabbit |
| CD11b | M1/70 | Human, mouse, cross reacts with rabbit |
| CD11c | 3/22 | Rabbit |
| CD14 | K4 | Rabbit |
| CD14 | TÜK4 | Human, cross reacts with rabbit |
| CD20 | B9E9 | Human, cross reacts with rabbit |
| CD21 | BL13 | Human, cross reacts with rabbit |
| CD23 | 9P25 | Human, cross reacts with rabbit |
| CD24 | M1/169 | Mouse, cross reacts with rabbit |
| CD25 | Kei-α1 | Rabbit |
| CD27 | LT27 | Human, cross reacts with rabbit |
| CD38 | IB6 | Human, cross reacts with rabbit |
| CD43 | L11/43 | Rabbit |
| CD44 | W4/86 | Rabbit |
| CD62L | LAM-1 | Human, cross reacts with rabbit |
| CD79a | HM47 | Human, cross reacts with rabbit |
| CD90 | 5E10 | Human, cross reacts with rabbit |
| BAFF | Polyclonal | Human, cross reacts with rabbit |
| BCL6 | BL6.02 | Human, cross reacts with rabbit |
| BR3 | Polyclonal | Human, cross reacts with rabbit |
| Complement C3 | Polyclonal | Rabbit |
| Caspase 3 | C92-605 | Human, mouse, cross reacts with rabbit |
| Ki67 | B56 | Human, cross reacts with rabbit |
| MHC II | 2C4 | Rabbit |
| Anti-Macrophage | RAM11 | Rabbit |
| IgM | 367 | Rabbit |
| IgA | 102 | Rabbit |
| IgG | 359 | Rabbit |
| Ig Light Chain | Polyclonal | Rabbit |
B lymphopoiesis declines in rabbit bone marrow early in life (2–4 months of age)
Bone marrow fills with fat simultaneous to the decline in B lymphopoiesis
Rabbit is a unique model for studying the decline of B lymphopoiesis in humans
Acknowledgments
Funding
Work for this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (AI068390), and by the National Institute on Aging of the National Institutes of Health (F31AG047817).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler BJ, Green DE, Pagnotti GM, Chan ME, Rubin CT. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One. 2014;9:e90639. doi: 10.1371/journal.pone.0090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitheen NB, McClure S, McCullagh P. B-cell development: one problem, multiple solutions. Immunol Cell Biol. 2010;88:445–450. doi: 10.1038/icb.2009.119. [DOI] [PubMed] [Google Scholar]
- Bainton DF, Maloney MA, Patt HM, Stern R. Characterization of rabbit stromal fibroblasts derived from red and yellow bone marrow. J Exp Med. 1986;163:400–413. doi: 10.1084/jem.163.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2:8-2395-2-8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RS, Knight KL. Somatic diversification of immunoglobulin heavy chain VDJ genes: evidence for somatic gene conversion in rabbits. Cell. 1990;63:987–997. doi: 10.1016/0092-8674(90)90502-6. [DOI] [PubMed] [Google Scholar]
- Becker RS, Suter M, Knight KL. Restricted utilization of VH and DH genes in leukemic rabbit B cells. Eur J Immunol. 1990;20:397–402. doi: 10.1002/eji.1830200224. [DOI] [PubMed] [Google Scholar]
- Bigelow CL, Tavassoli M. Fatty involution of bone marrow in rabbits. Acta Anat (Basel) 1984;118:60–64. doi: 10.1159/000145823. [DOI] [PubMed] [Google Scholar]
- Bilwani FA, Knight KL. Adipocyte-derived soluble factor(s) inhibits early stages of B lymphopoiesis. J Immunol. 2012;189:4379–4386. doi: 10.4049/jimmunol.1201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystryn JC, Jacobsen JS, Liu P, Heaney-Kieras J. Comparison of cell-surface human melanoma-associated antigens identified by rabbit and murine antibodies. Hybridoma. 1982;1:465–472. doi: 10.1089/hyb.1.1982.1.465. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Case N, Thomas J, Xie Z, Sen B, Styner M, Rowe D, Rubin J. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone. 2013;52:454–464. doi: 10.1016/j.bone.2012.08.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case N, Xie Z, Sen B, Styner M, Zou M, O’Conor C, Horowitz M, Rubin J. Mechanical activation of beta-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28:1531–1538. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra JJ, Colberg JE, Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966;123:547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Astle CM, Harrison DE. Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp Hematol. 1999;27:928–935. doi: 10.1016/s0301-472x(99)00018-1. [DOI] [PubMed] [Google Scholar]
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Crane MA, Kingzette M, Knight KL. Evidence for limited B-lymphopoiesis in adult rabbits. J Exp Med. 1996;183:2119–2121. doi: 10.1084/jem.183.5.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diascro DD, Jr, Vogel RL, Johnson TE, Witherup KM, Pitzenberger SM, Rutledge SJ, Prescott DJ, Rodan GA, Schmidt A. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. IL-1 inhibits B cell differentiation in long term bone marrow cultures. J Immunol. 1988;141:531–538. [PubMed] [Google Scholar]
- Dray S. Effect of maternal isoantibodies on the quantitative expression of two allelic genes controlling gamma-globulin allotypic specificities. Nature. 1962;195:677–680. doi: 10.1038/195677a0. [DOI] [PubMed] [Google Scholar]
- Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol. 2003;31:1284–1291. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol. 2011;186:697–707. doi: 10.4049/jimmunol.1002987. [DOI] [PubMed] [Google Scholar]
- Eskinazi DP, Knight KL, Dray S. Kinetics of escape from suppression of Ig heavy chain allotypes in multiheterozygous rabbits. Eur J Immunol. 1979;9:276–283. doi: 10.1002/eji.1830090406. [DOI] [PubMed] [Google Scholar]
- Feinstein A. Character and Allotypy of an Immune Globulin in Rabbit Colostrum. Nature. 1963;199:1197–1199. doi: 10.1038/1991197b0. [DOI] [PubMed] [Google Scholar]
- Fleischman JB, Porter RR, Press EM. The Arrangement of the Peptide Chains in Gamma-Globulin. Biochem J. 1963;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster I, Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Friedman ML, Tunyaplin C, Zhai SK, Knight KL. Neonatal VH, D, and JH gene usage in rabbit B lineage cells. J Immunol. 1994;152:632–641. [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathings WE, Mage RG, Cooper MD, Lawton AR, Young-Cooper GO. Immunofluorescence studies on the expression of VH a allotypes by pre-B and B cells of homozygous and heterozygous rabbits. Eur J Immunol. 1981;11:200–206. doi: 10.1002/eji.1830110308. [DOI] [PubMed] [Google Scholar]
- Gathings WE, Mage RG, Cooper MD, Young-Cooper GO. A subpopulation of small pre-B cells in rabbit bone marrow expresses kappa light chains and exhibits allelic exclusion of b locus allotypes. Eur J Immunol. 1982;12:76–81. doi: 10.1002/eji.1830120114. [DOI] [PubMed] [Google Scholar]
- Gibson LF, Piktel D, Landreth KS. Insulin-like growth factor-1 potentiates expansion of interleukin-7-dependent pro-B cells. Blood. 1993;82:3005–3011. [PubMed] [Google Scholar]
- Gilman-Sachs A, Mage RG, Young GO, Alexander C, Dray S. Identification and genetic control of two rabbit immunoglobulin allotypes at a second light chain locus, the c locus. J Immunol. 1969;103:1159–1167. [PubMed] [Google Scholar]
- Green KA, Cook WJ, Green WR. Myeloid-derived suppressor cells in murine retrovirus-induced AIDS inhibit T- and B-cell responses in vitro that are used to define the immunodeficiency. J Virol. 2013;87:2058–2071. doi: 10.1128/JVI.01547-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Wang L, Noelle RJ, Green WR. Selective Involvement of the Checkpoint Regulator VISTA in Suppression of B-Cell, but Not T-Cell, Responsiveness by Monocytic Myeloid-Derived Suppressor Cells from Mice Infected with an Immunodeficiency-Causing Retrovirus. J Virol. 2015;89:9693–9698. doi: 10.1128/JVI.00888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci U S A. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR, Stall AM, Herzenberg LA, Herzenberg LA. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Hayward AR, Simons MA, Lawton AR, Mage RG, Cooper MD. Pre-B and B cells in rabbits. Ontogeny and allelic exclusion of kappa light chain genes. J Exp Med. 1978;148:1367–1377. doi: 10.1084/jem.148.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg LA, Kantor AB. B-cell lineages exist in the mouse. Immunol Today. 1993;14:79–83. doi: 10.1016/0167-5699(93)90063-Q. discussion 88–90. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, Stall AM, Lalor PA, Sidman C, Moore WA, Parks DR, Herzenberg LA. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Hirayama F, Clark SC, Ogawa M. Negative regulation of early B lymphopoiesis by interleukin 3 and interleukin 1 alpha. Proc Natl Acad Sci U S A. 1994;91:469–473. doi: 10.1073/pnas.91.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R, Zuniga-Pflucker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.prot5156. pdb.prot5156. [DOI] [PubMed] [Google Scholar]
- Jacobsen K, Osmond DG. Microenvironmental organization and stromal cell associations of B lymphocyte precursor cells in mouse bone marrow. Eur J Immunol. 1990;20:2395–2404. doi: 10.1002/eji.1830201106. [DOI] [PubMed] [Google Scholar]
- Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J Immunol. 2003;171:6372–6380. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- Jensen CT, Boiers C, Kharazi S, Lubking A, Ryden T, Sigvardsson M, Sitnicka E, Jacobsen SE. Permissive roles of hematopoietin and cytokine tyrosine kinase receptors in early T-cell development. Blood. 2008;111:2083–2090. doi: 10.1182/blood-2007-08-108563. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kalis SL, Zhai SK, Yam PC, Witte PL, Knight KL. Suppression of B lymphopoiesis at a lymphoid progenitor stage in adult rabbits. Int Immunol. 2007;19:801–811. doi: 10.1093/intimm/dxm048. [DOI] [PubMed] [Google Scholar]
- Kennedy DE, Knight KL. Inhibition of B Lymphopoiesis by Adipocytes and IL-1-Producing Myeloid-Derived Suppressor Cells. J Immunol. 2015;195:2666–2674. doi: 10.4049/jimmunol.1500957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Knight KL, Becker RS. Molecular basis of the allelic inheritance of rabbit immunoglobulin VH allotypes: implications for the generation of antibody diversity. Cell. 1990;60:963–970. doi: 10.1016/0092-8674(90)90344-e. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie JE, 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004a;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie JE, 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004b;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- Lalor PA, Stall AM, Adams S, Herzenberg LA. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989;19:501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- Lanning D, Zhu X, Zhai SK, Knight KL. Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol Rev. 2000;175:214–228. [PubMed] [Google Scholar]
- Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New insights from an “old” molecule. Cell Cycle. 2010;9:3648–3654. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GW, Xu Z, Chen QW, Chang SX, Tian YN, Fan JZ. The temporal characterization of marrow lipids and adipocytes in a rabbit model of glucocorticoid-induced osteoporosis. Skeletal Radiol. 2013;42:1235–1244. doi: 10.1007/s00256-013-1659-7. [DOI] [PubMed] [Google Scholar]
- Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- Maeda K, Baba Y, Nagai Y, Miyazaki K, Malykhin A, Nakamura K, Kincade PW, Sakaguchi N, Coggeshall KM. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106:879–885. doi: 10.1182/blood-2005-02-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes LY, York JL, Soderberg LS. A soluble factor produced by bone marrow natural suppressor cells blocks interleukin 2 production and activity. Cell Immunol. 1988;116:35–43. doi: 10.1016/0008-8749(88)90207-9. [DOI] [PubMed] [Google Scholar]
- Mage RG, Lanning D, Knight KL. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Dev Comp Immunol. 2006;30:137–153. doi: 10.1016/j.dci.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mareschi K, Rustichelli D, Calabrese R, Gunetti M, Sanavio F, Castiglia S, Risso A, Ferrero I, Tarella C, Fagioli F. Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cells Int. 2012;2012:920581. doi: 10.1155/2012/920581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy PJ, Willcox N, Catty D. Early precursors of B lymphocytes. I Rabbit/mouse species differences in the physical properties and surface phenotype of pre-B cells, and in the maturation sequence of early B cells. Eur J Immunol. 1981;11:76–85. doi: 10.1002/eji.1830110203. [DOI] [PubMed] [Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MA, Fu WW, Green KA, Green WR. Subpopulations of M-MDSCs from mice infected by an immunodeficiency-causing retrovirus and their differential suppression of T- vs B-cell responses. Virology. 2015;485:263–273. doi: 10.1016/j.virol.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Oudin J. The allotype of certain blood protein antigens. C R Hebd Seances Acad Sci. 1956;242:2606–2608. [PubMed] [Google Scholar]
- Pernis B, Chiappino G, Kelus AS, Gell PG. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965;122:853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B, Forni L, Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970;132:1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman C, Knight KL. CD5+ B cells predominate in peripheral tissues of rabbit. J Immunol. 1992;149:3858–3864. [PubMed] [Google Scholar]
- Raman C, Spieker-Polet H, Yam PC, Knight KL. Preferential VH gene usage in rabbit Ig-secreting heterohybridomas. J Immunol. 1994;152:3935–3945. [PubMed] [Google Scholar]
- Ratcliffe MJ. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev Comp Immunol. 2006;30:101–118. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud CA, Anquez V, Grimal H, Weill JC. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud CA, Garcia C, Hein WR, Weill JC. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 1995;80:115–125. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Rosen CJ. What’s the matter with MAT?. Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol. 2013;25:504–510. doi: 10.1016/j.coi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal D, Johnson G, Wu TT, Mage RG. Generation of the primary antibody repertoire in rabbits: expression of a diverse set of Igk-V genes may compensate for limited combinatorial diversity at the heavy chain locus. Immunogenetics. 1999;50:31–42. doi: 10.1007/s002510050683. [DOI] [PubMed] [Google Scholar]
- Sell S, Gell PG. Studies on Rabbit Lymphocytes in Vitro. I Stimulation of Blast Transformation with an Antiallotype Serum. J Exp Med. 1965;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe BT, Kalis SL, Esteves PJ, Zhou T, Knight KL. A novel functional rabbit IL-7 isoform. Dev Comp Immunol. 2010;34:828–836. doi: 10.1016/j.dci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewe BT, Kalis SL, Le PT, Witte PL, Choi S, Conway SJ, Druschitz L, Knight KL. In vitro requirement for periostin in B lymphopoiesis. Blood. 2011;117:3770–3779. doi: 10.1182/blood-2010-08-301119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons MA, Hayward AR, Gathings WE, Lawton AR, Young-Cooper GO, Cooper MD, Mage RG. Expression of b 4 and b 5 chi light chain allotypes by B and pre-B cells in allotype-suppressed and neutralized b4b5 rabbits. Eur J Immunol. 1979;9:887–891. doi: 10.1002/eji.1830091110. [DOI] [PubMed] [Google Scholar]
- Sitnicka E, Brakebusch C, Martensson IL, Svensson M, Agace WW, Sigvardsson M, Buza-Vidas N, Bryder D, Cilio CM, Ahlenius H, Maraskovsky E, Peschon JJ, Jacobsen SE. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. J Exp Med. 2003;198:1495–1506. doi: 10.1084/jem.20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg LS. Rabbit bone marrow suppressor cells block the production or release of a soluble bone marrow growth factor. Cell Immunol. 1985;92:313–320. doi: 10.1016/0008-8749(85)90012-7. [DOI] [PubMed] [Google Scholar]
- Soderberg LS. Differential activities of rabbit bone marrow suppressor cells. Int Arch Allergy Appl Immunol. 1984a;74:341–346. doi: 10.1159/000233570. [DOI] [PubMed] [Google Scholar]
- Soderberg LS. Regulation of constitutive bone marrow cell proliferation by bone marrow suppressor cells. Int Arch Allergy Appl Immunol. 1984b;74:305–310. doi: 10.1159/000233565. [DOI] [PubMed] [Google Scholar]
- Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc Natl Acad Sci U S A. 1995;92:9348–9352. doi: 10.1073/pnas.92.20.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. [PubMed] [Google Scholar]
- Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, Case N, Xie Z, Sen B, Romaine A, Pagnotti GM, Rubin CT, Styner MA, Horowitz MC, Rubin J. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976;100:16–18. [PubMed] [Google Scholar]
- Tavassoli M. Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia. 1974;30:424–425. doi: 10.1007/BF01921701. [DOI] [PubMed] [Google Scholar]
- Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- Todd CW. Allotypy in rabbit 19S protein. Biochem Biophys Res Commun. 1963;11:170–175. doi: 10.1016/0006-291x(63)90329-2. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tsapogas P, Zandi S, Ahsberg J, Zetterblad J, Welinder E, Jonsson JI, Mansson R, Qian H, Sigvardsson M. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118:1283–1290. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar SR, McGuire TR, Brusnahan SK, Jackson JD, Garvin KL, Kessinger MA, Lane JT, O’Kane BJ, Sharp JG. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anat. 2011;219:574–581. doi: 10.1111/j.1469-7580.2011.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunyaplin C, Knight KL. Fetal VDJ gene repertoire in rabbit: evidence for preferential rearrangement of VH1. Eur J Immunol. 1995;25:2583–2587. doi: 10.1002/eji.1830250927. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C, Paul S, Haller C, Gassmann M, Rodewald HR. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- Wei C, Zeff R, Goldschneider I. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. J Immunol. 2000;164:1961–1970. doi: 10.4049/jimmunol.164.4.1961. [DOI] [PubMed] [Google Scholar]
- Weiss L. The hematopoietic microenvironment of the bone marrow: an ultrastructural study of the stroma in rats. Anat Rec. 1976;186:161–184. doi: 10.1002/ar.1091860204. [DOI] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009a;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009b;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171:5091–5099. doi: 10.4049/jimmunol.171.10.5091. [DOI] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Munzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]