Abstract
Purpose
All trans retinoic acid (ATRA) is successful in treating acute promyelocytic leukemia (APL) by inducing terminal differentiation-mediated cell death, but it has limited activity in non-APL acute myeloid leukemia (AML). We aim to improve ATRA therapy of AML by enhancing apoptosis through repression of the anti-apoptotic proteins Bcl-2 and Mcl-1.
Experimental Design
APL and AML cell lines, as well as primary AML samples, were used to explore the mechanisms regulating differentiation and apoptosis during ATRA treatment. Stable transfection and gene silencing with siRNA were used to identify the key factors that inhibit apoptosis during induction of differentiation and drugs that accelerate apoptosis.
Results
In differentiation responsive AML cells, ATRA treatment induces long-lasting repression of Bcl-2 while first up-modulating and then reducing the Mcl-1 level. The Mcl-1 level appears to serve as a gatekeeper between differentiation and apoptosis. During differentiation induction, activation of MEK/ERK and PI3K/Akt pathways by ATRA leads to activation of p90RSK and inactivation of glycogen synthase kinase 3β (GSK3β), which increase Mcl-1 levels by increasing its translation and stability. Sorafenib blocks ATRA-induced Mcl-1 increase by reversing p90RSK activation and GSK3β inactivation, maintains the repressed Bcl-2 level, and enhances ATRA induced apoptosis in non-APL AML cell lines and in primary AML cells.
Conclusion
Inhibition of Mcl-1 is required for apoptosis induction in ATRA differentiation responsive AML cells. ATRA and Sorafenib can be developed as a novel drug combination therapy for AML patients because this drug combination augments apoptosis by inhibiting Bcl-2 and Mcl-1.
Keywords: all trans retinoic acid, Mcl-1, glycogen synthase kinase-3β, p90RSK, acute myeloid leukemia, sorafenib
Introduction
All trans retinoic acid (ATRA) together with chemotherapy used as the first-line treatment for the newly diagnosed acute promyelocytic leukemia (APL) patients achieves ~75% cures (1). However, all APL patients treated with ATRA alone relapse, most likely because cells that escape terminal differentiation (2–3) and also escape death. Although, ATRA can induce differentiation in most non-APL AML cell lines and primary AML cells in culture, its clinical activity in non-APL AML patients is very limited (4). Past efforts have focused on the search of agents that can enhance ATRA induced differentiation (4–5). These have been proven to be unsuccessful in clinical testing (6).
The main pathway through which chemotherapy kills leukemia cells is induction of apoptosis. Apoptosis is mediated through intrinsic (mitochondrial) and extrinsic (death receptor) signaling pathways. The extrinsic pathway affected by TRAIL and death receptor 5 (DR5) is thought to be responsible for apoptosis of terminally differentiated APL cells after ATRA treatment (3). The mitochondrial apoptotic pathway is controlled by Bcl-2 family of anti-apoptotic proteins. ATRA treatment was shown to have variable effects on the transcription of the Bcl-2 family of anti-apoptotic proteins; it decreases Bcl-2, increases Bfl-1, while showing no effect on the Mcl-1 mRNA level (7–8). However, we and others have shown that Mcl-1 protein is up-regulated by ATRA and that this regulation is time dependent (9–10). Mcl-1 is a key pro-survival protein that protects mature neutrophils from cell death (11) and its ATRA-induced increase might save AMLs from apoptosis. We propose that a rapid AML cell death can be induced by blocking Mcl-1 in cells in which Bcl-2 is repressed by ATRA. Mcl-1 has a short half-life which is regulated through translation and protein degradation (12). We explored the mechanism of Mcl-1 upregulation by ATRA in APL cells and found that ATRA increased Mcl-1 levels at translational and posttranslational levels associated with p90RSK activation and GSK3β inactivation. We also studied the role of Mcl-1 in differentiation and apoptosis using cells in which Mcl-1 was either forced expressed or silenced and found that Mcl-1 plays a gate keeper role that protects cells from apoptosis. We then looked for an agent that, by reducing Mcl-1, will eliminate the block to apoptosis. We found that Sorafenib, a multi-kinase inhibitor, counteracts ATRA-induced Mcl-1 increase and augments apoptosis induction in non-APL AML cell lines and in primary cells.
Materials and methods
Reagents
ATRA, rapamycin, LY290024, SB216763 and cycloheximide (CHX) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). U0126, PD184352 and Sorafenib were obtained from LC Laboratories (Woburn, MA, USA). Antibody to Mcl-1, ERK1, Bcl-2, β-actin, and USP9X were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); to PARP was from BD Biosciences (San Diego, CA, USA ); to DR5 was from ProSci, Inc. (Poway, CA, USA); to p-MEK (Ser217/221), p-ERK (Thr202/Tyr204), Akt, p-Akt (Ser473), p-mTOR, p70S6K, p-p70S6K (Thr389), p-p70S6K (Thr421/Ser424), p-p90RSK (Ser380), p90RSK, p-rpS6 (Ser235/236), rpS6, GSK-3β, p-GSK-3β (Ser9), caspase-8, -9, -3 and Trail were from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Cell lines and treatments
APL derived NB4 and its ATRA-resistance clone R4 were obtained from Dr. Willson Miller Jr. (13), AML derived HL-60 and THP-1 were obtained from ATCC and ME-1 cells were obtained from Dr. Shigeru Fujita (14). MOLM13 cells were obtained from DSMZ. All cell lines were not authenticated by us once received in our laboratory and were cultured in RPMI 1640 medium supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine, and 10% (v/v) heat-inactivated fetal bovine serum (FBS). HL-60/Bcl2 cells transfected with Bcl-2 were obtained from Dr. Michael Cleary (15). Mcl-1 expressing HL-60/M15 cells were generated in our laboratory (16). These cells were seeded at 105/ml and treated with ATRA alone or in combination with other agents for 2 days. For the time course studies, the cell number was adjusted and new medium with ATRA was changed every 2 days.
Detection of apoptosis, differentiation, protein levels and silencing of Mcl-1
Apoptotic cells were determined by the annexin V assay according to the manufacturer’s instructions (annexin V–FITC Apoptosis Detection Kit (BD Biosciences). The nitroblue tetrazolium (NBT) reduction assay and CD11b expression were used to evaluate differentiation using FACS. Western blot analysis was used to measure protein levels. To silence Mcl-1 we used siRNA. All these methods were done as we previously reported (8, 17).
Isolation of patient-derived leukemic blasts
Leukemic blasts were obtained from a bone marrow and three peripheral blood samples of AML patients with FAB subtypes of M2 and M4. These studies have been sanctioned by the Investigation Review Board of Mount Sinai School of Medicine and all patients provided informed consent. Whole blood or bone marrow collected in heparinized tubes were subjected to Ficoll-Hypaque density gradient separation, the blasts were washed and resuspended in RPMI-1640 with 20% FBS at 1 × 107 cells/ml.
Animal survival study
Twenty NOD/SCI/IL-2Rg (NSG) mice (age, 8 weeks) were injected with 5 × 106 log-phase MOLM13 cells via the lateral tail vein. Five days after leukemic cell injection, the mice were divided randomly into 4 groups. A Cremophor EL/ethanol/water solution (12.5% Cremophor EL/12.5% ethanol/75%water) containing sorafenib was prepared and 20 mg/kg was administered by oral gavage. ATRA was prepared in DMSO and diluted with PBS to containing 10% DMSO and 10 mg/kg was administered intraperitoneally. Both drugs were given alone or as a combination daily for five continuous days a week until the mouse died. Control mice received the vehicle through the same route and on the same schedule. Survival times were compared with Kaplan–Meier survival analysis.
Statistical analysis
Data were analyzed for statistical significance using student’s t test (Microsoft Excel, Microsoft Corp., Redmond, WA, USA). A p-value of <0.05 was considered statistically significant.
Results
Mcl-1 protein is increased while Bcl-2 protein is decreased in ATRA differentiation responsive NB4 cells
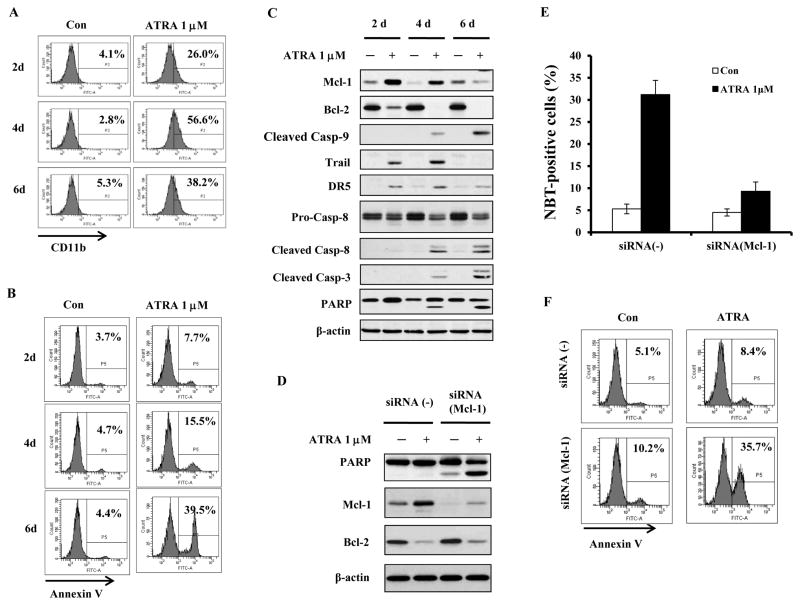
Treatment of NB4 cells with 1 μM ATRA for 2, 4 and 6 days produced on the respective days ~26%, ~57% and ~38% of differentiated cells (Fig. 1A) and ~8%, ~15% and ~40% of apoptotic cells (Fig. 1B). The parallel reduction in the percent of differentiated cells, accompanied by the rise in apoptotic cells, suggests that terminally differentiated cells eventually undergo apoptosis. Both the extrinsic and the intrinsic apoptotic pathways were examined by measuring the cleavage of caspase-8 and caspase-9 during the 2–6 day treatment (Fig. 1C). The cleaved fragments of caspase-3,-8 and -9 were not detected on day 2. PARP cleavage, detected on day 4, was associated with the extrinsic pathway caspase-8 cleavage but only minimally with caspase-9 cleavage. Consistent with the previous report we found that the levels of both TRAIL and DR5 were induced by ATRA on day 2 and 4 (3). Increased PARP cleavage associated with cleaved fragments of caspase-3, -8 and -9 was detected in cells treated with ATRA for 6 days (Fig. 1C), indicating activation of both apoptotic pathways after prolonged treatment.
Figure 1. ATRA induced NB4 cell differentiation coincides with increase in Mcl-1 protein and reduction in Bcl-2 protein.
NB4 cells were treated with 1μM ATRA for 2, 4 and 6 days. Percent of differentiated cells was determined using CD11b expression (A) and percent of apoptotic cells using Annexin V staining (B). Lysates of cells treated with ATRA for 2, 4 and 6 days were analyzed for apoptosis associated proteins by Western blotting (C). Mcl-1 was knocked down in NB4 cells using siRNA and the cells were treated with 1μM ATRA for 2 days. Silencing of Mcl-1 increased apoptosis as determined by enhanced PARP cleavage (D) and increased Annexin-V-FITC staining (F), but decreased the percent of differentiated cells as determined by NBT assay (E). Con, Control.
We also tested the levels of Bcl-2 and Mcl-1 protein in NB4 cells treated with 1 μM ATRA for 2, 4 and 6 days. The Bcl-2 protein level was decreased after 2 days of ATRA-treatment and remained repressed throughout the treatment (Fig. 1C). The level of Mcl-1 was increased on day 2 and gradually declined during day 4 and 6, at the time when the proportion of the differentiated cells declined and of the apoptotic cells rose. These data suggest that increased Mcl-1 protein protects differentiated cells from apoptosis and that Mcl-1 protein needs to be reduced for maximal apoptosis to occur. To test the role of Mcl-1 protein in ATRA-induced NB4 differentiation process, Mcl-1 was knocked down using siRNA (Fig. 1D). Although silencing of Mcl-1 decreased the ability of ATRA to induce differentiation at day 2 (Fig. 1E) it strongly enhanced ATRA-induced apoptosis as determined by PARP cleavage (Fig. 1D) and Annexin V staining (Fig. 1F). These data suggest that Mcl-1 protein is required for induction of differentiation by ATRA and that it functions as a main pro-survival protein protecting differentiated cells from death.
Increase in Mcl-1 protein levels by ATRA is associated with p90RSK activation and GSK3β inactivation
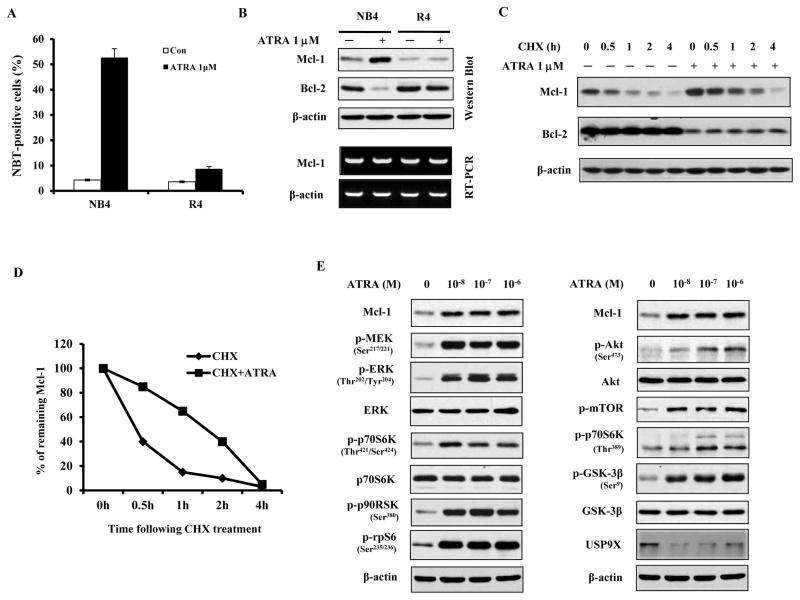
Utilizing ATRA differentiation sensitive NB4 and its resistant R4 subclone, the protein levels of Mcl-1 and Bcl-2 were determined during ATRA-induced differentiation defined as the ability to reduce NBT. After 4 days of treatment with 1μM ATRA there were 52.7% NBT-positive cells in NB4 and only 8.7% in R4 cells (Fig. 2A). Treatment with ATRA for 2 days increased the level of Mcl-1 protein but reduced Bcl-2 in NB4 cells (Fig. 2B). Previously it has been shown that ATRA represses Bcl-2 at both mRNA and protein levels (7). ATRA did not increase Mcl-1 mRNA level in NB4 cells as measured using RT-PCR (Fig. 2B), suggesting a posttranscriptional regulation. MCL-1 is an unstable protein with a very short-half life. To distinguish between ATRA effect on translation and stability of Mcl-1 protein, NB4 cells were treated with cycloheximide (CHX) for 1–4 hrs and with or without ATRA. ATRA treatment increased the Mcl-1 half-life in presence of CHX from ~20 min to ~80 min (Figs. 2C and 2D), indicating that ATRA stabilizes Mcl-1 protein. In contrast, ATRA effect on Bcl-2 was not influenced by CHX treatment (Fig. 2C).
Figure 2. Increase in Mcl-1 protein level after ATRA treatment coincides with activation of both PI3K/Akt/GSK3β and MEK/ERK/p90RSK signalings in NB4 cells.
NB4 cells and ATRA-resistant R4 cells were treated with 1μM ATRA for 4 days and percentage of differentiated cells was determined by NBT reduction assay (A). The data shown are the mean of three independent experiments ± SD. Lysates of ATRA-treated (2 days, 1μM) NB4 and ATRA-resistant R4 cells were analyzed by Western blotting for Mcl-1 and Bcl-2. β-actin was used as a loading control (B). The effect of ATRA treatment on the rate of degradation of Mcl-1 and Bcl-2 protein was tested in NB4 cells treated with cycloheximide (CHX, 10μg/ml) in the absence or presence of 1μM ATRA for the indicated time, and protein levels were analyzed with Western blot (C). Protein band intensity was analyzed using Image J software. Mcl-1 protein level at time 0 h was set at 100% and the decline in protein level with time was expressed as percent of time zero (D). NB4 cells were treated for 2 days with increasing concentrations (10−8–10−6M) of ATRA, lysed and analyzed for activation of MEK/ERK pathway (E, left panel) and Akt pathway (E, right panel) using specific antibodies to the phosphorylated proteins.
Raf/MEK/ERK and Akt/mTOR signaling pathways are activated during ATRA-induced differentiation process (18–19) and ATRA treatment of NB4 cells increases the levels of phosphorylated p70S6K and rpS6 (20). Both Raf/MEK/ERK and Akt/mTOR pathways phosphorylate p70S6K and then rpS6, increasing protein translation (21). ERK phosphorylates p70S6K at Thr421/Ser424 sites while mTOR phosphorylates it at Thr389 site (22). The existence of a link between rise in Mcl-1 and these factors was tested in ATRA treated NB4 cells. NB4 cells treated with 10−8 –10−6M of ATRA had elevated levels of phosphorylated MEK, ERK, Akt, mTOR, p70S6K and rpS6 as well as an increased level of Mcl-1 protein. Importantly, we found that at the lower (10−8 M) concentration of ATRA there was an increase in ERK pathway phosphorylated Thr421/Ser424 p-p70S6K level, while p-p70S6K (Thr389) (the target of mTOR pathway) was only induced by ATRA at concentrations higher that 10−7 M (Fig. 2E). The up-regulation of p-p70S6K (Thr421/Ser424) and p-rpS6, correlated with an increase in Mcl-1 level, most likely through an increase in Mcl-1 protein translation.
The stability of Mcl-1 protein is regulated by phosphorylation by ERK and GSK3β and by ubiquitination. GSK3β increases Mcl-1 degradation while deubiquitinase USP9X stabilizes Mcl-1 (23–24). GSK3β has been found to be inactivated by phosphorylation in ATRA differentiated NB4 cells (25–26). GSK3β can be phosphorylated by AKT and p90RSK, the down-stream target of ERK (27). We found that ATRA treatment of NB4 cells increased the phosphorylation of p90RSK, GSK3β, and ERK, and that those are associated with the increased levels of Mcl-1 (Fig. 2E). Because the level of USP9X was decreased by ATRA treatment (Fig. 2E), we concluded that the ATRA induced increase in Mcl-1 stability proceeds through activation of p90RSK, ERK and inactivation of GSK3β.
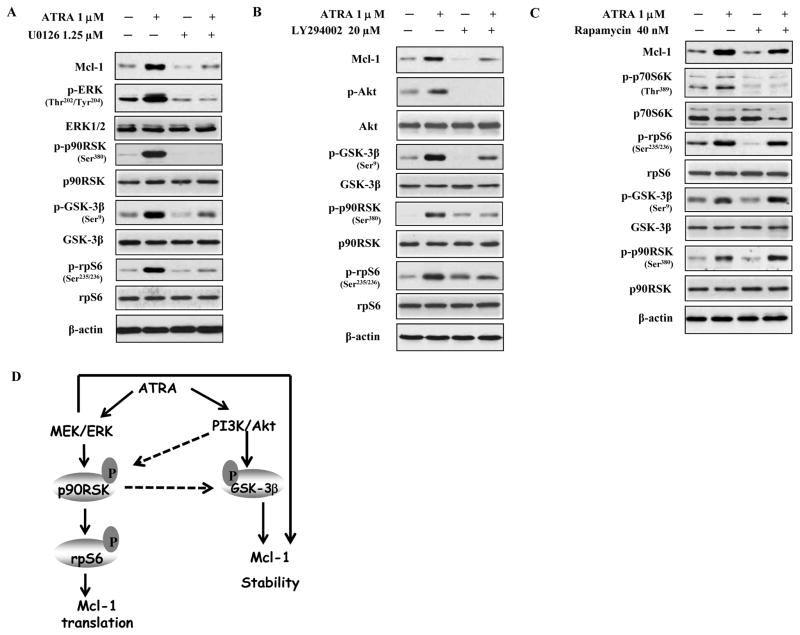
To further identify the specific ATRA up-regulated kinase pathways which mediate the Mcl-1 induction we treated cells with MEK inhibitor U0126, or PI3K inhibitor LY294002, or mTOR inhibitor rapamycin. Inhibition of MEK and PI3K blocked the increase in phosphorylated -p90RSK, -GSK3β and -rpS6 as well as the level of Mcl-1 protein (Fig. 3A, 3B). Rapamycin (40 nM) decreased the level of phosphorylated p70S6K at Thr389 (Fig. 3C) but neither blocked ATRA-induced phosphorylation of p90RSK, GSK3β and rpS6 nor Mcl-1 increase. These data suggest that ATRA-activated MEK/ERK and PI3K/Akt increase Mcl-1 protein by both enhanced translation and increased stability involving activation of p90RSK and inactivation of GSK3β (Fig. 3D).
Figure 3. The effects of MEK inhibitor U0126, PI3K inhibitor LY294002 and mTOR inhibitor rapamycin, on ATRA-induced Mcl-1 increase in NB4 cells.
NB4 cells were treated with 1.25μM MEK inhibitor, U0126 (A), 20μM PI3K inhibitor, LY294002 (B), and 40 nM mTOR inhibitor, rapamycin (C). The treatment was for 2 days with inhibitor alone or with 1μM ATRA. The protein levels of the indicated phosphorylated and unphosphorylated proteins were determined using specific antibodies in Western blot analysis. (D) Working model of ATRA-induction of Mcl-1. ATRA activates the MEK/ERK/p90RSK pathway which increases Mcl-1 translation and stability. ATRA also activates PI3K/Akt/GSK3β which increases Mcl-1 stability. p90RSK can directly inactivate GSK3β by phosphorylation and Akt can activate p90RSK by phosphorylation. Both pathways contribute to the Mcl-1 protein increase after ATRA treatment.
Mcl-1 level is crucial for ATRA-induced differentiation and apoptosis in a FAB-M2-HL-60 cells
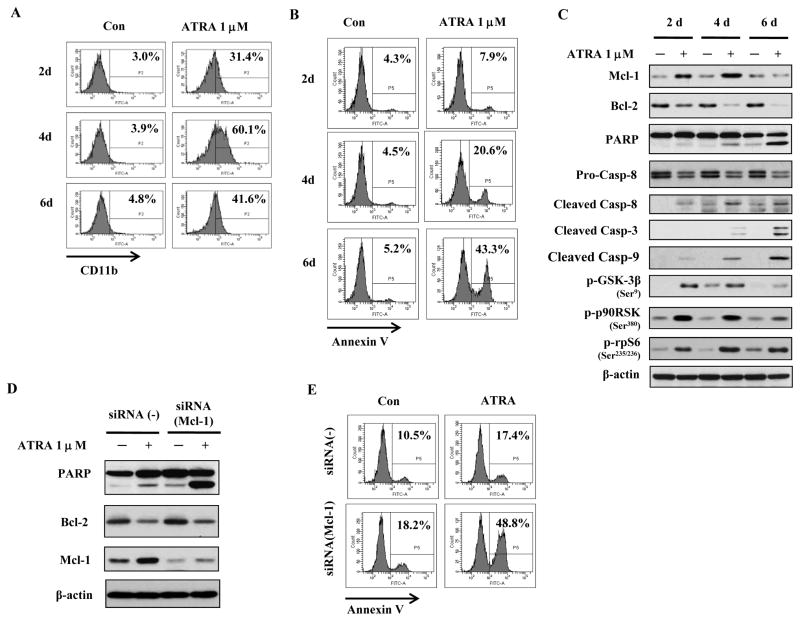
We tested the effect of 1μM of ATRA for 2, 4 and 6 days in differentiation responsive FAB-M2 HL-60 cells. We found that, similarly to NB4 cells, the percent of differentiated cells rose from 31% on day 2 to 60% on day 4 (Fig. 4A) and, like in NB4, it dropped to 40% on day 6; this drop was accompanied by a sharp rise (to ~43%) in apoptotic cells (Fig. 4B). Moreover, like in NB4, the Mcl-1 protein levels increased on day 2 and dropped on day 6 to the level in untreated cells (Fig. 4C). ATRA treatment of HL60 cells increased the levels of phosphorylated p90RSK, phosphorylated (inactive) GSK3β, and phosphorylated rpS6 (Fig. 4C). Compared to NB4 cells (Fig. 1C), there was a difference in the intensity of Mcl-1 induction by ATRA in HL-60 cells; this difference is correlated with the levels of p-GSK3β. The declining level of the phosphorylated GSK3β day 6, indicating its increased activity, led to a reduction in Mcl-1 in both HL-60 and NB4 cells (Fig. 4C and data not shown). As in NB4 cells (Fig. 1), Bcl-2 protein was repressed on day 2 and 4, but this was not accompanied by substantial degree of apoptosis. Silencing of Mcl-1 with siRNA (Fig. 4D) produced increased PARP cleavage (Fig. 4D), and Annexin V staining (Fig. 4E), which rose to 48.8% in cells treated with ATRA for only 2 days. The fact that this profound apoptosis inducing effect was not observed in absence of ATRA treatment (Figs. 4D and 4E), suggests that both, the repressed Bcl-2 and the silenced Mcl-1, were crucial.
Figure 4. Mcl-1 plays an important role in ATRA-induced differentiation and apoptosis in HL-60 cells.
HL-60 cells were treated with 1μM ATRA for the indicated times and the percent of differentiated cells was determined by measuring CD11b expression (A) and of apoptotic cells using Annexin V (B). HL-60 cells were treated with 1μM ATRA for the indicated times and the levels of Mcl-1, Bcl-2, cleaved caspase-9,-8,-3, PARP and β-actin were determined using specific antibodies with Western blot (C). HL-60 cells transfected with Mcl-1 siRNA or control siRNA were treated with 1μM ATRA for 2 days. The protein levels of PARP, Mcl-1, Bcl-2 and β-actin were determined using specific antibodies with Western blot (D) and the percent of apoptotic cells was determined using FACS of cells stained with Annexin V-FITC (E).
To conclusively test the role of Mcl-1 and Bcl-2 in differentiation and apoptosis, we used an Mcl-1 overexpressing clone of HL-60 (HL60/M15 cells) and a clone with overexpressed Bcl-2 (HL-60/Bcl2 cells) and respectively compared ATRA-induced differentiation and apoptosis to those in HL-60 clones transfected with empty vector (HL-60/V5 and HL-60/neo) (Sup. Fig. 1A, 1D). ATRA treatment of HL-60/M15 cells enhanced differentiation but blocked apoptosis (Sup. Fig. 1B, 1C). Bcl-2 overexpression had no effect on differentiation (Sup. Fig. 1E), but protected HL-60 cells from apoptotic cell death (Sup. Fig. 1F). These data show that although, both Bcl-2 and Mcl-1 block apoptosis, probably only Mcl-1 participates in ATRA-induced differentiation.
Enhancement of ATRA-induced apoptosis by MEK inhibitors is associated with blockade of Mcl-1 induction in FAB-M2 HL-60 cells
MEK inhibitors can enhance ATRA-induced apoptosis in AML cells but the mechanism has not been elucidated (28). The combined effects of ATRA with either of the two MEK inhibitors, U0126 and PD184352, were tested in HL-60 cells. ATRA combined with either U0126 or PD184352 induced more than 50% of the treated cells to undergo apoptosis (Sup. Fig. 2A). The increased levels of p-ERK, p-p90RSK, p-rpS6, p-GSK3β and Mcl-1 proteins by ATRA were blocked by either U0126 or PD184352 (Sup. Fig. 2B). ATRA-mediated repression of Bcl-2 protein was not reversed either by U0126 or by PD184352. At the concentrations used these agents alone did not induce apoptosis in HL-60 cells, even with repressed Bcl-2 or Mcl-1, respectively (Sup. Fig. 2B). These data suggest that ATRA-induced Mcl-1 could be counteracted by inhibiting MEK/ERK signaling.
Sorafenib augments ATRA-induced apoptosis in AML cell lines and primary cells
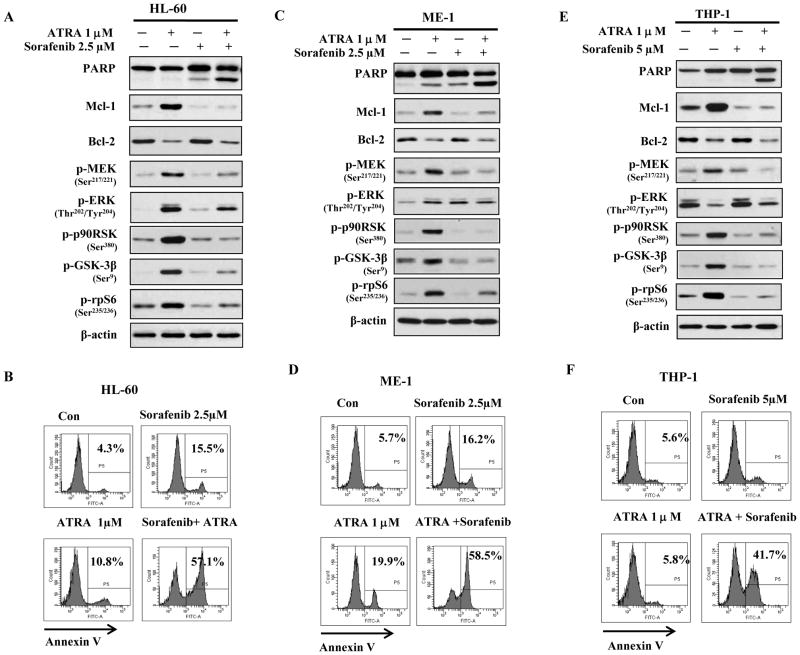
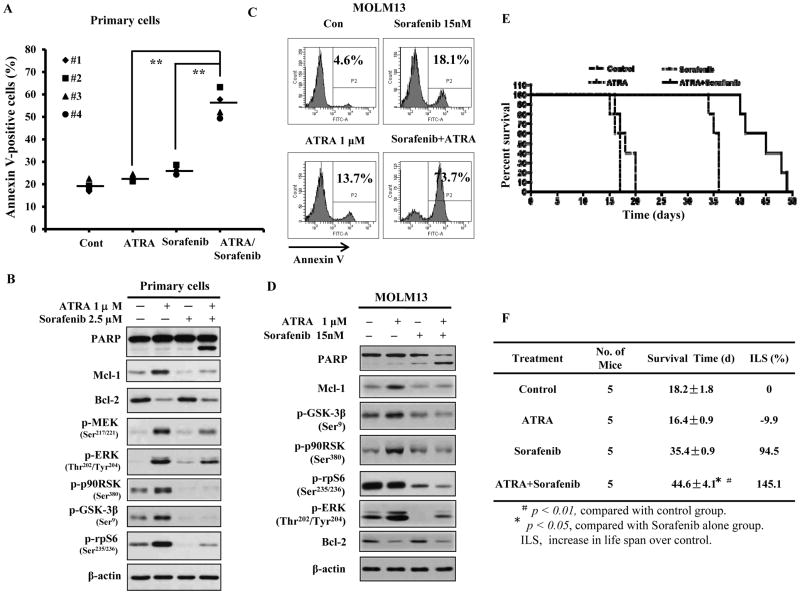
Sorafenib, an inhibitor of B-raf, the upstream activator of the MEK/ERK pathway, was approved for clinical use (29) and it has been reported to inhibit Mcl-1 translation in leukemia cells (30–31). We tested the combined effects of ATRA and Sorafenib in FAB-M2 HL-60 cells, additional AML cell lines, FAB-M4 ME-1 cells and FAB-M5 THP-1 cells as well as primary AML cells from four AML patients. Like HL-60 cells (Fig. 4A), both ME-1 and THP-1 cells are responsive to ATRA-induced differentiation (Sup. Fig. 3). ATRA increased the levels of p-MEK, p-90RSK, p-GSK3β and p-rpS6 as well as Mcl-1 protein while repressing Bcl-2 protein in HL-60 cells (Fig. 5A), ME-1 cells (Fig. 5C), and THP-1 cells (Fig. 5E). Sorafenib at 2.5 μM blocked the ATRA-induced rise in the levels of p-MEK, p-GSK3β, p-p90RSK and p-rpS6 as well as in Mcl-1 protein (Fig. 5A, C, E) and together with ATRA induced apoptosis in more than 40–50% of cells; each agent alone induced only 5–20% apoptosis (Fig. 5B, D, F). The four primary samples were isolated from two FAB-M2 AML patients and two FAB-M4 AML patients. Three cases had normal genetic phenotype and one case had inv(16) like ME-1 cells. Neither of the patient’s sample had FLT3 and (or/) NPM mutations. Treatment of these samples showed that ATRA or Sorafenib alone induced less than 10% apoptosis above the untreated control; the combination of ATRA with Sorafenib induced apoptosis in more than 30–40% of cells, suggesting a synergistic effect (Fig. 6A). ATRA increased the levels of p-MEK, p-90RSK, p-GSK3β and p-rpS6 as well as Mcl-1 proteins in one case of tested primary AML cells (Fig. 6B); these effects were blocked by the addition of Sorafenib. We found that Sorafenib blocking of ATRA-induced Mcl-1 levels always coincided with inhibition of ATRA-induced phosphorylation of p90RSK, GSK3β and rpS6 (Figs. 5A, B, C, 6B). Although, the regulation on p-ERK levels by either ATRA or Sorafenib in the different cell lines was inconsistent, the combination of ATRA with Sorafenib consistently augmented apoptosis in all cell lines and primary cells tested and this was always associated with down-regulation of Bcl-2 and Mcl-1 protein (Figs. 5A, B, C, 6B).
Figure 5. Sorafenib enhances ATRA-induced apoptosis in HL-60, ME-1 and THP-1 cells and this effect is linked with repression of both Bcl-2 and Mcl-1 proteins.
Sorafenib blocked ATRA-induced Mcl-1, p-p90RSK, p-GSK3β, and p-MEK and enhanced ATRA-induced apoptosis in HL-60 (A, B), ME-1(C, D) and THP-1(E, F) cells. HL-60, ME-1 and THP-1 cells were treated with 1μM ATRA plus 2.5 or 5 μM Sorafenib for 2 days. The levels of the indicated proteins were determined using Western blot analysis (A,C,E) and the apoptotic cells were determined using FACS after staining with Annexin V (B, D, F).
Figure 6. ATRA combined with Sorafenib has enhanced apoptosis induction ability in primary AML cells and MOML13 cells.
The combined effect of ATRA and Sorafenib on apoptosis induction in primary cells isolated from 4 cases of AML patients was examined after treatment with 1 μM ATRA and 2.5 μM Sorafenib for 2 days (A). The apoptotic cells were detected with Annexin V-FITC assay using flow cytometry. ** P <0.01 compared with cells treated with either ATRA or Sorafenib alone. The combined effects of ATRA and Sorafenib on Mcl-1 protein and its potential regulators in primary AML cells isolated from #2 patient were examined using specific antibodies with Western blot (B). The combined effect of ATRA and Sorafenib on apoptosis induction (C) and protein levels (D) in MOLM13 cells was examined after treatment with 1 μM ATRA and 15 nM Sorafenib for 2 days. Mice survival after treatment with Sorafenib and ATRA, as described in Material and Methods, is shown in Kaplan-Meyer survival plot (E) and the average survival times of each group and increase of life span over control was calculated (F). Five mice were used in each group.
Sorafenib is currently being tested as a FLT3-ITD inhibitor for AML treatment (32) and FLT3-ITD AML cells are responsive to Sorafenib-induced apoptosis at lower concentrations (33). Recently, Sorafenib together with ATRA was used to treat 3 patients with FLT3-ITD AML, achieving durable responses (34). In MOML13 cells, which have FLT3-ITD, Sorafenib, at lower concentrations, was able to induce apoptosis (35). This cell line undergoes differentiation in response to ATRA (36). We found that ATRA induced differentiation of MOLM13 cells (Sup. Fig. 3) increased the levels of phosphorylated pRSK, phosphorylated GSK3β and Mcl-1 (Fig. 6D). ATRA combined with lower concentrations of Sorafenib was more effective in apoptosis induction than Sorafenib alone (Fig. 6C). The effects of ATRA, Sorafenib and their combination were tested in vivo using MOML13 cells xenografted into NSG mice. ATRA alone did not increase survival while, as compared to control, Sorafenib prolonged the survival of mice xenografted with MOLM13. Importantly, ATRA significantly enhanced the Sorafenib-induced survival (Fig. 6E and 6F). These data show that the anti-leukemia effect of the two drugs is mediated by Sorafenib but greatly improved by the addition of ATRA.
Discussion
We found that, similarly to normal neutrophils (37), APL cells terminally differentiated by ATRA treatment, die through apoptosis and that this process is controlled, at least in part, by Mcl-1 protein. Silencing of Mcl-1 appears to accelerate the apoptosis of the differentiation responsive AML cells. This observation provides a rationale for achieving an accelerated apoptosis induction by combining ATRA with an Mcl-1 inhibitor in ATRA differentiation responsive AML cells.
Mcl-1 has been found to be essential for the development and survival of AML (38–40). We found that Mcl-1 protein levels were differently regulated in differentiated and apoptotic APL NB4 cells. The ratio of differentiated to apoptotic cells was ~4:1 on day 4 with elevated Mcl-1; while on day 6 of ATRA treatment, the ratio dropped to ~1:1 and Mcl-1 was strongly reduced (Fig. 1C). Although it is unclear how Mcl-1 is decreased in the terminally differentiated cells undergoing apoptosis, the observation that Mcl-1 knockdown increased apoptosis of ATRA treated cells (Figs. 1D, 4D) indicates that Mcl-1 protein is needed for survival of differentiated cells. Knockdown of Mcl-1 in control cells, which had high levels of Bcl-2, increased apoptosis, but only slightly (Figs. 1F, 4E), indicating that both anti-apoptotic proteins might play an important role in protecting these cells from death. This is supported by the observations that overexpression of either Bcl-2 or Mcl-1 blocks apoptosis of HL-60 cells treated with ATRA for 6 days (Sup. Fig. 1C, 1F). However, our results show that it is the upregulated Mcl-1 that is the crucial pro-survival protein in NB4 and HL-60 cells during ATRA treatment since Bcl-2 level is suppressed by this treatment (Fig. 1C, 4C).
Mcl-1 is regulated by a large number of pathways at translational and post-translational levels (12, 41). We found that both MEK/ERK and Akt/mTOR pathways are activated in ATRA treated APL cells (Fig. 2E) and that this leads to increase in phosphorylation of p70S6K, p90RSK, and p-rpS6 levels and in Mcl-1 protein (Fig. 2E). Pathway-specific inhibitors showed that both MEK/ERK/RSK and PI3K/Akt, but not mTOR, pathways regulate Mcl-1 protein levels in NB4 cells (Fig. 3A, B, C). Based on these observations we propose that ATRA increases the rate of Mcl-1 translation by activating p90RSK and rpS6 and stabilizes Mcl-1 protein by inactivating GSK3β and/or activating ERK (summarized in Fig. 3D). However, it is possible that other unexplored factors also participate in Mcl-1 protein regulation during ATRA treatment.
Could targeting Mcl-1 improve the ATRA therapy for other AMLs? In an earlier publication (28), ATRA/MEK inhibitor treated AML cells were shown to undergo accelerated apoptosis without signs of differentiation and through an unknown mechanism. We propose that the apoptotic effect of this combination is due to dual inhibition of Bcl-2 and Mcl-1. Treatment of HL-60 cells with either of the two MEK inhibitors, U0126 or PD184352, inhibited both the basal level and the ATRA-induced level of phosphorylated ERK, p90RSK, GSK3β and rpS6 as well as Mcl-1, while also maintaining Bcl-2 repression by ATRA, leading to a profoundly increased apoptosis (Sup. Fig. 2). As a preclinical approach we tested a combination of ATRA with Sorafenib, a multi-kinase inhibitor with a transient effect in resistant and relapsed AML patients (29, 42). We showed that in FAB-M2 HL60 cells, a short (2 days) treatment with Sorafenib was almost as effective as MEK inhibitors in reversing ATRA-induced Mcl-1 increase while allowing for ATRA-repression of Bcl-2 to continue (Fig. 5A) and strongly increasing apoptosis (Fig. 5B). Relevant to our goal of expanding this treatment to other AMLs, Sorafenib reversed ATRA-induced Mcl-1 level and maintained the low ATRA-repressed Bcl-2 level in FAB-M4 ME-1 cells and FAB-M5 THP-1 cells, in a sample of primary human AML cells from a FAB-M2 patient and in MOLM13 cells with FLT3-ITD mutation (Fig. 5, 6). The pathways regulating Mcl-1 by ATRA in these cells were in general similar to those in APL with induced p-p90RSK/p-rpS6 and p-GSK3β. Moreover, like in APL, the level of Bcl-2 was reduced by ATRA. The only difference from that observed in APL was the effect on ERK phosphorylation. While in the primary AML and HL-60 cells, ATRA treatment increased p-ERK level and Sorafenib reduced it by ~50%, the effect in ME-1 and THP-1 was unexpected. ATRA induced pERK in ME-1 cells but Sorafenib did not block it, while in THP-1 cells ATRA strongly reduced the level of pERK (Fig. 5, 6). All the kinases in this pathway, including ERK, are subject to multiple regulatory controls and rapid phosphorylation and dephosphorylation (43–44). We did not further study the reasons for the differences in phosphorylation status of ERK after treatment with ATRA and/or Sorafenib but the kinetics of ERK phosphorylation and de-phosphorylation might be different in these cells lines which would require more detailed kinetic study to prove it (45). We also did not resolve the question of p90RSK up-regulation in absence of pERK up-regulation but possibly, the active p90RSK persists longer. Importantly, the apoptosis augmenting effect of Sorafenib when added to ATRA is present in all 8 samples tested; 4 non-APL AML cell lines and 4 primary AMLs (Fig. 6) and, it is consistently accompanied by reduction of both Mcl-1 and Bcl-2 proteins. Several reports showed that ATRA repressed Bcl-2 mRNA in differentiated AML cells (7, 46) and we found the cell lines used to be responsive to ATRA induced differentiation (Fig. 4A, Sup. Fig. 3). Therefore, the down- regulation of Bcl-2 by ATRA might be transcriptional and secondary to differentiation. ATRA increases Mcl-1 protein, but not its mRNA (Fig. 2B). The Mcl-1 protein has a short half-life and it can be regulated by many factors such as cytokines, growth factors and reactive oxygen species as well as activated caspase due to spontaneous apoptosis of cells (11). Therefore the regulation of Mcl-1 by ATRA may be cell line specific and influenced by the culture conditions. However differentiated AML cells with repressed Bcl-2, like normal neutrophils without Bcl-2 expression, rely on Mcl-1 to survive (47). Although we found that ATRA-increased phosphorylation of GSK3β and p90RSK, which contributes to Mcl-1 induction by ATRA (Fig. 3D), is inhibited by Sorafenib, this inhibitor alone also represses the basal level of Mcl-1 in the tested cell lines. The inhibition of basal Mcl-1 levels by Sorafenib alone might be due to other mechanisms such as inhibition of FLT3-ITD and/or eIF4E phosphorylation, which are known to increase Mcl-1 translation (31, 48–50). Overall, we found that ATRA together with Sorafenib augments apoptosis in vitro and prolongs survival of AML-xenografted mice, providing a translational rationale for studying this combination in AML cells and patients.
Supplementary Material
Translational Relevance.
Cytotoxic chemotherapy induces acute myeloid leukemia (AML) cell death by apoptosis through mitochondrial pathway which is blocked in part by Bcl-2 and Mcl-1, the two main anti-apoptotic proteins. All trans retinoic acid (ATRA) induces terminal differentiation-mediated cell death causing complete remission in patients with acute promyelocytic leukemia (APL), but only limited effect in other AML sub-types. In APL cells Bcl-2 is repressed while Mcl-1 is induced during ATRA induced differentiation process. Inhibition of Mcl-1 accelerates apoptosis of differentiated cells. Increase in the ATRA-induced Mcl-1 protein is due to increased translation and stability associated with RSK activation and GSK3β inactivation. Sorafenib, a multikinase inhibitor, currently in clinical trials in AML patients, counteracts Mcl-1 induction by ATRA, together with ATRA augments apoptosis in cell culture, and in vivo extends the survival time of mice xenografted with human AML cells. This work provides a rationale for utilizing ATRA and Sorafenib as a combination therapy for AML patients by repressing both Bcl-2 and Mcl-1 proteins and augmenting apoptosis.
Acknowledgments
We thank Dr. Liliana Ossowski for editing of this manuscript. This work was supported by NIH grant R01-CA93533 and The Samuel Waxman Cancer Research Foundation
Footnotes
Conflict of interest disclosure:
There is no conflict of interest to disclose.
References
- 1.Stein EM, Tallman MS. Provocative pearls in diagnosing and treating acute promyelocytic leukemia. Oncology (Williston Park) 2012;26:636–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011;117:5795–802. doi: 10.1182/blood-2011-02-329367. [DOI] [PubMed] [Google Scholar]
- 3.Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7:680–6. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- 4.Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113:3655–65. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara FF, Fazi F, Bianchini A, Padula F, Gelmetti V, Minucci S, et al. Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 2001;61:2–7. [PubMed] [Google Scholar]
- 6.Koeffler HP. Is there a role for differentiating therapy in non-APL AML? Best Pract Res Clin Haematol. 2010;23:503–8. doi: 10.1016/j.beha.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monczak Y, Trudel M, Lamph WW, Miller WH., Jr Induction of apoptosis without differentiation by retinoic acid in PLB-985 cells requires the activation of both RAR and RXR. Blood. 1997;90:3345–55. [PubMed] [Google Scholar]
- 8.Jing Y, Wang L, Xia L, Chen GQ, Chen Z, Miller WH, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97:264–9. doi: 10.1182/blood.v97.1.264. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Ikezoe T, Nishioka C, Yokoyama A. Over-expression of Mcl-1 impairs the ability of ATRA to induce growth arrest and differentiation in acute promyelocytic leukemia cells. Apoptosis. 2013;18:1403–15. doi: 10.1007/s10495-013-0872-0. [DOI] [PubMed] [Google Scholar]
- 10.Xia L, Wurmbach E, Waxman S, Jing Y. Upregulation of Bfl-1/A1 in leukemia cells undergoing differentiation by all-trans retinoic acid treatment attenuates chemotherapeutic agent-induced apoptosis. Leukemia. 2006;20:1009–16. doi: 10.1038/sj.leu.2404198. [DOI] [PubMed] [Google Scholar]
- 11.Edwards SW, Derouet M, Howse M, Moots RJ. Regulation of neutrophil apoptosis by Mcl-1. Biochem Soc Trans. 2004;32:489–92. doi: 10.1042/BST0320489. [DOI] [PubMed] [Google Scholar]
- 12.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–9. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 13.Shao W, Benedetti L, Lamph WW, Nervi C, Miller WH., Jr A retinoid-resistant acute promyelocytic leukemia subclone expresses a dominant negative PML-RAR alpha mutation. Blood. 1997;89:4282–9. [PubMed] [Google Scholar]
- 14.Yanagisawa K, Horiuchi T, Fujita S. Establishment and characterization of a new human leukemia cell line derived from M4E0. Blood. 1991;78:451–7. [PubMed] [Google Scholar]
- 15.Naumovski L, Cleary ML. Bcl2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemia cells. Blood. 1994;83:2261–7. [PubMed] [Google Scholar]
- 16.Wang R, Liu C, Xia L, Zhao G, Gabrilove J, Waxman S, et al. Ethacrynic acid and a derivative enhance apoptosis in arsenic trioxide-treated myeloid leukemia and lymphoma cells: the role of glutathione S-transferase p1-1. Clin Cancer Res. 2012;18:6690–701. doi: 10.1158/1078-0432.CCR-12-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Xia L, Gabrilove J, Waxman S, Jing Y. Downregulation of Mcl-1 through GSK-3beta activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia. 2013;27:315–24. doi: 10.1038/leu.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billottet C, Banerjee L, Vanhaesebroeck B, Khwaja A. Inhibition of class I phosphoinositide 3-kinase activity impairs proliferation and triggers apoptosis in acute promyelocytic leukemia without affecting atra-induced differentiation. Cancer Res. 2009;69:1027–36. doi: 10.1158/0008-5472.CAN-08-2608. [DOI] [PubMed] [Google Scholar]
- 19.Glasow A, Prodromou N, Xu K, von Lindern M, Zelent A. Retinoids and myelomonocytic growth factors cooperatively activate RARA and induce human myeloid leukemia cell differentiation via MAP kinase pathways. Blood. 2005;105:341–9. doi: 10.1182/blood-2004-03-1074. [DOI] [PubMed] [Google Scholar]
- 20.Lal L, Li Y, Smith J, Sassano A, Uddin S, Parmar S, et al. Activation of the p70 S6 kinase by all-trans-retinoic acid in acute promyelocytic leukemia cells. Blood. 2005;105:1669–77. doi: 10.1182/blood-2004-06-2078. [DOI] [PubMed] [Google Scholar]
- 21.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 2003;278:28130–8. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- 23.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–7. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 25.Gupta K, Gulen F, Sun L, Aguilera R, Chakrabarti A, Kiselar J, et al. GSK3 is a regulator of RAR-mediated differentiation. Leukemia. 2012;26:1277–85. doi: 10.1038/leu.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Si J, Mueller L, Collins SJ. GSK3 inhibitors enhance retinoic acid receptor activity and induce the differentiation of retinoic acid-sensitive myeloid leukemia cells. Leukemia. 2011;25:1914–8. doi: 10.1038/leu.2011.171. [DOI] [PubMed] [Google Scholar]
- 27.De Mesquita DD, Zhan Q, Crossley L, Badwey JA. p90-RSK and Akt may promote rapid phosphorylation/inactivation of glycogen synthase kinase 3 in chemoattractant-stimulated neutrophils. FEBS Lett. 2001;502:84–8. doi: 10.1016/s0014-5793(01)02669-2. [DOI] [PubMed] [Google Scholar]
- 28.Milella M, Konopleva M, Precupanu CM, Tabe Y, Ricciardi MR, Gregorj C, et al. MEK blockade converts AML differentiating response to retinoids into extensive apoptosis. Blood. 2007;109:2121–9. doi: 10.1182/blood-2006-05-024679. [DOI] [PubMed] [Google Scholar]
- 29.Zauli G, Voltan R, Tisato V, Secchiero P. State of the art of the therapeutic perspective of sorafenib against hematological malignancies. Curr Med Chem. 2012;19:4875–84. doi: 10.2174/092986712803341548. [DOI] [PubMed] [Google Scholar]
- 30.Huber S, Oelsner M, Decker T, zum Buschenfelde CM, Wagner M, Lutzny G, et al. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25:838–47. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- 31.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–27. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 32.Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–45. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 33.Secchiero P, Melloni E, Voltan R, Norcio A, Celeghini C, Zauli G. MCL1 down-regulation plays a critical role in mediating the higher anti-leukaemic activity of the multi-kinase inhibitor Sorafenib with respect to Dasatinib. Br J Haematol. 2012;157:510–4. doi: 10.1111/j.1365-2141.2012.09042.x. [DOI] [PubMed] [Google Scholar]
- 34.Guenounou S, Delabesse E, Recher C. Sorafenib plus all-trans retinoic acid for AML patients with FLT3-ITD and NPM1 mutations. Eur J Haematol. 2014;93:533–6. doi: 10.1111/ejh.12334. [DOI] [PubMed] [Google Scholar]
- 35.Lierman E, Lahortiga I, Van Miegroet H, Mentens N, Marynen P, Cools J. The ability of sorafenib to inhibit oncogenic PDGFRbeta and FLT3 mutants and overcome resistance to other small molecule inhibitors. Haematologica. 2007;92:27–34. doi: 10.3324/haematol.10692. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto K, Imamura T, Yano M, Yoshida H, Fujiki A, Hirashima Y, et al. Sensitivity of MLL-rearranged AML cells to all-trans retinoic acid is associated with the level of H3K4me2 in the RARalpha promoter region. Blood Cancer J. 2014;4:e205. doi: 10.1038/bcj.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabrini M, Nahmod K, Geffner J. New insights into the mechanisms controlling neutrophil survival. Curr Opin Hematol. 2010;17:31–5. doi: 10.1097/MOH.0b013e3283333b29. [DOI] [PubMed] [Google Scholar]
- 38.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–5. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose P, Grant S. Mcl-1 as a Therapeutic Target in Acute Myelogenous Leukemia (AML) Leuk Res Rep. 2013;2:12–4. doi: 10.1016/j.lrr.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gores GJ, Kaufmann SH. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 2012;26:305–11. doi: 10.1101/gad.186189.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mojsa B, Lassot I, Desagher S. Mcl-1 ubiquitination: unique regulation of an essential survival protein. Cells. 2014;3:418–37. doi: 10.3390/cells3020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei A, Tan P. Limitations of targeted therapy with sorafenib in elderly high-risk myelodysplastic syndrome and acute myeloid leukemia. Leuk Lymphoma. 2013;54:675–6. doi: 10.3109/10428194.2012.731604. [DOI] [PubMed] [Google Scholar]
- 43.Lara R, Seckl MJ, Pardo OE. The p90 RSK family members: common functions and isoform specificity. Cancer Res. 2013;73:5301–8. doi: 10.1158/0008-5472.CAN-12-4448. [DOI] [PubMed] [Google Scholar]
- 44.Poulikakos PI, Solit DB. Resistance to MEK inhibitors: should we co-target upstream? Sci Signal. 2011;4:pe16. doi: 10.1126/scisignal.2001948. [DOI] [PubMed] [Google Scholar]
- 45.Pratilas CA, Solit DB. Targeting the mitogen-activated protein kinase pathway: physiological feedback and drug response. Clin Cancer Res. 2010;16:3329–34. doi: 10.1158/1078-0432.CCR-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grillier I, Umiel T, Elstner E, Collins SJ, Koeffler HP. Alterations of differentiation, clonal proliferation, cell cycle progression and bcl-2 expression in RAR alpha-altered sublines of HL-60. Leukemia. 1997;11:393–400. doi: 10.1038/sj.leu.2400575. [DOI] [PubMed] [Google Scholar]
- 47.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–502. [PubMed] [Google Scholar]
- 48.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–43. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraljacic BC, Arguello M, Amri A, Cormack G, Borden K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia. 2011;25:1197–200. doi: 10.1038/leu.2011.57. [DOI] [PubMed] [Google Scholar]
- 50.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.