Abstract
Background
In light of recent progress toward pharmacologic interventions to treat adolescent cannabis use disorder, it is important to consider which adolescent characteristics may be associated with a favorable response to treatment. This study presents secondary analyses from a parent randomized controlled trial of N-acetylcysteine (NAC) in adolescents with cannabis use disorder. We hypothesized high pretreatment impulsivity and medication non-adherence would be associated with reduced abstinence rates.
Methods
Participants were treatment-seeking adolescents (N = 115) who met criteria for cannabis use disorder and were assessed for pretreatment impulsivity. They received 1200 mg NAC or placebo orally twice daily for 8 weeks. An intent-to-treat analysis using a repeated-measures logistic regression model was used to relate pretreatment impulsivity (Barratt Impulsiveness Scale) and treatment group to abstinence rates, measured by urine cannabinoid tests. To explore mechanisms by which NAC may reduce cannabis use, relationships between impulsivity, adherence, and abstinence were assessed in a second statistical model using data from participants with recorded adherence and urine cannabinoid test results (n = 54).
Results
In the intent-to-treat analysis, low pretreatment impulsivity, NAC treatment, and negative baseline urine cannabinoid test results independently increased the odds of having negative urine cannabinoid tests during treatment (OR = 2.1, 2.3, 5.3 respectively). In the sample of participants with adherence data (n = 54), adherence tripled the odds of abstinence. Notably, the effect of adherence on abstinence was only observed in the NAC treatment group. Lastly, although the highly impulsive participants had reduced rates of abstinence, highly impulsive individuals adherent to NAC treatment had increased abstinence rates compared to non-adherent individuals.
Conclusion
Low impulsivity, NAC treatment, medication adherence, and baseline negative cannabinoid testing were associated with increased rates of abstinence in adolescents seeking treatment for cannabis use disorder. Efforts to optimize pharmacotherapy adherence may be particularly crucial for highly impulsive individuals. Understanding and addressing factors, such as impulsivity and adherence, which may affect outcomes, may aid in the successful evaluation and development of potentially promising pharmacotherapies.
Keywords: Impulsivity, Non-adherence, Abstinence, Cannabis, Marijuana, Adolescent, Youth, Pharmacotherapy, N-acetylcysteine
1. Introduction
Cannabis is the most commonly used illicit drug by adolescents in the United States, and the percentage of 12–17 year-olds who perceive great risks from its frequent use is steadily decreasing (Substance Abuse and Mental Health Services, 2014a). Cannabis also accounts for the majority of adolescent substance use-related treatment admissions (Substance Abuse and Mental Health Services, 2014b). Effective treatment for adolescent cannabis use disorder has become especially pressing in light of the escalating use of cannabis among this age group, recent changes in legalization, and findings linking heavy cannabis use in adolescence to behavioral problems and persistent cognitive deficits into adulthood, even after cessation of use (Meier et al., 2012; Randolph, Turull, Margolis, & Tau, 2013).
Psychosocial intervention is the current mainstay in treating adolescent substance use disorders (SUDs), including cannabis use disorder. However, this modality has only modest effect sizes and has failed to yield robust abstinence outcomes, driving the search for efficacious augmentative pharmacological agents (Budney, Vandrey, & Stanger, 2010; Waldron & Turner, 2008). In light of recent promising pharmacologic interventions to treat adolescent SUDs (Gray et al., 2012), an important question to ask is: Which group of adolescents will have a favorable response to a specific type of SUD treatment?
The pursuit to identify pretreatment factors that affect SUD treatment outcomes has persisted for decades, with genetic polymorphisms, comorbid conditions, environmental factors, and neurobehavioral traits heavily investigated (Bauer, Soares, & Nielsen, 2014; Rounds-Bryant, Kristiansen, & Hubbard, 1999). Impulsivity is a neurobehavioral trait that has been repeatedly linked to SUD predisposition, severity, and treatment outcomes (Bickel, Koffarnus, Moody, & Wilson, 2014; MacKillop et al., 2011; Perkel, Bentzley, Andrzejewski, & Martinetti, 2015). In humans, common measures of impulsivity include behavioral tasks, (i.e., delayed reward discounting paradigms) and self-report measures (i.e., Barratt Impulsiveness Scale [BIS-11]). The BIS-11 is a reliable self-report measure of impulsivity (Berg, Ahluwalia, & Cropsey, 2013) with external validity such that those known to be highly impulsive (e.g., individuals with Attention-Deficit/Hyperactivity Disorder) tend to score higher on the BIS-11 (Stanford et al., 2009).
Highly impulsive individuals may represent an important subgroup for which treatment outcomes differ. There is evidence supporting impulsivity’s relationship with SUD treatment non-completion and failure (Loree, Lundahl, & Ledgerwood, 2014; Stevens et al., 2014; Winhusen et al., 2013). Impulsivity has also been associated with poor treatment outcome in short-term treatment trials for smoking and cannabis use in adolescents (Krishnan-Sarin et al., 2007; Stanger et al., 2012). Furthermore, impulsivity may also be related to non-adherence in those seeking treatment for SUDs. Non-adherence may contribute to sub-optimal therapeutic response and confounding of results in clinical trials, greatly impacting pharmacotherapy development and translation into clinical use (Vrijens & Urquhart, 2014). A recent study found young adult heavy drinkers with co-occurring cannabis use exhibit more non-planning impulsivity and medication non-adherence than heavy drinkers without co-occurring cannabis use (Peters et al., 2012). However, adherence to medications may have multiple determinants (Kardas, Lewek, & Matyjaszczyk, 2013) and may be unassociated with impulsivity in those with psychotic or mood disorders (Liraud & Verdoux, 2001).
N-acetylcysteine (NAC) is a promising pharmacological agent being investigated for adolescent cannabis use disorder treatment (Gray et al., 2012). It is presently unknown whether NAC has different efficacy in highly impulsive individuals seeking treatment for cannabis use disorder. In a sample of individuals with cocaine use disorder, Schmaal and colleagues (2012) found NAC to be more effective at reducing dorsal anterior cingulate cortex (dACC) glutamate levels in individuals with high levels of self-reported impulsivity assessed by BIS-11. Based on these findings, highly impulsive adolescents seeking treatment for cannabis use disorder may respond differently to NAC than adolescents with lower impulsivity scores.
This study presents secondary analyses from an intent-to-treat parent randomized controlled trial of NAC in adolescents with cannabis use disorder (Gray et al., 2012). The goals of the current report were to determine the effects of impulsivity and adherence on abstinence rates in adolescents enrolled in a placebo-controlled trial of NAC for cannabis use disorder. We hypothesized that high pretreatment impulsivity (HI) and non-adherence would be associated with reduced abstinence rates.
2. Materials and methods
2.1 Participants
Participants were 115 treatment-seeking adolescents, aged 15–21 (mean = 18.9 ± 1.5 years) who met criteria for cannabis use disorder (DSM-IV cannabis dependence), were enrolled in the parent trial (Gray et al., 2012), and had pretreatment impulsivity scores. Exclusion criteria included allergy to NAC, pregnancy or lactation, use of carbamazepine or nitroglycerin within 14 days of enrollment, enrollment in additional substance abuse treatment, DSM-IV substance dependence other than cannabis or tobacco, and significant medical or psychiatric illness that may increase risk in the judgment of the study physician. Participants were assessed at pretreatment for eligibility (which included a history and physical examination) and eligible individuals were then randomized to receive 1200 mg NAC or placebo orally twice daily for 8 weeks. All participants received a contingency management intervention and weekly brief (≤10 minute) cessation counseling. Further details of the parent trial are described elsewhere (Gray et al., 2012). All participants provided informed consent and parental consent was also obtained if participants were <18 years of age. The study procedures were approved by the university institutional review board and were in accord with the Helsinki Declaration of 1975.
2.2 Measurements
2.21 Abstinence
Urine cannabinoid testing at baseline and during weekly clinic visits served as the primary biological measure of cannabis use. Tests were analyzed as positive or negative (cutoff 50 ng/mL, U.S. Screening Source, Inc., Louisville). 2.22 Impulsivity. The 30-item self-report Barratt Impulsiveness Scale, BIS-11 (Barratt, 1959; Patton & Stanford, 1995) was used to assess global impulsivity (Patton & Stanford, 1995; Stanford et al., 2009). In a review, Stanford and colleagues (2009) reported that the mean BIS-11 score for adults is 62.3 ± 10, and BIS-11 scores above 72 are highly impulsive. The mean BIS-11 total score in our sample at pretreatment was 67.5 ± 10.1 and the median was 66. We performed a median split of pretreatment impulsivity scores within our sample to designate pretreatment high (HI) and low impulsivity (LI) groups, an approach with ample precedence in the literature (Kiluk, Nich, & Carroll, 2010; Papachristou, Nederkoorn, Havermans, Horst, & Jansen, 2011).
2.23 Adherence
Medication adherence was calculated as number of capsules taken during each week of treatment (determined by blister pack pill counts reviewed by research staff) divided by number of capsules prescribed to obtain ratios (0.0–1.0). Participants with ratios of 1.0 were considered adherent and those with ratios of <1.0 were considered non-adherent for analysis purposes. Medication adherence data at one or more study visits were available for 54 participants.
2.3 Statistical analysis
Primary aims of this report included assessing how pretreatment impulsivity and adherence may be related to abstinence outcomes. For the primary analysis, an intent-to-treat (ITT) approach including all 115 randomized participants with baseline impulsivity data was used such that all participants who were lost to follow-up or were absent from visits were coded as having a positive urine cannabinoid test at every missed visit. However, when treatment adherence was included in the analysis, only individuals with adherence data and recorded urine cannabinoid test results were included.
Demographic and clinical characteristics were tabulated for all participants and compared between groups prior to statistical analyses. Standard descriptive statistics were used to summarize the demographic and clinical data. Differences in pretreatment characteristics were calculated using chi square tests, the Fischer exact text, t-test or Wilcoxon rank sum test, as appropriate.
The primary outcome measure was the odds of negative weekly urine cannabinoid test results during treatment. Repeated-measures logistic regression models using the methods of generalized estimating equations was used to assess the effects of pretreatment impulsivity on urine cannabinoid test results during active treatment. All study models were adjusted for baseline urine cannabinoid test results and assessed for possible confounding of baseline demographic and clinical characteristics. Chi square, Wilcoxon rank sum, and t tests were used to detect variables that differed by pretreatment impulsivity grouping (covariates were included in the model if p<0.10), and possible predictors of abstinence (covariates were included in the model if p<0.20). Results are presented as odds ratios with 95% confidence intervals (CIs).
Significance for all planned comparisons was set at a 2-sided p-value of 0.05 and no correction for multiple testing was applied to reported p-values to avoid Type II error in these exploratory secondary analyses. Statistical analyses were conducted using SPSS version 21.
3. Results
Demographics for high vs. low impulsive individuals are presented in Table 1. In our sample, the impulsivity groups differed in gender and racial makeup. More females were categorized as high impulsive (HI) and more males as low impulsive (LI), and a greater proportion of Black participants were LI than HI (Table 1). Additionally, LI individuals had significantly lower pretreatment craving than HI individuals and fewer years of cannabis use (Table 1).
Table 1.
Characteristics of Low and High Pretreatment Impulsivity Groups (N = 115)
| Low Impulsivity (n = 57) | High Impulsivity (n = 58) | Significance | |
|---|---|---|---|
| Gender | 47 Male | 37 Male | p = 0.024 |
| Race | 45 White, 11 Black, 1 Other | 51 White, 3 Black, 3 Other | p = 0.034 |
| Education | 15 7–12th; 10 HS Degree; 32 Some College | 14 7–12th; 14 HS Degree; 30 Some College | ns |
| Age (Years) | M = 18.8 ± 1.5 | M = 18.9 ± 1.6 | ns |
| Years of Cannabis Use | M = 3.9 ± 1.6 | M = 4.6 ± 2.0 | p = 0.037 |
| Treatment Assignment | 31 Placebo; 26 NAC | 27 Placebo; 31 NAC | ns |
| Baseline Urine Cannabinoid Test Results | 52 Positive | 52 Positive | ns |
| Pretreatment Marijuana Craving (MCQ Score) | M = 45 ± 15 | M = 51 ± 13 | p = 0.024 |
| Pretreatment Global Impulsivity (BIS-11 Total Score) | M = 59 ± 5 | M = 76 ± 7 | p < 0.001 |
MCQ: Marijuana Craving Questionnaire
BIS-11: Barratt Impulsiveness Scale
M: mean
Continuous impulsivity scores did not change over the course of the study (F(2, 131) = 0.113, p = 0.893); thus pretreatment HI and LI groups were used for all analyses. Pretreatment BIS-11 total scores were significantly different between dichotomized pretreatment impulsivity groups (Table 1).
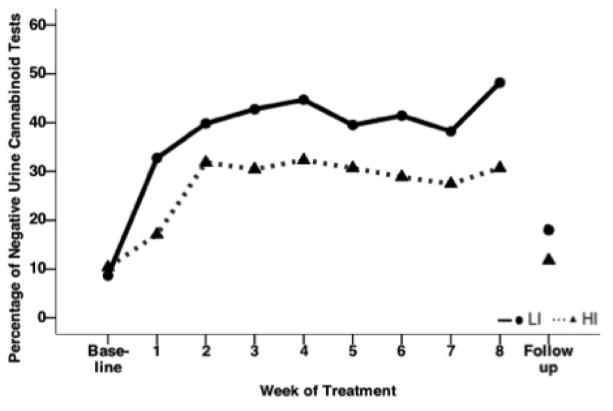
3.1 Low pretreatment impulsivity was associated with abstinence
Proportions of negative urine cannabinoid tests at each visit in the LI and HI groups are presented in Figure 1 (ITT sample, N = 115). LI individuals had double the odds of having negative urine cannabinoid tests during treatment compared to HI participants after adjusting for gender, race, pretreatment craving, and baseline years of cannabis use (OR = 2.14 95% CI = 1.01–4.54 χ2 = 3.9, p = 0.049). In this model, treatment and baseline urine cannabinoid testing were also significantly associated with abstinence over treatment. Those who received NAC had double the odds of having negative urine cannabinoid tests during treatment compared to the placebo group (OR = 2.27 95% CI = 1.10–4.76 χ2 = 4.9, p = 0.028). Additionally, those with baseline negative urine cannabinoid testing had 5 times the odds of having negative cannabinoid tests during treatment (OR = 5.26 95% CI = 1.74–16.67 χ2 = 8.6, p = 0.003).
Figure 1.
Proportion of Negative Urine Cannabinoid Tests Over Time by Pretreatment High and Low Impulsivity Groups Among Adolescents with Cannabis Use Disorder in a Randomized Controlled Trial of N-Acetylcysteine (NAC)a
LI: Low impulsivity
HI: High impulsivity
aIn this intent-to-treat analysis, all randomized participants with pretreatment impulsivity scores (N = 115) were included, and urine cannabinoid tests were assumed to be positive for all missed visits. With adjustment for years of cannabis use, baseline urine cannabinoid test results, pretreatment craving scores, gender, and race, odds ratio = 2.14 95% CI = 1.01–4.54 χ2 = 3.9, p = 0.049.
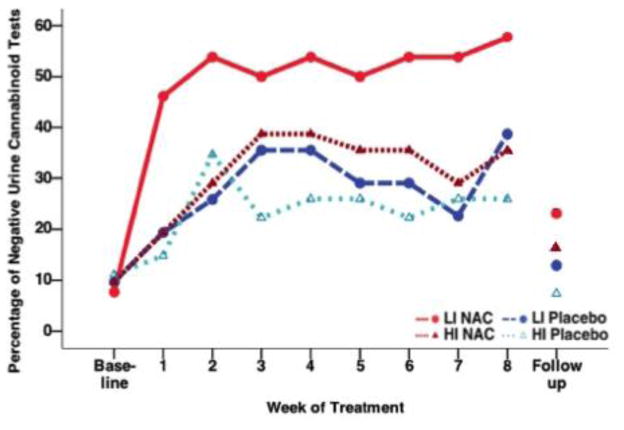
3.2. NAC was found to be efficacious in both LI and HI groups
Abstinence rates at each visit by impulsivity and treatment group are presented in Figure 2 (ITT sample, N = 115). Although NAC appears to be more effective in the LI group, there was no significant interaction between pretreatment impulsivity group and treatment in the full, adjusted statistical model (Impulsivity×Treatment, χ2 = 0.33, p = 0.856).
Figure 2.
Proportion of Negative Urine Cannabinoid Tests Over Time by Treatment and Pretreatment High and Low Impulsivity Groups Among Adolescents with Cannabis Use Disorder in a Randomized Controlled Trial of N-Acetylcysteine (NAC)a
LI: Low impulsivity
HI: High impulsivity
aIn this intent-to-treat analysis, all randomized participants with pretreatment impulsivity scores (N = 115) were included, and urine cannabinoid tests were assumed to be positive for all missed visits. There was no significant interaction between pretreatment impulsivity group and treatment in the full, adjusted statistical model (Impulsivity×Treatment, χ2 = 0.33, p = 0.856).
3.3 High pretreatment impulsivity was not associated with adherence
We tested whether there were different proportions of HI and LI individuals in subgroups of participants with versus without recorded adherence and urine cannabinoid test results. We did not find different proportions of HI and LI individuals in these groups over the 8-week treatment period (χ2 = 0.4, p = 0.501). There were 54 participants (25 LI, 29 HI) with recorded adherence and urine cannabinoid test results.
We then examined whether adherence differed by pretreatment impulsivity group in the 54 participants with recorded data. We did not find different proportions of HI vs. LI individuals in the adherent vs. non-adherent groups over the 8-week treatment period (χ2 = 0.64, p = 0.422).
3.4 Non-adherence was linked to reduced abstinence rates in the NAC group
We next assessed how adherence may have influenced urine cannabinoid test results over the 8 weeks of treatment. For these analyses, the ITT sample was not used, as this mechanistic approach required known adherence and abstinence outcomes at each time point. In the 54 participants with recorded adherence and urine cannabinoid test results, LI individuals had greater odds of having abstinent test results relative to HI individuals after adjusting for baseline cannabinoid test results and covariates (OR = 4.87 95% CI 1.27–18.71, χ2 = 5.3, p = 0.021). Additionally, adherence conferred doubled the odds of abstinence over the course of treatment compared to non-adherence (OR = 2.57 95% CI 1.12–5.92 χ2 = 4.9, p = 0.027).
We also assessed whether the relationship between adherence and abstinence was observed for both placebo and NAC groups. In the placebo group (n = 23), adherence had no relationship with abstinence; those who were non-adherent were just as likely to become abstinent as those who were adherent to the placebo (OR = 1.45 95% CI = 0.50–4.21 χ2 = 0.5, p = 0.49). However, within the NAC group (n = 31), those adherent had 4 times the odds of having negative urine cannabinoid test results compared to those non-adherent to NAC (OR = 4.49 95% CI = 1.24–16.23, χ2 = 8.65, p = 0.022).
3.5 Highly impulsive individuals adherent to NAC had improved abstinence rates
We tested if highly impulsive individuals had different abstinence rates if they were adherent or non-adherent with NAC over the 8-week treatment period. HI individuals adherent to NAC had increased odds of having negative urine cannabinoid test results compared to HI individuals non-adherent to NAC over treatment (OR = 8.08 95% CI = 1.43–45.70, χ2 = 5.6, p = 0.018, n = 18). In contrast, for LI individuals, adherence to NAC did not have the same effect on abstinence rates (χ2 = 0.2, p = 0.892).
4. Discussion
The purpose of this study was to assess if pretreatment impulsivity and medication adherence were linked to abstinence in adolescents enrolled in a placebo-controlled trial of NAC for cannabis use disorder. We hypothesized that high pretreatment impulsivity and non-adherence would be associated with reduced abstinence rates.
Low pretreatment impulsivity was associated with improved abstinence rates. This finding complements other studies suggesting impulsivity influences SUD treatment outcomes (Krishnan-Sarin et al., 2007; Stanger et al., 2012). Importantly, treatment and baseline urine cannabinoid testing also independently affected abstinence. Thus, low pretreatment impulsivity, NAC treatment, and negative baseline cannabinoid testing each increased odds of abstinence in this sample of adolescents seeking treatment for cannabis use disorder. Self-reported impulsivity is an easily measurable pretreatment factor that may be an important neurobehavioral trait to screen for when enrolling participants in clinical trials and when treating this population in the clinical arena.
We hypothesized that highly impulsive adolescents seeking treatment for cannabis use disorder may respond differently to NAC compared to adolescents with lower impulsivity scores. This hypothesis was based on evidence suggesting NAC more effectively suppresses glutamate levels in the dACC in patients with high self-reported impulsivity (Schmaal et al., 2012). In the current report, highly impulsive individuals adherent to NAC had higher abstinence rates than non-adherent individuals. However, this analysis was performed on a small subsample of our data with adherence and abstinence data. Further research is needed to explore the effect of NAC on highly impulsive individuals seeking treatment for cannabis disorder and to investigate whether degree of suppression in dACC glutamate improves treatment outcomes. Conceivably, glutamate suppression in the dACC may not be related to abstinence rates. Of note, there are several differences between the sample and procedure in Schmaal et al.’s study and the current study. Schmaal and colleagues used a sample of adult (mean age = 35 years) cocaine users and administered a single dose of NAC (2400 mg) to all participants, eliminating the possibility of non-adherence. Future research that integrates neuroimaging data within a randomized control trial will be needed to investigate if the efficacy of NAC is different for highly impulsive individuals.
In this report, medication adherence did not differ by pretreatment impulsivity group. However, non-adherence was an independent factor decreasing abstinence rates. This finding, that non-adherence may contribute to sub-optimal therapeutic response, is consistent with reports seen in heart disease and other chronic diseases (Bitton, Choudhry, Matlin, Swanton, & Shrank, 2013; Han, Suh, Lee, & Jang, 2014) as well as other substance use disorders, particularly the use of naltrexone in alcohol use disorders (Swift, Oslin, Alexander, & Forman, 2011).
Importantly, the relationship between medication adherence and abstinence was observed for the NAC group, and not the placebo group, further highlighting the promising efficacy of NAC in treating adolescent cannabis use disorder. Additionally, in highly impulsive individuals receiving NAC, adherence was particularly important for abstinence. Further research is necessary to clarify the effects of impulsivity and non-adherence on abstinence. It may be that impulsivity impacts outcome through its effect on adherence (i.e., mediation model). However, given that there was no difference between HI and LI individuals in terms of adherence in the current study, we propose that pretreatment impulsivity and adherence interact to affect abstinence (i.e., moderation effect). For example, high impulsivity plus non-adherence may create cumulative risk for non-abstinence, though the current study was underpowered to test this interaction. Regardless, the finding of superior abstinence outcomes among NAC-adherent HI individuals, versus those non-adherent with NAC, highlights the potentially crucial role of efforts to optimize pharmacotherapy adherence among HI individuals.
An interesting finding among this sample of adolescents with cannabis use disorder was a disproportionally larger number of females who fell into the highly impulsive category relative to males. A similar observation was seen by Lejuez and colleagues (2007), who found that among crack cocaine users, females had higher total BIS-11 scores than their male counterparts. Of note, there are twice as many male 12th grade daily cannabis users (8.9%) than there are female 12th grade daily users (3.8%), with a similar pattern holding for college students (Johnston, O’Malley, Bachman, Schulenberg, & Miech, 2014). Thus, given that daily cannabis use is relatively less common among female adolescents, frequent female users may exhibit heightened neurobehavioral risk factors.
There were several limitations of the present report. First, we conducted exploratory secondary analyses derived from a parent clinical trial that were not specifically powered to detect effects of impulsivity. Future studies should be specifically powered to detect the effect of impulsivity on abstinence rates. Second, the relationship between impulsivity and outcome is complicated by the “chicken or the egg phenomenon.” Pretreatment neurobehavioral trait impulsivity precedes cannabis use, and cannabis use exacerbates impulsivity. It is unknown how this entwining of cannabis use and impulsivity affects treatment outcomes, especially in light of several studies linking impulsivity with cannabis use (Churchwell, Lopez-Larson, & Yurgelun-Todd, 2010; Dougherty et al., 2013; Wrege et al., 2014), yet others failing to find differences in impulsivity between adult users and non-users after acute cannabis use (Johnson et al., 2010; McDonald, Schleifer, Richards, & de Wit, 2003; Vadhan et al., 2007).
There are important implications if impulsivity and adherence affect SUD treatment outcomes. First, clinicians may be able to provide individualized treatment options for highly impulsive individuals that target their impulsivity. Promising work has shown working-memory training decreases impulsivity measured as delay discounting (Bickel et al., 2011). Further research is needed to determine whether reduced impulsivity associated with such neurocognitive training could translate into improved SUD treatment outcomes. Second, efforts towards improving medication adherence may be warranted if indeed adherence is “the key mediator between medical practice and patient outcomes” (Kravitz & Melnikow, 2004, p. 197). Understanding and addressing factors, such as impulsivity, which may affect adherence and outcomes, may aid in the successful evaluation and development of potentially promising pharmacotherapies.
Highlights.
We conducted a secondary analysis of adolescents enrolled in a placebo-controlled trial of NAC for cannabis use disorder.
Pretreatment impulsivity was assessed using the Barratt Impulsiveness Scale.
Low pretreatment impulsivity was associated with increased abstinence rates.
High medication adherence was associated with increased abstinence rates.
Acknowledgments
Dataset: “A Controlled Trial of N-Acetylcysteine (NAC) in Cannabis-Dependent Adolescents” (National Institute on Drug Abuse grant R01 DA026777).
Abbreviations
- BIS-11
Barratt Impulsiveness Scale
- dACC
dorsal anterior cingulate cortex
- NAC
N-acetylcysteine
- SUD
substance use disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills. 1959;9(3):191–198. [Google Scholar]
- Bauer IE, Soares JC, Nielsen DA. The role of opioidergic genes in the treatment outcome of drug addiction pharmacotherapy: A systematic review. The American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2014 doi: 10.1111/j.1521-0391.2014.12172.x. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Ahluwalia JS, Cropsey K. Predictors of adherence to behavioral counseling and medication among female prisoners enrolled in a smoking cessation trial. Journal of Correctional Health Care: the Official Journal of the National Commission on Correctional Health Care. 2013;19(4):236–247. doi: 10.1177/1078345813499307. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton A, Choudhry NK, Matlin OS, Swanton K, Shrank WH. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. The American Journal of Medicine. 2013;126(4):357.e7–357.e27. doi: 10.1016/j.amjmed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Stanger C. Pharmacological and psychosocial interventions for cannabis use disorders. Revista Brasileira De Psiquiatria. 2010;32(Suppl 1):S46–55. [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008;35(4):362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Frontiers in Psychology. 2010;1 doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addictive Behaviors. 2008;33(11):1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacology. 2007;194(2):279–288. doi: 10.1007/s00213-007-0832-x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, et al. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. 2013;226(2):307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. American Journal of Psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E, Suh DC, Lee SM, Jang S. The impact of medication adherence on health outcomes for chronic metabolic diseases: a retrospective cohort study. Research in Social & Administrative Pharmacy: RSAP. 2014;10(6):e87–98. doi: 10.1016/j.sapharm.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence. 2009;102(1–3):35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18(1):99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Frontiers in Pharmacology. 2013;4 doi: 10.3389/fphar.2013.00091/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Carroll KM. Relationship of cognitive function and the acquisition of coping skills in computer assisted treatment for substance use disorders. Drug and Alcohol Dependence. 2010;114(2):169–176. doi: 10.1016/j.drugalcdep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz RL, Melnikow J. Medical adherence research: time for a change in direction? Medical Care. 2004;42(3):197–199. doi: 10.1097/01.mlr.0000115957.44388.7c. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Reynolds EK, Daughters SB, Curtin JJ. Risk factors in the relationship between gender and crack/cocaine. Experimental and Clinical Psychopharmacology. 2007;15(2):165–175. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liraud F, Verdoux H. Association between temperamental characteristics and medication adherence in subjects presenting with psychotic or mood disorders. Psychiatry Research. 2001;102(1):91–95. doi: 10.1016/s0165-1781(01)00240-2. [DOI] [PubMed] [Google Scholar]
- Loree AM, Lundahl LH, Ledgerwood DM. Impulsivity as a predictor of treatment outcome in substance use disorders: Review and synthesis. Drug and Alcohol Review. 2014 doi: 10.1111/dar.12132. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28(7):1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences. 2012;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Havermans R, Bongers P, Beunen S, Jansen A. Higher levels of trait impulsiveness and a less effective response inhibition are linked to more intense cue-elicited craving for alcohol in alcohol-dependent patients. Psychopharmacology. 2013;228(4):641–649. doi: 10.1007/s00213-013-3063-3. [DOI] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Havermans R, Horst M, Jansen A. Can’t stop the craving: The effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology. 2011;219(2):511–518. doi: 10.1007/s00213-011-2240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perkel JK, Bentzley BS, Andrzejewski ME, Martinetti MP. Delay discounting for sucrose in alcohol-preferring and nonpreferring rats using a sipper tube within-sessions task. Alcoholism: Clinical and Experimental Research. 2015;39(2):232–238. doi: 10.1111/acer.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Leeman RF, Fucito LM, Toll BA, Corbin WR, O’Malley SS. Co-occurring marijuana use is associated with medication nonadherence and nonplanning impulsivity in young adult heavy drinkers. Addictive Behaviors. 2012;37(4):420–426. doi: 10.1016/j.addbeh.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph K, Turull P, Margolis A, Tau G. Cannabis and cognitive systems in adolescents. Adolescent Psychiatry. 2013;3(2):135–147. [Google Scholar]
- Rounds-Bryant JL, Kristiansen PL, Hubbard RL. Drug abuse treatment outcome study of adolescents: a comparison of client characteristics and pretreatment behaviors in three treatment modalities. The American Journal of Drug and Alcohol Abuse. 1999;25(4):573–591. doi: 10.1081/ada-100101880. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: A randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37(9):2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47(5):385–395. doi: 10.1016/j.paid.2009.04.008. [DOI] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and Clinical Psychopharmacology. 2012;20(3):205–212. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. Journal of Substance Abuse Treatment. 2014;47(1):58–72. doi: 10.1016/j.jsat.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Treatment Episode Data Set (TEDS): 2002–2012. BHSIS Series S-71, HHS Publication No. (SMA) 14-4850. [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. Journal of Studies on Alcohol and Drugs. 2011;72(6):1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzis D, Mahoney JJ, Kalechstein AD, Newton TF, de la Garza R. The relationship between impulsivity and craving in cocaine- and methamphetamine-dependent volunteers. Pharmacology Biochemistry and Behavior. 2011;98(2):196–202. doi: 10.1016/j.pbb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clinical Pharmacology and Therapeutics. 2014;95(6):617–626. doi: 10.1038/clpt.2014.59. [DOI] [PubMed] [Google Scholar]
- Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. Journal of Clinical Child and Adolescent Psychology: the Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2008;37(1):238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, et al. Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. Journal of Substance Abuse Treatment. 2013;44(5):541–547. doi: 10.1016/j.jsat.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrege J, Schmidt A, Walter A, Smieskova R, Bendfeldt K, Radue EW, et al. Effects of cannabis on impulsivity: a systematic review of neuroimaging findings. Current Pharmaceutical Design. 2014;20(13):2126–2137. doi: 10.2174/13816128113199990428. [DOI] [PMC free article] [PubMed] [Google Scholar]