Abstract
Previous studies report that acquired prosopagnosia is frequently associated with topographic disorientation. Whether this is associated with a specific anatomic subtype of prosopagnosia, how frequently it is seen with the developmental variant, and what specific topographic function is impaired to account for this problem are not known.
We studied ten subjects with acquired prosopagnosia from either occipitotemporal or anterior temporal lesions and seven with developmental prosopagnosia. Subjects were given a battery of topographic tests, including house and scene recognition, the road map test, a test of cognitive map formation, and a standardized self-report questionnaire.
House and/or scene recognition were frequently impaired after either occipitotemporal or anterior temporal lesions in acquired prosopagnosia. Subjects with occipitotemporal lesions were also impaired in cognitive map formation: an overlap analysis identified right fusiform and parahippocampal gyri as a likely correlate. Only one subject with acquired prosopagnosia had mild difficulty with directional orientation on the road map test. Only one subject with developmental prosopagnosia had difficulty with cognitive map formation, and none were impaired on the other tests. Scores for house and scene recognition correlated most strongly with the results of the questionnaire.
We conclude that topographic disorientation in acquired prosopagnosia reflects impaired place recognition, with a contribution from poor cognitive map formation when there is occipitotemporal damage. Topographic impairments are less frequent in developmental prosopagnosia.
Keywords: navigation, face recognition, landmark, places, orientation
Navigating through the environment is a complex function that, like all high-level abilities, involves a number of cognitive skills. This ability is impaired in subjects with topographic disorientation, a disorder first described 150 years ago (Barrash, 1998), which leads invariably to the complaint that subjects get lost in surroundings that should be familiar. Given the multiplicity of cognitive operations involved, topographic disorientation is a heterogeneous collection of conditions, with different subjects varying in the reasons for their failure to find their way. In recognition of this, several taxonomies of the various problems that can lead to topographic disorientation have been proposed (de Renzi, 1982; Aguirre et al., 1999; Arnold et al., 2013). For example, in some subjects the main deficit may be failure to recognize landmarks (Takahashi et al., 2002; McCarthy et al., 1996), while in others, landmark recognition may be intact but there is difficulty in learning or recognizing scenes (Mendez et al., 2003; Epstein et al., 2001). Others who can also recognize landmarks may not accurately represent the spatial relationship between locations (Takahashi et al., 1997), or derive the spatial layout of the environment (De Renzi et al., 1977; Pai, 1997) and therefore cannot form a cognitive map (Iaria et al., 2009). The evaluation of these different abilities is reflected in the evolving design of test batteries for topographic skills (Liu et al., 2011; Arnold et al., 2013).
Topographic disorientation has also had a long-known relationship to prosopagnosia, the inability to recognize familiar faces. In fact, LL, one of the first cases of prosopagnosia described (Quaglino et al., 1867), had impaired spatial orientation and could not remember the facades of houses or familiar scenes (Benton, 1990; Della Sala et al., 2003). Also, the first large series of 22 subjects with acquired prosopagnosia noted a close association of this condition with “vestibular and directional disturbances” p. 28 of (Hecaen et al., 1962). Since then there have been numerous anecdotal reports (Landis et al., 1986; Takahashi et al., 1995; Clarke et al., 1997; Malone et al., 1982; Bauer, 1984; Martins et al., 1999; Barton et al., 2002; Uttner et al., 2002) and a recent review of acquired prosopagnosia found explicit mention of topographic problems in 29% of 147 cases (Schmidt, 2015). A relevant observation is that, in 31 patients with posterior cerebral arterial infarction, half of patients with impairments on tests of face recognition were also impaired on house recognition (Martinaud et al., 2012). One potential explanation of this association is the anatomic proximity of the parahippocampal place area, a region activated by viewing scenes (Epstein et al., 1998), to the fusiform face area (Kanwisher et al., 1997), and important component of the face processing network (Haxby et al., 2000).
These observations raise a number of questions. First, the type of topographic disorientation in these subjects has rarely been investigated or clarified. WF was impaired on a maze test (Henke et al., 1998) and VH, a subject with progressive right temporal atrophy, developed slight difficulty with a test of famous place identification as her disorder progressed (Evans et al., 1995). Some of the anecdotes reported would suggest that subjects had trouble recognizing places (Landis et al., 1986; Clarke et al., 1997). However, at this point the nature of topographic impairment associated with prosopagnosia must be considered unknown.
Second, it is not clear whether topographic impairments are linked to a specific type of prosopagnosia, which, being a complex task like topographic disorientation, is not a single entity but a family of disorders with different functional and structural bases (Barton, 2008). Anatomically, face processing involves a network of regions (Haxby et al., 2000). While prosopagnosia is classically associated with bilateral medial occipitotemporal lesions (Meadows, 1974; Damasio et al., 1982), it has since been described with other lesions, such as right occipitotemporal and right or bilateral anterior temporal lesions (Barton, 2008). These structural variants may also correspond to functional subtypes, with occipitotemporal lesions causing apperceptive face processing deficits and right anterior temporal lesions impairing access to facial memories (Damasio et al., 1990; Barton, 2008; Davies-Thompson et al., 2014). Since not all prosopagnosic subjects have topographic disorientation (Schmidt, 2015), a natural question is whether topographic impairments co-segregate with certain anatomic variants of prosopagnosia. Furthermore, a developmental form of prosopagnosia has been described recently (Duchaine et al., 2006b; Susilo et al., 2013). The nature of the functional deficits and structural anomalies of the developmental variant are still being clarified (Stollhoff et al., 2011; Avidan et al., 2014a). Whether or how frequently topographic disorientation is associated with this form is also uncertain.
In this study we evaluated spatial orientation skills in a large cohort of these rare subjects, both those with acquired prosopagnosia from a variety of lesions, as well as a second group with developmental prosopagnosia. Our goals were two-fold. The first was to determine if topographic impairments were present in all groups or specific to certain types of prosopagnosia. The second was to evaluate the type of the topographic impairments present, by using a selected battery of tests for different navigational skills. These included place recognition, directional orientation on map reading, and cognitive map formation. In one taxonomy of topographic disorientation (Aguirre et al., 1999), these can be considered approximately as tests for the subtypes of landmark agnosia, heading disorientation, anterograde disorientation.
METHODS
Subjects
Acquired prosopagnosia
This cohort included 10 subjects (5 female, age range 24 – 70 years, mean 43.4 years, s.d. 17.1 years). All subjects had a detailed neuro-ophthalmologic history and examination, with best-corrected acuity of 20/60 or better, as well as Goldmann perimetry and the Farnsworth-Munsell 100-hue test of colour discrimination. All complained of impaired face recognition in daily life and were impaired on both a famous faces test of recognition (Barton et al., 2001) and on at least one of either the Cambridge Face Memory test (Duchaine et al., 2006a) or the faces component of the Warrington Recognition Memory test (Warrington, 1984), while performing normally on the word component of the latter (Table 1). None had complaints of mistaking one type of object for another in daily life, and all were able to recognize objects or their drawings during the neuro-ophthalmogic exam. They were also administered a battery of standard neuropsychologic tests to exclude more general problems of attention, memory and vision (Table 2). During the clinical interview, several acknowledged difficulties with recognizing places or getting lost in familiar places, while others were not aware of such problems (Table 3).
Table 1.
demographic, inclusion, and imaging data
| fMRI results | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WRMT | famous | OFA | FFA | pSTS | ||||||||
| subject | age (yr) | gender | CFMT/72 | face/50 | word/50 | face (d') | R | L | R | L | R | L |
| Acquired prosopagnosia, Inferior Occipitotemporal | ||||||||||||
| R-IOT1 | 56 | M | 44 | 33 | 41 | 1.96 | x | - | x | - | - | - |
| R-IOT4 | 62 | M | 27 | 39 | 50 | 1.29 | x | - | - | - | - | - |
| B-IOT2 | 62 | M | 24 | 21 | 42 | 1.31 | - | x | x | x | - | - |
| B-ATOT1 | 46 | F | 30 | 27 | 50 | x | - | x | - | - | - | |
| B-ATOT2 | 24 | F | 24 | 19 | 39 | -0.15 | - | - | x | - | - | - |
| Acquired Prosopagnosia, Anterior Temporal | ||||||||||||
| R-AT1 | 33 | F | 38 | 17 | 41 | 1.28 | - | - | - | - | - | - |
| R-AT2 | 36 | F | 40 | 27 | 47 | 0.65 | - | - | - | - | - | - |
| R-AT3 | 41 | M | 31 | 29 | 45 | 0.9 | - | - | - | - | - | - |
| R-AT5 | 61 | F | 35 | 28 | 46 | 1.52 | - | - | - | - | x | - |
| B-AT1 | 32 | M | 39 | 23 | 48 | -0.36 | - | - | - | - | - | - |
| Developmental Prosopagnosia | ||||||||||||
| DP008 | 61 | F | 36 | 36 | 49 | 2.32 | - | - | - | - | - | - |
| DP014 | 42 | M | 32 | 30 | 48 | 1.39 | - | - | - | - | - | - |
| DP016 | 52 | F | 41 | 37 | 49 | 1.71 | - | - | - | - | - | - |
| DP024 | 35 | F | 41 | 38 | 50 | 0.97 | - | - | - | - | - | - |
| DP033 | 46 | F | 29 | 39 | 50 | 1.37 | c | c | c | c | c | c |
| DP035 | 40 | M | 36 | 35 | 49 | 0.61 | - | - | - | - | - | - |
| DP044 | 36 | F | 40 | 34 | 49 | 1.29 | - | - | - | - | - | - |
CFMT = Cambridge Face Memory Test, WRMT = Warrington Recognition Memory Test
OFA = occipital face area, FFA = fusiform face area,
pSTS = posterior superior temporal sulcus, R = right, L = left
x' = no activation on MRI, '-' = activation present on MRI, 'c' = MRI contraindicated bold and underlined = abnormal score
Table 2.
Neuropsychological test results, acquired prosopagnosia.
| Test | Max R-IOT1 | R-IOT4 | B-IOT2 | B-ATOT1 B-ATOT2 R-AT1 R-AT2 | R-AT3 | R-AT5 B-AT1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Attention | |||||||||||
| Trails A | - | 39 | 48# | 80 | 24 | 30 | 39 | 21 | 22 | 43 | 18 |
| Trails B | - | 61 | 102# | 142 | 60 | 93# | 61 | 44 | 37 | 78 | 25 |
| Star Cancellation | 54 | 54 | 54 | 53 | 54 | 54 | 54 | 54 | 54 | 54 | 54 |
| Visual Search | 60 | 54 | n/a | 56 | 52 | 59 | 54 | 59 | 59 | 52 | 59 |
| Memory | |||||||||||
| Digit span-forward | 16 | 12 | 8 | 14 | 12 | 7 | 12 | 13 | 16 | 10 | 12 |
| Spatial span-forward | 16 | 9 | 10 | 8 | 11 | 8# | 9 | 9 | 12 | 6 | 10 |
| Word list | 48 | 28 | 37 | 35 | 37 | 27 | 28 | 35 | 31 | 24 | 27 |
| Visuo-perceptual | |||||||||||
| Hooper Visual Organization | 30 | 27 | 22 | 22.5 | 17.5 | 12 | 27 | 28 | 27.5 | 22 | 20 |
| Benton Judgment of Line Orientation | 30 | 29 | 24 | 29 | 26 | 22 | 29 | 28 | 30 | 21 | 28 |
| Visual Object and Spatial Perception | |||||||||||
| Object: Screening | 20 | 20 | 18 | 20 | 20 | 20 | 20 | 20 | 20 | 17 | 20 |
| Incomplete Letters | 20 | 19 | 19 | 19 | 19 | 19 | 19 | 20 | 19 | 20 | 19 |
| Silhouettes | 30 | 21 | 18 | 12 | 9 | 4.5 | 21 | 18 | 22 | 19 | 10 |
| Object Decision | 20 | 16 | 19 | 14 | 9 | 10 | 16 | 20 | 17 | 14 | 16 |
| Progressive Silhouettes | 20 | 9 | 13 | 15 | 11 | 4 | 9 | 10 | 11 | 17 | 17 |
| Spatial: Dot Counting | 10 | 10 | 9 | 10 | 10 | 9 | 10 | 10 | 10 | 10 | 10 |
| Position Discrimination | 20 | 20 | 19 | 19 | 20 | 15 | 20 | 20 | 19 | 18 | 19 |
| Number Location | 10 | 10 | 10 | 10 | 10 | 8 | 10 | 9 | 10 | 10 | 10 |
| Cube Analysis | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 | 10 | 8 | 10 |
| Imagery | |||||||||||
| Mental Rotation | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 | 10 |
underline denotes impaired,
denotes borderline performance
Table 3.
results.
| group | subject | topographic complaints |
place recognition house scene |
road map test /32 |
cognitive map formation /21 |
Santa Barbara direction /7 |
|
|---|---|---|---|---|---|---|---|
| A' | A' | ||||||
| controls | |||||||
| mean | 0.95 | 0.96 | 31.55 | 10.71 | 4.26 | ||
| s.d. | 0.03 | 0.04 | 0.93 | 4.60 | 1.44 | ||
| n | 33 | 32 | 11 | 34 | 434 | ||
| 95 predict interval | 0.88 | 0.88 | 29 | 20.21 | 1.43 | ||
| acquired prosopagnosia, inferior occipitotemporal | |||||||
| R-IOT1 | n | 0.95 | 0.92 | 32 | 12 | 3.53 | |
| R-IOT4 | y | 0.84 | 0.94 | 31 | 21 | 4.13 | |
| B-IOT2 | y | 0.82 | 0.67 | 27 | 21 | 2.66 | |
| B-ATOT1 | n | 0.77 | 0.79 | 31 | 21 | 5.13 | |
| B-ATOT2 | y | 0.79 | 0.30 | 32 | 21 | 2.06 | |
| acquired prosopagnosia, anterior temporal | |||||||
| R-AT1 | n | 0.90 | 0.88 | 32 | 14 | 3.20 | |
| R-AT2 | y | 0.93 | 0.95 | 32 | 11 | 3.60 | |
| R-AT3 | y | 0.86 | 0.94 | 32 | 6 | 5.73 | |
| R-AT5 | n | 0.82 | 0.91 | 29 | 21 | 3.53 | |
| B-AT1 | y | 0.79 | 0.85 | 32 | 12 | 3.73 | |
| developmental prosopagnosia | |||||||
| DP008 | n | 0.99 | 1.00 | 31 | 21 | 4.20 | |
| DP014 | n | 0.99 | 0.99 | 32 | 20 | 5.66 | |
| DP016 | y | 0.99 | 0.98 | 31 | 7 | 4.33 | |
| DP024 | n | 0.99 | 0.98 | 32 | 2 | 5.66 | |
| DP033 | n | 1.00 | 0.98 | 32 | 11 | 5.26 | |
| DP035 | n | 0.90 | 0.94 | 32 | 9 | 4.33 | |
| DP044 | n | 0.99 | 0.99 | 32 | 20 | 5.66 | |
Developmental prosopagnosia
This cohort consisted of 7 adult subjects (5 female, age range 35 – 61 years, mean 44.6 years, s.d. 9.3 years). These were local residents recruited from www.faceblind.org. All reported life-long difficulty in face recognition. Diagnostic criteria confirming impaired face recognition (Table 1) included a score at least 2 standard deviations below the control mean on the Cambridge Face Memory Task (Duchaine et al., 2006a) and a discordance between preserved word memory and impaired face memory on the Warrington Recognition Memory Test for Faces and Words (Warrington, 1984) that was in the bottom 5th percentile. In addition, all but one (DP008) had a d’ below the 95% prediction limit of controls for a test of familiarity with famous faces (Barton et al., 2001). All subjects had best corrected visual acuity of better than 20/60, normal visual fields on Goldmann perimetry, and normal color vision on the Farnsworth-Munsell hue discrimination test. To exclude autism spectrum disorders, all subjects had a score less than 32 on the Autism Questionnaire (Baron-Cohen et al., 2001). While neither this nor the acquired cohort were selected on the basis of topographic complaints, during the diagnostic interview all subjects were asked two direct questions: “Do you have difficulty recognizing places?” and “Do you believe that your navigational abilities are poor compared to others?” Only one of the developmental prosopagnosic subjects, DP016, answered yes to either question, and did so for both (Table 3).
Control subjects
For house and scene recognition tests, this consisted of 32 subjects, 16 females and 16 males, ranging in age from 20 to 63 years. The Road map test had 11 controls, 6 female and 5 male, ranging in age from 30 to 63 years.
The on-line test of cognitive map formation had data for 568 control subjects. From these we excluded those who acknowledged neurologic or psychiatric conditions, including autism spectrum disorders, and those who reported use of psychoactive medications. From the remaining subjects we identified 2 age- and gender-matched subjects for each prosopagnosic subject. The resulting group of 34 control subjects consisted of 20 females and 14 males with mean age of 45.6 years (s.d. 12.9, range 24 to 71 years).
Results of the Santa Barbara Sense of Direction Scale were compared to the online database as a whole. Exclusion of those with psychiatric conditions or disorders of the central nervous system (other than migraine) and those on psychoactive medications left 434 subjects (102 male), with mean age of 44.5 years (s.d. 18.8, range 15 to 92 years). The average score of this cohort was 4.26 (s.d. 1.44), which is comparable to the results of a large on-line study of 1552 adults (Brunye et al., 2015), and a large study of 1425 older adults (Turano et al., 2009).
Imaging
Data were acquired on a Philips Achieva 3.0 Tesla MRI scanner with an 8-channel head coil. High-resolution (1mm3) T1-weighted three-dimensional structural images were collected for lesion analysis and anatomical localization of functional data. All prosopagnosic subjects had structural and functional magnetic resonance imaging to localize the core components of the face processing network, using the HVEM dynamic face localizer protocol (Fox et al., 2009). T2*-weighted echo-planar imaging (EPI) functional scans were used to collect data from 36 interleaved axial slices (TR = 2000ms, TE = 30ms, FOV = 240×216mm, 3mm thickness with 1mm gap, voxel size 3×3mm, 128 reconstruction matrix, reconstructed voxel size = 1.875×1.65mm). Functional runs comprised 198 volumes of acquisition. The functional slices were co-registered onto the T1-weighted anatomical image for each subject and transformed to standard space.
The HVEM Dynamic Face Localizer scan consisted of grayscale video clips of faces and objects. Each stimulus block included 6 video clips lasting 1.5s each, separated by a 500ms blank screen. Stimulus blocks were separated by a 12s fixation block. Each condition (faces or objects) was repeated 8 times per run. This localizer was presented to participants via an LCD projector to a screen placed in the front of the scanner, which was visible to the subject via a mirror mounted on the head coil. Attention was sustained by asking the subjects to press a button on an MRI-compatible button-box when the same video was presented twice in a row.
Acquired Prosopagnosia
Functional data were analyzed using BrainVoyager QX software. Preprocessing steps included slice time correction (cubic spline interpolation), 3D motion correction (trilinear/sinc interpolation), and high-pass temporal filtering (GLM-Fourier, 2 sines/cosines). Using a False-Discovery-Rate of q < 0.05 (corrected for multiple comparisons), we identified the core regions of face perception, bilaterally, within each participant (Haxby et al., 2000). Contiguous clusters of at least five face-selective voxels located on the lateral temporal portion of the fusiform gyrus were designated as the fusiform face area, while clusters located on the lateral surface of the inferior occipital gyrus were designated as the occipital face area. Face-selective clusters located on the posterior segment of the superior temporal sulcus were designated as the superior temporal sulcus.
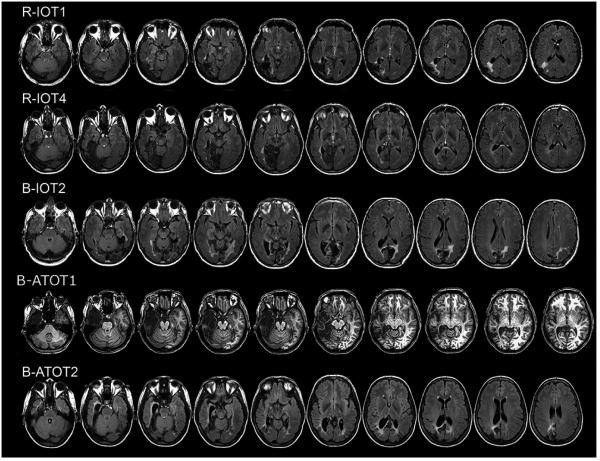
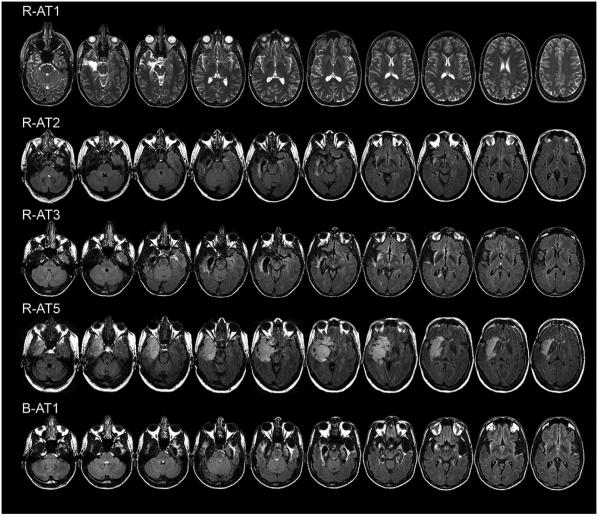
The nomenclature for our prosopagnosic subjects follows the evidence for tissue loss or hypointensity on T1-weighted images (Figures 1 and 2). The anterior tip of the middle fusiform sulcus (Weiner et al., 2014), at the approximate midpoint between the anterior temporal and occipital poles, served as a boundary (Talairach y = −30). Lesions mainly anterior to this line were designated as anterior temporal (AT) and those posterior to it as inferior occipitotemporal (IOT). Some subjects had additional complexities to their lesions. FLAIR sequences in R-AT3 revealed additional left medial temporal lobe and insula hyperintensities (Figure 1). B-ATOT2 had bilateral fusiform lesions and a right anterior temporal lesion, as well as posterior periventricular hyperintensities on FLAIR sequences.
Figure 1.
Axial MRI scans of subjects with acquired prosopagnosia, with inferior occipitotemporal lesions. FLAIR images are shown with the exception of T1-weighted imge for B-ATOT1.
Figure 2.
Axial FLAIR MRI scans of subjects with acquired prosopagnosia, with anterior temporal lesions.
The functional imaging data aligned with the T1-weighted structural nomenclature (Table 1). Subjects with an IOT designation did not show activation by faces of the right fusiform face area, whereas those with an AT designation alone showed activation of all three core areas in each hemisphere, with the exception of R-AT5, who did not show activation of the right superior temporal sulcus. Images of the functional imaging as well as volumetric lesion data are provided in another report on these patients (Liu et al., 2014).
Developmental Prosopagnosia
All but one subject (DP033) in whom it was contraindicated had magnetic resonance brain imaging with T1-weighted sequences to exclude structural lesions that would be indicative of early acquired rather than developmental prosopagnosia (Barton et al., 2003). In addition, these subjects also completed the functional localizer described above.
Functional data were analyzed using the tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL) (Woolrich et al., 2009). Preprocessing steps included high-pass filtering, motion correction with MCFLIRT (Jenkinson et al., 2002) and smoothing using a 6-mm full-width at half-maximum Gaussian kernel. We identified core face-selective areas (faces > objects) of contiguous clusters of at least 5 face-selective voxels (p < 0.001, uncorrected) as in the acquired prosopagnosia subjects. All subjects with developmental prosopagnosia who underwent MR imaging showed activation of the right and left fusiform face area, occipital face area, and superior temporal sulcus (Table 1).
Topographic tests
The Road Map test and place recognition tests were done in the laboratory on separate days in the same week. Place recognition stimuli were presented on a computer monitor, while the Road Map test was shown on paper. Subjects were also administered the Santa Barbara Sense of Direction questionnaire. After an interval that ranged from weeks to months, tests of cognitive map formation were done on-line, through the website www.gettinglost.ca.
1. Place recognition: Houses and scenes
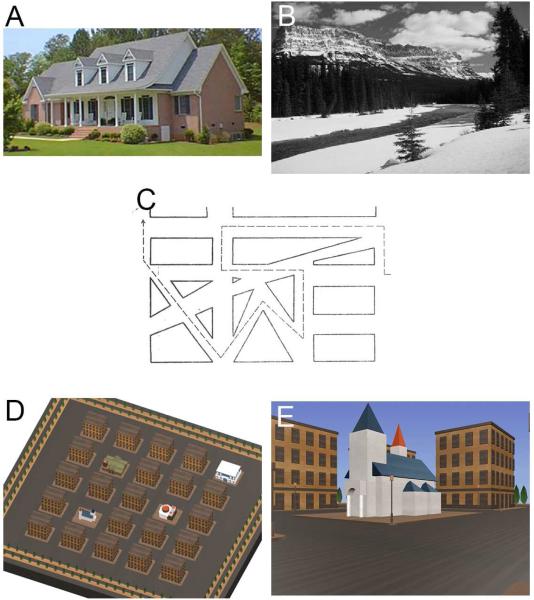
We tested place recognition with two components, one for houses and one for scenes. Two tests were used because of evidence that the recognition of landmarks such as buildings and objects may be dissociable from the recognition of scenes, which convey information about the environment in which landmarks may be situated (Epstein, 2008; Mendez et al., 2003). These two tests used an established protocol reported in prior studies of developmental prosopagnosia (Duchaine et al., 2005; Duchaine et al., 2006c). Test instructions were given both verbally and written on the monitor. Participants were seated approximately 40 cm away from a 24-inch monitor. For the house recognition test, ten examples of houses photographed in colour from the front were shown twice during an encoding phase, each image shown for 3 seconds, and each spanning approximately 24° horizontally and 11° vertically (Figure 3A). In a test phase, subjects were shown a series of 50 houses in random order, 20 of which were two repetitions each of the 10 houses that had been studied, and 30 of which were new houses. Each image remained visible until the subject responded, to a maximum duration of 7 seconds. If the subject did not respond within 7 seconds, the trial was scored as “no response.” The task was to indicate with a keypress whether a particular item had been seen in the encoding phase or not. An identical strategy was used for the scenes test, with 10 grey-scale images of scenes presented twice during an encoding phase, each image spanning about 30° horizontally and 14° vertically (Figure 3B), which were presented again twice with 30 new scenes in random order during the test phase. The ten target scenes consisted of two scenes each from five different categories: beaches, lakes, meadows, mountains, and deserts. The 30 distractor scenes consisted of six images from each of those five categories. As in Duchaine and Nakayama (2005), we calculated A’, a criterion-free measure of discrimination for both houses and scenes (MacMillan et al., 1991).
Figure 3.
Illustration of tests. A. House Recognition test, example image. B. Scene Recognition test, example image. C. Road Map test, a portion of the map. Subjects follow the dotted line in the direction shown by the arrowhead at the end, describing at each change of direction whether they are turning right or left. D. Cognitive Map Formation test, aerial view of the correct map. E. Cognitive Map Formation test, example of street-level view of travel near one of the landmarks as portrayed in a video-clip. From such clips subjects must deduce the correct location of the landmarks in the aerial map.
2. Road Map test
This test assessed directional orientation while following a route. Subjects were presented with a line drawing on paper illustrating a path that contained 32 turns (Money et al., 1965; Iaria et al., 2005). Subjects were to follow the path visually, without touching the map, and describe at each change of direction whether they would be making a right or left turn (Figure 3C). Scores were given out of 32.
3. Cognitive Map Formation
This test resembles those used in undirected way-finding tasks in a previous taxonomy (Wiener et al., 2009) and in other studies of cognitive map formation (Iaria et al., 2007; Iaria et al., 2009; Liu et al., 2011). It showed a series of 1-minute video clips of street-level view of movement through a virtual environment consisting of a five-by-five grid of streets and featureless rectangular buildings, in which four unique landmarks were located (Figure 3F). In each clip at least two of the four landmarks were encountered. The same path was never travelled twice. At the end of each video-clip, participants were presented with an aerial map of the environment in which they are asked to place icons for each landmark in its correct location, with unlimited time (Figure 3E). Following this, the next video-clip was presented. This test ended when participants successfully placed all four landmark icons correctly on the map, or when they had reached 20 trials. The number of trials participants required to reach this criterion was recorded: if they failed to do so by the 20th trial, they were given a default score of 21.
4. Santa Barbara Sense of Direction Scale
This is a standardized self-reported questionnaire containing 15 questions on environmental spatial skills, on which subjects indicate on a 7-point Likert scale their agreement with a statement relating to their own experience (Hegarty et al., 2002). The subject’s score is the average of these 15 ratings after adjustment of all items so that a higher score indicates better sense of direction.
Analysis
The place recognition tests for scenes and houses and the cognitive map formation test showed non-normal distribution in their data. Therefore for consistency we used a non-parametric approach and applied the Kruskal-Wallis test (http://vassarstats.net/kw3.html). For each test we performed two analyses. Our primary analysis included three groups: control subjects, acquired prosopagnosia, and developmental prosopagnosia. Our secondary analysis included four groups, as we divided the acquired prosopagnosia group into an anterior temporal group and an occipitotemporal group. Each of these two subgroups had five subjects, the minimum number required for this type of analysis.
When the results of the primary or secondary analyses were significant, we examined pair-wise comparisons of ranks against a calculation of the least significant difference between mean ranks, at an alpha level of 0.05 (http://department.obg.cuhk.edu.hk/researchsupport/Least_sig_diff_rank.asp).
In addition, to classify the data of each prosopagnosic subject as normal or abnormal, we computed 95% prediction intervals from the control data.
Two post-hoc analyses were conducted to examine the independence of the processes being tested in the acquired prosopagnosic cohort. First, to assess whether differences in topographic skill could be explained by more general object processing mechanisms, we compared house, scene, and cognitive map formation scores in those with and without mild object recognition impairments on the Visual Object Recognition Battery. Second, we compared the house and scene recognition scores of those with versus those without impaired cognitive map formation. These comparisons were conducted using a Mann-Whitney test for independent samples.
Finally, the results of house recognition, scene recognition, and cognitive map formation across the entire prosopagnosic cohort were correlated against the results of the Santa Barbara Sense of Direction scale.
RESULTS
1. House and scene recognition
The primary analysis for houses showed a significant difference in A’ between the groups (H(2) = 20.1, p<.0001). A’ was lower for acquired prosopagnosic subjects than either controls or developmental prosopagnosic subjects, but did not differ between controls and developmental prosopagnosic subjects (Table 3). The secondary analysis was also significant (H(3) = 20.1, p=.0002): both the anterior temporal and the occipitotemporal acquired subgroups had lower A’ than either controls or the developmental prosopagnosia group.
A similar result was obtained for scenes. There was a significant difference in A’ between groups (H(2) = 16.7, p <.0002) and the group with acquired prosopagnosia had lower A’ than either controls or developmental prosopagnosic subjects, while the latter did not differ from controls. The secondary analysis was also significant (H(3) = 17.0, p=.0007). Both the anterior temporal and the occipitotemporal subgroups of acquired prosopagnosia had lower A’ than developmental prosopagnosic subjects. The A’ of the occipitotemporal subgroup was also lower than that of the controls, with a trend for the anterior temporal group.
At the individual level, seven subjects with acquired prosopagnosia (three with anterior temporal and four with occipitotemporal lesions) were classified as impaired on house recognition, while for scene recognition five subjects with acquired prosopagnosia (two with anterior temporal and three with occipitotemporal lesions) were impaired. Combining these two place recognition tests, only two acquired prosopagnosic subjects (R-IOT1 and R-AT2) performed normally with both houses and scenes. Four subjects were impaired on both and four on one of the two tests: R-IOT4, R-AT3 and R-AT5 were impaired on house but not scene discrimination, while R-AT1 showed the converse pattern. However, inferences about dissociability must be tempered by the fact that only in R-IOT4 did the difference between house and scene scores exceeded the 95% prediction limit for differences for controls.
No developmental prosopagnosic subject was impaired on either test.
2. Road Map test
No group effect was seen with either the primary analysis (H(2) = 0.49, p = .78) or the secondary analysis with acquired prosopagnosia divided into subgroups (H(3) = 1.52, p = .68). At the individual subject level, only B-IOT2 with acquired prosopagnosia fell below the 95% prediction limit for controls.
3. Cognitive map formation
There was an effect of group in the primary analysis (H(2) = 7.19, p < .03). Subjects with acquired prosopagnosia needed more trials to create a cognitive map than controls, but this was not found for developmental prosopagnosia. In the secondary analysis of subgroups, there was also an effect of group (H(3) = 9.63, p< .02). The occipitotemporal subgroup took longer to form a map than controls, but this was not found for the anterior temporal subgroup. At the individual level, four of five subjects with occipitotemporal lesions but only one of five subjects with anterior temporal lesions failed to form a map by the maximum number of attempts allowed (i.e. default score of 21, which likely underestimates the severity of their deficit). The difference in the number of subjects failing to form a cognitive map was marginally significant between lesion location groups [χ(1) = 3.6, p = .058]. Only one subject with developmental prosopagnosia failed to do so, and only two of the 34 controls.
5. The relationship to basic visual processing in acquired prosopagnosia
This analysis asked whether topographic impairments were more severe in those subjects with mild impairments on neuropsychological tests of object recognition, namely the Visual Object and Spatial Perception and Hooper Visual Organization tests (see Table 2). A Mann-Whitney independent samples test revealed worse recognition of both houses (p = .008) and scenes (p = .016) in those with mild object recognition impairments. However, dissociations were evident at the individual level: importantly, three subjects with entirely normal object recognition scores were impaired on either house (R-IOT4 and R-AT3) or scene (R-AT1) recognition.
On the other hand, a Mann-Whitney independent samples test yielded no difference in cognitive map formation scores (p = .206) between those with and those without mild object recognition impairments.
Last, a Mann-Whitney independent samples test also yielded no difference in the recognition of houses (p = .206) or scenes (p = 1.0) between those with and without cognitive map formation impairment.
6. The relationship to the Santa Barbara Sense of Direction Scale
Across the entire prosopagnosic cohort, the results of the self-report questionnaire were correlated with house recognition (r = 0.50, p < 0.035) and scene recognition (r = 0.59, p < 0.01; with omission of the outlying score of B-ATOT2, r = 0.55, p < 0.022) but did not reach significance with cognitive map formation (r = 0.32, p = .18).
Lesion Analysis
On the Cognitive Map Formation test, five subjects with acquired prosopagnosia were impaired, with a score of 21 meaning that they failed to achieve a correct map by the maximum number of attempts permitted, and five performed in the normal range. This provided us an opportunity to perform a lesion overlap analysis to determine if there was a candidate anatomic substrate for impaired cognitive map formation in this population.
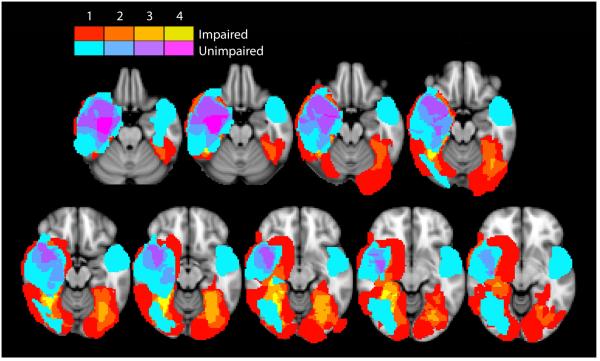
From the T1-weighted structural images we created a lesion mask for each of the 10 subjects with acquired prosopagnosia, using tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL) (Woolrich et al., 2009). Then, the subjects’ T1-weighted structural images were preprocessed with lesion filling when necessary (Battaglini et al., 2012), and linear-registration (Jenkinson et al., 2001; Jenkinson et al., 2002) or non-linear registration to the MNI-152 stereotaxic space. Within the standard space, lesion masks were summed within the two groups to give an overlap image. Those with impaired cognitive map formation were depicted in a red-yellow spectrum and those with intact cognitive map formation in a blue-fuchsia spectrum (Figure 4). The areas involved by the lesions of at least four subjects with impaired cognitive map formation and by none of the lesions of the five with intact performance were identified using the Harvard-Oxford Cortical and Subcortical Atlases as the right mid- to posterior-fusiform gyrus, and adjacent parahippocampal gyrus and hippocampus.
Figure 4.
Overlap lesion analysis for cognitive map formation. The overlap axial image of lesions from those with intact performance is shown as a color spectrum from blue to fuchsia, with blue indicating regions involved by the lesion in only one subject, and fuchsia indicating regions where the lesions of four subjects overlapped. This image is placed over the overlap image of lesions from those with impaired performance, shown as a color spectrum from red to fellow, with red indicating regions involved by the lesion in only one subject, and yellow indicating regions where the lesions of four subjects overlapped. Hence visible yellow regions represent regions with lesions in at least 4 subjects with impaired cognitive map formation and not involved by the lesions of any subjects with intact cognitive map formation.
DISCUSSION
We found that problems relevant to topographic orientation were common in acquired prosopagnosia, validating prior anecdotal reports of such difficulties in this group of subjects (Landis et al., 1986; Takahashi et al., 1995; Clarke et al., 1997; Malone et al., 1982; Bauer, 1984; Martins et al., 1999; Barton et al., 2002; Uttner et al., 2002). Impaired house and scene recognition were frequent. While house and scene recognition problems had a similar frequency among those with anterior temporal and inferior occipitotemporal lesions, problems with cognitive map formation were mainly found among those with inferior occipitotemporal lesions, specifically involving the right mid- to posterior-fusiform gyrus and adjacent parahippocampal gyrus and hippocampus. This localization for impaired cognitive map formation in subjects selected for prosopagnosia compares with a recent report implicating the right cuneus and calcarine sulcus in topographic disorientation in subjects with posterior cerebral arterial infarcts (Busigny et al., 2014), which underlines the distributed nature of topographic processing.
In contrast to these deficits in acquired prosopagnosia, none of the seven subjects with developmental prosopagnosia had impaired recognition of scenes or houses. As a group, the developmental cohort did not differ from controls on cognitive map formation, though one subject failed on the cognitive map formation test.
Acquired prosopagnosia
An association between acquired prosopagnosia and landmark agnosia has been reported (Takahashi et al., 2002). Landmark agnosia has been reported with lesions of right ventral temporo-occipital cortex (Pai, 1997; McCarthy et al., 1996), more specifically of the right parahippocampal, anterior lingual and fusiform gyri (Takahashi et al., 2002). Functional imaging has revealed a region activated by viewing buildings and places, the parahippocampal place area, which is medial to the fusiform face area (O'Craven et al., 2000), leading others to speculate that damage to the parahippocampal place area could impair landmark recognition, with or without accompanying prosopagnosia (Takahashi et al., 2002). However, others argue that this region may be more involved in encoding the appearance and layout of scenes than landmarks (Epstein et al., 1999). This may account for an agnosia for scenes, described as the inability to recognize scenes lacking salient landmarks, which been described in a man who also made semantic errors in face recognition after a right medial occipitotemporal infarct (Mendez et al., 2003). In support, two patients with damage to the parahippocampal place area had intact recognition of famous landmarks and could produce maps of routes known to them, but were unable to learn new scenes (Epstein et al., 2001). This has led to suggestions that the parahippocampal place area is involved in encoding the geometry of local scenes from the viewer’s perspective (Epstein, 2008), with landmarks encoded in adjacent fusiform or inferior occipital cortex.
These proposals could account for impaired house or scene recognition in our subjects with fusiform damage. Another potential candidate region would be the transverse occipital sulcus, or ‘occipital place area’, which is also involved in scene categorization (Dilks et al., 2013; Ganaden et al., 2013; Bettencourt et al., 2013) and could be involved by the posterior aspect of the lesions in at least some of our subjects with acquired prosopagnosia. Interestingly, though, our results show that impaired house or scene recognition is also found with anterior temporal lesions that spare fusiform and occipital cortex. This adds to a previous observation of impaired place recognition causing topographic disorientation in a patient with anterior temporal damage from encephalitis (McCarthy et al., 1996). This would suggest that, as with face recognition, place recognition for houses and scenes likely involves a larger network of cortical areas that extends into anterior temporal regions.
Cognitive map formation is the ability to create a mental map of the environment, by integrating the positions of landmarks and routes into a schema: it represents the transition from a viewer perspective of the spatial layout of scenes to an allocentric representation in a map that is independent of the location and viewpoint of the observer (Epstein, 2008). Functional MRI studies show that it involves activation of the right and left hippocampi as well as retrosplenial cortex (Iaria et al., 2007). Previous neuropsychological studies on cognitive map formation are limited. Learning a real-life route was impaired with damage to either right or left medial occipital and posterior parahippocampal damage, right hippocampus and right inferotemporal cortex (Barrash et al., 2000), but this report could not establish whether the problem lay in poor scene recognition or poor formation of a cognitive map. Retrosplenial damage can impair the ability to draw or describe routes (Pai, 1997) and to represent the spatial relationships between locations (Takahashi et al., 1997), which some consider is part of the transition from the viewer’s perspective to an ‘allocentric’ spatial representation, as in a cognitive map. In our series, we found that cognitive map formation was impaired in acquired prosopagnosia after unilateral or bilateral inferior occipitotemporal lesions. While one might suggest that this could be secondary to impaired landmark recognition, cognitive map formation was normal in most of the group with anterior temporal lesions, despite the fact that they too had similar difficulties on recognition of scenes and houses. (This may be because the landmarks used in the Cognitive Map Formation test are easier to recognize and differentiate than those used in the house recognition test.) Furthermore, acquired prosopagnosic subjects impaired in cognitive map formation did not perform more poorly on house and scene recognition than acquired prosopagnosia subjects without impairments in cognitive map formation. Hence we would conclude that impaired cognitive map formation is an independent contributing deficit to topographic disorientation in prosopagnosic subjects with medial occipitotemporal lesions, which may reflect the potential for such lesions to impact the hippocampi and/or their connections. Our overlap analysis implicated lesions of the right mid- and posterior fusiform, parahippocampal and hippocampal areas as correlates of impaired cognitive map formation. We would note also that our results likely underestimated the deficit in these subjects, given that a score of 21 is given to those who fail to form an accurate map by the maximal allowable number of trials of 20.
In contrast, our subjects appeared intact on directional orientation during map reading, with only one acquired prosopagnosic subject being mildly impaired. Caution is required for this result, given that there is a ceiling effect for our controls, although this has not been the case in other studies (Kurylo et al., 1996; Rainville et al., 2002). Performance on the Road Map test is considered to involve a number of functions, such as spatial attention, right-left orientation - which is classically associated with Gerstmann’s syndrome following left angular gyrus lesions (Gold et al., 1995) - direction discrimination in personal (i.e. egocentric) spatial orientation, and mental rotation (Rainville et al., 2002). In neuropsychology, the Road Map test has been studied mainly in subjects with Alzheimer’s dementia, where some consider it a test of the dorsal visual stream (Kurylo et al., 1996) or an index of attention and mental rotation (Rainville et al., 2002), while others find a correlation with impaired radial optic flow perception that they attribute to hippocampal and posterior cortical degeneration (O'Brien et al., 2001). Localization evidence from focal lesions is limited. An older study found that impairments were most marked after left frontal lesions, modest with right parietal or left temporal lesions, and not apparent with right temporal lesions (Butters et al., 1972). A more recent study of epileptic subjects found no impairment on the Road Map test after either right or left temporal lobectomies (Worsley et al., 2001). Again, our study would concur, in that all but one of our subjects with medial occipitotemporal or anterior temporal lesions performed the Road Map test well.
The results of our testing generally aligned with the daily experience reported by our subjects with acquired prosopagnosia. Many reported difficulties with getting lost and recognizing places, and stated that they relied on verbal cues such as street signs, building names and room numbers. R-IOT4 reported that when he was discharged from hospital, he recognized his own house only by the pillars at its entrance - an interesting parallel to anecdotal observations that prosopagnosic subjects can recognize certain faces by distinctive features - and often got lost inside the long-familiar homes of old friends. The family of B-ATOT2 put balloons on her bedroom door so she could find it. B-IOT2 followed a well-worn path from his house to reach the center of town, but if he deviated from it he would get lost and wander until he found a familiar intersection. Even the two subjects who scored normally on our house and scene tests reported some problems with place recognition in daily life. R-AT2 recalled being dropped off by mistake at a neighbour’s house instead of hers and not realizing it until she was inside. R-IOT1 recognized the Gothic church across the street from his apartment building but not the building itself. This discordance could reflect improvement over time, given that testing occurred years after onset, or a lack of sensitivity of our tests. Against the latter, though, are results like those of B-ATOT1, who was unaware of topographic difficulties and yet was impaired on both house and scene recognition and exceeded the maximum number of attempts allowed on our test of cognitive map formation.
The Santa Barbara Sense of Direction Scale is a standardized tool to capture people’s impression of their own topographic skills. Across the entire cohort of both acquired and developmental prosopagnosia, house and scene recognition scores correlated with the ratings on this scale, but not that of cognitive map formation. This could suggest that place recognition is a more significant determinant of people’s subjective sense of their navigational ability. However, the analysis for cognitive map formation suffers from a non-linearity imposed by the truncation of scores at 21, indicating that the subjects had failed to form a map by the twentieth trial. Since the definition of abnormality was this failure, the results for subjects with abnormal performance do not reflect the severity of their deficit, as all received a score of 21.
Developmental prosopagnosia
While acquired prosopagnosia has a long history in the neuropsychological literature, developmental prosopagnosia has only been recognized in recent decades (Susilo et al., 2013). Whether it is associated with other deficits in visual recognition continues to be debated (Behrmann et al., 2005; Duchaine et al., 2005), and its neural basis remains unsettled. While some report structural or functional anomalies in the fusiform region (Garrido et al., 2009; Furl et al., 2011; Dinkelacker et al., 2011; Zhang et al., 2015) others do not find this but point to subtler morphologic abnormalities in anterior fusiform cortex and the anterior and middle temporal lobes (Behrmann et al., 2007; Avidan et al., 2005; Avidan et al., 2009; Avidan et al., 2014b). Our results for acquired prosopagnosia following either inferior occipital or anterior temporal lesions would suggest that either developmental scenario could theoretically be associated with topographic problems. Recent studies show that developmental topographic disorientation does exist (Iaria et al., 2010), which in at least some subjects is due to impaired cognitive map formation and linked to failure to activate the hippocampi during this task (Iaria et al., 2009). However, we did not find evidence of impairments in place recognition or cognitive map formation in developmental prosopagnosia. Hence any temporal or occipital pathology in the developmental form may be more circumscribed and selective than that of the acquired disorder.
As in adult-onset acquired prosopagnosia, anecdotes of getting lost appear to be frequent among the rare subjects with early-onset acquired prosopagnosia described elsewhere (Barton et al., 2003; Young et al., 1989; Michelon et al., 2003). However, in the literature on developmental prosopagnosia, reports regarding associated topographic disorientation are mixed (Kress et al., 2003; Grueter et al., 2007). Among cases of developmental prosopagnosia, YT “easily identified…familiar locations” p. 824 (Bentin et al., 1999), BC “has no navigational difficulties and in fact is an avid hiker “ p. 80 (Duchaine, 2000), and EP could draw map of his own home and trace his route from home to work on a map (Nunn et al., 2001). On the other hand, NM “describes her traveling as ‘bumbling’ from place to place, and reports that she has trouble understanding how the neighborhoods that she has lived in are laid out “ p. 828 (Duchaine et al., 2003), while TA “was unable to use nonverbal landmarks to negotiate routes, and thus was occasionally lost.” p.266 (Jones et al., 2001), and Dr. S kept getting lost and had trouble recognizing her house (Temple, 1992). The first developmental prosopagnosic subject described, AB, gave instances of getting lost in her neighbourhood and relied on street names to identify her route on a map (McConachie, 1976).
As with acquired prosopagnosia, formal testing of topographic skills in developmental prosopagnosia has been limited to date. EP, whose navigation appeared unimpaired, was normal on short-term recognition of houses and naming of famous buildings (Nunn et al., 2001). On the same tests we used, Edward was normal on the houses test but impaired on the scenes test despite not having topographic complaints in real life (Duchaine et al., 2006c). There are reports of two small series. In one, two of seven developmental prosopagnosic subjects were impaired on scene but not house recognition (Duchaine et al., 2005). Another of six developmental prosopagnosic subjects examined perception of blurred and scrambled faces and houses, and found normal perception of blurred houses (Lobmaier et al., 2010). The only study that had a test using any kind of map reported that AB was poor at identifying right and left turns for a route (McConachie, 1976). In contrast, none of our developmental prosopagnosic subjects had problems with right and left turns on the Road Map Test. Finally, in a related vein, a recent study of white matter tracts in developmental prosopagnosia found that mean diffusivity in ventral temporal pathways was reduced in projections involving face-selective regions in the medial fusiform gyrus, but not in those involving place-selective regions in the collateral sulcus (Gomez et al., 2015), a finding that supports our observation of intact house and scene recognition and other topographic skills in developmental prosopagnosia.
Summary
Impaired place (i.e. house and scene) recognition was a common finding in our subjects with acquired prosopagnosia regardless of lesion location, while those with occipitotemporal lesions had additional problems with cognitive map formation. Neither group had difficulty with directional orientation during map reading. This work thus clarifies the mechanisms of the navigational impairment that forms part of the constellation of deficits associated with acquired prosopagnosia. In contrast, while previous case reports in the literature provide anecdotal evidence that some developmental prosopagnosic subjects may have topographic problems, the results from our cohort with developmental prosopagnosia and a prior one (Duchaine et al., 2005) show that most of these subjects perform normally on tests of topographic-related functions, a conclusion that aligns with recent neuroimaging findings (Gomez et al., 2015). Thus, although there may be heterogeneity and variation in developmental prosopagnosia (Stollhoff et al., 2011), its dominant form may be a more face-selective disorder than acquired prosopagnosia.
ACKNOWLEDGMENTS
This work was supported by CIHR operating grant (MOP-102567) to JB, and NSERC Discovery grant (RT735872) to GI. JB was supported by a Canada Research Chair and the Marianne Koerner Chair in Brain Diseases. BD was supported by grants from the Economic and Social Research Council (UK) (RES-062-23-2426) and the Hitchcock Foundation. SC was supported by National Eye Institute of the National Institutes of Health under award number F32 EY023479-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- AGUIRRE GK, D'ESPOSITO M. Topographical disorientation: A synthesis and taxonomy. Brain. 1999;122:1613–28. doi: 10.1093/brain/122.9.1613. Pt 9. [DOI] [PubMed] [Google Scholar]
- ARNOLD AE, BURLES F, KRIVORUCHKO T, LIU I, REY CD, LEVY RM, IARIA G. Cognitive mapping in humans and its relationship to other orientation skills. Exp Brain Res. 2013;224(3):359–72. doi: 10.1007/s00221-012-3316-0. [DOI] [PubMed] [Google Scholar]
- AVIDAN G, BEHRMANN M. Functional mri reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Curr Biol. 2009;19(13):1146–50. doi: 10.1016/j.cub.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVIDAN G, BEHRMANN M. Impairment of the face processing network in congenital prosopagnosia. Front Biosci (Elite Ed. 2014a;6:236–57. doi: 10.2741/E705. [DOI] [PubMed] [Google Scholar]
- AVIDAN G, HASSON U, MALACH R, BEHRMANN M. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Functional neuroimaging findings. J Cogn Neurosci. 2005;17(7):1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- AVIDAN G, TANZER M, HADJ-BOUZIANE F, LIU N, UNGERLEIDER LG, BEHRMANN M. Selective dissociation between core and extended regions of the face processing network in congenital prosopagnosia. Cereb Cortex. 2014b;24(6):1565–78. doi: 10.1093/cercor/bht007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON-COHEN S, WHEELWRIGHT S, SKINNER R, MARTIN J, CLUBLEY E. The autism-spectrum quotient (aq): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- BARRASH J. A historical review of topographical disorientation and its neuroanatomical correlates. J Clin Exp Neuropsychol. 1998;20(6):807–27. doi: 10.1076/jcen.20.6.807.1114. [DOI] [PubMed] [Google Scholar]
- BARRASH J, DAMASIO H, ADOLPHS R, TRANEL D. The neuroanatomical correlates of route learning impairment. Neuropsychologia. 2000;38(6):820–36. doi: 10.1016/s0028-3932(99)00131-1. [DOI] [PubMed] [Google Scholar]
- BARTON J. Structure and function in acquired prosospagnosia: Lessons from a series of ten patients with brain damage. J Neuropsychology. 2008;2:197–225. doi: 10.1348/174866407x214172. [DOI] [PubMed] [Google Scholar]
- BARTON J, CHERKASOVA M, O'CONNOR M. Covert recognition in acquired and developmental prosopagnosia. Neurology. 2001;57:1161–7. doi: 10.1212/wnl.57.7.1161. [DOI] [PubMed] [Google Scholar]
- BARTON J, CHERKASOVA M, PRESS D, INTRILIGATOR J, O’CONNOR M. Developmental prosopagnosia: A study of three patients. Brain and Cognition. 2003;51:12–30. doi: 10.1016/s0278-2626(02)00516-x. [DOI] [PubMed] [Google Scholar]
- BARTON J, PRESS D, KEENAN J, O'CONNOR M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58:71–8. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- BATTAGLINI M, JENKINSON M, DE STEFANO N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012;33(9):2062–71. doi: 10.1002/hbm.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER R. Autonomic recognition of names and faces in prosopagnosia: A neuropsychological application of the guilty knowledge test. Neuropsychologia. 1984;22:457–69. doi: 10.1016/0028-3932(84)90040-x. [DOI] [PubMed] [Google Scholar]
- BEHRMANN M, AVIDAN G, GAO F, BLACK S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex. 2007 doi: 10.1093/cercor/bhl144. epub. [DOI] [PubMed] [Google Scholar]
- BEHRMANN M, AVIDAN G, MAROTTA JJ, KIMCHI R. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. J Cogn Neurosci. 2005;17(7):1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- BENTIN S, DEOUELL L, SOROKER N. Selective visual streaming in face recognition: Evidence from developmental prosopagnosia. Neuroreport. 1999;10:823–7. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- BENTON A. Facial recognition 1990. Cortex. 1990;26:491–99. doi: 10.1016/s0010-9452(13)80299-7. [DOI] [PubMed] [Google Scholar]
- BETTENCOURT KC, XU Y. The role of transverse occipital sulcus in scene perception and its relationship to object individuation in inferior intraparietal sulcus. J Cogn Neurosci. 2013;25(10):1711–22. doi: 10.1162/jocn_a_00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNYE TT, MAHONEY CR, TAYLOR HA. Paths with more turns are perceived as longer: Misperceptions with map-based and abstracted path stimuli. Percept Mot Skills. 2015;120(2):438–61. doi: 10.2466/22.PMS.120v11x2. [DOI] [PubMed] [Google Scholar]
- BUSIGNY T, PAGES B, BARBEAU EJ, BLED C, MONTAUT E, RAPOSO N, ALBUCHER JF, CHOLLET F, PARIENTE J. A systematic study of topographical memory and posterior cerebral artery infarctions. Neurology. 2014;83(11):996–1003. doi: 10.1212/WNL.0000000000000780. [DOI] [PubMed] [Google Scholar]
- BUTTERS N, SOELDNER C, FEDIO P. Comparison of parietal and frontal lobe spatial deficits in man: Extrapersonal vs personal (egocentric) space. Percept Mot Skills. 1972;34(1):27–34. doi: 10.2466/pms.1972.34.1.27. [DOI] [PubMed] [Google Scholar]
- CLARKE S, LINDEMANN A, MAEDER P, BORRUAT F-X, ASSAL G. Face recognition and postero-inferior hemispheric lesions. Neuropsychologia. 1997;35:1555–63. doi: 10.1016/s0028-3932(97)00083-3. [DOI] [PubMed] [Google Scholar]
- DAMASIO A, TRANEL D, DAMASIO H. Face agnosia and the neural substrates of memory. Ann Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- DAMASIO AR, DAMASIO H, VAN HOESSEN GW. Prosopagnosia: Anatomic basis and behavioral mechanisms. Neurology. 1982;32:331–41. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- DAVIES-THOMPSON J, PANCAROGLU R, BARTON J. Acquired prosopagnosia: Structural basis and processing impairments. Front Biosci (Elite Ed) 2014;6:159–74. doi: 10.2741/e699. [DOI] [PubMed] [Google Scholar]
- DE RENZI E. Wiley; Chichester: 1982. Disorders of splace exploration and cognition. [Google Scholar]
- DE RENZI E, FAGLIONI P, VILLA P. Topographical amnesia. J Neurol Neurosurg Psychiatry. 1977;40(5):498–505. doi: 10.1136/jnnp.40.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLA SALA S, YOUNG AW. Quaglino's 1867 case of prosopagnosia. Cortex. 2003;39(3):533–40. doi: 10.1016/s0010-9452(08)70263-6. [DOI] [PubMed] [Google Scholar]
- DILKS DD, JULIAN JB, PAUNOV AM, KANWISHER N. The occipital place area is causally and selectively involved in scene perception. J Neurosci. 2013;33(4):1331–6a. doi: 10.1523/JNEUROSCI.4081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINKELACKER V, GRUTER M, KLAVER P, GRUTER T, SPECHT K, WEIS S, KENNERKNECHT I, ELGER CE, FERNANDEZ G. Congenital prosopagnosia: Multistage anatomical and functional deficits in face processing circuitry. J Neurol. 2011;258(5):770–82. doi: 10.1007/s00415-010-5828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCHAINE B, NAKAYAMA K. Dissociations of face and object recognition in developmental prosopagnosia. J Cogn Neurosci. 2005;17:249–61. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- DUCHAINE BC. Developmental prosopagnosia with normal configural processing. Neuroreport. 2000;11(1):79–83. doi: 10.1097/00001756-200001170-00016. [DOI] [PubMed] [Google Scholar]
- DUCHAINE BC, NAKAYAMA K. The cambridge face memory test: Results for neurologically intact individuals and an investigation of its validityt using inverted face stimuli and prosopagnosic patients. Neuropsychologia. 2006a;44:576–85. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- DUCHAINE BC, NAKAYAMA K. Developmental prosopagnosia: A window to content-specific face processing. Curr Opin Neurobiol. 2006b;16(2):166–73. doi: 10.1016/j.conb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- DUCHAINE BC, PARKER H, NAKAYAMA K. Normal recognition of emotion in a prosopagnosic. Perception. 2003;32(7):827–38. doi: 10.1068/p5067. [DOI] [PubMed] [Google Scholar]
- DUCHAINE BC, YOVEL G, BUTTERWORTH EJ, NAKAYAMA K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cogn Neuropsychol. 2006c;23(5):714–47. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- EPSTEIN R, DEYOE EA, PRESS DZ, ROSEN AC, KANWISHER N. Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cogn Neuropsychol. 2001;18(6):481–508. doi: 10.1080/02643290125929. [DOI] [PubMed] [Google Scholar]
- EPSTEIN R, HARRIS A, STANLEY D, KANWISHER N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23(1):115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- EPSTEIN R, KANWISHER N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- EPSTEIN RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12(10):388–96. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS J, HEGGS A, ANTOUN N, HODGES J. Progressive prosopagnosia associated with selective right temporal lobe atrophy. Brain. 1995;118:1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- FOX CJ, IARIA G, BARTON JJ. Defining the face processing network: Optimization of the functional localizer in fmri. Hum Brain Mapp. 2009;30:1637–51. doi: 10.1002/hbm.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURL N, GARRIDO L, DOLAN RJ, DRIVER J, DUCHAINE B. Fusiform gyrus face selectivity relates to individual differences in facial recognition ability. J Cogn Neurosci. 2011;23(7):1723–40. doi: 10.1162/jocn.2010.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANADEN RE, MULLIN CR, STEEVES JK. Transcranial magnetic stimulation to the transverse occipital sulcus affects scene but not object processing. J Cogn Neurosci. 2013;25(6):961–8. doi: 10.1162/jocn_a_00372. [DOI] [PubMed] [Google Scholar]
- GARRIDO L, FURL N, DRAGANSKI B, WEISKOPF N, STEVENS J, TAN GC, DRIVER J, DOLAN RJ, DUCHAINE B. Voxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosics. Brain. 2009;1321:3443–55. doi: 10.1093/brain/awp271. Pt 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD M, ADAIR JC, JACOBS DH, HEILMAN KM. Right-left confusion in gerstmann's syndrome: A model of body centered spatial orientation. Cortex. 1995;31(2):267–83. doi: 10.1016/s0010-9452(13)80362-0. [DOI] [PubMed] [Google Scholar]
- GOMEZ J, PESTILLI F, WITTHOFT N, GOLARAI G, LIBERMAN A, POLTORATSKI S, YOON J, GRILL-SPECTOR K. Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron. 2015;85(1):216–27. doi: 10.1016/j.neuron.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUETER M, GRUETER T, BELL V, HORST J, LASKOWSKI W, SPERLING K, HALLIGAN PW, ELLIS HD, KENNERKNECHT I. Hereditary prosopagnosia: The first case series. Cortex. 2007;43(6):734–49. doi: 10.1016/s0010-9452(08)70502-1. [DOI] [PubMed] [Google Scholar]
- HAXBY JV, HOFFMAN EA, GOBBINI MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- HECAEN H, ANGELERGUES R. Agnosia for faces (prosopagnosia) Arch Neurol. 1962;7:92–100. doi: 10.1001/archneur.1962.04210020014002. [DOI] [PubMed] [Google Scholar]
- HEGARTY M, RICHARDSON AE, MONTELLO DR, LOVELACE K, SUBBIAH I. Development of a self-report measure of environmental spatial ability. Intelligence. 2002;30:425–447. [Google Scholar]
- HENKE K, SCHWEINBERGER S, GRIGO A, KLOS T, SOMMER W. Specificity of face recognition: Recognition of exemplars of non-face objects in prosopagnosia. Cortex. 1998;34:289–96. doi: 10.1016/s0010-9452(08)70756-1. [DOI] [PubMed] [Google Scholar]
- IARIA G, BARTON JJ. Developmental topographical disorientation: A newly discovered cognitive disorder. Exp Brain Res. 2010;206(2):189–96. doi: 10.1007/s00221-010-2256-9. [DOI] [PubMed] [Google Scholar]
- IARIA G, BOGOD N, FOX CJ, BARTON JJ. Developmental topographical disorientation: Case one. Neuropsychologia. 2009;47(1):30–40. doi: 10.1016/j.neuropsychologia.2008.08.021. [DOI] [PubMed] [Google Scholar]
- IARIA G, CHEN JK, GUARIGLIA C, PTITO A, PETRIDES M. Retrosplenial and hippocampal brain regions in human navigation: Complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25(3):890–9. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- IARIA G, INCOCCIA C, PICCARDI L, NICO D, SABATINI U, GUARIGLIA C. Lack of orientation due to a congenital brain malformation: A case study. Neurocase. 2005;11(6):463–74. doi: 10.1080/13554790500423602. [DOI] [PubMed] [Google Scholar]
- JENKINSON M, BANNISTER P, BRADY M, SMITH S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- JENKINSON M, SMITH S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- JONES RD, TRANEL D. Severe developmental prosopagnosia in a child with superior intellect. J Clin Exp Neuropsychol. 2001;23(3):265–73. doi: 10.1076/jcen.23.3.265.1183. [DOI] [PubMed] [Google Scholar]
- KANWISHER N, MCDERMOTT J, CHUN M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRESS T, DAUM I. Developmental prosopagnosia: A review. Behav Neurol. 2003;14(3-4):109–21. doi: 10.1155/2003/520476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURYLO DD, CORKIN S, RIZZO JF, GROWDON JH. Greater relative impairment of object recognition than of visuaospatial abilitieis in alzheimer's disease. Neuropsychology. 1996;10(1):74–81. [Google Scholar]
- LANDIS T, CUMMINGS JL, BENSON DF, PALMER EP. Loss of topographic familiarity. An environmental agnosia. Arch Neurol. 1986;43(2):132–6. doi: 10.1001/archneur.1986.00520020026011. [DOI] [PubMed] [Google Scholar]
- LIU I, LEVY RM, BARTON JJ, IARIA G. Age and gender differences in various topographical orientation strategies. Brain Res. 2011;1410:112–9. doi: 10.1016/j.brainres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- LIU RR, PANCAROGLU R, HILLS CS, DUCHAINE B, BARTON JJ. Voice recognition in face-blind patients. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOBMAIER JS, BOLTE J, MAST FW, DOBEL C. Configural and featural processing in humans with congenital prosopagnosia. Adv Cogn Psychol. 2010;6:23–34. doi: 10.2478/v10053-008-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACMILLAN N, CREELMAN C. Cambridge University Press; Cambridge: 1991. Detection theory: A user's guide. [Google Scholar]
- MALONE D, MORRIS H, KAY M, LEVIN H. Prosopagnosia: A double dissociation between recognition of familiar and unfamiliar faces. J Neurol Neurosurg Psychiatry. 1982;45:802–2. doi: 10.1136/jnnp.45.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINAUD O, POULIQUEN D, GERARDIN E, LOUBEYRE M, HIRSBEIN D, HANNEQUIN D, COHEN L. Visual agnosia and posterior cerebral artery infarcts: An anatomical-clinical study. PLoS ONE. 2012;7(1):e30433. doi: 10.1371/journal.pone.0030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINS I, CUNHA E, SA M. Loss of topographic memory and prosopagnosia during migraine aura. Cephalalgia. 1999;19:841–3. doi: 10.1046/j.1468-2982.1999.1909841.x. [DOI] [PubMed] [Google Scholar]
- MCCARTHY R, EVANS J, HODGES J. Topographic amnesia: Spatial memory disorder, perceptual dysfunction, or category specific semantic memory impairment? J Neurol Neurosurg Psychiatry. 1996;60:318–25. doi: 10.1136/jnnp.60.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCONACHIE H. Developmental prosopagnosia, a single case report. Cortex. 1976;12:76–82. doi: 10.1016/s0010-9452(76)80033-0. [DOI] [PubMed] [Google Scholar]
- MEADOWS JC. The anatomical basis of prosopagnosia. J Neurol Neurosurg Psychiatry. 1974;37:489–501. doi: 10.1136/jnnp.37.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDEZ MF, CHERRIER MM. Agnosia for scenes in topographagnosia. Neuropsychologia. 2003;41(10):1387–95. doi: 10.1016/s0028-3932(03)00041-1. [DOI] [PubMed] [Google Scholar]
- MICHELON P, BIEDERMAN I. Less impairment in face imagery than face perception in early prosopagnosia. Neuropsychologia. 2003;41(4):421–41. doi: 10.1016/s0028-3932(02)00176-8. [DOI] [PubMed] [Google Scholar]
- MONEY J, ALEXANDER D, WALKER HT. The Johns Hopkins press; Baltimore: 1965. A standardized road map test of direction sense. [Google Scholar]
- NUNN JA, POSTMA P, PEARSON R. Developmental prosopagnosia: Should it be taken at face value? Neurocase. 2001;7(1):15–27. doi: 10.1093/neucas/7.1.15. [DOI] [PubMed] [Google Scholar]
- O'BRIEN HL, TETEWSKY SJ, AVERY LM, CUSHMAN LA, MAKOUS W, DUFFY CJ. Visual mechanisms of spatial disorientation in alzheimer's disease. Cereb Cortex. 2001;11(11):1083–92. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- O'CRAVEN KM, KANWISHER N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci. 2000;12(6):1013–23. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- PAI MC. Topographic disorientation: Two cases. J Formos Med Assoc. 1997;96(8):660–3. [PubMed] [Google Scholar]
- QUAGLINO A, BORELLI G. Emiplegia sinistra con amaurosi - guarigone - perdita totale della percezione dei colori e della memoria della configurazione degli oggetti. Giornale d'Oftalmologia Italiano. 1867;10:106–117. [Google Scholar]
- RAINVILLE C, MARCHAND N, PASSINI R. Performances of patients with a dementia of the alzheimer type in the standardized road-map test of direction sense. Neuropsychologia. 2002;40(5):567–73. doi: 10.1016/s0028-3932(01)00133-6. [DOI] [PubMed] [Google Scholar]
- SCHMIDT D. Neuro-ophthalmological findings in patients with acquired prosopagnosia. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):333–4. doi: 10.1007/s00417-014-2890-1. [DOI] [PubMed] [Google Scholar]
- STOLLHOFF R, JOST J, ELZE T, KENNERKNECHT I. Deficits in long-term recognition memory reveal dissociated subtypes in congenital prosopagnosia. PLoS ONE. 2011;6(1):e15702. doi: 10.1371/journal.pone.0015702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSILO T, DUCHAINE B. Advances in developmental prosopagnosia research. Curr Opin Neurobiol. 2013;23(3):423–9. doi: 10.1016/j.conb.2012.12.011. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI N, KAWAMURA M. Pure topographical disorientation--the anatomical basis of landmark agnosia. Cortex. 2002;38(5):717–25. doi: 10.1016/s0010-9452(08)70039-x. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI N, KAWAMURA M, HIRAYAMA K, SHIOTA J, ISONO O. Prosopagnosia: A clinical and anatomic study of four patients. Cortex. 1995;31:317–29. doi: 10.1016/s0010-9452(13)80365-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI N, KAWAMURA M, SHIOTA J, KASAHATA N, HIRAYAMA K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49(2):464–9. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- TEMPLE C. Developmental memory impairment: Faces and patterns. In: Campbell R, editor. Mental lives. Case studies in cognition. Blackwell; Oxford, UK: 1992. pp. 199–215. [Google Scholar]
- TURANO KA, MUNOZ B, HASSAN SE, DUNCAN DD, GOWER EW, ROCHE KB, KEAY L, MUNRO CA, WEST SK. Poor sense of direction is associated with constricted driving space in older drivers. J Gerontol B Psychol Sci Soc Sci. 2009;64(3):348–55. doi: 10.1093/geronb/gbp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTNER I, BLIEM H, DANEK A. Prosopagnosia after unilateral right cerebral infarction. J Neurol. 2002;249(7):933–5. doi: 10.1007/s00415-002-0710-8. [DOI] [PubMed] [Google Scholar]
- WARRINGTON E. Warrington recognition memory test. Western Psychological Services; Los Angeles: 1984. [Google Scholar]
- WEINER KS, GOLARAI G, CASPERS J, CHUAPOCO MR, MOHLBERG H, ZILLES K, AMUNTS K, GRILL-SPECTOR K. The mid-fusiform sulcus: A landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage. 2014;84:453–65. doi: 10.1016/j.neuroimage.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIENER JM, BÜCHER SJ, HÖLSCHER C. Taxonomy of human wayfinding tasks: A knowledge-based approach. Spat Cog Comp. 2009;9:152–165. [Google Scholar]
- WOOLRICH MW, JBABDI S, PATENAUDE B, CHAPPELL M, MAKNI S, BEHRENS T, BECKMANNC, JENKINSON M, SMITH SM. Bayesian analysis of neuroimaging data in fsl. Neuroimage. 2009;45(1 Suppl):S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- WORSLEY CL, RECCE M, SPIERS HJ, MARLEY J, POLKEY CE, MORRIS RG. Path integration following temporal lobectomy in humans. Neuropsychologia. 2001;39(5):452–64. doi: 10.1016/s0028-3932(00)00140-8. [DOI] [PubMed] [Google Scholar]
- YOUNG A, ELLIS H. Childhood prosopagnosia. Brain Cogn. 1989;9:16–47. doi: 10.1016/0278-2626(89)90042-0. [DOI] [PubMed] [Google Scholar]
- ZHANG J, LIU J, XU Y. Neural decoding reveals impaired face configural processing in the right fusiform face area of individuals with developmental prosopagnosia. J Neurosci. 2015;35(4):1539–48. doi: 10.1523/JNEUROSCI.2646-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]