Abstract
Background
The aversive properties of ethanol that limit its intake are poorly understood. There is increasing interest in the role of the rostromedial tegmental nucleus (RMTg), because it encodes aversion signals and inhibits motivated behaviors. It is also a major source of inhibitory GABAergic inputs to the midbrain dopamine neurons. Up to this time, the role of the RMTg in ethanol drinking behaviors has not been well explored.
Methods
Male Long-Evans rats were trained either to drink ethanol under the intermittent two bottle choice protocol or to self-administer ethanol in operant chambers under fixed-ratio-3 schedules. Changes in drinking behaviors induced by the bilateral infusion into the RMTg of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), an agonist of AMPA-type glutamate receptors, or muscimol, an agonist of GABAA receptors were measured.
Results
Consumption and preference for ethanol, numbers of active lever pressing, and head entrance to the ethanol port, were all significantly decreased upon activation of the RMTg by the infusion of AMPA, but were increased upon inhibition of the RMTg by the infusion of muscimol. By contrast, intra-RMTg infusion of these agents did not change sucrose consumption.
Conclusions
This data shows for the first time that ethanol drinking and seeking behaviors of rats changed inversely with RMTg function, supporting the idea that the RMTg plays a crucial role in ethanol drinking behaviors.
Keywords: AMPA, muscimol, stereotaxic surgery, microinjection
INTRODUCTION
The neurobiological mechanism underlying alcohol use disorders (AUDs) remains unclear. Like many other drugs of abuse, alcohol can be aversive as well as rewarding. While the rewarding properties promote, the aversive properties limit ethanol intake (Verendeev and Riley, 2013). Ethanol’s rewarding properties may be associated with its ability to increase the activity of dopamine neurons in the ventral tegmental area (VTA) and the release of dopamine in their target areas, such as the nucleus accumbens (Thomas et al., 2001, Nicola and Malenka, 1997, Di Chiara, 1998). Ethanol activates many signaling systems within diverse brain regions, which could contribute to its rewarding effects. Relatively little is known about the mechanisms that mediate ethanol-related aversion, although recent evidence has linked aversion to synaptic inhibition of VTA-dopamine neurons (Tan et al., 2012, van Zessen et al., 2012). The mesopontine rostromedial tegmental nucleus (RMTg), also named tail of the VTA (Kaufling et al., 2009, Kaufling et al., 2010, Perrotti et al., 2005) is a recently identified structure that contains primarily γ-aminobutyric acid (GABA) neurons, which are a major source of inhibitory GABAergic input to midbrain dopamine neurons in the substantia nigra pars compacta and the VTA (Jhou et al., 2009a, Kaufling et al., 2010).
RMTg neurons are activated by aversive events (Jhou et al., 2009a, Lecca et al., 2012), including foot shocks, shock-predictive cues, food deprivation, or reward omission (Barrot et al., 2012, Jhou et al., 2009a, Hong et al., 2011). The RMTg receives a strong glutamate input from the lateral habenula (LHb) (Jhou et al., 2009a, Kaufling et al., 2009, Balcita-Pedicino et al., 2011, Stamatakis and Stuber, 2012). LHb neurons are activated by aversive stimuli (Lecourtier and Kelly, 2007, Matsumoto and Hikosaka, 2009, Hikosaka, 2010, Stamatakis and Stuber, 2012) and convey anti-reward and aversive information.
Activation of the LHb-RMTg neural circuit disrupts positive reinforcement (Stamatakis and Stuber, 2012), and a LHb lesion increases voluntary ethanol consumption (Haack et al., 2014). Thus, the RMTg might contribute importantly to the aversive properties of ethanol. In an attempt to test this hypothesis in the current study, the RMTg function was manipulated by directly injecting into the RMTg either the glutamate receptor agonist AMPA, which increases the activity of RMTg, or the GABAA receptor agonist, muscimol, which inhibits the RMTg. Changes in ethanol self-administration in rats that were trained to drink ethanol either in the intermittent access to 20% ethanol two-bottle free choice drinking procedure or in the operant self-administration paradigm were then measured. Here we provide the first evidence that ethanol drinking and seeking behaviors of rats changed inversely with RMTg function, supporting the idea that the RMTg plays a crucial role in ethanol drinking behaviors possibly through the change in ethanol-related aversion.
MATERIALS AND METHODS
Animal handling
The Animal Care and Utilization Committee of Rutgers, the State University of New Jersey, in accordance with National Institutes of Health guidelines minimizing the number of animals used and their suffering, approved all procedures. All experiments were performed in male Long-Evans rats (250–350 g at the start of the experiments). The rats were individually housed. Food and water were available ad libitum unless indicated otherwise. The experimental groups, group sizes and treatment conditions throughout each experimental procedure are summarized in Table 1.
Table 1. Summary of experimental groups and timeline of procedures.
There were 10 rats in each of the six groups, and only 7–8 rats in each group had the correct cannula tip placements within the RMTg and were included in the data statistics.
| Rat group | Number of rats | Experiments |
|---|---|---|
| 1 | 10 (8) | Test effects of AMPA with intermittent access to 20% ethanol at 30 minute and 24 hour. |
| 2 | 10 (8) | Test effects of muscimol with intermittent access to 20% ethanol at 30 minute, 24 and 48 hour. |
| 3 | 10 (7) | Test effects of AMPA with intermittent access to 0.125% sucrose at 24 hour. |
| 4 | 10 (7) | Test effects of muscimol with intermittent access to 0.125% sucrose at 24 hour. |
| 5 | 10 (8) | Test effects of AMPA with operant ethanol self-administration during 30 minute. |
| 6 | 10 (8) | Test effects of muscimol with operant ethanol self-administration during 30 minute. |
Intermittent-access to 20% ethanol two-bottle free choice drinking procedure (I2BC)
We used the I2BC paradigm as described previously (Li et al., 2011). Briefly, after acclimating to the homecage environment, rats had 24-hour concurrent access to two bottles, one with 20% ethanol (v/v) and another with water, starting on Monday afternoon. After 24 hours, the ethanol bottle was replaced with a second water bottle that was available for the next 24 hours. This pattern was repeated on Wednesdays and Fridays. On all other days the rats had unlimited access to two bottles of water. In each ethanol drinking session, the placement of the ethanol bottle was alternated to control for side preferences. The amount of ethanol or water consumed was determined by weighing the bottles before access and after 24 hours of access. Ethanol consumption was determined by calculating grams of alcohol consumed per kilogram of body weight. Two bottles, one containing water and one containing 20% ethanol in a cage without rats, was used to evaluate the spillage due to experimental manipulations during the test sessions. The spillage was always < 1.0 ml (< 2.5% of the total fluid intake) during 24 hours. Body weight of all rats was recorded weekly. Rats under this paradigm escalated their ethanol intake and preference (Li et al., 2011).
Sucrose self-administration
A separate group of naïve rats were trained to drink 0.125% sucrose solution (wt/vol) under intermittent 2 bottle free choice drinking paradigm similar to that described above. Since a previous study reported that rats presented a ~50% preference to 0.125% sucrose as compared to water (Wallace et al., 2008), administration of such low concentration of sucrose could reduce the possible ceiling effect usually observed when a higher concentration of sucrose is used. In addition, the similar preference to 0.125% sucrose could well simulate the preference to 20% ethanol (vs. water) observed in Long-Evans rats that trained under the intermittent two bottle free choice drinking paradigm (Carnicella et al., 2014). These rats received intra-RMTg cannula implantation surgery when a stable baseline level of sucrose intake was reached after 6 drinking sessions. After one-week recovery from surgery, these rats resumed sucrose drinking until a stable level was reached again, then the influence of intra-RMTg injections of aCSF, AMPA or muscimol on the intake of and preference to sucrose in 24 hours was measured.
Operant self-administration after intermittent access to ethanol
The conditioning chambers were 30 cm wide and 29 cm high and contained within larger sound-attenuating chambers. Two levers were located against the right wall, 7 cm from the floor and 1 cm from the right or left edge of the right wall. A 2.5-cm white stimulus light was located above each lever. A rectangular recess (3 cm in diameter) was located between the two levers, 3 cm above the floor. Syringe pumps delivered fluid into a fluid receptacle within this recess (ethanol port). A house light, located on the right wall 14 cm from the floor, was on for the duration of each behavioral session. In addition, the operant chambers contained infrared head poke detectors that recorded how many times an animal’s head entered the ethanol reward port. All behavioral equipment (MED Associates, St. Albans, VT) was computer-controlled via software (MED Associates) that also recorded the responses and reinforcer deliveries during behavioral sessions. This experiment was conducted similarly to that described previously (Li et al., 2012, Seif et al., 2013). Briefly, an independent group of rats under the I2BC paradigm for 16–20 sessions received three overnight (12–14 h) sessions with 0.1 ml of 20% ethanol available on a fixed-ratio 1 (FR1) schedule after responses to the active lever. After shaping, subjects began daily 45-min sessions, 5 days a week. One week later, the response requirement was increased to a FR3 schedule in 30-min sessions for two weeks. After 6 drinking sessions, when a stable baseline level of drinking was reached, these rats received cannula implantation into the RMTg under stereotaxic surgery. One week after recovery from surgery, they resumed operant self-administration of ethanol. After 3 weeks of responding for 20% ethanol on a FR3 schedule, drug testing began. The calculation for ethanol consumption was based on previous studies (June and Gilpin, 2010):
Implantation of cannulae
Stereotaxic surgery was performed on rats as described (Li et al., 2012). Briefly, a bilateral guide cannula (1.2 mm width, 26 gauge, Plastics One, Wallingford, CT, USA) was inserted dorsally into the RMTg (A/P: −7.4 mm from Bregma and D/V: −7.5 mm from skull surface) based on previous work (Kaufling et al., 2009, Jhou et al., 2009b, Huff and LaLumiere, 2015). Before microinjection, animals were taken from the colony, brought to the experimental room, and handled for 5 min per day until the experimental day. During this phase, animals became accustomed to the experimenter, the experimental room and the manipulation procedure, with a total of four to five sessions to decrease the stress of and habituate the subjects to the microinjection procedures.
At the end of the behavior tests, animals were sacrificed and the brains were dissected. Nissl-staining was used to verify the coordination of the cannula tip. Rats with injection sites outside the RMTg were excluded from further analysis.
Microinjection procedure
Drugs, including the AMPA-type glutamate receptor specific agonist AMPA (16.8 ng/300 nl/side, 0.3 mM) or the GABAA receptor agonist muscimol (18.5 ng/125 nl/side, 1.3 mM) or vehicle (artificial cerebral spinal fluid, aCSF) were infused through a 28-gauge internal cannula (Plastics One) connected to a Hamilton 1.0 μl syringe driven by a syringe pump (Harvard Instruments, South Natick, MA). Rats in the operant-self-administration experiment received each of the three treatments (aCSF, AMPA, muscimol) in a counterbalanced order using a Latin square design. There was a minimum of 7 days between successive drug tests. We selected the doses of AMPA and muscimol based on previous work on the RMTg (Lavezzi et al., 2015, Jhou et al., 2012, Jhou et al., 2013).
On the test days, microinjections were given approximately 10 min before access to ethanol. Obstructers were gently removed and injectors were inserted bilaterally to a depth of 1 mm beyond the end of the guide cannulae. Vehicle (aCSF) control or drug was infused over 1 min into the RMTg of gently restrained rats via the internal cannulae. The injectors were left in place for an additional 60 s to allow for diffusion. After removal of the injector, a new sterile obstructer was inserted. After 10 min, rats were placed in homecage or operant chamber and the ethanol drinking session began.
The mean body weight was 200±10 g at the start of the experiments when rats were approximately 2 months old, and was 475±13 g at the first drug test session when rats were approximately 3 and 1/2 month old.
Chemicals and application
We purchased AMPA, muscimol and other common salts from Sigma-Aldrich Corporation, and ethanol from Pharmco Products (Brookfield, CT). AMPA and muscimol were dissolved in sterile aCSF at a stock concentration of 1 μg/μl and 5 μg/μl, respectively.
Statistical analysis
All data were expressed as mean ± S.E.M. (standard error of the mean). The baseline ethanol drinking data was measured at 30 minutes and at 24 hours after the start of a drinking session, over 3 consecutive sessions before intra-RMTg infusion. Ethanol or sucrose drinking data after drug treatment (AMPA or muscimol) were compared to the baseline and/or to aCSF injection using a One-way repeated-measures analysis of variance (RM ANOVA) to extract the significant main effects followed by post hoc comparisons using the Bonferroni t-test. Lever pressing for ethanol self-administration in the operant chamber was analyzed using a Two-way RM ANOVA on lever type (active versus inactive) and drug treatment. The number of active lever press within a 30-minute session was analyzed by a Two-way RM ANOVA on time and drug effect. The ethanol consumption and ethanol port head entry data were analyzed with a One-way RM ANOVA to extract the significant main effect of drug followed by post hoc analysis using Bonferroni, Student-Newman-Keuls or Fisher LSD method. Statistical significance was declared at p < 0.05.
RESULTS
Intra-RMTg injection of AMPA decreases voluntary ethanol consumption in rats
Long–Evans rats in the intermittent-access to 20% ethanol 2-bottle-choice drinking paradigm escalated their ethanol consumption, and reached a stable baseline consumption of 4.7±0.5g/kg/day, with 53.5±4.6% preference to ethanol in ~ 2 months (data not shown), in keeping with previous reports (Simms et al., 2008, Li et al., 2011).
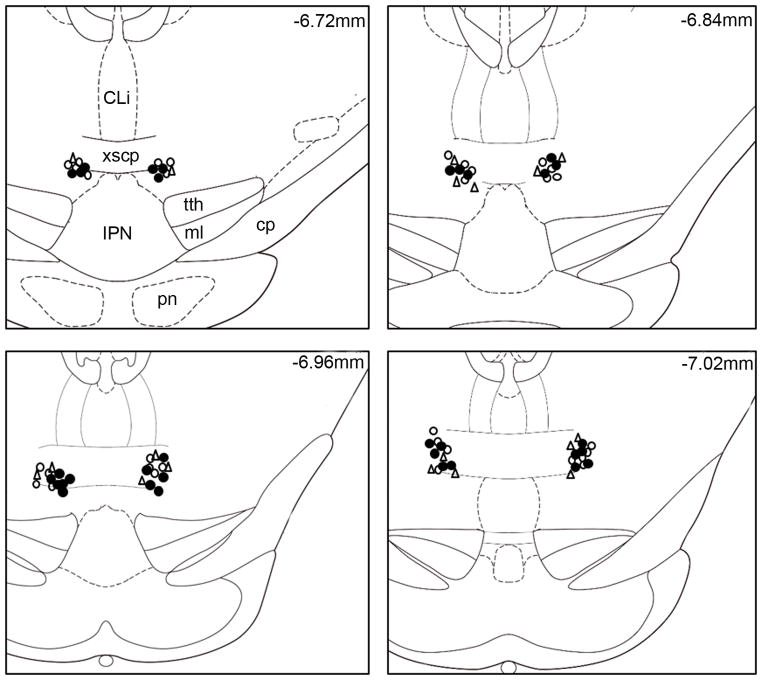
To examine the effect of activation of RMTg on ethanol consumption, we injected AMPA, an agonist of AMPA-type glutamate receptors, bilaterally into the RMTg 10 minutes before the start of an ethanol drinking session. Histological verification revealed correct cannula placement in 8 of the 10 rats (Fig. 1). Two rats in this experiment showing cannula placements outside of the RMTg were excluded from statistical analysis.
Fig. 1.
Schematic drawings of coronal sections of the rat brain showing the tip of injector placements from individual rats with accurate bilateral placements in the RMTg. Solid circles represent injector sites of animals that were under the voluntary ethanol drinking paradigm; blank triangles represent injector sites of animals that were under the voluntary sucrose drinking paradigm; blank circles represent injector sites of animals that were in operant self-administration of ethanol (adapted from (Paxinos and Watson, 2007).
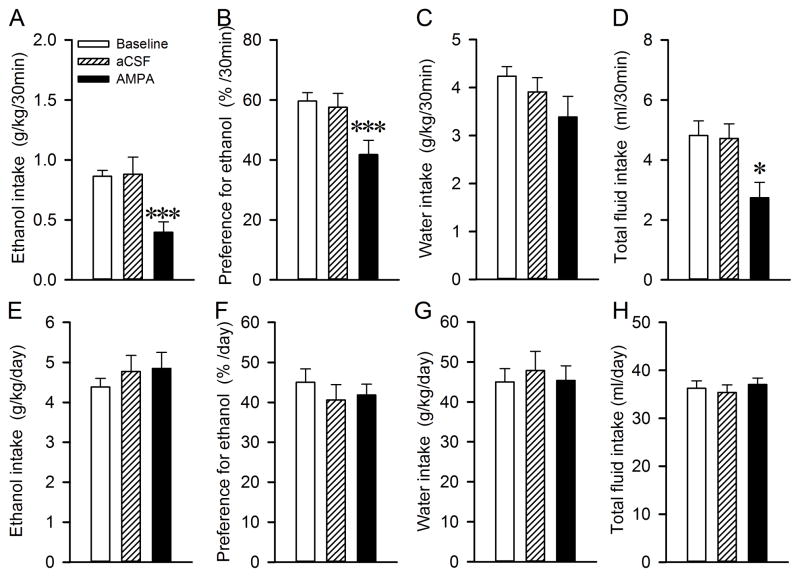
During the initial 30 minutes, there was an overall main effect of AMPA injection (F(2,14)=18.7, p<0.001). Post hoc analysis revealed that intra-RMTg AMPA injection substantially lowered ethanol intake compared with either baseline or aCSF-treated group (baseline vs. AMPA, t=5.2, p<0.001; aCSF vs. AMPA, t=5.4, p<0.001; Fig. 2A). There was no significant difference between baseline and aCSF injection, indicating that intra-RMTg injection of aCSF (300 nl) did not affect ethanol consumption. In addition, intra-RMTg AMPA injection significantly decreased preference for ethanol (F(2,14)=13.1, p<0.001) and the total fluid intake (F(2,14)=6.4, p=0.011), but not water intake (F(2,14)=1.7, p=0.213). Post hoc analysis revealed AMPA significantly decreased preference for ethanol (baseline vs. AMPA, t=4.7, p<0.001; aCSF vs. AMPA, t=4.1, p<0.001; Fig. 2B) and total fluid intake (baseline vs. AMPA, t=3.2, p=0.02; aCSF vs. AMPA, t=3.0, p=0.027; Fig. 2D). At 24 h, AMPA did not significantly alter ethanol intake, ethanol preference, water intake and total fluid intake (all p>0.05, Fig. 2E–H).
Fig. 2.
Intra-RMTg AMPA injection significantly decreases ethanol consumption and preference. Bilateral intra-RMTg AMPA (16.8 ng/300 nl/side, 0.3 mM) injection significantly decreased ethanol consumption (A), preference for ethanol (B) and total fluid intake (D), but not the water intake (C) measured 30 minutes after the onset of the ethanol drinking session, compared to Baseline or aCSF treatment group. Conversely, there was no significant change in ethanol intake (F), preference for ethanol (G), water intake (H) and total fluid intake (I) measured 24 h after the onset of ethanol drinking. The values are mean ethanol ± SEM (One-way RM ANOVA by Bonferroni post hoc test). * p < 0.05; *** p < 0.001 significant difference compared with Baseline or aCSF injection, n=8.
Intra-RMTg injection of muscimol increases voluntary ethanol consumption
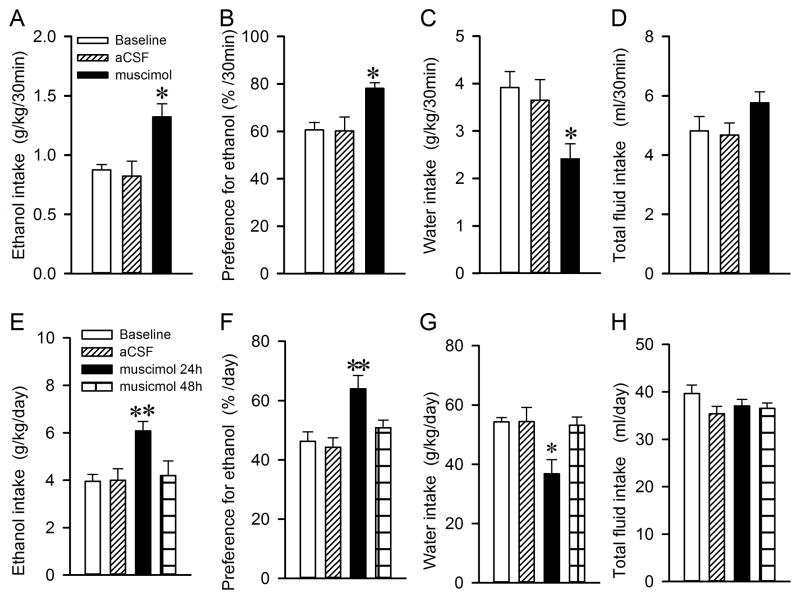
To examine the effect of RMTg inhibition on voluntary ethanol consumption, we injected muscimol, a GABAA receptor agonist bilaterally into the RMTg 10 minutes before the start of the drinking session. Within the initial 30 minutes of the drinking session, there was an overall main effect of muscimol injection (F(2,14)=7.2, p=0.007). Post hoc analysis revealed that intra-RMTg muscimol injection significantly increased ethanol intake (baseline vs. muscimol, t=3.1, p=0.024; aCSF vs. muscimol, t=3.4, p=0.012; Fig. 3A). There was no significant difference between baseline and aCSF injections, indicating that intra-RMTg injection of aCSF (125 nl) did not affect ethanol consumption. Additionally, intra-RMTg muscimol had significant main effects on ethanol preference (F(2,14)=5.264, p=0.02) and water intake (F(2,14)=5.853, p=0.014), without changing the total fluid intake (F(2,14)=2.671, p=0.104). Post hoc analysis found that muscimol significantly elevated ethanol preference (baseline vs. muscimol, t=32.7, p=0.045; aCSF vs. muscimol, t=2.8, p=0.039; Fig. 3B) and decreased water intake (g/kg/30min) (baseline vs. muscimol, t=32.7, p=0.04; aCSF vs. muscimol, t=3.1, p=0.032; Fig. 3C).
Fig. 3.
Intra-RMTg muscimol injection significantly increases ethanol consumption and preference. Muscimol (18.5 ng/125 nl/side, 1.3 mM) significantly increased ethanol consumption (A and E) and preference (B and F), but decreased water intake (C and G), without changing the total fluid intake (D and H) at either 30 min or 24 h after the onset of the ethanol drinking session. Muscimol did not alter ethanol intake 48 h after the onset of ethanol drinking. Values are mean ± S.E.M., (One-way repeat measure ANOVA followed by bonferroni post hoc test; n=8 for 30 min and 24h test). * p < 0.05 and ** p < 0.01 vs. Baseline, aCSF or 48 hours.
After 24 h access to ethanol, there was an overall main effect of muscimol injection compared with the baseline, aCSF, muscimol and at 48 h after the start of ethanol access (F(3, 21) = 6.132, p=0.004). Intra-RMTg muscimol injection also had significant main effects on ethanol preference (F(3, 21) = 16.001, p<0.001) and water intake (F(3, 21) = 4.727, p=0.011), but did not significantly change the total fluid intake (F(3, 21) = 2.03, p=0.14). Post hoc analysis revealed that muscimol significantly elevated ethanol intake (Baseline vs. muscimol, t=3.6, p=0.009; aCSF vs. muscimol, t=3.6, p=0.01; 48h vs. muscimol, t=3.2, p=0.004; Fig. 3E), ethanol preference (Baseline vs. muscimol, t=4.2, p=0.002; aCSF vs. muscimol, t=3.8, p=0.006; 48h vs. muscimol, t=3.7, p=0.004; Fig. 3F) and decreased water intake (Baseline vs. muscimol, t=3.1, p=0.029; aCSF vs. muscimol, t=3.1, p=0.031; 48h vs. muscimol, t=3.02, p=0.03; Fig. 3G).
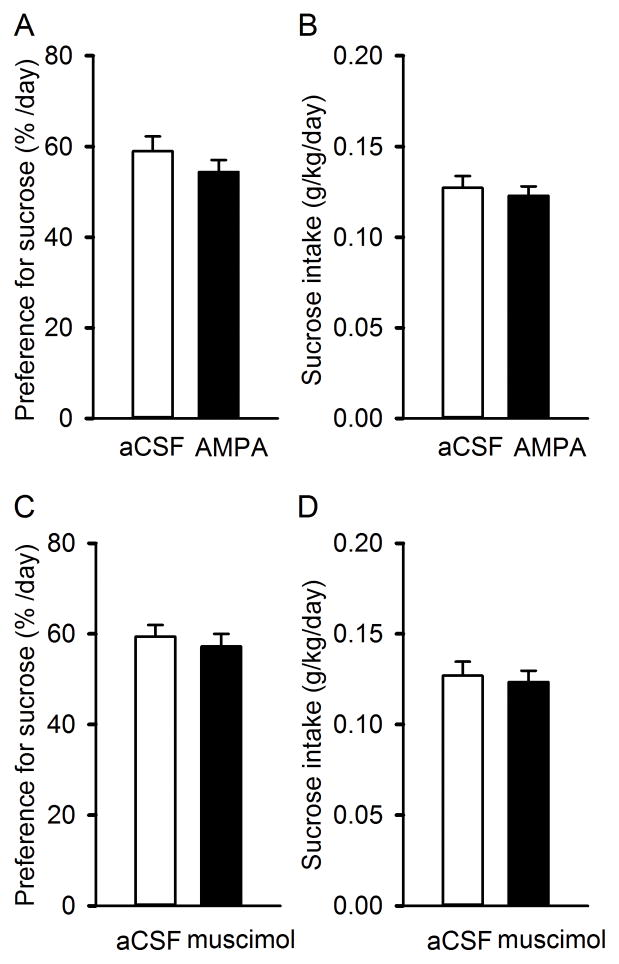
Next, to determine whether the alteration in ethanol self-administration by modulating RMTg function is specific to ethanol, the intake of and the preference to 0.125% sucrose was measured using intermittent two-bottle free choice paradigm as described in the Method section. Histological verification showed that only seven rats in each group had the correct cannula tip placements in the RMTg (Fig. 1). As shown in Figure 4, intra-RMTg AMPA did not significantly alter either the intake of (F(1, 6) = 0.251; p=0.634) or the preference for sucrose solution (F(1, 6) = 1.04; p=0.347), compared to aCSF respectively (all p > 0.05). Similarly, intra-RMTg muscimol did not significantly change either the intake of (F(1, 6) = 0.808; p=0.479) or the preference for sucrose (F(1, 6) = 0.227; p=0.65) compared to intra-RMTg aCSF injection (all p > 0.05).
Fig. 4.
Intra-RMTg injection of AMPA or muscimol does not alter the intake or preference for sucrose. AMPA or muscimol did not alter the intake (A) of and preference (B) for 0.125% (w/v) sucrose. Values are mean ± S.E.M., (unpaired t-test; n=7, for each drug test).
Intra-RMTg infusion of AMPA or muscimol robustly change operant ethanol self-administration
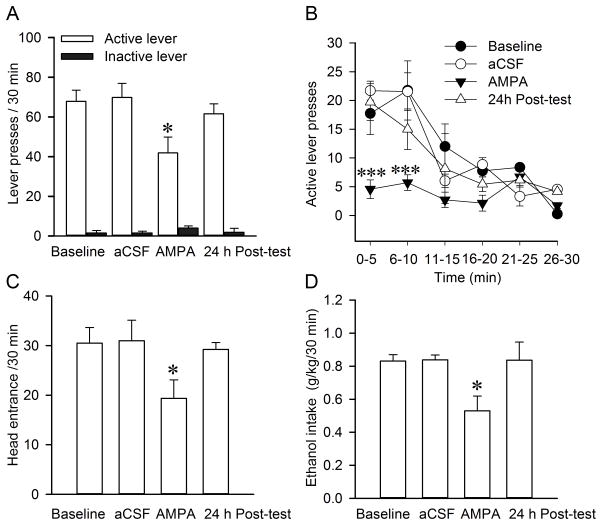
Next, we tested the effect of intra-RMTg AMPA/muscimol on ethanol seeking by using an operant self-administration model, as described (Li et al., 2012). Briefly, in this model, the delivery of the 20% ethanol reward was contingent on responses of the active lever under an FR3 schedule (see Material and Methods). No reward was received if the rats pressed the inactive lever, and the event was merely recorded as a measure of nonspecific behavioral activity. When the rats had maintained a stable level of responding over 20 sessions (3 weeks) on the FR3 schedule, aCSF, AMPA or muscimol were administered 10 minutes before the session. Histological verification showed that only eight rats in each group had the correct cannula tip placements in the RMTg (Fig. 1). Two rats with cannula placements missed the RMTg and were excluded from statistical analysis. Figure 5 depicts operant responding for ethanol by rats after injection of AMPA into the RMTg. Two-way RM ANOVA revealed significant main effects of AMPA injection (F(3, 21) = 3.976; p=0.02) and lever (F(1, 7) = 107.881; p < 0.001) with a significant interaction between the two main factors (F(3, 21) = 4.419; p=0.019). Post hoc analysis by Bonferroni t-test revealed that AMPA injection significantly decreased the number of active lever pressing compared to any other groups (aCSF vs. AMPA, t=4.449, p<0.001; Baseline vs. AMPA, t=4.059, p=0.001; 24 h posttest vs. AMPA, t=2.879, p=0.038, Fig. 5A). There was no significant difference among aCSF, baseline and 24 h posttest (all p>0.05). There were no significant changes for inactive lever responding. The data analysis within 30 minutes (bin=5 minutes) revealed a significant main effect of time (F(5, 35) = 26.156; p<0.001) and treatment (F(3, 21) = 10.398; p<0.001), with a significant interaction between the two main factors (F(15, 105) = 4.194; p<0.001). As illustrated in Fig. 5B, the operant response to the active lever mainly occurred during the initial 15 minutes. Thereafter, rats decreased the number of active lever pressing in a time-dependent manner, which is consistent with a previous study (Haack et al., 2014). Post hoc analysis by Bonferroni t-test revealed AMPA significantly decreased the number of active lever pressing during the first 10 minutes compared with Baseline, aCSF injection and 24 h posttest (Within 1–5 minutes: Baseline vs. AMPA, t=5.707, p<0.001; aCSF vs. AMPA, t=3.891, p<0.001; 24 h posttest vs. AMPA, t=5.855, p<0.001; Within 6–10 minutes: Baseline vs. AMPA, t=4.495, p<0.001; aCSF vs. AMPA, t=3.738, p=0.002; 24h posttest vs. AMPA, t=4.82, p<0.001; Fig. 5B). One way RM ANOVA revealed significant main effects of AMPA in the head entries into the ethanol port (F(3, 21) = 3.108; p=0.048) and operant ethanol consumption (F(3, 21) = 3.108; p = 0.019) compared to other groups. Post hoc comparisons using the Fisher LSD test and Student-Newman-Keuls method revealed that AMPA significantly decreased the number of head entries into the ethanol port (Baseline vs. AMPA, p=0.023; aCSF vs. AMPA, p=0.012; 24 h posttest vs. AMPA, p=0.047, Fig. 5C) and operant ethanol consumption (g/kg/30 min) (Baseline vs. AMPA, q=3.954, p=0.012; aCSF vs. AMPA, q=4.310, p=0.032; 24 h posttest vs. AMPA, q =4.163, p=0.023, Fig. 5D).
Fig. 5.
Intra-RMTg AMPA injection decreases ethanol seeking. AMPA was administered 10 minutes before the start of the ethanol-drinking session. AMPA (16.8 ng/300 nl/side, 0.3 mM), decreased the number of active lever pressing, but not inactive lever pressing during the 30-min test session (A). The data analysis within 30 mins (bin= 5 min) revealed that AMPA significantly decreased the active lever pressing during the initial 10 mins (B). AMPA injection also reduced the number of head entries into the ethanol port (C) as well as ethanol intake (D). Values are mean ± S.E.M. (one-way or two-way RM ANOVA followed by Bonferroni, Fisher LSD test or Student-Newman-Keuls post hoc test; n=8). * p < 0.05 and *** p<0.001, there was a significant difference compared with Baseline, aCSF injection or 24 h post-test., n=8.
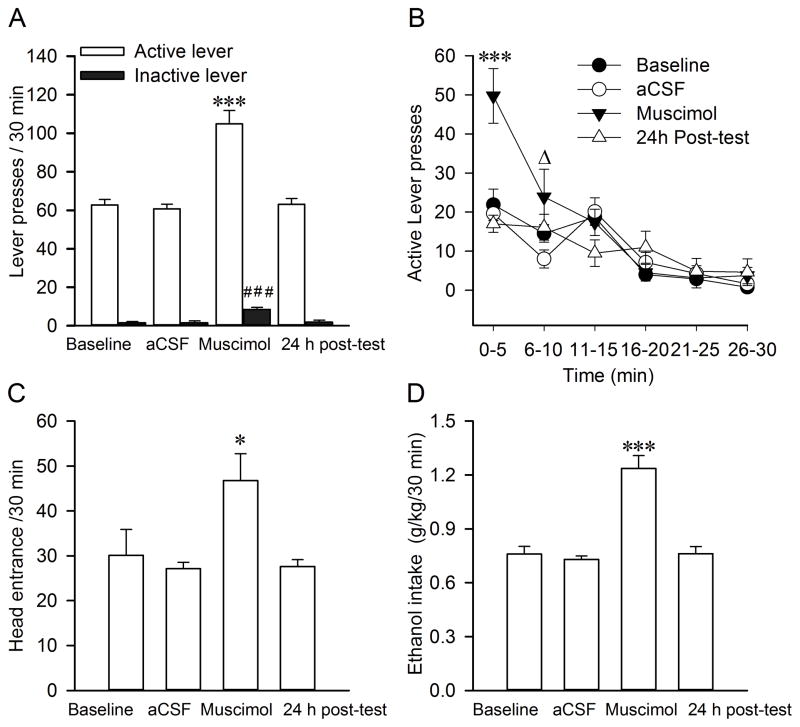
Figure 6 shows operant responding for ethanol in rats with intra-RMTg muscimol injection. Two-way RM ANOVA revealed significant main effects of muscimol injection (F(3, 21) = 39.143; p<0.001) and lever (F(1, 7) = 691.021; p<0.001) with a significant interaction between the two main factors (F(3, 21) = 22.54; p<0.001). Post hoc analysis revealed that muscimol robustly increased the number of active-lever pressing compared to any other groups (Baseline vs. muscimol, p<0.001; aCSF vs. muscimol, t=11.406, p<0.001; 24h posttest vs. muscimol, t=10.792, p<0.001, Fig. 6A). No significant difference was observed among Baseline, aCSF injection and 24 h posttest (all p > 0.05). Interestingly, muscimol also had a significant main effect on inactive lever responding (F(1, 7) = 17.947, p<0.001; Fig. 6A). Bonferroni t-test revealed muscimol significantly increased the number of inactive lever pressing compared to any other groups (Baseline vs. muscimol, t=6.03, p<0.001; aCSF vs. muscimol, t=6.092, p<0.001; 24h posttest vs. muscimol, t =5.766, p<0.001). The data analysis within 30 minutes (bin=5 min) revealed a significant main effect of time (F(5, 35) = 25.76; p<0.001) and treatment (F(3, 21) = 8.371; p<0.001), with a significant interaction between the two main factors (F(15, 105) = 4.355; p<0.001). Post hoc analysis revealed muscimol significantly increased the number of active lever pressing during the first 10 min compared with aCSF injection (Within 1–5 minutes: Baseline vs. muscimol, t=5.683, p<0.001; aCSF vs. muscimol, t=3.875, p=0.001; 24 h posttest vs. muscimol, t=5.83, p<0.001; within 6–10 minutes: Baseline vs. muscimol, t=4.476, p<0.001; aCSF vs. muscimol, t=3.722, p=0.001; 24 h posttest vs. muscimol, t=4.799, p<0.001 Fig. 6B). One way RM ANOVA revealed significant main effects of muscimol for the number of head entries into the ethanol port (F(3, 21) = 6.94, p=0.002) as well as ethanol consumption (F(3, 21) = 47.504, p<0.001) compared to other groups. Post hoc comparisons using Bonferroni t-test showed muscimol increased head entries into the ethanol port (aCSF vs. muscimol, t=3.922, p=0.005; Baseline vs. muscimol, t=3.322, p=0.019; 24 h posttest vs. muscimol, t =3.822, p=0.006, Fig. 6C) as well as ethanol intake (g/kg/30 min) (Baseline vs. muscimol, t=9.463, p<0.001; aCSF vs. muscimol, t=10.138, p<0.001; 24 h posttest vs. muscimol, t =9.589, p<0.001, Fig. 6D).
Fig. 6.
Intra-RMTg muscimol injection increases ethanol seeking. Muscimol (18.5 ng/125 nl/side, 1.3 mM) robustly elevated the number of active lever pressing (A). Data analysis within 30 min (bin=5 min) revealed that muscimol significantly increased active lever pressing during the initial 10 min (B). Muscimol injection increased the number of head entries into the ethanol port (C) and ethanol intake (D) during the 30-min session. Values are mean ±S.E.M. (one-way or two-way RM ANOVA followed by Bonferroni post hoc test; n=8). * p < 0.05 and *** p<0.001, compared with Baseline, aCSF or 24 h post-test; Δp <0.001, compared with aCSF; ### p<0.001, compared with baseline, aCSF or 24 h post-test on inactive lever pressing.
DISCUSSION
In the present study, we demonstrated that activation or inhibition of the RMTg in Long-Evans rats by intra-RMTg infusion of the glutamate receptor agonist AMPA or the GABAA receptor agonist muscimol selectively reduced or increased ethanol intake in two different drinking models; intermittent access in home cages and operant self-administration. By contrast, intra-RMTg infusion of these agents did not change sucrose consumption. This finding indicates that the RMTg plays a critical role in modulating ethanol self-administration.
Increased RMTg excitability reduces ethanol intake
Most of the neurons in the RMTg received a strong glutamatergic input from the LHb (Jhou et al., 2009a, Kaufling et al., 2009, Balcita-Pedicino et al., 2011, Stamatakis and Stuber, 2012, Hong et al., 2011). AMPARs, belong to the ionotropic glutamate receptor family and are mainly found on the postsynaptic membrane on dendritic spines and produce relatively fast actions in many brain areas (Chater and Goda, 2014), including the RMTg (Huff and LaLumiere, 2014, Jhou et al., 2013). It has been shown that local injection of AMPA (300 nl 0.3 mM) activated the RMTg in rats (Jhou et al., 2013). In the present study, intra-RMTg injection of the same dose of AMPA led to a significant reduction in ethanol consumption, both in home cages and in operant chambers. Mechanisms underlying RMTg activation reducing ethanol consumption are unclear. However, given that the RMTg is a major source of inhibitory GABAergic input to midbrain dopamine neurons, increased RMTg activity will likely inhibit VTA dopamine neurons. Therefore, the reduction of ethanol consumption induced by intra-RMTg infusion of AMPA may be resulted from the reduction of VTA dopamine neuronal activity and of dopamine release in their target areas. This could correspond to a reduction of the euphoric effect of ethanol. This possibility is supported by evidence that direct excitation of VTA-GABA neurons disrupts reward-related behaviors (van Zessen et al., 2012). Conversely, RMTg activation induced inhibition of ethanol consumption may be a result of an increased aversive effect of ethanol, since the RMTg is known to play a critical role in aversion, and is activated by aversive stimuli. This idea is supported by previous evidence that activation of the RMTg (by AMPA) induced conditioning aversion (Jhou et al., 2013), and stimulation of VTA-GABA neurons or inhibition of VTA-dopamine neurons promotes aversion (Tan et al., 2012). Anatomically, the RMTg is an intermediate structure situated between the LHb and the VTA, mediates LHb-induced inhibition on dopamine neurons (Hong et al., 2011, Lecca et al., 2012). Functionally, activation of the LHb-to-RMTg circuit disrupts positive reinforcement (Stamatakis and Stuber, 2012), and activation of LHb terminals in the RMTg promotes behavioral avoidance, suggesting that endogenous activity of LHb glutamatergic inputs to the RMTg conveys information related to aversion (Stamatakis and Stuber, 2012). All of these evidences indicate that strong aversive stimuli can inhibit drug-seeking behavior (Jhou et al., 2013, Fields, 2007, Leknes and Tracey, 2008).
Inhibition of the RMTg increases ethanol intake
Previous studies have shown that VTA-DA neurons are under tonic inhibitory control originating in the RMTg (Jalabert et al., 2011), and that muscimol injection within the RMTg inhibited the RMTg (Jhou et al., 2012, Lavezzi et al., 2014, Jalabert et al., 2011). In the current study, we used muscimol to explore the effect of RMTg inhibition on ethanol consumption and found that intra-RMTg injection of muscimol robustly increased the intake of and the preference for ethanol. Interestingly, the effect of muscimol lasts longer than that of AMPA. Specifically, the effect of muscimol remained at 24 h after injection, when the effect of AMPA disappeared. This is in general agreement with a previous study showing that muscimol injection into the dorsal raphe nucleus could elevate ethanol intake for 24 h in Wistar rats (Tomkins et al., 1994). Moreover, the current study showed that muscimol substantially increased the number of active lever pressing and head entrance during operant ethanol self-administration. These results suggest that RMTg neurons may normally be responsible for the aversive effect of ethanol and act as a break for ethanol drinking. Conversely, these neurons may normally inhibit the euphoric effect of ethanol, and their inhibition may make ethanol drinking more rewarding. Interestingly, intra-RMTg muscimol injection increased the inactive lever pressing in the operant chamber, suggesting that RMTg neurons may normally play an inhibitory role over locomotion, which is consistent with the observation that pharmacological inhibition of the RMTg increases locomotion (Huff and LaLumiere, 2015, Lavezzi et al., 2015). This is not surprising given that the RMTg projects to both the VTA and the substantial nigra, which control locomotion (Jhou et al., 2009a). The results from the combination of voluntary and operant ethanol drinking suggest that the RMTg may contribute importantly to the balance between the rewarding and aversive properties of ethanol.
Interestingly, we did not observe a significant change in sucrose intake or preference after intra-RMTg infusion of AMPA or muscimol. This appears to contradict with the result of a previous study showing that intra-RMTg infusion of AMPA produces a conditional place aversion (Jhou et al., 2013), suggesting a general role for RMTg in regulating reward and aversion. The reasons for this discrepancy are not clear, but we offer the following possible interpretations.
First, the current study used 0.125% sucrose solution and these rats showed no more than 60% preference to it over water. It is possible that sucrose of low concentration lacks a strong reward property. Another possibility is that the alternation of RMTg plasticity induced by ethanol drinking is different from that by sucrose drinking. Indeed, the discovery of the RMTg as a distinct region began with the observation that psychostimulants induced a significant increase in Fos expression in a neuroanatomically distinct area that was eventually described as the RMTg(Jhou et al., 2009b, Kaufling et al., 2010). Considering the RMTg mediates aversive, reward prediction error or omission of rewards signals, ethanol might act on the RMTg through its aversive properties. Our recent work has shown that LHb neurons encode the aversive property of ethanol (Zuo et al., 2015), and RMTg is known to receive LHb projections directly (Jhou et al., 2009b). We propose that the LHb–RMTg pathway might mediate the aversive property of ethanol. Thus, ethanol may have a particular ability to influence plasticity in these structures, in contrast to the non-aversive sucrose.
In summary, this study demonstrates that activation or inhibition of the RMTg led to the decrease or increase of the voluntary and operant ethanol self-administration in rats. Conversely, alteration of RMTg function does not change the preference for or consumption of the naturally rewarding sucrose. These findings indicate that the RMTg may play a crucial role in the regulation of ethanol consumption, implicating that dysfunction of these neurons likely plays a critical role in the pathogenesis of alcohol use disorders. Collectively, these data suggest that RMTg neurons may encode the aversive signals of ethanol, which may prevent the development of ethanol addiction and thus represent a pivotal element in the neurobiological processes underlying ethanol addiction. Manipulating activity of the RMTg may be of therapeutic value in the prevention and treatment of alcohol abuse.
Acknowledgments
This work was supported by grants (AA022292 and AA021657) from the National Institute of Health.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
RF and JHY were responsible for the study concept and design. RF performed the stereotaxic surgery and histological experiment. RF and DG performed behavioral experiment. RF and JHY analyzed the data and drafted the manuscript. JL,WHZ helped on the data analysis and provided critical comments on the design and manuscript. JHY supervised the study and provided critical revision of the manuscript for important intellectual content. Dennis G provided critical revision of the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater TE, Goda Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci. 2014;8:401. doi: 10.3389/fncel.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. Journal of psychopharmacology. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Fields H. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007 May-Jun;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PloS one. 2014;9:e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nature reviews Neuroscience. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, LaLumiere RT. The Rostromedial Tegmental Nucleus Modulates Behavioral Inhibition Following Cocaine Self-Administration in Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, LaLumiere RT. The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:861–873. doi: 10.1038/npp.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F. Neuronal circuits underlying acute morphine action on dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16446–16450. doi: 10.1073/pnas.1105418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. The Journal of comparative neurology. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Xu SP, Lee MR, Gallen CL, Ikemoto S. Mapping of reinforcing and analgesic effects of the mu opioid agonist endomorphin-1 in the ventral midbrain of the rat. Psychopharmacology. 2012;224:303–312. doi: 10.1007/s00213-012-2753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Gilpin NW. Operant self-administration models for testing the neuropharmacological basis of ethanol consumption in rats. Curr Protoc Neurosci. 2010;Chapter 9(Unit 9 12):11–26. doi: 10.1002/0471142301.ns0912s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. The Journal of comparative neurology. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. gamma-Aminobutyric acid cells with cocaine-induced DeltaFosB in the ventral tegmental area innervate mesolimbic neurons. Biological psychiatry. 2010;67:88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DS. Modulation of Locomotor Activation by the Rostromedial Tegmental Nucleus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DS. Modulation of locomotor activation by the rostromedial tegmental nucleus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:676–687. doi: 10.1038/npp.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nature reviews Neuroscience. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addiction biology. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. The Journal of pharmacology and experimental therapeutics. 2012;341:196–204. doi: 10.1124/jpet.111.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nature neuroscience. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7. 2007. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. The European journal of neuroscience. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature neuroscience. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism, clinical and experimental research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature neuroscience. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature neuroscience. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Sellers EM, Fletcher PJ. Effect of dorsal raphe injections of the GABAA agonist, muscimol, on ethanol intake and measures of intoxication in Wistar rats. Alcohol Alcohol Suppl. 1994;2:551–558. [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behavioural pharmacology. 2013;24:363–374. doi: 10.1097/FBP.0b013e32836413d5. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Fu R, Hopf FW, Xie G, Krnjevic K, Li J, Ye JH. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. 2015 doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]