Abstract
The importance of vaccine-induced T-cell immunity in conferring protection with prototype and commercial FIV vaccines is still unclear. Current studies performed adoptive transfer of T cells from prototype FIV-vaccinated cats to partial-to-complete feline leukocyte antigen (FLA)-matched cats a day before either homologous FIVPet or heterologous-subtype pathogenic FIVFC1 challenge. Adoptive-transfer (A–T) conferred a protection rate of 87% (13 of 15, p<0.001) against FIVPet using FLA-matched T cells, whereas all 12 control cats were unprotected. Furthermore, A-T conferred protection rate of 50% (6 of 12, p<0.023) against FIVFC1 using FLA-matched T cells, whereas all 8 control cats were unprotected. Transfer of FLA-matched T and B cells demonstrated that T cells are needed to confer A-T protection. In addition, complete FLA-matching and addition of T-cell numbers >13×106 cells were required for A-T protection against FIVFC1 strain, reported to be a highly pathogenic virus resistant to vaccine-induced neutralizing-antibodies. The addition of FLA-matched B cells alone was not protective. The poor quality of the anti-FIV T-cell immunity induced by the vaccine likely contributed to the lack of protection in an FLA-matched recipient against FIVFC1. The quality of the immune response was determined by the presence of high mRNA levels of cytolysin (perforin) and cytotoxins (granzymes A, B, and H) and T helper-1 cytokines (interferon-γ [IFNγ] and IL2). Increased cytokine, cytolysin and cytotoxin production was detected in the donors which conferred protection in A-T studies. In addition, the CD4+ and CD8+ T-cell proliferation and/or IFNγ responses to FIV p24 and reverse transcriptase increased with each year in cats receiving 1X-3X vaccine boosts over 4 years. These studies demonstrate that anti-FIV T-cell immunity induced by vaccination with a dual-subtype FIV vaccine is essential for prophylactic protection against AIDS lentiviruses such as FIV and potentially HIV-1.
Keywords: Feline immunodeficiency virus, vaccine, T cell immunity, adoptive transfer
1. Introduction
The development of an effective HIV-1 vaccine for prophylaxis and immunotherapy is critically needed in light of the inability of the antiretroviral therapy alone to cure HIV-1 infection [1–6]. After the failure of the STEP trial [7] which was designed to target cell-mediated immunity, the major emphasis in phase-II to phase-III clinical HIV-1 vaccine trials focused on vaccines formulated to induce antibody immunity against HIV-1, and in particular HIV-1 neutralizing antibodies (NAbs) (IAVI Report, clinical trials database, http://www.iavireport.org/Trials-Database/Pages/default.aspx, accessed 05/13/2015). Even though the focus has moved away from the generation of T-cell immunity, many of the known HIV NAbs consists of the IgG isotype. Therefore, the importance of T-cell immunity cannot be ignored since the production of IgG antibodies generally requires CD4+ T-cell helper-2 (TH2) activity via production of the appropriate cytokines [8]. Only token efforts in developing a T-cell based HIV-1 vaccine have been made without major success (reviewed in [9]). However, numerous findings evaluating HIV-1 infected subjects indicated the importance of T-cell immunity, especially CD8+ T-cell immunity, in the control of HIV-1 infection [10–12]. Similar findings were also demonstrated in the simian immunodeficiency virus (SIV)/macaque model where CD8+ T cells were important in the control of SIV load in SIV-infected macaques [13–15]. More recently, a vaccine consisting of a SIV gag/pol/env construct in a CMV vector conferred 50% protection with homologous SIV challenge in rhesus macaques [16,17]. The mechanism of such protection was reported to be mediated by an unconventional major histocompatibility complex (MHC)-II restricted CD8+ T-cell immunity rather than anti-SIV NAb immunity [17]. Furthermore, the importance of antiviral T-cell immunity against feline immunodeficiency virus (FIV), the feline counterpart of HIV, has been demonstrated [18–20].
In the FIV/cat model of HIV/AIDS, the prototype (inactivated whole virus [IWV]) and the commercial (inactivated whole cell lysate) dual-subtype FIV vaccines, composed of subtypes A and D, conferred protection against the heterologous subtype-B FIVFC1 isolate [21]. Furthermore, this FIV isolate was resistant to vaccine-induced FIV NAbs based on in vitro testing and an in vivo passive-transfer study using vaccine-induced purified antibodies. Hence, the most likely mechanism of such protection was reported to be the vaccine-induced cellular immunity such as T-cell immunity [18–21]. This observation was also supported by an earlier study which determined high levels of T-cell immunity generated by cats vaccinated with the prototype FIV vaccine [19]. In addition, complete protection against FIV challenge was observed in 36% (4 of 11) of recipients of adoptive transfer with Ab-free peripheral blood mononuclear cells (PBMC) from vaccinated parental donors prior to homologous FIV challenge [18]. Since no vaccine antibodies were transferred, such protection was thought to be mediated by cellular immunity such as antiviral T-cell immunity [19].
Current studies have been undertaken to decisively determine if feline leukocyte antigen (FLA)-restricted T-cell immunity induced by the prototype FIV vaccine is indeed conferring protection against a challenge with vaccine-induced NAb-resistant, pathogenic FIVFC1. The following studies utilized adoptive transfer of T-cell preparations from vaccinated cats to FLA-matched and unmatched naïve cats a day before challenge. The A-T approach is based on a well-established concept that T cells are presented with viral peptides by MHC-restricted antigen presenting cells and/or MHC-restricted virus-infected cells [22]. Thus, the protection conferred between vaccinated donors and MHC-matched A-T T-cell recipients further confirms that the vaccine immunity is mediated by anti-FIV T-cell immunity.
2. Materials and methods
2.1. MHC-matched animals and adoptive-transfer (A–T) studies
In order to develop MHC-matched laboratory cats, three lines of semi-inbred cats were developed over 15 years (described in [20,23]). Each donor-recipient pair in the A-T study was first matched by mixed leukocyte reaction (MLR) [18] from semi-inbred cats of the same colony. Donors were vaccinated subcutaneously (400 µg) and intradermally (100 µg) with the prototype dual-subtype FIV vaccine 4X in the first year and 1X-3X per year thereafter. For example, a 2-year vaccinated donor refers to any cat that received the prototype dual-subtype FIV vaccine 4X in the first year and 1X-3X in second year, placing the total number of vaccinations at 5X-7X for a 2-year vaccinated donor. The prototype FIV vaccine consists of 250 µg each of inactivated whole viruses (IWV) of subtype-A FIVPet and subtype-D FIVShi in FD-1 adjuvant (kindly provided by Fort Dodge Animal Health, Fort Dodge, IO) supplemented with 5 µg of recombinant feline IL2 (FD-1 adjuvant/FeIL12) (R&D Systems, Minneapolis, MN).
The control group in these studies was represented by any cat that did not receive T cells from vaccinated donors, and thus consisted of T-cell or PBMC transfer from non-vaccinated cats, B-cell transfer from vaccinated cats, and/or only PBS. Controls in previous studies directly immunized with uninfected vaccine cell line (e.g., FeT-J cell lysate as non-specific antigen) in adjuvant alone and adjuvant/HuIL12 afforded no protection [21]. Therefore, the addition of a control group consisting of recipients of A-T of T cells from donors vaccinated with non-specific antigen, such as uninfected vaccine cell antigen devoid of FIV antigen, was not included.
In Studies 3, 4 and 5, the protected recipient cats from previous A-T study, Study 2, were vaccinated and used as A-T donors. A-T studies were performed with small group sizes in order to perform the blood collection from the donors, analysis of the donor cells, T-cell purifications, and adoptive transfers in a single day. A total of five AT studies using T-cell preparations were performed. The recipients of A-T were challenged with homologous (i.e., vaccine strain) FIVPet in the first three studies and heterologous-subtype FIVFC1 in the last two studies. The T-cell preparation was administered intravenously (IV) 24 hours prior to IV challenge with 25× median 50% cat infectious doses (CID50) of either in vitro-derived FIVPet or in vivo-derived pathogenic FIVFC1 as previously described [21]. As a final confirmation of FLA-matching, both the protected and unprotected recipients and their corresponding donors were further evaluated for FLA class-I and -II matching by an FLA-specific PCR method. All procedures on cat studies including inbreeding and maintenance were completed in accordance with the University of Florida IACUC.
2.2. Purification of T-cell enriched population
The T-cell enriched population was produced by sorting PBMC using the DynaMag™-15 magnetic separation system (Life Technologies, Oslo, Norway). Briefly, ficoll-gradient purified PBMC from vaccinated A-T donors [18] were treated with rabbit polyclonal Abs to feline IgG and IgM (Bethyl Laboratories, Montgomery, TX), and subsequently mixed with magnetic beads conjugated with goat anti-rabbit IgG (Life Technologies) and then passed twice through a magnetic field to deplete B cells. The resulting flow through was used as the T-cell enriched population. The B-cell population for A-T Study 1 consisted of the positively selected B cells eluted from the magnetic beads. To obtain the CD8+ T-cell enriched population (CD8+ T-cell population) the T-cell enriched population was incubated with a combination of murine IgG MAbs to feline CD4 (hybridoma clone kindly provided by Dr. Nazareth Gengozian, described in [24]) and to canine CD21 for further B-cell depletion (AbD Serotec, Raleigh, NC; cross-reactivity to feline CD21 described in [25]). Cells were then washed 3X with PBS, and treated with magnetic beads conjugated anti-mouse IgG before exposure to the magnetic field. To obtain the CD4+ T-cell enriched population (CD4+ T-cell population), the murine IgG MAb to feline CD4 was replaced with murine IgG MAb to feline CD8 (SouthernBiotech, Birmingham, AL). The final T-cell preparations were washed 3X and resuspended in PBS at total concentrations of 5–10×106 cells/mL. The phenotypes of these cell preparations were determined by fluorescence-activated cell sorting (FACS) as previously described [21].
2.3. PCR-based MHC matching
MHC-matching was determined by PCR using primer sets specific for FLA class-I and class-II alleles as previously described [26] (Table 1, Supplemental Table 1). All amplified products were sequenced by Eurofins MWG Operon LLC (Louisville, KY) and determined to be specific for the sequence of the corresponding FLA allele.
Table 1.
Feline MHC class-I and -II allele specific primers for semi-inbred cats.
| Allele Specific Primer Set* | Primer Sequence | Product Length |
|---|---|---|
| A1 (FLA Class-I) | F: 5’-CGAGGAGACGCGGAACAT-3’ | 127 bp |
| R: 5’-CACGTCACAGCCATACATTG-3’ | ||
| A2 (FLA Class-I) | F: 5’-CCCGAATCCCAGGGAAGA-3’ | 128 bp |
| R: 5’-GGAGCAACGTGTTCAGGTT-3’ | ||
| B3 (FLA Class-I) | F: 5’-CTCCCACTCCCTGAGGTATTT-3’ | 256 bp |
| R: 5’-GTTGTAGTAGCGGAGGAC-3’ | ||
| B4 (FLA Class-I) | F: 5’-TGTGACATCGGACCGAACA-3’ | 178 bp |
| R: 5’-GGTAGTTCCTGTAGTGCTCT-3’ | ||
| B5 (FLA Class-I) | F: 5’-GCCCCGAATCCGAGGTAT-3’ | 297 bp |
| R: 5’-CCAGGTAGTTCCTGATGTCT-3’ | ||
| B6 (FLA Class-I) | F: 5’-GCGGAAGGTGAAGAACAC-3’ | 168 bp |
| R: 5’-CCGTCATAGGAGTCCTGATT-3’ | ||
| D1 (FLA-DRB) | F: 5’-GAGTTCCGAGCGGTATCA-3’ | 556 bp |
| R: 5’-GAAGTCCAGAGTGTCCTTTCT-3’ | ||
| D2 (FLA-DRB) | F: 5’-GACACCTCATCACATTTCTTAG-3’ | 207 bp |
| R: 5’-CTGCTCCAGGTGGTCCTT-3’ | ||
| D3 (FLA-DRB) | F: 5’-GACACCTCACCACATTTCTTGTT-3’ | 186 bp |
| R: 5’-CTCGTTCATGTACTTGGCGT-3’ | ||
| D4 (FLA-DRB) | F: 5’-GTGGAAGTTCGAGTGTCATTATC-3’ | 184 bp |
| R: 5’-CTTCCGCTCCAGGACCT-3’ | ||
| D5 (FLA-DRB) | F: 5’-GACACCTCACCACATTTCTTGTT-3’ | 186 bp |
| R: 5’-CTCGTTCATGTACTTGGCAATG-3’ | ||
| D6 (FLA-DRB) | F: 5’-ACATTTCTTAACCATGTGGAAGTTC-3’ | 237 bp |
| R: 5’-CCGTAGTTGTGTCTGCAGTA-3’ | ||
| D7 (FLA-DRB) | F: 5’-TGATGGCAGCTCTGATGGTA-3’ | 182 bp |
| R: 5’-GCTGTCGAAGCGCAAGTT-3’ |
2.4. Monitoring for FIV infection
FIV infection of the challenged cats was determined by virus isolation and proviral PCR of PBMC at 3, 6, 9, 12, and 21–27 weeks post challenge (wpc) [21]. These analyses were also performed on the bone marrow, lymph node, and thymus at the termination of each study. The sera collected at corresponding timepoints were tested for FIV antibodies by immunoblot analysis as previously described [27]. As the focus of these studies were to determine the ability of T cells from vaccinated donors to confer complete protection, plasma and proviral virus loads were not measured.
2.5. Monitoring cellular immunity of the vaccinated donors
The donor cats were evaluated for vaccine-induced cellular immunity 6–8 weeks after the blood collection for the A-T procedure. This evaluation of anti-FIV cellular immunity was delayed to allow donor cats to recover from blood draws used entirely for the preparation of T-cell enriched populations for A-T to maximize T cells transferred. The feline IFNγ and IL-2 ELISpot analyses of PBMC to overlapping peptide pools for FIV p24 and reverse transcriptase (RT) were performed as previously described [28]. CD3+CD4+ and CD3+CD8+ T-cell proliferation was determined by FACS using the carboxyfluorescein diacetate succinimide ester (CFSE) method as previously described [29]. Cytokine and cytotoxin mRNA analysis was performed using PBMC and FACS-sorted CD3+CD4+ and CD3+CD8+ T cells from donors as previously described [19].
2.6. Statistics
The various groups in the A-T studies were compared using pairwise Mann-Whitney Rank Sum test (SigmaPlot version 11.0, San Jose, CA). The recipients in the experimental group received T-cell enriched populations from cats vaccinated with prototype FIV vaccine. However, the recipients in the control group did not receive T-cell transfer from cats vaccinated with non-specific antigen in identical vaccine formulations devoid of FIV antigen, but rather consisted of T-cell or PBMC transfer from non-vaccinated cats, B-cell transfer from vaccinated cats, and/or only PBS. All statistical results are shown in the right most column on Tables 2 and 3.
Table 2.
A-T studies using FLA-matched and unmatched cats against homologous FIVPet challenge.
| Study- Group |
Immune Status a |
A-T |
FLA Match bc |
A-T Cell Typed |
A-T Cell (×106/A-T)cde |
FIV Antibodies/Isolation/PCR g |
B/L/Th |
Protection Rate (%) |
p-value h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Recipient | I / II / MLR | CD4 | CD8 | Tf | B | 3wpc | 6wpc | 9wpc | 12wpc | 21wpc | 21–27wpc | |||||
| 1A | Vac | 3EC | 3EA | N / N / + | T | 19 | 8 | 30 | 2 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | 3/4 (75%) | 0.114 |
| (Y-1) | Vac | 3HA | 3HB | N / N / + | T | 27 | 10 | 45 | 20 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | ||
| Vac | 6DB | 6DA | N / N / + | T | 2 | 0.2 | 2 | 0.4 | −/+/− | +/+/± | +/+/+ | +/+/+ | +/+/+ | +/+/N | |||
| Vac | 9QD | 9QK | N / N / + | T | 22 | 5 | 31 | 3 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| 1B | Vac | 3EC | 3EB | N / N / + | B | 4 | 2 | 11 | 13 | ±/−/− | +/+/− | +/+/+ | +/+/+ | +/+/+ | +/+/N | 0/4 (0%) | |
| Vac | 6DB | 6DC | N / N / + | B | 13 | 3 | 19 | 13 | −/+/+ | −/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/N | |||
| Non-Vac | 901 | 803 | N / N / − | PBMC | 33 | 10 | 52 | N | −/+/− | +/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/N | |||
| Non-Vac | 807 | 805 | N / N / − | PBMC | 23 | 8 | 39 | 42 | −/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/N | |||
| 2A | Vac | BDB | BDI | +/ + / + | T | 22 | 17 | 42 | 4 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | 8/10 (80%) | 0.048i |
| (Y-1) | Vac | BDB | BDK | ± / − / + | CD8+ T | 5 | 14 | 25 | 3 | −/−/− | −/+/+ | −/+/+ | +/+/− | +/+/+ | +/+/+ | ||
| Vac | BDF | BDH | +/ ± / + | CD8+ T | 5 | 17 | 33 | 2 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| Vac | BDF | BDJ | + / + / + | CD4+ T | 24 | 3 | 42 | 2 | −/−/− | −/−/− | −/−/− | +/+/+ | +/+/+ | +/−/− | |||
| Vac | BDC | BDA | + / + / + | T | 42 | 19 | 65 | 3 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| Vac | BDC | BDD | ± / −/ + | CD4+ T | 38 | 1 | 44 | 1 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| Vac | QVB | QVE | + / + / + | T | 56 | 26 | 82 | 7 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| Vac | QVB | QVF | + / + / + | CD4+ T | 47 | 1 | 68 | 2 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| Vac | QVC | QVD | + / + / + | T | 13 | 17 | 36 | 2 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| Vac | QVC | QVG | + / + / + | CD8+ T | 6 | 24 | 33 | 1 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/N | |||
| 2B | Non-Vac | BDG | BDE | N / N / + | T | 127 | 28 | 157 | 4 | −/−/− | −/+/+ | +/+/+ | +/+/+ | +/+/+ | +/−/− | 0/4 (0%) | |
| Non-Vac | QVA | QWA | N / N / − | PBMC | 65 | 33 | 98 | 21 | −/−/− | +/−/− | +/+/+ | +/+/+ | +/+/+ | −/−/+ | |||
| Non-Vac | None | 271 | N | PBS | N | N | N | N | −/−/− | −/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ | |||
| Non-Vac | None | 350 | N | PBS | N | N | N | N | −/−/− | +/+/+ | +/+/+ | +/+/+ | +/+/+ | −/−/− | |||
| 3A | Vac | BDAj | VSF | ±/ − / + | T | 36 | 16 | 65 | 0.6 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | 6/7 (86%) | 0.024 |
| (Y-5) | Vac | BDM | DVA | ± / − / + | T | 16 | 4 | 28 | 0.2 | −/−/− | −/−/− | +/+/+ | +/+/− | +/+/− | −/+/− | ||
| Vac | QVD | j VSD | + / +/ + | T | 44 | 11 | 63 | 1.7 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| Vac | QVF j | VSE | + / + / + | T | 57 | 23 | 89 | 6.4 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| Vac | QVZ | VVC | + / + / + | T | 12 | 2 | 21 | 0.1 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| Vac | VOG | VON | + / + / + | T | 66 | 27 | 106 | 2.4 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| Vac | QWF | QWH | + / + / + | T | 35 | 23 | 65 | 0.8 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| 3B | Non-Vac | VWG | VVA | N | T | 40 | 24 | 68 | 0.7 | −/−/− | −/−/+ | +/+/+ | +/+/− | +/+/− | +/+/+ | 0/4 (0%) | |
| NU | NU | QVO | N | PBS | N | N | N | N | −/−/− | −/−/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ | |||
| NU | NU | 9HJ | N | PBS | N | N | N | N | −/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ | −/+/+ | |||
| NU | NU | 5HE | N | PBS | N | N | N | N | −/−/− | −/−/+ | +/+/+ | +/+/− | +/+/+ | +/+/+ | |||
| T cells only from Group 1A+2A+3A vs. Control Group 1B+2B+3B: | <0.001 | ||||||||||||||||
Vaccinated donor (Vac), non-vaccinated donor (Non-Vac), or donor not used (NU).
Donor-recipient FLA compatibility of 90–100% (+), ≥50% (±), and <50% (−) (see Supplemental Table 1). MLR results are shown as either compatible (+) or not compatible (−)
Not done (N).
Enriched for CD4+ T cells (CD4+ T), CD8+ T cells (CD8+ T), T cells (T), and B cells (B). The bolded number multiplied by 106 represents the total number of CD4+ T cells, CD8+ T cells, T cells, or B cells transferred. The bolded T-cell count plus bolded B-cell count multiplied by 106 approximates the total PBMC transferred. PBS added (PBS) instead of cells.
The non-B and non-T lymphocyte contamination accounted for 0.1%-3.0% (absolute number of 0.4×105-1.3×106 transferred) compared to the total T- and B-cell population of the T-cell preparation transferred with each A-T. The non-T and non-B populations are negative for CD3, CD4, CD8, CD21, IgG, and IgM.
T-cell counts were higher than the total of CD4+ T-cell plus CD8+ T-cell counts due to CD3+CD4−CD8− and CD3+CD4+CD8+ T-cell counts. In Study 3, T-cell counts are higher than the combined count for CD3+CD4+ and CD3+CD8+ T cells mainly due to the CD3+CD4−CD8 T cells.
FIV antibodies, virus isolation (isolation), and proviral PCR (PCR) are shown as either positive (+) or negative (−). Virus isolation on lymph node (L), bone marrow cells (B), and thymocytes (Th) performed at the termination of each study.
P-value of FLA matched group vs. control group unless stated otherwise. Bolded values indicate significance.
Total population of T cells, CD4+ T-cells and CD8+ T cells (p=0.048); only whole T-cells (p=0.029).
The protected cats from Study 2 vaccinated and used as A-T donors in Study 3.
Table 3.
A-T protection against heterologous subtype-B FIVFC1.
| Study- Group |
Immune Status a |
A-T |
FLA Matchbc |
A-T Cell Type c |
A-T Cells (×106) cde |
FIV Antibodies/Isolation/PCR cg |
B/L/Th |
Protection Rate (%) |
p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Recipient | I / II / MLR | CD4 | CD8 | Tf | B | 3wpc | 6wpc | 9wpc | 12wpc | 16wpc | 25wpc | 25–40wpc | |||||
| 4A | Vac | BDA | BDD | ± / −/ + | T | 5 | 3 | 9 | 2 | −/−/− | −/−/− | −/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ | 3/6 (50%) | 0.257 |
| (Y-1) | Vac | BDM | BDI | + / + / + | T | 13 | 10 | 23 | 6 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | ||
| Vac | QVF | QVE | + / + / + | T | 52 | 22 | 83 | 5 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| Vac | QVD | QVG | + / + / + | T | 86 | 30 | 129 | 11 | −/−/− | −/+/+ | +/+/+ | +/+/+ | N | +/+/+ | +/+/+ | |||
| Vac | QWC | BDR | + / +/ + | T | 37 | 7 | 46 | 7 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | |||
| Vac | QWE | BDQ | ± / − / + | T | 20 | 3 | 24 | 5 | −/−/− | −/−/+ | −/−/+ | −/+/+ | +/+/+ | +/+/+ | +/+/+ | |||
| 4B | Non-Vac | 228 | 128 | N / N / − | T | 82 | 21 | 109 | 15 | −/−/− | −/−/− | +/+/+ | +/+/− | N | +/+/+ | +/+/+ | 0/4 (0%) | |
| NU | NU | 130 | N | PBS | N | N | N | N | −/−/− | −/−/− | −/+/+ | −/+/+ | +/−/+ | +/+/+ | +/+/+ | |||
| NU | NU | 228 | N | PBS | N | N | N | N | −/−/− | +/+/+ | +/+/− | +/+/+ | N | +/+/+ | +/+/+ | |||
| 5A | Vac | QVD | SBC | + / + / + | T | 11 | 6 | 17 | 0.3 | −/−/− | −/−/− | −/−/− | N | −/−/− | N | −/−/N | 3/6 (50%) | 0.257 |
| (Y-6) | Vac | QVF | SBD | + / + / + | T | 34 | 4 | 47f | 0.1 | −/−/− | −/−/− | −/−/− | N | −/−/− | N | −/−/N | ||
| Vac | QVZ | OCB | + / + / + | T | 18 | 6 | 25 | 0.0 | −/−/− | −/−/− | −/−/− | N | −/−/− | N | −/−/N | |||
| Vac | VOG | WFA | + / + / + | T | 9 | 3 | 13 | 0.0 | −/−/+ | +/+/− | +/+/+ | N | +/+/+ | N | +/+/N | |||
| Vac | QWF | OCE | + / + / + | T | 5 | 3 | 8 | 0.0 | −/−/− | +/+/+ | +/+/+ | N | +/+/+ | N | −/−/N | |||
| Vac | BDA | OCD | ± / − / + | T | 10 | 2 | 13 | 0.1 | −/−/− | +/+/− | +/+/+ | N | +/−/+ | N | +/−/N | |||
| 5B | Non-Vac | QVQ | SBE | N / N / + | T | 8 | 4 | 13 | 0.3 | −/+/+ | +/+/+ | +/+/+ | N | +/+/+ | N | +/+/N | 0/4 (0%) | |
| NU | NU | OCC | N | N | N | N | N | N | −/+/+ | +/+/+ | +/+/+ | N | +/+/+ | N | +/−/N | |||
| NU | NU | OLA | N | N | N | N | N | N | −/+/+ | +/+/+ | +/+/+ | N | +/+/+ | N | −/+/N | |||
| NU | NU | SBB | N | N | N | N | N | N | +/+/+ | +/+/− | +/+/+ | N | +/+/+ | N | −/+/N | |||
| Group 4A+5 A vs. Control Group 4B+5B: | 0.023 | |||||||||||||||||
Vaccinated donor (Vac), non-vaccinated donor (Non-Vac), or donor not used (NU).
Donor-recipient FLA compatibility of 90–100% (+), ≥50% (±), and <50% (−) (see Supplemental Table 1). MLR results are shown as either compatible (+) or not compatible (−).
Not done (N).
Enriched for T cells (T). The bolded number multiplied by 106 represents the total number of T cells transferred. PBS added (PBS) instead of cells. Numbers listed for all populations denoted in the column header display actual numbers for each cell phenotype transferred.
The non-B and non-T lymphocyte contamination accounted for 0.1%-3.0% (absolute number of 0.4×105-1.3×106 transferred) compared to the total T- and B-cell population of the T-cell preparation transferred with each A-T. The non-T and non-B populations are negative for CD3, CD4, CD8, CD21, IgG, and IgM.
T-cell count also includes CD3+CD4−CD8− and CD3+CD4+CD3+ T-cell counts. Total T-cell count for donor QVF in Study 5 is higher than the combined count for CD3+CD4+ and CD3+CD8+ T cells mainly due to the presence of CD3+CD4+CD8+ T cells.
FIV antibodies, virus isolation (Isolation), and proviral PCR (PCR) shown as either positive (+) or negative (−). Virus isolation on lymph node (L), bone marrow cells (B), and thymocytes (Th) performed at the termination of each study.
3. Results
3.1. A-T studies against homologous FIVPet challenge
Adoptive transfer studies began with Study 1 (Table 2), which was a small pilot study to determine if A-T of the T-cell enriched population from vaccinated semi-inbred cats could confer protection in the semi-inbred siblings against homologous FIVPet challenge. These donor-recipient pairs were FLA matched based solely on MLR analysis. Although not statistically significant, A-T protection was observed in 75% (3 of 4) of T-cell recipients, whereas no (0 of 2) A-T protection was observed in B cell recipients.
In Study 2 (Table 2), T cells were separated into CD4+ and CD8+ T cells to determine which population confers A-T protection. Complete protection was observed in all T cells recipients from vaccinated/MHC-matched siblings. In comparison, only 2 of 3 (67%) recipients receiving either CD4+ T cells or CD8+ T cells were protected. Notably, the unprotected cats received the lowest number of CD4+ (24×106) or CD8+ (14×106) T-cells and were either partially matched (CD8+ T-cell recipient [BDK]) in both FLA class-I and -II or completely FLA matched (CD4+ T-cell recipient [BDJ]). Additionally, partially-to-completely FLA-matched recipients of CD8+ or CD4+ T cells in these groups were protected against the same challenge (Table 2, Study 2A, [BDH, BDD, QVF, and QVG]).
Studies 1 and 2 used 4X vaccinated donors in Year 1, whereas Study 3 (Table 2) used vaccinated donors which were immunized annually for 5 years (1X-3X per year). Six of 7 recipients of the T-cell enriched population from vaccinated donors were protected against homologous FIVPet whereas all control cats were infected. The one unprotected cat (DVA) had only 50% and 25% compatibility with donor at FLA class-I and class II, respectively. In contrast, the protected cat (VSF), which received more donor T cells than the unprotected cat (DVA), had 50% FLA class-I and 33% FLA class-II compatibility with the donor. Nevertheless, these three studies demonstrated that T cells are important in dual-subtype vaccine protection against homologous FIVPet. Moreover, such anti-FIV T-cell immunity was conferred by donor cats receiving 1X-3X boosts per year for 5 years.
3.2. A-T studies against heterologous-subtype FIVFC1 challenge
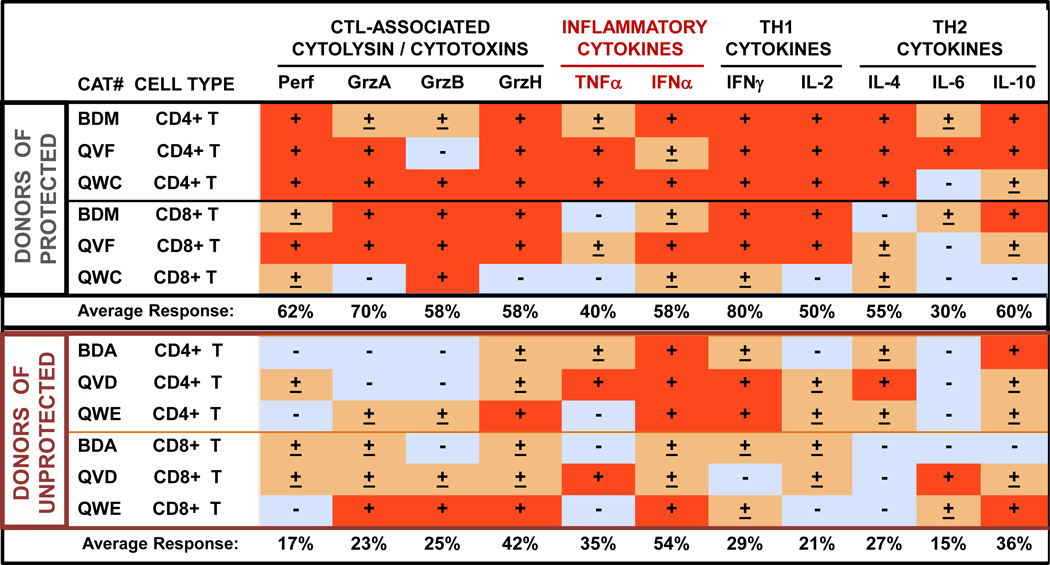
In order to determine that T cells are essential in conferring protection against both homologous and heterologous subtype viruses, two A-T studies against vaccine-induced NAb-resistant, heterologous-subtype FIV challenge were performed using a highly-divergent heterologous subtype FIVFC1. T cells from 2-year vaccinated donors were used in Study 4, and those from 6-year vaccinated donors were used in Study 5 (Table 3). Three of 6 cats in Study 4 and 3 of 6 cats in Study 5 receiving partially-to-completely FLA-matched T-cell population resisted challenge (Table 3). However, when the recipients of partially FLA-matched T-cell transfer were removed, the protection rates increased to 3 of 4 for Study 4 and 3 of 5 for Study 5. In Study 4, donor CD4+ and CD8+ populations were monitored for CTL-activity specific to FIV through mRNA expression of cytolysin, cytotoxins, and cytokines. High levels of cytolysin (perforin) and cytotoxins (perforin, GrzA, GrzB, and GrzH) and Th1 cytokines were observed in CD8+ T cells and/or CD4+ T cells in donors (BDM, QVF, QWC) of protected A-T recipients from Study 4 (Fig. 1, top set). In contrast, the T cells from donors (BDA, QVD, QWE) of unprotected recipients produced minimal levels of both IL2 and CTL-associated mRNAs with high IFNα and IFNγ mRNA levels in CD4+ T cells (Fig. 1, bottom set). All donors in Study 4 received 4 vaccinations before their T cells were used for A-T. However, T cells from donor QVD at Years 5 and 6 of vaccination conferred A-T protection to recipients VSD and SBC in Studies 3 and 5, respectively. After Study 4 in Year 1 of vaccination, QVD had received prototype FIV vaccinations 2X annually prior to Studies 3 and 5.
Fig. 1.
Cytokine and cytotoxin mRNA levels of vaccinated A-T donors. The chart (A) consists of three A-T donors of protected recipients from Study 4 (Year 1) (top set) and three A-T donors of unprotected recipients (bottom set). All % relative densitometric values are considered negative (blue box) or positive (red box) when the media control-subtracted values are <3% or >10%, respectively. Those values between 3%-10% are assigned (±) in an orange box. The relative densitometric value of each band is compared to the corresponding β-actin band and the media control for the corresponding target mRNA is subtracted. Abreviations: cytotoxic T lymphocyte-associated (CTL-assoc.), T helper-1 (Th1), T helper-2 (Th2), media control (M), inactivated dual-subtype FIV viruses (F), staphylococcal enterotoxin A ( (S), perforin (Perf), granzyme A (GrzA), granzyme B (GrzB), granzyme H (GrzH), tumor necrosis factor-α, interferon-α (IFNα), interferon-γ (IFNγ), interleukin (IL). Average response in percentage refers to the mean of the CD4+ and CD8+ T-cell response for the two groups of 3 cats each (Donors of Protected and Donors of Unprotected. Note that donors QVD, BDA, and QVF were used in Studies 3–5, whereas donor BDM was used in Studies 3 and 4. The mRNA results of all donors are from Study 4 performed in Year 1 of vaccination.
Furthermore, the quality of anti-FIV T-cell immunity improved with long-term vaccination (section 3.3 below). Hence, only those cats that received at least 17×106 T cells, received donor T cells with strong anti-FIV activities, and received 100% FLA-matched donor T cells were protected against heterologous-subtype pathogenic FIVFC1 challenge (Table 3).
3.3. The quality of T-cell immunity induced over 4 years
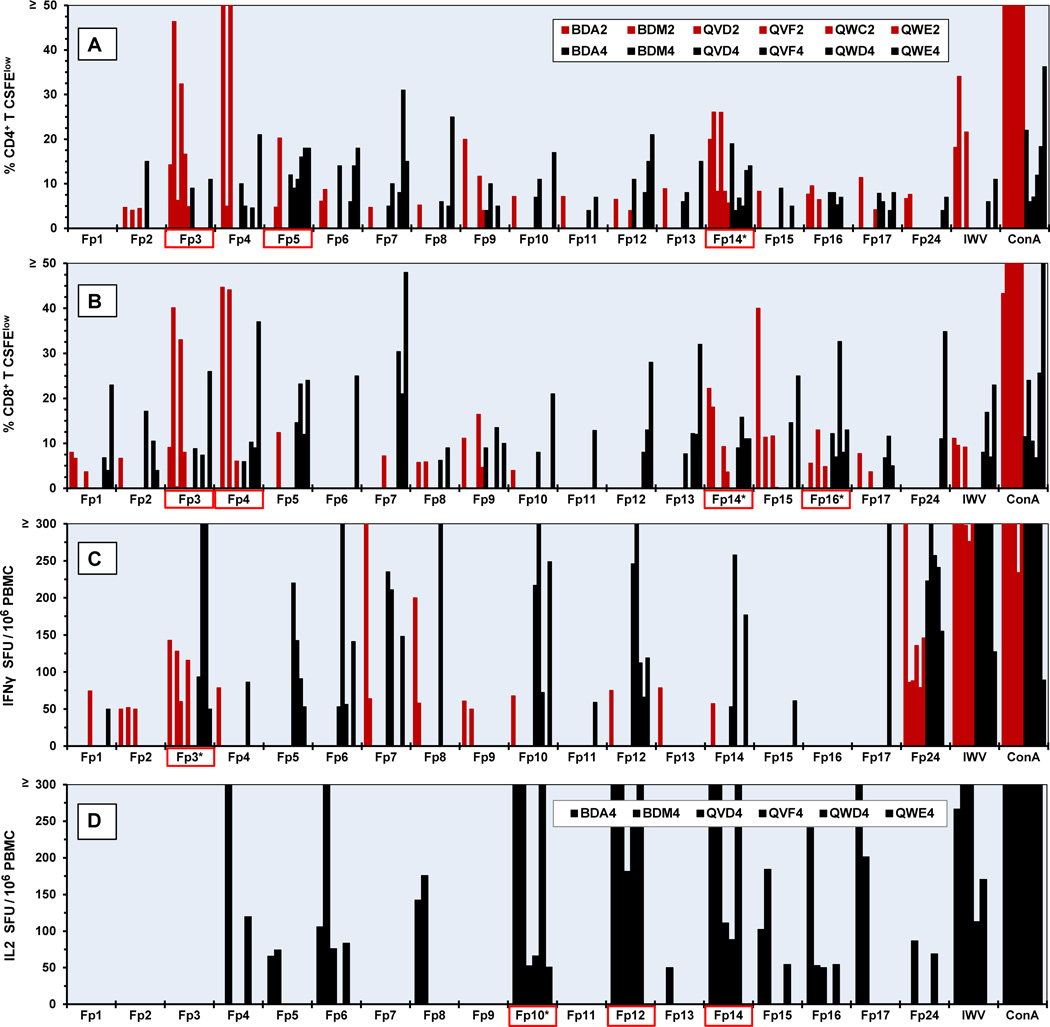
Proliferation of CD4+ and CD8+ T cells were observed against multiple regions of FIV p24 using peptide pools (Fig. 2A and 2B). Six vaccinated donors were used in Year 2 and the same five donors with the exception of the replacement of cat QWC by long-term vaccinated littermate QWD due to death of QWC before year 4. These cats were vaccinated annually and their responses to overlapping peptide pools were evaluated in Years 2 and 4 (Fig 2). Notably, more proliferation responses were detected in Year 4 than Year 2 of vaccination (CD4+ T-cell responses: 56 vs. 38; CD8+ T-cell responses: 50 vs. 31). A slightly higher magnitude and greater numbers of IFNγ responders were observed in the PBMC of vaccinated A-T donors in Year 4 than Year 2 (IFNγ responses: 33 vs. 19) (Fig. 2C). The IL2 assay was unavailable in Year 2 of vaccination and analyzed only for Year 4. High number and magnitude of IL2 responses were observed to peptide-pools Fp10, Fp12, and Fp14 (Fig. 2D).
Fig. 2.
Cellular immunity to FIV p24 peptide pools on Years 2 and 4 of vaccination. CD4+ (A) and CD8+ (B) T-cell proliferation in response to overlapping FIV p24 peptide pools (Fp1-Fp17) are shown for six A-T donors in Year 2 (red bar) and Year 4 (black bar). The IFNγ (C) and IL2 (D) production in response to the p24 peptide pools are shown for Years 2 and 4 for IFNγ and Year 4 for IL2. Cat identification codes are depicted in A (inset) for panels A-C. Each donor is designated as either 2 for Year 2 or 4 for Year 4 following the cat identification code (i.e., BDA2 represents BDA’s responses at Year 2 and BDA4 represents BDA’s responses at Year 4). The order of donors in the inset from left to right corresponds to the order of the bars shown from left to right. Each peptide pool contains 3–4 peptides with an overlap of 8 amino acids. Those peptide pools denoted with an asterisk have the highest number of combined Year-2 and Year-4 responses and those with red-lined box have ≥80% of the total number of possible responses per peptide pool. The threshold of IL2 and IFNγ production and T-cell proliferation are set at 50 SFU/ 106 PBMC and ≥4% CFSElow, respectively. Abbreviations: inactivated dual-subtype FIV viruses (IWV), T-cell mitogen concanavalin A (ConA), spot forming unit (SFU), CFSE-based proliferation of CD4+ T cells (% CD4+ T CFSElow), CFSE-based proliferation of CD8+ T cells (% CD8+ T CFSElow).
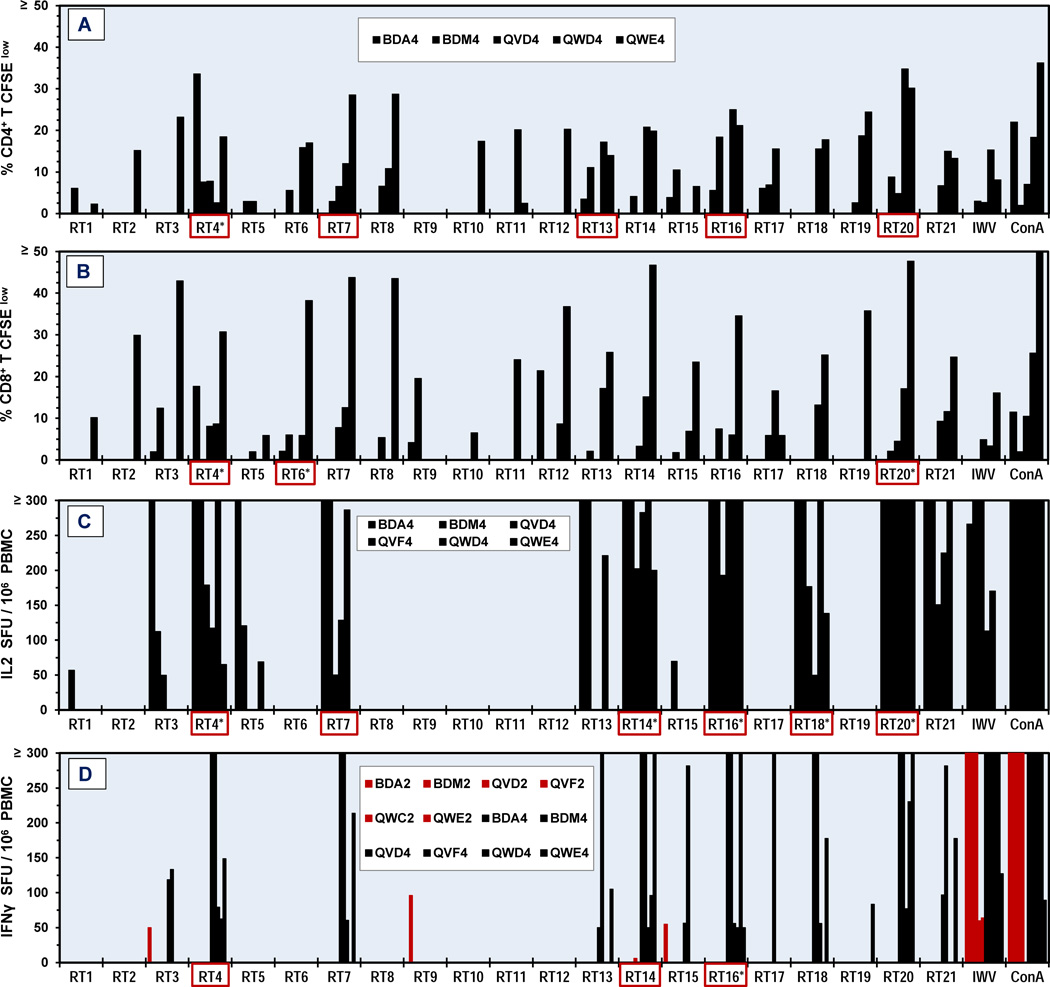
Since IFNγ responses to the overlapping FIV RT peptide pools in Years 1 and 2 (Fig. 3D, Year 2 shown) of vaccination were minimal, T-cell proliferation assays were not performed until Year 4. Both the CD4+ and CD8+ T cells had moderate-to-high responses to multiple RT pools (Fig. 3A and 3B). The PBMC from Year 4 had IL2 and IFNγ responses to multiple RT pools (Fig. 3C and 3D). Remarkably, no responses were detected to pools RT8 or RT12 when these peptide pools induced substantial T-cell proliferation.
Fig. 3.
Cellular immunity to FIV RT peptide pools on Year 4 of vaccination. CD4+ (A) and CD8+ (B) T-cell proliferation and IL2 (C) and IFNγ (D) production in response to overlapping FIV RT peptide pools (RT1-RT21) are shown for five (A,B) to six (C,D) A-T donors from Year 4 (black bar). The IFNγ production for Year 2 is also shown (D, red bar) but one donor (QWC2) in Year 2 has been replaced with a different donor (QWD4) in Year 4. Each peptide pool contains 4–5 peptides with an 8-amino acid overlap. The insert for A is also for B. Those peptide pools with asterisk have the highest number of Year 4 responses and those with a red box have ≥80% of the total number of possible responses per peptide pool. The threshold of IL2 and IFNγ production and T-cell proliferation are 50 SFU/106 PBMC and ≥2% CFSElow, respectively. Abbreviations are the same as Fig. 2 legend.
4. Discussion
In a previous A-T study using parent-to-offspring half-matched FLA, only 36% protection was observed against medium-to-high (20–50 CID50) challenge doses with homologous FIVPet [18]. However, in a completely FLA-matched system produced by bone marrow transplantation (BMT), adoptive transfer of washed whole blood from vaccinated/BMT recipient to BMT donor or BMT-recipient sibling conferred 100% protection against a high dose (100 CID50) of homologous FIVPet challenge [18]. Since these studies used either purified PBMC or washed whole blood, the current studies performed with T cells achieved significant protection in 87% (13 of 15, p<0.001; Studies 1–3, Table 2) of recipients receiving partial-to-complete FLA-matched T cells against homologous FIVPet.
In comparison, only A-T of T cells from 100% FLA-matched, vaccinated donors conferred protection to recipients against vaccine-induced NAb-resistant, heterologous-subtype FIVFC1 (Table 3). In Study 4, the lack of FLA class-I and/or class-II compatibility in recipients BDD and BDQ as well as the low number of T cells for adoptive transfer in recipient BDD may have contributed to the lack of protection observed. However, in Study 5 (Table 3), 2 of 3 unprotected cats (WFA and OCE) were completely FLA matched with their donors but received the lowest numbers of T cells which may indicate that the number of T cells transferred was also an important factor in protection against FIVFC1. The other unprotected cat (OCD, Study 5) received a low number of T cells but from a FLA-unmatched donor.
Most importantly, anti-FIV T-cell activities with high cytokine and cytotoxin production induced by the prototype FIV vaccine were vital in conferring protection. Since the commercial FIV vaccine also conferred protection against FIVFC1 and other moderately NAb-resistant heterologous FIV strains [21], the anti-FIV T-cell immunity generated by the commercial vaccine was most likely the immunity that conferred protection. For example, although unprotected recipient QVG received the highest amount of completely FLA-matched T cells from donor QVD, this donor’s CD4+ and CD8+ anti-FIV T-cell immunity, as measured by cytolysin and cytotoxin mRNA production to FIV antigen, appeared similar to the profile (Fig. 1, bottom set) of other donors (BDA, QWE) which failed to confer protection.
T-cell immune responses to FIV p24 peptide pools were detected as early as Year 1 of vaccination in previous publications [20,28] and Year 2 of vaccination in the current study (Fig. 2, Year 2) which increased with annual boosts (Fig. 2, Year 4). Although IFNγ responses to p24 peptide pools were detected as early as Year 1, IFNγ responses to RT peptide pools were not detected until Year 3 (Fig. 3D). The reason for the difference in responses between the p24 and RT peptide pools is most likely due to the major difference in the amount of these proteins expressed by the virus present in the vaccine. Both prototype and commercial FIV vaccines contain a large amount of p24 and a very low amount of RT [30]. Thus, T-cell epitopes on p24 may be involved in protection throughout Years 1–6 of vaccination but those on RT may be involved in protection starting in Years 3–4.
While a large number of recipients of A-T of T cell enriched populations from FIV-vaccinated cats were performed in Studies 1–5 (Tables 2 and 3), recipients of A-T consisting of B cells from FIV vaccinated cats was limited to Study 1 (Table 2). Although the result from Study 1 is not statistically significant, T cells appeared to be involved in conferring A-T protection, but the number of B-cell recipients was too small to determine whether B cells alone can confer A-T protection. In regards to the potential of B cells to afford protection to recipients of A-T, studies by others have shown that the commercial dual-subtype FIV vaccine [31] as well as priming T cells with FIVPet Env pDNA [32] induced high NAbs to FIVPet. However, in current studies, all recipients of A-T from vaccinated cats, except for the unprotected recipients, had no NAbs to FIVPet or FIVFC1 (data not shown) and no FIV Abs (Tables 2 and 3, immunoblot). Furthermore, both NAbs and FIV Abs were not detected in the sera from the protected recipients tested on day 3 or 7 after A-T (data not shown). Similarly, protection against FIV challenges in the absence of NAbs has been reported with FIV-ΔRT pDNA vaccine and inactivated FIV-infected cell vaccine [33,34].
Limitations imposed on control group selection were primarily a consequence of resource availability as the number of semi-inbred cats that could be used for vaccination, including control vaccinations, was limited. Due to the timing required to use consistent age-matched recipients for the adoptive transfer studies, these studies were completed in smaller, manageable sized groups. Load limitations of magnetic sorting also limited the number of cats which could be processed for adoptive transfer in a setting of one full day. In our previous vaccine studies, active vaccination of naïve cats with uninfected vaccine cell line (FeT-J) in FD-1 adjuvant, FD-1 adjuvant/HuIL12, and FD-1 adjuvant containing FeIL12 conferred no protection [21]. Therefore, the potential for protection against FIV challenge in a control group, consisting of recipients by A-T of T-cell enriched populations from donors immunized with vaccine formulations identical to the commercial and prototype vaccines but with non-specific antigen (e.g., FeT-J without FIV antigen), was reasonably low. Resource availability was also an issue with blood draws, as 20% blood volume was collected from vaccinated donors solely to support the sorting of PBMC into the largest T-cell enriched population possible for A-T.The subsequent blood draw for analysis of T-cell mediated immunity was delayed 6–8 weeks to allow for an adequate refractory period per University of Florida IACUC protocols.
The recent CMV-vectored SIV vaccine study demonstrated the importance of anti-SIV CD8+ T cells [16,17]. In human vaccine trials, the CD4+ CTL activities to the V2 region of HIV-1 surface envelope were observed in vaccinated subjects of the most successful human vaccine trial RV144 [35,36]. These studies using HIV-positive subjects and the SIV/macaque model demonstrate the critical role played by the T cells in lentiviral protection [10–16]. Current A-T Study 2 also demonstrated the importance of both anti-FIV CD4+ and CD8+ T cells in conferring protection with prototype FIV vaccine and most likely with commercial FIV vaccine [21]. Thus, efforts should be made in developing a T-cell based vaccine against HIV-1 and FIV.
Supplementary Material
Highlights.
23/33 recipients of adoptive T-cell transfer from vaccinated donors were protected.
81% (17/21) protection was against homologous FIV (vaccine strain).
50% (6/12) protection was against neutralizing Ab-resistant, different-subtype FIV.
Protection required >60% to 100% MHC-matching between each donor-recipient pair.
T cells mediate protection conferred by prototype and commercial FIV vaccines.
Acknowledgments
This work was supported by NIH R01-AI03904 (JKY), Miscellaneous Donors Fund (JKY), and the Clinical and Translational Science Award RL1 TR00066 (SRR). JKY is the inventor of record on a patent held by the University of Florida and may be entitled to royalties from companies developing commercial products related to the research described in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mylvaganam GH, Silvestri G, Amara RR. HIV therapeutic vaccines: moving towards a functional cure. Curr Opin Immunol. 2015;35:1–8. doi: 10.1016/j.coi.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ensoli B, Cafaro A, Monini P, Marcotullio S, Ensoli F. Challenges in HIV Vaccine Research for Treatment and Prevention. Front Immunol. 2014;5:417. doi: 10.3389/fimmu.2014.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Goede AL, Vulto AG, Osterhaus AD, Gruters RA. Understanding HIVinfection for the design of a therapeutic vaccine. Part II: Vaccination strategies for HIV. Ann Pharm Fr. 2015;73(3):169–179. doi: 10.1016/j.pharma.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mcllroy D. Do HIV-specific CTL continue to have an antiviral function during antiretroviral therapy? If no, why not, and what can be done about it? Frontiers Immunol. 2013;4:52. doi: 10.3389/fimmu.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siliciano RF. Targeting reservoirs to clear and cure. Nat. Med. 20(5):480–481. doi: 10.1038/nm.3550. [DOI] [PubMed] [Google Scholar]
- 7.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 6th. Philadelphia: Saunders; 2010. B cell activation and antibody production; pp. 215–241. [Google Scholar]
- 9.Sanou MP, De Groot AS, Murphey-Corb M, Levy JA, Yamamoto JK. HIV-1 Vaccine Trials: Evolving Concepts and Designs. The Open AIDS J. 2012;6:274–288. doi: 10.2174/1874613601206010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279(5359):2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 11.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75(24):11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76(5):2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;89(6):991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 15.Wick WD, Yang OO. Biologically-directed modeling reflects cytolytic clearance of SIV-infected cells in vivo in macaques. PLoS One. 2012;7(9):e44778. doi: 10.1371/journal.pone.0044778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(469):100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340(6135):1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu R, Omori M, Okada S, Rine SL, Lewis BA, Lipton E, et al. MHC-restricted protection of cats against FIV infection by adoptive transfer of immune cells from FIV-vaccinated donors. Cellular Immunol. 1999;198(1):30–43. doi: 10.1006/cimm.1999.1574. [DOI] [PubMed] [Google Scholar]
- 19.Omori M, Pu R, Tanabe T, Hou W, Coleman JK, Arai M, et al. Cellular immune responses to feline immunodeficiency virus (FIV) induced by dual-subtype FIV vaccine. Vaccine. 2004;23(3):386–398. doi: 10.1016/j.vaccine.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Uhl EW, Martin M, Coleman JK, Yamamoto JK. 2008 Advances in FIV vaccine technology. Veterinary Immunol Immunopathol. 2008;123(1–2):65–80. doi: 10.1016/j.vetimm.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman JK, Pu R, Martin MM, Noon-Song EN, Zwijnenberg R, Yamamoto JK. Feline immunodeficiency virus (FIV) vaccine efficacy and FIV neutralizing antibodies. Vaccine. 2014;32(6):746–754. doi: 10.1016/j.vaccine.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto JK, Sanou MP, Abbott JR, Coleman JK. Feline immunodeficiency virus model for designing HIV/AIDS vaccines. Curr HIV Res. 2010;8(1):14–25. doi: 10.2174/157016210790416361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gengozian N, Reyes L, Pu R, Homer BL, Bova FJ, Yamamoto JK. Fractionation of feline bone marrow with the soybean agglutinin lectin yields populations enriched for erythroid and myeloid elements: transplantation of soybean agglutinin-negative cells into lethally irradiated recipients. Transplantation. 1997;64(3):510–518. doi: 10.1097/00007890-199708150-00022. [DOI] [PubMed] [Google Scholar]
- 25.Dean GA, Reubel GH, Moore PF, Pedersen NC. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J Virol. 1996;70(8):5165–5169. doi: 10.1128/jvi.70.8.5165-5169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brick C, Atouf O, Bouayad A, Essakalli M. Moroccan study of HLA (−A, −B, −C, −DR, −DQ) polymorphism in 647 unrelated controls: Updating data. Mol Cell Probes. 2015 doi: 10.1016/j.mcp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Coleman JK, Pu R, Martin M, Sato E, Yamamoto JK. HIV-1 p24 vaccine protects cats against feline immunodeficiency virus infection. AIDS. 2005;19(14):1457–1466. doi: 10.1097/01.aids.0000183627.81922.be. [DOI] [PubMed] [Google Scholar]
- 28.Abbott JR, Pu R, Coleman JK, Yamamoto JK. Utilization of feline ELISPOT for mapping vaccine epitopes. Methods Mol Biol. 2012;792:47–63. doi: 10.1007/978-1-61779-325-7_4. [DOI] [PubMed] [Google Scholar]
- 29.Roff SR, Sanou MP, Rathore MH, Levy JA, Yamamoto JK. Conserved epitopes on HIV-1, FIV and SIV p24 proteins are recognized by HIV-1 infected subjects. Hum Vaccin Immunother. 2015;11:6. doi: 10.1080/21645515.2015.1026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto JK, Okuda T, Ackley CD, Louie H, Pembroke E, Zochlinski H, et al. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Human Retroviruses. 1991;7(11):911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- 31.Beczkowski PM, Harris M, Techakriengkrai N, Beatty JA, Willet BJ, Hosie MJ. Neutralising antibody response in domestic cats immunised with a commercial feline immunodeficiency virus (FIV) vaccine. Vaccine. 2015;33(8):977–984. doi: 10.1016/j.vaccine.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pistello M, Bonci F, Zabogli E, Conti F, Freer G, Maggi F, et al. Env-expressing autologous T lymphocytes Induce neutralizing antibody and afford marked protection against feline immunodeficiency virus. J Virol. 2010:3845–3856. doi: 10.1128/JVI.02638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro L, et al. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing Antibodies. J Virol. Jan. 1996:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosie MJ, Flynn N, Rigby MA, Cannon C, Dunsford T, Mackay N, Argyle D, Willett BJ, Miyazawa T, Onions DE, Jarrett O, Neil JC. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1998;72:7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 36.de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, Nuntapinit B, et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol. 2012;188(10):5166–5176. doi: 10.4049/jimmunol.1102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.