Abstract
Background
Prophylactic cranial irradiation (PCI) has become a standard option for extensive stage small cell lung cancer (ES-SCLC) patients. CALGB 30504 was a randomized phase II study of sunitinib vs placebo in ES-SCLC patients responding to platinum-based therapy requiring pre-enrollment brain imaging. PCI was at the discretion of treating physicians. We performed a secondary analysis of CALGB 30504 to determine the impact of PCI on ES-SCLC patients.
Methods
Fisher’s exact and Wilcoxon rank-sum tests were conducted to test the differences between PCI and non-PCI patients. Kaplan-Meier analyses described PFS and OS for PCI and non-PCI patients.
Results
85 patients received maintenance (41 placebo, 44 sunitinib). 41 received PCI, 44 did not. Characteristics were balanced between PCI and no-PCI patients. PCI patients receiving sunitinib had non-significant 2.7-month PFS improvement (5.0 months vs. 2.3 months, p=0.14, HR=0.62 (95% CI: 0.33–1.18)), trending toward improved OS (8.9 months vs. 5.4 months, p=0.053, HR: 0.47 (0.22–1.03)). PCI was associated with a trend toward improved median PFS (2.9 months vs. 2.2 months, p=0.096, HR=0.69 (95% CI 0.45–1.07)), but not median OS (PCI 8.3 months vs. no PCI 8.7 months, p=0.76, HR=1.07 (95% CI 0.67–1.71)). Placebo patients had no PFS or OS difference.
Conclusions
Trends for improved PFS and OS were seen in patients receiving PCI and sunitinib supporting the need for further prospective research evaluating the integration of maintenance systemic therapy and PCI in ES-SCLC. Improved outcomes for ES-SCLC patients after induction chemotherapy may require PCI and systemic therapy to achieve control of both intracranial and extracranial disease.
Keywords: Prophylactic cranial irradiation, extensive stage small cell lung cancer, sunitinib, maintenance chemotherapy, survival
Introduction
Prophylactic cranial irradiation (PCI) is an established treatment for small cell lung cancer (SCLC). While initially shown to provide a survival benefit for extensive stage (ES) patients who had a complete response after chemotherapy[1], further studies found similar benefits in those who had any favorable response to chemotherapy in one trial and stable disease or better in a pooled analysis[2, 3]. However, some oncologists have questioned the value of PCI in ES-SCLC since one of these studies did not require brain imaging prior to enrollment[2], raising the possibility that the benefit may have been due to treatment of occult brain metastases. Additionally, others have questioned these data because of the use of a wide range of radiation dose-fractionation regimens and non-platinum-based systemic therapy. In fact, one randomized study, specifically undertaken to address these concerns, that included a standardized PCI dose, pre-PCI brain imaging, and platinum-based systemic therapy was closed early due to futility. In that study, ES-SCLC patients randomized to the PCI arm had a trend towards worse survival than those patients who received no PCI (10.1 months vs. 15.1 months, p=0.091 (HR=1.38, 95% CI: 0.95–2.01)[4].
Cancer and Leukemia Group B (CALGB) 30504 was a double-blinded randomized phase II trial comparing maintenance sunitinib to placebo for untreated ES-SCLC that had disease control after up to six cycles of standard platinum-based chemotherapy. The CALGB 30504 protocol stated that patients having a partial response (PR) or complete response (CR) after chemotherapy should be offered PCI and almost half of patients with responding tumors received PCI. Given these uncertainties as to the role of PCI for ES-SCLC patients, we conducted a secondary analysis of survival outcomes in relation to PCI for CALGB 30504. We hypothesized that the patients on CALGB 30504, who received brain imaging prior to registration, standard platinum-based chemotherapy and a standardized PCI regimen, were more representative of ES-SCLC patients receiving standard clinical care compared to some clinical trials evaluating PCI in ES-SCLC. Furthermore, while PCI was recommended for all patients responding to systemic therapy on CALGB 30504, it was not administered to approximately half of patients achieving partial response with chemotherapy for undocumented reasons. Therefore, we hypothesized that an analysis of the cohorts of patients who received and did not receive PCI in CALGB30504 could contribute to a better understanding of the impact of PCI in ES-SCLC.
Methods
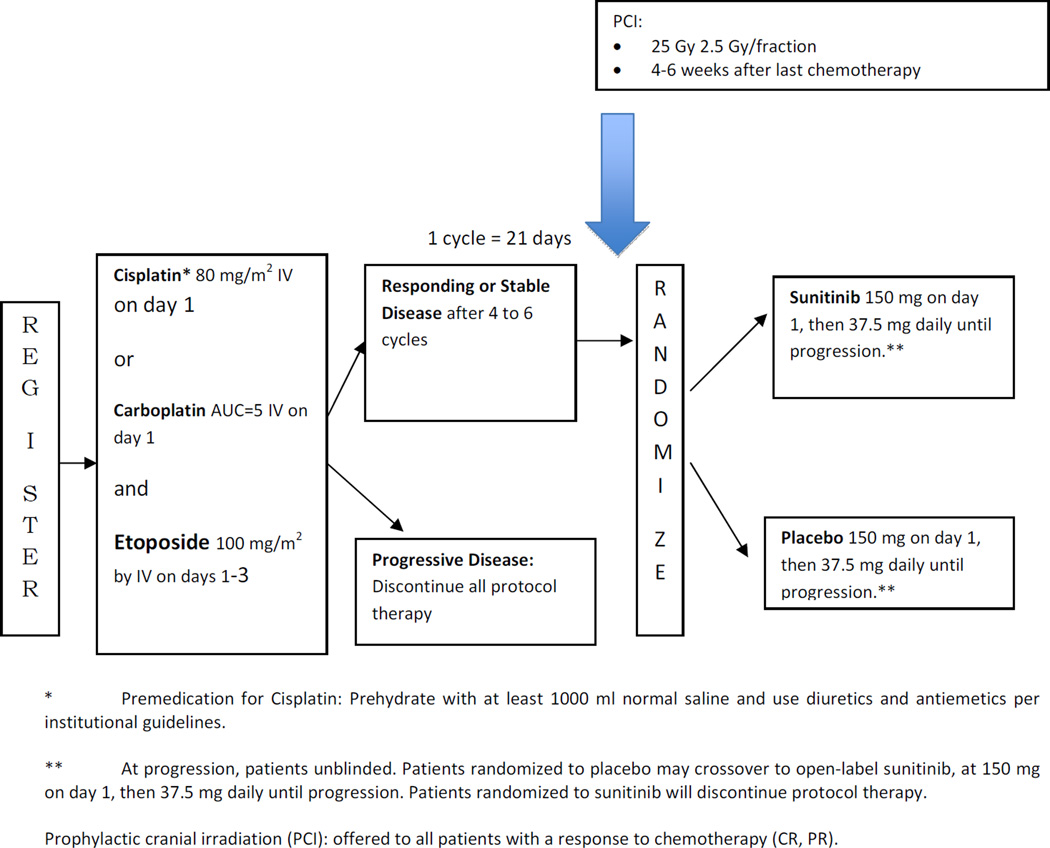
The methods of CALGB 30504 have been published [5]. Briefly, each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. Four to six cycles of etoposide 100 mg/m2 day 1–3 and either carboplatin AUC=5 or cisplatin 80 mg/m2 day 1 were administered in 21-day cycles, followed by maintenance sunitinib versus placebo in patients with stable disease or a response. The trial schema is shown in Figure 1. Prior to registration, patients were staged with contrast-enhanced CT or MRI of the brain, CT or MRI of the chest (including liver and adrenals), and either a bone scan or PET scan (all within 42 days prior to registration). Patients achieving disease control after at least 4 and no more than 6 cycles of chemotherapy were randomized double-blind to receive either placebo or sunitinib. Sunitinib was given at 150 mg PO day 1 followed by 37.5 mg PO daily until progression. Patients were evaluated every 2 cycles (every 6 weeks)on maintenance therapy with CT of the chest (including liver and adrenals). At time of progression, patients randomized to placebo were allowed to cross over to sunitinib within 14 days. The primary and secondary objectives of the trial were to determine if maintenance sunitinib would improve progression-free survival (PFS) and overall survival (OS), as was recently reported[5].
Figure 1.
CAGB 30504 schema
PCI was recommended, but not required, for all patients with a PR or CR at the completion of 4–6 cycles of chemotherapy. The recommended dose was 25 Gy in ten 2.5-Gy fractions to a standard whole brain volume, within 4–6 weeks following the last cycle of chemotherapy. Sunitinib was to be held for 2 days prior, during, and 2 days after the completion of PCI.
Statistical Methods
We performed this retrospective secondary analysis to investigate the effect of PCI in this prospectively selected cohort of patients treated uniformly with standard brain imaging obtained prior to treatment. We also examined the possibility of an interaction between maintenance sunitinib and PCI in an ES-SCLC population. Fisher’s exact test for categorical variables[6], and Wilcoxon rank-sum test[7] for continuous variables were conducted to test the differences between patients who received and who did not receive PCI in terms of demographics and clinical characteristics prior to chemotherapy, and the patterns of progression following all therapy. Kaplan-Meier curves were provided to visually describe PFS and OS for patients who received and who did not receive PCI treatment[8]. PFS was defined as the time from randomization to disease progression and/or death from any cause, whichever came first. OS was defined as the time from randomization to death from any cause; living patients were censored at the date of last follow-up. Two-sided p values were reported if otherwise stated. All data collection and statistical analyses were performed by the statisticians at the Alliance Statistics and Data Center on the platform of SAS (version 9.3; SAS Institute Inc., Cary, North Carolina). Differences between Kaplan-Meier curves were identified using the log-rank test. P values of <0.05 were considered statistically significant. Data lock was on January 12, 2015.
Results
For the entire study, enrollment was 144; 85 patients were randomized between November 2008 and December 2011 and received maintenance therapy. Forty-one patients (48%) received PCI, while 44 (52%) did not. There was no statistical difference between the deliveries of PCI in either the sunitinib or placebo arm (p=1.00). All patient characteristics were balanced between patients who received PCI and those that did not, except for a numerically, but not statistically significant lower, median age (58 vs. 62.5 years, p=0.17) in the PCI cohort as shown in Table 1. The number and distribution of metastases at registration were also not statistically different between patients who were treated with PCI and those who were not, as shown in Table 2. The median PCI dose used was 25 Gy (range: 25–37.5 Gy). The median time to PCI delivery from randomization was 16 (12 –49) days, although 6 patients started PCI before (n=5) or on (n=1) the randomization date.
Table 1.
Patients demographics and initial clinical characteristics
| PCI Not Received (N=44) |
PCI Received (N=41) |
Total (N=85) |
p value | |
|---|---|---|---|---|
| Arm | 1.0000 | |||
| Sunitinib | 23 (52.3%) | 21 (51.2%) | 44 (51.8%) | |
| Placebo | 21 (47.7%) | 20 (48.8%) | 41 (48.2%) | |
| Age (years) | 0.1694 | |||
| Median | 62.5 | 58.0 | 60.0 | |
| Range | (39.0–77.0) | (44.0–72.0) | (39.0–77.0) | |
| Sex | 0.8291 | |||
| Male | 19 (43.2%) | 19 (46.3%) | 38 (44.7%) | |
| Female | 25 (56.8%) | 22 (53.7%) | 47 (55.3%) | |
| ECOG Performance Status | 0.4785 | |||
| 0 | 22 (50.0%) | 15 (36.6%) | 37 (43.5%) | |
| 1 | 16 (36.4%) | 18 (43.9%) | 34 (40.0%) | |
| 2 | 6 (13.6%) | 8 (19.5%) | 14 (16.5%) | |
| Best response to chemotherapy | 0.6388 | |||
| CR | 2 (4.5%) | 3 (7.3%) | 5 (5.9%) | |
| PR | 37 (84.1%) | 36 (87.8%) | 73 (85.9%) | |
| Stable | 5 (11.4%) | 2 (4.9%) | 7 (8.2%) | |
| Grade 3+ AEs from chemotherapy | 34 (77.3%) | 29 (70.7%) | 63 (74.1%) | 0.6213 |
PCI, prophylactic cranial irradiation; ECOG, Eastern Cooperative Oncology Group; CR, complete response; PR, partial response; AE: adverse event.
Table 2.
Disease at baseline prior to chemotherapy
| PCI Not Received (N=44) |
PCI Received (N=41) |
Total (N=85) |
p value | |
|---|---|---|---|---|
| No. of sites | 0.6367 | |||
| Median | 4.5 | 5.0 | 5.0 | |
| Range | (1–10) | (2–8) | (1–10) | |
| No. of sites | 0.3615 | |||
| 1–2 | 4 (9.1%) | 1 (2.4%) | 5 (5.9%) | |
| 3–10 | 40 (90.9%) | 40 (97.6%) | 80 (94.1%) | |
| Sites specific | ||||
| Primary lung | 41 (93.2%) | 38 (92.7%) | 79 (92.9%) | 1.0000 |
| Contralateral lung | 10 (22.7%) | 5 (12.2%) | 15 (17.6%) | 0.2601 |
| Ipsilateral hilar nodes | 24 (54.5%) | 26 (63.4%) | 50 (58.8%) | 0.5091 |
| Contralateral hilar nodes | 7 (15.9%) | 7 (17.1%) | 14 (16.5%) | 1.0000 |
| Mediastinal nodes | 32 (72.7%) | 33 (80.5%) | 65 (76.5%) | 0.4509 |
| Ipsilateral supraclavicular/scalene nodes | 7 (15.9%) | 10 (24.4%) | 17 (20.0%) | 0.4188 |
| Contralateral supraclavicular/scalene | 5 (11.4%) | 5 (12.2%) | 10 (11.8%) | 1.0000 |
| Pleura | 5 (11.4%) | 7 (17.1%) | 12 (14.1%) | 0.5405 |
| Liver | 22 (50.0%) | 22 (53.7%) | 44 (51.8%) | 0.8290 |
| Adrenals | 9 (20.5%) | 5 (12.2%) | 14 (16.5%) | 0.3860 |
| Bone | 21 (47.7%) | 17 (41.5%) | 38 (44.7%) | 0.6636 |
| Other nodal | 11 (25.0%) | 10 (24.4%) | 21 (24.7%) | 1.0000 |
| Other sites | 8 (18.2%) | 9 (22.0%) | 17 (20.0%) | 0.7879 |
PCI, prophylactic cranial irradiation.
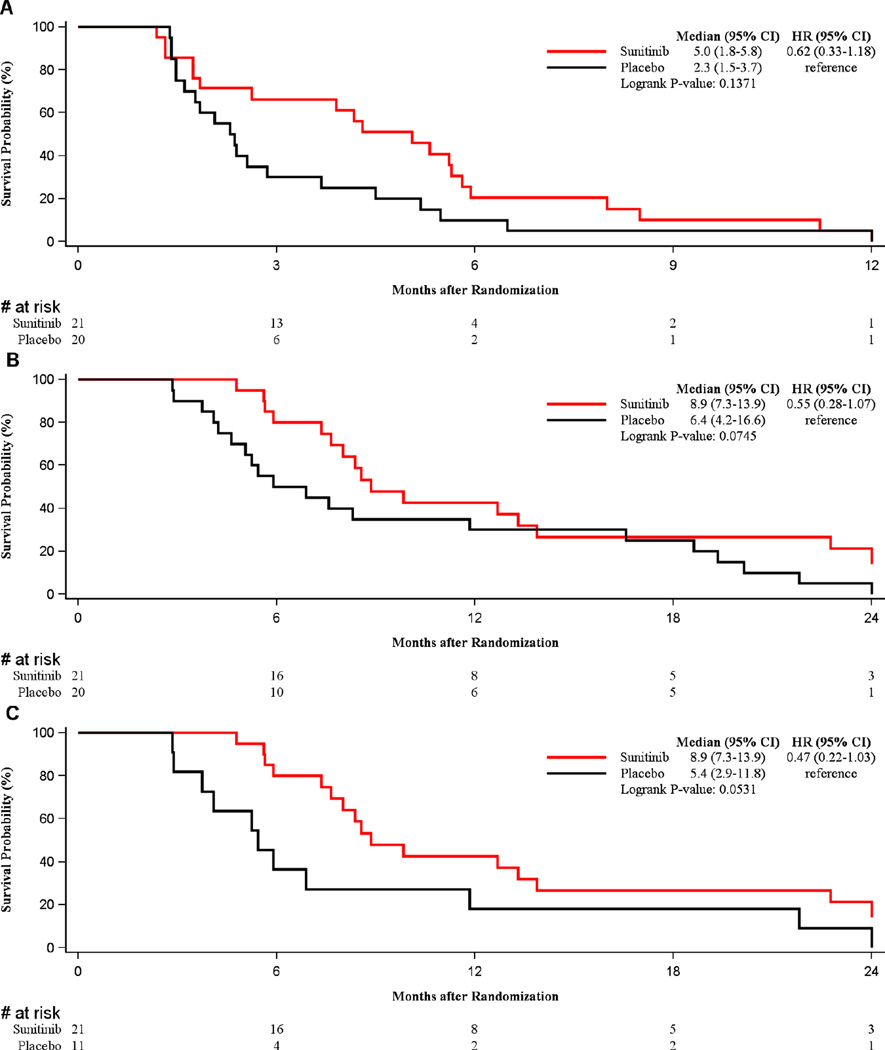
The impact of sunitinib in patients who had received PCI was evaluated. Interestingly, in patients receiving PCI, those who were randomized to the sunitinib arm had a doubling of PFS with a 2.7-month improvement that was not statistically significant (5.0 vs. 2.3 months, p=0.14, HR=0.62 (95% CI 0.33–1.18)) (Figure 2A). There was also a trend toward an improvement in OS (8.9 vs. 6.4 months, p=0.075, HR=0.55 (95% CI 0.28–1.07)) (Figure 2B). Eighteen patients on the placebo arm crossed over to sunitinib at time of progression. When crossover patients were excluded, this trend toward improvement in OS was magnified for PCI patients treated with sunitinib and approached statistical significance (8.9 months vs. 5.4 months, p=0.053, HR: 0.47 (95% CI 0.22–1.03)), as shown in Figure 2C. When restricted to patients who did not receive PCI, there was no difference in PFS or OS between patients randomized to receive sunitinib or placebo.
Figure 2.
A:Trend for PFS improvement in patients receiving PCI who were randomized to sunitinib compared to those receiving placebo
B & C: Improved OS in patients receiving PCI who were randomized to sunitinib compared to those receiving placebo.
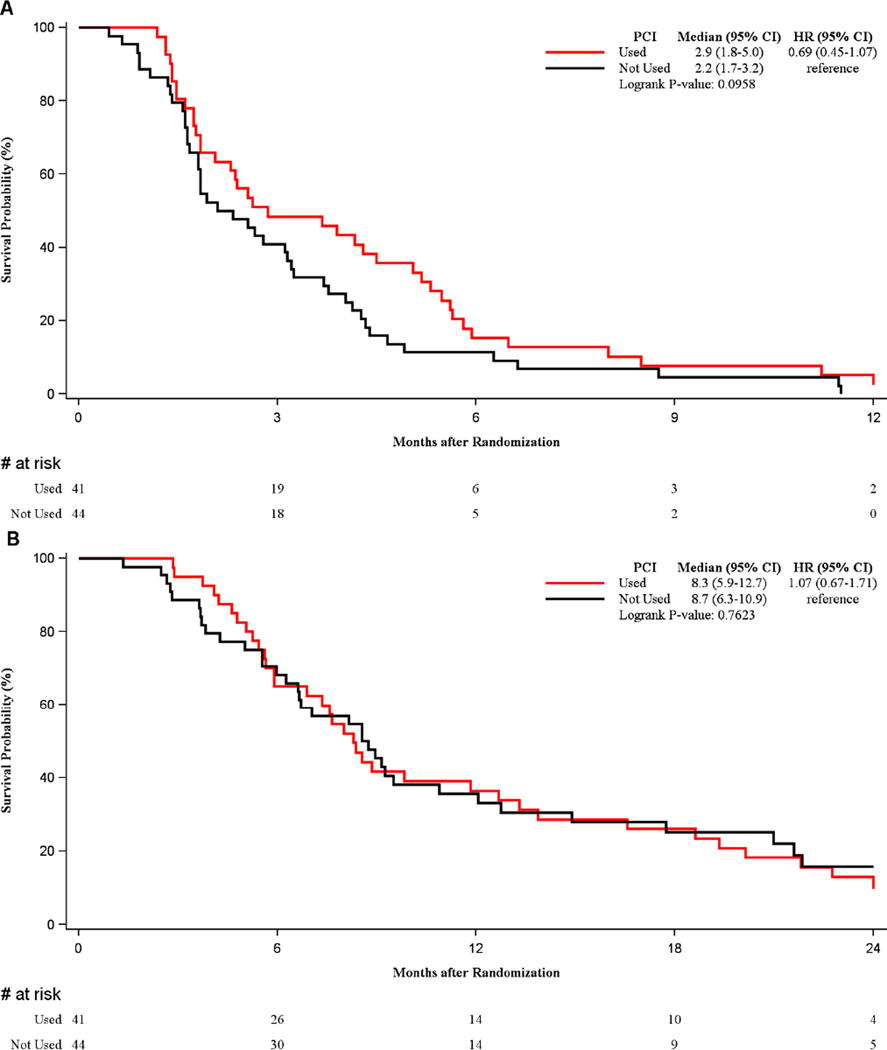
There was a trend for improved PFS in patients receiving PCI (median 2.9 months vs. median 2.2 months, p=0.096, HR=0.69 (95% CI 0.45–1.07)), but not OS as shown in Figure 3. In the patients randomized to placebo, there was no PFS or OS benefit associated with the use of PCI.
Figure 3.
A: PFS benefit seen with the use of PCI
B. Lack of OS improvement with the use of PCI
We next evaluated patterns of first progression to identify factors that might explain the outcome differences noted above. Central nervous system (CNS) progression was more than twice as common in the non-PCI group compared to those receiving PCI (27% vs. 12%, p=0.11), and was amongst the most common sites of progression in the non-PCI group as shown in Table 3. In patients who received PCI, the most common pattern of progression was in the thorax, with significantly increased hilar nodal progression (51% vs. 30%, p=0.05) and primary tumor progression (61% vs. 30%, p=0.005) compared to those who did not receive PCI.
Table 3.
Cumulative patterns of progression following all therapy.
| PCI Not Received (N=44) |
PCI Received (N=41) |
p value | |
|---|---|---|---|
| No. of sites | 0.1278 | ||
| Median | 2 | 2 | |
| Range | (0–8) | (0–5) | |
| No. of sites | 0.2575 | ||
| 0 | 3 (6.8%) | 4 (9.7%) | |
| 1–2 | 26 (59.1%) | 17 (41.5%) | |
| 3–8 | 15 (34.1%) | 20 (48.8%) | |
| Sites of Progression | |||
| Hilar nodes | 13 (29.5%) | 21 (51.2%) | 0.0489 |
| Mediastinal nodes | 13 (29.5%) | 16 (39.0%) | 0.3716 |
| Supraclavicular/scalene nodes | 6 (13.6%) | 5 (12.2%) | 1.0000 |
| Primary lung | 13 (29.5%) | 25 (61.0%) | 0.0047 |
| Contralateral lung | 3 (6.8%) | 1 (2.4%) | 0.6169 |
| Pleura | 2 (4.5%) | 4 (9.8%) | 0.4227 |
| Liver | 14 (32.6%) | 9 (21.4%) | 0.3383 |
| Adrenals | 4 (9.1%) | 4 (9.8%) | 1.0000 |
| Bone | 3 (6.8%) | 5 (12.2%) | 0.4738 |
| Bone marrow | 0 | 0 | -- |
| CNS-brain | 12 (27.3%) | 5 (12.2%) | 0.1065 |
| Skin | 0 | 0 | -- |
| Other nodal | 13 (29.5%) | 10 (24.4%) | 0.6326 |
| Other sites | 6 (13.6%) | 6 (14.6%) | 1.0000 |
PCI, prophylactic cranial irradiation; CNS, central nervous system
Finally, adverse events were compared between patients receiving PCI and not receiving PCI by randomization (sunitinib vs. placebo). Grade 4 toxicities were similar and not likely related to administration of PCI. While the group receiving PCI and sunitinib had 2 grade 4 events compared to 0 in sunitinib no PCI and 1 each in placebo groups with and without PCI, these were pancreatitis and GI bleeding in one patient each, which were probably not related to PCI administration. Patients receiving PCI were more likely to have grade 3 fatigue compared to patients not receiving PCI. However the grade 3 fatigue was similar in both the sunitinib (24%, n=5) and placebo arms (20%, n=4).
Discussion
In this post-hoc unplanned analysis of ES-SCLC patients followed from pre-enrollment with intracranial imaging, we identified that patients treated with PCI and sunitinib had trends for improved PFS and OS compared to those who received PCI alone, without increased toxicity. Furthermore, in patients randomized to the placebo group, we did not detect a measurable benefit to PCI. We found that the patterns of progression in patients treated with PCI were most commonly locoregional.
Although our findings are contrary to the overall survival benefit identified in some[2, 3], but not all[4] earlier studies of PCI in ES-SCLC, the difference between our results and those of others may be due to a number of factors, including relatively small sample size, differences in radiation dose,, and patient selection. While CALGB 30504 recommended a PCI dose established as standard of care for limited stage small cell lung cancer patients[9], the most common doses used in the EORTC study, 20 Gy in five 4-Gy fractions (62%) or 30 Gy in ten 3-Gy fractions (16%) are established and effective regimens for the treatment of brain metastases[10]. Therefore, patients included on the EORTC study, who did not have routine brain imaging, may have had occult brain metastases treated with an established therapeutic regimen. However, no evidence exists to prove differing effects for various PCI dose schemes, which are intended to treat microscopically occult metastases.
The interpretation of these data for a possible interaction of PCI and maintenance sunitinib in ES-SCLC is purely hypothesis generating, since the analysis is unplanned, retrospective, and the sample sizes are small. Potential bias could be introduced by the selection of patients that were treated with PCI, and although patients were screened for brain metastases prior to study entry, the lack of restaging brain imaging following systemic therapy in CALGB 30504 may have resulted in a subset of patients that actually had asymptomatic brain metastases at the time of cranial RT receiving PCI doses (25 Gy in 2.5-Gy fractions). The usual total whole brain radiation dose used to treat brain metastases in this fractionation is 35–37.5 Gy. The addition of sunitinib to 25 Gy in 2.5-Gy fractions may have stabilized intracranial disease that may have been undertreated by radiation, since sunitinib use has been reported to stabilize[11] progression of or delay in appearance[12] of brain metastases by some, but not others[13].
Alternatively, these data are also consistent with the hypothesis that the combination of PCI followed by sunitinib has an additive effect beneficial for the survival of ES-SCLC patients. CALGB 30504 showed that sunitinib has activity in SCLC, and that maintenance sunitinib was better tolerated than sunitinib given at crossover in the setting of progressive SCLC. CALGB 30504 also found a CNS progression rate of 27% in patients who did not receive PCI which was lower than that reported by Slotman 40% and Seto 58%. This sequential combination of PCI and maintenance sunitinib in CALGB 30504 may have blunted the two driving forces of mortality in ES-SCLC patients, with PCI limiting intracranial progression, and maintenance sunitinib limiting extracranial progression. This hypothesis is supported by the differing patterns of progression with patients receiving PCI having decreased intracranial progression in CALGB 30504. Although progression was delayed, patients receiving PCI on CALGB 30504 were more likely to progress in locoregional sites within the thorax, compared to patients who did not receive PCI who were more likely to progress intracranially. These data are also consistent with previously reported studies demonstrating improvements in OS (2-year 13% vs. 3%, p=0.004) with the addition of thoracic radiotherapy to PCI compared to PCI alone[14] as well another study using concomitant chemotherapy and thoracic radiotherapy in addition to PCI for ES-SCLC with CR to systemic therapy[15]. The pattern of increased locoregional progression in the thorax supports including thoracic radiotherapy (TRT) in future trial design.
The potential for synergy between PCI and sunitinib is supported by both preclinical and clinical reports. It has been previously established that small cell lung cancer expresses VEGF receptors[16], and that VEGF is required for the development of brain metastases[17]. In murine models, the delivery of antiangiogenic agents alone in the brain has been shown to slow brain metastasis growth[18]. Case reports have demonstrated single agent activity of sunitinib against brain metastases [19]. Clinical trials testing the combination of sunitinib and radiotherapy has demonstrated safety and potential efficacy in patients with brain metastases [20].
Integrating the combination of maintenance systemic therapy, PCI, and TRT as a clinical research treatment strategy for ES-SCLC is the next logical step for further study. While already some therapeutic gains have been achieved with radiotherapy used as PCI[2, 3] and TRT[14, 15] for ES-SCLC patients, studies looking to expand radiotherapy beyond the thorax have been associated with grade 5 toxicity[21]. Recently, RTOG 0937, a randomized phase II study comparing PCI alone vs. PCI + radiation to both thoracic and extracranial metastases was closed to accrual due to excess deaths and increased grade 4–5 toxicity in the experimental arm. Maintenance systemic therapy in addition to PCI and TRT may be able to further inhibit extrathoracic extracranial progression, improving the survival of ES-SCLC patients.
In conclusion, in this unplanned post hoc analysis of a prospectively conducted randomized study of ES-SCLC patients, we found that patients who received PCI were found to benefit in terms of progression-free and overall survival when they also received maintenance sunitinib. On the other hand, patients who did not receive PCI had little benefit from maintenance therapy.
We hypothesize that these improvements are due to additive effects of improved intracranial control with PCI and extracranial control with sunitinib. Prospective trials studying the role of PCI, thoracic radiation therapy, and maintenance systemic therapy are warranted in ES-SCLC patients.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 16th World Lung Conference, September 9, 2015, Denver, CO.
References
- 1.Auperin A, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 2.Slotman B, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 3.Schild SE, et al. Prophylactic cranial irradiation in small-cell lung cancer: findings from a North Central Cancer Treatment Group Pooled Analysis. Ann Oncol. 2012;23(11):2919–2924. doi: 10.1093/annonc/mds123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takashi S, et al. Prophylactic cranial irradiation (PCI) has a detrimental effect on the overall survival (OS) of patients (pts) with extensive disease small cell lung cancer (ED-SCLC): Results of a Japanese randomized phase III trial. J Clin Oncol. 2014;32(5s) (suppl;abstr 7503). [Google Scholar]
- 5.Ready NE, et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double- Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance) J Clin Oncol. 2015;33(15):1660–1665. doi: 10.1200/JCO.2014.57.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85(1):87–94. [Google Scholar]
- 7.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1(6):80–83. [Google Scholar]
- 8.Kaplan E, M P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Le Pechoux C, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10(5):467–474. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 10.Borgelt B, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6(1):1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 11.Chevreau C, et al. A phase II trial of sunitinib in patients with renal cell cancer and untreated brain metastases. Clin Genitourin Cancer. 2014;12(1):50–54. doi: 10.1016/j.clgc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Dudek AZ, et al. Brain metastases from renal cell carcinoma in the era of tyrosine kinase inhibitors. Clin Genitourin Cancer. 2013;11(2):155–160. doi: 10.1016/j.clgc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanhuyse M, et al. Do anti-angiogenic therapies prevent brain metastases in advanced renal cell carcinoma? Bull Cancer. 2012;99(12):100–106. doi: 10.1684/bdc.2012.1672. [DOI] [PubMed] [Google Scholar]
- 14.Slotman BJ, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 15.Jeremic B, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol. 1999;17(7):2092–2099. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 16.Tanno S, et al. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer. 2004;46(1):11–19. doi: 10.1016/j.lungcan.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Yano S, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60(17):4959–4967. [PubMed] [Google Scholar]
- 18.Pishko GL, et al. Vascular endothelial growth factor blockade alters magnetic resonance imaging biomarkers of vascular function and decreases barrier permeability in a rat model of lung cancer brain metastasis. Fluids Barriers CNS. 2015;12:5. doi: 10.1186/2045-8118-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah AY, et al. Clinical and pathological complete remission in a patient with metastatic renal cell carcinoma (mRCC) treated with sunitinib: Is mRCC curable with targeted therapy? Urol Case Rep. 2015;3(2):18–20. doi: 10.1016/j.eucr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wuthrick EJ, et al. A pilot study of hypofractionated stereotactic radiation therapy and sunitinib in previously irradiated patients with recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2014;90(2):369–375. doi: 10.1016/j.ijrobp.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonner JA, et al. Long term results of a phase I/II study of aggressive chemotherapy and sequential upper and lower hemibody radiation for patients with extensive stage small cell lung cancer. Cancer. 1995;76(3):406–412. doi: 10.1002/1097-0142(19950801)76:3<406::aid-cncr2820760310>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]