Abstract
Aging results in a natural decline in social behavior, yet little is known about the processes underlying these changes. Engaging in positive social interaction is associated with many health benefits, including reduced stress reactivity, and may serve as a potential buffer against adverse consequences of aging. The goal of these studies was to establish a tractable model for the assessment of social behavior deficits associated with late aging. Thus, in Exp. 1, 1.5-, 3-, and 18-month-old male Fischer 344 (F344) rats were assessed for object investigation, and social interaction with a same-aged partner (novel/familiar), or a different-aged partner, thereby establishing working parameters for studies that followed. Results revealed that 18-month-old males exhibited reductions in social investigation and social contact behavior, with this age-related decline not influenced by familiarity or age of the social partner. Subsequently, Exp. 2 extended assessment of social behavior to both male and female F344 rats at multiple ages (3, 9, 18, and 24 months), after which a series of sensorimotor performance tests were conducted. In this study, both males and females exhibited late aging-related reductions in social interactions, but these changes were more pronounced in females. Additionally, sensorimotor performance was shown to be impaired in 24-month-olds, but not 18-month-olds, with this deficit more evident in males. Finally, Exp. 3 examined whether aging-related inflammation could account for declines in social behavior during late aging by administering naproxen (0, 7, 14, and 28 mg/kg; s.c.)—a non-steroidal anti-inflammatory drug—to 18-month-old females. Results from this study revealed that social behavior was unaffected by acute or repeated (6 days) naproxen, suggesting that aging-related social deficits in females may not be a consequence of a general aging-related inflammation and/or malaise. Together, these findings demonstrate that aging-related declines in social behavior are (i) specific to social stimuli and (ii) not indicative of a general state of aging-related debilitation. Thus, these findings establish working parameters for a highly tractable model in which the neural and hormonal mechanisms underlying aging-related declines in social behavior can be examined.
Keywords: Rat, Fischer 344, Development, Aging, Social Behavior, Sex Differences, Senescence
1.0 Introduction
Social interaction is a dynamic process that can be influenced by a number of factors, including motivation to engage in social behavior, familiarity of the testing situation, as well as key features of would-be social partners (e.g. familiarity, presence of social bonds, health status) (Arakawa et al., 2009; Carstensen, 1992). One natural, yet unsettling, consequence of aging in humans is an overall reduction in social interaction that occurs across the lifespan(Carstensen, 1992). This is potentially detrimental to older individuals, because engaging in positive social interaction (relative to social deprivation) produces significant health benefits evidenced by reduced stress reactivity (Carter, 2007, 1998; Nomura and Okuma, 1999), enhanced resilience (Charuvastra and Cloitre, 2008), and faster recovery from neurological deficits induced by stroke (DeVries et al., 2007). Interestingly, prior studies have shown that elderly people gradually narrow the number of people with whom they interact; yet interactions with these individuals deepens across time (Amore, 2005; Lang, 2001; Schiffman, 1997). Indeed, elderly people seem to show a distinct preference for interacting with highly familiar individuals relative to establishment of new social relationships. While this trend is not problematic in and of itself, the narrowing of social interests becomes a significant threat to overall well-being as aging-associated debilitation (i.e., reduced ability to travel and/or communicate with social partners) and bereavement (death of social partners) compromises the depth and quantity of social engagement (Caruso et al., 2004; Lang, 2001). When combined with other findings showing that the intensity and breadth of positive social interactions are primary indicators of overall happiness and life satisfaction, it is clear that preservation of a broad repertoire of social partners could play a key role in maintaining and/or improving quality of life for aging populations. It is therefore crucial to develop and validate strong preclinical models that can be utilized to better understand the neural mechanisms underlying changes in social behavior that occur across the lifespan.
Studies have demonstrated that aged rodents exhibit alterations in a number of behavioral domains, including impaired cognitive function (Barrientos et al., 2012; Foster, 2012; Gallagher and Rapp, 1997; Nomura and Okuma, 1999; Rosenzweig and Barnes, 2003), increased anxietylike (Boguszewski and Zagrodzka, 2002; Darwish et al., 2001; Frussa-Filho et al., 1991; Hunt et al., 2011; Imhof et al., 1993; Miyagawa et al., 1998) and depressive-like behaviors (Kiss et al., 2012; Moretti et al., 2011), decreased locomotor activity (Gage et al., 1984; Godbout et al., 2008; Hunt et al., 2011), and importantly, impaired social interaction (Andersen et al., 1999; Hunt et al., 2011; Markel et al., 1995; Mencio-Wszalek et al., 1992; Salchner et al., 2004; Shoji and Mizoguchi, 2011; Soffié and Bronchart, 1988). Aged male rats (14–30-months-old) have been reported to exhibit reduced play behavior (Soffié and Bronchart, 1988), fewer social interactions (Markel et al., 1995; Salchner et al., 2004), less social investigation (Andersen et al., 1999), and to engage in less contact with conspecifics (Hunt et al., 2011) relative to adolescent or young adult rats (1.5–6-months-old). Importantly, these aging-related social deficits are ubiquitous across many rat strains, including the Wistar (Andersen et al., 1999; Hunt et al., 2011; Markel et al., 1995; Soffié and Bronchart, 1988), Sprague-Dawley (Salchner et al., 2004), and Fischer 344/Brown Norway cross strains rats (Shoji and Mizoguchi, 2011), yet to our knowledge, only one study has examined potential sex differences in social behavior in aged animals. Hunt et al. (2011) found that aged male and female Wistar rats (22–30 months) exhibited similar reductions in interaction with a novel age- and sex- matched social partner. Furthermore, age-related alterations in social interaction have not been assessed in the Fischer 344 rat strain. Although adult F344 rats have been shown to differ in frequency and duration of social interaction, from other strains (Berton et al., 1997; Ramos et al., 1997; Rex et al., 1999), none of these studies examined social interaction in aged F344 rats. Given that F344 rats have an 80–90% survival rate at the desired ages (Turturro et al., 1999), and have been under-represented in previous studies of aging-related declines in social beahvior (particularly with respect to potential sex differences) in social behavior), this strain of rat was selected for the current series of experiments. F344 rats are also a commonly used strain in aging studies, evidenced by the fact that F344 is one of only a few select rat lines maintained by the National Institute on Aging (NIA) for use in NIA-funded projects.
When examining reduced social interaction in aged animals, some studies have reported concomitant reductions in locomotor activity (Hunt et al., 2011), whereas others showed no such hypoactivity (Andersen et al., 1999; Salchner et al., 2004; Shoji and Mizoguchi, 2011). Furthermore, few studies have manipulated features of the dyadic interaction (i.e., familiarity and age of social partner (Yates et al., 2013), and to our knowledge, no studies have manipulated these features in aged animals to determine what aspect(s) of social behavior are impacted in aging. Since social interaction is a dynamic process that can be influenced by a number of factors, more detailed assessments of social behavior are required to disentangle other, off-target processes that may influence social functioning. For instance, reduced social interaction in aged rats might result from aging-related inflammation that may be associated with a general state of achiness or malaise, reduced motivation to engage in social behavior (Varlinskaya et al., 1999), the inability to detect, remember, and/or recognize conspecifics (Mencio-Wszalek et al., 1992; Guan and Dluzen, 1994; Prediger et al., 2006, 2005; Terranova et al., 1994), or aging-related increases in anxiety that may suppress social interaction (Darwish et al., 2001; File, 1990; Hunt et al., 2011; Miyagawa et al., 1998).
This report presents our initial working model of aging-associated alterations in social behavior. Overall, we considered 4 key variables that might influence social behavior assessments: (i) general exploratory activity, (ii) motor function, (iii) familiarity of the social partner, and (iv) age of the social partner. In Exp. 1, aging-related alterations in object exploration and social interaction were assessed in male rats. Exp. 2 then evaluated social interaction across the lifespan in males and females, while also conducting detailed assessments of sensorimotor function. Lastly, Exp. 3 tested whether administration of naproxen, a non-steroidal anti-inflammatory drug (NSAID), would attenuate the aging-related decline in social interaction, with the idea that NSAID treatment might alleviated aging-related inflammation and temper any general achiness and/or malaise that could account for declines in social behavior during late aging in females. Naproxen—a non-selective cyclooxygenase (COX) inhibitor—was selected as an initial pharmacological approach because it has been classically used to reduce fever, pain, and inflammation (Clarke et al., 1994), and more recently to alleviate illness-related changes in social behavior in guinea pigs (Hennessy et al., 2015). Together, these experiments represent our development of a tractable working model to assess mechanisms underlying late-aging associated social deficits.
2.0 Materials & Methods
2.1 Subjects
F344 rats were used in all experiments, with the vendor and source of the animals explained for each experiment below. Animals were provided ad libitum access to both food and water throughout all experiments. At all times, animals were maintained and treated in accordance with the guidelines set forth by the Institute of Laboratory Animal Resources, (1996) and in accordance with the protocol approved by the IACUC at Binghamton University.
2.1a Experiment 1
Male F344 rats (n = 6–9/group) of different ages (1.5, 3, and 18 months) were obtained from the NIA colonies at Taconic (Germantown, NY). All rats were given at least 1 week to acclimate to the colony conditions before the onset of experimentation. Colony conditions were maintained at 22 ± 1°C with 14:10 light:dark cycle (lights on 0600). Rats remained pair-housed except during the brief (30–40 min) sessions during which behavioral testing occurred.
2.1b Experiment 2
Male and female F344 rats (n = 6–10/group) of different ages (3, 9, 18, and 24 months) were obtained from the NIA colony at Charles River Laboratories. Due to federal restrictions on the number of animals that can be obtained from the NIA colony, a separate group of 3-month-old F344 rats (males and females) were purchased from Charles River to serve as social partners. This approach had the added advantage of ensuring that social partners were truly unfamiliar to the test subjects. All rats were given at least 1 week to acclimate to the colony conditions (22 ± 1°C; 12:12 light:dark cycle with lights on at 0700) before the onset of experimentation. On the day prior to the start of behavioral testing, rats were handled for 3 min. Additionally, in this study, experimental subjects and social partners were single-housed for five days prior to the onset of behavioral testing, and remained individually housed throughout the experiment, as previous research has demonstrated that brief periods of social deprivation increase the motivation to engage in social interaction (Arakawa et al., 2011, 2009; Varlinskaya et al., 1999).
2.1c Experiment 3
Eighteen-month-old female F344 rats (n = 7–10/group) were obtained from the Charles River NIA colony. As in Exp. 2, social partners were also female F344 rats and were obtained from a separate Charles River stock. Since short-term social isolation did not appear to increase social interaction (Exp. 2 and also in other unpublished pilot experiments), in Exp. 3 rats remained pair-housed throughout the entire experiment. Rats were handled for 3 min per day for 2 days prior to the onset of behavioral testing.
2.2 Social Interaction Testing
2.2a Apparatus
Testing was conducted between 0800 and 1200 h, under dim light (15–20 lux). The experimenter was not present in the room, and all sessions were recorded for later scoring by a trained observer. Males and females were tested in separate, but adjacent, rooms. Animals were placed in Plexiglas chambers (Binghamton Plate Glass, Binghamton, NY) that measured 30 × 20 x 20 cm for adolescents and 45 × 30 x 30 cm for adults. Clean pine shavings lined the bottoms of the apparatuses. Each test chamber was divided into two compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm for adolescents; 9 × 7 cm for adults) that allowed for movement of animals between the compartments. After testing of each subject, the soiled wood shavings were removed, chambers were cleaned with a 3% hydrogen peroxide solution (Exp.1) or were wiped with water (Exp. 2 and 3), and clean shavings were added for the next subject.
2.2b Behavioral Measures
Object exploration was defined as sniffing of the object or contact with the object (cotton ball). Social investigation was defined as the sniffing of any part of the body of the partner, whereas frequency of contact behavior was scored as the sum of crawling over and under the partner and social grooming. The total number of crossovers (movement through the aperture) demonstrated by the experimental subjects was also recorded for each session. Total crossovers were used as an index of general locomotor activity.
2.3 Experimental Design and Procedure
2.3a Experiment 1
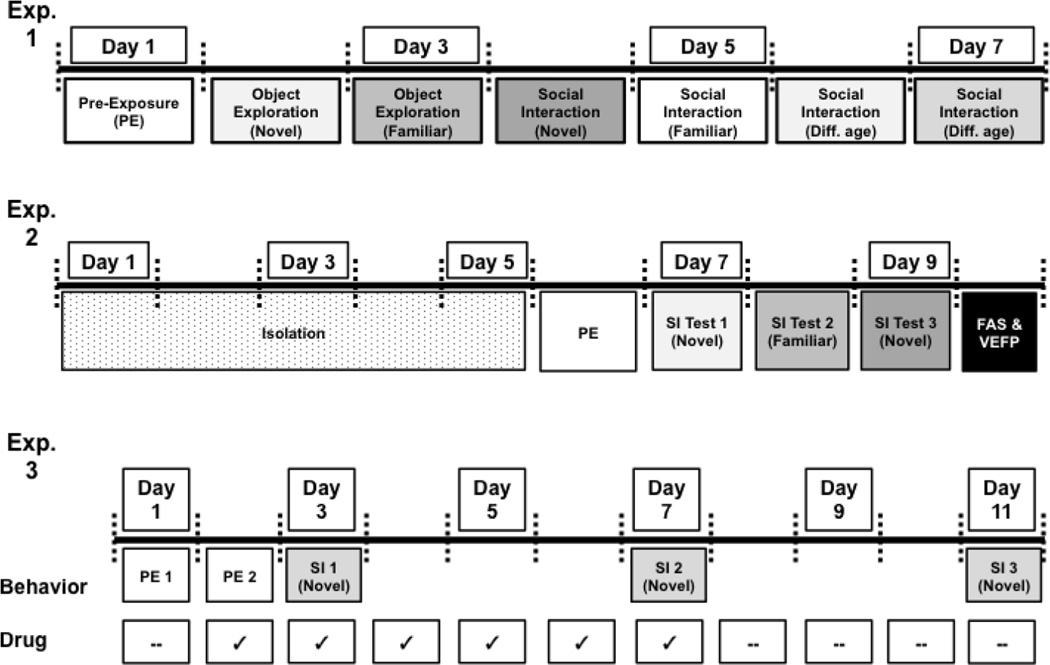
Object exploration and social behavior were assessed in male rats at three ages: 1.5 (late adolescence (Spear, 2015, 2013; Zernig and Pinheiro, 2015), 3 (adulthood), and 18 (senescence (Coleman, 2004; Turturro et al., 1999) months, using a between subjects design (n = 8–9/group). Figure 1 (top panel) contains a schematic representation of the behavioral testing procedures used in Exp. 1. On Day 1, experimental subjects were placed individually into the testing apparatus for a 30-min acclimation session (Pre-Exposure; PE). On the following day (Day 2), rats were again exposed to the testing apparatus, but with the opportunity to interact with a novel object—a white cotton ball (approximately 10 × 2 x 1.5 cm; l x w x h) for 30 min. Since investigation of both context and novel objects declines rapidly after the first 10 min of exposure, our analyses focused on behavior emitted during the first 10-min time bin. On Day 3, rats were again placed in the testing apparatus with a cotton ball, thus allowing assessment of interaction with a familiar object.
Figure 1.
Schematic diagram of experimental timelines.
Starting on Day 4, social behavior with an age-matched partner was assessed. Rats were first exposed to the testing apparatus alone for 30 min, after which time an age- and sex-matched novel social partner was placed into the testing context for a 10 min social interaction session. On Day 5, social behavior towards a familiar partner was investigated, using a similar protocol to Day 4, with the exception that the same social partner from Day 4 was re-introduced. When interacting with an age-matched partner, weight differences between experimental and partner animals were minimized, in an effort to reduce any aggression towards the social partner (Lucion et al., 1996). Additionally, social partners were initially unfamiliar with the chamber on the first day of social behavior testing. On Days 6 and 7, social behavior towards a partner of a different age was assessed. On these days, rats were allowed to interact with a novel partner from one of the other two age groups for 10 min, following the usual 30-min pre-exposure to the apparatus. The order in which the rats interacted with an animal from another age group was counterbalanced. For the purposes of these experiments, the term “familiar” refers to a social partner that was not novel.
2.3b Experiment 2
Previous studies have used brief social isolation to enhance the motivation to engage in social interaction (Arakawa et al., 2011, 2009; Varlinskaya et al., 1999). Rats were single-housed for five days prior to the onset of behavioral testing (Figure 1, middle panel). On Day 6, PE occurred, with subjects and partners acclimated to the testing environment for 30 min. Social interaction testing occurred over the next three days, and consisted of the experimental subject being placed into the testing environment for 20 min (Acclimation phase; AC), after which a sex-matched partner (~ 3 months old) was placed into the apparatus for a 10-min interaction session (social interaction phase; SI). On Day 7, all animals interacted with a novel, sex-matched partner (Novel 1), followed by the same partner on Day 8 (Familiar). Then, on Day 9, a different, sex-matched partner was introduced (Novel 2).
Given that altered social behavior in aged animals could be related to aging-associated alterations in sensorimotor function, on Day 10, sensorimotor performance was assessed using the Forepaw Adjustment Steps (FAS) and Vibrissae-Evoked Forelimb Placing (VEFP) tests. The FAS test was used to measure rodent forelimb motor performance (Chang et al., 1999; Conti et al., 2014; Olsson et al., 1995). An experimenter held the rat’s hindlimbs and one forepaw so that weight would be imposed on the other forepaw. Rats were moved laterally across a table at a rate of 90 cm/10 s. Each test consisted of 6 trials for each forepaw: 3 trials each to assess forehand (adjusting for movement toward the body) and backhand (adjusting for movement away from the body) adjustments. Data are presented as mean total steps, where forehand and backhand stepping for the right and left forepaws were added together for each animal. Higher stepping scores indicate better motor performance when comparing age and sex differences.
Due to the VEFP’s sensitivity to sensorimotor performance (Anstrom et al., 2007; Woodlee et al., 2005), it was used along with the FAS test assess sensorimotor function across ages of male and female rats. An experimenter held the animal by the torso, restrained a forelimb, and brushed its vibrissae against the edge of a table to elicit a forelimb placement from the ipsilateral limb. Animals were then turned sideways so that vibrissae were perpendicular to the table and the downwardly oriented limb was restrained. The vibrissae were brushed against the tabletop to evoke forelimb placement from the contralateral limb. There were a total of ten trials for each ipsilateral and contralateral limb, and trials when the animal struggled were not counted. The total number of steps for ipsilateral and contralateral placing was used, where higher numbers indicate better performance.
2.3c Experiment 3
Previous studies have reported that late aging is associated with a pro-inflammatory state (Barrientos et al., 2015; Jurgens and Johnson, 2010; Norden et al., 2014; Norden and Godbout, 2013), with the aging brain thought to reside in a chronic state of neuroinflammation that is characterized by increased concentrations of central cytokines and an exaggerated response to immune stimulation (Henry et al., 2009; Huang et al., 2008; Norden et al., 2014; Norden and Godbout, 2013). It is therefore possible that this heightened inflammatory state occurring during senescence could result in a general aging-related malaise that contributes to social deficits in late aging. To investigate this possibility, Exp. 3 examined the potential for a general anti-inflammatory drug to reverse age-associated declines in social behavior. This approach was based on prior research, which reported attenuation of negative affective behaviors induced by illness (or an illness-like) state. For example, ibuprofen (another NSAID) was able to attenuate depressive-like behavior in ill tumor-bearing mice (Norden et al., 2015). Additionally, Hennessy and colleagues (Hennessy et al., 2015) reported that naproxen ameliorated social separation-induced depressive-like behavior in guinea pigs, with this pattern of separation-induced depressive-like behavior strikingly similar to sickness behavior exhibited during a state of illness (Hennessy et al., 2014), and which also involves a decline in social behavior (Henry et al., 2008).
Thus, Exp. 3 investigated the effects of acute and/or repeated naproxen (Cat. No. N8280; Sigma)-a commonly used NSAID that is a non-selective COX inhibitor—on the social behavior of aged18-month-old female F344 rats (n = 7–10/group). Females were selected for this study, given that Exp. 2 demonstrated more pronounced social deficits in this sex. Naproxen was suspended fresh daily in a working solution of 5 mg/kg, using 0.25% carboxymethylcellulose (CMC; Cat. No. C5678; Sigma) as vehicle. Because a drug suspension was utilized, the volume of injectate was varied to achieve the desired doses of naproxen (7, 14, 28 mg/kg; s.c.), with vehicle-injected rats receiving equivolume vehicle (0.25% CMC). Drug administration on behavioral testing days always occurred 1 h before the onset of the testing. For all social interaction tests, the animals were placed into the testing apparatus for a 20-min acclimation period, after which time a social partner was introduced and behavior was assessed for 10 min. Social partners were always novel 3-month-old females. On Day 1 of the experiment, animals were pre-exposed to the testing environment for 30 min, prior to the administration of any drug. On Day 2, drug was administered in the home cage and animals were pre-exposed to the testing environment again for 30 min. Two pre-exposure sessions were used so that animals were able to acclimate to the testing apparatus with and without drug. On Day 3, drug was administered and social interaction against a novel 3-month-old female partner was assessed. Days 4–6 consisted of daily drug administration in the home cage, with no behavioral testing occurring on those days. On Day 7, social interaction was assessed again, to determine the effects of prolonged naproxen administration on social behavior. On days 8–10, no drugs were administered and no behavioral testing occurred. Finally, on Day 11, social behavior was assessed (Figure 1c) such that the impact of repeated testing on overall social behavior could be assessed on this final test day.
2.4 Data Analysis
All data were analyzed using Statistica (Version 12, StatSoft, Inc, Tulsa, OK). Data were assessed for normality using Shapiro-Wilks test. Data that violated normality were subjected to a square root transformation. For ease of presentation and interpretation, all data in the figures represent untransformed data. A One-way ANOVA with Age as a factor was used to assess the total number of crossovers exhibited during PE in Exp. 1. Aging-related differences in object exploration and social interaction were analyzed with repeated-measures ANOVAs with Age as the between-subjects measure and Familiarity or Partner Age as the within-subjects measure. Object exploration, social interaction with a same-aged partner, and social interaction with a different-aged partner, and crossovers during those sessions were assessed by separate repeated-measures ANOVAs. Crossovers exhibited toward a different-aged partner were assessed with Friedman ANOVA with Wilcoxon signed rank post-hoc tests. For Exp. 2, 4 (Age) x 2 (Sex) x 3 (Day) mixed ANOVAs, were used to analyze social investigation. Social contact and total crossovers exhibited during each phase of testing (AC and SI; Days 7–9) were assessed with Friedman ANOVA. Crossovers exhibited during PE (Day 6) were assessed with Kruskal-Wallis ANOVA with Mann-Whitney U post-hoc tests. Sensorimotor performance on the FAS and VEFP tests were assessed using 4 (Age) x 2 (Sex) ANOVAs. For Exp. 3, a one-way ANOVA with Dose as a factor was used to assess total crossovers exhibited during PE and social behavior on each day individually. Furthermore, the impact of repeated testing on social behavior and total crossovers during PE was assessed using 4 (Dose) x 3 (Day) mixed ANOVAs. Social contact was not normally distributed, and was analyzed using Friedman ANOVA and Kruskal-Wallis ANOVA. In all parametric analyses, Fisher’s Least Significant Difference (LSD) post-hoc tests were used to determine the loci of significant differences between groups in the event of significant main effects or interaction.
3.0 Results
3.1 Experiment 1
3.1a Pre-Exposure
The total number of crossovers during the acclimation session decreased with age [F(2,21) = 20.17, p < 0.001]. Aged rats had fewer crossovers than adult and adolescent rats (Table 1).
Table 1.
Activity data (total number of crossovers; mean ± SEM) from Experiment 1.
| Experiment 1 | |||||
|---|---|---|---|---|---|
| Age of Experimental Animal (months) | |||||
| Test Day |
Testing Conditions |
Scoring Interval | 1.5 | 3.0 | 18.0 |
| 1 | Pre-Exposure | 30 min. | 33.13 ± 3.51* | 22.63 ± 2.86 | 8.88 ± 1.22* |
| 2 | Novel Object |
First 10 min. (with object) |
12.75 ± 1.90 | 11.63 ± 1.43 | 5.75 ± 0.94* |
| 3 | Familiar Object |
First 10 min. (with object) |
10.13 ± 1.87 | 6.88 ± 1.13 | 3.75 ± 1.13 |
| 4 | Novel (age- matched) |
10 min. (with social partner) |
32.75 ± 2.86* | 13.75 ± 1.50 | 3.13 ± 0.44* |
| 5 | Familiar (age- matched) |
10 min. (with social partner) |
26.38 ± 2.46* | 12.00 ± 1.32 | 4.38 ± 0.82* |
|
6 & 71 |
1.5 month partner (Novel) |
10 min. (with social partner) |
32.75 ± 2.86 | 11.13 ± 1.75# | 4.38 ± 1.05# |
| 3 month partner (Novel) |
10 min. (with social partner) |
20.63 ± 1.46 | 13.75 ± 1.50 | 3.75 ± 0.67 | |
| 18 month partner (Novel) |
10 min. (with social partner) |
15.88 ± 2.37 | 11.75 ± 2.66 | 3.13 ± 0.44 | |
Data for the same-aged unfamiliar partner were obtained from Day 4 of testing.
On days 1, 4, and 5 of testing, an asterisk denotes a significant difference from the 3-month-old group.
On days 6 and 7 of testing, a pound sign indicates a significant deviation from the crossovers exhibited by an animal interacting with an age-matched unfamiliar partner (value highlighted in gray).
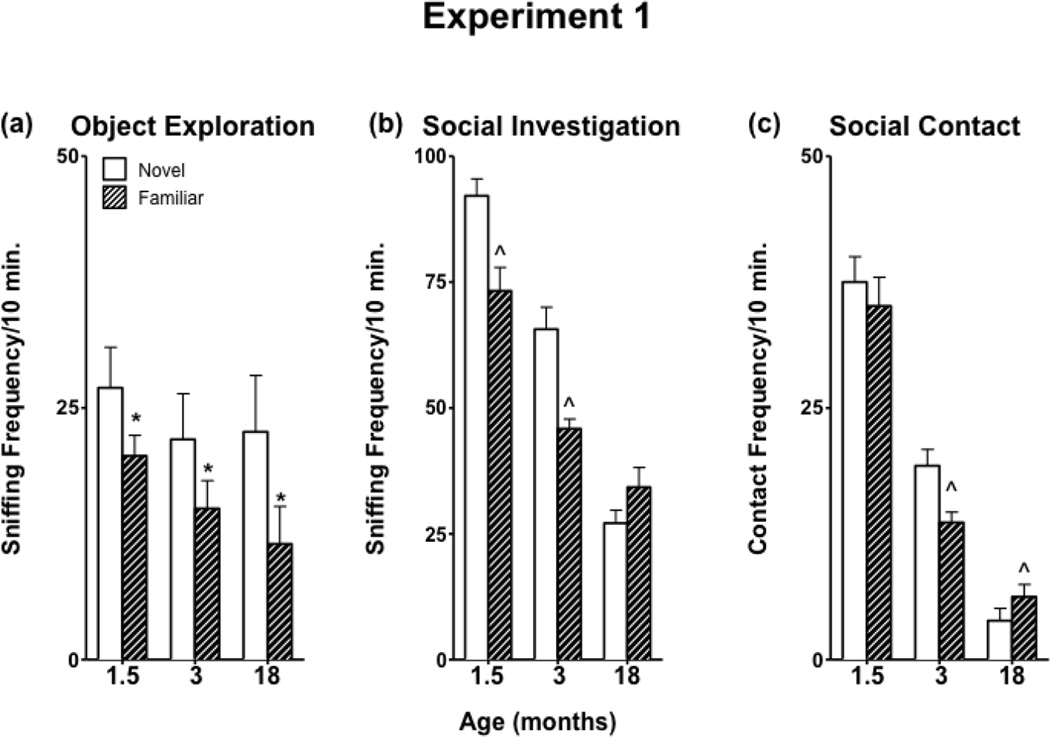
3.1b Object Exploration
No significant age differences were observed in object exploration, although rats of all ages spent more time investigating a novel versus a familiar object [F(1,21) = 9.17, p < 0.01] (Figure 2a). The total number of crossovers exhibited during the 10-minute session was influenced by object familiarity [F(1,21) = 12.51, p < 0.01] and age of the experimental animal [F(2,21) = 7.73, p < 0.01] (Table 1), such that both aging and familiarity of the object resulted in fewer crossovers.
Figure 2.
Object exploration and social interaction directed towards a same-aged social partner in 1.5-, 3-, and 18-month-old males in Experiment 1.
3.1c Social behavior towards an age-matched partner
Adolescents and young adults investigated a novel partner more than a familiar partner, whereas aged animals displayed similar levels of investigation, regardless of partner familiarity [Age x Familiarity interaction, F(2,21) = 16.82, p < 0.0001] (Figure 2b). Contact behavior was not normally distributed, so data were transformed prior to ANOVA. In adolescents, contact behavior was similar toward novel and familiar social partners, while in adults, contact was higher for a novel partner, and in aged animals, contact was higher for a familiar partner [Age x Familiarity interaction], F(2,21) = 5.73, p < 0.05. The total number of crossovers exhibited by experimental animals during the social interaction was transformed due to non-normality. As the age of the rats increased, the total number of crossovers decreased [F(2,21) = 120.49, p < 0.0001] (Table 1).
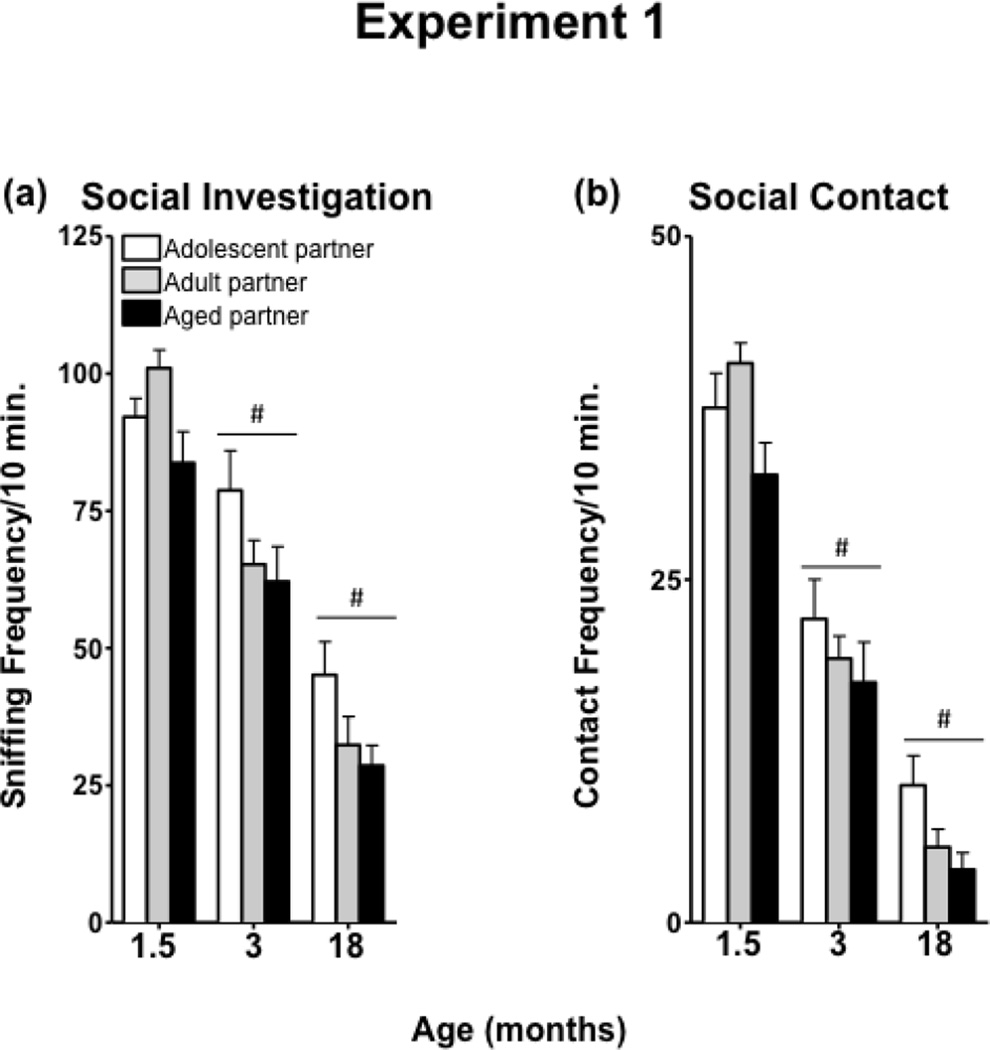
3.1d Social behavior towards a different-aged partner
Overall, investigation of a different-aged partner decreased with age of experimental animal [F(2,21) = 99.74, p < 0.0001] (Figure 3a). In addition, experimental animals spent less time investigating a partner as the partner’s age increased [F(2,42) = 5.15, p < 0.05]. Contact behavior directed toward a different-aged partner was transformed due to non-normality. Social contact behavior decreased across age [F(2,42) = 96.94, p < 0.00001] (Figure 3b). Contact with the partner animal was also attenuated as the age of the partners increased [F(2,42) = 4.25, p < 0.05]. Since data were not normally distributed, even after transformation, Friedman ANOVA was used to assess the impact of age on total crossovers exhibited when interacting with a different-aged partner. Separate ANOVAs were used for each age, with age of the social partner as the within-subjects variable. Wilcoxon signed-rank tests with Bonferroni corrections for multiple comparisons were used to assess the loci of significant differences following Friedman ANOVA. Adolescents exhibited fewer crossovers as the age of the social partner increased [χ2 (2) = 13.00, p < 0.01], while there was no difference in crossovers exhibited toward different-aged partners in adult or aged animals (Table 1).
Figure 3.
Social investigation and contact of a different-aged partner in 1.5-, 3-, and 18-month-old males in Experiment 1.
3.2 Experiment 2
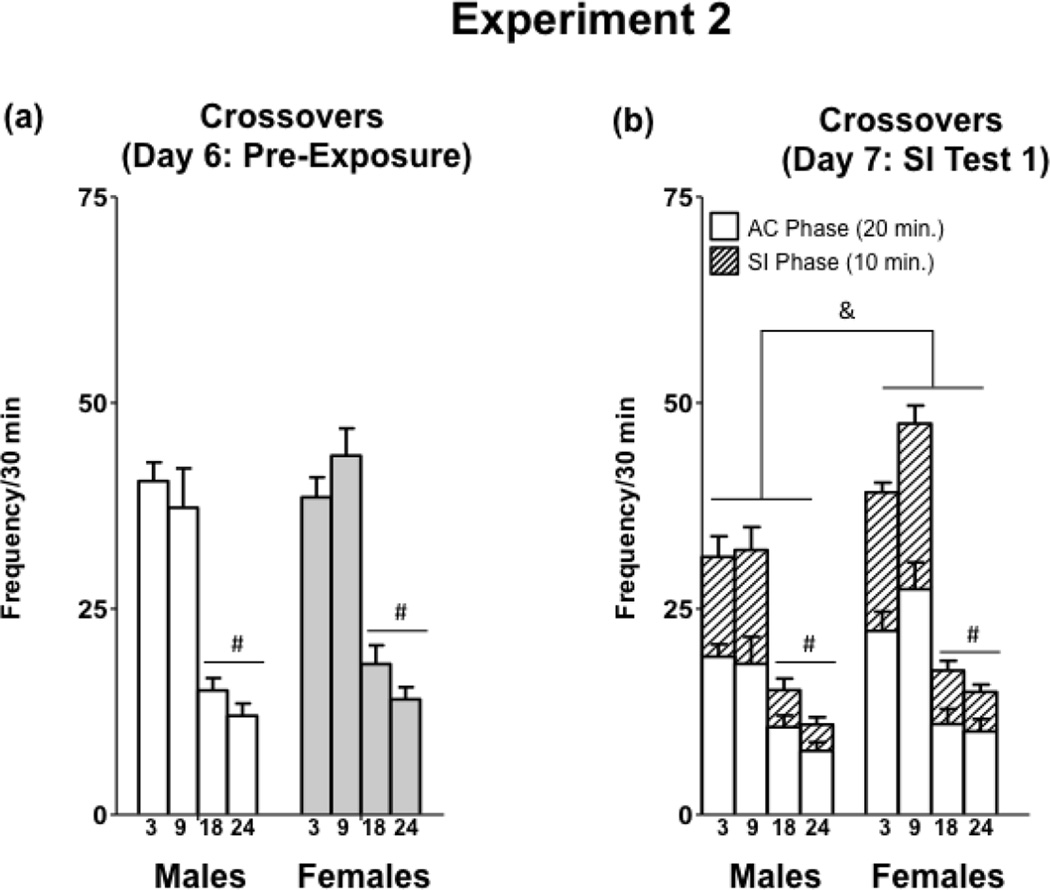
3.2a Pre-Exposure
The total number of crossovers exhibited by subjects during the 30-min pre-exposure session was not normally distributed, even after transformation. Thus, Kruskal-Wallis ANOVA was used to assess total crossovers. Kruskal-Wallis ANOVA does not allow for factorial designs, so age and sex were assessed separately. Crossovers were significantly impacted by age [H(3,69) = 51.48, p < 0.0001]. Mann-Whitney U tests with Bonferroni corrections for multiple comparisons revealed that 18- and 24-month-old rats exhibited a reduction in crossovers compared to 3- and 9-month old rats. Total crossovers during PE were not impacted by sex [H(1,69) = 2.71, p > 0.05] (Figure 4a)
Figure 4.
Total number of crossovers exhibited during pre-exposure and the first social interaction test in 3-, 9-, 18- and 24-month-old males and females in Experiment 2.
3.2b Social Interaction
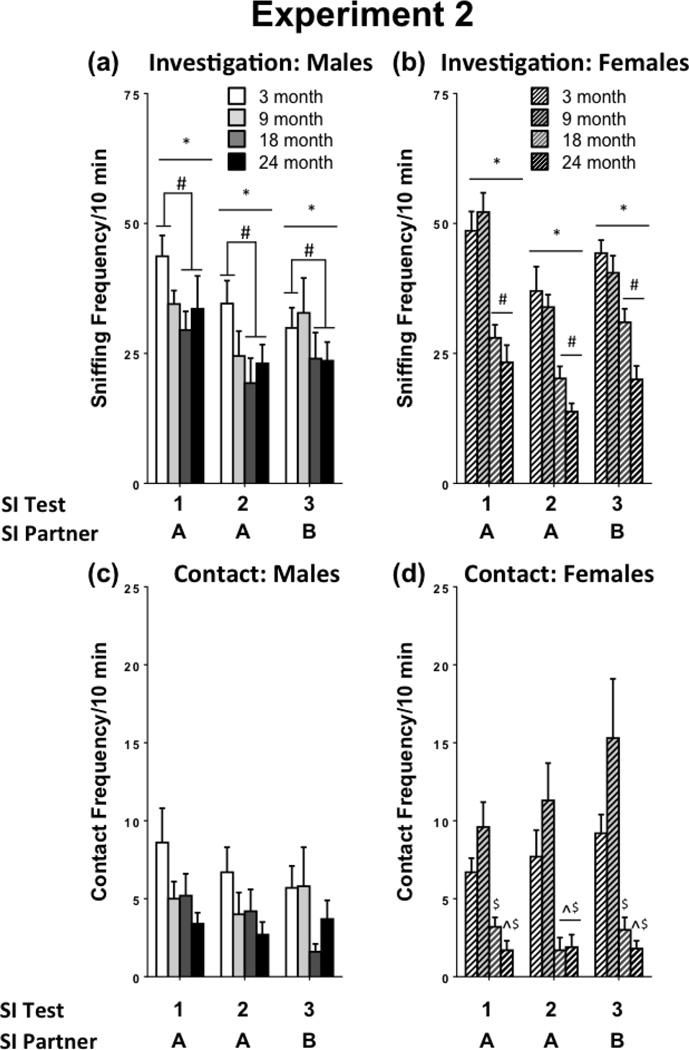
Investigation was assessed with a mixed-design ANOVA, where test day was the within-subjects measure and age and sex were between-subjects measures. There was a main effect of test day, [F(2,130) = 26.40, p < 0.01], and post-hoc tests revealed that there were significant differences between all days. Social investigation declined between tests 1 and 2. In addition, there was a slight increase in social investigation upon presentation of a novel partner in test 3. Investigation (mean ± SEM) for social interaction tests 1–3 were 36.85 ± 1.76, 25.92 ± 1.57, and 30.68 ± 1.58, respectively, collapsed across age and sex. Thus, this pattern shows habituation to the same conspecific, then dishabituation when a novel conspecific was presented in the third social interaction test. In males, 18- and 24-month olds exhibited reduced social investigation compared to 3-month olds. 9-month-old males were not different from males at any other age (Figure 5a). In females, 18- and 24-month olds exhibited significant reductions in investigation compared to 3- and 9-month olds [Age x Sex interaction, F(3,130) = 3.67, p < 0.05] (Figure 5b).
Figure 5.
Social investigation and contact in 3-, 9-, 18-, and 24-month-old males and females in Experiment 2.
To analyze social contact, a separate GROUP variable was used, which combined sex and age (e.g. 3-month old male = 3M, 3-month-old female = 3F). Social contact was assessed using Friedman ANOVA for each group separately. There were no differences in social contact across days in any group. To assess the impact of age and sex on contact behavior, separate Kruskal-Wallis ANOVAs were conducted for males and females on each test day. Regardless of test day, males of different ages did not show differences in contact behavior. However, in females, contact behavior was significantly impacted by age on all test days [Novel 1: H(3,38) = 23.65, p < 0.001; Familiar: H(3,38) = 19.24, p < 0.001; Novel 2: H(3,38) = 22.92, p < 0.001]. Mann-Whitney U tests with a Bonferroni correction revealed that when interacting with a novel partner (Novel 1), 24-month-old females exhibited fewer contacts than 3- and 9-month old females, whereas 18-month-old females exhibited fewer contacts than 9-month-old females. When interacting with a familiar conspecific, 18- and 24- month old females exhibited fewer contacts, compared to 9-month-old females. When another novel conspecific was presented on the third test day, 24-month-old females had fewer contacts compared to 3- and 9-month old females, while 18-month-old females had fewer contacts than 9-month-old females (Figure 5d).
The total number of crossovers were assessed separately using Friedman ANOVA for the AC and SI phases across the three days of social interaction testing (Novel 1, Familiar, and Novel 2) (Table 2). Total crossovers during the AC phase were impacted by test day in 3-month-old males [χ2(2) = 7.40, p < 0.05] and 3-month-old females [χ2(2) = 8.22, p < 0.05]. Wilcoxon signed-rank tests with a Bonferroni correction were used to assess differences between days in each group. In 3-month-old females, there were no differences between days, while in 3-month-old males, there was a significant decline from test day 1 (Novel 1) to test day 2 (Familiar) (Data for SI Test 1 are shown in Figure 4b). Crossovers did not decline across days during the social interaction phase (Table 2). To assess the influence of sex on total crossovers during the SI phase on each day, separate Mann-Whitney U tests were conducted, with Bonferroni corrections for multiple comparisons. Crossovers were higher in females, compared to males, on the third test day (Novel 2). To analyze the impact of age on total crossovers exhibited during the social interaction tests, separate Kruskal-Wallis ANOVAs were conducted for each test day. There was a significant effect of age on total crossovers on all test days [Novel 1:H(3,73) = 41.56, p < 0.001; Familiar: H(3,73) = 40.55, p < 0.001; Novel 2: H(3,73) 39.97, p < 0.001]. Mann-Whitney U tests with Bonferroni corrections confirmed that 18- and 24-month old rats exhibited a reduction in crossovers, compared to 3- and 9-month-old rats.
Table 2.
Total number of crossovers (mean ± SEM) during the 10-minute social interaction test for Experiment 2.
| Experiment 2 | |||||
|---|---|---|---|---|---|
| Age of Experiment Animal (Months) | |||||
| Test Day | Sex | 3 | 9 | 18 | 24 |
| Novel 1 | Males | 12.10 ± 2.47 | 13.83 ± 2.81^ | 4.50 ± 1.40^$ | 3.22 ± 0.88^$ |
| Females | 16.78 ± 1.20 | 20.10 ± 2.23^ | 6.50 ± 1.18^$ | 4.78 ± 0.91^$ | |
| Familiar | Males | 12.20 ± 2.52 | 15.67 ± 4.14^ | 4.20 ± 1.35^$ | 3.11 ± 1.22^$ |
| Females | 14.44 ± 1.20 | 18.80 ± 2.94^ | 4.90 ± 1.17^$ | 3.11 ± 0.73^$ | |
| Novel 2 | Males | 8.90 ± 1.77* | 15.50 ± 3.17*^ | 3.40 ± 1.27*^$ | 1.89 ± 0.56*^$ |
| Females | 17.44 ± 1.75 | 18.30 ± 1.97^ | 5.90 ± 1.15^$ | 3.22 ± 0.88^$ | |
indicates a main effect of sex,
indicates a significant difference from 3-month-olds.
indicates a significant difference from 9-month-olds. Females exhibited more crossovers compared to males at all ages. Regardless of sex, 3- and 9-month-olds had more crossovers 18- and 24-month olds had fewer crossovers, whereas 18- and 24-month olds were not different from one another.
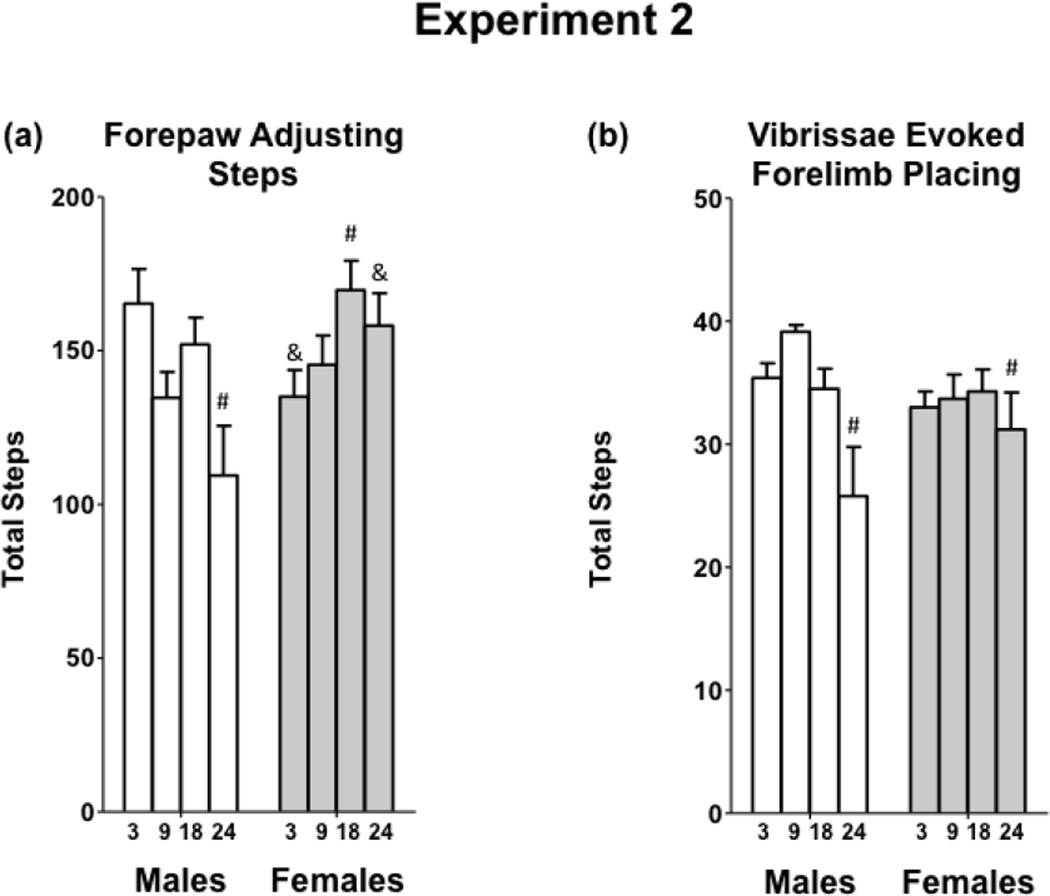
3.2c Forepaw Adjustment Steps and Vibrissae-Evoked Forelimb Placement Tests
The FAS and VEFP tests assess sensorimotor performance, and in both tests, higher total step counts indicate better performance. FAS performance was significantly impacted by both age and sex [Age x Sex interaction, F(3,66) = 4.62, p < 0.01] (Figure 6a). 24-month-old males had the fewest number of steps, and had poorer performance than 3- and 18- month-old males and 9-, 18-, and 24-month-old females. 3-month-old males performed better than 3-month-old females, while 24-month-old females performed better than 24-month-old males. Aged (24-month-old) males and females showed impaired VEFP performance [F(3,66) = 4.58, p < 0.01] (Figure 6b).
Figure 6.
Sensorimotor function in 3-, 9-, 18-, and 24-month-old males and females in Experiment 2.
3.3 Experiment 3
3.3a Pre-Exposure
There were two pre-exposure sessions, one prior to administration of any drug, and one that occurred 1h after drug administration. There were no effects of drug on total crossovers exhibited during either of these test sessions [PE1: F(3,29) = 0.14, p > 0.90; PE2: F(2,31) = 0.28, p > 0.80]. Crossovers declined from PE1 to PE2 [F(1,27] = 94.98, p < 0.0001], indicating that there was habituation to the testing apparatus (Table 3).
Table 3.
Total number of crossovers (mean ± SEM) during the 30-minute pre-exposure sessions for Experiment 3.
| Experiment 3 | |||||
|---|---|---|---|---|---|
| Naproxen Dose (mg/kg) | |||||
| Test Day | Drug | 0 | 7 | 14 | 24 |
| PE 1 | No | 20.88 ± 1.47& | 20.13 ± 1.75& | 21.60 ± 1.82& | 20.43 ± 2.17& |
| PE 2 | Yes | 14.20 ± 0.85 | 14.40 ± 1.51 | 14.88 ± 1.78 | 12.86 ± 1.98 |
indicates a significant effect of Day.
3.3b Social Interaction
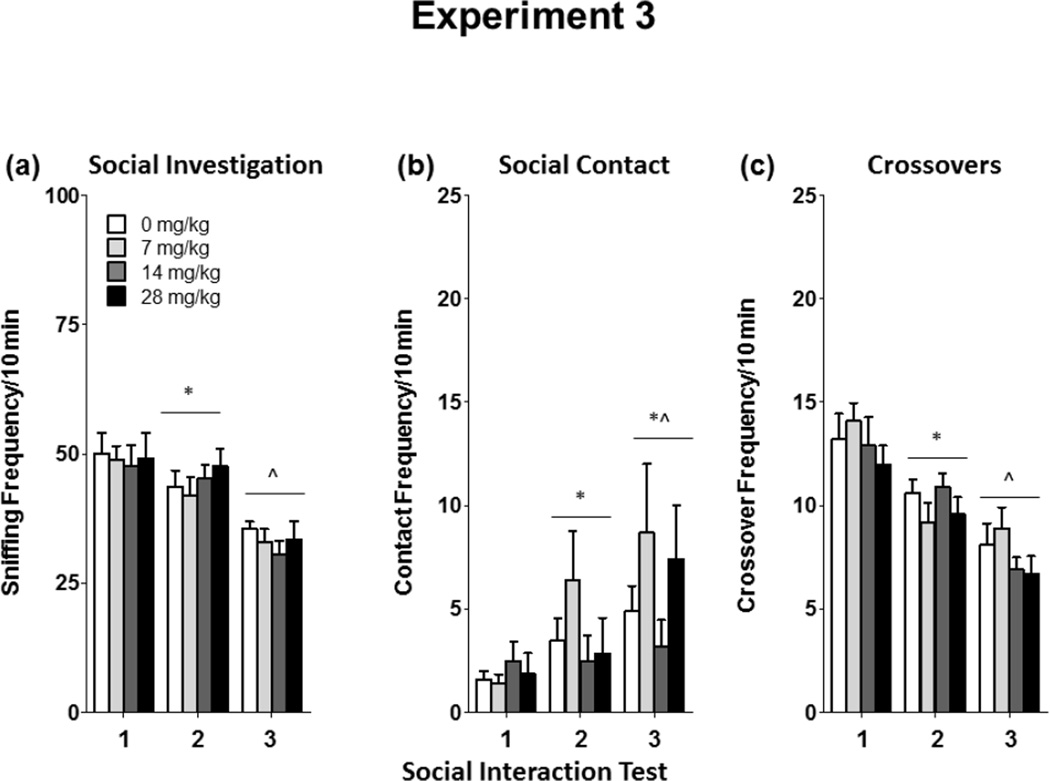
Naproxen administration, regardless of dose, did not affect social investigation or social contact on any day of testing (Figure 7a–c). Social investigation declined across test day [F(2,66) = 36.62, p < 0.0001]. Friedman ANOVA was used to assess the impact of naproxen on social contact, since it was not normally distributed. Social contact increased across testing days [χ2(2) = 17.28, p < 0.001], but was not impacted by dose. Total crossovers during the social interaction test also declined across test day [F(2,66] = 32.5, p < 0.0001], but were not impacted by naproxen administration.
Figure 7.
Social investigation, contact, and total crossovers in 18-month-old females administered naproxen (0–28 mg/kg) in Experiment 3.
4.0 Discussion
Overall, the findings reported here are consistent with the literature in which social behavior has been studied in aged rats (Andersen et al., 1999; Hunt et al., 2011; Markel et al., 1995; Salchner et al., 2004; Shoji and Mizoguchi, 2011; Soffié and Bronchart, 1988), thereby replicating a substantial decline in overall social behavior during senescence (Exps. 1 and 2) and extending those findings to female Fischer 344 rats (Exp. 2). Thus, the present findings contribute to our knowledge on the relationship between diminished social behavior and several other aging-related changes. Specifically, our experimental approach examined the specificity of aging-related declines in social behavior through (i) assessment of other factors, such as general exploratory activity, exploration of an inanimate object, and motor performance, that could influence aging-related changes in social behavior; (ii) examination of how sex influences aging-related alterations in social behavior; and (iii) systematic variation of the characteristics of the social partner (familiarity and age) as a means to determine whether nonspecific deficits in conspecific recognition might account for aging-related declines in social behavior.
Aging was associated with a significant decline in general activity (as measured by total crossovers), compared to adolescent and adult rats. This aging-related decrease in activity was observed in both males (Exp. 1 and 2) and females (Exp. 2). This is consistent with studies showing a reduction in overall locomotor activity in aged rats (Hunt et al., 2011; Mencio-Wszalek et al., 1992). A reduction in the total number of crossovers exhibited by aged animals was consistently observed during exploration of a novel object and all phases of social interaction. In addition, social deficits were observed in both 18- and 24- month old male (Figure 5a, and 5c) and female rats (Figure 5b, and 5d), suggesting that aging-related declines in social behavior observed in males and females are not simply due to impaired ability to move about the testing environment, since a decline in social interaction was observed in both ages. Additionally, our results confirm previous findings showing a decline in motor performance with increased age in rodents (Altun et al., 2007; Gage et al., 1984; Spangler et al., 1994).
Another possible explanation for age-related differences in social interaction is that aged animals have impaired cognitive abilities that may manifest in a deficit in object and/or social recognition. Here, we provide evidence that aged males are able to discriminate between a novel and familiar object (Exp. 1). The present data also suggest that the decline in social behavior observed in aged rats is not simply a matter of an inability to recognize or remember conspecifics. This is not a trivial issue since natural aging is often accompanied by general declines in cognitive function (Barrientos et al., 2012). Indeed, previous studies have demonstrated that aged rats show impaired social recognition (Guan and Dluzen, 1994; Prediger et al., 2006, 2005) and olfactory discrimination (Prediger et al., 2006, 2005; Terranova et al., 1994), although some studies have shown no impairment (Kraemer and Apfelbach, 2004). In a study conducted by Mencio-Wszalek et al. (1992), aged male Sprague-Dawley rats (20-months-old) exhibited reductions in olfactory investigation of non-receptive females and immature males, although investigation of receptive females was similar between young (2–5 months), middle-aged (11–15 months), and aged rats (20+ months), suggesting that the decline in investigation may be due to a reduction in motivation to engage in social investigation, rather than an inability to detect conspecifics. Even with low levels of general activity throughout behavioral testing, aged males were able to discriminate the novel and familiar objects (Exp. 1). Furthermore, in Exp. 2, there was a decline in investigation of a familiar conspecific, indicative of habituation, across all ages. Then, when a novel conspecific was presented on the third day, social investigation increased, indicating dishabituation. This suggests that aged animals are able to recognize a previously encountered conspecific, and adjust behavior accordingly. However, further analysis of conspecific recognition and memory would be needed to test this more specifically. There was a slight decrease in social investigation in 18- and 24-month old males, but no differences in social contact. On the other hand, there was a significant and consistent decrease in social investigation and contact in aged females (18 and 24 months; Figure 5). These findings are important because previous studies of aging-related changes in social behavior have used exclusively males or found no difference between aged male and females in social behavior (20–30 month old Wistar rats; Hunt et al., 2011). When these findings are combined with our results on sensorimotor performance, it appears as if the decline in social investigation in 24-month-old males could be due, in part, to impaired movement around the testing environment, while in 24-month-old females these abilities remain largely intact.
In the final experiment, neither acute nor prolonged naproxen administration affected social interaction in 18-month-old females. Thus, aging-related changes in social behavior in females may represent an actual decline in social motivation, rather than a decline in general health that would be secondary to aging-related achiness or malaise. Previous work by Godbout and colleagues (Huang et al., 2008) has demonstrated sickness-associated declines in social behavior, which are more pronounced in aged mice. Thus, it is possible that low-grade inflammation, as occurs in aging, could be responsible for aging-related social deficits. What we show here is that peripheral administration of naproxen, which reduces inflammation, did not affect social interaction in aged females. As naproxen is a non-selective COX-2 inhibitor (Mitchell et al., 1993), we cannot rule out that drugs targeting other inflammatory pathways (e.g. prostaglandins, cytokines) could influence social behavior in aged rats.
The vast majority of rodent studies examining social behavior have done so in young rodents, during early developmental periods in which social behavior often manifests as rough-and-tumble play (Panksepp et al., 1984; Bredewold et al., 2014; van Kerkhof et al., 2013; Kummer et al., 2011; Pellis et al., 1997; Smith, 2012; Strickland and Smith, 2015; Trezza et al., 2011; Vanderschuren and Trezza, 2014; Veenema et al., 2012; Yates et al., 2013; Zakharova et al., 2009; Zernig and Pinheiro, 2015). Indeed, social processes in young rodents are richly expressed at high levels and lend themselves toward ready delineation of neural substrates underlying social play behavior. Other rodent models, such as prairie voles, have been utilized extensively to better understand brain mechanisms underlying social bonding in young adults (Wang et al., 1998; Young et al., 2011). Social interaction in young adults is also commonly used as a reporter of sickness-like behavior as well as adverse consequences of stressor exposure (Arakawa et al., 2009; Christianson et al., 2009). However, substantially fewer studies have examined social processes in aged rodent models, perhaps because aged rodents express considerably reduced social behavior (than their younger counterparts) that can often be contaminated by other social (aggression, sexual behavior) or nonsocial (general activity, motor function, responses to novelty, etc.) factors. Thus, the studies here provide an important behavioral foundation for the pursuit of neural mechanisms contributing to the decline in social behavior during senescence. Future research may focus on age-related differences in social reward that may help to explain aging-related deficits in social behavior, given that the rewarding value of social interactions declines across ontogeny, with adolescent rats being more responsive to social reward than their older counterparts (Douglas et al., 2004; Trezza et al., 2011; et al., 2010; Trezza et al., 2009). Furthermore, not only age, but also the housing condition and sex of an experimental subject as well as its partner can play a substantial role in the rewarding value of social interactions (Douglas et al., 2004).
The overall low levels of social investigation/contact among aged rats makes studying such behaviors quite difficult, and investigators often elect to isolate rats for a few hours to a few days prior to social behavior testing (Arakawa et al., 2011, 2009; Varlinskaya et al., 1999). Indeed, the housing conditions in the days leading up to social behavior testing is one variable that changed across experiments (30 min of isolation in Exp. 1; 5 days in Exp. 2; 20 min of isolation in Exp. 3). These procedural differences represented our attempts to optimize the sensitivity of our social behavior testing procedures for aged subjects. Although housing condition was not systematically evaluated in these experiments, a cautious comparison of data across experiments suggests that short-term isolation (5 days) does not appear to affect social processes in aged subjects, indicating that perhaps aged rats may be resistant to the influence of short term social deprivation (unlike younger counterparts). We make this inference based largely on differences in ambient social behavior observed between Exps. 2 and 3, since subjects from Exp. 1 were derived from a different vendor (Taconic) relative to subsequent experiments (Charles River; i.e., NIA switched their primary vendor amidst the execution of these studies). Nevertheless, the aging-related decline in social behavior appears to be highly reproducible regardless of other variables that may have changed (source of rats, pre-testing isolation), underscoring the importance of delineating the neural substrates underlying social behavior regulation in aged rats. In this way, the studies here provide an important behavioral foundation for the pursuit of neural mechanisms contributing to the decline in social behavior during senescence.
When comparing these findings to what has been observed in aged humans, these data support the observation that humans show a natural reduction in social interaction with increased age (Carstensen, 1992). In addition, the manipulation of the familiarity of the social partner in these experiments show that aged animals do not necessarily show an increase in social interaction with a familiar conspecific, as would be expected from data in humans showing a gradual narrowing of social circles, with a preference for engaging in social interaction with familiar individuals (Amore, 2005; Lang, 2001; Schiffman, 1997). However, it could be argued that 1 day of social interaction with a novel conspecific is not sufficient interaction to constitute the depth of familiarity achieved by humans engaged in enduring social relationships. This represents a minor limitation to the model of brief social interaction utilized here, and future studies should target the development of pre-clinical models that might be valuable for assessment of how durable social relationships impact the response to novel partners among aged subjects. Regardless, engagement in positive social interaction has positive health benefits, and understanding the factors that contribute to the decline in social behavior observed in aging can inform potential treatment options, leading to a better quality of life for the aging population.
Highlights.
Natural aging is accompanied by a decline in social behavior in rodent models.
Conspecific recognition was intact in 18-month old Fischer 344 rats of both sexes.
Social behavior deficits are not attributable to sensorimotor dysfunction in aging.
Acute or chronic NSAID treatment had no effect on aging-related social deficits.
Aging-associated social deficits likely arise from disruption in social motivation.
Acknowledgments
Supported by NIH grant number R01AG043467 to T.D. and the Center for Development and Behavioral Neuroscience at Binghamton University. We would like to thank Eric. M. Truxell and Jackie E. Paniccia for their technical assistance. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies.
Abbreviations
- AC
Acclimation Phase
- CMC
Carboxymethylcellulose
- COX
Cyclooxygenase
- F344
Fischer 344
- FAS
Forepaw Adjusting Steps
- NIA
National Institute on Aging
- NSAID
Non-Steroidal Anti-Inflammatory Drug
- PE
Pre-Exposure
- SI
Social Interaction Phase
- VEFP
Vibrissae-Evoked Forelimb Placing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- Altun M, Bergman E, Edström E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol. Behav. 2007;92:911–923. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Amore M. Partial androgen deficiency and neuropsychiatric symptoms in aging men. J. Endocrinol. Invest. 2005;28:49–54. [PubMed] [Google Scholar]
- Andersen MB, Zimmer J, Sams-Dodd F. Specific behavioral effects related to age and cerebral ischemia in rats. Pharmacol. Biochem. Behav. 1999;62:673–682. doi: 10.1016/s0091-3057(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Schallert T, Woodlee MT, Shattuck A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson’s disease. Behav. Brain Res. 2007;179:183–191. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blandino P, Deak T. The role of neuroinflammation in the release of aversive odor cues from footshock-stressed rats: Implications for the neural mechanism of alarm pheromone. Psychoneuroendocrinology. 2011;36:557–568. doi: 10.1016/j.psyneuen.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Deak T. Acute illness induces the release of aversive odor cues from adult, but not prepubertal, male rats and suppresses social investigation by conspecifics. Behav. Neurosci. 2009;123:964–978. doi: 10.1037/a0017114. [DOI] [PubMed] [Google Scholar]
- Barrientos R, Frank M, Watkins L, Maier S. Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition. Hormones and behavior. 2012;62:219–227. doi: 10.1016/j.yhbeh.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R, Kitt M, Watkins L, Maier S. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Ramos A, Chaouloff F, Mormde P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behav. Genet. 1997;27:155–166. doi: 10.1023/a:1025641509809. [DOI] [PubMed] [Google Scholar]
- Boguszewski P, Zagrodzka J. Emotional changes related to age in rats--a behavioral analysis. Behav. Brain Res. 2002;133:323–332. doi: 10.1016/s0166-4328(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL. Motivation for social contact across the life span: a theory of socioemotional selectivity. Nebraska Symposium on Motivation. Nebraska Symposium on Motivation. 1992;40:209–254. [PubMed] [Google Scholar]
- Carter C. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioural Brain Research. 2007;176:170186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter SC. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, Longevity, Inflammation, and Cancer. Annals of the New York Academy of Sciences. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Charuvastra A, Cloitre M. Social Bonds and Posttraumatic Stress Disorder. Annu. Rev. Psychol. 2008;59:301–328. doi: 10.1146/annurev.psych.58.110405.085650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–450. doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GD, MacPherson IS, Petrone G, Spangler RS. Antinociceptive effects of non-steroidal anti-inflammatory drugs in a rat model of unilateral hindpaw inflammation. Eur. J. Pharmacol. 1994;257:103–108. doi: 10.1016/0014-2999(94)90700-5. [DOI] [PubMed] [Google Scholar]
- Coleman P. How old is old? Neurobiology of Aging. 2004;25:1. doi: 10.1016/j.neurobiolaging.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Conti MM, Ostock CY, Lindenbach D, Goldenberg AA, Kampton E, Dell’isola R, Katzman AC, Bishop C. Effects of prolonged selective serotonin reuptake inhibition on the development and expression of L-DOPA-induced dyskinesia in hemi-parkinsonian rats. Neuropharmacology. 2014;77:1–8. doi: 10.1016/j.neuropharm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish M, Korányi L, Nyakas C, Almeida OF. Exposure to a novel stimulus reduces anxiety level in adult and aging rats. Physiol. Behav. 2001;72:403–407. doi: 10.1016/s0031-9384(00)00424-8. [DOI] [PubMed] [Google Scholar]
- DeVries A, Craft T, Glasper E, Neigh G, Alexander J. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32 doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- File SE. New strategies in the search for anxiolytics. Drug Des. Deliv. 1990;5:195–201. [PubMed] [Google Scholar]
- Foster T. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Progress in Neurobiology. 2012;96:283303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frussa-Filho R, Otoboni JR, Uema FT, Sá-Rocha LC. Evaluation of memory and anxiety in rats observed in the elevated plus-maze: effects of age and isolation. Braz. J. Med. Biol. Res. 1991;24:725–728. [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol. Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp P. The use of animal models to study the effects of aging on cognition. Psychology. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O’Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav. Brain Res. 1994;61:87–90. doi: 10.1016/0166-4328(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml PA. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav. Immun. 2014;37:15–20. doi: 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Stafford NP, Yusko-Osborne B, Schiml PA, Xanthos ED, Deak T. Naproxen attenuates sensitization of depressive-like behavior and fever during maternal separation. Physiol. Behav. 2015;139:34–40. doi: 10.1016/j.physbeh.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol. Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G, Nieuwenhuijzen P, Chan-Ling T, McGregor I. “When an old rat smells a cat”: A decline in defense-related, but not accessory olfactory, Fos expression in aged rats. Neurobiology of Aging. 2011;32:737749. doi: 10.1016/j.neurobiolaging.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav. Brain Res. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Jurgens H, Johnson R. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental neurology. 2010;233:40–48. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof LW, van Damsteegt R, Trezza V, Voorn P, Vanderschuren LJMJ. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology. 2013;38:1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SMM, Ferraz AC. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behavioural brain research. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol. Behav. 2004;81:435–442. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. Conditioned place preference for social interaction in rats: contribution of sensory components. Front Behav Neurosci. 2011;5:80. doi: 10.3389/fnbeh.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F. Regulation of Social Relationships in Later Adulthood. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2001;56:P321–P326. doi: 10.1093/geronb/56.6.p321. [DOI] [PubMed] [Google Scholar]
- Lucion AB, De-Almeida RM, Da-Silva RS. Territorial aggression, body weight, carbohydrate metabolism and testosterone levels of wild rats maintained in laboratory colonies. Braz. J. Med. Biol. Res. 1996;29:1657–1662. [PubMed] [Google Scholar]
- Markel E, Felszeghy K, Luiten PG, Nyakas C. Beneficial effect of chronic nimodipine treatment on behavioral dysfunctions of aged rats exposed to perinatal ethanol treatment. Arch Gerontol Geriatr. 1995;21:75–88. doi: 10.1016/0167-4943(95)00653-3. [DOI] [PubMed] [Google Scholar]
- Mencio-Wszalek T, Ramirez VD, Dluzen DE. Age-dependent changes in olfactory-mediated behavioral investigations in the male rat. Behav. Neural Biol. 1992;57:205–212. doi: 10.1016/0163-1047(92)90164-y. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa H, Hasegawa M, Fukuta T, Amano M, Yamada K, Nabeshima T. Dissociation of impairment between spatial memory, and motor function and emotional behavior in aged rats. Behav. Brain Res. 1998;91:73–81. doi: 10.1016/s0166-4328(97)00105-8. [DOI] [PubMed] [Google Scholar]
- Moretti de Souza, de Chaves de Andrade, Romao P, Gavioli Boeck. Emotional behavior in middle-aged rats: Implications for geriatric psychopathologies. Physiology & behavior. 2011;102:115–120. doi: 10.1016/j.physbeh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Okuma Y. Age-related defects in lifespan and learning ability in SAMP8 mice. Neurobiology of aging. 1999;20:111–115. doi: 10.1016/s0197-4580(99)00006-8. [DOI] [PubMed] [Google Scholar]
- Norden D, Muccigrosso M, Godbout J. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, McCarthy DO, Bicer S, Devine RD, Reiser PJ, Godbout JP, Wold LE. Ibuprofen ameliorates fatigue- and depressive-like behavior in tumor-bearing mice. Life Sci. 2015;143:65–70. doi: 10.1016/j.lfs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden Godbout. Review: Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol. Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Prediger RD, De-Mello N, Takahashi RN. Pilocarpine improves olfactory discrimination and social recognition memory deficits in 24 month-old rats. Eur. J. Pharmacol. 2006;531:176–182. doi: 10.1016/j.ejphar.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormède P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav. Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. Behavioral and neurochemical differences between Fischer 344 and Harlan-Wistar rats raised identically. Behav. Genet. 1999;29:187–192. doi: 10.1023/a:1021644002588. [DOI] [PubMed] [Google Scholar]
- Rosenzweig E, Barnes C. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Progress in Neurobiology. 2003;69:143179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Salchner P, Lubec G, Singewald N. Decreased social interaction in aged rats may not reflect changes in anxiety-related behaviour. Behavioural Brain Research. 2004;151:18. doi: 10.1016/j.bbr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278:1357–1362. [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K. Aging-related changes in the effects of social isolation on social behavior in rats. Physiology & behavior. 2011;102:58–62. doi: 10.1016/j.physbeh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl.) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffié M, Bronchart M. Age-related scopolamine effects on social and individual behaviour in rats. Psychopharmacology (Berl.) 1988;95:344–350. doi: 10.1007/BF00181945. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol. Aging. 1994;15:319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. Animal models of social contact and drug self-administration. Pharmacol. Biochem. Behav. 2015;136:47–54. doi: 10.1016/j.pbb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JPP, Pério A, Worms P, Fur G, Le Soubrié P. Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav Pharmacol. 1994;5:90–98. [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011;1:444–458. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro Witt, Lewis Hass, Lipman Hart. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. The journals of gerontology. Series A, Biological sciences and medical sciences. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Trezza V. What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. The Neurobiology of Childhood. 2014;16:189–212. doi: 10.1007/7854_2013_268. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E, Spear L, Spear N. Social Behavior and Social Motivation in Adolescent Rats: role of housing conditions and partner’s activity. Physiology & Behavior. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61:50–56. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Young LJ, De Vries GJ, Insel TR. Voles and vasopressin: a review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog. Brain Res. 1998;119:483–499. doi: 10.1016/s0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-García AM, Zhao X, Liu S-JJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp. Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alcohol Depend. 2013;129:240–246. doi: 10.1016/j.drugalcdep.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Frontiers in neuroendocrinology. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Pinheiro BS. Dyadic social interaction inhibits cocaine-conditioned place preference and the associated activation of the accumbens corridor. Behav Pharmacol. 2015;26:580–594. doi: 10.1097/FBP.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]