Abstract
Background
The World Health Organization (WHO) aims to reduce mortality from chronic diseases including cardiovascular disease (CVD) by 25% by 2025. High blood pressure (BP) is a leading CVD risk factor. We sought to compare three strategies for treating BP in China and India: a treat-to-target (TTT) strategy emphasizing lowering BP to a target, a benefit-based tailored treatment (BTT) strategy emphasizing lowering CVD risk, or a hybrid strategy currently recommended by the WHO.
Methods and Results
We developed a microsimulation model of adults aged 30–70 years old in China and in India to compare the two treatment approaches across a 10-year policy-planning horizon. In the model, a BTT strategy treating adults with a 10-year CVD event risk ≥10% used similar financial resources but averted about 5 million more DALYs in both China and India than a TTT approach based on current U.S. guidelines. The hybrid strategy in current WHO guidelines produced no substantial benefits over TTT. BTT was more cost-effective at $205–$272/ DALY averted, which was $142–$182 less per DALY than TTT or hybrid strategies. The comparative effectiveness of BTT was robust to uncertainties in CVD risk estimation or to variations in the age range analyzed, the BTT treatment threshold, or rates of treatment access, adherence, or concurrent statin therapy.
Conclusions
In model-based analyses, a simple BTT strategy was more effective and cost-effective than TTT or hybrid strategies in reducing mortality.
Keywords: hypertension, myocardial infarction, stroke, epidemiology, prevention
The World Health Organization’s General Assembly has adopted a target of reducing chronic disease mortality, including mortality from cardiovascular disease (CVD), by 25% by 2025 among adults 30 to 70 years old.1 Achieving such a large reduction in CVD mortality will likely require more extensive blood pressure (BP) treatment, as high BP is a leading modifiable risk factor for CVD mortality.2 Particularly in rapidly-developing countries that face the greatest burden of CVD, a key question is how to maximize CVD mortality reduction within limited budgets.
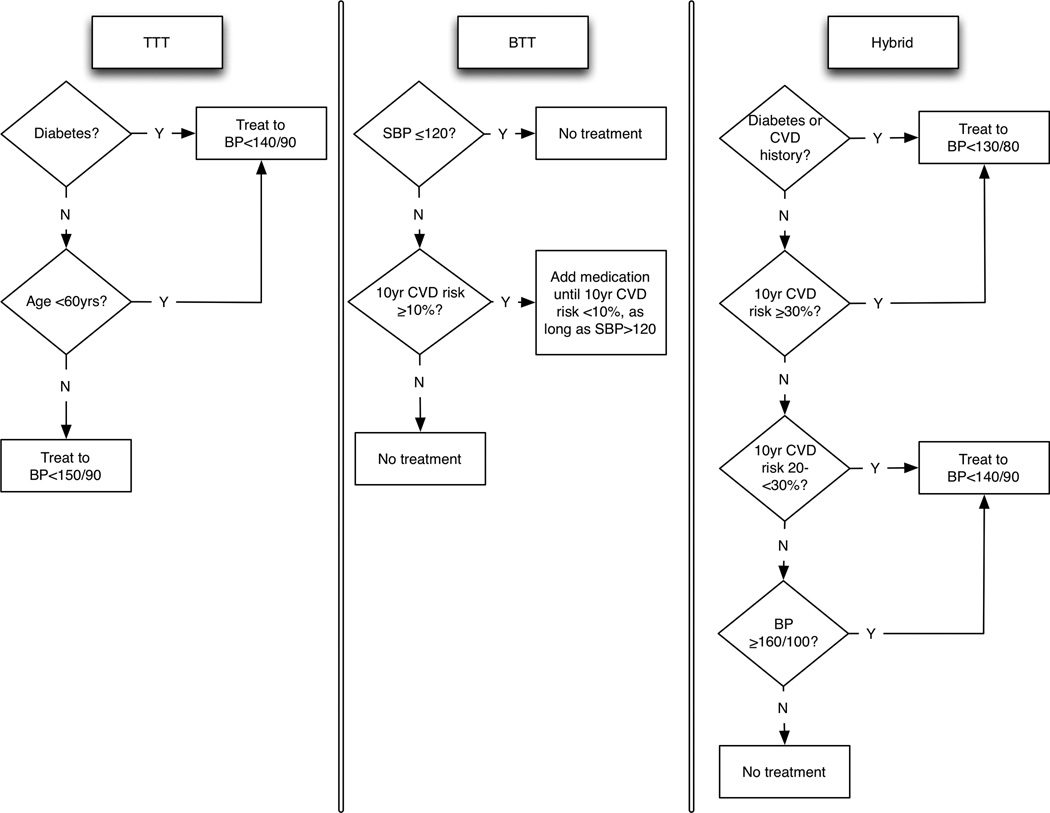
Most international BP treatment guidelines are based on U.S. or European guidelines. U.S. guidelines have emphasized a “treat-to-target” (TTT) strategy, in which BP therapy is titrated until blood pressures fall below a threshold (i.e., ≤140/90 mmHg).3 Conversely, European guidelines have emphasized a “benefit-based, tailored” treatment (BTT) strategy in which BP therapy is initiated for patients with high estimated CVD risk (i.e., for myocardial infarctions [MI] or strokes).4 Both US and European guidelines acknowledge that treating high BP is not to lower BP values per se, but to reduce CVD events. The World Health Organization (WHO) has proposed a hybrid approach that recommends treating patients with both high CVD risk and high BP levels (Figure 1),5 and has distributed “risk charts” to aid clinicians.6
Figure 1.
Treat-to-target (TTT, left), benefit-based tailored treatment (BTT, middle), and hybrid (right) alternatives. The hybrid strategy is from the World Health Organization (WHO) guidelines for hypertension treatment.5 10-year risks in calculated via the WHO risk equations.6 CVD: cardiovascular disease; BP: blood pressure; SBP: systolic blood pressure. All blood pressure treatment targets are in units of mmHg.
Here, we sought to compare population benefits and cost-effectiveness of BTT, TTT, and hybrid strategies, using data from China and India—two countries expected to face the greatest burden of CVD deaths over the next decade.7
Methods
Study design
We developed and validated two microsimulation models (one for China, one for India; Supplemental Figure 1). Microsimulation models estimate risk among individuals by repeatedly sampling from correlated probability distributions of risk factors to capture the distribution of risk and treatment benefit among diverse populations. The models simulated nationally-representative populations of adults 30–70 years old in each country; each individual was characterized by age, sex, location (urban/rural), blood pressure treatment access, systolic and diastolic blood pressure, total cholesterol, history of diagnosed diabetes, and current tobacco smoking. As detailed in the Supplemental Material, individuals were assigned these features using a multivariate sampling algorithm for each country, accounting for demography-specific risk factors, their inter-correlations and trends.8 We calculated the 10-year risk of MI or stroke for each individual before and after BP treatment using the WHO risk equations, which overcome ethnic biases in traditional risk equations through regional calibration.6,9
We then applied each of three alternative treatment strategies: (1) a TTT strategy based on current U.S. guidelines, targeting BP <150/90 mmHg (or <140/90 mmHg if having diabetes or being younger than 60 years old);3 (2) a simple CVD risk-based BTT strategy based on current UK guidelines,10 recommending treatment for a 10-year combined risk of MI and stroke ≥10% (where the 10% threshold was chosen because it approximately matches the budget expenditure for the TTT strategy, for fair comparison); and (3) the hybrid approach recommended in current WHO guidelines (Figure 1).5 For each treatment strategy, we estimated the reduction in CVD morbidity (in MI and stroke events, and disability-adjusted life years, DALYs) and mortality over a 10-year policy-planning horizon. A DALY is a standard measure of health, where one DALY refers to the loss of one year of healthy life. The number of DALYs lost to a disease are calculated as the sum of the years of life lost due to premature mortality, plus the sum of years of life lived with disability due to the disease.11 To produce fair comparisons, the same proportion of the population is assumed to have access to treatment, and the same proportion is assumed to adhere to treatment, in our simulations of the TTT, BTT, and hybrid strategies. In sensitivity analyses, we explored variations in treatment strategies, CVD risk estimation, treatment access, adherence, and concomitant statin treatment.5
Data sources
Input parameters to the model are summarized in Table 1.11–23 Three types of input data were incorporated into the models: demographic, population risk factor, and treatment effect data. Population-level demographic data for the simulations (age, sex, and urban/rural location) were obtained from the United Nations (Supplemental Tables 1 and 2).24 Population CVD risk factor distributions and their inter-correlations were obtained from the WHO Study on Global Aging and Adult Health (SAGE)12 and associated country-specific data sources such as the China Health and Nutrition Survey25 (Supplemental Tables 3–13). Estimates of the BP reduction achieved through pharmacologic therapy were obtained through a comprehensive meta-analysis of BP reduction by medication class, assessed from randomized trials (ranging from 7.9 to 9.9 mmHg of systolic BP reduction from standard doses of each drug, Table 1);13 a PubMed database search using the terms “systematic review” and “blood pressure treatment” found no more recent estimates that would alter these estimates.
Table 1.
Input parameters for the model. 95% confidence intervals are in parentheses.
| Parameter | China data | India data | Source(s) |
|---|---|---|---|
| Population with access to blood pressure treatment (%) | Urban 84%, rural 81% | Urban 74%, rural 65% | World Health Organization12 |
| Systolic blood pressure reduction from standard doses of each drug (mmHg)* | Thiazide: 8.8 (8.3–9.4); Angiotensin converting enzyme inhibitors: 8.5 (7.9–9.0); Calcium channel blockers: 8.8 (8.3–9.2); Beta-blockers: 9.2 (8.6–9.9) |
Meta-analysis of randomized trials13 | |
| Diastolic blood pressure reduction from standard doses of each drug (mmHg)* | Thiazide: 4.4 (4.0–4.8); Angiotensin converting enzyme inhibitors: 4.7 (4.4–5.0); Calcium channel blockers: 5.9 (5.6–6.2); Beta-blockers: 6.7 (6.2–7.1) |
Meta-analysis of randomized trials13 | |
| Population prescribed blood pressure treatment who adhere to treatment (%) | 50% (varied in sensitivity analyses) | Observational cohort studies14–16 | |
| Relative risk of myocardial infarction from each mmHg of systolic blood pressure reduction | RRMI = 2α×(β1γ2+β2γ+β3), where α = post-treatment minus pre-treatment blood pressure in mmHg (i.e., a negative number), β1 = −1.1009×10−5, β2 = 8.6305×10−4, β3 = 3.5176×10−2, γ = age in years. |
Risk function derived previously17 from meta-analysis of randomized trials (R2 = 0.995 for MI and 0.997 for stroke),18 validated against independent meta-analyses (<5% absolute difference)19,20 | |
| Relative risk of stroke from each mmHg of systolic blood pressure reduction | RRstroke = 2α×(β1γ2+β2γ+β3), where α = post-treatment minus pre-treatment blood pressure in mmHg (i.e., a negative number), β1 = −2.5946×10−5, β2 = 2.3052×10−3, β3 = 2.2168×10−2, γ = age in years. |
||
| Cost of standard doses of each drug (2015 $US/person/year)* | Thiazide: $1.6 ($1.2–$2.0); Angiotensin converting enzyme inhibitors: $6.0 ($3.0–$10.3); Calcium channel blockers: $9.2 ($5.7–$13.7); Beta-blockers: $3.8 ($2.6–$6.0); Statin: $19.4 ($8.4–$30.4) |
International Drug Price Indicator Guide21 | |
| Cost of medical services including patient-borne costs (2015 $US/person/year) | Blood pressure therapy annual care: $12 ($8–$16); MI event: $645 ($363–$927); Post-MI annual care: $79 ($73–$86); Stroke event: $883 ($543–$1,223); Post-stroke annual care: $795 ($548–$1,041); Diabetes testing (fasting blood glucose): $2 ($1–$3); Lipid testing (total cholesterol): $1 ($0.5–$2) |
Prior cost estimates updated to 2015 USD11,22 | |
| Rate of adverse side-effects attributable to treatment from standard doses of each drug (%)* | Thiazide: 9.9% (6.6–13.2%); Angiotensin converting enzyme inhibitors: 3.9% (0.5–8.3%); Calcium channel blockers: 8.3% (4.3–11.8%); Beta-blockers: 7.5% (4.0–10.9%) |
Meta-analysis of randomized trials13 | |
| Relative risk reduction when adding statin therapy | 25% for MI, 19% for stroke | Meta-analysis of randomized trials23 | |
Standard doses for thiazide is hydrochlorothiazide 25mg, for angiotensin converting enzyme inhibitor is enalapril 5mg, for calcium channel blocker is amlodipine 5mg, for beta-blocker is atenolol 50mg, and for statin is simvastatin 20mg.
We applied the effect of BP medication to the subset of the population having access to treatment according to the most recent WHO survey (ranging from 65% to 84% access among subpopulation cohorts, Table 1)12. We also applied the treatment benefit to the subset of the population who typically adhere to BP treatment, which was estimated by conducting a PubMed search using the terms “adherence” and “blood pressure treatment”, revealing three relevant estimates (averaging ~50% adherence, Table 1).14–16 For those treated and adhering, we applied the relative risk reduction from BP reduction estimated in three meta-analyses and one cost-effectiveness analysis including meta-regression of randomized trials quantifying blood pressure treatment effectiveness by age (ranging from relative risks of 0.79 to 0.89 across age groups for a 5 mmHg reduction in systolic BP, Table 1); through a PubMed database search using the terms “systematic review” and “blood pressure treatment”, no more recent estimates were found that would alter these estimates (Table 1).17–20
Treatment approaches
Therapy was prescribed per the algorithms depicted in Figure 1, based on the systolic and diastolic averages of two clinically-observed blood pressure measurements, simulated by applying a coefficient of variation of 0.09 per measurement to each individual’s untreated systolic and diastolic blood pressure to reflect measurement error and biological variation.26,27 Choice and dose of drug therapy followed WHO guidelines (see Supplemental Material).5 We accounted for the lower blood pressure reduction achieved from the third or fourth medication (16% lower) added to the first or second medication in a series,28,29 and variation in treatment response (3%).30 As shown in Figure 1, the BTT algorithm included the recommended strategy of having a minimum safe level of systolic blood pressure below which therapy should not be prescribed regardless of the risk calculation (clinically-observed systolic BP <120 mmHg), to avoid possibly increased mortality below this level.31
In each year of the simulation, individuals potentially experienced CVD events (MI or stroke) based on their calculated risk estimates from the WHO risk equations, which make use of the estimated relative risk of CVD events from systolic and diastolic blood pressure, total cholesterol, history of diagnosed diabetes, current tobacco smoking, and a prior history of MI or stroke (risk factors chosen because of data availability in developing countries; Supplemental Table 14). Individuals survived or died based on their demographic-specific case fatality rate, capturing local treatment infrastructure and quality (Supplemental Table 12), and were additionally subject to non-CVD mortality using a competing risks algorithm (Supplemental Table 13).32 The microsimulation used discrete-time annual probabilities of CVD events and incorporated annually-updated continuous risk factors adjusted for age-related and secular trends, as detailed in the Supplemental Material. To check the convergent validity of the model prior to conducting simulations, we ensured that our projected estimates of demographic size were within 5% absolute error of the United Nations estimates,24 and that projected estimates of CVD mortality had <5% absolute error from the most recent projections by age, sex, and location from the Global Burden of Disease Project (Supplemental Figure 2).33
Cost-effectiveness analysis
Because data on non-medical costs (i.e., lost work hours) were unavailable, we used a medical perspective for cost-effectiveness analysis, including costs of pharmaceuticals and medical service delivery for patients and other payers (Table 1). The costs include screening and monitoring at the frequencies observed among patients receiving BP drugs in each country, including laboratory costs, personnel costs, overhead expenses, and costs of MI and stroke in the year of the event as well as in subsequent years (e.g., rehabilitation).11,22 For the BTT and hybrid strategies, we included costs of both cholesterol and diabetes testing for those patients who have not yet received such testing (costs shown in Table 1); for the TTT strategy, only persons who do not meet treatment criteria for therapy based on age or blood pressure alone also incurred costs of diabetes testing. Drug costs included standard doses for generic (off patent) drugs at doses recommended in the WHO essential medicines list (Table 1).21,34
Disability weights for MI and stroke were obtained from the Global Burden of Disease Project35 (Supplemental Tables 14–16). We also included the disutility of receiving pharmacotherapy (0.001 per pill per year),36 and an additional probabilistic disutility (0.01 per person per year) for patients who incur significant side-effects conditional on age, drug and dosage (Table 1).13 All costs were expressed in 2015 US Dollars; cost-effectiveness ratios compared to the counterfactual of no treatment were calculated after discounting both costs and DALYs at a 3% annual rate and compared to the threshold for cost-effectiveness of three-times the gross national index per person ($7,380 in China and $1,610 in India) per DALY averted.11,37 Consistent with WHO guidelines, we estimated DALYs and costs that would accumulate over the life-course of each person alive or born during the period 2016–2025.11
Alternative treatment strategies and sensitivity analyses
In further analyses, we varied the risk threshold for treatment in the BTT strategy to 15%, or to 5% among adults <60 years old; lowered the threshold for BP therapy down to a systolic BP of 120mmHg for all persons under the TTT strategy, given results of the Systolic Blood Pressure Intervention Trial (SPRINT);38 simulated a 5% rise in access or adherence to therapy; modified the BTT strategy to incorporate a “safety valve” of treating everyone with systolic BP>150mmHg; increased the upper age for analysis to 85 years; and simulated concurrent statin therapy prescribed per WHO guidelines.5,23
In probabilistic sensitivity analyses, we repeated all simulations 10,000 times while simultaneously sampling from normal distributions estimated from the means and standard deviations of all input parameters, including the CVD risk estimation coefficients, to estimate means and standard deviations around all results. The number of iterations was chosen as 10,000 as this level of repeated sampling generated stable standard deviation estimates to within rounding error. The models were implemented in R (version 3.1.2, R Foundation for Statistical Computing, Vienna).
Results
At current treatment access levels, the TTT strategy (Table 2) would recommend treatment for 9.0% of 30 to 70 year olds in China (64.4 million people, 95% CI: 63.0–65.9) and 11.0% in India (54.9 million people, 95% CI: 53.9–55.9). 81.9% of those recommended for treatment in China and 71.4% of those recommended for treatment in India would receive 1 to 2 medications, with a mean of 1.8 medications per person treated in China and 2.2 in India. At 50% medication adherence, 1.1 million MI and stroke events would be averted in China (95% CI: 1.0–1.2 million) and 0.8 million in India (95% CI: 0.7–0.9 million), saving 8.2 million DALYs in China (95% CI: 7.5–8.9) and 6.5 million in India (95% CI: 6.1–6.9). While CVD risk was higher in India than in China, fewer events were prevented in India given lower treatment access. The costs of TTT would be $3.6 billion in China for a cost-effectiveness ratio of $435.9 per DALY averted (95% CI: $390.4–$480.8), and $2.6 billion in India for a cost-effectiveness ratio of $399.2 per DALY (95% CI: $352.2–445.7). The TTT strategy would avert 391.8 thousand deaths in China (a 4.0% reduction, 95% CI: 3.7–4.4%) and 325.0 thousand deaths in India (a 3.3% reduction, 95% CI: 3.2–3.6%).
Table 2.
Outcomes of treatment for adults aged 30 to 70 in China and India, comparing the treat-to-target (TTT), benefit-based tailored treatment (BTT), and a hybrid strategy (based on current World Health Organization Guidelines), utilizing current estimates of blood pressure levels, blood pressure treatment access, other risk factors, and treatment benefit for populations in China and India. Uncertainty intervals are in parentheses.*
| Outcome | China (N=716 million) | India (N=499 million) | |||||
|---|---|---|---|---|---|---|---|
| TTT | BTT | Hybrid | TTT | BTT | Hybrid | ||
| Medications used: | |||||||
| per 100 persons age 30–85 | 15.1 (14.9–15.3) | 14.8 (11.2–18.4) | 13.8 (13.0–14.6) | 22.2 (22–22.4) | 18.5 (13.8–23.2) | 15.4 (14.6–16.2) | |
| per person treated | 1.8 (1.8–1.8) | 2.8 (2.7–2.9) | 2.5 (2.4–2.6) | 2.2 (2.2–2.2) | 3.0 (2.9–3.1) | 2.9 (2.9–2.9) | |
| Adults who receive treatment, %, n millions: | |||||||
| Total | 9.0%, 64.4 (8.8%–9.2%) | 5.4%, 38.7 (3.8%–7%) | 5.8%, 41.5 (5.2%–6.4%) | 11.0%, 54.9 (10.8%–11.2%) | 6.6%, 32.9 (4.2%–9%) | 5.1%, 25.4 (4.6%–5.6%) | |
| 1 medication | 5%, 35.9 (4.9%–5.1%) | 0.5%, 3.2 (0%–1%) | 1.9%, 13.4 (1.6%–2.2%) | 4.9%, 24.6 (4.8%–5%) | 0.5%, 2.4 (0.1%–1.1%) | 0.9%, 4.5 (0.7%–1.1%) | |
| 2 medications | 2.4%, 16.9 (2.3%–2.5%) | 2.2%, 15.9 (1.8%–2.6%) | 1.3%, 9.5 (1.1%–1.5%) | 2.9%, 14.6 (2.8%–3%) | 2.6%, 12.9 (1.7%–3.5%) | 0.7%, 3.6 (0.6%–0.8%) | |
| 3 medications | 0.9%, 6.4 (0.9%–0.9%) | 0.9%, 6.5 (0.6%–1.2%) | 1.1%, 8.2 (1%–1.2%) | 1.4%, 7.1 (1.4%–1.4%) | 1%, 5.0 (0.7%–1.3%) | 1.1%, 5.7 (1%–1.2%) | |
| 4 medications | 0.7%, 4.8 (0.7%–0.7%) | 1.8%, 12.9 (1.4%–2.2%) | 1.5%, 10.5 (1.5%–1.5%) | 1.8%, 8.9 (1.8%–1.8%) | 2.5%, 12.4 (1.9%–3.1%) | 2.4%, 12.1 (2.3%–2.5%) | |
| Initial SBP among treated, in mmHg | 153.7 (153.6–153.8) | 144.6 (142.3–146.9) | 151.4 (150.8–152.0) | 160.3 (160.2–160.4) | 147.6 (146.0–149.2) | 158.8 (158.5–159.1) | |
| Final SBP among treated, in mmHg | 146.2 (146.0–146.4) | 133.2 (130.3–136.1) | 141.0 (140.5–141.5) | 151.1 (150.9–151.3) | 135.1 (133.4–136.8) | 146.9 (146.5–147.3) | |
| Pre-treatment 10-year CVD risk among treated, mean % | 9.1 (8.2–10) | 16.7 (14.8–18.6) | 11.5 (9.6–13.4) | 13.2 (11.1–15.3) | 21.2 (15.7–26.7) | 15.8 (12.4–19.2) | |
| Post-treatment 10-year CVD risk among treated, mean % | 7.1 (6.4–7.8) | 11.8 (10.6–13) | 8.3 (6.8–9.8) | 10.1 (8.5–11.7) | 15.4 (11.3–19.5) | 11.8 (9.2–14.4) | |
| MI and stroke events prevented per 10 years: | |||||||
| Total, millions | 1.1 (1.0–1.2) | 1.9 (1.5–2.3) | 1.1 (0.9–1.3) | 0.8 (0.7–0.9) | 1.6 (1.3–1.9) | 0.9 (0.7–1.1) | |
| MI, millions | 0.3 (0.3–0.3) | 0.5 (0.4–0.6) | 0.3 (0.2–0.4) | 0.5 (0.4–0.6) | 1 (0.8–1.2) | 0.5 (0.4–0.6) | |
| Stroke, millions | 0.8 (0.7–0.9) | 1.4 (1.1–1.7) | 0.8 (0.6–1) | 0.3 (0.3–0.3) | 0.6 (0.4–0.8) | 0.3 (0.2–0.4) | |
| Number needed to treat to prevent one event | 58.5 (52.5–65.9) | 20.4 (11.8–33.4) | 37.7 (28.6–50.9) | 68.6 (59.9–79.9) | 20.6 (11.1–34.5) | 28.2 (20.9–39.9) | |
| Total costs, million $US 2015 | $3575 (2928–4280) | $3710 (2511–5150) | $3519 (2612–4557) | $2595 (2149–3076) | $2436 (1705–3323) | $2228 (1711–2817) | |
| Total DALYs averted, millions | 8.2 (7.5–8.9) | 13.2 (10.2–16.2) | 8.1 (6.6–9.6) | 6.5 (6.1–6.9) | 11.4 (9.1–13.7) | 6.2 (5.2–7.2) | |
| $/DALY averted | $435.9 (390.4–480.8) | $281.0 (246.1–317.9) | $434.4 (395.7–474.6) | $399.2 (352.2–445.7) | $213.6 (187.3–242.5) | $359.3 (329.0–391.2) | |
| MI and stroke deaths averted, %, n thousands | 4.0% (3.7%–4.4%), 391.8 | 7.0% (5.5%–8.4%), 689.4 | 4.0% (2.9%–5.1%), 399.1 | 3.3% (3.2%–3.6%), 325.0 | 6.4% (4.9%–8.0%), 637.0 | 3.3% (2.4%–4.0%), 329.4 | |
Uncertainty intervals (in parentheses) were determined by 10,000 iterations with multivariable Monte Carlo sampling from normal distributions constructed from the mean and standard deviation of estimated values of all input parameters, including the CVD risk estimation equations. Note: The uncertainty range for results of the BTT strategy are greater than for the TTT strategy because BTT depends on overall CVD risk estimation, which can be impacted by errors in measurement of BP, cholesterol, and knowledge of diabetes diagnosis and prior CVD history; by contrast, the TTT treatment decision was only affected by clinical errors in measurement of BP and knowledge of diabetes diagnosis.
By contrast, the BTT strategy (Table 2) would recommend treatment to fewer people, 5.4% of 30 to 70 year old adults in China (38.7 million, 95% CI: 27.2–50.1, 25.7 million fewer than TTT) and 6.6% in India (32.9 million, 95% CI: 21.0–44.9, 22.0 million fewer than TTT). Among those treated, however, a greater number of medications would be prescribed per person; on average, 2.8 medications in China (1.0 more than under TTT), and 3.0 in India (0.8 more than under TTT). The BTT strategy would avert more CVD events and deaths: 0.8 million more MIs and strokes prevented in China (95% CI: 0.5–1.1) and 0.8 million more in India (95% CI: 0.5–1.0) than with TTT, and 298 thousand more deaths in China (a 7.0% reduction, 95% CI: 5.5–8.4%) and 317 thousand more deaths in India (a 6.4% reduction, 95% CI: 4.9–8.0%). The treatment costs of BTT would be $3.7 billion in China for a cost-effectiveness ratio $281.0 per DALY averted in China (95% CI: $246.1–317.9), and $2.4 billion in India for a ratio of $213.6 per DALY averted (95% CI: $187.3–242.5). The hybrid strategy would avert similar numbers of events and DALYs to the TTT strategy, at a similar cost, but reduce the number of people on treatment by between one-third and one-half (Table 2). Supplemental Figure 3 provides breaks down the three treatment algorithms to detail which subpopulations are differentially treated by them in each country.
Because many people would be treated similarly under different strategies, it is informative to examine effectiveness and efficiency for those people treated differently by the three approaches. We estimated the cost-effectiveness of treatment among patients who would be treated most intensively by BTT and the cost-effectiveness of treatment among those who would be most intensively by TTT. The population who would be treated most intensively by one approach refers to the group prescribed more antihypertensive medications under that approach than under the alternative treatment approach. We identified this population in the analysis by tracking the number of medications prescribed to each simulated individual under both of the alternative treatment strategies. As shown in Table 3, the cost-effectiveness for those who would be treated most intensively by BTT was much better in both countries—at $271.7/DALY, versus $438.2 for those who would be treated most intensively by TTT in China, and $192.9/DALY for BTT in India, versus $404.4/DALY for TTT. In China, despite almost 28 million fewer people being treated most intensively by BTT than by TTT (36.6M vs. 62.3M, respectively), the group most intensively treated by BTT would have over 65% more total DALYs averted (12.4M vs. 7.4M). Similarly, in India, 22 million fewer people would be treated most intensively by BTT than by TTT (28.8M vs. 50.8M), yet the group most intensively treated by BTT would have roughly twice as many total DALYs averted (10.0M vs. 5.1M). Those most intensively treated by BTT would also use pharmacotherapy more efficiently than the TTT strategy (~22 more DALYs per 1000 person-years of pharmacotherapy in China, and ~25 more DALYs per 1000 person-years in India; Table 3). Those treated most intensively by the hybrid strategy would have lower total DALYs saved and cost-effectiveness than the BTT group. Supplemental Figure 4 illustrates incremental cost-effectiveness plots of BTT versus the two other treatment strategies.
Table 3.
Relative efficiency of the treat-to-target (TTT), benefit-based tailored treatment (BTT), or hybrid approach (based on current World Health Organization guidelines), utilizing current estimates of utilizing current estimates of blood pressure levels, blood pressure treatment access, other risk factors, and treatment benefit for populations in China and India. Uncertainty intervals are in parentheses.*
| Outcome | People treated identically by all three strategies |
People treated most intensively by TTT |
People treated most intensively by BTT |
People treated most intensively by Hybrid |
|---|---|---|---|---|
| China | ||||
| Number of people treated (million n) | 2.1 (1.1–3.1) | 62.3 (59–65.6) | 36.6 (27.8–45.4) | 39.4 (36.8–42) |
| Total DALYs averted (millions) | 0.8 (0.5–1.1) | 7.4 (7.1–7.7) | 12.4 (10.2–14.6) | 7.3 (6.9–7.7) |
| $/DALY among treated | 401.4 (222.7–581.7) | 438.2 (401.6–475) | 271.7 (216.6–328.6) | 436.9 (317.9–558.8) |
| DALYs averted per 1000 patient-years of pharmacotherapy | 38.1 (37.7–38.5) | 11.9 (10.9–12.9) | 33.9 (28.2–39.6) | 18.5 (17.8–19.2) |
| India | ||||
| Number of people treated (million n) | 4.1 (3.2–5) | 50.8 (47.8–53.8) | 28.8 (13.9–43.7) | 21.3 (19.6–23) |
| Total DALYs averted (millions) | 1.4 (1.2–1.6) | 5.1 (4.8–5.4) | 10 (7.6–12.4) | 4.8 (4.6–5) |
| $/DALY among treated | 359.1 (293.2–427) | 404.4 (294.5–514.4) | 192.9 (165.4–223.5) | 359.8 (85.3–637.1) |
| DALYs averted per 1000 patient-years of pharmacotherapy | 34.1 (31.3–36.9) | 10.0 (9.1–10.9) | 34.7 (30.2–39.2) | 22.5 (21.8–23.2) |
Uncertainty intervals (in parentheses) were determined by 10,000 iterations with multivariable Monte Carlo sampling from normal distributions constructed from the mean and standard deviation of estimated values of all input parameters, including the CVD risk estimation equations. Note: The uncertainty range for results of the BTT strategy are greater than for the TTT strategy because it depends on CVD risk estimation, which can be impacted by errors in measurement of BP, cholesterol, and knowledge of diabetes diagnosis and prior CVD history; by contrast, the TTT treatment decision was only affected by clinical errors in measurement of BP and knowledge of diabetes diagnosis.
Detailed subgroup analyses (Supplemental Table 17) revealed that TTT strategy would more intensively treated younger people and people with CVD event risks of less than 5% over 10 years in both China and India. The hybrid strategy would also be more likely than the other strategies to focus therapy in people with diabetes. Supplemental Table 18 provides characteristics of persons averted from a CVD event across strategies, while Supplemental Table 19 provides examples of patients who would be treated differently under each strategy.
Alternative treatment strategies and sensitivity analyses
Lowering the risk threshold for BTT treatment from 10% to 5% for persons <60 years old would increase the total DALYs averted from 13.2 to 19.4 million in China and from 11.4 to 12.9 million in India, and the total number of CVD deaths averted from 684.6 thousand to 1.05 million in China and 634.4 thousand to 788 thousand in India, but worsen the cost-effectiveness ratio to $406/DALY in China and $278 in India—still better than TTT or hybrid strategies (Supplemental Table 20).
Lowering the target for TTT strategy to a systolic blood pressure of 120mmHg or less for all persons would increase DALYs averted by 3- to 4-fold in proportion to the larger population treated, but at a worse cost-effectiveness ratio (to $542/DALY averted in China, 95% CI: $453–626; and $490/DALY averted in India, 95% CI: $405–573) (Supplemental Table 21). The same budget and cost-effectiveness ratio would be achieved by lowering the BTT threshold to 3.1% in China and 2.4% in India (Supplemental Table 21). Over and above the BTT strategy of treating <60 year olds for a risk of at least 5% and ≥60 years old for a risk of at least 10%, the incremental gains of treating to a target systolic blood pressure of 120mmHg would have a cost-effectiveness of $2,400 per DALY averted in China and $1,300 per DALY averted in India.
Conversely, raising the BTT treatment initiation threshold to 15% for all adults would reduce the benefits of BTT substantially, and also worsen cost-effectiveness ratios as compared to the 10% threshold scenario, but the cost-effectiveness ratios were nevertheless still be better than the TTT or hybrid strategies (Supplemental Table 22).
Expectedly, improving treatment access and adherence would substantially improve each treatment strategy’s absolute benefits. A 5 percentage point increase in treatment access would increase the number of deaths averted by 1–9% in China and 12–24% in India compared to the baseline estimates, while not significantly impacting the relative benefits or cost-effectiveness among strategies (Supplemental Table 23). A 5 percentage point increase in adherence would increase the number of deaths averted by between 8% and 28% in either country, without significantly impacting the relative benefits or cost-effectiveness among strategies (Supplemental Table 24).
Including a “safety valve” in the BTT strategy by treating everyone with systolic BP>150mmHg would increase the BTT strategy’s comparative effectiveness, averting ~25% more deaths than in the baseline assessment, but required treating 53% of people more intensively (Supplemental Table 25).
Similarly, expanding the age range for analysis to 30 to 85 years old would increase the comparative effectiveness of BTT given the higher risk among older adults, reducing numbers of CVD deaths by two (India) to five (China) times the baseline assessment when accounting for the large population of older adults without significantly worse cost-effectiveness (Supplemental Table 26). This assumes the benefit of treatment and of a life-year remains consistent as people age.
Including access to statins (Supplemental Table 27) would reduce the benefits of all three hypertension treatment strategies by only 1–4% from the baseline assessment; some CVD deaths previously averted by hypertensive therapy were instead averted by the statin therapy, and total CVD deaths averted increased by ~21%.
In addition to our pre-specified sensitivity analyses, we performed a post-hoc examination to find the level of adherence to BTT that would maintain its comparative advantage over TTT and hybrid strategies. If TTT and hybrid strategies maintained 50% adherence, the BTT strategy would still be superior in terms of overall DALYs, mortality and cost-effectiveness if adherence to BTT were as low as 26% in China and 21% in India.
Discussion
We found that a simple BTT strategy was always more effective than currently recommended TTT strategies in reducing CVD events and mortality in both China and India, even when accounting for uncertainties in CVD risk estimation, BP levels, other risk factors, treatment access and adherence, and concurrent statin therapy. BTT’s relative advantage was achieved by treating high-risk, high-benefit individuals more intensively. Further, the total DALYs saved by BTT can be greatly improved, without a substantial loss in cost-effectiveness, by lowering the treatment initiation threshold in those <60 years old from a 10% to a 5% 10-year CVD risk. We estimate that this strategy on its own could achieve over one third of the WHO’s CVD mortality target. BTT’s comparative benefits require maintaining adherence to a greater pill burden, but adherence would need to be much worse to fully negate its greater benefits. Surprisingly, the hybrid strategy in current WHO recommendations did not succeed in combining the relative advantages of TTT and BTT, but minimally improved upon the TTT approach. The hybrid strategy concentrated therapy in population subgroups such as people with diabetes, but also treated such individuals only at higher levels of BP, reducing the overall population benefits of BP therapy.
As with any model-based analysis, our results are limited by assumptions and input data. First, our analysis used WHO risk equations, which are the basis for global recommendations and are regionally calibrated. Some alternative risk scores do not require laboratory data,39 but require sparsely-available dietary and physical activity data. Second, our sensitivity analyses found substantial improvements in total deaths averted if those age 70 to 85 are also treated with BTT, but older adults often require more nuanced clinical judgment than can be easily modeled. Our analysis also focused on the two outcomes of MI and stroke, which reflect over 90% of BP-associated outcomes,2 and have parallel relationships to BP as other outcomes such as renal disease.40–42 Hence, our analysis does not reflect all-cause benefits of BP treatment, but rather addresses the question of relative benefits among the treatment strategies. Finally, we were only able to account for medical costs of treatment and not larger societal costs (such as lost work) from CVD, which could lead to conservative estimates BP therapeutic benefits.
Despite these needs for future research, our study revealed that a simple BTT strategy may greatly increase population health benefits and improve the cost-effectiveness of BP treatment in the two middle-income countries facing the greatest total burden of CVD – China and India. Achieving the Sustainable Development Goal of reducing chronic disease globally would be better advanced through a BTT strategy than alternative TTT and hybrid strategies currently suggested in common guidelines.
Supplementary Material
Clinical Perspective.
In May 2012, the World Health Organization’s (WHO) General Assembly adopted a target of reducing cardiovascular disease (CVD) mortality by 25% by 2025, setting the template for the global “Sustainable Development Goals”. Achieving such a large reduction in CVD mortality will likely require more extensive blood pressure (BP) treatment. Particularly in rapidly-developing countries, a key question is how to achieve the greatest CVD mortality reduction within limited budgets. Here, we sought to compare the population benefits and cost-effectiveness of three alternative proposed strategies to treat high BP, using data from China and India on treatment access, BP levels, and other risk factors for CVD. Our research, which uses a modeling strategy, found that a simple treatment approach using calculations of CVD risk to inform treatment decisions was more effective and cost-effective than the common strategy of using BP levels to decide treatment. The risk-based approach recommends treatment for patients with a 10-year combined risk of myocardial infarction and stroke of at least 10%. We also found that the current WHO guidelines were worse than the simple risk-based approach; these guidelines recommend treating individuals with high CVD risk (≥30% risk over 10 years) to a BP of <130/80 mmHg, and individuals with lower risk (20–30% over 10 years) to BP <140/90 mmHg. Even with poor treatment adherence, the risk-based approach to BP treatment could on its own succeed in achieving between one-quarter and one-third of the CVD mortality goal set by the WHO.
Acknowledgments
We thank the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, Carolina Population Center (R24 HD-050924), the University of North Carolina at Chapel Hill, the U.S. National Institutes of Health (R01 HD-30880, R01 DK-056350, R24 HD-050924, and R01 HD-38700), the Fogarty International Center at the NIH, and the China-Japan Friendship Hospital, Ministry of Health for the China Health and Nutrition Survey (CHNS) data collection and analysis files that were used in this study. We also thank the U.S. National Institute on Aging Division of Behavioral and Social Research (R01 AG-034479) and the World Health Organization's Department of Health Statistics and Information Systems for the Study on Global Aging and Adult Health (SAGE) data collection and analysis files that were used in this study.
Funding Sources: This work was supported by the U.S. National Institutes of Health (SB, grant nos. K08 HL-121056 and DP2 MD-010478; RH, JS, grant no. P60 DK-20572), the Veterans Affairs Health Services Research and Development Service (JS, Career Development Award 13-021), the Rosenkranz Prize for Healthcare Research in Developing Countries (SB), the International Development Research Center of Canada (SB), the NIHR Research Professorship award (CM), and the Wellcome Trust Capacity Strengthening Strategic Award (CM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures: None.
References
- 1.World Health Organization. Geneva: WHO; 2015. Towards a monitoring framework with targets and indicators for the health goals of the post-2015 Sustainable Development Goals. [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT-A, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y-H, Khatibzadeh S. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Geneva: WHO; 2013. Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings. [Google Scholar]
- 6.Mendis S, Lindholm LH, Mancia G, Whitworth J, Alderman M, Lim S, Heagerty T. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25:1578–1582. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Geneva: WHO; 2015. [cited 2015 Jun 15]. Projections of mortality and causes of death, 2015 and 2030 [Internet] Available from: http://www.who.int/healthinfo/global_burden_disease/projections/en/ [Google Scholar]
- 8.Hofert M, Mächler M. Nested Archimedean copulas meet R: The nacopula package. J Stat Softw. 2011;39:1–20. [Google Scholar]
- 9.Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, Rodgers A. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 10.Rabar S, Harker M, O’Flynn N, Wierzbicki AS. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: summary of updated NICE guidance. BMJ. 2014;349:g4356. doi: 10.1136/bmj.g4356. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva: WHO; 2010. Choosing interventions that are cost effective (WHO-CHOICE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S, Millett C. Social epidemiology of hypertension in middle-income countries determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension. 2013;62:18–26. doi: 10.1161/HYPERTENSIONAHA.113.01374. [DOI] [PubMed] [Google Scholar]
- 13.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newby LK, LaPointe NMA, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-Term Adherence to Evidence-Based Secondary Prevention Therapies in Coronary Artery Disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 16.Thomas Dennis NM. Medication adherence and associated barriers in hypertension management in India. CVD Prev Control. 2011;6:9–13. [Google Scholar]
- 17.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost-effectiveness analysis. Ann Intern Med. 2010;152:481–487. W170–W173. doi: 10.7326/0003-4819-152-8-201004200-00212. [DOI] [PubMed] [Google Scholar]
- 18.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 19.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 21.Management Sciences for Health. International drug price indicator guide [Internet] Cambridge, MA: Management Sciences for Health; 2014. [cited 2015 Jan 28]. Available from: http://www.msh.org/sites/msh.org/files/international-drug-price-indicator-guide.pdf. [Google Scholar]
- 22.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368:679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 24.United Nations. World Population Prospects: The 2012 Revision. Geneva: UN; 2013. [Google Scholar]
- 25.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2010;39:1435–1440. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Clinical Guideline Centre. Hypertension: The clinical management of primary hypertension in adults, Clinical Guideline 127. London: Naitonal Institute for Health and Clinical Excellence; 2011. [Google Scholar]
- 27.Powers BJ, Oddone EZ, Bosworth HB. Measuring blood pressure for decision making and quality reporting. Ann Intern Med. 2011;155:565. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 28.Timbie JW, Hayward RA, Vijan S. Variation in the net benefit of aggressive cardiovascular risk factor control across the US population of patients with diabetes mellitus. Arch Intern Med. 2010;170:1037–1044. doi: 10.1001/archinternmed.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel J-M, Turner ST, Hunt SC, et al. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18:935–942. doi: 10.1016/j.amjhyper.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Bell KJ, Hayen A, Macaskill P, Craig JC, Neal BC, Irwig L. Mixed models showed no need for initial response monitoring after starting antihypertensive therapy. J Clin Epidemiol. 2009;62:650–659. doi: 10.1016/j.jclinepi.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. J-Shaped Relationship between Blood Pressure and Mortality in Hypertensive Patients: New Insights from a Meta-Analysis of Individual-Patient Data. Ann Intern Med. 2002;136:438–448. doi: 10.7326/0003-4819-136-6-200203190-00007. [DOI] [PubMed] [Google Scholar]
- 32.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978:541–554. [PubMed] [Google Scholar]
- 33.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 19th. Geneva: WHO; 2015. WHO Model List of Essential Medicines. [Google Scholar]
- 35.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2013;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–2317. doi: 10.1161/CIRCULATIONAHA.113.002290. Epub 2013 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Bank. World Development Indicators 2013 [Internet] Washington D.C.: World Bank Publications; 2014. [cited 2015 Jan 28]. Available from: https://books.google.com/books?hl=en&lr=&id=YLXzAI_oVmcC&oi=fnd&pg=PR5&dq=world+bank+world+development+indicators&ots=dbxEP9Q7Jw&sig=K4O-INdwoBG_ZhMQA7FlX1ebrgo. [Google Scholar]
- 38.SPRINT Research Group. Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 40.Echouffo-Tcheugui JB, Kengne AP. Risk models to predict chronic kidney disease and its progression: a systematic review. [cited 2015 Jun 17];2012 doi: 10.1371/journal.pmed.1001344. Available from: http://dx.plos.org/10.1371/journal.pmed.1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 42.Murabito JM, D’Agostino RB, Silbershatz H, Wilson PW. Intermittent claudication a risk profile from the Framingham heart study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.