Abstract
Norovirus is the primary cause of viral gastroenteritis in humans with multiple genotypes currently circulating worldwide. The development of a successful norovirus vaccine is contingent on its ability to induce both systemic and mucosal antibody responses against a wide range of norovirus genotypes. Norovirus virus like particles (VLPs) are known to elicit systemic and mucosal immune responses when delivered intranasally. Incorporation of these VLPs into an intranasal powder vaccine offers the advantage of simplicity and induction of neutralizing systemic and mucosal antibodies. Nasal immunization, which provides the advantage of ease of administration and a mucosal delivery mechanism, faces the real issue of limited nasal residence time due to mucociliary clearance. Herein, we describe a novel dry powder (GelVac™) formulation of GI or GII.4 norovirus VLPs, two dominant circulating genotypes, to identify the optimal antigen dosages based on systemic and mucosal immune responses in guinea pigs. Systemic and mucosal immunogenicity of each of the VLPs was observed in a dose dependant manner. In addition, a boosting effect was observed after the second dosing of each VLP antigen. With the GelVac™ formulation, a total antigen dose of ≥15 µg was determined to be the maximally immunogenic dose for both GI and GII.4 norovirus VLP based on evaluation for 56 days. Taken together, these results indicate that norovirus VLPs could be used as potential vaccine candidates without using an immunostimilatory adjuvant and provides a basis for the development of a GelVac™ bivalent GI/GII.4 norovirus VLP vaccine.
Keywords: Vaccine, Norovirus, Intranasal, Virus Like Particles, Guinea Pigs
INTRODUCTION
Norovirus, a single-stranded RNA virus in the Caliciviridae family, is the primary cause of nonbacterial gastroenteritis worldwide, accounting for 96% of all cases of viral gastroenteritis [1–5]. It is distributed among five different genogroups GI, GII, GIII, GIV, and GV [3, 6, 7]. Only genogroups I, II, and IV are infectious to humans, with GI and GII being most prevalent [8, 9]. Recently, genogroup II has become the most prevalent, accounting for 81.4% of norovirus outbreaks worldwide [10]. Each genogroup is subdivided further into genoclusters. Full-length genomic sequencing of various norovirus strains indicate that norovirus can vary by 3–31% within genogroups and 44–49% between genogroups [11]. Due to this wide variation, development of a broadly effective vaccine remains a challenge as the antibodies from humans immunized against one genogroup do not cross react with noroviruses from other genogroups [12].
The success of virus-like particles (VLPs) as vaccine antigens has been demonstrated by the licensure of hepatitis B virus VLP and human papilloma-virus VLP vaccines. Extensive research has focused on the development of norovirus VLPs as vaccine antigens that can be delivered parenterally, orally, or mucosally [13, 14]. Clinical evidence has demonstrated that norovirus VLPs administered orally or intranasally were well tolerated and modestly immunogenic [15, 16]. The lack of a clear immune correlate of protection has been an obstacle for the development of such vaccine candidates. A recent study has shown that antibodies that block the binding of norovirus VLPs to histo-blood group antigens correlate with clinical protection against norovirus-induced gastroenteritis [17]. Additional studies have employed recombinant expression techniques to create norovirus VLPs using baculovirus and tobacco mosaic virus demonstrating that VLPs produced by both production systems have similar structure and immunogenicity [18, 19].
Previous studies have shown that administration of a norovirus vaccine through the nasal cavity is able to induce systemic immunity as well as both local and distal mucosal immunity [20, 21]. Furthermore, the incorporation of VLP with GelVac™ nasal dry powder formulation elicits a greater immune response than antigen alone [21]. GelVac™ is the dry powder formulation with GelSite®, which is an Aloe vera L.-derived polysaccharide polymer with mucoadhesive properties. In the presence of divalent cations, GelVac™ is capable of in-situ gelation which improves mucosal residence time of intranasally administered vaccines [22]. Intranasal immunization of guinea pigs with the GelVac™ norovirus vaccine showed high levels of mucosal IgA antibodies along with high levels of serum IgG antibodies [21]. The present study extends the previous work [21] and demonstrates GelVac™ formulated norovirus GI and GII.4 VLPs induce high levels of antigen-specific systemic and mucosal antibodies in a dose-dependent manner. The GelVac™ norovirus vaccine formulation also induced neutralizing antibodies that have been shown as a surrogate marker for efficacy in humans [17, 18]. Based on the results presented herein, future studies are recommended to investigate a bivalent GelVac™ GI/GII.4 norovirus vaccine formulation. This bivalent vaccine could result in the prevention of norovirus-induced gastroenteritis in humans.
MATERIALS AND METHODS
GI and GII.4 Vaccine Formulation
Recombinant norovirus GI and GII.4 VLPs expressed in Nicotiana benthamiana were obtained from Kentucky Bioprocessing (Owensboro, KY) as previously described [23]. Endotoxins and remaining small molecules were removed by Q Column fractionation. Electron microscopy was performed to confirm the presence of VLPs (Supplementary Figure 1). Stability evaluation of the VLPs was also conducted based on SYPRO Orange binding and antigenicity, which showed that the VLPs were stable up to 65°C (Supplementary Figures 2 and 3).
The GelVac™ vaccine powders were produced using a lyophilization-milling method. Liquid formulations were first prepared using a proprietary formulation comprising the recombinant VLP in a solution with GelSite® polymer and then lyophilized. Following lyophilization, dried formulation contained 0.25 %(w/w) GelSite® and 0–100µg (based on Enzyme-linked immunosorbent assay (ELISA) data) of VLP per 20mg of formulation, depending on the desired dose. Each dried formulation was milled using a mortar and pestle under a controlled, low-humidity (<10% RH) environment and passed through a 70µm filter. Powders were stored in sealed containers under desiccation at room temperature until use.
Laser Diffraction Particle Size Distribution
A 50 mg sample of each powder was suspended in 100% isopropanol and particle size distribution, by volume, was determined using a laser diffraction particle size analyzer with a liquid module (Beckman Coulter LS13 320, Pasadena, CA). Performance of the instrument was verified using a 35 µm garnet reference standard.
Capture ELISA
Mouse monoclonal IgG2 anti-norovirus antibodies (Maine Biotech, MAB228 (GI); MAB227 (GII)) diluted 1:2000 in PBS were coated on Nunc MaxiSorp 96-well plates (Fisher Scientific, Pittsburgh, PA) overnight at 4°C. The wells were washed 5 times with wash buffer, and then blocked for 1 h at room temperature in blocking buffer. Norovirus VLPs at indicated concentrations were diluted in blocking buffer, and allowed to incubate on the plate at room temperature for 1 h. The wells were washed 3 times with wash buffer, followed by incubation with corresponding mouse monoclonal IgG1 anti-norovirus antibodies (Millipore, MAB80143 (GI); Maine Biotech MAB226 (GII)) diluted 1:2000 in blocking buffer for 1 h at room temperature. The wells were washed 3 times with wash buffer, followed by incubation with a polyclonal anti-mouse IgG1:HRP (Abcam, Cambridge, MA) diluted 1:2000 in blocking buffer for 1 h at room temperature. Finally, the wells were washed 3 times with wash buffer and were developed using 1-step Ultra TMB according to manufacturer’s protocol (Thermo Scientific, Waltham, MA). The OD at 450 nm was measured and plotted against known VLP concentrations.
Animal Studies
All animal study protocols were approved by the Institutional Animal Care and Use Committee at Battelle Memorial Institute. General procedures for animal care and housing were in accordance with Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) recommendations. Female (250 g) Hartley guinea pigs (Harlan Laboratories) were selected as the model animal for evaluation of dry power delivery. Two separate studies were conducted to examine the dose response of guinea pigs to both GelVac™ GI and GelVac™ GII.4 VLP vaccines independently (Table 1). Each study was designed identically with the exception of the antigen present during immunization.
Table 1.
Animal Experimental Design
| Monovalent Guinea Pig Studies | ||
|---|---|---|
| Group # | n | Total Antigen per Vaccination(µg)* |
| 1 | 4 | 100 |
| 2 | 4 | 50 |
| 3 | 4 | 15 |
| 4 | 4 | 5 |
| 5 | 4 | 1 |
| 6 | 4 | 0.1 |
| 7 | 4 | 0 |
Animals were immunized with a total of 20 mg of powder via both nares. Each nare received 10 mg of powder or half of the total antigen dose.
Prior to immunization, animals were randomly distributed into study groups (n=4) and allowed to acclimate for one week. Each study consisted of 7 study groups, one for placebo powders and six for GelVac™ VLP vaccines at different antigen dose levels. Guinea pigs were anaesthetized with 5 % isoflurane prior to immunization. Powder vaccines were administered intranasally on days 0 and 21 with 10 mg/nare of dry powder formulation. Total antigen exposure for each group is described in Table 1. For intranasal powder delivery, Aptar Unit Dose Spray (UDS) Devices (Aptar Pharma, Congers, NY) were each loaded with 10 mg of vaccine powder. Small animal adapters were attached to the end of each device for immunization. While under anesthesia, the end of the adapter was inserted into the nare and the device was actuated. Each animal was given two administrations of VLP powders, one per nare with half of the total antigen dose per nare (10 mg total powder per nare). The control group was administered the same amount of a placebo powder formulation.
Guinea pig serum and vaginal lavage samples were collected on days 0 (preimmunization), 21, 42, and 56. Blood (1 ml/collection period) was collected from the superior vena cava and allowed to coagulate in serum separation tubes. Serum was collected as the supernatant after centrifugation for 10 minutes at 6000 rpm. Vaginal lavages were collected by lavaging 300 µL of PBS for 60 seconds in the vaginal tract with an oral feeding tube. On day 56, guinea pigs were maintained under 5 % isoflurane and exsanguinated by cardiocentesis. All samples were clarified by centrifugation and stored at −20° C prior to analysis.
ELISA for Serum and Mucosal Antibodies
Norovirus GI or GII.4 VLPs (2µg/mL) in PBS were incubated on Nunc MaxiSorp 96-well plates (Fisher Scientific) for 4 hours at room temperature. The plates were blocked overnight at 4°C in blocking buffer. All samples were diluted in blocking buffer and serially diluted 2-fold down the plate. Samples were allowed to incubate at room temperature for 1hr. The wells were washed 5 times with wash buffer, followed by incubation with anti-guinea pig IgG-HRP (Southern Biotech, Birmingham, AL) at 1:1000, anti-guinea pig IgA (Creative Diagnostics, Shirley, NY) at 1:1000, anti-guinea pig IgG1-HRP (Antibodies Online) at 1:1000, or anti-guinea pig IgG2-HRP (Antibodies Online) at 1:2000 for 1 h at room temperature. The wells were washed 5 times with wash buffer and developed using 1-step Ultra TMB according to manufacturer’s protocol. End-point titers were reported as the reciprocal of the highest dilution that produced an OD of 0.1 above background. A positive control serum generated in guinea pigs against GI or GII.4 VLP was included in each test run to confirm reproducibility.
Gastric Mucin Ligand-Binding Serum Neutralization Assay
Porcine Gastric Mucin Type III (PGM) (Sigma Aldrich) has been previously used as a substrate for norovirus VLP antibody-blockade assays [24, 25]. For blockade assays, PGM was dissolved in PBS to a final concentration of 5mg/mL. The PGM solution (100 µL) was added to each well of a 96 well Nunc MaxiSorp plate (Fisher Scientific) and incubated for 4 h at room temperature. The wells were then blocked with blocking buffer overnight at 4° C. Serum was serially diluted in blocking buffer. Norovirus GI or GII.4 VLPs were added to the hyperimmune serum at a final concentration of 0.25 µg/mL and incubated for 1 h at room temperature. The blocking buffer was then removed from the plate and replaced with 100 uL of the VLP-hyperimmune serum solution and incubated for 1 h at room temperature. The wells were then washed 5 times with wash buffer. The bound VLPs were detected using strain specific monoclonal antibodies (Maine Biotech, MAB228 (GI); MAB227 (GII)) diluted 1:2000 in blocking buffer and incubated for 1 h at room temperature. The wells were washed 5 times in wash buffer followed by the addition of Goat anti-mouse-HRP conjugate antibody (Millipore, 12-349) diluted (1:2000) in blocking buffer for 1 h at room temperature. Plates were developed using 1-step Ultra TMB Substrate according to manufacturer’s protocol. Each serum sample was tested in two-fold serial dilutions to complete a 4-Parameter Logistics (4-PL) curve. Neutralizing titer was reported as the inflection of the curve (50 % reduction) as indicated by the 4-PL fit.
Statistics
Two-way Analysis of variance (ANOVA) models were fit separately to each sample type (serum, neutralizing, and vaginal lavage). Each titer was transformed by taking the base-10 logarithm of the measurement values. Dunnett’s multiple comparisons analysis was used to compare each dose group to the control. All results are reported based on the p≤0.05 level of significance.
RESULTS
GelVac™ Vaccine Powder Characterization
The GelVac™ vaccine powder was manufactured through a manual milling process under nitrogen gas. Laser diffraction particle size distribution confirmed the volumetric mean particle size to be 24–37 µm for all powders. Furthermore, the d10 for the powders was approximately 5 µm for all powders, thus minimizing the amount of powders (< 5 µm). A representative particle distribution result for each antigen can be found in Table 2. The mean particle diameter for the GI VLP formulation was 29.73 µm and for GII.4 VLP formulation was 25.2 µm.
Table 2.
Representative volumetric particle size distribution of GI and GII.4 monovalent vaccine powders
| Vaccine powders | Mean | d10 | d50 | d90 |
|---|---|---|---|---|
| GI | 29.73 µm | 5.08 µm | 25.23 µm | 59.25 µm |
| GII.4 | 25.20 µm | 5.49 µm | 22.97 µm | 49.32 µm |
Capture ELISAs were used to confirm the GI or GII VLP dose content of each vaccine powder. GI and GII.4 VLP capture ELISAs were performed with a 15 µg dose formulated vaccine. A 10 mg sample of each 15 µg VLP dose powders was dissolved in 1mL of water. Each sample was tested in the capture ELISA and compared to the VLP reference standard. The results showed that the powders contained the expected amount of VLPs (Supplementary Table 1). The capture ELISA was established using the GI- and GII.4 - specific monoclonal antibodies. No cross-reactivity was observed between these two genogroups (data not shown). The presence of VLPs in vaccine powders were further confirmed by Western blot (Supplementary Figure 4).
Immunogenicity of GelVac GI and GII.4 Powders
The immunogenicity of a GelVac™ vaccine powder formulated with GI VLP has been reported previously [21]. To further these studies, antigen dose-dependent immune responses were investigated with GelVac™ vaccine powders with GI or GII.4 VLPs. Animals were dosed with varying amounts of norovirus GI or GII.4 VLPs (Table 1) on days 0 and 21. Serum and vaginal lavage samples were collected from the animals on days 0 (preimmunization), 21, 42 and 56. One animal in the 100 µg dose group for GI antigen was lost after the second immunization on day 21. Upon autopsy examination, death was attributed to anesthesia and/or the blood collection procedure.
Serum Antibody Response
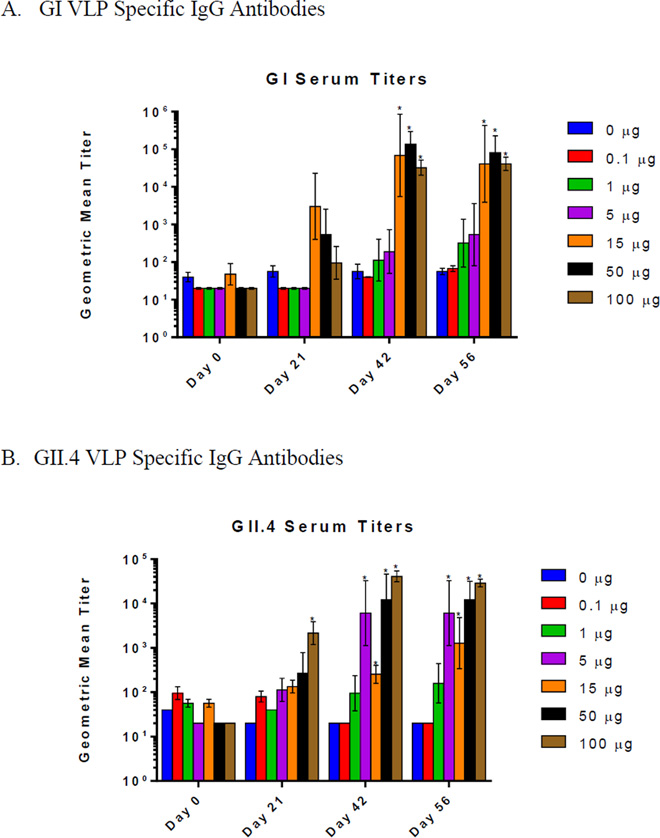
Serum samples were analyzed for norovirus VLP specific IgG by ELISA. The total antigen specific IgG antibodies present in the serum exhibited a dose-dependent increase with both GI and GII.4 vaccine powders (Figure 1). Compared to the control group, serum IgG titers increased on day 21 and a further increase was observed by day 42 at all doses greater than 1 µg. By day 42, GI IgG titers increased by >600-fold compared to day 21 for all dose groups of ≥15 µg and GII.4 IgG titers increased by >300-fold compared to day 21 for all dose groups of ≥5 µg. There were no differences between 15µg and 100µg doses for GI and between 5 µg and 100 µg doses for GII.4. The lowest dose that elicited an antigen specific IgG response was 1 µg for both GI and GII.4 which corresponded to a titer of 495 and 320 on day 56, respectively. As expected, all doses above 0.1 µg exhibited a boosting effect after the second dosing on day 21 with both GI and GII.4 powders. Antigen specific IgA serum levels were also investigated. At day 56, anti-GI and anti-GII.4 VLP IgA antibodies were observed at all doses that were administered when compared to the mock dose controls, except for the 1.0 µg dose group with GII.4 (Figure 1). They also showed an overall trend of higher levels at higher antigen doses. These results showed that the VLP formulations with GelVac™ nasal powder were highly immunogenic and significant antibody production can be induced with GelVac™ nasal powders with GI VLP at 15 µg and GII.4 VLP at 5 µg.
Figure 1.
Serum norovirus-specific IgG and IgA production following intranasal immunization with GelVac™ vaccine powders. Female Hartley guinea pigs were immunized intranasally with 20mg of powder formulation containing various amounts of GI or GII VLP on days 0 and 21. Serum samples were collected on days 0, 14, 21, 42, and 56 and analyzed for specific IgG antibodies GI (A) and GII.4 (B) Norovirus. Day 56 samples were analyzed in pooled samples for specific IgA antibodies against GI (C) and GII.4 (D) Norovirus. Error bars are provided as geometric standard error. *P<0.05 as compared to the placebo control group. Exact adjusted p-values following Dunnett’s multiple comparison test following two-way ANOVA is shown in Supplementary Table 2sA–B.
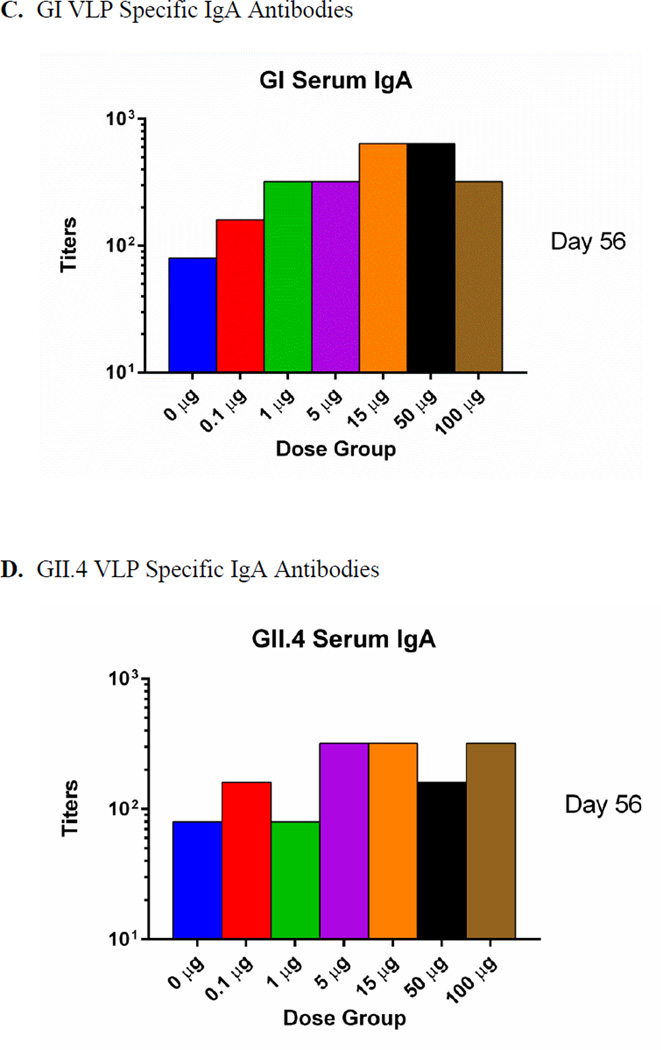
The IgG1 and IgG2 subclasses were also analyzed using the pooled serum samples from each group (Figure 2). Both IgG1 and IgG2 exhibited similar response profiles as the total IgG described above. The IgG2 titers were apparently higher than IgG1 titers with both GI and GII VLP powders, especially at low dose levels (1 and 5 µg) with a difference of up to 100 fold (Figure 2).
Figure 2.
Serum norovirus-specific IgG1 and IgG2 production following intranasal immunization with GelVac vaccine powders. Serum samples described in Figure 5 were pooled for each group and analyzed for GI (A) and GII.4 (B) norovirus-specific IgG1 and IgG2 antibodies.
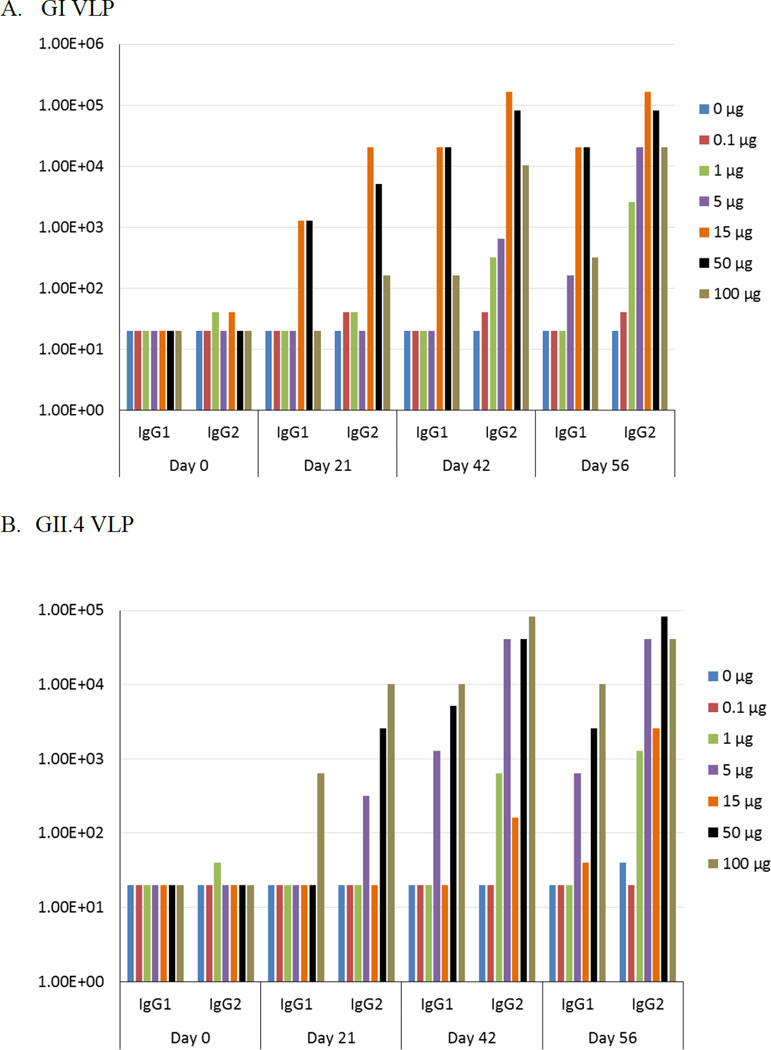
Serum Neutralization Antibody Response
Previous studies have demonstrated that norovirus VLP-specific antibodies can block the binding of norovirus VLP to ABH histo-blood group antigen (HBGA) in a strain specific manner [26]. HBGAs are carbohydrates ubiquitously expressed on mucosal tissues and red blood cells that have been implicated as natural receptors for norovirus binding and entry, suggesting that blockade of HBGA interactions with VLPs may prevent norovirus infection [27]. To this end, antigen specific antibodies were investigated for their ability to inhibit the binding of the norovirus VLPs to porcine gastric mucin. The neutralizing antibodies present in the serum exhibited a dose-dependent response similar to that observed for antigen specific IgG antibody titers (Figure 3). Compared to the control, GI neutralizing antibody titers were elevated in the 15 µg dose group by day 21, in 15 µg, 50 µg, and 100 µg dose groups by day 42, and in all dose groups greater than 1µg by day 56. GII.4 neutralizing antibody titers were elevated for the 100 µg dose group by day 21, elevated by day 42 in the 5 µg, 50 µg and 100 µg dose groups, and elevated in all dose groups greater than 1 µg by day 56. By day 42, GI neutralizing antibody titers increased by >5-fold for all dose groups of >5 µg and GII.4 neutralizing antibody titers increased by >10-fold for all dose groups >1 µg, consistent with the findings with serum IgG titers. There were no significant differences between 5 µg and 100 µg doses for both GI and GII.4 at day 56. The lowest dose that produced a detectable neutralization titer at day 56 was 5 µg for both GI and GII.4. The highest neutralizing antibody titers at day 56 occurred in the 15 µg dose group for GI and 100 µg dose group for GII.4. As expected, all groups above 5 µg for both GI and GII.4 exhibited a boosting effect after the second dose on day 21. These results showed that the neutralizing antibody titers followed a similar dose-dependent response to that observed for the total serum IgG titers.
Figure 3.
Neutralizing antibody production following intranasal immunization with GelVac™ vaccine powder. Female Hartley guinea pigs were immunized intranasally with 20 mg of powder formulation containing various amounts of VLP on days 0 and 21. Serum samples were collected on days 0, 14, 21, 42, and 56 and analyzed for GI (A) and GII.4 (B) neutralizing antibodies. Error bars are provided as geometric standard error. *P<0.05 as compared to the placebo control group. Exact adjusted p-values following Dunnett’s multiple comparison test following two-way ANOVA is shown in Supplementary Tables 2C–D.
Mucosal Antibody Response
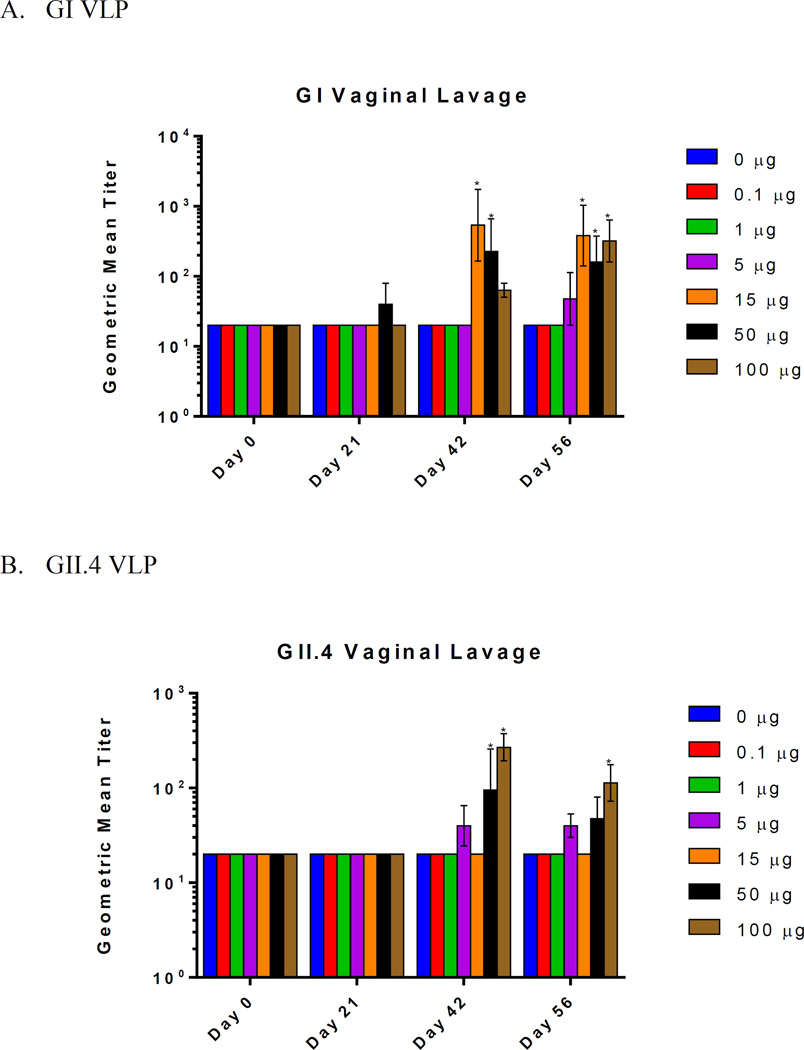
To investigate the mucosal immune response at various antigen doses, mucosal antibody titers were evaluated in the reproductive tracts with vaginal lavage (Figure 4). GI vaginal antibody titers were elevated in 50 µg dose group by day 21 and in all dose groups greater than 1 µg by day 56. GII.4 vaginal antibody titers were elevated in the 5 µg, 50 µg, and 100 µg dose groups by day 42. The lowest dose that elicited a mucosal IgG response was 5 µg for both GI and GII.4. The highest vaginal antibody titers occurred at 15 µg for GI and 100 µg for GII.4. These results showed that vaginal IgG antibody titers exhibited a dose-dependent response that reached a significantly higher level at 15 µg and 50 µg for GI and GII.4, respectively.
Figure 4.
Vaginal Norovirus-specific IgG production following intranasal immunization with GelVac™ vaccine powder. Female Hartley guinea pigs were immunized intranasally with 20 mg of powder formulation containing various amounts of VLP on days 0 and 21. Vaginal lavages samples were collected on days 0, 14, 21, 42, and 56 and analyzed for GI (A) and GII.4 (B) Norovirus-specific IgG antibodies. Error bars are provided as geometric standard error. *P<0.05 as compared to the placebo control group. Exact adjusted p-values following Dunnett’s multiple comparison test following two-way ANOVA is shown in Supplementary Tables 2E–F.
DISCUSSION
Human noroviruses are the leading cause of epidemic non-bacterial gastroenteritis worldwide. Various candidate norovirus vaccines are under development [28–33]. It was shown previously that a norovirus VLP GelVac™ vaccine produces a robust systemic and mucosal immune response [21]. In the present study, we describe the systemic, mucosal, and neutralizing antibody responses to varying amounts of GI or GII.4 norovirus VLP antigens formulated as a monovalent GelVac™ vaccine powder.
Overall, both GI and GII.4 vaccine powders induced a dose dependent antibody response. Serum antigen specific IgG antibody production was correlated with amounts of both GI and GII.4 VLP antigens present in the powders and reached a maximal level at 15–50 µg of VLP antigen. Administration of higher doses of VLPs did not result in significantly higher levels of antigen specific IgGs. It is important to note that the boosting effect on systemic and mucosal IgGs was observed for each VLP antigen after the second dose on day 21.
The production of serum antibodies that neutralize the HBGA binding sites has been largely accepted as a surrogate marker for efficacy and correlates well with protection in humans and chimpanzees [17, 34, 35]. Porcine Gastric Mucin Type III has been previously used as a substrate for norovirus VLP antibody-blockade assays [24]. In this study we have demonstrated that the GelVac™ vaccine powder containing either GI or GII.4 VLPs administered intranasally was capable of producing antibodies in guinea pigs that inhibited the binding of the VLPs to pig gastric mucin. The serum levels of these neutralizing antibodies correlated with the amount of GI or GII.4 VLP administered to the guinea pigs. In a similar fashion that was observed for serum IgG antibodies, a boosting effect in the neutralizing antibody titers after the second dose was also observed. However, a larger amount of VLP antigen was required for the production of neutralizing antibodies as compared to the induction of total specific IgG antibodies. A dose of 15 µg for both GI and GII.4 VLP antigen was required to maximize the production of neutralizing antibodies. These results correlated well with the serum IgG titers and further support a maximally efficacious dose of 15 µg for each genotype.
Finally, mucosal antibody titers were measured through vaginal lavage sampling. Antigen specific IgG antibody production in the vaginal tract showed similar trends to what was observed for both antigen specific IgG antibodies and neutralizing antibodies detected in serum. Presence of mucosal IgG antibodies is most likely conferred through transudation of serum IgG antibodies [36]. These results suggest that our GelVac™ vaccine powder is capable of inducing a mucosal response along with a neutralizing antibody response.
Here we sought to establish the effect of antigen content on the production of systemic and mucosal anti-norovirus specific antibodies with GelVac nasal powder. A dose content of at least 15µg GI or GII.4 VLP antigen appeared to be required to produce a robust response. Future studies will seek to examine this trend with GelVac™ GI and GII bivalent vaccine formulations made of just one powder or an admixture of two individual (GI and GII) powders. The results will help to define an optimal antigen dose range for the bivalent vaccine that could be used in future human clinical trials.
Supplementary Material
Table 3.
Testing of GelVac™ GI and GII.4 vaccine powders for antigen content
| Vaccine Powders | Expected Antigen Dose (µg VLP/mg Powder) |
Observed Antigen Dose (µg VLP/mg Powder) |
|---|---|---|
| GI VLP | 15 µg/10 mg | 15.46 µg/10mg |
| GII VLP | 15 µg/10mg | 16.16 µg/10mg |
Acknowledgments
This research was funded by a grant from the National Institutes of Allergy and Infectious Disease (4R44AI094919-03). The authors would also like to thank Nanotherapeutics, Inc. for their continued support of this research.
The authors of this paper are either employees of Nanotherapeutics or were paid by Nanotherapeutics via the grant to perform this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
We do not have any conflicts of interest.
REFERENCES CITED
- 1.Hedberg C. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:840–842. doi: 10.3201/eid0506.990624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caul EO. Small round structured viruses: airborne transmission and hospital control. Lancet. 1994;343:1240–1242. doi: 10.1016/s0140-6736(94)92146-6. [DOI] [PubMed] [Google Scholar]
- 3.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mead PS, Slutsker L, Griffin PM, Tauxe RV. Food-related illness and death in the united states reply to dr. hedberg. Emerg Infect Dis. 1999;5:841–842. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansman GS, Natori K, Shirato-Horikoshi H, Ogawa S, Oka T, Katayama K, et al. Genetic and antigenic diversity among noroviruses. The Journal of general virology. 2006;87:909–919. doi: 10.1099/vir.0.81532-0. [DOI] [PubMed] [Google Scholar]
- 7.Yun SI, Kim JK, Song BH, Jeong AY, Jee YM, Lee CH, et al. Complete genome sequence and phylogenetic analysis of a recombinant Korean norovirus, CBNU1, recovered from a 2006 outbreak. Virus Res. 2010;152:137–152. doi: 10.1016/j.virusres.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Blanton LH, Adams SM, Beard RS, Wei G, Bulens SN, Widdowson MA, et al. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000–2004. J Infect Dis. 2006;193:413–421. doi: 10.1086/499315. [DOI] [PubMed] [Google Scholar]
- 9.Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, Parashar UD, et al. Epidemiologic and molecular trends of "Norwalk-like viruses" associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- 10.Zheng DP, Widdowson MA, Glass RI, Vinjé J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol. 2010;48:168–177. doi: 10.1128/JCM.01622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev. 2008;225:190–211. doi: 10.1111/j.1600-065X.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 12.LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24:5220–5234. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 13.Sundararajan A, Sangster MY, Frey S, Atmar RL, Chen WH, Ferreira J, et al. Robust mucosal-homing antibody-secreting B cell responses induced by intramuscular administration of adjuvanted bivalent human norovirus-like particle vaccine. Vaccine. 2015;33:568–576. doi: 10.1016/j.vaccine.2014.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Herbst-Kralovetz M, Mason HS, Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010;9:299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 17.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santi L, Huang Z, Mason H. Virus-like particles production in green plants. Methods. 2006;40:66–76. doi: 10.1016/j.ymeth.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White LJ, Hardy ME, Estes MK. Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J Virol. 1997;71:8066–8072. doi: 10.1128/jvi.71.10.8066-8072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, et al. Immunogenicity and specificity of norovirus Consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine. 2012;30:3580–3586. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasquez LS, Shira S, Berta AN, Kilbourne J, Medi BM, Tizard I, et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29:5221–5231. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni YaY, K M. In: In-situ Gel Formation of Pectin. USPTO, editor. United States: Carrington Laboratories, Inc.; 2004. [Google Scholar]
- 23.Santi L, Batchelor L, Huang Z, Hjelm B, Kilbourne J, Arntzen CJ, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindesmith LC, Debbink K, Swanstrom J, Vinjé J, Costantini V, Baric RS, et al. Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J Virol. 2012;86:873–883. doi: 10.1128/JVI.06200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindesmith LC, Costantini V, Swanstrom J, Debbink K, Donaldson EF, Vinjé J, et al. Emergence of a norovirus GII.4 strain correlates with changes in evolving blockade epitopes. J Virol. 2013;87:2803–2813. doi: 10.1128/JVI.03106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol. 2002;76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marionneau S, Ruvoën N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, et al. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, et al. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis. 2014;209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, et al. Norovirus Vaccine Against Experimental Human GII.4 Virus Illness: A Challenge Study in Healthy Adults. J Infect Dis. 2015;211:870–878. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czako R, Atmar RL, Opekun AR, Gilger MA, Graham DY, Estes MK. Experimental human infection with Norwalk virus elicits a surrogate neutralizing antibody response with cross-genogroup activity. Clinical and vaccine immunology: CVI. 2015;22:221–228. doi: 10.1128/CVI.00516-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindesmith LC, Ferris MT, Mullan CW, Ferreira J, Debbink K, Swanstrom J, et al. Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial. PLoS Med. 2015;12:e1001807. doi: 10.1371/journal.pmed.1001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker TD, Kitamoto N, Tanaka T, Hutson AM, Estes MK. Identification of Genogroup I and Genogroup II broadly reactive epitopes on the norovirus capsid. J Virol. 2005;79:7402–7409. doi: 10.1128/JVI.79.12.7402-7409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, Ferreira J, et al. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate--reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis. 2014;210:1763–1771. doi: 10.1093/infdis/jiu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atmar RL, Bernstein DI, Lyon GM, Treanor JJ, Al-Ibrahim MS, Graham DY, et al. Serological Correlates of Protection against a GII.4 Norovirus. Clinical and vaccine immunology: CVI. 2015 doi: 10.1128/CVI.00196-15. {Lindesmith, 2012 #52} [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A. 2011;108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotkin SA, Orenstein WA, Offit PA. Vaccines. 5th. Philadelphia, Pa: Saunders/Elsevier; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.