Abstract
The relation between insulin resistance and coronary artery disease (CAD) in patients with HIV infection remains incompletely defined. Fasting serum insulin and glucose measurements from 448 HIV-infected and 306 uninfected men enrolled in the Multicenter AIDS Cohort Study (MACS) were collected at semi-annual visits between 2003 and 2013 and used to compute the homeostatic model assessment of insulin resistance (HOMA-IR). Coronary computed tomographic angiography (CTA) was performed at the end of the study period to characterize coronary pathology. Associations between HOMA-IR (categorized into tertiles and assessed near the time of the CTA and over the 10 year study period) and the prevalence of coronary plaque or stenosis ≥ 50% were assessed with multivariable logistic regression. HOMA-IR was higher in HIV-infected men than HIV-uninfected men when measured near the time of CTA (3.2 vs. 2.7, P = 0.002) and when averaged over the study period (3.4 vs. 3.0, P < 0.001). The prevalence of coronary stenosis ≥ 50% was similar between both groups (17% vs. 15%, P = 0.41). Both measures of HOMA-IR were associated with greater odds of coronary stenosis ≥ 50% in models comparing men with values in the highest versus the lowest tertiles, though the effect of mean HOMA-IR was stronger than the single measurement of HOMA-IR prior to CTA (OR 2.46, 95% CI 1.95–3.11, vs. OR 1.43, 1.20–1.70). This effect was not significantly modified by HIV serostatus. In conclusion, insulin resistance over nearly a decade was greater in HIV-infected men than HIV-uninfected men, and among both groups, was associated with significant coronary artery stenosis.
Keywords: Insulin resistance, Subclinical coronary atherosclerosis, HIV infection
Independent of the deleterious effects of hyperglycemia, insulin resistance (IR) is thought to promote atherosclerosis by impairing normal endothelial cell function and altering macrophage function in arterial plaques.1 Despite the well-known associations between IR and CAD in the general population, relatively few studies have examined this association in HIV-infected populations. We sought to investigate the relation between HIV infection, IR, and subclinical CAD using a well-established marker of insulin resistance (HOMA-IR)2 and a highly accurate imaging technique (coronary CTA)3 to detect atherosclerotic disease and characterize it more specifically than with coronary artery calcium (CAC) scores or carotid intima-media thickness (cIMT) alone, as other studies have done previously.4,5 Taking advantage of the longitudinal data from the Multi-Center AIDS Cohort Study (MACS), we measured HOMA-IR at the study visit closest to the CTA, and also averaged HOMA-IR measurements over a 10 year period prior to the CTA in order to capture any cumulative effect of IR on CAD. We hypothesized that HOMA-IR was greater among HIV-infected than uninfected men and, consequently, the presence and extent of CAD would be amplified in men with more IR.
Methods
The MACS is an ongoing, prospective observational study investigating the sequela of HIV infection in men who have sex with men (MSM), in 4 large metropolitan areas in the United States: Baltimore, Maryland/Washington DC; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania. Data from these men, including a clinical evaluation and laboratory testing, are obtained semi-annually. Men enrolled in the MACS were entered into an ancillary investigation focusing on the cardiovascular effects of HIV infection if they were 40 to 70 years old, weighed less than 300 lbs., and had no history of cardiac surgery or percutaneous transluminal coronary angioplasty. All participants underwent coronary CTA if they had no iodinated contrast allergy, atrial fibrillation, or renal insufficiency (defined as an estimated glomerular filtration rate under 60 ml/min/1.73m2). This analysis was restricted to men who participated in the ancillary investigation and underwent CTA. Each individual provided informed consent, and the Institutional Review Board at each site approved the study protocol.
At each semi-annual visit, study participants underwent a physical examination and submitted fasting blood samples. Data regarding demographic information, comorbidities, and medication use (including anti-retroviral therapy [ART], anti-hypertensives, and lipid-lowering agents) were collected via standardized questionnaires. Anthropometric data, including weight, waist circumference, waist to hip ratio, and body mass index (BMI), were measured using a standardized protocol at each visit, as previously described.6 Fasting lipid profiles, comprising total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol, and triglycerides, were obtained according to a pre-specified protocol, and LDL was directly measured if triglycerides exceeded 400 mg/dL and the Friedewald equation could not be used.7 Comorbidities such as hypertension were defined as a systolic blood pressure (SBP) > 140 mmHg, diastolic blood pressure (DBP) > 90 mmHg, or anti-hypertensive medication use. Diabetes mellitus was defined as a fasting glucose level ≥ 126 mg/dL, non-fasting glucose level ≥ 200 mg/dL, hemoglobin A1c ≥ 6.5%, or use of medications to treat diabetes at more than 1 follow-up visit at any point during the study period. HIV disease activity was assessed through laboratory data (i.e. CD4+ T-cell count, CD4+ T-cell count nadir, plasma HIV RNA levels), or via the participant’s medical records and questionnaire responses (i.e. history of AIDS, duration of ART, including protease inhibitor [PI] use). HCV infection was identified by enzyme immunoassay (anti-HCV; ADVIA Centaur HCV assay, Siemens Healthcare Diagnostics, Tarrytown, NY, USA) followed by quantitative real time PCR (COBAS AmpliPrep COBAS TaqMan HCV assay, Roche Molecular Systems, Pleasanton, CA, USA). The aforementioned data was collected prior to CTA acquisition.
The median duration between the date of the CTA and the date of the closest MACS visit with fasting glucose and insulin data, which were measured at each semi-annual visit, was 2.6 months (interquartile range [IQR] 1.0–5.3). The 10 years of longitudinal data prior to the CTA included a median of 14 study visits among HIV-uninfected men (range 1–21 visits) and 13 visits among HIV-infected men (range 1–22 visits). Fasting glucose levels were measured using the combined hexokinase/glucose-6-phosphate dehydrogenase method (coefficient of variation [CV] 1.8%),8 and fasting insulin levels were measured using a radioimmunoassay technique (CV 2.6%; Lincoln Research, St. Charles, Missouri, USA)9 at a centralized laboratory (Heinz Laboratory, Pittsburgh, Pennsylvania, USA).10 These measurements were used to calculate the HOMA-IR with the following equation: (fasting glucose [mg/dL] x fasting insulin [mU/L]) ÷ 405.11 The mean HOMA-IR was calculated from all semi-annual visits between 2003 and 2013 before the CTA was performed.
The protocol for measurement of coronary atherosclerosis with CTA has been described previously.12 In brief, all men underwent an ECG-gated coronary CTA using multi-detector scanners (64-slice multidetector at 3 sites, 320-row multidetector at 1 site) to characterize coronary lesions. Plaques were localized according to the American Heart Associations’ 15-segment classification of coronary anatomy.13 Plaque size in a given segment was graded semi-quantitatively on a 0 to 3 scale using the following scoring: plaque absent – 0, mild plaque – 1, moderate plaque – 2, and severe plaque – 3. Plaque composition was also categorized as calcified (≥ 50% of the plaque density exceeding 130 Hounsfield units [HU]), mixed (< 50% of the plaque exceeding 130 HUs), and non-calcified. Stenoses measuring ≥ 50% of the pre-lesion vessel diameter were also identified. Each participant was assigned a calcified plaque score (CPS), mixed plaque score (MPS), and non-calcified plaque score (NCPS) by summing the scores from the 15 coronary artery segments. Total plaque score (TPS), a reflection of the extent of plaque burden, was then calculated as the combination of the CPS, MPS, and NCPS. Two blinded readers in a central location (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center) used 3-dimensional image analysis workstations (GE Advantage Workstations, GE Healthcare, Bethesda, Maryland, USA) to interpret the CTAs, and this method of image analysis is reliable and highly reproducible.14
Demographic and metabolic parameters at the time of the CTA were compared by HIV serostatus using the Wilcoxon rank-sum and Pearson Chi-square tests for continuous and categorical variables, respectively. Using the longitudinal HOMA-IR measures, we used a mixed-effects multinomial logistic regression model to evaluate the associations between HOMA-IR tertiles as the dependent variable and concurrent measures of HIV and HCV serostatus, demographic parameters (age, race [non-Hispanic White vs. non-White]), and traditional CVD risk factors (BMI, SBP, use of anti-hypertension medications, total cholesterol concentrations, HDL cholesterol concentrations, the use of lipid-lowering medications, and smoking status [current vs. former vs. never]). All measurements available between 2003 and the time of a participant’s CTA were included, and the mixed-effects model accounted for correlation between the repeated measures within individual subjects. To evaluate whether HOMA-IR was associated with plaque presence or severity, we performed multivariable regression analyses (both logistic and linear) with the different plaque measures as the dependent variables and HOMA-IR as the primary independent variable, adjusting for HIV and HCV serostatus as well as the demographic parameters and CVD risk factors specified above. One set of models used the HOMA-IR measure at a time most proximally but prior to the CTA, while the second set used the mean of all HOMA-IR measurements available between 2003 and the time the CTA was performed. Both HOMA-IR variables were modeled as tertiles and compared the effect of the highest vs. lowest tertile and the effect of the middle vs. the lowest tertile. Tertiles were chosen to categorize the data given the absence of well-established cut-offs for HOMA-IR measurements that correspond to clinically meaningful endpoints. Logistic models were used to assess the relation of HOMA-IR with the presence of each plaque type and coronary artery stenosis ≥ 50%, and linear regression to assess the association with plaque extent among men with plaque present. To assess whether the associations between HOMA-IR and the CTA findings differed by HIV serostatus, an HIV-serostatus*HOMA-IR tertile interaction term was added to the multivariable models. We also examined the associations between HOMA-IR and the CTA findings in multivariable models restricted to the HIV-infected population with additional adjustment for CD4+ T-cell count and undetectable plasma HIV RNA (defined as < 50 copies/mL). A small proportion of participants were missing data, so for the analyses between HOMA-IR and plaque, missing data were imputed ten times based on the distribution of covariates using a Markov chain Monte Carlo method, assuming multivariable normality. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA). Statistical significance was established with a P value < 0.05.
Results
Data from 754 men who underwent CTA were included in this analysis (Table 1). Median follow-up time between the first HOMA-IR calculation and when CTA was performed was 8.4 years (fz of 7.8–9.3 years). HIV-infected individuals had higher fasting glucose and insulin concentrations compared to HIV-uninfected men at measurements from the study visit closest to the CTA, resulting in a higher median HOMA-IR. Similarly, mean HOMA-IR over a nearly 10-year period was greater in the HIV-infected vs. HIV-uninfected men.
Table 1.
Participant characteristics
| Variable | HIV-uninfected (n =306) |
HIV-infected (n = 448) |
P-value* |
|---|---|---|---|
| Age (years) | 54 (50–61) | 51 (47–57) | <0.001 |
| White | 69% | 50% | <0.001 |
| Hispanic | 6% | 15% | |
| Black | 24% | 34% | |
| Other | 2% | <1% | |
| BMI (kg/m2) | 26.6 (24.0–29.8) | 25.6 (23.3–28.4) | <0.01 |
| Diabetes mellitus | 9% | 12% | 0.21 |
| Hypertension | 42% | 45% | 0.43 |
| Antihypertensive medication use | 29% | 32% | 0.41 |
| Lipid-lowering therapy use | 31% | 33% | 0.55 |
| Anti-diabetic therapy use | 6% | 8% | 0.31 |
| Smoker | <0.01 | ||
| Current | 22% | 30% | |
| Former | 54% | 43% | |
| Never | 23% | 26% | |
| Cumulative cigarette pack years | 3.3 (0–22.3) | 5.4 (0–21.3) | 0.36 |
| Current HOMA-IR | 2.7 (2.0–3.9) | 3.2 (2.2–4.5) | <0.01 |
| Mean HOMA-IR# over study period | 3.0 (2.2–4.1) | 3.4 (2.5–5.0) | <0.001 |
| Fasting glucose (mg/dL) | 96 (89–104) | 98 (90–107) | 0.04 |
| Fasting insulin (μU/mL) | 11.5 (8.9–16.0) | 12.9 (9.8–17.6) | <0.01 |
| Hemoglobin A1c (%) | 5.6 (5.4–5.8) | 5.5 (5.3–5.8) | 0.01 |
| Total cholesterol (mg/dL) | 194 (168–218) | 185 (159–212) | <0.01 |
| LDL cholesterol (mg/dL) | 114 (91–139) | 103 (82–133) | <0.001 |
| HDL cholesterol (mg/dL) | 52 (43–62) | 45 (38–55) | <0.001 |
| Triglycerides (mg/dL) | 106 (74–147) | 127 (93–193) | <0.001 |
| Serum creatinine (mg/dL) | 1.00 (0.89–1.07) | 0.99 (0.85–1.10) | 0.69 |
| HCV infection | 2.8% | 10.6% | <0.001 |
| Viral load undetectable¶ | – | 79% | – |
| CD4+ T-cell count (cells/mm3) | – | 599 (426–774) | – |
| CD4+ T-cell count nadir (cells/mm3) | – | 287 (176–413) | – |
| Time on HAART (years) | – | 12.3 (8.5–14.0) | – |
| Protease inhibitor use | – | 42% | – |
| History of AIDS | – | 11% | – |
All values presented as medians with IQR in parenthesis, or percent.
Demographic and metabolic parameters at the time of the CTA were compared by HIV serostatus using the Wilcoxon rank-sum and Pearson Chi-square tests for continuous and categorical variables, respectively.
Mean HOMA-IR averages all HOMA-IR measurements over the 10 years prior to the participant’s CTA.
Defined as <50 copies/mL
AIDS = Acquired Immune Deficiency Syndrome, BMI = Body Mass Index, HAART = Highly Active Anti-Retroviral Therapy, HCV = Hepatitis C Virus, HDL = High-density lipoprotein, HIV = Human Immunodeficiency Virus, HOMA-IR = Homeostatic Model Assessment of Insulin Resistance, IQR = interquartile range, LDL = Low-density lipoprotein
HIV infection demonstrated a graded association with HOMA-IR in the multivariable analysis (Figure 1), with 1.5 fold greater odds of having a HOMA-IR value in the middle compared to the lowest tertile and 2.5 fold greater odds of having a HOMA-IR value in the highest compared to the lowest tertile. Greater HOMA-IR was also associated with advancing age, and the association between age and HOMA-IR did not differ by HIV serostatus (data for interaction not shown). Additionally, increased HOMA-IR was associated with variables like greater BMI, SBP, use of anti-hypertensive medications, higher total cholesterol levels, use of lipid-lowering agents, and lower HDL-c levels. Over the 10-year study interval, HOMA-IR did not significantly change among the HIV-infected or HIV-uninfected men after multivariable adjustment (Figure 2).
Figure 1.
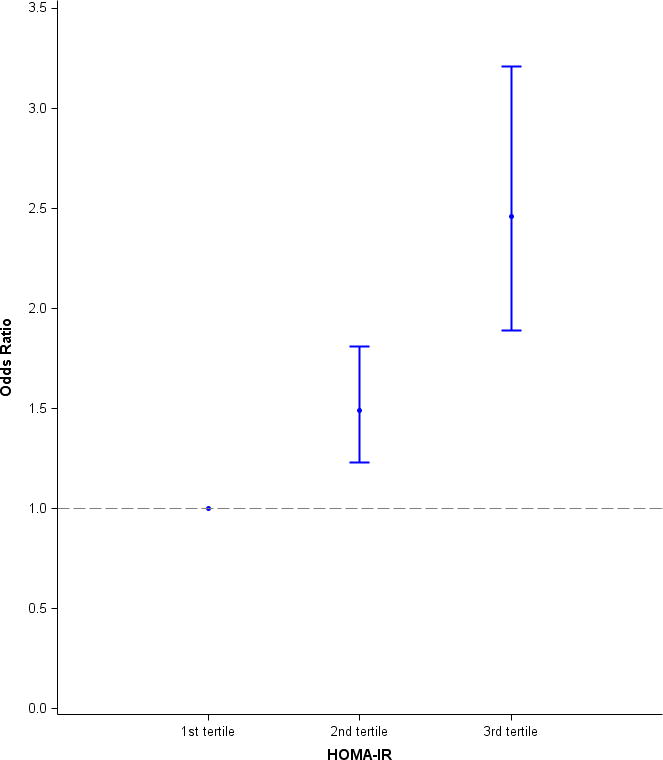
The effect of HIV-infection on HOMA-IR tertile, presented as odds ratios relative to first, and lowest, tertile of HOMA-IR. Among 8,033 person-visits included in this analysis, 37% belonged to the first tertile, 32% to the second tertile, and 30% to the third tertile. Using a multivariable model, a graded association between HIV-infection and HOMA-IR measured near the time the CTA was performed was observed. HIV-infected men had 1.5 fold greater odds of having a HOMA-IR value in the second compared to the first tertile and 2.5 fold greater odds of having a HOMA-IR value in the third compared to the first tertile.
Figure 2.
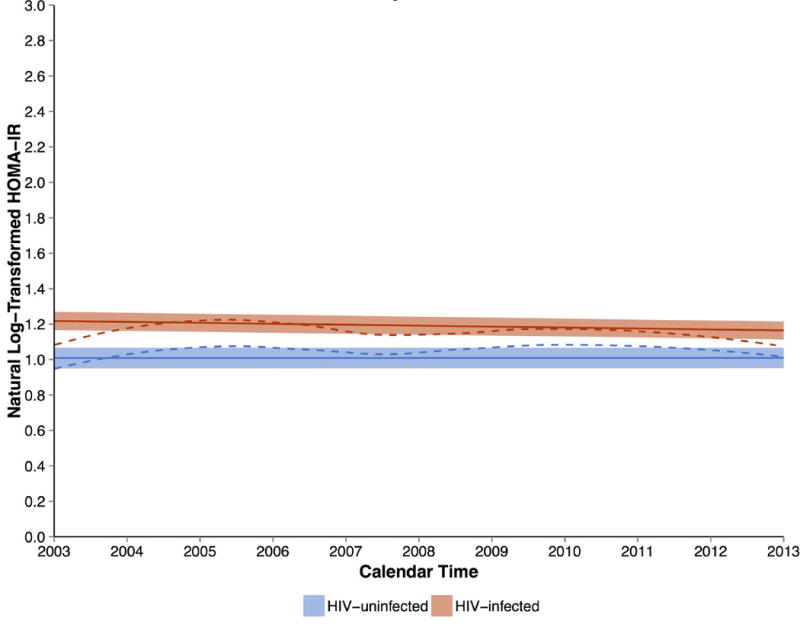
HOMA-IR (log transformed) in HIV-infected and HIV-uninfected men over the study interval. The solid lines represent the average change of log-transformed HOMA-IR over calendar time with the confidence interval estimated from the multivariate models, and dashed lines represent the same outcome estimated from the Loess fitting of raw data. Results from HIV-infected men include the red dashed and solid lines, while the blue dashed and solid lines represent uninfected men. There was no significant change in HOMA-IR levels among both HIV-infected and uninfected men, but HOMA-IR levels were higher among HIV-infected men than in uninfected men.
Coronary plaque was present in a majority of men: 78% of HIV-infected men and 75% of HIV-uninfected men (Table 3). HIV-infected men had a higher prevalence of non-calcified plaque as well as a higher NCPS. However, the CPS and MPS were not statistically different between infected and uninfected individuals, nor was the prevalence of a stenosis ≥ 50%.
Table 3.
Imaging characteristics from coronary computed tomographic angiography
| Parameter | HIV-uninfected (n = 306) |
HIV-infected (n = 448) |
P-value |
|---|---|---|---|
| Presence of coronary plaque | 75% | 78% | 0.29 |
| Presence of calcified coronary plaque | 41% | 35% | 0.11 |
| Presence of non-calcified plaque | 53% | 64% | <0.01 |
| Presence of mixed coronary plaque | 32% | 35% | 0.41 |
| Presence of coronary stenosis ≥ 50% | 15% | 17% | 0.41 |
| Total plaque score, median (IQR) | 2 (0–5) | 2 (1–5) | 0.35 |
| Calcified plaque score, median (IQR) | 0 (0–2) | 0 (0–1) | 0.04 |
| Non-calcified plaque score, median (IQR) | 1 (0–2) | 1 (0–3) | 0.001 |
| Mixed plaque score, median (IQR) | 0 (0–1) | 0 (0–1) | 0.27 |
IQR = Interquartile range
Men with mean HOMA-IR values in the highest tertile demonstrated a 2.8 fold greater odds of coronary stenosis ≥ 50% when compared to men in the lowest tertile in multivariable regression models adjusted for HIV serostatus, demographic parameters, and CVD risk factors (Table 4). These associations remained significant even when men with a history of diabetes and those taking anti-glycemic medications were excluded from the analysis (Table 5). The middle tertile had a moderate, but non-significant association with stenosis ≥ 50%. For the alternative measurement of HOMA-IR from a single time point closest to the CTA, men with values in the highest tertile had 82 percent increased odds of coronary stenosis ≥ 50% when compared with men in the lowest tertile. There was no significant difference in odds of stenosis between the middle and lowest tertile.
Table 4.
Associations between current (defined as the measurement closest to the time of CTA) and mean HOMA-IR and the presence of coronary artery stenosis ≥ 50% in men
| Total (n = 754) |
HIV-uninfected (n = 306) |
HIV-infected (n = 448) |
HIV* HOMA-IR Interaction P-value | |
|---|---|---|---|---|
| Current HOMA-IR, Model 1 | ||||
|
| ||||
| HOMA-IR, lowest tertile (reference) | 1.00 | 1.00 | 1.00 | – |
| HOMA-IR, middle tertile | 0.97 (0.55–1.70) |
0.75 (0.30–1.89) |
1.13 (0.56–2.28) |
0.50 |
| HOMA-IR, highest tertile | 1.82 (1.02–3.26) |
2.69 (1.10–6.58) |
1.50 (0.74–3.03) |
0.29 |
|
| ||||
| Mean HOMA-IR, Model 2 | ||||
|
| ||||
| HOMA-IR, lowest tertile (reference) | 1.00 | 1.00 | 1.00 | – |
| HOMA-IR, middle tertile | 1.75 (0.98–3.15) |
1.56 (0.64–3.80) |
1.91 (0.89–4.07) |
0.73 |
| HOMA-IR, highest tertile | 2.76 (1.50–5.09) |
3.43 (1.36–8.66) |
2.50 (1.19–5.25) |
0.58 |
Models 1 and 2 are adjusted for HIV and HCV serostatus, age, race (non-Hispanic White vs. non-White), BMI, SBP, use of anti-hypertension medications, total and HDL cholesterol, use of lipid-lowering medications, and smoking status (current and former vs. never).
HIV = Human Immunodeficiency Virus, HOMA-IR = Homeostatic Model Assessment of Insulin Resistance, IQR = Interquartile range
Table 5.
Associations between current (defined as the measurement closest to the time of CTA) and mean HOMA-IR and the presence of coronary artery stenosis ≥ 50% among non-diabetic men
| Total (n = 633) |
HIV-uninfected (n = 371) |
HIV-infected (n = 262) |
HIV* HOMA-IR Interaction P-value | |
|---|---|---|---|---|
| Current HOMA-IR, Model 1 | ||||
|
| ||||
| HOMA-IR, lowest tertile (reference) | 1.00 | 1.00 | 1.00 | – |
| HOMA-IR, middle tertile | 0.99 (0.54–1.84) |
0.79 (0.29–2.12) |
1.16 (0.54–2.50) |
0.54 |
| HOMA-IR, highest tertile | 2.05 (1.07–3.95) |
3.19 (1.15–8.91) |
1.68 (0.77–3.69) |
0.30 |
|
| ||||
| Mean HOMA-IR, Model 2 | ||||
|
| ||||
| HOMA-IR, lowest tertile (reference) | 1.00 | 1.00 | 1.00 | – |
| HOMA-IR, middle tertile | 2.00 (1.07–3.71) |
1.87 (0.73–4.79) |
2.11 (0.95–4.67) |
0.85 |
| HOMA-IR, highest tertile | 3.06 (1.51–6.19) |
4.46 (1.47–13.51) |
2.64 (1.15–6.06) |
0.58 |
Models 1 and 2 are adjusted for HIV and HCV serostatus, age, race (non-Hispanic White vs. non-White), BMI, SBP, use of anti-hypertension medications, total and HDL cholesterol, use of lipid-lowering medications, and smoking status (current and former vs. never).
HIV = Human Immunodeficiency Virus, HOMA-IR = Homeostatic Model Assessment of Insulin Resistance, IQR = Interquartile range
When stratified by HIV serostatus, the association between the highest HOMA-IR tertile and stenosis remained significant among HIV-uninfected men (OR 3.43, 95% CI 1.36–8.66) and HIV-infected men (OR 2.50, 95% CI 1.19–5.25) even after adjusting for HIV clinical parameters (data not shown, OR 2.80, 95% CI 1.30–6.01). However, when IR was defined using the single HOMA-IR measurement, the association with coronary stenosis among HIV-infected men was not significant. The association between HOMA-IR and stenosis was not significantly modified by HIV serostatus. Comparisons between the middle and lowest tertiles regarding associations with HOMA-IR and presence of stenosis ≥ 50% were non-significant in the entire population as well as when the population was stratified by serostatus.
Despite the increased prevalence of non-calcified plaque in HIV-infected participants, mean HOMA-IR was not associated with the presence of non-calcified plaque among all studied men or among HIV-infected men. Similarly, there were no significant associations between mean HOMA-IR and presence of any coronary plaque, calcified plaque, or mixed plaque. In the linear regression models adjusted for CVD risk factors, we observed no associations between HOMA-IR and measures of the extent of coronary plaque, including the total-, calcified-, non-calcified-, or mixed-plaque scores (see supplementary tables).
Discussion
In this large cohort of well-characterized and prospectively followed HIV-infected and uninfected men, 3 key findings emerged. First, HIV-infected men were more insulin resistant than HIV-uninfected men. This finding was observed with measurements of IR at a single time point prior to CTA as well as with measurements averaged over a nearly 10 year period. Second, IR was associated among all men with common CVD risk factors like hypertension and hyperlipidemia, and also with HCV-infection. Third, coronary artery stenosis was associated with IR in HIV-infected and uninfected men, particularly when IR values assessed over the previous 10 years rather than at the time of the CAD assessment were analyzed. These findings suggest that, while IR has not worsened among HIV-infected persons over time, long-standing IR is an important contributor to CVD among HIV-infected persons.
This is the first study to our knowledge that has examined the relation between IR and subclinical coronary atherosclerosis as assessed by CT angiography in an HIV-infected population. Our finding of a positive relationship between IR and coronary artery stenosis among HIV-infected men is novel, but consistent with previous observations using other modalities to assess CVD. Rossi found that IR, as assessed by HOMA-IR, was significantly associated with vascular remodeling of the brachial artery in 570 HIV-infected patients after adjustment for age, sex, and BMI.15 Similar findings were reported in another cohort for both cIMT and brachial flow-mediated vasodilation among 50 HIV-infected persons.16 However, neither of these studies simultaneously adjusted for traditional coronary artery disease risk factors. Similarly, Mangili showed that IR was greater in HIV-infected persons with metabolic syndrome than those without the metabolic syndrome, and that the presence of metabolic syndrome was associated with greater cIMT and the presence of coronary artery calcium. The study did not, however, determine whether IR was associated with these measures of subclinical atherosclerosis independent of other CVD risk factors.5 In our study, the association between HOMA-IR and coronary artery stenosis remained after adjustment for multiple CVD risk factors, as well as HIV-related variables including CD4+ T-cell count and detectable HIV RNA levels, suggesting that the association is independent of the severity of immune suppression or HIV control. In contrast to our finding of a relationship between IR and significant CAD (stenosis ≥ 50%), we did not detect any association between IR and the presence of coronary plaque, regardless of composition. We and others have described an increased prevalence of non-calcified plaque among HIV-infected participants compared to HIV-uninfected controls.7,17,18 Our data suggest that the increased burden of insulin resistance in HIV-infected person does not underlie this finding. Similarly, we observed no association between IR and the extent of these plaque subtypes, which is consistent with the study by Guaraldi and colleagues that failed to demonstrate any relationship between HOMA-IR and progression of CAC.4
Although IR has been linked to atherosclerotic disease in the general population, the mechanisms underlying this association remain incompletely understood. The most robust findings have related IR to cardiovascular events,19,20 whereas the relationships between IR and plaque burden and progression have been inconsistent. In a large study of 986 patients from the general population undergoing coronary angiography, for example, IR was related to the presence of metabolic syndrome but not the burden of atherosclerosis.21 It has been suggested that IR may be more related to endothelial dysfunction through a direct effect on endothelial cells or plaque rupture related to macrophage apoptosis and resulting plaque friability rather than the progression of atherosclerosis.1,21 These finding may explain the closer association of IR with CVD events compared to subclinical atherosclerosis in the general population.22 Investigations regarding the relationship between IR and CVD events in HIV-infected populations are needed.
One unique feature of our study was the assessment of IR over a 10 year period prior to the CTA, using prospectively collected data in the MACS on a semi-annual basis. This allowed us to characterize IR at both a single time point close to when the CTA was performed (current HOMA-IR), as well as longitudinally over the course of the study period (mean HOMA-IR). We found that mean HOMA-IR was more closely associated with coronary artery stenosis, compared to HOMA-IR at the time of the CTA among HIV-infected and uninfected men. These findings suggest that a greater degree of IR in the past may contribute to the pathogenesis of atherosclerosis.
Our findings suggest that targeting IR may be an important strategy to reduce CVD events in HIV-infected populations. Exercise, metformin, and thiazolidenedione therapy have all been shown to decrease IR in HIV-infected populations,23 and metformin, in particular, has been shown to halt the progression of calcified plaque in HIV-infected individuals.24 Whether these strategies will also decrease CVD events in HIV-infected persons is unknown.
Our study had several limitations. First, it was restricted to men and our conclusions cannot be extended to women, who now constitute up to 36.5% of newly HIV-infected individuals in some areas in the United States.25 Second, this analysis is observational in nature, so we cannot rule out unmeasured confounding of the reported association between IR and significant CAD. Third, although we demonstrated a greater degree of IR in HIV-infected individuals, we did not explore in this analysis whether this was related to HIV-infection or ART use. While we have previously explored this question early in the ART era10 it will be important to repeat these studies in the modern ART era.
Supplementary Material
Table 2.
Factors associated in a multivariable model with the middle and highest tertiles of HOMA-IR over the 10 year study period.
| Effect | Middle Tertile of HOMA-IR | Highest Tertile of HOMA-IR | ||
|---|---|---|---|---|
|
| ||||
| Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | |
|
|
||||
| HIV-infection | 1.49 (1.23–1.81) | <0.001 | 2.46 (1.89–3.21) | <0.001 |
| HCV-infection | 1.27 (0.85–1.92) | 0.24 | 2.20 (1.34–3.64) | 0.002 |
| Age (per 1 year change) | 1.02 (1.01–1.03) | 0.003 | 1.02 (1.01–1.04) | 0.003 |
| Race (Non-Hispanic White vs. Other) | 0.93 (0.75–1.15) | 0.51 | 0.67 (0.50–0.89) | 0.006 |
| Current vs. never smoking | 0.96 (0.76–1.22) | 0.75 | 0.95 (0.70–1.30) | 0.76 |
| Former vs. never smoking | 0.97 (0.78–1.21) | 0.79 | 0.95 (0.72–1.27) | 0.75 |
| BMI (per 1 kg/m2 change) | 1.16 (1.13–1.19) | <0.001 | 1.33 (1.29–1.37) | <0.001 |
| Systolic blood pressure (per 10 mmHg) | 1.08 (1.02–1.14) | 0.006 | 1.11 (1.04–1.19) | 0.001 |
| Anti-hypertensive use | 1.24 (1.02–1.51) | 0.029 | 1.47 (1.18–1.84) | <0.001 |
| Total cholesterol (per 5mg/dL) | 1.01 (1.00–1.02) | 0.047 | 1.01 (1.00–1.02) | 0.14 |
| HDL cholesterol (per 5mg/dL) | 0.89 (0.87–0.92) | <0.001 | 0.88 (0.84–0.91) | <0.001 |
| Lipid-lowering therapy use | 1.31 (1.08–1.58) | 0.005 | 1.43 (1.14–1.78) | 0.002 |
The highest and middle tertiles are compared to the lowest tertile. Covariates were measured at the time of the HOMA-IR determination.
BMI = Body Mass Index, HCV = Hepatitis C Virus, HIV = Human Immunodeficiency Virus, HDL = High-density lipoprotein
Acknowledgments
The authors would like to thank Sandra Reynolds for her assistance with the study’s data analysis.
Funding
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS Locations (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; Harbor-UCLA, CTSI UL1TR000124; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The MACS Cardiovascular Substudy is funded by an NIH Grant Number: RO1 HL095129 (Post). Dr. Todd Brown is supported in part by K24AI120834. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
References
- 1.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 2.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7:e52036. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Cardiology Foundation Task Force on Expert Consensus D. Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, Hecht HS, Hlatky MA, Hodgson JM, Lauer MS, Miller JM, Morin RL, Mukherjee D, Poon M, Rubin GD, Schwartz RS. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2509–2543. doi: 10.1161/CIR.0b013e3181d4b618. [DOI] [PubMed] [Google Scholar]
- 4.Guaraldi G, Zona S, Orlando G, Carli F, Ligabue G, Fiocchi F, Rossi R, Modena MG, Raggi P. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2012;28:935–941. doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 5.Mangili A, Jacobson DL, Gerrior J, Polak JF, Gorbach SL, Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis. 2007;44:1368–1374. doi: 10.1086/516616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Chu H, Wang Z, Palella FJ, Kingsley L, Witt MD, Dobs AS. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS. 2007;21:1731–1738. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 7.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20:586–590. [PubMed] [Google Scholar]
- 9.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 10.Brown TT, Li X, Cole SR, Kingsley LA, Palella FJ, Riddler SA, Chmiel JS, Visscher BR, Margolick JB, Dobs AS. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–1383. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Hacioglu Y, Gupta M, Choi TY, George RT, Deible CR, Jacobson LP, Witt MD, Palella FJ, Post WS, Budoff MJ. Use of cardiac CT angiography imaging in an epidemiology study – the Methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu Kardiyol Derg. 2013;13:207–214. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 14.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr. 2013;64:51–57. doi: 10.1097/QAI.0b013e31829ed726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi R, Nuzzo A, Guaraldi G, Squillace N, Orlando G, Esposito R, Lattanzi A, Modena MG. Metabolic disorders induced by highly active antiretroviral therapy and their relationship with vascular remodeling of the brachial artery in a population of HIV-infected patients. Metabolism. 2009;58:927–933. doi: 10.1016/j.metabol.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Mondy KE, las Fuentes L de, Waggoner A, Onen NF, Bopp CS, Lassa-Claxton S, Powderly WG, Davila-Roman V, Yarasheski KE. Insulin resistance predicts endothelial dysfunction and cardiovascular risk in HIV-infected persons on long-term highly active antiretroviral therapy. AIDS. 2008;22:849–856. doi: 10.1097/QAD.0b013e3282f70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitch KV, Lo J, Abbara S, Ghoshhajra B, Shturman L, Soni A, Sacks R, Wei J, Grinspoon S. Increased coronary artery calcium score and noncalcified plaque among HIV-infected men: relationship to metabolic syndrome and cardiac risk parameters. J Acquir Immune Defic Syndr. 2010;55:495–499. doi: 10.1097/QAI.0b013e3181edab0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, Nasir K, Grinspoon SK. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Runzo C, Prina Cerai S, Pagano G, Cavallo-Perin P, Casale Monferrato S. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2004;27:2689–2694. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 20.Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Gemma L, Santi L, Bonadonna RC, Muggeo M. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med. 2004;21:52–58. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 21.Vonbank A, Saely CH, Rein P, Beer S, Breuss J, Boehnel C, Drexel H. Insulin resistance is associated with the metabolic syndrome and is not directly linked to coronary artery disease. Clin Chim Acta. 2011;412:1003–1007. doi: 10.1016/j.cca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Saely CH, Aczel S, Marte T, Langer P, Hoefle G, Drexel H. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J Clin Endocrinol Metab. 2005;90:5698–5703. doi: 10.1210/jc.2005-0799. [DOI] [PubMed] [Google Scholar]
- 23.Mulligan K, Yang Y, Wininger DA, Koletar SL, Parker RA, Alston-Smith BL, Schouten JT, Fielding RA, Basar MT, Grinspoon S. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS. 2007;21:47–57. doi: 10.1097/QAD.0b013e328011220e. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17148967. [DOI] [PubMed] [Google Scholar]
- 24.Fitch K, Abbara S, Lee H, Stavrou E, Sacks R, Michel T, Hemphill L, Torriani M, Grinspoon S. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. AIDS. 2012;26:587–97. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall HI, Espinoza L, Benbow N, Hu YW, Urban Areas HIVSW Epidemiology of HIV infection in large urban areas in the United States. PLoS One. 2010;5:e12756. doi: 10.1371/journal.pone.0012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


