Abstract
Haematopoiesis is a process that is responsible for generating sufficient numbers of blood cells in the circulation and in tissues. It is central to maintenance of homeostasis within an animal, and is critical for defense against infection. While haematopoiesis is common to all animals possessing a circulatory system, the specific mechanisms and ultimate products of haematopoietic events vary greatly. Our understanding of this process in non-vertebrate organisms is primarily derived from those species that serve as developmental and immunological models, with sparse investigations having been carried out in other organisms spanning the metazoa. As research into the regulation of immune and blood cell development advances, we have begun to gain insight into haematopoietic events in a wider array of animals, including the molluscs. What began in the early 1900’s as observational studies on the morphological characteristics of circulating immune cells has now advanced to mechanistic investigations of the cytokines, growth factors, receptors, signalling pathways, and patterns of gene expression that regulate molluscan haemocyte development. Emerging is a picture of an incredible diversity of developmental processes and outcomes that parallels the biological diversity observed within the different classes of the phylum Mollusca. However, our understanding of haematopoiesis in molluscs stems primarily from the three most-studied classes, the Gastropoda, Cephalopoda and Bivalvia. While these represent perhaps the molluscs of greatest economic and medical importance, the fact that our information is limited to only 3 of the 9 extant classes in the phylum highlights the need for further investigation in this area. In this review, we summarize the existing literature that defines haematopoiesis and its products in gastropods, cephalopods and bivalves.
Keywords: haematopoiesis, haemocyte, mollusc, gastropod, cephalopod, bivalve, proliferation, mitosis, cell development, differentiation
Introduction
Molluscs are a large and morphologically diverse group of animals, many of which are known for their economic and biomedical importance. Some are excellent model organisms for studying neurobiology [1, 2], while several others, such as clams, oysters, squids and abalones, are economically important food sources that can be reared in aquaculture [3–7]. Notably, gastropods are obligatory intermediate hosts for the vast majority of digenean trematodes, parasites that infect and cause disease in an incredible diversity of animals, including humans and livestock [8–10]. For these reasons, immune responses in molluscs, and the processes that govern them, are important areas of active research. These immunological processes are centrally coordinated by a group of cells known as haemocytes or amebocytes, which may act directly or in concert with humoral factors in the haemolymph to defend the animal against infection.
Haemocytes constitute the cellular component of the haemolymph, but they are also resident in other sites such as the connective and vascular tissues [8, 11]. Among the important functions they perform in molluscs, haemocytes are best known for their primary role in phagocytosis [12], encapsulation [13] and production of cytotoxic molecules (for example, nitric oxide and hydrogen peroxide) [14, 15] involved in pathogen killing and elimination. However, they also participate in other vital processes such as wound healing [16], nerve repair, [17] and shell formation [18]. Some of these roles often may deplete the number of circulating haemocytes, which then must be replenished by haematopoiesis. Moreover, the normal turnover of haemocytes, through senescence or migration across external epithelia, requires their continuous replacement. Thus, the haematopoietic processes that regulate haemocyte proliferation and differentiation are important not only for immune function but for the survival of the animal as a whole.
Haematopoiesis in molluscs is not a well-understood process; however, progress is being made in this area to define the networks of signalling pathways important for haemocyte development, as well as the endogenous factors that regulate haemocyte numbers [19–22]. Evidence so far indicates that major differences exist among the various molluscan classes in terms of haemocyte lineages and haematopoietic sites. Haemocytes in molluscs can be classified into at least two main types – the granulocytes and the agranular hyalinocytes [11, 23–26]. However, within these two general types, there is variability in the number of haemocyte lineages described in various molluscs. This diversity may be in part due to some true differences, but also results from the use of different criteria (extent of granularity, cell size, ultrastructural features, cell surface/biochemical markers) and nomenclatures adopted by various researchers due to lack of biological markers for differentiating specific cell lineages or states of maturation. Thus, a unified classification system for haemocytes in molluscs would help to better organize this area of research.
The location where haematopoiesis takes place in molluscs varies greatly. In many gastropods, haemocyte production occurs in the pericardial region, for example in the anterior pericardial wall [27] or in histologically and anatomically equivalent structures [24, 28, 29]. Other reported pericardial sites of gastropod haemocyte production include the external surface of the ctenidial/pulmonary and renal veins in the pericardial cavity [30]. In cephalopods, haemocytes are thought to originate from the white body, which is a multi-lobed organ that wraps around the optic bundle [22, 31], while in the bivalves, the irregularly folded structure (IFS) of the gills has been proposed as the site of haemocyte formation [21]. In many members of these molluscan groups, a haematopoietic site is yet to be described, and it is possible that more than one primary haematopoietic site exists within each of these groups.
Numerous cytokines, growth factors, receptors, intracellular signalling components, and homologs of transcription factors known to be involved in vertebrate haematopoiesis have been identified as part of genomic or transcriptional studies [19, 22, 32], or are assumed to be present in various molluscan groups [33]. These assumptions are based on sequence identity or immunoreactivity with antibodies raised against their vertebrate counterparts and sometimes a demonstration that they perform similar functions [16, 34]. However, there is no described mechanism detailing the sequence of haematopoietic events for haemocyte proliferation, differentiation and maturation or cell fate determination in any molluscan group. Although our understanding of stages of development and types of terminally differentiated cells would be greatly aided by a haemocyte cell line, to date only short-lived primary cultures of these cells have been established, and in fact only a single immortalized cell line has been derived from molluscs, an embryonic, non-haemocyte cell line (Bge) from Biomphalaria glabrata [126].
While a great deal of information on haemocytes now exists, there is still much to be learned about their origin and the molecules and mechanisms that guide their proliferation and differentiation. In this review, we present current knowledge of haematopoietic processes from three molluscan classes: Gastropoda, Cephalopoda and Bivalvia. These represent the only molluscan classes for which such information currently exists.
Gastropod haematopoiesis and haemocyte function
Gastropoda is the most highly diversified class within the phylum Mollusca, composed of over 60,000 species of snails and slugs [35]. From land to sea, gastropods live in a large variety of habitat types and face a number of challenges from both pathogenic and non-pathogenic stressors, requiring, as in all other animals, both defense mechanisms and the ability to maintain homeostasis. This diversity presents both an interesting and challenging research problem in that numerous biological processes, such as haematopoiesis, can differ from species to species, reflecting their unique ecological requirements.
The majority of what we know about haematopoiesis in gastropods is derived from a few species of medical or veterinary importance, especially the freshwater planorbid B. glabrata. This snail is the obligatory intermediate host for Schistosoma mansoni, one of 3 species of trematodes that cause the human disease schistosomiasis, mainly in sub-Saharan Africa, South America, and parts of Asia, and it can also be infected with several other species of larval trematodes. The life cycles of nearly all of the ~18,000 species of digenean trematode require the use of a snail intermediate host to undergo larval development [36]. The relationship between snails and larval trematodes has been key to our current understanding of haematopoiesis and haemocyte functions in gastropods.
The Amebocyte-Producing Organ
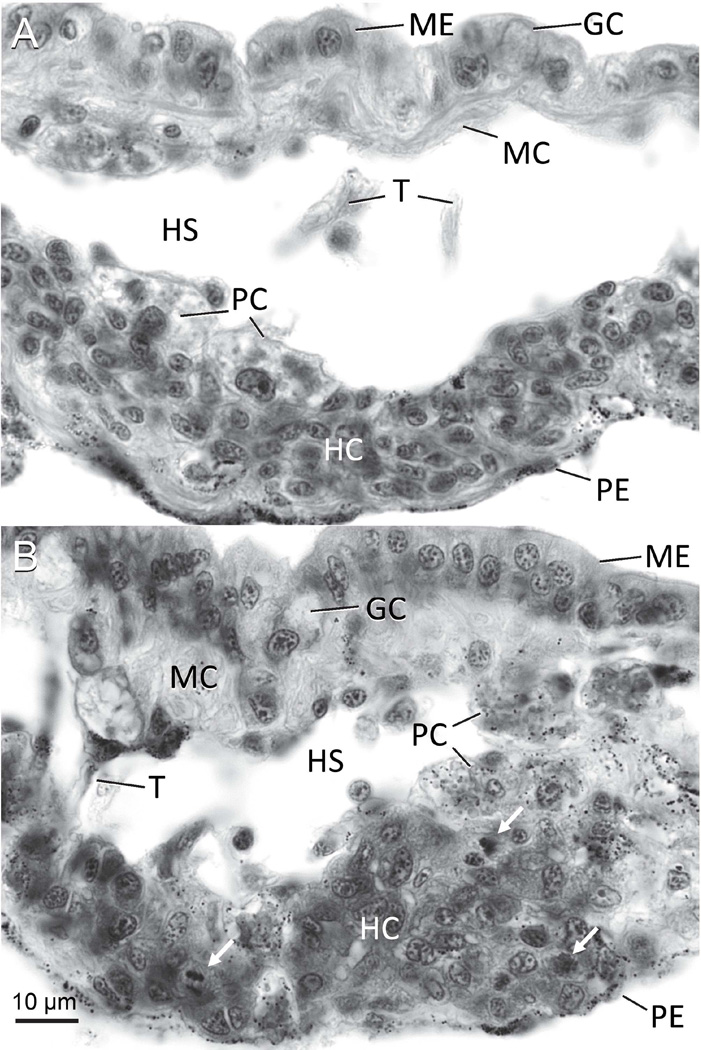
In B. glabrata the anterior pericardial wall (APW) has been proposed as a site in which haemocytes are produced [27]. The APW is a flat, roughly triangular sheet of tissue located ventral to the saccular kidney at the rear of the mantle cavity [37], and is covered posteriorly by simple squamous pericardial epithelium and anteriorly by simple cuboidal mantle epithelium [38]. Between the mantle and pericardium of the APW lie loose connective tissue and a haemolymph sinus. Typical cells of the connective tissue [39, 40] are present, including fibroblast-like cells, pore cells (rhogocytes), and haemocytes (Fig. 1A). Additionally, isolated or confluent nodules of small, mitotically active basophilic cells are found attached to the basal surface of the pericardial epithelium, and on the basis of histological, histochemical, and ultrastructural evidence appear to be haemocyte precursors [27, 41, 42]. Consequently, this structure has been named the amebocyte-producing organ or APO [27].
Figure 1.
Histological 7-µM sections of the anterior pericardial wall or amebocyte-producing organ (APO) of Biomphalaria glabrata. A. Unstimulated APO, showing normal histology. B. APO from snail injected with 0.05 mg fucoidan 24 hours previously, with several mitotic figures (arrows). Abbreviations: GC, goblet cell; HC, hematopoietic cells; HS, haemolymph sinus; MC, muscle cell; ME, mantle epithelial cell; PC, pore cell; PE, pericardial epithelium; T, trabecula formed by a muscle cell. Delafield’s hematoxylin and eosin stain. Photographs by JTS.
In addition to structural evidence, transplantation studies support a haematopoietic function of the APO. Heterotopic allografts of anterior pericardial wall, but not other organs, from schistosome-resistant to schistosome-susceptible snails confer increased non-susceptibility to infection and increased capacity to encapsulate schistosome sporocysts [43–45], although some of this transferred resistance may be due to soluble factors produced by transplanted cells [46]. Perhaps the strongest evidence for a haematopoietic function of the APO is the observation that B. tenagophila recipients of anterior pericardial wall allografts possess circulating haemocytes with a unique molecular marker from the donor [44].
The haematopoietic cells of the APO undergo increased and spontaneous mitotic division in vivo in certain genetic strains of snails [47], and as soon as 24 hours after infection with larval S. mansoni, Ribieroia marini, or Echinostoma sp. trematodes [27, 48–51]. Injection with various non-self substances, including excretory-secretory products or freeze-thaw extracts of larval and adult trematodes, lipopolysaccharide (LPS) from Escherichia coli, or the sulfated polysaccharide fucoidan, also stimulate cell proliferation in the APO [20, 52–54] (Fig. 1B). In vitro, when the APO is exposed to freeze-thaw extracts of S. mansoni or phorbol myristate acetate (PMA), proliferation can also be induced [55, 56]. The increased mitotic activity or mitotic burst in the APO results in its distinct enlargement, due to both hypertrophy and hyperplasia [27]. Jeong et al. (1980) [57] reported that exposure of B. glabrata to miracidia of Echinostoma lindoense, a treatment that increases cell division in the APO [38], elicited elevated concentrations of haemocytes in the haemolymph beginning at 3 days post infection, and these authors proposed the APO as a source for this leukocytosis. As expected, immersion of snails in colchicine results in a significant increase in the number of mitotic figures observed in histological sections of the APO [48].
Although the mitotic burst in the APO of B. glabrata seems to be a response to challenge with some types of non-self, the immune-protective function of the APO, if any, is unclear. On the one hand, resistance to a challenge with normal miracidia of E. lindoense in snails first exposed to irradiated miracidia is always preceded by this mitotic response [49], suggesting its role in so-called acquired resistance. However, at the same time, schistosome-resistant snails infected with echinostome miracidia (and having an enlarged APO) actually lose their resistance to S. mansoni [58, 59], probably as a result of interference with haemocyte function by excretory-secretory products of the larval echinostome [60]. Moreover, the APO is mitotically unresponsive to most foreign materials that have been injected into the haemocoel, including concentrated suspensions of live Gram positive and Gram negative bacteria, and the increased cell division in the APO of susceptible snails following penetration by compatible schistosome or echinostome miracidia does not prevent infection [51]. Finally, the 24-hour delay in the onset of the mitotic response following challenge with non-self suggests that this response may not be important in the initial haemocytic encapsulation and killing of sporocysts in schistosome-resistant snails, which typically begins within several hours post infection [13]. Thus, rather than a protective response, the mitotic burst may represent a “second line of defense” against certain pathogens or their molecules [53], with the APO normally functioning to help maintain (along with peripheral sites of haematopoiesis) homeostasis of haemocyte numbers. Whether the APO has any immune function beyond haematopoiesis is unknown.
A structure that is anatomically and histologically similar to the APO of Biomphalaria sp. has also been reported in a number of other gastropods, e.g., Bulinus truncatus [28], Helisoma trivolvis [29], Lymnaea palustris [61], and Planorbarius corneus [24]. Although the anterior pericardial wall appears to be a site of haemocyte production, peripheral haemocytes seem to also retain the capacity to divide, and possible haematopoietic events have also been observed in other locations in snails, including the kidney, mantle connective tissue, haemolymph, and head foot [29, 39, 40, 61, 62]. The APO of Marisa cornuarietis is a thickened band in the roof of the lung rather than in the pericardial wall [62], and Physa virgata does not appear to possess a defined haematopoietic organ, instead forming haemocytes in the connective tissues of the mantle [29].
In Pomacea canaliculata, haematopoiesis occurs within the pericardial cavity, based on the observation of dividing cells along the external surface of the ctenidial/pulmonary and renal veins near their junction with the heart, and within the pericardial fluid [30]. Newly formed haemocytes are thought to be stored in a saccular organ connected to the anterior aorta and loosely adherent to the heart called the ampulla. The ampulla is hypothesized to function in homeostasis of haemocyte numbers in P. canaliculata, by serving as a haemocyte reservoir, rather than as a site of haematopoiesis. When haemolymph was withdrawn repeatedly from this snail, haemocytes were lost from the ampulla, but dividing cells were not observed in this organ.
The specific nature and source of the stimulus eliciting mitotic division among the haemocyte precursors in the APO is not known. While it is known that certain non-self substances stimulate these cells, it is unclear whether this is in a direct or indirect manner. It is also likely that other types of cells, such as mature haemocytes or even the cells of the APO itself, are capable of producing endogenous factors that are able to induce cell proliferation and differentiation. Receptors for mitogenic non-self substances such as LPS or for putative growth factors produced endogenously have not yet been identified in B. glabrata, or any other gastropod to date.
Souza and Andrade (2006, 2012) [63, 64] have proposed an alternative function for the APO in B. glabrata, i.e., renal absorption and filtration. This hypothesis is based in part on ultrastructural observations that there are no “transitional forms” between mantle epithelial cells and haemocytes [64], and that the APO “vaguely resembled the juxtaglomerular apparatus” of the vertebrate kidney [63]. However, although haemocyte progenitor cells have been reported from oyster gill epithelium [21], we are not aware of any reports of mantle epithelial cells differentiating into haemocytes, and, therefore, transitional cells would not be expected. Moreover, the analogy with the vertebrate juxtaglomerular complex is difficult to reconcile with its known components and functions, which have no counterparts in gastropods (notwithstanding the superficial resemblance of mantle epithelium to macula densa and underlying haemocytes to juxtaglomerular and lacis cells). Therefore, additional evidence may be needed in support of this hypothesis.
Haemocytes, Effectors and Drivers of Haematopoiesis
Currently, more is known about effector functions of mature haemocytes than the process of their production, stages of development, or the identity and mechanistic basis of involvement of haematopoietic drivers - cytokines, growth factors, receptors, signalling pathways and transcription factors. Almost nothing is understood about haemocyte precursors, particularly in terms of the growth factors driving haematopoiesis, gene expression profiles, and how commitment to specific haemocyte subsets is determined. Blast-like cells have been described in some species such as B. glabrata, Lymnaea stagnalis, Lymnaea truncatula and Littorina littorea [27, 41, 65–67] but these are mainly based on ultrastructure of the cells, which may be localized in the APO or in circulation, and not on any observed pluripotency or molecular markers.
Morphological characterizations of gastropod haemocytes initially described two unique cell types, granulocytes and hyalinocytes. Granulocytes in B. glabrata are characterized by extensive production of pseudopodia in all directions and conspicuous granules, which are limited mainly to the endoplasm. These cells are adherent and measure about 24 and 16 micrometers in their longest and widest dimensions. They constitute about 87 % of the haemocyte population [68]. Hyalinocytes are smaller in size compared to the granulocytes and are generally spherical or slightly oval. Granules are sparse and the only pseudopodia formed are lobose and not extensive. Hyalinocytes measure about 6.9 and 6.6 micrometers and constitute 13 % of the haemocyte population [68]. In Viviparus ater, only one haemocyte type has been described, the equivalent of the granulocyte. This cell is characterized by irregular form, pseudopodia formation, round or oval nucleus and abundant cytoplasm, with inclusions and numerous vacuoles [69]. However, more recent studies describe three cell types based on size, ultrastructure and internal complexity. In B. glabrata, three haemocyte subpopulations have been described: large haemocytes (> 8µm), medium haemocytes (~8µm) and small haemocytes (5–6µm) [70]. Large haemocytes are characterized by an asymmetrical shape, small nucleus:cytoplasm ratio, cytoplasm with numerous mitochondria, dense particles of glycogen and prominent extensions. Medium haemocytes in comparison to the large ones are more symmetrical and have a higher nucleus:cytoplasm ratio. They have fewer organelles around the nucleus and few aggregates of glycogen particles. Small haemocytes have a high nucleus:cytoplasm ratio, are organelle-rich, with few secretory granules. In terms of haemolymph proportions, the large and medium haemocytes are almost equally numerous while small haemocytes are comparatively fewer [70]. B. glabrata and B. tenagophila haemocytes have also been categorized into three (large, medium and small) categories based on flow cytometric analysis [71]. Each category can also be divided into a low or high granular haemocyte based on side scatter. Small and medium haemocytes were found to be the most numerous in non-infected B. glabrata and B. tenagophila, respectively, while large haemocytes were the least numerous for both species.
Gastropod haemocytes are thought to be functionally heterogenous in measures such as binding to parasite glycan epitopes [127, 128], enzyme content [74, 129, 130] and cell surface markers [131, 132]. For example, a study investigating the function of one of the fibrinogen-related proteins (FREP3) in B. glabrata indicates that not all haemocytes express FREP3. The number of haemocytes expressing FREP3 was found to increase with increased haematopoietic activity induced by exposure to larval S. mansoni parasites [72]. FREP3 acts as an opsonin, enhancing the rate of phagocytosis by the newly produced haemocytes. As part of the encapsulation process that is used for parasite elimination, haemocytes are known to attack and phagocytose portions of the tegument of digenean sporocyts [73]. Haemocyte abundance (mainly of the adherent granulocytes) is also correlated with resistance of B. glabrata snails to S. mansoni along with the expression of genes for production of humoral factors such as reactive oxygen and nitrogen species [74].
A number of studies have assessed the presence of mammalian cytokine homologs or impact of mammalian recombinant cytokines on haemocyte function and development in Planobarius corneus, V. ater (IL-1α, IL-1β IL-2, IL-6 and TNF-α) [33], B. glabrata (IL-1) [34] and the slug, Limax maximus (IL-1, IL-8 and TNF-α) [16]. Detection of these homologs was largely based on immunoassays, utilizing antibodies raised against their mammalian counterparts, thus limiting our ability to interpret the true identity and function of the recognized proteins. Moreover, reports of antibody-based detection of putative cytokines in B. glabrata should be viewed with caution, inasmuch as a lectin-like protein in snail plasma non-specifically binds antibodies from several vertebrates, potentially leading to false positive results [75]. However, the identification and characterization of some of these factors in other invertebrates indicate that they may be ancestrally and functionally conserved [76–78].
Migration inhibitory factor (MIF) is perhaps the only endogenous cytokine to be cloned and functionally characterized in gastropods. MIF is a cytokine with pleiotropic functions in mammals, including the stimulation of cell proliferation and suppression of p53-mediated apoptosis [79]. In gastropods, it has been identified both in haliotid (Haliotis diversicolor) and planorbid (B. glabrata) snails [32, 80]. Just as its mammalian ortholog, B. glabrata MIF, which is expressed in haemocytes, stimulates cell proliferation and inhibits nitric oxide-dependent, p53-mediated apoptosis in Bge cells. Moreover, knockdown of B. glabrata MIF altered haemocytic behaviour in a manner that led to a significant increase in parasite burden in infected snails [32].
Bona fide growth factors have begun to be identified in gastropods, largely because of gene sequence comparisons to known vertebrate growth factors. In Haliotis tuberculata, porcine insulin and recombinant human epidermal growth factor (EGF) have been shown to influence the primary capacity of haemocytes to incorporate labelled leucine or thymidine in a concentration-dependent manner [81], an observation which suggests that homologs of these growth factors might be present in this gastropod. However, the interpretation of the results was complicated by the fact that the possible proliferative effect of concanavalin A, which was used to promote the attachment of cells in the assays, could not be ruled out. More recent studies have since provided more definitive evidence; an insulin receptor has been cloned from the Bge cell line, which also responds to bovine insulin by incorporating labelled methionine and thymidine [82], and a B. glabrata EGF-related protein is one of the transcripts upregulated early on in the course of infection with S. mansoni [83]. An EGF and its receptor have also been identified in L. stagnalis (L-EGF) and they are thought to be associated with the activation and survival of resident endoneurial phagocytes, which are important for neuronal regeneration [17].
In terms of signal transduction during haematopoiesis, evidence so far indicates involvement of the mitogen-activated protein kinase (MAPK) pathway. A number of other immune relevant processes in haemocytes also appear to be mediated by the MAPK pathway. These include cellular adhesion, motility and spreading required for phagocytosis and encapsulation [85], and regulation of the release of the cytotoxic molecules, hydrogen peroxide and nitric oxide [14, 86, 87]. The MAPK/ERK pathway can be activated by protein kinase C (PKC). In vitro treatment of APOs from B. glabrata with the PKC activator phorbol myristate acetate (PMA) induced cell division in the APO in a concentration-dependent manner and this effect was blocked when ERK1/2 inhibitor (U0126) was added [56]. However, some cell division was still observed in the presence of the ERK1/2 inhibitor, indicating that other signalling pathways are likely involved in gastropod haematopoiesis.
Another MAPK (p38) has also been implicated in the induction of hydrogen peroxide production in B. glabrata haemocytes in response to PMA or galactose-conjugated BSA [14], making it a potential candidate for signal transduction during haemocyte proliferation in gastropods. Toll-like receptor (TLR) signalling, which can also activate the MAPKs (p38 and JUN) or NF-kB is another pathway through which mitotic activity can be achieved. In L. littorea, treatment of haemocytes with the TLR4 ligand, LPS, resulted in the activation of MAPKs, ERK2, and p38 [88]. As reviewed above, LPS induces cell proliferation in the APO of B. glabrata [54]. Although the receptor-ligand relationship has not been established in this snail, analysis of its genome shows that it contains several TLRs and leucine-rich repeat-containing molecules. A TLR has also been identified in the disk abalone Haliotis discus [89]. This TLR is expressed in haemocytes more than in other tissues and its transcript is upregulated significantly upon bacterial and viral challenges. Further studies are needed in order to elucidate the signal transduction pathways involved in gastropod haematopoiesis.
Transcriptional regulation of haematopoiesis is the least understood aspect of haemocyte development in gastropods. There are no studies dealing with the transcription factor profile of haemocyte precursors that we are aware of to date. A few transcription factors have been cloned, and the pattern of expression determined, but there is no direct evidence for their functional involvement in specific proliferation, development and maturation events in gastropods. The cAMP response element-binding protein (CREB) is a transcription factor better known for its role in memory [90], but also functions in cell proliferation and survival [91]. CREB has been identified in some gastropod species [92–94] but has only been studied extensively in Aplysia and Lymnaea in the context of memory. The nuclear factor, kappa-light-chain enhancer of activated B cells (NF-kB), is an evolutionarily conserved family of transcription factors that function in immune cell activation, transcription of pro-inflammatory genes and learning and memory in the nervous system. NF-kB homologs have been cloned in B. glabrata [93] and the abalone H. diversicolor [95]. B. glabrata NF-kB was upregulated during S. mansoni challenge, whereas H. diversicolor NF-kB was downregulated during viral haemorrhagic septicaemia virus challenge, but expression of its transcript changed inconsistently with bacterial challenge over 48 hours. NF-kB-like protein has been detected in the axons of Aplysia, but its function there is thought to be communication, linking axons and synapses with the nuclear synthetic machinery [96].
Other transcription factors playing important roles in vertebrate haematopoiesis or cell proliferation that have been identified in gastropods include STAT1 and 2 [93] and CCAAT-enhancer binding protein (C/EBP) [97]. The functionality of the STATs has not been explored in these organisms, while C/EBP is associated with facilitation of sensory-to-motor neuron synapses in Aplysia. Further studies are needed in order to determine the specific roles of these transcription factors in the context of haematopoiesis in gastropods.
A recent study [84] has examined the transcriptomic response in the APO of B. glabrata snails at 24 hours post-challenge with three substances having mitogenic activity in the APO: the bacterial PAMPs lipopolysaccharide (LPS) and peptidoglycan, and fucoidan, a sulfated polysaccharide that may mimic fucosylated glycan PAMPs on the sporocysts of S. mansoni. Using a 60-nt oligonucleotide B. glabata microarray with 30,647 probes, this study revealed that genes involved in cellular proliferation were among the most differentially expressed, along with immune-related and detoxification genes, as well as genes with no known homologs in other organisms. Changes in gene expression were elicited by all three PAMPS with LPS having the most potent effect. Checkpoint kinase 1, a serine/threonine-specific kinase and key regulator of mitosis was found to be highly upregulated in the APO of LPS-challenged snails, indicating that it plays a role in cellular proliferation in the APO. The authors proposed that the expression of this kinase might serve as a potential genetic marker for identifying sites of haemocyte production in the snail [84].
Cephalopod haematopoiesis and haemocyte function
Cephalopods are the largest of all molluscs, and included within this group are the octopus, squid, nautilus, and cuttlefish. Cephalopods differ from other molluscs in that they have a closed circulatory system that consists of a central heart, two branchial hearts, and a system of blood vessels throughout the tissues [31].
The White Body
Our understanding of haematopoiesis in cephalopods is rudimentary; however the site of haematopoiesis in coleoid cephalopods, including octopus (Octopus vulgaris, O. briareus) cuttlefish (Sepia officinalis) and squid (Euprymna tasmanica), is the white body. The white body is a multi-lobed organ that wraps around the optic bundle [22, 31]. Haematopoietic development in the white body was first described in the common octopus O. vulgaris by Cowden (1972) [98], who identified primary, secondary and tertiary leukoblasts in histological sections. These cells were found near the surface of the organ, in a loose connective tissue network that followed the blood vessels. He observed that the mature leukocytes had an irregularly shaped nucleus [98].
Since this early study, two major haemocyte developmental stages have been identified in the white body of O. vulgaris, O. briareus and in two unidentified squid species: haemocytoblasts and leukoblasts. Haemocytoblasts have a large cytoplasmic volume and abundant rough endoplasmic reticulum and nucleoli. They are found in the reticulum of the white body’s lobes, and give rise to leukoblasts, which are characterized by a reduced cytoplasmic volume and nuclear size compared to their progenitors [99]. With the use of transmission and scanning electron microscopy, Claes (1996) [100], found haemocytoblasts, leukoblasts, and mature haemocytes in the cuttlefish S. officianalis, and proposed a schematic of haemocyte development similar to that described for octopus and squid species [99]. He observed the number of each cell type undergoing mitosis, and proposed that haemocytoblasts undergo mitosis and differentiate to produce leukoblasts, and that the primary leukoblasts undergo another round of mitosis to produce secondary leukoblasts. He did not observe mitosis in the secondary leukoblasts, suggesting that these cells differentiate into mature circulating haemocytes [100]. Mature haemocytes are larger and have a folded nucleus, and a well-developed Golgi apparatus [99].
Cephalopod Haemocytes
Recent work has begun to characterize haemocyte populations and their functions in immunity among cephalopods. Castellanos-Martinez et al. (2014) [26], sought to morphologically characterize circulating, mature haemocytes and elucidate their function in O. vulgaris. Using light and electron microscopy and flow cytometry, they identified two morphologically distinct haemocyte populations in the haemolymph. One cell type was large, and had high granularity. When these cells were observed under the microscope, the cells were round, and had numerous pseudopodia, with an abundant cytoplasm and a U-shaped, eccentric nucleus. The chromatin was observed in clumps at the periphery of the nucleus, and the cells had small endoplasmic reticulum, closely associated with the nucleus. They had large and small membrane-bound cytoplasmic granules. The granules were of medium and high electron densities. A smaller, less numerous cell type was also observed. The smaller cells were less granular, had fewer pseudopodia, were irregularly shaped and had a rounded nucleus. The most distinctive ultrastructural detail of these cells were fewer and smaller cytoplasmic inclusions. The larger cells were more phagocytic and produced a more intense respiratory burst than the smaller cells [26]. However, a similar study found three haemocyte types in O. vulgaris, which were described as hyalinocytes, granulocytes, and haemoblast-like cells [22]. Such discrepancies demonstrate a need for a standardized characterization of the haemocyte populations in cephalopods.
Cephalopod haemocytes share similar functions with immune cells in other animals. Haemocytes in octopus and cuttlefish can phagocytose bacteria, zymosan, and synthetic beads [26, 31, 99]. Cytotoxic activity, characterized by the production of reactive oxygen species and reactive nitrogen species, has been demonstrated in octopus, cuttlefish and squid haemocytes (Reviewed in [31]). Haemocytes of the curled octopus, Eledone cirrhosa, have been implicated in wound healing. They have been found to increase in number after wounding, and have been observed forming a plug to prevent blood loss. Similar behaviour has been observed in the cuttlefish, S. officinalis, in response to arm amputation (Reviewed in [31]).
Only one study to date has sought to characterize the molecular basis for haematopoiesis in cephalopods. In the squid Euprymna tasmanica, Salazar et al. (2015) [22] used a transcriptome analysis followed by a gene ontogeny analysis of the white body to identify 8 gene transcripts known to be involved in haematopoiesis in other organisms. The authors found transcripts of Cleavage and polyadenylation specificity factor complex (CPSF1), transcription factor GATA 2, Induced myeloid leukemia cell differentiation protein (MCL-1), the ribosomal protein s7, the transcription initiation factor TFIID subunit 3 (TAF3), fibroblast growth factor receptor 2 (FGFR2), CD109 antigen and ferritin. The expression of these developmental genes, which have also been implicated in hematopoiesis in vertebrates, in the white body supports the hypothesis that this organ is the site of haematopoiesis in squid, as well as in other cephalopods. Future studies should continue to functionally characterize gene expression profiles that are involved in haematopoiesis in cephalopods. The recently published octopus genome (O. bimaculoides) identified several other genes that may be associated with haematopoietic control, including fibroblast growth factor and TGFβ homologs [101].
Bivalve haematopoiesis and haemocyte function
Bivalves (class Bivalvia) include oysters, cockles, clams, mussels and scallops. Bivalves differ from other molluscs in that their shell consists of two valves joined by a hinge. Bivalves are sediment and suspension feeding organisms, and this trait, in addition to their sedentary lifestyle, has resulted in the loss of the typical head and radula organs of the other molluscan classes. Instead, some bivalves have a pronounced foot used for digging into sediment.
Neoplasia and Haemocyte Development
Since its initial description in Crassostrea sp. oysters, neoplasia has been described in numerous marine bivalve species in oceans worldwide. This condition is characterized by abnormal and excessive cell replication. The neoplastic cells may be malignant, continue replicating and spreading to distant sites causing death, but the cells may also be benign and have less severe effects on the organism. Malignant neoplasia is, therefore, especially of interest to the aquaculture industry. Generally, there are two types of neoplasia in bivalves, one type affecting the gonads, and the second type systemic. Many studies have emerged on systemic neoplasia, and these have demonstrated interesting links to haematopoiesis. The specific characteristics of systemic neoplasia in bivalves have been reviewed in detail by Carballal et al. (2015) [102]. Briefly, there is an increase in the infiltration of atypical mitotic cells in the connective tissue and in the haemolymph sinuses and tissues of the circulatory system. To date, there have been five neoplastic cell types described, and they share general characteristics with cells observed in vertebrate cancers. Specifically, they are anaplastic, have few organelles in the cytoplasm, and have enlarged ribosomes and mitochondria. Due to the cytological characteristics of these cells, most notably a high nucleus to cytoplasm ratio and phagocytic activity, it has been suggested that these infiltrating cells are immature haemocytes [104]. In support of this hypothesis, several studies have compared phagocytosis between haemocytes and neoplastic cells in various mussel and cockle species, and noted lower phagocytic activity in neoplastic cells [105–107]. However, it has also been demonstrated that there is measurably higher ROI production in neoplastic cells compared to haemocytes [107]. Such comparisons are interesting, and ultimately may be useful in advancing the understanding of haematopoiesis in bivalve species, as well as the origins of neoplasia, in bivalve species [102].
A definitive haematopoietic site common to all bivalves has yet to be identified, and is a focus of investigation in a number of species. In the clam Tapes philippinarum, evidence suggests that at least some of the newly formed haemocytes originate from the division of circulating cells called haemoblasts. Matozzo et al. (2008) [103] observed through cytochemical analysis the formation of mitotic spindles in these cells which their previous observations suggested had stem cell features. One study, using the oyster C. gigas, found a transcription factor, Cg-tal (Tal1/SCL), which is important in vertebrate embryonic haematopoiesis, expressed only in cells that were attached to the blood vessel endothelium, which led the authors to hypothesize that the site of haematopoiesis is in blood vessel or arterial endothelial cells [108]. However, Jemaa et al. (2014) [21] have recently provided evidence of adult haemocyte progenitor cells in specialized regions of gill epithelium in the pacific oyster C. gigas. The gill is comprised of two structures, a regularly folded epithelium of tightly knit, non-ciliated cells, and irregularly folded structures (IFS), consisting of tubules embedded in a thick extracellular matrix. Using immunohistochemistry, the authors stained for Sox2 transcription factor, a stem cell marker, and Zn/Cu superoxide dismutase (SOD), an enzyme specifically expressed in oyster haemocytes. They found cells in tubules of the IFS were Sox2 positive, and that cells in the underlying haemolymph vessels were SOD positive. The authors noted a population of cells in the sub-epithelial connective tissue and vessels that were both Sox2 and SOD positive, and they argued that these cells are haemocyte progenitor cells. They also found evidence of DNA synthesis and cell proliferation in this cell population. Specifically, they found BrdU incorporation in the gill, and used flow cytometry to demonstrate a higher percentage of gill cells in the S and G2/M stages of the cell cycle than cells in the mantle. This paper is the first report on adult somatic progenitor cell in bivalves, and represents an essential first step in elucidating the haematopoietic process [21].
Haemocyte Classification in Bivalves
Haemocyte populations in bivalves vary greatly, both in observed morphologies, the proportions of haemocyte types observed at any given time, and total haemocyte abundance. Generally, bivalve haemocytes are classified as granulocytes (sometimes differentiated by size into large granulocytes and small granulocytes) and hyalinocytes. Despite these general categories, there is currently no unified nomenclature used to describe bivalve haemocytes, nor is there any one method used for their identification [109]. In the blue mussel Mytilus edulis [110] and the California mussel, M. californianus[111], three primary populations of haemocytes have been described in the haemolymph: hyalinocytes and two populations of granulocyte, acidophilic and basophilic. These different haemocyte populations are functionally distinct, in that the granulocytes have phagocytic activity, with acidophilic granulocytes being the most phagocytic, and in contrast, no phagocytic activity is observed in hyalinocytes [110]. Ruddell et al. (1971) [112] reported agranulocytes and basophilic and eosinophilic granulocytes in the haemocytes of the pacific oyster C. gigas, while others identified two granular populations of haemocyte and one agranular population of haemocyte in the eastern oyster, C. virginica [113]. In the scallop Argopecten irradians, studies of cell size and morphology have identified four main types of haemocytes: small hyalinocytes, large hyalinocytes, small granulocytes and large granulocytes [114]. Morphological and functional characterization of haemocytes in the giant clam Tridacna crocea have revealed three haemocyte populations, eosinophilic granular haemocytes, agranular cells, and morula-like cells. One study sought to functionally characterize these populations in T. crocea and observed that the eosinophilic granulocytes were phagocytic, while the agranular and morula-like cells were unable to phagocytose latex beads. All three cell types were observed to participate in clot formation upon wounding and exposure to seawater [115]. In the clam Scrobicularia plana, Wootton et al. (2003) described three different haemocyte types with the use of light and electron microscopy: granular eosinophils, granular basophils and agranular basophils [116]. Overall, the haemocytes from this species are much smaller in size than any other known bivalve species and are predominantly granular [116, 117].
Phagocytosis and Pathogen Killing Mechanisms
Several studies have shown that bivalve haemocytes have the ability to chemotactically respond to and phagocytose exogenous particles [118] by active rearrangement of the cytoskeleton [119]. However, studies have shown that many factors including relative haemocyte size, stress, and environment may play important roles in the ability of haemocytes to phagocytose efficiently and effectively. For instance, the haemocytes of S. plana were able to phagocytose three different species of heat-killed bacteria, but could not phagocytose zymosan. This inability is likely due to their smaller size relative to haemocytes of other bivalve species. The authors were not able to distinguish which specific sets of haemocytes were responsible for phagocytosing the bacteria, as only those in suspension were able to complete the task, while the adherent cells were not phagocytic [116].
It is known that the haemocytes of shellfish can generate a typical respiratory burst. ROI production has been determined in haemocytes from numerous bivalve species, such as the oysters C. gigas, C. virginica, the scallop Pecten maximus, and the flat oyster Ostrea edulis [120, 121]. Pipe et al. (1992), found that rhodamine B −123 elicits superoxide production by haemocytes of M. edulis [121]. Anderson et al. (1992), found that superoxide dismutase can inhibit the reduction of nitro blue tetrazolium reduction (NBT) in a stationary state or during the process of phagocytosis [120]. In field and laboratory studies, NBT reduction was greater in haemocytes as water temperature increased (2°C −29°C). No significant difference in NBT reduction was observed when a range of salinity was tested. These results suggest that NBT reduction is associated with a temperature dependent or seasonal phenomenon, a factor which should be considered in the design and interpretation of experimental studies of ROI production in bivalves [120].
The production of nitric oxide (NO) by haemocytes of bivalve molluscs has been reported in M. galloprovincialis [122], M. edulis [33], V. ater [123, 124], and the carpet shell clam Ruditapes decussatus [125]. Though the haemocytes of these species are able to produce NO in response to various stimuli, such as LPS, zymosan, and live bacterial challenge by Vibrio tapetis, this production is independent of phagocytosis [125].
Concluding remarks
Haematopoiesis plays an important role in maintaining the homeostasis of haemocytes, which in molluscs perform several vital functions required for survival such as defending against infection. Several decades of research have steadily advanced the field of molluscan haematopoiesis from one of observational studies into the morphological characteristics of circulating immune cells, to the point where we can now conduct mechanistic investigations into the cytokines, growth factors, receptors, signalling pathways and transcriptional events that are involved in molluscan haemocyte development. However, as yet, we do not have a complete description for the entire process in any mollusc. There are absolute knowledge gaps in some areas while others are in need of further investigations. For instance, our knowledge of haematopoiesis in molluscs primarily stems from studies of key species of economic and medical importance from 3 out of 9 extant classes. Given that molluscs are incredibly diverse both in form and the habitats they dwell in, there is the need to extend investigations of the haematopoietic processes beyond these key species and into the other 6 extant classes of molluscs. As is obvious from the above review, the nomenclature and method of identification for haemocytes need standardization in order to better organize this area of research.
As in many areas of comparative biology, the study of haematopoiesis in molluscs will benefit from the completion of mollusc genome sequencing projects. This information will facilitate gene identification and provide evidence for the existence of relevant haematopoietic factors. Efforts could then be directed at characterization of molecules important for haemocyte development and defining the regulatory mechanisms that control haematopoiesis in molluscs.
Highlights.
Reviews current literature on mollusc haematopoiesis.
Covers haematopoiesis in gastropods, cephalopods and bivalves.
Discusses haemocyte development and function
Acknowledgements
This paper was supported by funding from NSFC (NO. 31272682) (XZW and JF), The Fletcher Jones Foundation and NIH Grant AI097967 (JTS), and NSERC 418540 (PCH, EAP, MAG, SPR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Chen S, Cai D, Pearce K, Sun PYW, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. eLife. 2014;3:e03896. doi: 10.7554/eLife.03896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochner B. How nervous systems evolve in relation to their embodiment: what we can learn from octopuses and other molluscs. Brain, Behavior & Evolution. 2013;82:19–30. doi: 10.1159/000353419. [DOI] [PubMed] [Google Scholar]
- 3.Guerra A, Sanchez P, Rocha F. The Spanish fishery for Loligo: recent trends. Fisheries Res (Netherlands) 1994:217. [Google Scholar]
- 4.Uriarte I, Iglesias J, Domingues P, Rosas C, Viana MT, Navarro JC, et al. Current status and bottle neck of octopod aquaculture: the case of American species. J World Aquacult Soc. 2011;42:735–752. [Google Scholar]
- 5.Lafarga de lC, Gallardo-Escárate C. Intraspecies and interspecies hybrids in Haliotis: natural and experimental evidence and its impact on abalone aquaculture. Rev Aquacult. 2011;3:74–99. [Google Scholar]
- 6.Rojas R, Miranda CD, Opazo R, Romero J. Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck, 1819) J Invertebr Pathol. 2015;124:61–69. doi: 10.1016/j.jip.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Richards GP, Watson MA, Needleman DS, Church KM, Hase CC. Mortalities of Eastern and Pacific oyster larvae caused by the pathogens Vibrio coralliilyticus and Vibrio tubiashii. Appl Environ Microbiol. 2015;81:292–297. doi: 10.1128/AEM.02930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loker ES. Gastropod immunobiology. Adv Exp Med Biol. 2010;708:17–43. doi: 10.1007/978-1-4419-8059-5_2. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson JD, Fairbanks WS, Cornicelli L. Ecology of gastropod and bighorn sheep hosts of lungworm on isolated, semiarid mountain ranges in Utah, USA. J Wildl Dis. 2008;44:28–44. doi: 10.7589/0090-3558-44.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Hung NM, Duc NV, Stauffer JR, Jr, Madsen H. Use of black carp (Mylopharyngodon piceus) in biological control of intermediate host snails of fish-borne zoonotic trematodes in nursery ponds in the Red River Delta, Vietnam. Parasit Vectors. 2013;6:1–9. doi: 10.1186/1756-3305-6-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng TC. Bivalves. In: Ratcliffe NA, Rowley AF, editors. Invertebrate Blood Cells. New York: Academic Press; 1981. pp. 233–300. [Google Scholar]
- 12.Hanington PC, Forys MA, Dragoo JW, Zhang SM, Adema CM, Loker ES. Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection. Proc Natl Acad Sci U S A. 2010;107:21087–21092. doi: 10.1073/pnas.1011242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loker ES, Bayne CJ, Buckley PM, Kruse KT. Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J Parasitol. 1982;68:84–94. [PubMed] [Google Scholar]
- 14.Humphries JE, Yoshino TP. Regulation of hydrogen peroxide release in circulating hemocytes of the planorbid snail Biomphalaria glabrata. Dev Comp Immunol. 2008;32:554–562. doi: 10.1016/j.dci.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacchini AH, Davies AJ, Mackintosh D, Walker AJ. Beta-1, 3-glucan modulates PKC signalling in Lymnaea stagnalis defence cells: a role for PKC in H2O2 production and downstream ERK activation. J Exp Biol. 2006;209:4829–4840. doi: 10.1242/jeb.02561. [DOI] [PubMed] [Google Scholar]
- 16.Franchini A, Ottaviani E. Repair of molluscan tissue injury: role of PDGF and TGF-beta. Tissue Cell. 2000;32:312–321. doi: 10.1054/tice.2000.0118. [DOI] [PubMed] [Google Scholar]
- 17.Hermann PM, Nicol JJ, Nagle GT, Bulloch AG, Wildering WC. Epidermal growth factor-dependent enhancement of axonal regeneration in the pond snail Lymnaea stagnalis: role of phagocyte survival. J Comp Neurol. 2005;492:383–400. doi: 10.1002/cne.20732. [DOI] [PubMed] [Google Scholar]
- 18.Mount AS, Wheeler AP, Paradkar RP, Snider D. Hemocyte-mediated shell mineralization in the Eastern oyster. Science. 2004;304:297–300. doi: 10.1126/science.1090506. [DOI] [PubMed] [Google Scholar]
- 19.Ma H, Wang J, Wang B, Zhao Y, Yang C. Characterization of an ETS transcription factor in the sea scallop Chlamys farreri. Dev Comp Immunol. 2009;33:953–958. doi: 10.1016/j.dci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JT, Belloir JA, Beltran RV, Grivakis A, Ransone KA. Fucoidan stimulates cell division in the amebocyte-producing organ of the schistosome-transmitting snail Biomphalaria glabrata. J Invertebr Pathol. 2014;123:13–16. doi: 10.1016/j.jip.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jemaa M, Cavelier P, Cau J, Morin N. Adult somatic progenitor cells and hematopoiesis in oysters. J Exp Biol. 2014:3067–3077. doi: 10.1242/jeb.106575. [DOI] [PubMed] [Google Scholar]
- 22.Salazar KA, Joffe NR, Dinguirard N, Houde P, Castillo MG. Transcriptome analysis of the white body of the squid Euprymna tasmanica with emphasis on immune and hematopoietic gene discovery. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0119949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hine PM. The inter-relationships of bivalve haemocytes. Fish Shell Immunol. 1999;9:367–385. [Google Scholar]
- 24.Ottaviani E. Molluscan immunorecognition. Invert Surviv J. 2006;3:50–63. [Google Scholar]
- 25.Yoshino TP, Coustau C. Immunobiology of Biomphalaria-trematode interactions. In: Toledo R, Fried B, editors. Biomphalaria Snails and Larval Trematodes. New York: Springer; 2011. pp. 159–189. [Google Scholar]
- 26.Castellanos-Martínez S, Prado-Alvarez M, Lobo-da-Cunha A, Azevedo C, Gestal C. Morphologic, cytometric and functional characterization of the common octopus (Octopus vulgaris) hemocytes. Dev Comp Immunol. 2014;44:50–58. doi: 10.1016/j.dci.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Lie KJ, Heyneman D, Yau P. Origin of amebocytes in Biomphalaria glabrata. J Parasitol. 1975;61:574–576. [Google Scholar]
- 28.Kinoti GK. Observations on the infection of bulinid snails withSchistosoma mattheei. II. The mechanism of resistance to infection. Parasitology. 1971;62:161–170. doi: 10.1017/s0031182000071389. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan JT. Hematopoiesis in three species of gastropods following infection with Echinostoma paraensei (Trematoda, Echinostomatidae) Trans Am Microsc Soc. 1988;107:355–361. [Google Scholar]
- 30.Accorsi A, Ottaviani E, Malagoli D. Effects of repeated hemolymph withdrawals on the hemocyte populations and hematopoiesis in Pomacea canaliculata. Fish Shellfish Immunol. 2014;38:56–64. doi: 10.1016/j.fsi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Castillo MG, Salazar KA, Joffe NR. The immune response of cephalopods from head to foot. SI: Mollusc Immun. 2015;46:145–160. doi: 10.1016/j.fsi.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Baeza Garcia A, Pierce RJ, Gourbal B, Werkmeister E, Colinet D, Reichhart JM, et al. Involvement of the cytokine MIF in the snail host immune response to the parasite Schistosoma mansoni. PLoS Pathog. 2010;6:e10011156. doi: 10.1371/journal.ppat.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottaviani E, Franchini A, Franceschi C. Presence of several cytokine-like molecules in molluscan hemocytes. Biochem Biophys Res Commun. 1993;195:984–988. doi: 10.1006/bbrc.1993.2141. [DOI] [PubMed] [Google Scholar]
- 34.Granath WO, Jr, Connors VA, Tarleton RL. Interleukin 1 activity in haemolymph from strains of the snail Biomphalaria glabrata varying in susceptibility to the human blood fluke, Schistosoma mansoni: presence, differential expression, and biological function. Cytokine. 1994;6:21–27. doi: 10.1016/1043-4666(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 35.Bouchet P, Rocroi JP. Classification and nomenclature of gastropod families. Malacologia. 2005;47:1–397. [Google Scholar]
- 36.Littlewood DTJ, Bray RA. Interrelationships of the Platyhelminthes. Systematics Association Special Volume Series. 2001;60:i–xii. 1–356. [Google Scholar]
- 37.Sullivan JT. Long-term survival of heterotopic allografts of the amebocyte-producing organ in Biomphalaria glabrata (Mollusca, Pulmonata) Trans Am Microsc Soc. 1990;109:52–60. [Google Scholar]
- 38.Lie JK, Heyneman D, Jeong KH. Studies on resistance in snails 4. Induction of ventricular capsules and changes in the amebocyte-producing organ during sensitization of Biomphalaria glabrata snails. J Parasitol. 1976;62:286–291. [PubMed] [Google Scholar]
- 39.Sminia T. Structure and function of blood and connective-tissue cells of fresh water pulmonate Lymnaea stagnalis studied by electron-microscopy and enzyme histochemistry. Z Zellforsch Mikrosk Anat. 1972;130:497. doi: 10.1007/BF00307004. [DOI] [PubMed] [Google Scholar]
- 40.Pan CT. The general histology and topographic microanatomy of Australorbis glabratus. Bull Museum Comp Zool. 1958;119:235–299. [Google Scholar]
- 41.Jeong KH, Lie KJ, Heyneman D. The ultrastructure of the amebocyte-producing organ in Biomphalaria glabrata. Dev Comp Immunol. 1983;7:217–228. doi: 10.1016/0145-305x(83)90003-4. [DOI] [PubMed] [Google Scholar]
- 42.McKerrow JH, Jeong KH, Beckstead JH. Enzyme histochemical comparison of Biomphalaria glabrata amebocytes with human granuloma macrophages. J Leukoc Biol. 1985;37:341–347. doi: 10.1002/jlb.37.3.341. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan JT, Spence JV. Factors affecting adoptive transfer of resistance to Schistosoma mansoni in the snail intermediate host, Biomphalaria glabrata. J Parasitol. 1999;85:1065–1071. [PubMed] [Google Scholar]
- 44.Barbosa L, Caldeira RL, Carvalho OS, Vidigal TH, Jannotti-Passos LK, Coelho PM. Resistance to Schistosoma mansoni by transplantation of APO Biomphalaria tenagophila. Parasite Immunol. 2006;28:209–212. doi: 10.1111/j.1365-3024.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan JT, Spence JV, Nunez JK. Killing of Schistosoma mansoni sporocysts in Biomphalaria glabrata implanted with amoebocyte-producing organ allografts from resistant snails. J Parasitol. 1995;81:829–833. [PubMed] [Google Scholar]
- 46.Vasquez RE, Sullivan JT. Hematopoietic tissue allografts in Biomphalaria glabrata (Mollusca: Pulmonata) induce humoral immunity to Schistosoma mansoni. Dev Comp Immunol. 2001;25:561–564. doi: 10.1016/s0145-305x(01)00022-2. [DOI] [PubMed] [Google Scholar]
- 47.Richards CS. Genetic studies of pathologic conditions and susceptibility to infection in Biomphalaria glabrata. Ann N Y Acad Sci. 1975;266:394–410. doi: 10.1111/j.1749-6632.1975.tb35118.x. [DOI] [PubMed] [Google Scholar]
- 48.Joky A, Matricon-Gondran M, Benex J. Response to the amoebocyte-producing organ of sensitized Biomphalaria glabrata after exposure to Echinostoma caproni miracidia. J Invertebr Pathol. 1985;45:28–33. doi: 10.1016/0022-2011(85)90045-x. [DOI] [PubMed] [Google Scholar]
- 49.Lie KJ, Heyneman D, Lim HK. Studies on resistance in snails: specific resistance induced by irradiated miracidia of Echinostoma lindoense in Biomphalaria glabrata snails. Int J Parasitol. 1975;5:627–631. doi: 10.1016/0020-7519(75)90062-4. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan JT, Richards CS, Lie KJ, Heyneman D. Ribeiroia marini: irradiated miracidia and induction of acquired resistance in Biomphalaria glabrata. Exp Parasitol. 1982;53:17–25. doi: 10.1016/0014-4894(82)90088-1. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan JT, Cheng TC, Howland KH. Mitotic responses of the anterior pericardial wall of Biomphalaria glabrata (Mollusca) subjected to challenge. J Invertebr Pathol. 1984;44:114–116. [Google Scholar]
- 52.Noda S. Effects of excretory-secretory products of Echinostoma paraensei larvae on the hematopoietic organ of M-Line Biomphalaria glabrata snails. J Parasitol. 1992;78:512–517. [PubMed] [Google Scholar]
- 53.Sullivan JT, Pikios SS, Alonzo AQ. Mitotic responses to extracts of miracidia and cercariae of Schistosoma mansoni in the amebocyte-producing organ of the snail intermediate host Biomphalaria glabrata. J Parasitol. 2004;90:92–96. doi: 10.1645/GE-3266. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan JT, Bulman CA, Salamat Z. Effect of crude lipopolysaccharide from Escherichia coli O127:B8 on the amebocyte-producing organ of Biomphalaria glabrata (Mollusca) Dev Comp Immunol. 2011;35(11):1182–1185. doi: 10.1016/j.dci.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Salamat Z, Sullivan JT. In vitro mitotic responses of the amebocyte-producing organ of Biomphalaria glabrata to extracts of Schistosoma mansoni. J Parasitol. 2008;94:1170–1173. doi: 10.1645/GE-1554.1. [DOI] [PubMed] [Google Scholar]
- 56.Salamat Z, Sullivan JT. Involvement of protein kinase C signalling and mitogen-activated protein kinase in the amebocyte-producing organ of Biomphalaria glabrata (Mollusca) Dev Comp Immunol. 2009;33:725–727. doi: 10.1016/j.dci.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Jeong KH, Lie KJ, Heyneman D. Leucocytosis in Biomphalaria glabrata sensitized and resensitized to Echinostoma lindoense. J Invertebr Pathol. 1980;35:9–13. doi: 10.1016/0022-2011(80)90076-2. [DOI] [PubMed] [Google Scholar]
- 58.Lie KJ. Swellengrebel lecture: Survival of Schistosoma mansoni, other trematode larvae in the snail Biomphalaria glabrata. A discussion of the interference theory. Trop Geogr Med. 1982;34:111–122. [PubMed] [Google Scholar]
- 59.Hanington PC, Forys MA, Loker ES. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl Trop Dis. 2012;6:e1591. doi: 10.1371/journal.pntd.0001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loker ES, Cimino DF, Hertel LA. Excretory-secretory products of Echinostoma paraensei sporocysts mediate interference with Biomphalaria glabrata hemocyte functions. J Parasitol. 1992;78:104–115. [PubMed] [Google Scholar]
- 61.Rachford FW. Host-parasite relationship of Angiostrongylus cantonensis in Lymnaea palustris 2. Histopathology. Exp Parasitol. 1976;36:382–392. doi: 10.1016/0014-4894(76)90042-4. [DOI] [PubMed] [Google Scholar]
- 62.Yousif F, Roushdy M, El-Emam M. The host-parasite relationships of Angiostrongylus cantonensis in Egypt. 1. Natural and experimental infection of the snail intermediate host Lanistes carinatus. J Egypt Soc Parasitol. 1980;10:399–412. [Google Scholar]
- 63.Souza SD, Andrade ZA. The significance of the amoebocyte-producing organ in Biomphalaria glabrata. Mem Inst Oswaldo Cruz. 2012;107:598–603. doi: 10.1590/s0074-02762012000500005. [DOI] [PubMed] [Google Scholar]
- 64.Souza SD, Andrade ZA. On the origin of the Biomphalaria glabrata hemocytes. Mem Inst Oswaldo Cruz. 2006;101:213–218. doi: 10.1590/s0074-02762006000900033. [DOI] [PubMed] [Google Scholar]
- 65.Sminia T, Van der Knaap WPW, Van Asselt LA. Blood cell types and blood cell formation in gastropod molluscs. Dev Comp Immunol. 1983;7:665–668. [Google Scholar]
- 66.Monteil JF, Matricon-Gondran M. Hemocyte production in trematode-infected Lymnaea truncatula. Parasitol Res. 1991;77:491–497. doi: 10.1007/BF00928416. [DOI] [PubMed] [Google Scholar]
- 67.Gorbushin AM, Iakovleva NV. Haemogram of Littorina littorea. J Mar Biol Assoc UK. 2006;86:1175. [Google Scholar]
- 68.Cheng TC. Functional morphology and biochemistry of molluscan phagocytes. An NY Acad Sci. 1975;266:343–379. doi: 10.1111/j.1749-6632.1975.tb35116.x. [DOI] [PubMed] [Google Scholar]
- 69.Ottaviani E. Haemocytes of the freshwater snail Viviparus ater (Gastropoda, Prosobranchia) J Molluscan Stud. 1989;55:379–382. [Google Scholar]
- 70.Matricon-Gondran M, Letocart M. Internal defenses of the snail Biomphalaria glabrata. J Invertebr Pathol. 1999;74:224–234. doi: 10.1006/jipa.1999.4876. [DOI] [PubMed] [Google Scholar]
- 71.Martins-Souza RL, Pereira CA, Coelho PM, Martins-Filho OA, Negrao-Correa D. Flow cytometry analysis of the circulating haemocytes from Biomphalaria glabrata and Biomphalaria tenagophila following Schistosoma mansoni infection. Parasitology. 2009;136:67–76. doi: 10.1017/S0031182008005155. [DOI] [PubMed] [Google Scholar]
- 72.Gordy MA, Pila EA, Hanington PC. The role of fibrinogen-related proteins in the gastropod immune response. Fish Shell Immunol. 2015;46:39–49. doi: 10.1016/j.fsi.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Ataev GL, Coustau C. Cellular response to Echinostoma caproni infection in Biomphalaria glabrata strains selected for susceptibility/resistance. Dev Comp Immunol. 1999;23:187–198. doi: 10.1016/s0145-305x(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 74.Larson MK, Bender RC, Bayne CJ. Resistance of Biomphalaria glabrata 13-16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. Int J Parasitol. 2014;44:343–353. doi: 10.1016/j.ijpara.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hahn UK, Fryer SE, Bayne CJ. An invertebrate (Molluscan) plasma protein that binds to vertebrate immunoglobulins and its potential for yielding false-positives in antibody-based detection systems. Dev Comp Immunol. 1996;20:39–50. doi: 10.1016/0145-305x(95)00035-r. [DOI] [PubMed] [Google Scholar]
- 76.Buckley KM, Rast JP. Diversity of animal immune receptors and the origins of recognition complexity in the deuterostomes. Dev Comp Immunol. 2015;49:179–189. doi: 10.1016/j.dci.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300:349–365. doi: 10.1016/j.ydbio.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 78.Song LS, Wang LL, Zhang H, Wang MQ. The immune system and its modulation mechanism in scallop. Fish Shell Immunol. 2015;46:65–78. doi: 10.1016/j.fsi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang B, Zhang Z, Wang Y, Zou Z, Wang G, Wang S, et al. Molecular cloning and characterization of macrophage migration inhibitory factor from small abalone Haliotis diversicolor supertexta. Fish Shell Immunol. 2009;27:57–64. doi: 10.1016/j.fsi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Lebel JM, Giard W, Favrel P, Boucaud-Camou E. Effects of different vertebrate growth factors on primary cultures of hemocytes from the gastropod mollusc, Haliotis tuberculata. Biol Cell. 1996;86:67–72. [PubMed] [Google Scholar]
- 82.Lardans V, Coppin JF, Vicogne J, Aroca E, Delcroix M, Dissous C. Characterization of an insulin receptor-related receptor in Biomphalaria glabrata embryonic cells. Biochim Biophys Acta. 2001;1510:321–329. doi: 10.1016/s0005-2736(00)00364-3. [DOI] [PubMed] [Google Scholar]
- 83.Hanington PC, Lun CM, Adema CM, Loker ES. Time series analysis of the transcriptional responses of Biomphalaria glabrata throughout the course of intramolluscan development of Schistosoma mansoni and Echinostoma paraensei. Int J Parasitol. 2010;40:819–831. doi: 10.1016/j.ijpara.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang SM, Loker ES, Sullivan JT. Pathogen-associated molecular patterns activate expression of genes involved in cell proliferation, immunity and detoxification in the amebocyte-producing organ of the snail Biomphalaria glabrata. Dev Comp Immunol. doi: 10.1016/j.dci.2015.11.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Humphries JE, Elizondo L, Yoshino TP. Protein kinase C regulation of cell spreading in the molluscan Biomphalaria glabrata embryonic (Bge) cell line. Biochim Biophys Acta. 2001;1540:243–252. doi: 10.1016/s0167-4889(01)00136-7. [DOI] [PubMed] [Google Scholar]
- 86.Zelck UE, Gege BE, Schmid S. Specific inhibitors of mitogen-activated protein kinase and PI3-K pathways impair immune responses by hemocytes of trematode intermediate host snails. Dev Comp Immunol. 2007;31:321–331. doi: 10.1016/j.dci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Gorbushin AM, Iakovleva NV. Functional characterization of Littorina littorea (Gastropoda: Prosobranchia) blood cells. J Mar Biol Assoc UK. 2007;87:741. [Google Scholar]
- 88.Iakovleva NV, Gorbushin AM, Storey KB. Modulation of mitogen-activated protein kinases (MAPK) activity in response to different immune stimuli in haemocytes of the common periwinkle Littorina littorea. Fish Shell Immunol. 2006;21:315–324. doi: 10.1016/j.fsi.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Elvitigala DA, Premachandra HK, Whang I, Nam BH, Lee J. Molecular insights of the first gastropod TLR counterpart from disk abalone (Haliotis discus discus), revealing its transcriptional modulation under pathogenic stress. Fish Shell Immunol. 2013;35:334–342. doi: 10.1016/j.fsi.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 90.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trend Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Zhang CY, Wu YL, Boxer LM. Impaired proliferation and survival of activated B cells in transgenic mice that express a dominant-negative cAMP-response element-binding protein transcription factor in B cells. J Biol Chem. 2002;277:48359–48365. doi: 10.1074/jbc.M209329200. [DOI] [PubMed] [Google Scholar]
- 92.Lee SH, Lim CS, Park H, Lee JA, Han JH, Kim H, et al. Nuclear translocation of CAM-associated protein activates transcription for long-term facilitation in Aplysia. Cell. 2007;129:801–812. doi: 10.1016/j.cell.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 93.Zhang SM, Coultas KA. Identification and characterization of five transcription factors that are associated with evolutionarily conserved immune signaling pathways in the schistosome-transmitting snail Biomphalaria glabrata. Mol Immunol. 2011;48:1868–1881. doi: 10.1016/j.molimm.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sadamoto H, Sato H, Kobayashi S, Murakami J, Aonuma H, Ando H, et al. CREB in the pond snail Lymnaea stagnalis: Cloning, gene expression, and function in identifiable neurons of the central nervous system. J Neurobiol. 2004;58:455. doi: 10.1002/neu.10296. [DOI] [PubMed] [Google Scholar]
- 95.Jiang Y, Wu X. Characterization of a Rel\NF-κB homologue in a gastropod abalone, Haliotis diversicolor supertexta. Dev Comp Immunol. 2007;31:121–131. doi: 10.1016/j.dci.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 96.Povelones M, Tran K, Thanos D, Ambron RT. An NF-κB-like transcription factor in axoplasm is rapidly inactivated after nerve injury in Aplysia. J Neurosci. 1997;17:4915–4920. doi: 10.1523/JNEUROSCI.17-13-04915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamamoto N, Hegde AN, Chain DG, Schwartz JH. Activation and degradation of the transcription factor C/EBP during long-term facilitation in Aplysia. J Neurochem. 2002;73:2415–2423. doi: 10.1046/j.1471-4159.1999.0732415.x. [DOI] [PubMed] [Google Scholar]
- 98.Cowden RR. Some cytological and cytochemical observations on leukopoietic organs, white bodies, of Octopus vulgaris. J Invertebr Pathol. 1972;19:113. [Google Scholar]
- 99.Ford LA. Host defense mechanisms of cephalopods. Ann Rev of Fish Dis. 1992;2:25–41. [Google Scholar]
- 100.Claes MF. Functional morphology of the white bodies of the cephalopod mollusc Sepia officinalis. Acta Zool. 1996;77:173–190. [Google Scholar]
- 101.Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JR, Edsinger-Gonzales E, et al. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature. 2015;524:220–224. doi: 10.1038/nature14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carballal MJ, Barber BJ, Iglesias D, Villalba A. Neoplastic diseases of marine bivalves. J Invert Path. 2015 doi: 10.1016/j.jip.2015.06.004. pii: S0022-2011(15)00117-2 Epub. [DOI] [PubMed] [Google Scholar]
- 103.Matozzo V, Marin MG, Cima F, Ballarin L. First evidence of cell division in circulating haemocytes from the Manila clam Tapes philippinarum. Cell Biol Int. 2008;32:865–868. doi: 10.1016/j.cellbi.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 104.Barber BJ. Neoplastic diseases of commercially important marine bivalves. Aqua Liv Res. 2004;17:449–466. [Google Scholar]
- 105.Kent ML, Elston RA, Wilkinson MT, Drum AS. Impaired defense mechanisms in bay mussels, Mytilus edulis, with hemic neoplasia. J Invertebr Pathol. 1989;53:378–386. doi: 10.1016/0022-2011(89)90103-1. [DOI] [PubMed] [Google Scholar]
- 106.Diaz S, Villalba A, Insua A, Soudant P, Fernandez-Tajes J, Mendez J, et al. Disseminated neoplasia causes changes in ploidy and apoptosis frequency in cockles Cerastoderma edule. J Invertebr Pathol. 2013;113:214–219. doi: 10.1016/j.jip.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 107.Le Grand F, Soudant P, Marty Y, Le Goic N, Kraffe E. Altered membrane lipid composition and functional parameters of circulating cells in cockles (Cerastoderma edule) affected by disseminated neoplasia. Chem Phys Lipids. 2013;167:9–20. doi: 10.1016/j.chemphyslip.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 108.Tirape A, Bacque C, Brizard R, Vandenbulcke F, Boulo V. Expression of immune-related genes in the oyster Crassostrea gigas during ontogenesis. Dev Comp Immunol. 2007;31:859–873. doi: 10.1016/j.dci.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Shi AJ, Qiu AD, Tang M, Yu YP, Zhang HY, Machii A. Hemoculture of Anodonta woodiana pacifica. Acta Hydrobiologica Sinica. 2001;25:116–122. [Google Scholar]
- 110.Carballal MJ, Lopez C, Azevedo C, Villalba A. In vitro study of phagocytic ability of Mytilus galloprovincialis Lmk haemocytes. Fish Shellfish Immunol. 1997;7:403–416. [Google Scholar]
- 111.Bayne CJ, Moore MN, Carefoot TH, Thompson RJ. Hemolymph functions in Mytilus californianus: cytochemistry of hemocytes and their responses to foreign implants and hemolymph factors in phagocytosis. J Invertebr Pathol. 1979;34:1–20. [Google Scholar]
- 112.Ruddell CL. Fine structure of granular amebocytes of pacific oyster, Crassostrea gigas. J Invertebr Pathol. 1971;18:269. doi: 10.1016/0022-2011(71)90155-8. [DOI] [PubMed] [Google Scholar]
- 113.Ford SE, Ashtonalcox KA, Kanaley SA. Comparative cytometric and microscopic analyses of oyster hemocytes. J Invertebr Pathol. 1994;64:114–122. [Google Scholar]
- 114.Zhang WZ, Wu XZ, Sun JF, Li DF. Micro- and ultra-structural characterization of haemocytes in scallop Chlamys farreri. J Trop Oceanography. 2007;26:57–62. [Google Scholar]
- 115.Nakayama K, Nomoto AM, Nishijima M, Maruyama T. Morphological and functional characterization of hemocytes in the giant clam Tridacna crocea. J Invertebr Pathol. 1997;69:105–111. doi: 10.1006/jipa.1996.4626. [DOI] [PubMed] [Google Scholar]
- 116.Wootton EC, Pipe RK. Structural and functional characterisation of the blood cells of the bivalve mollusc, Scrobicularia plana. Fish Shellfish Immunol. 2003;15:249–262. doi: 10.1016/s1050-4648(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 117.Wootton EC, Dyrynda EA, Ratcliffe NA. Bivalve immunity: comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua) Fish Shellfish Immunol. 2003;15:195–210. doi: 10.1016/s1050-4648(02)00161-4. [DOI] [PubMed] [Google Scholar]
- 118.Cajaraville MP, Pal SG. Morphofunctional study of the hemocytes of the bivalve mollusk Mytilus galloprovincialis with emphasis on the endolysosomal compartment. Cell Struct Funct. 1995;20:355–367. doi: 10.1247/csf.20.355. [DOI] [PubMed] [Google Scholar]
- 119.Legall G, Chagot D, Mialhe E, Grizel H. Branchial Rickettsiales-like infection associated with a mass mortality of sea scallop Pecten maximus. Dis Aquat Organ. 1988;4:229–232. [Google Scholar]
- 120.Anderson RS, Oliver LM, Brubacher LL. Superoxide anion generation by Crassostrea virginica hemocytes as measured by nitroblue tetrazolium reduction. J Invertebr Pathol. 1992;59:303–307. [Google Scholar]
- 121.Pipe RK. Generation of reactive oxygen metabolites by the hemocytes of the mussel Mytilus edulis. Dev Comp Immunol. 1992;16:111–122. doi: 10.1016/0145-305x(92)90012-2. [DOI] [PubMed] [Google Scholar]
- 122.Arumugan M, Romestand B, Torreilles J. Nitrite released in haemocytes from Mytilus galloprovincialis Crassostrea gigas and Ruditapes decussatus upon stimulation with phorbol myristate acetate. Aquat Living Resour. 2000;13:173–177. [Google Scholar]
- 123.Franchini A, Conte A, Ottaviani E. Nitric oxide: an ancestral immunocyte effector molecule. Adv Neuroimmunol. 1995;5:463–478. doi: 10.1016/0960-5428(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 124.Franchini A, Fontanili P, Ottaviani E. Invertebrate immunocytes: relationship between phagocytosis and nitric oxide production. Comp Biochem Physiol B Biochem Mol Biol. 1995;110B:403–407. [Google Scholar]
- 125.Tafalla C, Gomez-Leon J, Novoa B, Figueras A. Nitric oxide production by carpet shell clam (Ruditapes decussatus) hemocytes. Dev Comp Immunol. 2003;27:197–205. doi: 10.1016/s0145-305x(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 126.Yoshino TP, Bickham U, Bayne CJ. Molluscan cells in culture: primary cell cultures and cell lines. Can J Zool. 2013;91:391–404. doi: 10.1139/cjz-2012-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoshino TP, Wu XJ, Gonzalez LA, Hokke CH. Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp Parasitol. 2013;133:28–36. doi: 10.1016/j.exppara.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Johnson LA, Yoshino TP. Larval Schistosoma mansoni excretory-secretory glycoproteins (ESPs) bind to hemocytes of Biomphalaria glabrata (Gastropoda) via surface carbohydrate binding receptors. J Parasitol. 2001;87:786–793. doi: 10.1645/0022-3395(2001)087[0786:LSMESG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 129.Goodall CP, Bender RC, Broderick EJ, Bayne CJ. Constitutive differences in Cu/Zn superoxide dismutase mRNA levels and activity in hemocytes of Biomphalaria glabrata (Mollusca) that are either susceptible or resistant to Schistosoma mansoni (Trematoda) Mol Biochem Parasitol. 2004;137:321–328. doi: 10.1016/j.molbiopara.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 130.Bender RC, Goodall CP, Blouin MS, Bayne CJ. Variation in expression of Biomphalaria glabrata SOD1: A potential controlling factor in susceptibility/resistance toSchistosoma mansoni. Dev Comp Immunol. 2007;31:874–878. doi: 10.1016/j.dci.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 131.Yoshino TP, Granath WO. Surface antigens of Biomphalaria glabrata (Gastropoda) hemocytes: functional heterogeneity in cell subpopulations recognized by a monoclonal antibody. J. Invert Pathol. 1985;45:174–186. doi: 10.1016/0022-2011(85)90007-2. [DOI] [PubMed] [Google Scholar]
- 132.Martins-Souza RL, Pereira CAJ, Martins-Filho OA, Coelho PMZ, Correa A, Negrao-Correa D. Differential lectin labelling of circulating hemocytes from Biomphalaria glabrata and Biomphalaria tenagophila resistant or susceptible to Schistosoma mansoni infection. Mem Inst Oswaldo Cruz. 2006;101:185–192. doi: 10.1590/s0074-02762006000900029. [DOI] [PubMed] [Google Scholar]