Abstract
Ammonium tetrathiomolybdate (TTM) was found to be a slow hydrogen sulfide (H2S) releasing agent. Its H2S generation capability in aqueous solutions was confirmed by UV-vis and fluorescence assays. TTM also showed H2S-like cytoprotective effects in hydrogen peroxide (H2O2)-induced oxidative damage in HaCaT cells.
Keywords: Ammonium Tetrathiomolybdate, Hydrogen Sulfide, Donor, Oxidative damage
Graphical Abstract
Hydrogen sulfide (H2S) is newly recognized as a nitric oxide (NO)-like gaseous transmitter that plays regulatory roles in many physiological and pathological processes.1–5 Endogenous production of H2S involves both enzymatic pathways (mediated by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST)) and non-enzymatic pathways (from the sulfane sulfur pools). Current knowledge strongly suggests that modulation of H2S could have potential therapeutic values for certain disease states, including vasodilation, anti-inflammation, anti-oxidation, and down regulation of cellular metabolism under stress.1–5 Because of this, the search for compounds that can release H2S and mimic the beneficiary activities of H2S has become an attractive area in medicinal chemistry.6–10 So far, a number of H2S releasing compounds (also known as H2S donors), such as GYY4137, dithiothiones, N-mercapto-based donors, persulfide-based donors, gem-dithiol-based donors, etc., have been reported (Figure 1).6–10 These compounds are normally small organic molecules which can be triggered by certain biologically relevant or compatible reactions to release H2S. Some of them have shown promising bioactivities.6–10 On the other hand, inorganic molecule-based donors, especially metal complexes, which can slowly hydrolyze to release H2S have not been reported. Inspired by the fact that sodium nitroprusside (SNP), with the formula Na2[Fe(CN)5NO], is a widely used NO donor,11–13 we suspected that certain sulfide-containing inorganic compounds might be able to serve as interesting H2S donors in aqueous solutions. Like SNP, the release of H2S from inorganic molecule-based donors is likely to be the result of simple hydrolysis. As such, the donors would be suitable for many biological studies. Herein we wish to report the discovery of ammonium tetrathiomolybdate (TTM) as an effective inorganic H2S donor.
Figure 1.
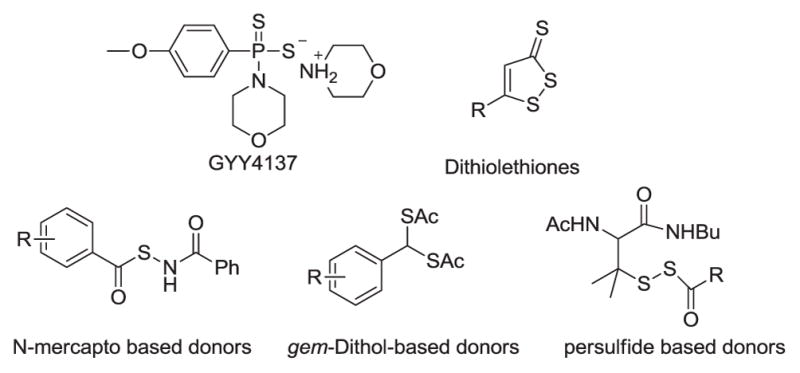
Representative H2S donors
TTM, with the formula (NH4)2MoS4, is a commonly used building block in the chemistry of molybdenum.14,15 As an excellent copper chelator, TTM has been used therapeutically in the treatment of copper toxicosis, especially for Wilson’s disease.16 It was previously noted that under strong acidic condition (5% H2SO4) H2S could be generated from TTM.17 We first wondered if TTM could produce H2S under mild and biologically friendly conditions, especially under physiological pH. To this end, we measured H2S release from TTM in four different pH buffers (5, 6, 7.4, and 8). Normally the release of H2S from the donors can be determined by the standard methylene blue (MB) method.18 However, a strong acidic condition was involved in this method. As it is known that acidic media would facilitate TTM hydrolysis, we envisioned that the standard MB method was not appropriate. In this study, a zinc-sulfide precipitation based MB method was used.19 This method should avoid the false positive signals caused by acid-promoted TTM hydrolysis. Briefly, the solutions of 500 μM TTM were freshly prepared in phosphate buffers under different pH. At different time intervals, aliquots were taken to Eppendorf vials containing a mixture of zinc acetate and NaOH solution. After 15 minutes, solid ZnS was formed and collected by centrifugation. ZnS was then treated by a MB cocktail. The resulted H2S concentrations were obtained by UV-vis measurements and calculated based on a calibration curve. As shown in Figure 2, we observed immediate H2S formation in all of these TTM solutions. The level of H2S in pH 5 was significantly higher than the levels in other pH values. In all solutions we found H2S concentrations were maintained in a stable level for a long time (up to 15 hours). These results indicate TTM is a stable and slow release H2S donor.
Figure 2.
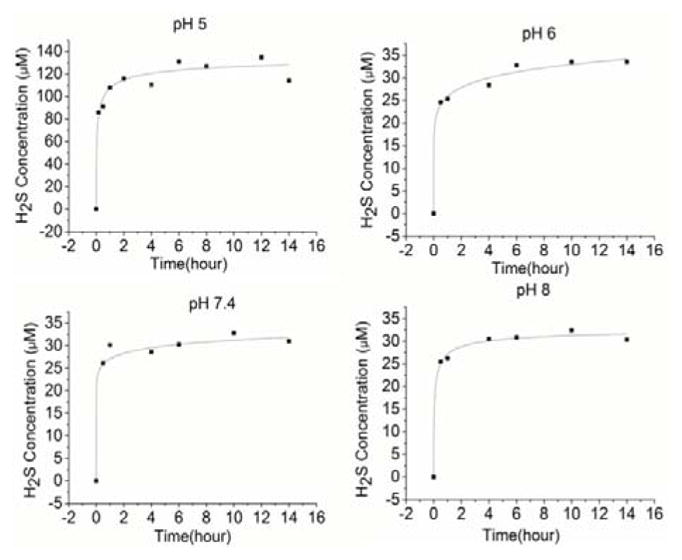
H2S release from TTM under different pHs
To further confirm the production of H2S from TTM in buffers, a H2S gas trapping experiment was designed. As shown in Figure 3, a solution of TTM was placed in a sealed glass vial. An Eppendorf vial containing a solution of WSP-5, a H2S specific fluorescent probe,20 was also placed in the vial to trap the evaporated H2S from the TTM solution. After incubation at 37 °C for 3 hours, the trapping solution was diluted and the fluorescence intensity (excitation at 502 nm, emission at 525 nm) was measured. Both the positive and negative controls (using the standard Na2S solution or pure buffer) were also carried out using the same procedure for comparison. As shown in Figure 4, TTM and Na2S led to significant fluorescence increases in the trapping solutions while the pure buffer did not cause any detectable fluorescence increase. The pH dependence of H2S release from TTM was also noted as higher fluorescence was observed in pH 5 than in other pHs. These results further demonstrated H2S generation from TTM in buffers.
Figure 3.

H2S gas trapping set-up
Figure 4.
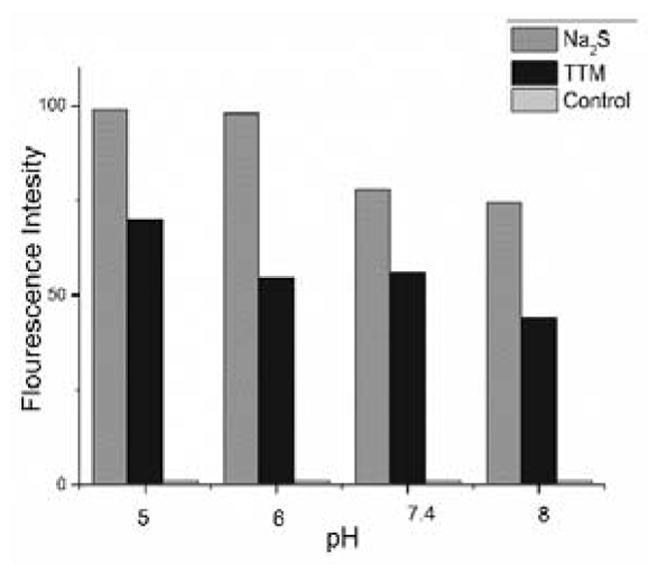
H2S release of TTM detected by indirect fluorescent assay
One of the most well-studied biological functions of H2S is its cytoprotective effects under oxidative stress. Being a H2S donor, TTM was expected to have similar effects. We then applied TTM in a cellular model of oxidative damage to study its cytoprotective ability, using protocols established in our previous works.21,22 As shown in Figure 5-A, hydrogen peroxide (H2O2) alone caused the decrease of cell (HaCaT) viability due to oxidative damage. When the cells were pre-treated with TTM (50, 100, 200 μM), a dose-dependent protective effect was observed. At a higher concentration (400 μM), TTM had no protection, presumably because of the slight toxicity of TTM under high concentration. In addition, lactate dehydrogenase (LDH) release assay was also carried out to validate TTM’s cytoprotective effects. As shown in Figure 5-B, the exposure of cells to H2O2 (400 μM) remarkably enhanced LDH release. However, LDH release was significantly reduced when cells were pretreated with TTM (200 μM).
Figure 5.
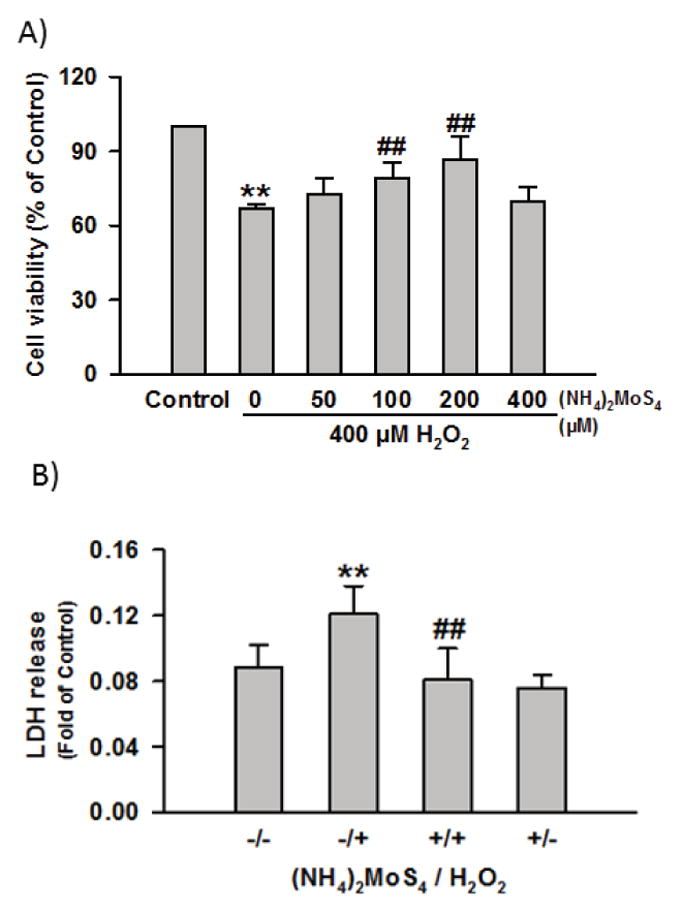
Effects of TTM on H2O2-induced cellular damage. a) Cell viability assay under the treatment of 400 μM H2O2 for 5 h in the absence or presence of various concentrations of TTM for 1h. b) LDH release assay of cells treated with 400 μM H2O2 in the absence or presence of 200 μM TTM. Data were shown as the mean ± SE. **P<0.01 vs Control group, ##P<0.01 vs H2O2 alone group.
Mitochondria membrane potential (MMP) usually reflects whether mitochondria are healthy, which further indicates if cells are suffering from noxious damage. Rhodamine 123 (Rh123) staining followed by fluorescence photography can be used to observe MMP. Figure 6-A shows that under normal conditions the cells had bright green fluorescence. When cells were treated with H2O2 (400 μM), a dramatic MMP loss was noted (Figure 6-B), evidenced by decreased fluorescence, suggesting that H2O2 damaged cells. However, preconditioning with 200 μM TTM greatly impeded MMP loss by preserving mitochondrial functions (Figure 6-C). TTM alone did not significantly alter MMP (Figure 6-D). These results confirmed that TTM could exhibit H2S-like cellular protection against oxidative injury in cells.
Figure 6.
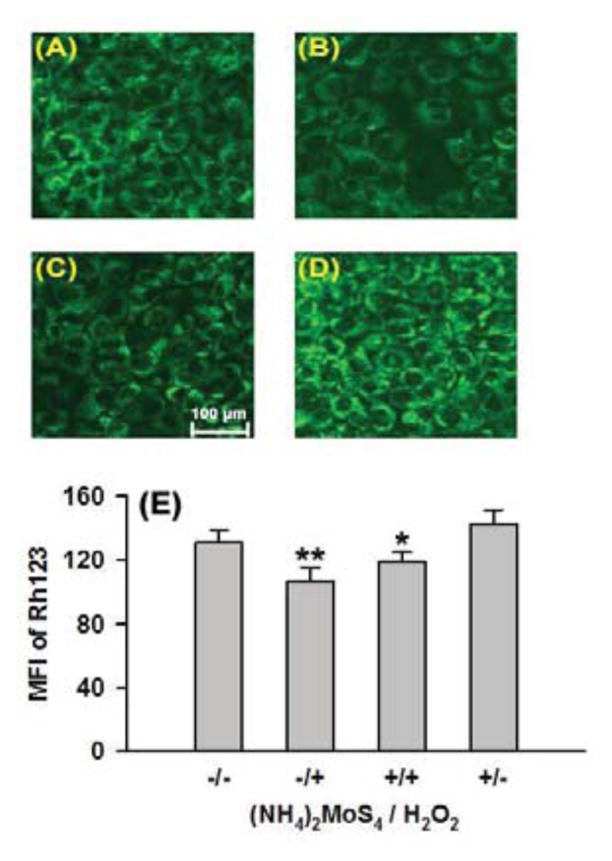
Effects of TTM on H2O2-induced MMP loss in HaCaT cells. Rh123 staining followed by photofluorography for the observation of MMP. (A) Control cells, (B) Cells treated with 400 μM H2O2 for 5h, (C) Cells treated with 400 μM H2O2 combined with pretreatment with 200 μM TTM for 1 h. (D) Cells treated with TTM alone. (E) Quantitative analysis of the mean fluorescence intensity (MFI) of Rh123 in group a–d using Image J software. Data were shown as the mean ± SE. **P<0.01 vs Control group, *P<0.05 vs H2O2 alone group.
In conclusion, our results demonstrate that ammonium tetrathiomolybdate (TTM) is an effective H2S donor in aqueous buffers. TTM’s release of H2S is pH-dependent. Under neutral pH, the release is a slow but sustained process. TTM can exhibit H2S-like cytoprotection against oxidative damage. These results should promote researchers to re-think the biological activities of TTM and apply it to other studies.
Supplementary Material
Acknowledgments
This work was supported by the NIH (R01HL116571) and Natural Science Foundation of Guangdong Province in China (No. 2015A030313458).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li L, Moore PK. Annu Rev Pharmacol Toxicol. 2011;51:169. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 2.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Chem Res Toxicol. 2012;25:769. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandiver MS, Snyder SH. J Mol Med. 2012;90:255. doi: 10.1007/s00109-012-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R. Physiol Rev. 2012;92:791. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 5.Kolluru GK, Shen X, Bir SC, Kevil CG. Nitric Oxide. 2013;35:5. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Pacheco A, Xian M. Medicinal chemistry: insights into the development of novel H2S donors. Part VIII. In: Moore PK, Whiteman M, editors. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide, Handbook of Experimental Pharmacology. Vol. 230. 2015. pp. 365–388. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Biggs TD, Xian M. Chem Commun. 2014;50:11788. doi: 10.1039/c4cc00968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace JL. Trends Pharmacol Sci. 2007;28:501. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Kashfi K. Antioxid Redox Signal. 2014;20:831. doi: 10.1089/ars.2013.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace JL, Wang R. Nat Rev Drug Discov. 2015;14:329. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 11.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ. Chem Rev. 2002;102:1091. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 12.Butler AR, Megson IL. Chem Rev. 2002;102:1155. doi: 10.1021/cr000076d. [DOI] [PubMed] [Google Scholar]
- 13.Hottinger DG, Beebe DS, Kozhimannil T, Prielipp RC, Belani KG. J Anaesth Clin Pharmacol. 2014;30:462. doi: 10.4103/0970-9185.142799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunasundari T, Chandrasekaran S. J Org Chem. 2010;75:6685. doi: 10.1021/jo1010125. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu KR, Devan N, Chandrasekaran S. Synlett. 2002;11:1762. [Google Scholar]
- 16.Brewer GJ. Matallomics. 2009;1:199. doi: 10.1039/b901614g. [DOI] [PubMed] [Google Scholar]
- 17.Wang HW, Skeldon P, Thompson GE, Wood GC. J Mater Sci. 1997;32:497. [Google Scholar]
- 18.Abdalla MA, Fogg AG, Burgess C. Analyst. 1982;107:213. doi: 10.1039/an9830800053. [DOI] [PubMed] [Google Scholar]
- 19.Ang AD, Konigstorfer A, Giles GI, Bhatia M. Adv Biol Chem. 2012;2:360. [Google Scholar]
- 20.Peng B, Chen W, Liu C, Rosser EW, Pacheco A, Zhao Y, Aguilar HC, Xian M. Chem Eur J. 2014;20:1010. doi: 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Yang C, Organ C, Li Z, Bhushan S, Otsuka H, Pacheco A, Kang J, Aguilar HC, Lefer DJ, Xian M. J Med Chem. 2015;58:7501. doi: 10.1021/acs.jmedchem.5b01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CM, Zhao Y, Zhu Z, Pacheco A, Peng B, Devarie-Baez N, Bagdon P, Zhang H, Xian M. Mol Bio Syst. 2013;9:2430. doi: 10.1039/c3mb70145j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


