Abstract
Bypassing inhibitors in hemophilia patients is limited to activated (a) Factor(F)VII products. We introduced “FVa activity augmentation” as another bypassing strategy and studied effects of an engineered FVa variant designated superFVa. Procoagulant and clot stabilizing properties of superFVa and recombinant human (rh)FVIa, either alone or in combination, were studied in thrombin generation and clot lysis assays in normal human plasma (NHP) with or without anti-FVIII inhibitors, in hemophilia plasma, and in FVIII-deficient mice or in wild-type mice with anti-FVIII inhibitors. superFVa was as effective as rhFVIIa to improve thrombin generation or clot lysis. Furthermore, procoagulant effects were significantly enhanced when these compounds were combined. RhFVIIa at 40 nM (a therapeutic concentration) improved thrombin generation mildly, but markedly improved thrombin generation when combined with a low concentration (e.g., 3 nM) of superFVa. In clot lysis studies, the concentration of rhFVIIa to normalize clot lysis times could be reduced by 100-fold (e.g., from 40 nM to 0.4 nM) when combined with a low concentration (0.37 nM) of superFVa. In hemostasis studies of FVIII-deficient mice, blood loss was dose-dependently reduced by either superFVa or rhFVIIa. SuperFVa (200 U/kg) corrected mean blood loss indistinguishably from rhFVIII. Blood loss correction by rhFVIIa was greatly improved when combined with superFVa. Similar blood loss correction results were observed for therapies in wild-type mice after infusion with anti-FVIII inhibitors. Thus, superFVa may be an effective procoagulant agent in the setting of hemophilia with inhibitors and it merits further evaluation for new bypassing strategies.
Keywords: Hemophilia, Factor VIII, Factor V, Bleeding, Hemostasis, Inhibitors
Introduction
Hemophilia is an X-linked bleeding disorder caused by either deficiency of Factor (F)VIII (Hemophilia A) or FIX (Hemophilia B). Regular prophylactic treatment with clotting factor concentrates is recommended to prevent severe bleeding episodes in patients with severe hemophilia, and is usually started in early childhood (1). Unfortunately, approximately 20–30% of patients with Hemophilia A and approximately 5% of patients with Hemophilia B develop neutralizing inhibitory antibodies (“inhibitors”) against exogenously administered FVIII or FIX (2). The development of inhibitors is the most devastating complication of treatment with clotting factor concentrates since it leaves patients unresponsive to FVIII- or FIX-treatment. There is no easy way to eradicate inhibitors. Treatment with Rituximab (Rituxan®, Genentech; South Francisco, USA) has shown variable success (3), and immune tolerance induction (ITI) with high doses of clotting factor, with or without concomitant immune modulating agents (4) can take up to 2 years with a treatment failure rate of approximately 30% (5). During this time and life-long thereafter, if ITI was not successful, patients remain vulnerable to fatal bleeding, and are at high risk of developing debilitating arthropathy with poor quality of life (6). While hemophilia patients usually died as infants or in young adulthood last century, they are now aging with life spans comparable to the general population (7). This presents an urgent need for improved or new strategies to decrease uncontrolled bleeding and maintain functional joints in patients with inhibitors.
Currently, activated (a) FVII-based clotting factor preparations, either recombinant human (rh) FVIIa (NovoSeven®, Novo Nordisk, Bagsvaerd, Denmark) or a plasma-derived product (FEIBA®, Baxter Biosciences, Westlake Village, USA), are the only available bypassing options for patients with inhibitors. Unfortunately, treatment with FVIIa-based agents remains suboptimal and less effective compared to FVIII-based or FIX-based clotting factor concentrates in patients without inhibitors (6, 8, 9). One reason is the missing amplification of thrombin generation when either FVIII or FIX is absent. However, the thrombin generation deficit not only impairs clot formation but also clot stabilization because of reduced activation of Thrombin-Activatable Fibrinolysis (TAFI) Inhibitor, an important inhibitor of fibrinolysis (10–12). Since impaired inhibition of fibrinolysis contributes to bleeding in hemophilia (10–12), and since rhFVIIa has not been uniformly effective to promote the activation of anti-fibrinolytic mechanisms (12, 13), the suboptimal efficacy of rhFVIIa may in part also be explained by suboptimal clot stabilization. Therefore, potential effects on clot stabilization are an important consideration when developing new bypassing strategies.
We recently proposed “FVa activity augmentation” as a new concept to bypass inhibitors. The concept was based on several previous observations implying that the prothrombotic FVLeiden mutation changed phenotypic bleeding in hemophilia patients and mice (14, 15), and that rhFVLeiden and rhFVCambridge, which are partially resistant against inactivation by activated protein C (APC), improved thrombin generation in hemophilia plasma (16, 17). This is because FVa is required as an important cofactor in the prothrombinase complex, where it enhances the rate of thrombin generation approximately 10,000-fold (18). However, FVa is also rapidly inactivated by APC, which inspired our hypothesis that strategies to augment FVa activity may enhance hemostasis in hemophilia. Towards that end we engineered several APC-inactivation resistant FVa variants and studied them for their degree of APC-resistance and their hemostatic properties in hemophilic plasma (19). We identified one lead candidate, denoted superFVa, that demonstrated near complete APC inactivation resistance and superior ability to enhance in vitro hemostasis when compared to the other FVa variants (19). In subsequent studies superFVa was not only able to control bleeding in a mouse model of Hemophilia A (19), but was also able to control bleeding and rescue survival in a mouse trauma model where bleeding was enhanced by exogenous APC (20). The inactivation resistance of superFVa is due to mutations of all three APC cleavage sites at Arg506, Arg306 and Arg679. In addition, an engineered interdomain disulfide bond (involving His609Cys-Glu1691Cys) connecting the A2 and A3 domains (A2-SS-A3) (21) appears to confer high specific activity presumably by preventing domain dissociation as the final step of FVa inactivation (19). SuperFV is being used in activated form since B domain-deleted FV has already some inherent cofactor activity, and because it was previously reported that clot formation with human plasma FV in FVIII-deficient mice required prior activation of the FV (14).
Here we investigated the effectiveness of superFVa and its combinatorial effects with rhFVIIa in the setting of hemophilia complicated by inhibitors. Studies included in vitro and in vivo analyses of superFVa’s effects on hemostasis in patient samples before and after treatment with rhFVIIa, and effects on tail bleeding in hemophilia mice and wild-type mice with inhibitors. We also studied the effects on improvement of TAFI-dependent fibrinolysis inhibition, given its pathophysiologic relevance in hemophilic bleeding. In summary, these studies demonstrate that superFVa is an attractive candidate for development as an effective bypassing agent, either alone or in combination with another agent such as rhFVIIa.
Materials and Methods
Materials
The following commercial reagents were used: thrombin (Enzyme Research Laboratories, South Bend, IN, USA), hirudin (Calbiochem, San Diego, CA, USA), corn trypsin inhibitor (CTI) (Enzyme Research Laboratories, South Bend, IN, USA) thrombin calibrator (Synapse BV, Maastricht The Netherlands), Substrate Z-Gly-Gly-Arg-AMC (Bachem, Bubendorf, Switzerland), recombinant human tissue factor (TF), Dade Innovin®, (Dade Behring, Newark, DE, USA), mouse anti-human FVIII (GMA8015) and isotype antibody (GMA650) (Green Mountain Antibodies, Burlington, VT, USA), rhFVIIa, NovoSeven® (Novo Nordisk, Bagsvaerd, Denmark), recombinant tissue plasminogen activator, Activase® (Genentech, San Francisco, CA, USA), FVIII, Kogenate® (Bayer Healthcare, Leverkusen, Germany), and carboxypeptidase inhibitor from potato tuber (CPI) (Sigma-Aldrich, USA). Pooled normal Human Plasma (NHP) and plasma from FVIII-deficient patients (FVIIIdP) with and without inhibitors (titer specified in Bethesda Units (BU)), were purchased from George King Bio-Medical (Overland Park, KS, USA). Phosphatidylcholine (PC), phosphatidylserine (PS) and phosphatidylethanolamine (PE) were purchased from Avanti Lipids (Alabaster, AL,USA) and phospholipid vesicles containing 80% PC and 20% PS or 60% PC, 20% PS, and 20% PE were prepared as described (22).
Clinical plasma samples
Plasma and clinical information was obtained from patients with severe FVIII-deficiency (<1% intrinsic FVIII-activity levels) and inhibitors (titers determined in BU by the clinical laboratory using the Nijmegen assay (23)), who are seen regularly at the Hemophilia Treatment Center at the University of California, San Diego (UCSD). Patient confidentiality safeguards and data acquisition methods were approved by the Human Research Protection Programs at UCSD. Patients provided written informed consent. Patient blood samples were drawn into 3.8% sodium citrate (9:1 v/v) and processed immediately. Plasma was collected by centrifugation at 2600 × g for 15 minutes and frozen at −80°C.
Protein preparation
Recombinant superFV (Molecular Weight (MW) 174 kDa) was made on a B-domain deleted S2183A FV background and purified from conditioned media of stable transfected BHK cells by a combination of affinity chromatography using anti-FV 3B1 and HV5101 monoclonal antibodies as described (21). SuperFV protein concentration was determined by absorbance at 280 nm (FV ε1% = 16.9) and ELISA (Enzyme Research Laboratories, South Bend, IN, USA). SuperFV was activated with 2 nM thrombin for 20 minutes at 37°C in prothrombinase buffer (50 mM HEPES, 150 mM NaCl, 0.5% BSA, 5 mM CaCl2 and 0.1 mM MnCl2). Activation was terminated by the addition of 1.1 molar equivalent of hirudin.
Recombinant mouse factor VII (mFVII) was cloned from mouse liver marathon-ready cDNA (Clonetech) using primers 5’-CCAAAGCTTCATCATGGTTCCACAGG-3’ and 5’-GGTCTAGACAAGGAGCTACAGTAGTGG-3’, ligated in pCR2.1-TOPO, and transferred to pcDNA3.1(+)-neo by restriction digest with HindIII and XbaI. The sequence of the mFVII-pcDNA3.1 construct was identical to the reference sequence of mFVII (NM_010172.2). Stable mFVII expressing HEK293 cells were generated and grown in DMEM/F12 with 10% fetal bovine serum and 10 µg/ml vitamin K1 (Sigma, St Louis, MO). Purified mFVII was obtained by passing conditioned serum-free media collected in 5 mM benzamidine and 8 mM EDTA over a fast flow Q Sepharose (Sigma) equilibrated in 20 mM Tris, 100 mM NaCl, pH 7.4. All buffers were prepared in HyPure cell culture grade water (Hyclone, Logan UT). After washing with the same buffer, mFVII was eluted in 20 mM Tris, 50 mM CaCl2, pH 7.4. Pooled fractions were brought to 60 mM EDTA, diluted with three volumes of H2O, and loaded on a HiTrap Q HP column (GE Lifesciences) equilibrated in 20 mM Tris, pH 7.4, washed with the same buffer and eluted with a gradient from 0 to 1M NaCl. Purified mFVII was dialyzed into HBS (50 mM Hepes, 150 mM NaCl, 5 mM CaCl2 pH 7.4) before activation with 1/100 (w/w) human FXa (Enzyme Research Laboratories) for 8 hours at room temperature. FXa was removed by two 30 min incubations with the FXa removal resin (Qiagen, Valencia, CA) equilibrated in HBS. Concentrations of mFVII and mFVIIa were determined by absorbance at 280 nm using ε1%, 1 cm = 13.9 and MW = 50 kDa (24).
Bethesda Unit titer determination
BU titers for FVIII antibodies were determined using the modified Nijmegen Bethesda assay (25). Briefly, antibody was serially diluted in HBS with 0.05 % Tween 80 (HBST) and mixed 1:1 with NHP supplemented with 100 mM Imidazole (pH 7.0). After incubation for 2 hours at 37 °C samples were assayed by an activated partial prothrombin time (APTT)) using APTT XL reagent (Thermo Scientific, Waltham, MA, USA). Percent FVIII activity was derived from an APTT standard curve (serial dilution of NHP into FVIIIdP with NHP defined as 100%) and plotted against the antibody concentration with 50% FVIII activity defined as 1 BU/ml (Supplemental Figure 1).
The Nijmegen Bethesda assay was used for calculation of the BU titer of FVIII antibodies (GMA-8015) injected in mice (26). Briefly, mouse plasma with antibody was incubated at 58°C for 10 minutes followed by centrifugation at 20,000g for 10 min. Serial dilutions of the supernatant in human FVIIIdP were mixed 1:1 with NHP supplemented with 100 mM Imidazole (pH 7.0) and incubated at 37 °C for 2 hours. Residual FVIII activity was determined by APTT and the BU titer was calculated as described above.
Thrombin generation assays
Thrombin generation assays were performed at 37 °C as described (19, 27). The final reaction mixture contained, 50% plasma (v/v), 0.2 pM TF, 4 µM PC:PS vesicles (80:20), 0.725 µM CTI, 0.5 mM Z-Gly-Gly-Arg-AMC and 10 mM CaCl2 in HBS/0.1% BSA in a total volume of 100 µL. In some experiments plasma was pre-incubated for 1 hour with anti-FVIII antibody (BU = 32,000/mg). Fluorescence was measured at excitation/emission wavelengths of 360/460 nM using the Gemini EM fluorescent plate reader (Molecular Devices Corporation, Menlo Park, CA, USA). The endogenous thrombin potential (ETP), defined as the area under the curve (AUC) and the thrombin peak height were determined as described (28).
Clot lysis assay
Plasma clot lysis was measured by the change in turbidity of a tissue factor-induced clot and tPA-mediated fibrinolysis as described (29, 30). The clot lysis time (CLT) was defined as the time difference between maximal coagulation and maximal fibrinolysis. The final reaction mixture contained 50% plasma (v/v), 0.1 pM TF, 10 µM PC/PS/PE vesicles (40:20:40), 17 mM CaCl2, and 0.06 µg/ml r-tPA in HBS/0.1% BSA in a total volume of 150 µL. After thorough mixing, 100 µl was transferred to a 96-well micro titer plate and the change in turbidity at 37°C was measured in time at 405 nm in a Thermomax microplate reader (Molecular Devices Corporation, Menlo Park, CA, USA). CPI (10 Tg/ml), anti-FVIII antibody (10 µg/ml; 1 hour preincubation), rhFVIII (10 µg/ml; Kogenate®, Bayer Healthcare, Leverkusen, Germany), rhFVIIa and superFVa were added to NHP or FVIIIdP as described below.
Animals
All described animal protocols were carried out as approved by the institutional animal and care committee of The Scripps Research Institute. Several breeding pairs of FVIII-deficient mice (BalbC background) were a generous gift of Dr. David Lillicrap. FVIII-deficient mice of both genders and female BALB/c mice, aged ≥ 8 weeks, were used for tail bleeding assays.
In vivo dosing of FVa variants
In vivo superFVa dosing was based on units/kg, determined as prothrombinase cofactor activity, whereby the activity of 20 nM wild-type FVa (FV plasma concentration) was defined as one Unit (19). Dosing by activity is the usual method for clinical administration of clotting factors (such as FVIII), thereby correcting for variations of specific activity in biological material. For these experiments 10, 40 or 200 U/kg corresponded to 0.035, 0.14, or 0.7 mg/kg superFVa, respectively.
Tail bleeding assays
Tail bleeding assays were performed as described (19). Mice were placed on temperature controlled heating pads (37°C) and anesthetized by isoflurane inhalation. The distal portion of the tail was cut at 1.5 mm diameter after which the tail was immersed in a predefined volume of 37°C saline (0.9% NaCl) for 20 minutes. Blood loss was determined after red cell lysis with 2% acetic acid by measuring the absorbance of hemoglobin at 490 nm and using a hemoglobin standard curve derived from defined blood volumes. Blood loss was expressed in µL/g body weight assuming a hematocrit of 46%. FVIII-deficient mice were injected retro-orbitally 5 minutes prior to tail cut with vehicle, superFVa, rhFVIIa, mFVIIa, or rhFVIII (200 units/kg; Xyntha®, Pfizer, NY, NY, USA) in an equal volume (200 µL) of sterile saline for injection (0.9%, Hospira Inc.).
For FVIII inhibitor mice, BalbC mice were injected with the GMA-8015 anti-FVIII antibody (100 µL, 0.25 mg/kg in sterile saline) retro-orbitally 2 hours before tail clip. Prohemostatic agents were injected retro-orbitally in the other eye 5 minutes before tail clip in an equal volume (100 µL) of sterile saline.
Statistical analysis
Student’s t-test, ANOVA with multiple comparison correction, or for bleeding Kruskal-Wallis followed by one-tailed Mann-Whitney test were used to assess statistical significance where appropriate. A p-value of ≤ 0.05 was considered statistically significant.
Results
Effects of superFVa and rhFVIIa on thrombin generation in FVIIIdP
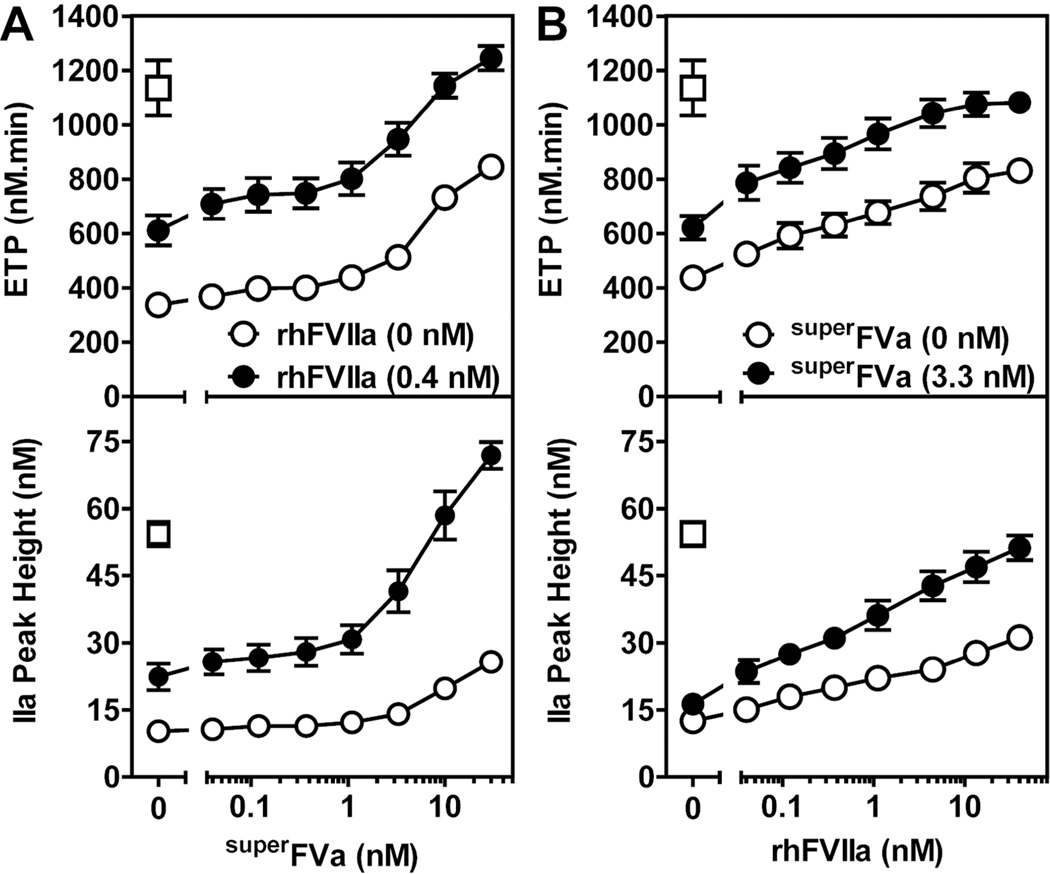
To study the manifestation of procoagulant effects of superFVa and FVIIa when both proteins are present at the same time, thrombin generation was determined in FVIIIdP at increasing concentrations of superFVa in the presence or absence of rhFVIIa (Figure 1A). Thrombin generation was enhanced dose-dependently by superFVa, and ETP and thrombin peak height were increased ~2.5-fold at 30 nM superFVa. The FVIIa therapeutic plasma concentration after intravenous administration of rhFVIIa at 90 µg/kg body weight was estimated to be 40 nM (2 µg/ml) based on a plasma-to-weight ratio of 45 ml/kg. Addition of rhFVIIa at a concentration of 0.4 nM (~100-fold below the FVIIa therapeutic plasma concentration), enhanced ETP and thrombin peak height also by ~ 2-fold. Subsequent titration of superFVa in the presence of rhFVIIa (0.4 nM) resulted in a left shift of the curves for ETP and thrombin peak height (Figure 1A and Supplemental Figure 1A/B). Similar interactions between superFVa and rhFVIIa were observed in the presence of increasing concentrations of rhFVIIa and a constant concentration of superFVa (Figure 1B). ETP and thrombin peak height were increased more efficiently by rhFVIIa when superFVa (3.3 nM) was present. In fact, superFVa reduced by 2-orders of magnitude the concentration of rhFVIIa required to achieve procoagulant effects similar to 40 nM rhFVIIa alone in FVIIIdP (Figure 1B). Similarly, rhFVIIa (0.4 nM) reduced by 1-order of magnitude the concentration of superFVa required to achieve procoagulant effects similar to 30 nM superFVa alone (Figure 1A). Thus, superFVa and rhFVIIa cooperate to restore thrombin generation in FVIIIdP.
Figure 1. Cooperative effects of rhFVIIa and superFVa on thrombin generation in FVIII-deficient plasma.
Thrombin generation was determined as ETP (top panel) and thrombin peak height (bottom panel) in FVIIIdP supplemented with (A) either increasing concentrations of superFVa in the absence (open circles) or presence (solid circles) of rhFVIIa (0.4 nM), or (B) increasing concentrations of rhFVIIa in the absence (open circles) or presence (solid circles) of superFVa (3.3 nM). Normalization of thrombin generation is indicated by the reconstitution of FVIIIdP with 2 U/ml FVIII (open squares). ETP denotes Endogenous Thrombin Potential; FVIIIdP, FVIII-deficient plasma. Error bars indicate SEM (n≥5).
Effects of superFVa and rhFVIIa on thrombin generation in NHP with FVIII-inhibiting antibodies
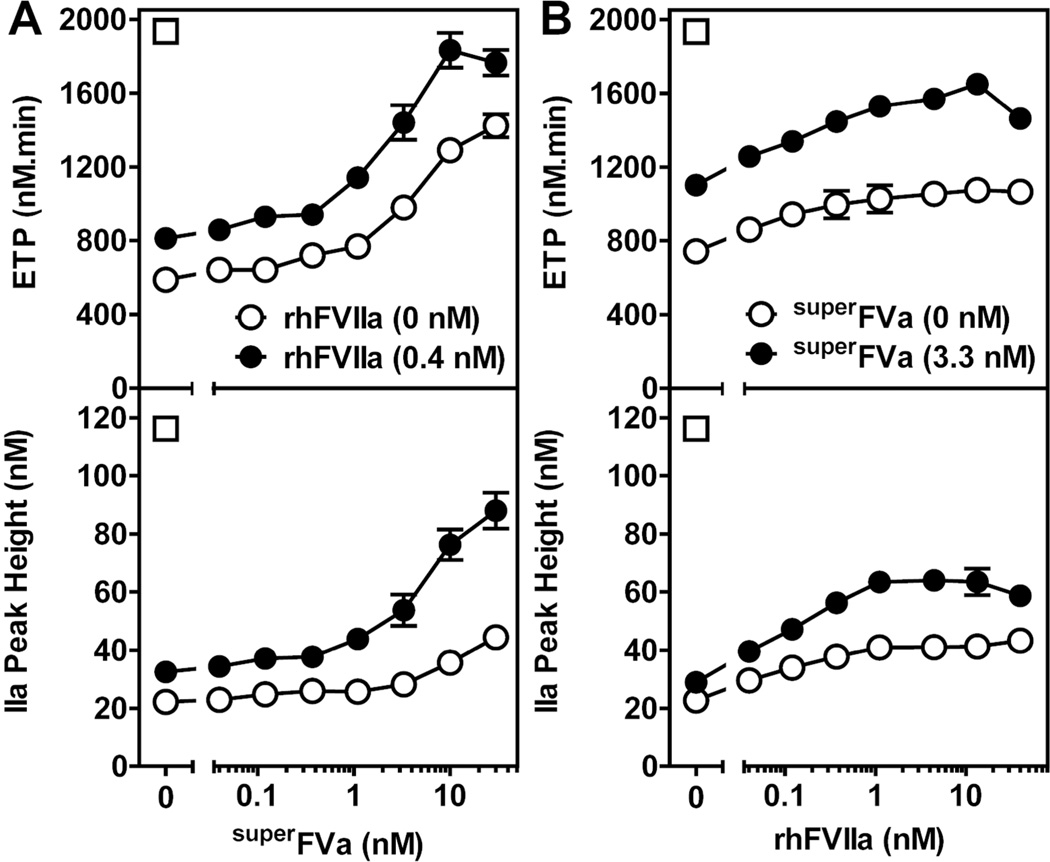
To provide additional evidence concerning the procoagulant effects of superFVa and rhFVIIa, an inhibitory anti-FVIII antibody (32,000 BU/mg) was added to NHP in order to mimic acquired Hemophilia A (Supplemental Figure 2A). Titrations of the anti-FVIII antibody (GMA8015) in NHP indicated that thrombin generation was effectively suppressed at 1.25 µg/mL antibody (40 BU) whereas an isotype control antibody did not interfere with thrombin generation (Supplemental Figure 2B & 2C). Both, rhFVIIa and superFVa were able to improve thrombin generation in NHP with the inhibitory anti-FVIII antibody. At 30 nM superFVa, ETP and thrombin peak height were increased ~2-fold (Figure 2A). Similar effects were observed with rhFVIIa, although effects of rhFVIIa on ETP and thrombin peak height seemed to plateau at ~4 nM rhFVIIa (Figure 2B). The effects of superFVa on ETP and thrombin peak height were markedly enhanced by relatively small amounts of rhFVIIa (0.4 nM), which is ~100-fold below the usual therapeutic plasma concentration of rhFVIIa (Figure 2A). Of note, ETP enhancement by superFVa in the presence of rhFVIIa plateaued at ~13 nM superFVa when ETP levels were normalized (e.g., for NHP without anti-FVIII antibody, the ETP was 1,935 nM.min). Moreover, small amounts of superFVa (3.3 nM) enhanced the efficacy of rhFVIIa (Figure 2B). On average, the dose-response curves for superFVa or rhFVIIa were shifted by approximately 1-order of magnitude in the presence of small amounts of either rhFVIIa (0.4 nM) or superFVa (3.3 nM), respectively. Thus, superFVa and rhFVIIa cooperated to restore thrombin generation in NHP with an inhibitory anti-FVIII antibody.
Figure 2. Cooperative effects of rhFVIIa and superFVa on thrombin generation in normal human plasma containing an anti-FVIII antibody.
Thrombin generation was determined as ETP (top panel) and thrombin peak height (bottom panel) in NHP supplemented with 1.25 µg/ml anti-human FVIII-antibody (40 BU). Increasing concentrations of either (A) superFVa in the absence (open circles) or presence (closed circles) of rhFVIIa (0.4 nM), or (B) increasing concentrations of rhFVIIa in the absence (open circles) or presence (closed circles) of a fixed concentration of superFVa (3.3 nM) were used. Thrombin generation in NHP in the absence of the inhibitory anti-FVIII antibody is indicated by the open squares. ETP denotes Endogenous Thrombin Potential; NHP, normal human plasma. Error bars represent standard error of the mean (n≥5).
Cooperative effects of superFVa and FVIIa on thrombin generation in hemophilia patient plasma with inhibitors
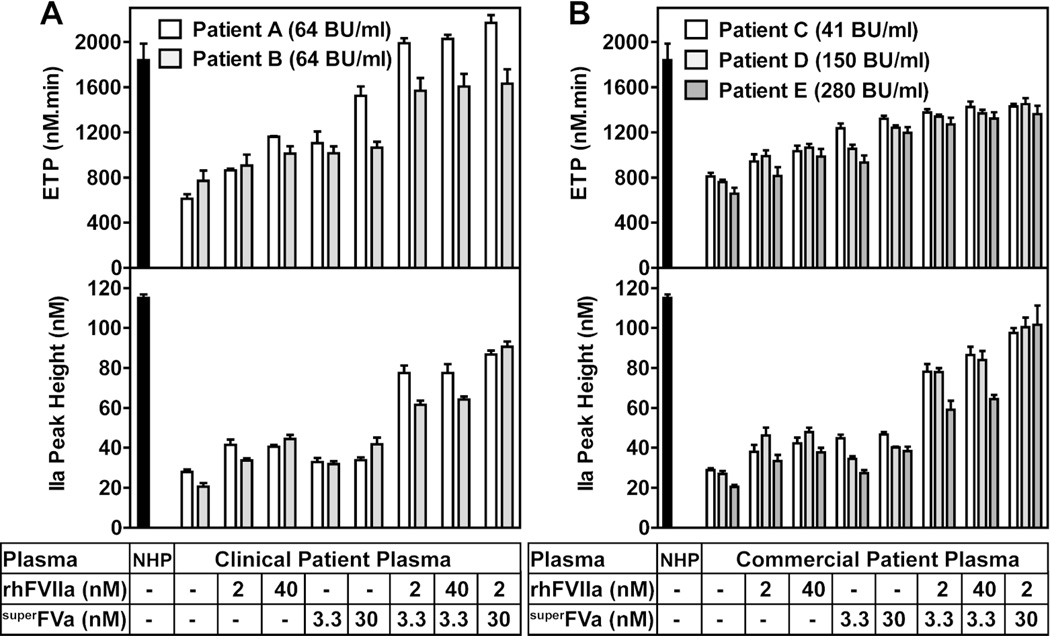
Next, the effects of superFVa and/or rhFVIIa were studied in plasma samples from patients with congenital FVIII deficiency and inhibitors. Plasma samples from five subjects were selected to represent a wide range of anti-FVIII titers from 41–280 BU (Figure 3A; Patients A & B from our clinic, and Figure 3B; Patients C, D & E from commercially obtained samples). Compared to NHP, the ETP and thrombin peak height were markedly reduced in all five patient samples in the absence of superFVa or rhFVIIa (Figure 3A & 3B). Addition of either superFVa or rhFVIIa increased ETP and thrombin peak height in all patient samples. ETP and thrombin peak height were comparable between plasmas spiked with 40 nM rhFVIIa, a therapeutic concentration, or with superFVa at 3.3 nM. Notwithstanding inter-individual responses, ETP and thrombin peak height increased noticeably for all patient samples when rhFVIIa (2 or 40 nM) and superFVa (3.3 nM) were combined, demonstrating an ~1.5-fold to 3-fold enhanced thrombin generation compared to either factor alone. Effects on ETP and peak height by a combination of 3.3 nM superFVa and 2 nM rhFVIIa could not be further improved by raising the concentration of rhFVIIa to 40 nM. However, increasing the concentration of superFVa to 30 nM did raise ETP and/or peak height in most patient samples, approaching the levels of thrombin generation that were observed in NHP.
Figure 3. Cooperative effects of rhFVIIa and superFVa on thrombin generation in plasma from patients with congenital FVIII-deficiency and high titer anti-FVIII antibodies.
Thrombin generation was determined as ETP (top panel) and peak height (bottom panel) in (A) plasma samples with inhibitors from two patients seen at the University of California San Diego Hemophilia Treatment Center or (B) commercial plasma samples with inhibitors from three different patients. ETP was determined at two different concentrations of superFVa or rhFVIIa, or combinations thereof. Inhibitor titers are expressed in BU. Pooled NHP served as control. ETP denotes Endogenous Thrombin Potential; NHP, normal human plasma; BU, Bethesda Unit. Error bars represent standard error of the mean (n≥3).
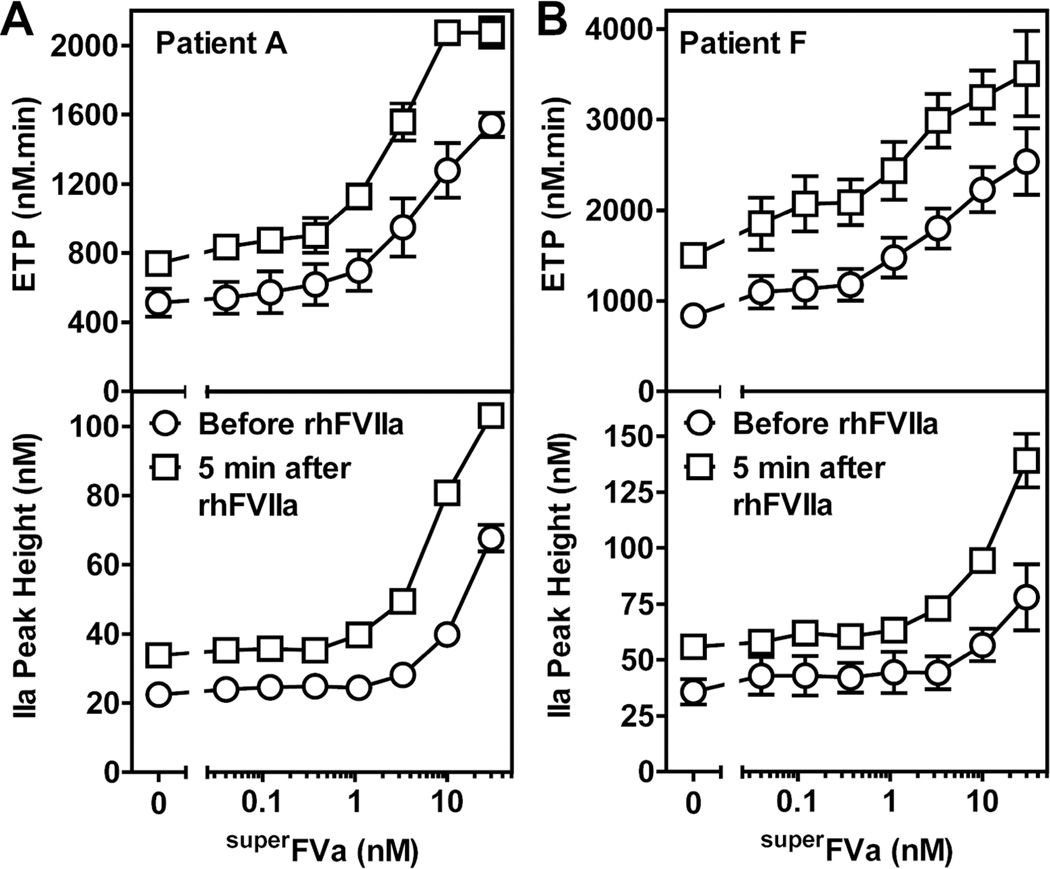
Additional insights into the effects of superFVa on thrombin generation during acute bleeding events were obtained from analyses of plasma from two patients with congenital FVIII-deficiency and anti-FVIII inhibitors (Patient A with 64 BU (Figure 4A) and Patient F with 32 BU (Figure 4B)) that received rhFVIIa for treatment of joint or muscle bleeding. Plasma was collected immediately before and 5 minutes after rhFVIIa (90 µg/kg) administration. Thrombin generation was determined prior to and after treatment with rhFVIIa with and without increasing concentrations of superFVa added ex vivo (Figure 4). Therapeutic administration of rhFVIIa increased the ETP and peak height for both patients by 1.4 to 1.8-fold. SuperFVa added to plasma collected prior to rhFVIIa administration resulted in a dose-dependent increase of ETP and peak height, and about 1 nM superFVa generated similar thrombin levels (ETP) compared to what was observed in the same plasma after treatment with rhFVIIa. Somewhat higher concentrations of superFVa were required to achieve similar enhancements of peak height (6 nM superFVa (patient A) and 9 nM superFVa (patient F)). When superFVa was titrated into patient plasma harvested 5 minutes after rhFVIIa infusion, thrombin generation could be significantly improved, as demonstrated by a marked left-shift of the dose-response curve for ETP and peak height. Concentrations of superFVa in the plasmas of the rhFVIIa treated patients could be reduced by ~ 10-fold for a similar augmentation of thrombin generation with superFVa before rhFVIIa treatment.
Figure 4. Cooperative effects of superFVa on thrombin generation in plasma of two patients with congenital FVIII-deficiency and high titer anti-FVIII antibodies treated with rhFVIIa during acute bleeding episodes.
Plasma samples were obtained just before (open circles) and 5 min post infusion of rhFVIIa at 90 µg/kg (open squares) from two patients with BU-titers of 64 U/mL (Patient A) and 32 U/ml (Patient F). Thrombin generation was determined as ETP (top panel) or peak height (bottom panel) with increasing concentrations of superFVa added to plasma samples ex vivo. ETP denotes Endogenous Thrombin Potential; BU, Bethesda Unit. Error bars represent standard error of the mean (n≥3).
Of note, values for ETP and peak height in the plasma of Patient A after infusion of 90 µg/kg rhFVIIa (Figure 4A) corresponded to ETP and peak height when this patient’s plasma was spiked ex vivo with rhFVIIa at 40 nM (Patient A, Figure 3A). This corroborates that ex vivo addition of 40 nM rhFVIIa to FVIIIdP accurately reflects the therapeutic concentration of rhFVIIa for in vitro experiments
Anti-fibrinolytic effects of superFVa in NHP with FVIII-inhibiting antibodies
The effects of superFVa and rhFVIIa on clot lysis were studied in NHP with and without anti-FVIII antibodies to assess their effects on TAFIa-mediated protection of the clot against premature fibrinolysis (12, 31, 32) (Figure 5). Based on a GMA8015 anti-FVIII dose-response titration, an antibody concentration of 10 Tg/mL (320 BU) impaired thrombin generation sufficiently to prevent thrombin-mediated activation of TAFI (Supplemental Figure 3). The requirement for a higher anti-FVIII concentration in the clot lysis assay compared to the thrombin generation assay is in agreement with previous observations that the concentration of FVIII in FVIIIdP required to normalize TAFIa-mediated anti-fibrinolytic effects is lower than that required for normalization of clotting (12).
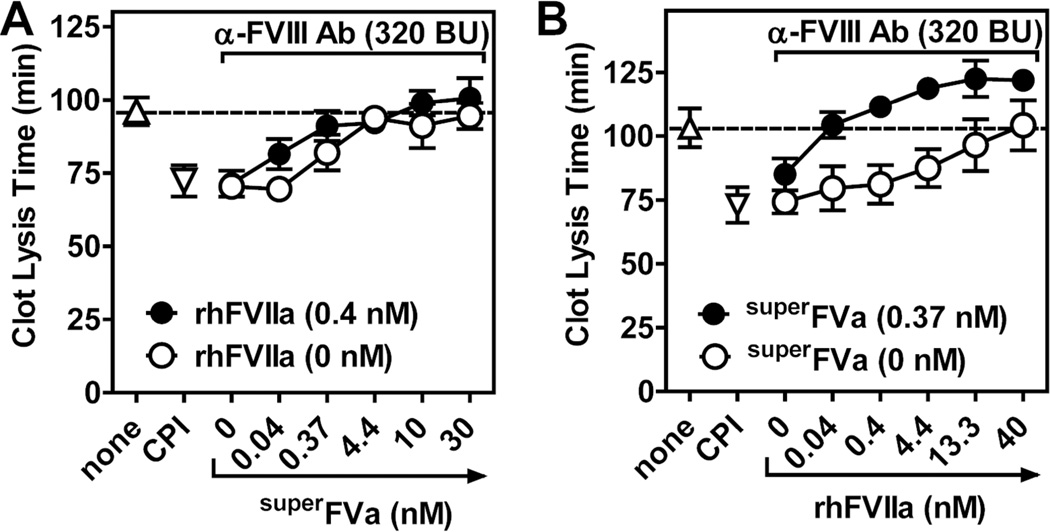
Figure 5. Cooperative effects of superFVa in combination with rhFVIIa on clot stabilization in NHP spiked with an anti-FVIII antibody.
Clot lysis time was determined by t-PA mediated fibrinolysis following tissue factor-induced clot formation in NHP spiked with an anti-FVIII antibody (10 µg/ml; 320 BU). (A) Increasing concentrations of superFVa were added in the absence (open circle) or presence (solid circle) of rhFVIIa (0.4 nM). (B) Increasing concentrations of rhFVIIa were added in the absence (open circle) or presence (solid circle) of superFVa (0.37 nM). Clot lysis time of NHP without the anti-FVIII antibody in the absence (triangle) or presence of CPI (inverted triangle) are indicated on the left. The first open circle on the left (0 nM superFVa (A) or 0 nM rhFVIIa (B)) represent the CLT of NHP with the anti-FVIII antibody but without superFVa or rhFVIIa. NHP denotes normal human plasma; CPI, carboxypeptidase inhibitor from potato tubers. Error bars represent standard error of the mean (n≥3).
Increasing concentrations of superFVa added to NHP with anti-FVIII antibody effectively prolonged the CLT, resulting in normalization of the CLT to that of NHP in the absence of anti-FVIII at 3.33 nM superFVa (Figure 5A). The CLT was also prolonged by rhFVIIa, and normalization to that of NHP without anti-FVIII antibody required 40 nM rhFVIIa (Figure 5B). Remarkably, the concentration of rhFVIIa to normalize the CLT could be reduced by 100-fold to ~0.4 nM in the presence of a low concentration (0.37 nM) of superFVa (Figure 5B and Supplemental Table 1). Similarly, the concentration of superFVa for CLT normalization could be reduced 10-fold in the presence of a low (0.4 nM) concentration of rhFVIIa (Figure 5A and Supplemental Table 1). Thus, superFVa and rhFVIIa synergize to restore the CLT of NHP that contains FVIII inhibitors.
Cooperation between superFVa and FVIIa to reduce bleeding in hemophilia A mice
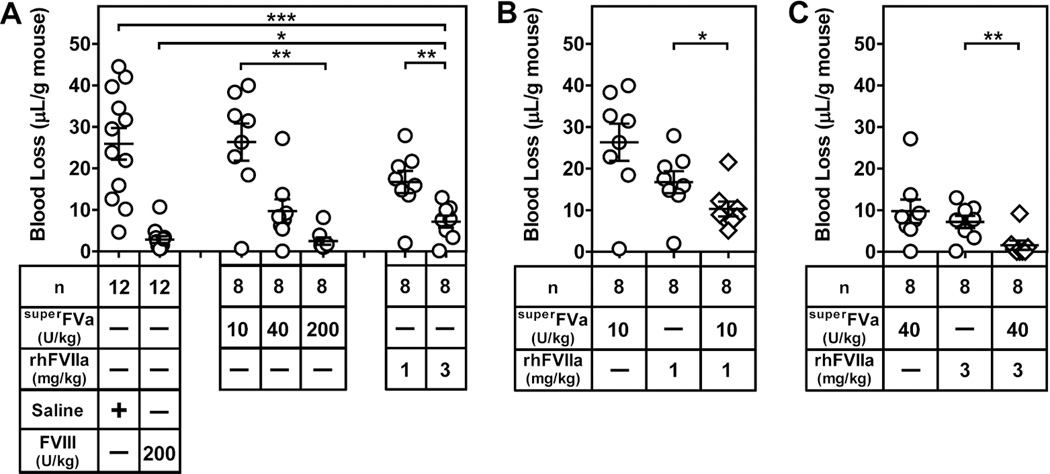
To determine the in vivo interactions between superFVa and rhFVIIa for bleed reduction in hemophilia, blood loss was determined in a tail transection model in FVIII-deficient mice during a 20-minute bleeding period. To establish conditions where combinatorial effects of superFVa and rhFVIIa can be revealed, individual dose titrations of superFVa and rhFVIIa were performed first. Mean blood loss from mice injected with the lowest dose of superFVa (10 U/kg) was similar to mean blood loss after injection of saline (26.3 µL/g vs. 25.9 µL/g), but it was reduced significantly to 9.7 µL/g at 40 U/kg superFVa and the effect of the highest dose of 200 U/kg superFVa (2.5 µL/g) was indistinguishable from rhFVIII (200 U/kg; 2.9 µL/g) (Figure 6A). Mean blood loss was reduced from 25.9 µL/g (saline) to 16.8 TL/g by 1 mg/kg rhFVIIa and to 7.2 µL/g by 3 mg/kg rhFVIIa (Figure 6A), consistent with a previously published report demonstrating that reduction of bleeding in FVIII-deficient mice requires 10 to 100-fold higher doses of rhFVIIa than in humans (33). When rhFVIIa (1 mg/kg) with an intermediate effect on bleeding was combined with the lowest dose of superFVa (10 U/kg), which by itself had no effect, a significant reduction of mean blood loss from 16.7 to 10.2 µL/g was observed (Figure 6B). Combination of rhFVIIa at 3 mg/kg with the medium dose of superFVa (40 U/kg) reduced bleeding (1.6 µL/g) similar to that achieved by injection of 200 U/kg rhFVIII (Figure 6C). Thus, superFVa and rhFVIIa showed marked cooperative effects to reduce bleeding in FVIII-deficient mice in vivo.
Figure 6. Combination therapy with superFVa and rhFVIIa enhances bleed correction in FVIII-deficient mice.
FVIII-deficient mice were injected intravenously with saline, rhFVIII, increasing doses of superFVa or rhFVIIa, as well as rhFVIIa in combination with superFVa. Bleeding was determined during 20 minutes after tail clip and expressed as Tl blood loss per gram mouse. A) Dose responses of bleed correction by either superFVa or rhFVIIa in comparison to untreated and FVIII treated FVIII-deficient mice. These conditions provide the reference points for the evaluation of efficacy of superFVa and rhFVIIa combinations in panels (B) and (C). B) Cooperation of “low” doses of superFVa (10 U/kg) and rhFVIIa (1 mg/kg) to reduce bleeding in FVIII-deficient mice. C) Cooperation of a “medium” dose of superFVa (40 U/kg) and rhFVIIa (3 mg/kg) to reduce bleeding in FVIII-deficient mice. Note: the open circles in panels (B) and (C) are the single-agent controls derived from the dose response in panel (A). Error bars represent mean ± SEM (n = 8–12 as indicated). 1 Unit superFVa = activity of 20 nM wild-type FVa in the prothrombinase assay. * p≤ 0.05; ** p≤ 0.005; *** p≤ 0.0005.
Bleed reduction by combinations of superFVa and FVIIa in wild-type mice with anti-FVIII inhibitors
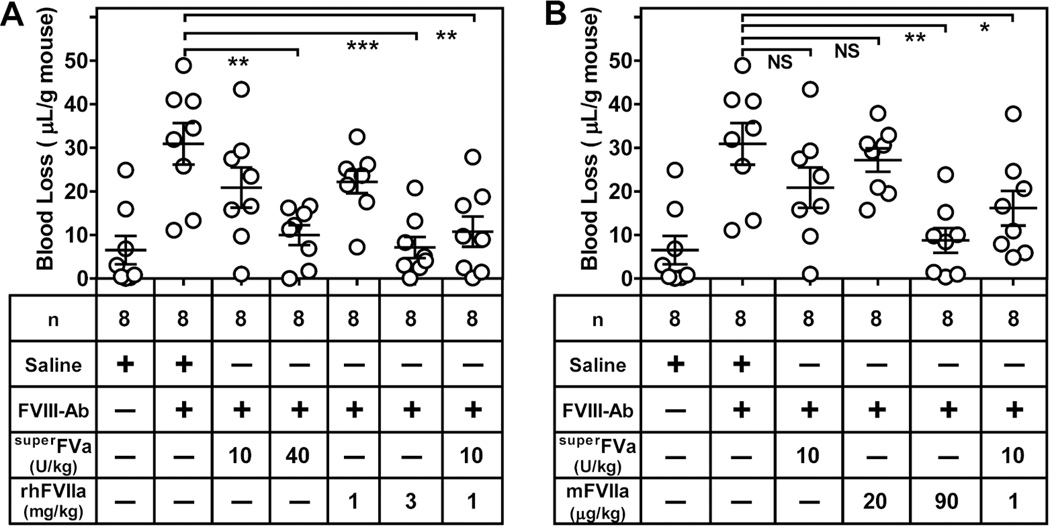
To mimic acquired Hemophilia A, an inhibitory anti-FVIII antibody that cross-reacts with mouse FVIII was injected into wild-type BalbC mice and bleeding was determined after tail transection during a 20-minute bleeding period. Injection of 0.25 mg/kg anti-FVIII resulted in an antibody titer of 35 ± 2 BU and caused significantly increased bleeding (mean blood loss 30.9 µL/g versus 6.54 µL/g in the absence of anti-FVIII) that was comparable to that observed in the FVIII-deficient mice (26.3 µL/g) (Figure 7A). As in the FVIII-deficient mouse, a significant dose dependent reduction of blood loss was observed with superFVa at 40 U/kg (10.0 TL/g) when compared to saline injected mice (30.9 µL/g) control (Figure 7A). Also similar to the FVIII-deficient mouse, a high dose of 3 mg/kg rhFVIIa was required to result in significant blood loss reduction (7.1 µL/g). The combination of 10 U/kg superFVa (19.5 µL/g) and 1 mg/kg rhFVIIa (22.2 µL/g) that individually did not significantly reduce bleeding, were effective to decrease blood loss significantly (10.8 µL/g) (Figure 7A).
Figure 7. Bleed correction with superFVa, rhFVIIa, mFVIIa and combinations thereof in BalbC mice injected with an inhibitory anti-FVIII antibody.
BalbC mice were injected intravenously with an inhibitory antibody against FVIII (0.25 mg/kg) resulting in an inhibitor titer of 35 BU. Bleeding was determined for 20 min after tail clip and expressed in Tl blood loss per gram mouse. (A) Blood loss was measured by intravenous injection of saline, increasing doses of superFVa or rhFVIIa and a combination of rhFVIIa and superFVa at low concentrations. (B) Blood loss was measured by intravenous injection of increasing doses of mFVIIa and a combination of mFVIIa and superFVa at low concentrations. Error bars represent mean ± SEM (n = 8). 1 Unit superFVa = activity of 20 nM wild-type FVa in the prothrombinase assay. * p≤ 0.05; ** p≤ 0.005; *** p≤ 0.0005; NS, not significant.
To overcome the known requirement for supratherapeutic dosing of rhFVIIa in the mouse and to shed additional light on combinatorial effects of superFVa and FVIIa on bleed reduction in vivo, we used recombinant mFVIIa to determine its prohemostatic effects in wild-type mice injected with the anti-FVIII-inhibitory antibodies after tail transection. A dose titration showed that mFVIIa at the human therapeutic dosing of 90 µg/kg reduced blood loss significantly (8.8 µl/g, compared to 30.9 µl/g with saline), similar to blood loss reduction with 3 mg/kg rhFVIIa (Supplemental Figure 4). Moreover, when mFVIIa (20 µg/kg) and superFVa (10 U/kg) were combined at concentrations that by themselves had no effects on bleed reduction, blood loss diminished significantly to 16.2 µl/g (Figure 7B). Thus, the blood loss reduction observed for the combination of lower concentrations of superFVa and rhFVIIa was similarly observed for superFVa in combination with mFVIIa.
Discussion
This study demonstrated that superFVa improves hemostasis effectively in hemophilia with inhibitors. SuperFVa increased thrombin generation and inhibition of fibrinolysis in the presence of inhibitors in patient plasma samples, collected at routine visits and during acute bleeding episodes. In hemophilia mouse models, FVIII-deficient mice and wild-type mice with and without inhibitors, superFVa corrected bleeding following tail transection. The study further demonstrated that hemostasis in vitro and in vivo could be significantly improved when FVIIa was combined with superFVa, or maintained at a certain level when either compound was given at a lower dose. As previously reported (33), we confirmed that rhFVIIa needs to be administered at supratherapeutic doses in mice to reduce bleeding after injury (>3 mg/kg, >30-fold higher than administered to hemophilic patients with inhibitors). Since this leaves some uncertainty as to applicability of murine studies for human conditions, murine recombinant FVIIa was prepared and its efficacy to reduce bleeding in the mouse was compared to rhFVIIa. We found that mFVIIa was vastly more potent than rhFVIIa as it stopped bleeding at human therapeutic dosing (90 µg/kg) and, similar to rhFVIIa, cooperated at subtherapeutic doses (20 µg/kg) with low doses of superFVa to reduce blood loss. These data give confidence that results from our mouse experiments using rhFVIIa may be extrapolated to the human situation, to the extent that our murine model is relevant to treatment of human disease.
RhFVIIa at therapeutic levels (~40 nM) increased thrombin generation but did not normalize it to the degree observed in NHP. This fits with the observations that usual dosing at 90 µg/kg does not always result in cessation of bleeding. Therefore, in clinical practice, dosing and treatment intervals of rhFVIIa are often increased by 3-fold (270 µg/kg per dose every 6 hours) (34), which is also more convenient for patients. Alternatively, combination therapy with or sequential administration of FEIBA is sometimes used as salvage therapy, and was deemed efficacious and safe by the European Hemophilia Standardization Board without reports of thromboembolic complications (35, 36). Synergistic effects on thrombin generation when combining those 2 products have been described (36), and may be facilitated by impurities of activated Vitamin K-dependent clotting factors contained in FEIBA. Our findings that trace amounts of superFVa combined with rhFVIIa were sufficient to normalize hemostasis parameters in hemophilia samples with inhibitors are consistent with these previous observations, and suggest that strategies to combine active clotting factors may be beneficial and safe in extreme hemophilic bleeding situations. In this context, the marked improvement in thrombin generation achieved by addition of superFVa ex vivo to plasma from the two patients in our study treated with rhFVIIa during bleeding is noteworthy.
Combinations of superFVa with FVIIa were synergistic in several vitro assays, e.g., in thrombin generation assays when low concentrations of superFVa (~1–10 nM) were combined with therapeutic doses of rhFVIIa. Accordingly, when concentrations of superFVa were increased (~20 to 30 nM), concentrations of rhFVIIa could be decreased by ~10- to 100-fold below therapeutic levels without compromising thrombin generation. Of note, synergistic effects were also present in respect to restoration of fibrinolysis inhibition, and here superFVa was also able to normalize clot lysis times as a single agent at concentrations lower than the normal plasma concentration of FV. This is important since the ability of a bypassing agent to improve normal inhibition of fibrinolysis may be a critical mechanistic gain for clot stabilization and prevention of re-bleeding (29, 32).
The cooperation between superFVa and rhFVIIa is most likely caused by the fact that each agent targets different aspects of the coagulation cascade. Following vascular injury, rhFVIIa contributes to thrombin generation mainly by direct activation of FX on platelet surfaces, thereby bypassing the requirement for FVIII and FIX in the tenase complex (37). SuperFVa then uses this FXa to promote the incorporation of FXa in the prothrombinase complex thereby enhancing the formation of thrombin. Furthermore, FXa is relatively protected against inactivation by serine protease inhibitors when incorporated in the prothrombinase complex (38), and thus superFVa may diminish the inactivation of free FXa. The superFVa resistance to inactivation by APC likely contributes to retaining FXa in the prothrombinase complex, supported by a study demonstrating that the transgenic expression of FVLeiden provided partial improvements of hemostasis in hemophilia mice (14). Consistent with this proposed mechanism, thrombin generation curves indicated that rhFVIIa was more effective to enhance the initial rate of thrombin generation and thrombin peak height, whereas the effects of superFVa were more pronounced on the duration of thrombin generation as evident by the effects on ETP. Also, the more efficient normalization of the CLT by superFVa compared to rhFVIIa are directly related to the ETP enhancements of superFVa because the activation of TAFI and its ability to down-regulate fibrinolysis are critically dependent on secondary thrombin generation that occurs inside the clot over a prolonged period of time (32). Further support for this mechanism of cooperation between superFVa and FVIIa is also provided by the previously published observation that exogenously added FVa, but not FV, improved hemostasis in hemophilia mice (14), suggesting that overcoming the activation of FV as a rate limiting step greatly facilitates thrombin generation initiated by FVIIa in hemophilia.
The potential development of superFVa as a bypassing agent offers considerable versatility as it is efficient as a single agent to improve prothrombinase activity, but it is even more efficient in combination with rhFVIIa where it may enable enhanced effects of rhFVIIa on thrombin generation and restoration of fibrinolysis by overcoming the activation of FV as the rate limiting step. In addition to its use as a single agent, potential therapeutic applications might involve simultaneous or sequential administrations of FVIIa and superFVa. Towards that end daily prophylaxis with rhFVIIa has been introduced into hemophilia care off-label and demonstrated reduced bleeding frequency and improved health related quality of life (39), thus indicating that frequent infusions are acceptable to many patients if clinical benefit is achieved. In summary, the results of these studies continue to support the concept that “FVa activity augmentation” alone or in combination with rhFVIIa deserves consideration as a new bypassing strategy for hemophilia patients with inhibitors.
Supplementary Material
Extra Table.
| What is known on this topic |
|
| What the paper adds |
|
Acknowledgements
Factor VIII-deficient mice (BALB/c background) were a generous gift of David Lillicrap, MD, (Queens University, Ontario, CAN).
Funding Sources
This work was funded by grant support from an Early Career Development Award from Bayer Hemophilia (A.v.D. and V.B.), by National Institutes of Health grants HL104165 (L.O.M.) and HL03195 and HL052246 (J.H.G.).
Disclosures
A.v.D. has received honoraria for participating in scientific advisory board panels, consulting, and speaking engagements for Baxalta, Pfizer, Biogen, CSL-Behring, Novo Nordisk, and Grifols. UCSD and TSRI hold intellectual property rights related to superFVa on which AvD, AJG, JHG, and LOM are listed as inventors. AvD, AJG, and LOM are founders of Hematherix LLC., a biotech company that is developing superFVa therapy for bleeding complications. AvD and LOM are members of the Board of Directors of Hematherix LLC.
Footnotes
Authorship Contributions
V.B. and A.v.D. contributed equally to design of the research, performed experiments, and drafted the manuscript. J.H.G. contributed to concept and supported the experimental work. A.J.G. performed experiments and provided experimental guidance. L.O.M. designed concept and research, provided project oversight and experimental guidance and contributed to manuscript drafting. All authors critically reviewed the manuscript and approved it in its final version.
References
- 1.Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379(9824):1447–1456. doi: 10.1016/S0140-6736(11)61139-2. [DOI] [PubMed] [Google Scholar]
- 2.Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231–239. doi: 10.1056/NEJMoa1208024. [DOI] [PubMed] [Google Scholar]
- 3.Laros-van Gorkom BA, Falaise C, Astermark J. Immunosuppressive agents in the treatment of inhibitors in congenital haemophilia A and B--a systematic literature review. Eur J Haematol Suppl. 2014;76:26–38. doi: 10.1111/ejh.12372. [DOI] [PubMed] [Google Scholar]
- 4.Berntorp E, Astermark J, Carlborg E. Immune tolerance induction and the treatment of hemophilia. Malmo protocol update. Haematologica. 2000;85(10 Suppl):48–50. [PubMed] [Google Scholar]
- 5.Hay CR, DiMichele DM International Immune Tolerance Study. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119(6):1335–1344. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 6.Hoots WK. Arthropathy in inhibitor patients: differences in the joint status. Semin Hematol. 2008;45(2) Suppl 1:S42–S49. doi: 10.1053/j.seminhematol.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 8.Astermark J, Donfield SM, DiMichele DM, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109(2):546–551. doi: 10.1182/blood-2006-04-017988. [DOI] [PubMed] [Google Scholar]
- 9.Astermark J, Santagostino E, Keith HW. Clinical issues in inhibitors. Haemophilia. 2010;16(Suppl 5):54–60. doi: 10.1111/j.1365-2516.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- 10.Broze GJJ, Higuchi DA. Coagulation-dependent inhibition of fibrinolysis: role of carboxypeptidase-U and the premature lysis of clots from hemophilic plasma. Blood. 1996;88(10):3815–3823. [PubMed] [Google Scholar]
- 11.Foley JH, Nesheim ME. Soluble thrombomodulin partially corrects the premature lysis defect in FVIII-deficient plasma by stimulating the activation of thrombin activatable fibrinolysis inhibitor. J Thromb Haemost. 2009;7(3):453–459. doi: 10.1111/j.1538-7836.2008.03261.x. [DOI] [PubMed] [Google Scholar]
- 12.Mosnier LO, Lisman T, van den Berg HM, et al. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb Haemost. 2001;86(4):1035–1039. [PubMed] [Google Scholar]
- 13.Lisman T, Mosnier LO, Lambert T, et al. Inhibition of fibrinolysis by recombinant factor VIIa in plasma from patients with severe haemophilia A. Blood. 2002;99(1):175–179. doi: 10.1182/blood.v99.1.175. [DOI] [PubMed] [Google Scholar]
- 14.Schlachterman A, Schuettrumpf J, Liu JH, et al. Factor V Leiden improves in vivo hemostasis in murine hemophilia models. J Thromb Haemost. 2005;3(12):2730–2737. doi: 10.1111/j.1538-7836.2005.01639.x. [DOI] [PubMed] [Google Scholar]
- 15.Franchini M, Lippi G. Factor V Leiden and hemophilia. Thromb Res. 2010;125(2):119–123. doi: 10.1016/j.thromres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Kalafatis M, Egan JO, van 't Veer C, et al. The regulation of clotting factors. Crit Rev Eukaryot Gene Expr. 1997;7(3):241–280. doi: 10.1615/critreveukargeneexpr.v7.i3.40. [DOI] [PubMed] [Google Scholar]
- 17.Bos MH, Meijerman DW, van der Zwaan C, et al. Does activated protein C-resistant factor V contribute to thrombin generation in hemophilic plasma? J Thromb Haemost. 2005;3(3):522–530. doi: 10.1111/j.1538-7836.2005.01181.x. [DOI] [PubMed] [Google Scholar]
- 18.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 19.von Drygalski A, Cramer TJ, Bhat V, et al. Improved hemostasis in hemophilia mice by means of an engineered factor Va mutant. J Thromb Haemost. 2014;12:363–372. doi: 10.1111/jth.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Drygalski A, Bhat V, Gale AJ, et al. An engineered factor Va prevents bleeding induced by anticoagulant wt activated protein C. PLoS One. 2014;9(8):e104304. doi: 10.1371/journal.pone.0104304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale AJ, Xu X, Pellequer JL, et al. Interdomain engineered disulfide bond permitting elucidation of mechanisms of inactivation of coagulation factor Va by activated protein C. Protein Sci. 2002;11(9):2091–2101. doi: 10.1110/ps.0210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesters RM, Houghten RA, Griffin JH. Identification of a sequence of human activated protein C (residues 390– 404) essential for its anticoagulant activity. J Biol Chem. 1991;266(36):24514–24519. [PubMed] [Google Scholar]
- 23.Lusher JMKC, Green D. Acquired Hemophilia. Princeton: Excerpta Medica, Inc.; 1995. Secon Edition ed. [Google Scholar]
- 24.Bajaj SP, Rapaport SI, Brown SF. Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem. 1981;256(1):253–259. [PubMed] [Google Scholar]
- 25.Barrow RT, Lollar P. Neutralization of antifactor VIII inhibitors by recombinant porcine factor VIII. J Thromb Haemost. 2006;4(10):2223–2229. doi: 10.1111/j.1538-7836.2006.02135.x. [DOI] [PubMed] [Google Scholar]
- 26.Duncan E, Collecutt M, Street A. Nijmegen-Bethesda assay to measure factor VIII inhibitors. Methods Mol Biol. 2013;992:321–333. doi: 10.1007/978-1-62703-339-8_24. [DOI] [PubMed] [Google Scholar]
- 27.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282(45):33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 28.Hemker HC, Giesen P, AlDieri R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32(5–6):249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 29.Mosnier LO, Von dem Borne PAK, Meijers JCM, et al. Plasma TAFI levels influence the clot lysis time in healthy individuals in the presence of an intact pathway of coagulation. Thromb Haemost. 1998;80(5):829–835. [PubMed] [Google Scholar]
- 30.Mosnier LO, Fernandez JA, Davis TP, et al. Influence of the 3K3A-activated protein C variant on the plasma clot lysis activity of tPA and of tPA on the variant's anticoagulant activity. J Thromb Haemost. 2013;11(11):2059–2062. doi: 10.1111/jth.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sixma JJ, van den Berg A. The haemostatic plug in haemophilia A: a morphological study of haemostatic plug formation in bleeding time skin wounds of patients with severe haemophilia A. Br J Haematol. 1984;58:741–753. doi: 10.1111/j.1365-2141.1984.tb06121.x. [DOI] [PubMed] [Google Scholar]
- 32.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arterioscler Thromb Vasc Biol. 2006;26(11):2445–2453. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]
- 33.Tranholm M, Kristensen K, Kristensen AT, et al. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood. 2003;102(10):3615–3620. doi: 10.1182/blood-2003-05-1369. [DOI] [PubMed] [Google Scholar]
- 34.Puetz J. Optimal use of recombinant factor VIIa in the control of bleeding episodes in hemophilic patients. Drug Des Devel Ther. 2010;4:127–137. doi: 10.2147/dddt.s6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinowitz U, Livnat T, Zivelin A, et al. Concomitant infusion of low doses of rFVIIa and FEIBA in haemophilia patients with inhibitors. Haemophilia. 2009;15(4):904–910. doi: 10.1111/j.1365-2516.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 36.Gringeri A, Fischer K, Karafoulidou A, et al. Sequential combined bypassing therapy is safe and effective in the treatment of unresponsive bleeding in adults and children with haemophilia and inhibitors. Haemophilia. 2011;17(4):630–635. doi: 10.1111/j.1365-2516.2010.02467.x. [DOI] [PubMed] [Google Scholar]
- 37.Monroe DM, Hoffman M, Oliver JA, et al. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99(3):542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 38.Brufatto N, Nesheim ME. The use of prothrombin(S525C) labeled with fluroscein to directly study the inhibition of prothrombinase by antithrombin during prothrombin activation. J Biol Chem. 2001;276(21):17663–17671. doi: 10.1074/jbc.M011586200. [DOI] [PubMed] [Google Scholar]
- 39.Hoots WK, Ebbesen LS, Konkle BA, et al. Secondary prophylaxis with recombinant activated factor VII improves health-related quality of life of haemophilia patients with inhibitors. Haemophilia. 2008;14(3):466–475. doi: 10.1111/j.1365-2516.2008.01654.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.