Abstract
Background
Advanced stage non-small cell lung cancer (NSCLC) patients with borderline performance status (PS2) are often excluded from clinical trials and platinum-based therapy. In light of the potential role for serum proteomics in predicting erlotinib benefit beyond that of EGFR mutational status, we conducted a trial of erlotinib +/− chemotherapy in a cohort of PS2 NSCLC patients enriched by the Veristrat proteomics assay.
Methods
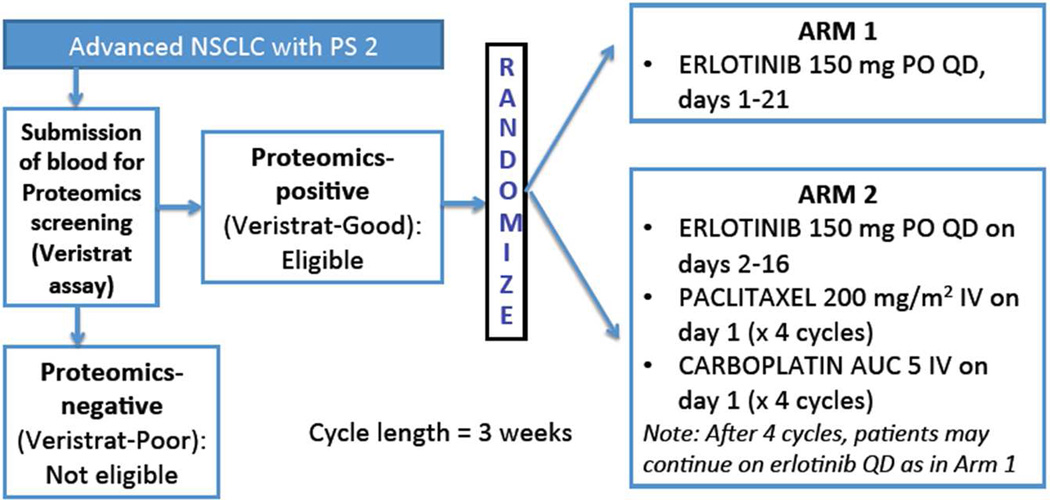
Metastatic NSCLC PS2 patients with acceptable end-organ function and Veristrat-good status were randomized to either erlotinib 150 mg orally daily (Arm 1) or erlotinib 150 mg orally daily on days 2–16 plus 4 cycles of carboplatin (AUC 5 day 1) and paclitaxel (200 mg/m2 IV day 1), followed by erlotinib 150 mg orally (Arm 2). Arm 2 agents were pharmacodynamically separated to mitigate potential antagonism. The arm with superior observed median progression free survival (PFS) would be selected for further evaluation, but only if ≥ 3 months.
Results
The trial terminated prior to the planned accrual of 98 patients for regulatory reasons. Of 156 patients screened, 83 (59%) were classified Veristrat-good; 59 met trial eligibility and were randomized (Arm 1– 33; Arm 2– 26). Arm 2 patients had higher response rate (23% vs. 6%, p=0.06), disease control rate (77% vs. 41%, p=0.0046), median PFS (4.6 vs. 1.6 months, p=0.06), and median overall survival (11 vs. 6 months, p=0.27). Treatment-related grade 4 adverse events were seen in 2 patients in Arm 1 (thrombosis, hypomagnesemia) and 5 patients in Arm B (neutropenia in 5, febrile neutropenia in 1, leukopenia in 1).
Conclusion
In a proteomics-enriched cohort of PS2 patients with NSCLC, pharmacodynamically-separated erlotinib plus chemotherapy, when compared to erlotinib alone, had better efficacy and surpassed the protocol-specified benchmark of PFS ≥ 3 months required for further study.
BACKGROUND
In patients with metastatic non-small cell lung cancer (NSCLC) whose tumors do not have an actionable driver mutation, palliative platinum-based therapy is considered frontline standard-of-care. In this group is a subset of patients with impaired performance status (defined as Zubrod PS2), representing up to 30–40%.[1] Patients with PS2 are capable of all self-care but unable to carry out any work activities and are usually confined to bed or chair < 50% of waking hours. PS2 patients have traditionally been excluded from clinical trials and from receiving standard platinum-based chemotherapy because of their expected high rate of toxicity following such therapy.
In the middle of the last decade, there were retrospective data suggesting a potential role for serum proteomics in predicting erlotinib benefit in NSCLC patients beyond that of epidermal growth factor receptor (EGFR) mutational status.[2–4] Subsequently, a laboratory-developed serum proteomics assay (VeriStrat, Biodesix) became commercially available. This assay has since been shown to significantly correlate with survival outcomes in NSCLC patients, particularly those treated with EGFR tyrosine kinase inhibitors (TKI).[5–8] In the PROSE trial - a randomized proteomic-stratified phase III study of second-line erlotinib versus chemotherapy - patients classified as VeriStrat-Poor demonstrated significantly improved OS when treated with single-agent chemotherapy versus erlotinib, while those classified as VeriStrat-Good demonstrated similar OS when given either erlotinib or single-agent chemotherapy.[9]
SWOG S0709 was designed to investigate the role of serum proteomics as enrichment factor for NSCLC patients with PS2 treated with erlotinib-based therapy. In this trial, erlotinib and chemotherapy were “pharmacodynamically separated” (i.e., intercalated) to mitigate hypothesized antagonism that occurs when these treatments are given concurrently. [10,11]
PATIENTS AND METHODS
Patients with metastatic NSCLC of any histologic subtype with Zubrod PS2 were eligible. EGFR mutational status was not required. In the screening step, potentially eligible patients were consented for baseline serum specimen submission for proteomics testing using the commercial Veristrat assay. Only patients who were classified as VeriStrat Good were eligible for the randomization step. No prior therapy for systemic disease was allowed. A signed written informed consent document was required. This study was reviewed and approved by the Institutional Review Board of each participating site. Study schema and treatment regimen are shown in Figure 1.
Figure 1. Study Schema.
Patients were seen prior to the start of every cycle for toxicity assessment. The CTCAE (NCI Common Terminology Criteria for Adverse Events) version 4.0 was utilized for serious adverse event reporting while version 3.0 was used for routine toxicity reporting. Response assessments were based on Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1.
Statistical Considerations
The primary objective for this Phase II selection design was to select one of the two regimens for further study in a Phase III trial. If neither arm had an observed median progression free survival (PFS) of at least 3 months, then neither arm will be selected for further study in this setting. If only one arm had an observed PFS of 3 months or greater, then that arm will be chosen. If both arms have an observed PFS of 3 months or greater, then Arm 1 will be chosen unless Arm 2 has an observed PFS at least 1 month greater than the observed PFS of Arm 1. Assuming 98 eligible patients accrued over 16 months (49 per arm) and 6 months of additional follow-up, Table 1 shows the probabilities that each arm is chosen under various scenarios.
Table 1.
Statistical assumptions for S0709
| True median PFS (months) Erlotinib alone |
True median PFS (months) Erlotinib + Chemo |
True underlying hazard ratio |
Probability Erlotinib arm is chosen |
Probability Erlotinib + Chemo arm is chosen |
Probability neither arm is chosen |
|---|---|---|---|---|---|
| 1.5 | 3 | 2 | < 0.001 | 0.49 | 0.51 |
| 2 | 3 | 1.5 | 0.01 | 0.48 | 0.51 |
| 2 | 4 | 2 | 0.01 | 0.96 | 0.03 |
| 2.5 | 3 | 1.2 | 0.10 | 0.43 | 0.47 |
| 2.5 | 3.5 | 1.4 | 0.09 | 0.76 | 0.15 |
| 2.5 | 4 | 1.6 | 0.08 | 0.89 | 0.03 |
| 3 | 3 | 1 | 0.49 | 0.24 | 0.27 |
| 3 | 4 | 1.33 | 0.43 | 0.55 | 0.02 |
| 3.5 | 2.5 | 0.71 | 0.83 | 0.02 | 0.15 |
| 4 | 2 | 0.5 | 0.96 | < 0.001 | 0.04 |
| 4 | 3 | 0.75 | 0.97 | 0.02 | 0.01 |
| 4 | 4 | 1 | 0.93 | 0.06 | 0.01 |
Study Conduct
The trial activated on 12/1/2008 but was prematurely closed prior to completion of accrual after the FDA determined following study activation that an IDE application was required for the Veristrat proteomics assay. Since SWOG had limited resources available for such filing, the study was administratively closed on 3/20/2013. All PFS and OS events have already occurred.
RESULTS
Patient characteristics
One hundred fifty six patients were screened by serum proteomics; of these, 83 (53%) were found to be Veristrat-Good and allowed to proceed to the randomization step. Thirty-three patients were randomized to Arm 1, 26 to Arm 2. One eligible patient did not receive any protocol treatment, coded as a major protocol deviation, and was not evaluable for adverse events. Patient characteristics are summarized in Table 2.
Table 2.
Patient Characteristics
| Arm 1: Erlotinib alone (n=33) |
Arm 2: Paclitaxel + Carboplatin + Erlotinib (n=26) |
|||
|---|---|---|---|---|
| AGE (years) | ||||
| Median | 74.9 45.2 84.9 |
70.8 40.9 85.9 |
||
| Minimum | ||||
| Maximum | ||||
| SEX | ||||
| Males | 14 | 42% | 10 | 38% |
| Females | 19 | 58% | 16 | 62% |
| HISPANIC | ||||
| Yes | 1 | 3% | 1 | 4% |
| No | 29 | 88% | 22 | 85% |
| Unknown | 3 | 9% | 3 | 12% |
| RACE | ||||
| White | 29 | 88% | 19 | 73% |
| Black | 1 | 3% | 3 | 12% |
| Asian | 2 | 6% | 2 | 8% |
| Pacific Islander | 1 | 3% | 0 | 0% |
| Unknown | 0 | 0% | 2 | 8% |
| HISTOLOGY | ||||
| Adenocarcinoma | 27 | 82% | 23 | 88% |
| Squamous | 5 | 15% | 2 | 8% |
| Large cell | 0 | 0% | 1 | 4% |
| Other | 1 | 3% | 0 | 0% |
| SMOKING HISTORY | ||||
| Current | 10 | 30% | 9 | 35% |
| Former | 17 | 52% | 11 | 42% |
| Never | 6 | 18% | 6 | 23% |
| STAGE | ||||
| IIIB (unresectable) | 1 | 3% | 1 | 4% |
| IV | 32 | 97% | 25 | 96% |
| WEIGHT LOSS PAST 6 MONTHS | ||||
| < 5% | 14 | 42% | 16 | 62% |
| 5 – < 10% | 14 | 42% | 7 | 27% |
| 10% – 20% | 3 | 9% | 1 | 4% |
| > 20% | 1 | 3% | 1 | 4% |
| Not reported | 1 | 3% | 1 | 4% |
Efficacy
Efficacy results are summarized in Table 3. In Arm 1, two patients had either a confirmed or unconfirmed partial response for an estimated response rate of 6% (95% confidence interval: 1% – 21%). Eleven patients had a best response of stable disease, for an estimated disease control rate of 41% (95% confidence interval: 24% – 59%). In Arm 2, six patients had either a confirmed or unconfirmed partial response for an estimated response rate of 23% (95% confidence interval: 9% – 44%). Fourteen patients had a best response of stable disease for an estimated disease control rate of 77% (95% confidence interval: 56% – 91%). A Fisher's exact test was used to compare response and disease control rate between the arms and the two-sided p-values were 0.06 and 0.0046 respectively.
Table 3.
Summary of Efficacy Results
| Arm 1: Erlotinib alone (N=33) |
Arm 2: Erlotinib + Carboplatin/Paclitaxel (N=26) |
p-value | |
|---|---|---|---|
| Response rate* (n, %) | 2/32 (6%) | 6/26 (23%) | 0.06 |
| Disease control rate* (n, %) | 13/32 (41%) | 20/26 (77%) | 0.0046 |
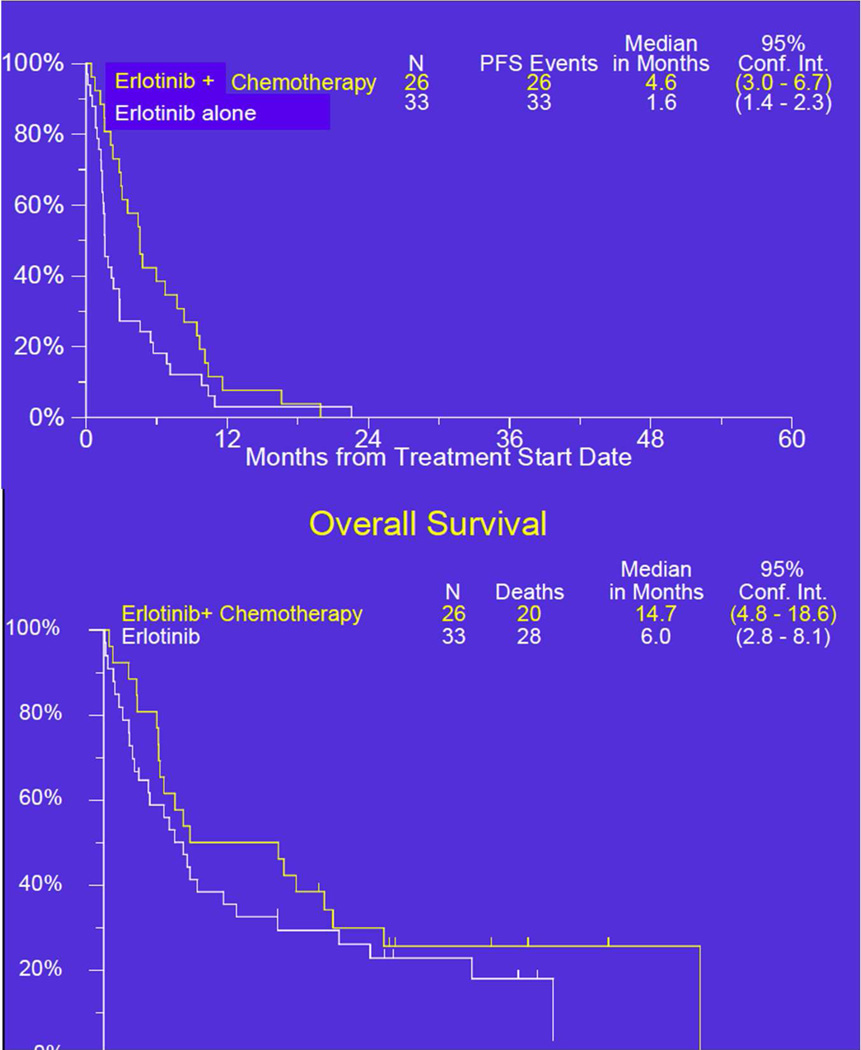
| Median PFS, months (95% CI) | 1.6 (1.4, 2.3) | 4.6 (3.0, 6.7) | 0.06 |
| Median OS, months (95% CI) | 6 (2.8, 8.1) | 11 (4.8, 18.5) | 0.27 |
subset of patients with measurable disease at baseline.
Kaplan Meier curves for PFS and OS are shown in Figure 2. The combination arm had the superior observed median PFS and also surpassed the protocol specified benchmark of 3 months. Additionally, the lower bound of the confidence interval was at 3.0, representing the benchmark value to recommend further investigation. The two-sided p-value (log-rank) was 0.06.
Figure 2. Kaplan Meier survival curves for progression-free and overall survival.
Toxicity
Of 32 patients in Arm 1 assessed for adverse events (AEs), two experienced treatment-related Grade 4 serious AEs (thrombosis/embolism, hypomagnesemia). Of 26 patients in Arm 2 assessed for AEs, five had treatment-related Grade 4 AEs due to neutropenia (5), febrile neutropenia (1), and leukopenia (1). There were no treatment related deaths. Toxicity results are summarized in Table 4.
Table 4.
Toxicity
| ADVERSE EVENT | Arm 1: Erlotinib (n=32) |
Arm 2: Erlotinib+ Chemotherapy (n=26) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 32 | 0 | 0 | 0 | 0 | 0 | 22 | 2 | 0 | 2 | 0 | 0 |
| Blood/Bone Marrow | 26 | 3 | 3 | 0 | 0 | 0 | 6 | 3 | 7 | 6 | 4 | 0 |
| Cardiac General | 29 | 1 | 1 | 1 | 0 | 0 | 23 | 0 | 1 | 2 | 0 | 0 |
| Constitutional symptoms | 18 | 3 | 10 | 1 | 0 | 0 | 10 | 9 | 6 | 1 | 0 | 0 |
| Dermatology/Skin | 7 | 9 | 16 | 0 | 0 | 0 | 6 | 4 | 15 | 1 | 0 | 0 |
| Gastrointestinal | 10 | 8 | 11 | 3 | 0 | 0 | 6 | 4 | 9 | 7 | 0 | 0 |
| Infection | 26 | 0 | 6 | 0 | 0 | 0 | 23 | 0 | 0 | 1 | 2 | 0 |
| Metabolic/Laboratory | 23 | 4 | 3 | 1 | 1 | 0 | 12 | 9 | 3 | 2 | 0 | 0 |
| Musculoskeletal/Soft Tissue | 29 | 0 | 2 | 1 | 0 | 0 | 21 | 3 | 1 | 1 | 0 | 0 |
| Neurology | 30 | 1 | 1 | 0 | 0 | 0 | 15 | 4 | 4 | 3 | 0 | 0 |
| Pain | 26 | 3 | 3 | 0 | 0 | 0 | 17 | 7 | 1 | 1 | 0 | 0 |
| Pulmonary/Upper Respiratory | 28 | 2 | 1 | 1 | 0 | 0 | 23 | 2 | 1 | 0 | 0 | 0 |
| Renal/Genitourinary | 30 | 1 | 1 | 0 | 0 | 0 | 24 | 0 | 1 | 1 | 0 | 0 |
| Vascular | 31 | 0 | 0 | 0 | 1 | 0 | 25 | 0 | 0 | 1 | 0 | 0 |
| MAX. GRADE ANY ADVERSE EVENT | 3 | 5 | 18 | 4 | 2 | 0 | 2 | 0 | 4 | 15 | 5 | 0 |
DISCUSSION
The optimal treatment for metastatic NSCLC patients with PS2 has not yet been fully defined. Nevertheless, in the past decade, several attempts have been made to prospectively evaluate the tolerability and efficacy of various systemic treatment approaches in PS2 patients in the context of modestly sized clinical trials. These trials have generally attempted to answer relevant treatment intensity questions, such as comparing one versus two drugs, or testing sequential therapy approaches. In CALGB 9730, the subgroup of PS2 patients had a higher response rate, PFS, and OS with carboplatin/paclitaxel as compared to single-agent paclitaxel. [2] In SWOG 0027, sequential single agent chemotherapy with vinorelbine followed by docetaxel in patients ≥ 70 years of age and/or had a PS of 2 yielded an overall response rate of 11%, median PFS of 2.6 months, and median OS of 5.5 months [12]. SWOG 0341 showed that erlotinib resulted in uniformly disappointing outcomes with PFS and OS of 2.1 and 5 months, respectively.[12] Lillenbaum, et. al. subsequently reported the results of a randomized phase II trial of erlotinib versus carboplatin/paclitaxel in PS2 patients which showed that outcomes with erlotinib appeared to be inferior to that of standard chemotherapy.[13] Notably, these two latter trials were both designed with an “all comers” strategy, with no form of molecular enrichment.
Until recently, there have been very few attempts to enrich the PS2 population using modern molecular techniques to find subsets of patients more likely to benefit from treatment. S0709 was designed to explore the possibility of enriching the PS2 population for patients most likely to benefit from EGFR TKI therapy. Proteomic profiling was selected for several reasons. Early reports had shown that a proteomic signature in serum was significantly associated with survival outcome in patients with advanced NSCLC [2,14] and it was associated with benefit from erlotinib therapy.[5,7] In one study, mass spectra from patient sera were independently obtained at two institutions, were found to be highly concordant, and were used to generate an algorithm predictive of time to progression and OS.[3] This prediction algorithm was then validated in a blinded manner in two independent cohorts of NSCLC patients treated with EGFR TKIs. Subsequently, proteomic profiling became highly feasible with the availability of a laboratory-developed, commercially available assay (Veristrat).[15] Convenient and accessible serum-based approaches were thought to be more optimal for PS2 patients who would otherwise not be amenable to additional tumor biopsies and would require a rapid turnaround of molecular enrichment results in order to initiate therapy more quickly.
Limitations of S0709 include its modest sample size due to early administrative closure preventing completion of full accrual and that the proteomics classifier employed was later found to be a “negative predictive biomarker” rather than one that selects for which patients preferentially benefit from erlotinib.
In conclusion, PS2 patients with advanced NSCLC and “good” classification by serum proteomics treated with pharmacodynamically-separated erlotinib plus chemotherapy experienced better observed median PFS/OS versus patients treated with erlotinib alone. The combination regimen also surpassed the protocol-specified benchmark of PFS >= 3 months required for further study. This trial provides no evidence to suggest that conventional doublet chemotherapy should not remain the standard of care even in highly selected PS2 patients. Finally, we were able to demonstrate the feasibility of developing and conducting a molecular enrichment trial in a unique subset of NSCLC patients with impaired performance status within the cooperative group setting.
Acknowledgements
This investigation was supported in part by the following PHS/DHHS grant numbers awarded by the National Cancer Institute (NCI), National Clinical Trials Network (NCTN): CA180888, CA180819, CA180846, CA180818, CA180830; by the NCI Community Oncology Research Program (NCORP): CA189821, CA189971, CA189953, CA189830, CA189822, CA 189872, CA 189858; and in part by Biodesix and Genentech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rodriguez E, Lilenbaum RC. New treatment strategies in patients with advanced non-small-cell lung cancer and performance status 2. Clinical lung cancer. 2008;9:326–330. doi: 10.3816/CLC.2008.n.047. [DOI] [PubMed] [Google Scholar]
- 2.Kikuchi T, Carbone DP. Proteomics analysis in lung cancer: challenges and opportunities. Respirology. 2007;12:22–28. doi: 10.1111/j.1440-1843.2006.00957.x. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi F, Solomon B, Gregorc V, et al. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. Journal of the National Cancer Institute. 2007;99:838–846. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 4.Stinchcombe TE, Roder J, Peterman AH, et al. A retrospective analysis of VeriStrat status on outcome of a randomized phase II trial of first-line therapy with gemcitabine, erlotinib, or the combination in elderly patients (age 70 years or older) with stage IIIB/IV non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:443–451. doi: 10.1097/JTO.0b013e3182835577. [DOI] [PubMed] [Google Scholar]
- 5.Carbone DP, Salmon JS, Billheimer D, et al. VeriStrat classifier for survival and time to progression in non-small cell lung cancer (NSCLC) patients treated with erlotinib and bevacizumab. Lung cancer. 2010;69:337–340. doi: 10.1016/j.lungcan.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone DP, Ding K, Roder H, et al. Prognostic and predictive role of the VeriStrat plasma test in patients with advanced non-small-cell lung cancer treated with erlotinib or placebo in the NCIC Clinical Trials Group BR.21 trial. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1653–1660. doi: 10.1097/JTO.0b013e31826c1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautschi O, Dingemans AM, Crowe S, et al. VeriStrat(R) has a prognostic value for patients with advanced non-small cell lung cancer treated with erlotinib and bevacizumab in the first line: pooled analysis of SAKK19/05 and NTR528. Lung cancer. 2013;79:59–64. doi: 10.1016/j.lungcan.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper JL, Lind JS, Groen HJ, et al. VeriStrat((R)) has prognostic value in advanced stage NSCLC patients treated with erlotinib and sorafenib. British journal of cancer. 2012;107:1820–1825. doi: 10.1038/bjc.2012.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. The lancet oncology. 2014;15:713–721. doi: 10.1016/S1470-2045(14)70162-7. [DOI] [PubMed] [Google Scholar]
- 10.Davies AM, Ho C, Lara PN, Jr, et al. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clinical lung cancer. 2006;7:385–388. doi: 10.3816/CLC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 11.Sangha R, Davies AM, Lara PN, Jr, et al. Intercalated erlotinib-docetaxel dosing schedules designed to achieve pharmacodynamic separation: results of a phase I/II trial. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:2112–2119. doi: 10.1097/JTO.0b013e31822ae061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesketh PJ, Chansky K, Wozniak AJ, et al. Southwest Oncology Group phase II trial (S0341) of erlotinib (OSI-774) in patients with advanced non-small cell lung cancer and a performance status of 2. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:1026–1031. doi: 10.1097/JTO.0b013e318183aa1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:863–869. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 14.Chung CH, Levy S, Chaurand P, et al. Genomics and proteomics: emerging technologies in clinical cancer research. Critical reviews in oncology/hematology. 2007;61:1–25. doi: 10.1016/j.critrevonc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Pinelo S, Pastor MD, Paz-Ares L. VeriStrat: a prognostic and/or predictive biomarker for advanced lung cancer patients? Expert review of respiratory medicine. 2014;8:1–4. doi: 10.1586/17476348.2014.861744. [DOI] [PubMed] [Google Scholar]