Abstract
Traumatic brain injury (TBI) is a devastating neurological disorder that usually presents in acute and chronic forms. Brain edema and associated increased intracranial pressure in the early phase following TBI are major consequences of acute trauma. On the other hand, neuronal injury, leading to neurobehavioral and cognitive impairments, that usually develop months to years after single or repetitive episodes of head trauma, are major consequences of chronic TBI. The molecular mechanisms responsible for TBI-induced injury, however, are unclear. Recent studies have suggested that early mitochondrial dysfunction and subsequent energy failure play a role in the pathogenesis of TBI. We therefore examined whether oxidative metabolism of 13C-labeled glucose, lactate or glutamine is altered early following in vitro mechanical percussion-induced trauma (5 atm) to neurons (4–24 h), and whether such events contribute to the development of neuronal injury. Cell viability was assayed using the release of the cytoplasmic enzyme lactate dehydrogenase (LDH), together with fluorescence-based cell staining (calcein and ethidium homodimer-1 for live and dead cells, respectively). Trauma had no effect on the LDH release in neurons from 1 h to 18 h. However, a significant increase in LDH release was detected at 24 h after trauma. Similar findings were identified when traumatized neurons were stained with fluorescent markers. Additionally 13C-labeling of glutamate showed a small, but statistically significant decrease at 14 h after trauma. However, trauma had no effect on the cycling ratio of the TCA cycle at any time-period examined. These findings indicate that trauma does not cause a disturbance in oxidative metabolism of any of the substrates used for neurons. Accordingly, such metabolic disturbance does not appear to contribute to the neuronal death in the early stages following trauma.
Keywords: Cell death, GC-MS, oxidative metabolism, neuron, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is an important clinical condition which is associated with a high morbidity and mortality. It is estimated that 7.7 million individuals who have experienced a TBI in the European Union have neurological disabilities [1]. TBI affects 3 out of every 1000 Americans accounting for as many as 56,000 deaths and hundreds of thousands hospital admissions per year [2]. Approximately 200,000 individuals in the US live with disabilities resulting from TBI [1, 3], which is associated with immense socio-economic consequences.
While the mechanisms responsible for tissue injury in TBI remain poorly understood, a number of factors have been implicated in its mechanisms; among these include the loss of plasma membrane integrity and depolarization [4,5]; loss of ion homeostasis (in particular Ca2+, K+ and Na+) [4, 6]; development of brain edema, ischemia, hyperthermia [7, 8]; inflammation [9]; glutamate-induced excitotoxicity [6, 10]; and the production of reactive oxygen species (ROS) [11]. However, current treatment strategies aimed at reducing or preventing TBI associated complications based on the above observations are unsatisfactory, as the precise pathophysiological events occurring after TBI remain incompletely understood.
In addition to the above mentioned factors observed after TBI, various studies indicate that neuronal energy metabolism is affected after TBI which may contribute to the development of neuronal injury [12–15]. Recently, Robertson et al. [16] and Casey et al., [17], showed mitochondrial dysfunction, as well as a decrease in the neuronal marker, N-acetylaspartate, early after traumatic insults (4 h). However, using [13C]glucose and NMR spectroscopy, Robertson et al. [16] reported that oxidative glucose metabolism was not altered at 24 h after trauma in rat brain. Additionally, Bartnik et al. [18], reported that the entry of [1,2-13 C]glucose into the TCA cycle was not inhibited, and that oxidative metabolism in glutamatergic neurons was maintained after TBI in adult rats as opposed to a decrease in oxidative metabolism 6 h post-injury in immature rat brains [15]. Our study was therefore designed to investigate the status of oxidative metabolism of glucose and other energy substrates early (6–24 h) after in vitro trauma to cultured neurons.
Since neurons are known to be able to employ glucose, lactate, and glutamine as metabolic substrates, cultured neurons were incubated in media containing 13C-labeled substrates post-trauma, and changes in the metabolism of glucose, lactate and glutamine were measured at different time-periods [19]. Cellular integrity and viability were also examined using the lactate dehydrogenase (LDH) release assay [20, 21] in combination with the LIVE/DEAD® viability/cytotoxicity assay [22] to correlate the possible changes in neuronal metabolism with cell injury.
Materials and Methods
Neuronal Cultures
Cerebral cortical neuronal cultures were prepared by a modification of the method described by Hertz et al. [23]. Briefly, cortices were removed from 16–18 day-old rat fetuses and placed in DMEM-high glucose (30 mM) containing 25 mM KCl and 10% horse serum. The tissue was minced and mechanically dissociated with a pipette. Approximately 1–2 × 106 cells per ml were seeded onto poly-D-lysine-coated 35 mm culture dishes. To prevent the proliferation of astrocytes, cytosine arabinoside (10 mM) was added to the culture medium 48 hours after seeding. These cultures consist of at least 90% neurons as determined by immunohistochemical staining for neurofilament protein; the remaining cells were principally astrocytes. Experiments were performed on cultures that were 10–12 days old.
Cytotoxicity assays
The activity of lactate dehydrogenase (LDH) was measured as previously described by Wroblewski and LaDue, [24] with modifications [21]. Released LDH was calculated as the percentage of LDH in the medium versus total LDH activity (cells plus medium).
Cytotoxicity was also measured by the LIVE/DEAD® Viability/Cytotoxicity assay using a kit (Cat# L-3224, Life Technologies). Briefly, at different time points after trauma (3, 6, 12, 18, 24 h), traumatized and non-traumatized neuronal cultures were washed twice with DMEM without phenol red and serum, and incubated with calcein-AM and ethidium homodimer-1 simultaneously for 20 min at 37°C. Cells were then visualized with a Nikon Diaphot inverted fluorescence microscope equipped with multi-variant fluorescence filters as described previously [22]. The number of calcein-AM-stained cells (green fluorescence, live cells), as well as ethidium homodimer-1-postive (red fluorescence, dead cells) nuclei were counted from 15 random fields of the culture plate (10x objective) using the Sigma Scan Pro 5 program [22]. The intensity of the green/red fluorescence ratio was also measured using the Sigma Scan Pro 5 program, and the results were expressed as a percent change over control.
In Vitro Trauma
Since cellular heterogeneity and differential cellular sensitivity to injury are important characteristics of the CNS, and as changes in neuronal energy metabolism have been implicated in the pathogenesis of TBI, we examined whether neuronal metabolism of glucose, lactate and glutamine might be affected after trauma. We therefore used a fluid percussion injury (FPI) model of in vitro cell injury capable of accurately delivering 1–5 atms of pressure [25–27]. The system is identical to the one used in numerous in vivo studies, but which has been modified for cell cultures [26–28]. The in vitro system has the unique advantage of clearly delineating mechanisms and analyzing the responses to trauma in different cell types (neurons and astrocytes). We have found that this system reliably produces consistent degrees of traumatic injury [28–30].
Additionally, many of the findings occurring in TBI in vivo, including neuronal death in the early stages following trauma (24 h) [31–33]; changes in neuronal proteins (i.e., altered synaptic proteins including PSD95, NMDA-R, synaptotagmin, synaptophysin and NADPH oxidase activity [34–38]; altered neuronal signaling systems (MAPKs activity and changes in transcription factor levels) [39, 30]; and characteristic morphologic changes [40] are also observed in these cultures following mechanical trauma. Additionally, we previously documented that following in vitro mechanical trauma to cultured astrocytes, changes in various intracellular signaling systems, as well as in cell swelling occurred [27–30], and that such changes correlated well with in vivo findings [41, 32]. Altogether, these findings strongly suggest that our in vitro cell injury system is indeed a highly suitable system for investigating mechanisms of trauma-induced injury to neural cells.
Briefly, the injury chamber was coupled to the fluid percussion injury device with non-distensible Tygon tubing, and the piston was percussed by the weighted pendulum at varying angles of incidence [27–30]. Five atmospheres of pressure were administered twice for a 25-milliseconds duration each. Pressures were continuously recorded with a PowerLab system (AD Instruments, Inc., Colorado Springs, CO) interfaced with a high-speed pressure transducer. Sham controls were treated exactly like the traumatized cells, except that fluid percussion was omitted.
Metabolic studies
Cultured neurons were incubated in media containing a combination of 13C-labeled and non-labeled substrates (glucose, lactate and glutamine), and gas chromatography-mass spectrometry (GC-MS) was used to obtain critical information about neuronal metabolism (for references see Walls et al. [19]). Accordingly, our study was designed to gain information about changes in the metabolic handling of glucose, lactate and glutamine after exposure of cultured cortical neurons to FPI. The culture dishes were filled with 1.5 ml fresh culture medium and subjected to trauma as described above. Following trauma, the medium was removed and the cultures were washed twice with PBS. The cultures were then provided with 1.5 ml of fresh medium, and returned to the incubator for 4, 12 or 16 hours, after which the culture media were exchanged with a similar medium containing uniformly 13C-labeled substrate in combination with the unlabeled co-substrate; i.e., one labeled substrate plus the unlabeled co-substrates glucose (2.5 mM), lactate (1 mM), or glutamine (0.5 mM), and then incubated for two hours. Thus, for all experiments, three substrates were present, but only one was labeled with 13C. The incubation was terminated by washing twice with ice-cold PBS, and the cultures were lysed in 70% (v/v) ethanol. Following centrifugation, the supernatants (the cell extracts) where adjusted to pH 1–2 with HCl, lyophilized and reconstituted in water for GC-MS analysis. The reconstituted cell extracts were derivatized and analyzed by GC-MS as described elsewhere [42]. The isotopic enrichment was adjusted for natural abundance and calculated according to Biemann [43], and the percentage amounts of the different isotopomers of glutamate (M + 1, M + 2, M + 3, M + 4 and M + 5) thus allowing for the calculation of the percent molecular labeling (MCL), and the cycling ratio of the TCA cycle [17, 29]. MCL (%) is defined as the content of 13C atoms within a metabolite pool, e.g., if glutamate has an MCL (%) of 50, it means that 50% of all carbon atoms in the glutamate pool are 13C [19]. The cycling ratio is defined as the sum of the glutamate isotopomers derived from α-ketoglutarate generated in the second or later turns of the cycle divided by that derived after the first turn [19].
Statistical analysis
Each experimental group consisted of 4 culture dishes per experiment for each time point examined. Experiments were performed in triplicate or quadruplicate, and the cultures were obtained from at least 5–6 separate seedings. The data were subjected to analysis of variance (ANOVA) followed by Tukey’s post-hoc comparisons. At each time point, the experimental cultures were compared to their respective controls.
Results
LDH release
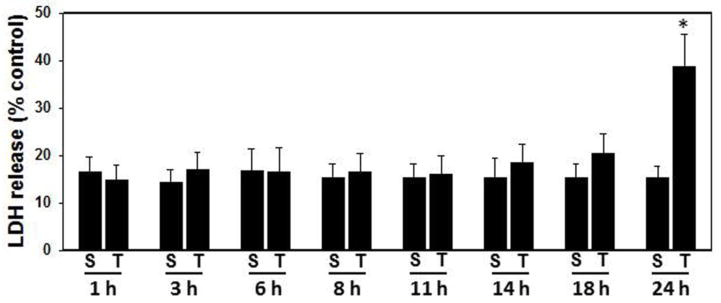
The integrity of the plasma membrane of cultured neurons was assessed by monitoring the release of the cytosolic enzyme lactic acid dehydrogenase (LDH) into the incubation medium. The level of LDH in the medium of sham-treated neurons was 15–16% of that observed in non-incubated control cultures, regardless of the time period after the sham-treatment (Fig. 1). As observed in Fig. 1, percussion-induced trauma (5 atms) had no effect on LDH release from 1–18 h after trauma. However, as shown in Fig. 1, a significant increase in LDH release was detected 24 h after trauma (38%, p<0.05).
Figure 1.
LDH release following trauma (5 atm) to cultured neurons. Percentage of LDH in the medium vs. total LDH activity (cells plus medium) was determined in control (non-traumatized sham) and traumatized neurons. Trauma had no effect on LDH release from 1–18 h after trauma. However, a significant increase in LDH release into the culture medium was detected at 24 after trauma.*p < 0.05 vs. control. S, sham; T, trauma.
Fluorescence labeling of cells
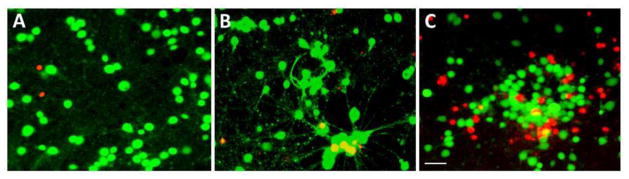
As an additional assay to monitor cell death, we used the fluorescent markers, calcein and ethidium homodimer-1, for identifying live/dead cells. As documented in Fig. 2, the sham-treated and traumatized neurons exhibited a comparable ratio of green and red fluorescing cells. This finding is in accordance with the results of the LDH release assay in that only a small population of cells had been damaged at 18 h after trauma.
Figure 2.
Live/Dead Assay after in vitro trauma (5 atm) in cultured neurons. A) Control (non-traumatized sham) shows baseline live (calcein-AM-positive green fluorescence) and dead (ethidium homodimer-1-postive red fluorescence) cells in non-traumatized control neurons. B). Traumatized neurons at 18 after trauma showed a similar live/dead cell population as observed in sham control. C) Traumatized neurons at 24 h after trauma showed significant cell death, as compared to sham control. Green (calcein-AM) and Red (ethidium homodimer-1). Scale bar = 35 μm.
Biochemical studies
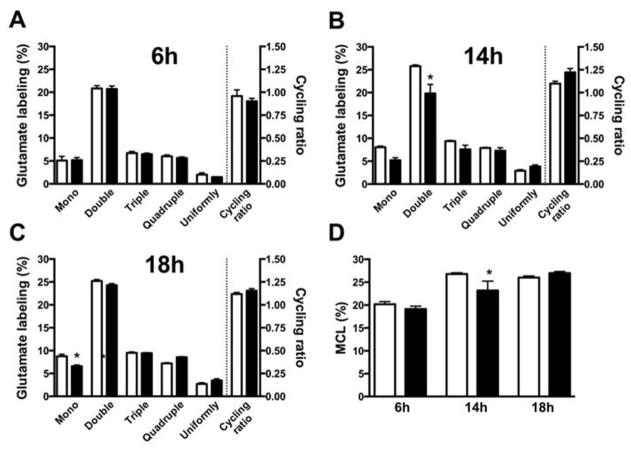
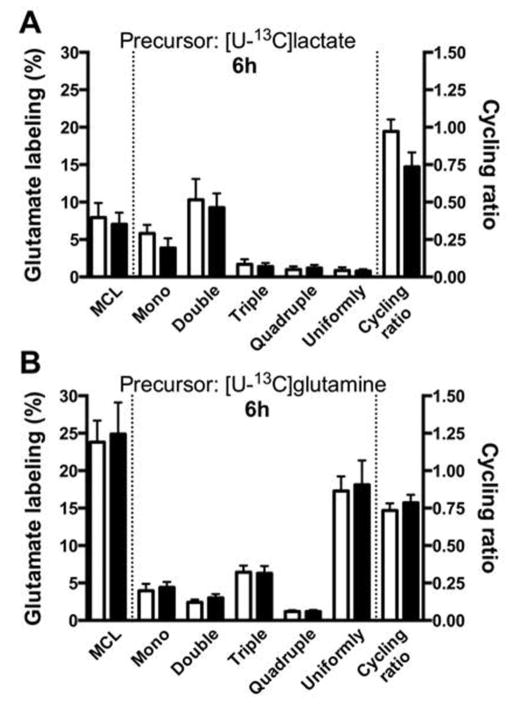
In order to assess the metabolic capacity of traumatized neurons relative to that of sham-treated cells, the cultures were incubated for 2 h in a medium containing a mixture of glucose, lactate and glutamine with one of the substrates labeled uniformly with 13C (see Materials and Methods for details). Results from the experiments using [U-13C]glucose as the labeled substrate are shown in Fig. 3A–D. Fig. 3 A–C show the distribution of isotopomers of glutamate (M + 1; M + 2; M + 3; M + 4 and M + 5) at 6, 14 and 18 hours after trauma in sham-treated and experimental cultures. Only marginal effects on the metabolic pattern were observed in traumatized neurons at 14 and 18 hours post-trauma, which showed a small, but statistically significant decreases in the M + 2 (14 h) and M + 1 (18 h) isotopomers of glutamate. Fig. 3 D summarizes the total molecular carbon labeling (MCL, %) of glutamate which was detected at 14 h after trauma with a small, but statistically significant decrease in the MCL in traumatized cells, likely reflecting the decrease in the M + 2 isotopomer (Fig. 3 B). No effects on the cycling ratio of the TCA cycle was observed at any of the time periods examined (Fig. 3 A–C). When the other substrates (e.g., lactate and glutamine) were 13C-labeled, no effects on labeling were observed in traumatized neurons as compared to sham-treated cultures (results not shown except for the 6 h incubation period, Fig. 4 A,B). To allow for a comparison of the extent of labeling between the different substrates, Fig. 4 A,B documents the isotopomer labeling, MCL, and the cycling ratios for [U-13C]lactate (Fig. 4 A) and [U-13C]glutamine (Fig. 4 B) after a 6 hour incubation period. The labeling intensity for lactate was found to be much lower than that for glucose (see Fig. 3 A, D), while the cycling ratio for lactate was similar to that seen for glucose. When glutamine was used as the labeled substrate (Fig. 4 B) the MCL was comparable to that observed when glucose was used as the labeled substrate (see Fig, 3 D). As the metabolism of glutamine to glutamate is very different from that of glucose, it was not possible to compare the isotopomer distribution between these two substrates.
Figure 3.
Glucose metabolism in cultured neurons is only marginally affected by in vitro trauma. Cultured neurons were subjected to in vitro trauma (see Methods section) and glucose metabolism was assayed by allowing the cultures to metabolize [U-13C]glucose (2.5 mM) for 2h (from 4–6 h post trauma) in the presence of lactate (1 mM) and glutamine (0.5 mM). Panels A, B, and C show the percent 13C-labeling of intracellular glutamate and the calculated TCA cycling ratios at 6, 14, and 18h post trauma, respectively, as determined by mass spectrometry (see Methods and Results). The labeling in glutamate reflects the combined process of glycolysis and oxidative metabolism in the TCA cycle. Panel D summarizes the labeling in intracellular glutamate as molecular carbon labeling (MCL, %) (see Methods section). Small decreases in double-labeled glutamate (B) and MCL (D) were observed after 14 h, but otherwise no effects were detected except for a small decrease in mono-labeled glutamate at 18 h (C), indicating that glucose metabolism is not affected by in vitro trauma. The data are presented as mean ± SEM. Statistics were calculated employing ANOVA, followed by Tukey’s multiple comparisons test. A p-value of 0.05 was considered statistically significant and indicated by an asterisk.
Figure 4.
Metabolism of lactate and glutamine in cultured neurons is not significantly affected by in vitro trauma. Cultured neurons were subjected to in vitro trauma (see Methods section) and (A) lactate and (B) glutamine metabolism were assayed at 6h post trauma by allowing the cultures to metabolize [U-13C]lactate (1 mM) or [U-13C]glutamine (0.5 mM) for 2 h (i.e., from 4–6h post-trauma), respectively. The experiments were performed in the presence of unlabeled glucose (2.5 mM), along with either unlabeled glutamine or lactate, and the labeling in intracellular glutamate was determined by mass spectrometry. The molecular carbon labeling (MCL, %) and TCA cycling ratio were determined as described in the Methods section. No differences were observed, suggesting that the metabolism of lactate or glutamine are not affected 6 h after in vitro trauma. The data are presented as mean ± SEM. Statistics were calculated employing ANOVA, followed by Tukey’s multiple comparisons test. No statistically significant changes were observed.
Discussion
Our findings demonstrate that fluid percussion injury to cultured neurons caused a significant increase in neuronal death at 24 h. However, trauma had no effect on the cycling ratio of the TCA cycle from 6–18 h when glucose was used as the labeled substrate, although a small, but statistically significant decrease in the MCL was observed at 14 h after trauma. The lack of effect on the cycling ratio was also observed for the other two substrates. These findings indicate that trauma did not cause a significant change in oxidative glucose, lactate and glutamine metabolism in neurons, and thus likely did not contribute to the neuronal death observed in the early stages following trauma. However, it should be noted that if ATP production is lower under the present experimental conditions, a certain degree of uncoupling may occur without being detectable in the 13C-labeling. It was further observed that glutamine was extensively metabolized in neurons, a finding similar to that of previous studies [44–47]. By contrast, lactate was metabolized to a much lesser extent than glucose, a finding reported previously by Bak et al. [48]. It may be noted, however, that Bouzier-Sore et al. [49] found that lactate was as efficient, if not better, than glucose as a neuronal energy substrate.
Mitochondrial dysfunction has been frequently reported in brains of animals after experimental TBI (for references, see Introduction). These changes have been identified early after brain injury (1–4 h) which persisted for days-weeks after TBI [50]. Further, changes in key proteins involved in glial and neuronal stress, oxidative metabolism, calcium uptake and neurotransmitter function, as well as significant reductions in the concentration of branched chain amino acids (BCAAs), and associated cognitive impairment were described following experimental TBI [51–52]. Similarly, neuronal death was reported to occur early post-trauma (24 h) [50]. However, it is not clear whether neuronal oxidative glucose metabolism is altered early post-trauma, and whether such alterations play any significant role in the neuronal death observed after trauma.
Brain metabolic changes have also been detected in patients with TBI by NMR spectroscopy [53–55], and such changes were shown to be associated with long-term behavioral and cognitive deficits [56]. Additionally, increased lactate level (from 4–24 h) in the ipsilateral hemisphere of the immature rat brain, as well as decreased N-acetylaspartate (24 h to 7 days), were identified in animal models of TBI [16]. Further, Scafidi et al. [15] showed a decrease in the cycling ratio in glutamate (labeled from glucose metabolism) after TBI in immature rat brain. The current study, however, demonstrates that trauma did not cause any significant change in oxidative metabolism of glucose, lactate or glutamine in neurons in vitro. In particular, we measured the cycling ratio which reflects the activity of the TCA cycle, and found that trauma had no effect on this ratio, indicating that the activity of the TCA cycle was not impaired at any of the time periods examined (6–18 h). On the contrary, the total MCL of glutamate, a parameter reflecting overall metabolism, exhibited a small, but statistically significant decrease at 14 h after trauma. Since neuronal death occurs 24 h after trauma and changes in oxidative glucose metabolism were not detected in neurons prior to this time period (6–18 h), our findings strongly suggest that neuronal oxidative glucose metabolism is not directly involved in the process of neuronal death in the early stages following trauma.
Our findings showing no change in oxidative metabolism 6–18 h post injury differ from those reported by Scafidi et al. [15], who showed a decrease in oxidative metabolism 6 h post-injury in immature rat brains. Bartnik et al. [18], however, found that at 3.5h and 24h after TBI in adult rats, the entry of [1,2-13 C]glucose into the TCA cycle was not inhibited, and that oxidative metabolism in glutamatergic neurons was maintained. It is therefore likely that the differences between the present study, and those reported by Scafidi et al. [15] are that the age of neurons (mature vs. immature), likely played a significant role, since our findings are almost identical to those observed in adult brains after TBI as reported by Bartnik et al. [18].
While the mechanisms by which mechanical trauma to neurons in the early time period caused cell death are unclear, it is possible that increased oxidative stress may have been involved, as we and others have reported that trauma to cultured neurons increased free radical production early on (30–60 min) [26, 57–59]. Since free radicals are well-known to increase a number of signaling systems [29, 30], including the stimulation of various cell death signaling pathways, it is possible that increased free radicals in the initial phase following trauma may have contributed to the neuronal death following TBI.
As noted above, it is also possible that changes (increases/decreases) in the expression of a number of neuronal genes following trauma [60], may have been induced, thereby affecting intracellular signaling systems in neurons, which may have contributed to neuronal death following TBI. Hence, acutely injured and degenerating neurons after TBI may die by a mechanism of cell death involving the activation of discrete signaling pathways. Such pathways include the pro-cell death regulatory pathways initiated by genotoxic (DNA) damage in neurons, possibly as a result of increased production of ROS [61]. Such cellular stresses are linked to the activation of numerous downstream signaling pathways, such as the cyclin-dependent and stress-activated kinases, DNA-dependent protein kinases, ATM and p53 [62]. Cell death signals can also be activated through cell membrane receptors such as the Fas and TNF receptors, as well as by pro-apoptotic factors (Bax, Bak, Bad, Bim Bok, Noxa, PUMA) [62]. Additionally, changes in the mitochondrial membrane permeability that occurs following trauma [63] may release numerous apoptotic mediators from the intra-mitochondrial space, including cytochrome c, endonuclease G and apoptosis-inducing factor, all of which may result in neuronal death [64 and references therein].
In summary, our findings demonstrate that mechanical injury to cultured neurons caused a significant increase in neuronal death; yet, no significant change in oxidative glucose, lactate or glutamine metabolism was identified immediately prior to cell death, indicating that a disturbance in oxidative metabolism in neurons per se does not contribute to the neuronal death in the early stages following trauma. We therefore propose that oxidative stress and the subsequent induction of intracellular signaling systems that are known to be activated in the early phase post-trauma, likely play a major role in the neuronal death in the early phase following CNS trauma.
Acknowledgments
This work was supported by a Merit Review from the US Department of Veterans Affairs and by a National Institutes of Health grant DK063311. The authors thank Alina Fernandez-Revuelta for the preparation of cell cultures and Ms. Anna Hansen for expert technical assistance performing the GC-MS analyses.
References
- 1.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 2.Dutton RP, McCunn M. Traumatic brain injury. Curr Opin Crit Care. 2003;9:503–509. doi: 10.1097/00075198-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 3.National Hospital Discharge Survey (NHDS), 2010; National Hospital Ambulatory Medical Care Survey (NHAMCS), 2010; National Vital Statistics System (NVSS), 2010. All data sources are maintained by the CDC National Center for Health Statistics
- 4.Young W, Yen V, Blight A. Extracellular calcium ionic activity in experimental spinal cord contusion. Brain Res. 1982;253:105–113. doi: 10.1016/0006-8993(82)90677-1. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson P, Hillered L, Ponten V, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10:331–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 6.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 7.Marion DW, Obrist WD, Carlier PM, Penrod LE, Darby JM. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993;79:354–362. doi: 10.3171/jns.1993.79.3.0354. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain in the rat. Acta Neuropathol (Berl) 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- 9.Ott L, McClain CJ, Gillesnie M, Young B. Cvtokines and metabolic dysfunction after& severe head-injury. J Neurotrauma. 1994;11:447–472. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- 10.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 11.Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6:303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 12.Keenan HT, Bratton SL. Epidemiology and outcomes of pediatric traumatic brain injury. Developmental Neuroscience. 2006;28:256–263. doi: 10.1159/000094152. [DOI] [PubMed] [Google Scholar]
- 13.Tilford JM, Aitken ME, Anand KJ, Green JW, Goodman AC, Parker JG, Killingsworth JB, Fiser DH, Adelson PD. Hospitalizations for critically ill children with traumatic brain injuries: a longitudinal analysis. Crit Care Med. 2005;33:2074–2081. doi: 10.1097/01.ccm.0000171839.65687.f5. [DOI] [PubMed] [Google Scholar]
- 14.Robertson CL, Saraswati M, Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- 15.Scafidi S, O’Brien J, Hopkins I, Robertson C, Fiskum G, McKenna M. Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J Neurochem. 2009;109(Suppl 1):189–197. doi: 10.1111/j.1471-4159.2009.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson CL, Saraswati M, Scafidi S, Fiskum G, Casey P, McKenna MC. Cerebral glucose metabolism in an immature rat model of pediatric traumatic brain injury. J Neurotrauma. 2013;30:2066–2072. doi: 10.1089/neu.2013.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey PA, McKenna MC, Fiskum G, Saraswati M, Robertson CL. Early and sustained alterations in cerebral metabolism after traumatic brain injury in immature rats. J Neurotrauma. 2008;25:603–614. doi: 10.1089/neu.2007.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- 19.Walls AB, Bak LK, Sonnewald U, Schousboe A, Waagepetersen Metabolic mapping of astrocytes and neurons in culture using stable isotopes and gas chromotography-mass spectrometry (GC-MS) Neoromethods. 2014;90:73–105. [Google Scholar]
- 20.Frandsen A, Schousboe A. Time and concentration dependency of the toxicity of excitatory amino acids on cerebral neurones in primary culture. Neurochem Int. 1987;10:583–591. doi: 10.1016/0197-0186(87)90088-x. [DOI] [PubMed] [Google Scholar]
- 21.Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochem Res. 1997;22:1443–1447. doi: 10.1023/a:1021994126329. [DOI] [PubMed] [Google Scholar]
- 22.Sinke AP, Jayakumar AR, Panickar KS, Moriyama M, Reddy PVB, Norenberg MD. NFκB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem. 2008;106:2302–2311. doi: 10.1111/j.1471-4159.2008.05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz L, Juurlink BH, Hertz E, Fosmark H, Schousboe A. Preparation of primary cultures of mouse (rat) astrocytes. In: Shahar A, de Vellis J, Vernandakis A, Haber B, editors. A dissection and tissue culture manual for the nervous system. Alan R. Liss; New York: 1989. pp. 105–108. [Google Scholar]
- 24.Wroblewski F, LaDue JS. Lactate dehydrogenase activity in blood. Proc Soc Exptl Biol Med. 1955;90:210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO. Fluid-percussion model of mechanical brain injury in the cat. J Neurosurg. 1976;45:521–534. [PubMed] [Google Scholar]
- 26.Shepard SR, Ghajar JBG, Gianuzzi R, Kupferman S, Hariri RJ. Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J Surg Res. 1991;51:417–424. doi: 10.1016/0022-4804(91)90144-b. [DOI] [PubMed] [Google Scholar]
- 27.Panickar KS, Jayakumar AR, Norenberg MD. Differential response of neural cells to trauma-induced free radical production in vitro. Neurochem Res. 2002;27:161–166. doi: 10.1023/a:1014875210852. [DOI] [PubMed] [Google Scholar]
- 28.Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol. 2008;67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- 29.Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD. Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J Neurochem. 2011;117:437–448. doi: 10.1111/j.1471-4159.2011.07211.x. [DOI] [PubMed] [Google Scholar]
- 30.Jayakumar AR, Tong XY, Ruiz-Cordero R, Bregy A, Bethea JR, Bramlett HM, Norenberg MD. Activation of NF-κB mediates astrocyte swelling and brain edema in traumatic brain injury. J Neurotrauma. 2014;31:1249–1257. doi: 10.1089/neu.2013.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark RS, Kochanek PM, Dixon CE, Chen M, Marion DW, Heineman S, DeKosky ST, Graham SH. Early neuropathologic effects of mild or moderate hypoxemia after controlled cortical impact injury in rats. J Neurotrauma. 1997;14:179–189. doi: 10.1089/neu.1997.14.179. [DOI] [PubMed] [Google Scholar]
- 32.Lu KT, Cheng NC, Wu CY, Yang YL. NKCC1-mediated traumatic brain injury-induced brain edema and neuron death via Raf/MEK/MAPK cascade. Crit Care Med. 2008;36:917–922. doi: 10.1097/CCM.0B013E31816590C4. [DOI] [PubMed] [Google Scholar]
- 33.Pleasant JM, Carlson SW, Mao H, Scheff SW, Yang KH, Saatman KE. Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: implications for mechanistic and therapeutic studies. J Neurotrauma. 2011;28:2245–2262. doi: 10.1089/neu.2010.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci U S A. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Zou L, Yuan X, Long Y, Yang K. N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. J Neurosci Res. 2002;67:781–786. doi: 10.1002/jnr.10181. [DOI] [PubMed] [Google Scholar]
- 36.Niesman IR, Schilling JM, Shapiro LA, Kellerhals SE, Bonds JA, Kleschevnikov AM, Cui W, Voong A, Krajewski S, Ali SS, Roth DM, Patel HH, Patel PM, Head BP. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolindeficient mice. J Neuroinflammation. 2014;11:39. doi: 10.1186/1742-2094-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobeissy FH, Ottens AK, Zhang Z, Liu MC, Denslow ND, Dave JR, Tortella FC, Hayes RL, Wang KK. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics. 2006;5:1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Ding JY, Kreipke CW, Schafer P, Schafer S, Speirs SL, Rafols JA. Synapse loss regulated by matrix metalloproteinases in traumatic brain injury isassociated with hypoxia inducible factor-1alpha expression. Brain Res. 2009;1268:125–134. doi: 10.1016/j.brainres.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Otani N, Nawashiro H, Fukui S, Nomura N, Yano A, Miyazawa T, Shima K. Differential activation of mitogen-activated protein kinase pathways after traumatic braininjury in the rat hippocampus. J Cereb Blood Flow Metab. 2002;22:327–334. doi: 10.1097/00004647-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 40.O’Dell DM, Raghupathi R, Crino PB, Eberwine JH, McIntosh TK. Traumatic brain injury alters the molecular fingerprint of TUNEL-positive cortical neurons In vivo: A single-cell analysis. J Neurosci. 2000;20:4821–4828. doi: 10.1523/JNEUROSCI.20-13-04821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu KT, Wu CY, Cheng NC, Wo YY, Yang JT, Yen HH, Yang YL. Inhibition of the Na+-K+-2Cl−-cotransporter in choroid plexus attenuates traumatic brain injury-induced brain edema and neuronal damage. Eur J Pharmacol. 2006;548:99–105. doi: 10.1016/j.ejphar.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 42.Voss CM, Pajęcka K, Stridh MH, Nissen JD, Schousboe A, Waagepetersen HS. AMPK activation affects glutamate metabolism in astrocytes. Neurochem Res. 2015 Apr 7; doi: 10.1007/s11064-015-1558-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Biemann K. Mass spectrometry. McGraw-Hill; New York: 1962. Organic chemistry applications; pp. 223–227. [Google Scholar]
- 44.Bradford HF, Ward HK, Thomas AJ. Glutamine - a major substrate for nerve endings. J Neurochem. 1978;30:1453–1459. doi: 10.1111/j.1471-4159.1978.tb10477.x. [DOI] [PubMed] [Google Scholar]
- 45.Hertz L, Drejer J, Schousboe A. Energy metabolism in glutamatergic neurons, GABAergic neurons and astrocytes in primary cultures. Neurochem Res. 1988;13:605–610. doi: 10.1007/BF00973275. [DOI] [PubMed] [Google Scholar]
- 46.Westergaard N, Sonnewald U, Petersen SB, Schousboe A. Glutamate and glutamine metabolism in cultured GABAergic neurons studied by 13C NMR spectroscopy may indicate compartmentation and mitochondrial heterogeneity. Neursci Lett. 1995;185:24–28. doi: 10.1016/0304-3940(94)11216-6. [DOI] [PubMed] [Google Scholar]
- 47.Waagepetersen HS, Sonnewald U, Schousboe A. Compartmentation of glutamine, glutamate and GABA metabolism in neurons and astrocytes: Functional implications. Neuroscientist. 2003;9:398–403. doi: 10.1177/1073858403254006. [DOI] [PubMed] [Google Scholar]
- 48.Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessasary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab. 2006;26:1285–1297. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- 49.Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 50.Robertson CL. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J Bioenerg Biomembr. 2004;36:363–368. doi: 10.1023/B:JOBB.0000041769.06954.e4. [DOI] [PubMed] [Google Scholar]
- 51.Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107:366–371. doi: 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochanek AR, Kline AE, Gao WM, Chadha M, Lai Y, Clark RS, Dixon CE, Jenkins LW. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev Neurosci. 2006;28(4–5):410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, Bloomer C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI) Magn Reson Med. 2009;61:548–559. doi: 10.1002/mrm.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabati M, Zhan J, Govind V, Arheart KL, Maudsley AA. Impact of reduced k-space acquisition on pathologic detectability for volumetric MR spectroscopic imaging. J Magn Reson Imaging. 2014;39:224–234. doi: 10.1002/jmri.24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maudsley AA, Govind V, Levin B, Saigal G, Harris L, Sheriff S. Distributions of magnetic resonance diffusion and spectroscopy measures with traumatic brain injury. J Neurotrauma. 2015;32:1056–1063. doi: 10.1089/neu.2014.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, Harris L, Jagid J, Maudsley AA. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKinney JS, Willoughby KA, Liang S, Ellis EF. Stretch-induced injury of cultured neuronal, glial, and endothelial cells. Effect of polyethylene glycol-conjugated superoxide dismutase. Stroke. 1996;27:934–940. doi: 10.1161/01.str.27.5.934. [DOI] [PubMed] [Google Scholar]
- 58.Zhang JR, Scherch HM, Hall ED. Direct measurement of lipid hydroperoxides in iron-dependent spinal neuronal injury. J Neurochem. 1996;66:355–613. doi: 10.1046/j.1471-4159.1996.66010355.x. [DOI] [PubMed] [Google Scholar]
- 59.Arundine M, Aarts M, Lau A, Tymianski M. Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci. 2004;24:8106–8123. doi: 10.1523/JNEUROSCI.1362-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michael DB, Byers DM, Irwin LN. Gene expression following traumatic brain injury in humans: analysis by microarray. J Clin Neurosci. 2005;12:284–290. doi: 10.1016/j.jocn.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Morrison RS, Kinoshita Y, Johnson MD, Ghatan S, Ho JT, Garden G. Neuronal survival and cell death signaling pathways. Molecular and cellular biology of neuroprotection in the CNS. Adv Exp Med Biol. 2002;513:41–86. doi: 10.1007/978-1-4615-0123-7_2. [DOI] [PubMed] [Google Scholar]
- 62.Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 63.Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- 64.Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]