Abstract
Myeloid-derived suppressor cells (MDSC) are one of the major components of the tumor microenvironment. The main feature of these cells is their potent immune suppressive activity. MDSC are generated in the bone marrow, and in tumor-bearing hosts, migrate to peripheral lymphoid organs and the tumor to contribute to the formation of the tumor microenvironment. Recent findings have revealed differences in the function and fate of MDSC in the tumor and peripheral lymphoid organs. We review these findings here, and in this context we discuss the current understanding as to the nature of these differences, the underlying mechanisms, and their potential impact on the regulation of tumor progression.
Introduction
Abnormal differentiation and function of myeloid cells is a hallmark of cancer. The accumulation of relatively immature and pathologically activated myeloid-derived suppressor cells (MDSC) with potent immunosuppressive activity is common in tumors. MDSC have the ability to support tumor progression by promoting tumor cell survival, angiogenesis, invasion of healthy tissue by tumor cells, and metastases (reviewed in [1]).
There are two different types of MDSC, as identified in studies in both mice and humans: polymorphonuclear MDSC (PMN-MDSC) are morphologically and phenotypically similar to neutrophils, whereas monocytic MDSC (M-MDSC) are similar to monocytes. The morphologic and phenotypic characteristics of both murine and human MDSC have been described in several recent reviews [2-4] and will not be discussed here. In tumor-bearing hosts, MDSC accumulate in peripheral lymphoid organs and tumor tissues, suggesting that the function and fate of MDSC depend on their localization. We are only beginning to elucidate the mechanisms regulating MDSC in different tissue compartments and we will discuss their potential implication on the fate and function of MDSC. The important question is whether those differences play an important role in the ability of MDSC to regulate tumor progression.
Available data strongly suggest that MDSC in peripheral lymphoid organs and the tumor have different functional specialization. MDSC in peripheral lymphoid organs are largely represented by PMN-MDSC with relatively modest suppressive activity and a major role in the regulation of tumor-specific immune responses culminating in the development of tumor-specific T-cell tolerance. Differentiation of M-MDSC to macrophages (MΦ) and dendritic cells (DC) in these tissues is inhibited. In the tumor, MDSC become more suppressive, M-MDSC are more prominent than PMN-MDSC, and M-MDSC rapidly differentiate to tumor associated macrophages (TAM). This suggests that targeting only one branch of myeloid cells (monocytes/macrophages or granulocytes) as well as only intratumoral populations may not be sufficient for achieving therapeutic benefits. It may also suggest that the differences in the mechanisms regulating MDSC function in tumors and peripheral lymphoid organs may affect therapeutic targeting of these cells. For example, a recent study demonstrated that inhibition of STAT3 in tumor-bearing mice resulted in depletion of MDSC in spleens but not in tumors [5].
Here we review evidence indicating different fates and functions for MDSC in tumors versus those in peripheral lymphoid organs. We discuss the current understanding on the mechanisms underlying these differences, including the contribution of the tumor microenvironment. In this context, we outline gaps in understanding and important areas of future research, and discuss the implications of these findings to therapeutic strategies targeting MDSC.
MDSC development and differentiation
MDSC are generated in the bone marrow (BM) from common myeloid progenitor cells. The development of MDSC is governed by a complex network of signals that can be divided into two categories: signals promoting accumulation of immature myeloid cells, and signals providing for the pathological activation of these cells (reviewed in [6]). Changes in the myeloid compartment in cancer are evident in BM, since accumulation of MDSC in BM of tumor-bearing hosts was reported in many studies [7-9]. Pathological activation of MDSC is the result of persistent stimulation of the myeloid compartment with relatively low-strength signals coming from tumors and is characterized by relatively poor phagocytic activity, continuous production of reactive oxygen species (ROS), nitric oxide (NO), and mostly anti-inflammatory cytokines [10]. This is in contrast to myeloid cell activation observed in response to bacteria and viruses, which is characterized by rapid activation of phagocytosis, respiratory burst, and release of proinflammatory cytokines. Normalization of myelopoiesis occurs when inflammation is resolved. MDSC are characterized by a number of biochemical and genomic features that distinguish these cells from neutrophils and monocytes. They include expression of a large amount of NADPH oxidase (Nox2), resulting in increased production of ROS in the form of superoxide anion (O2−), hydrogen peroxide (H2O2), and peroxynitrite (PNT) (ONOO−) [11, 12]; increased expression of arginase 1 (arg1) and nitric oxide synthase 2 (nos2) genes, resulting in increased production of Arg1 and NO [13, 14]; increased expression of the transcriptional regulators c/EBPβ [15] and STAT3 [16, 17]; decreased expression of IRF8[18]; and increased production of S100A8/9 proteins [9, 19]. Additionally, a specific role for miR-142-3p micro-RNA in MDSC was recently suggested [20]. As a result of these changes, MDSC have a decreased capacity to differentiate into mature myeloid cells (macrophages, DCs, mature neutrophils). Hyperproduction of ROS, NO, Arg1, and inhibitory cytokines are responsible for the immune suppressive effects of MDSC (reviewed in [21]).
Regulation of MDSC recruitment to tumors
MDSC are abundant in tumor tissues. Numerous reports describe the presence of MDSC in murine tumors (reviewed in [22, 23]) and recent studies demonstrated that tumor associated MDSC have an important role in tumor progression [24, 25]. In humans, recent studies described the presence of MDSC in glioblastoma [26], urothelial carcinoma [27], pancreatic adenocarcinoma [7], and breast cancer [28]. Monocytes and M-MDSC are actively recruited to primary and metastatic tumor sites [29]. This process is regulated by chemokines produced by the tumor with little specificity in the types of chemokines produced by different tumors (Table 1). CCL2 and CCL5 are the main chemokines implicated in monocyte/M-MDSC migration to tumors [30, 31]. Breast, ovarian and gastric human tumors cultured in vitro secrete CCL2, and MDSC from these patients express cognate CCR2 receptor and migrate towards these chemokines in vitro [32]. CCL2 expression increases with progression of colorectal cancer in humans and deletion of CCL2 in a spontaneous mouse model of colorectal cancer reduced the numbers of colonic MDSC [25]. In SMAD4-deficient colorectal cancer, increased CCL15 expression at the invasive front recruited CCR1-expressing MDSC [33]. The other chemokines reported to induce monocyte recruitment to tumors are CCL7, CXCL8, and CXCL12 [34]. Recently, the chemokine cascade involving CCL2 and CCL3 was described in a mouse model of breast cancer where CCL2 recruited monocytes to the tumor site and CCL3, produced by TAM differentiated from those monocytes, helped retain macrophages in the metastatic site [29]. However, somewhat different results were found in ovarian cancer where the expression of CCR2 was decreased on TAM, presumably as a mechanism to retain TAM in the tumor microenvironment [35]. It is not clear how these results can be reconciled. Apparently, as often occurs with experimental conditions, cell recruitment and retention is controlled by redundant mechanisms, which are prevalent in different tumor types and stages of tumor development. Neutrophils and PMN-MDSC are recruited primarily by CXC chemokines, which include CXCL1, CXCL5, CXCL6, CXCL8, and CXCL12 (Table 1). Moreover, there is evidence suggesting that PMN-MDSC could be recruited to the tumor site in response to CCL2 chemokines, which largely attract monocytes/M-MDSC [25]. It is known that CCL3, in addition to influencing TAM migration, [35] may attract neutrophils [36] and can, thus, regulate both lineages of myeloid cells. In addition to chemokines, sphingosine-1-phosphate (S1P), a bioactive phospholipid overexpressed in tumor tissues, has been shown to be a chemoattractant for PMN [37]. Hypoxia may play a role in the recruitment of PMN-MDSC. Sceneay et al. demonstrated in an orthotopic mammary tumor model that hypoxia in primary tumors promoted pre-metastatic niche formation in secondary organs (lung). PMN-MDSC and NK cells were found to be the major cell types infiltrating the pre-metastatic niche [38]. In a mouse model of hepatocellular carcinoma, large amounts of tissue inhibitor of metalloproteinases led to increased CXCL12 production, promoting CXCR4-mediated recruitment of PMN to sites for pre-metastatic niche formation [39]. In a xenograft model, CXCL8 mediated recruitment of PMN by human melanoma cells helped to tether the melanoma cells to the vascular endothelium and promoted lung metastasis [40]. S100A8 and S100A9 proteins drive the recruitment of PMN and PMN-MDSC to pre-metastatic sites in colon cancer [41] and PMN, via production of S100 proteins, can create a positive feedback loop resulting in the accumulation of more PMN in the pre-metastatic lung [42].
Table 1.
Chemokines recruiting monocytes, neutrophils and MDSC to the tumor
| Chemokine | Cancer | Leukocyte infiltrate | Citation |
|---|---|---|---|
| CCL2 (MCP-1) | Prostate carcinoma | Monocytes/TAM | [99] |
| Anaplastic thyroid carcinoma |
Monocytes/TAM | [100] | |
| Breast, gastric, ovarian cancer |
MDSC | [32] | |
| Colorectal cancer | MDSC | [25] | |
| CCL5 (RANTES) | Breast carcinoma | Monocytes | [101, 102] |
| CXCL5 | Mammary carcinoma (mouse) |
MDSC | [103] |
| Non small cell lung cancer | PMN | [104] | |
| Melanoma (mouse) | PMN-MDSC | [105] | |
| CXCL6 (GCP-2) | Gastrointestinal tumors | PMN | [106] |
| Tongue squamous cell carcinoma |
PMN | [107] | |
| CXCL8 (IL-8) | Head and neck squamous cell carcinoma |
PMN | [107, 108] |
| Gastric carcinoma | PMN | [109] | |
| Bronchioalveolar carcinoma | PMN | [110] | |
| Tongue squamous cell carcinoma |
PMN | [107] | |
| CCL15 (MIP-5) | Colorectal cancer | PMN-MDSC | [103] |
| CXCL12 (SDF-1) | Colorectal cancer | M-MDSC | [111] |
| Mammary carcinoma (mouse) |
MDSC | [26] |
MDSC – total population of MDSC without defining specific subset
Thus, MDSC recruitment to the tumor is controlled by many different factors. These factors are not specific for particular types of cancer and have a high degree of redundancy to support a constant supply of MDSC to the tumor in a constantly changing microenvironment. It suggests that a therapeutic blockade of MDSC recruitment to tumors with inhibition of one specific chemokine may only have limited effects. Targeting of chemokine receptors may be more promising since one receptor may interact with several chemokines. However, only clinical trials could clarify this possibility.
Mechanisms of MDSC-mediated immune suppression are localization dependent
Most studies on MDSC suppression have focused on cells in peripheral lymphoid organs (spleen, lymph nodes) and peripheral blood, mainly due to the technical challenges associated with MDSC isolation from tumors, which requires mechanical and enzymatic treatments that result in low recovery of MDSC and also impact cell function [23]. However, with technical advances in the isolation of myeloid cells from tumor tissues by using more gentle disaggregation of tissues with gentleMACS disaggregator® (Miltenyi) or optimized dissociation protocols (one example described in [43]), it became clear that the mechanisms of MDSC action in the tumor site are different from the ones employed by these cells in peripheral lymphoid organs.
Immune suppression by MDSC in peripheral lymphoid organs involves multiple mechanisms. These mechanisms include production of NO and ROS (including PNT) [12, 44], and elimination of key nutrition factors needed for T cell proliferation by depleting L-arginine (via Arg1) [45], sequestering L-cysteine [46], or reducing local tryptophan levels due to the activity of indole amine 2,3 dioxygenase (IDO) [28]. PNT produced by MDSC can nitrate chemokines and block entry of CD8+ T cells to tumors [47]. MDSC also can produce immunosuppressive cytokines like IL-10 and TGF-β [48], induce T regulatory cells (Tregs) [49], affect NK cell function [50] and use other suppressive mechanisms (Fig. 1). Most of the studies demonstrated that inhibition of T cells by MDSC in peripheral lymphoid organs required close cell-to-cell contact (reviewed in [21]). Evidently, not all of these mechanisms act simultaneously. The specific mechanism depends on the type of MDSC produced in different cancers. In most types of cancers, including lung, breast, colon, renal, head and neck, pancreatic and others, PMN-MDSC represent the major population of MDSC in mouse spleens or lymph nodes and in peripheral blood of cancer patients [51, 52]. However, there is substantial variability in the PMN-MDSC/M-MDSC ratio in different types of cancers, and is especially evident in human cancers. Patients with melanoma and prostate cancer, for example, have a substantially higher proportion of M-MDSC in the peripheral blood than PMN-MDSC [53]. The ratio between PMN-MDSC and M-MDSC is important because these cells use different mechanisms for immunosuppression. PMN-MDSC produce large amount O2-, H2O2, and PNT and much less NO than M-MDSC. Because ROS are very unstable and active only for very short period of time, PMN-MDSC need close cell-to-cell contact to exert their effect on T cells. This contact is provided via antigen-specific interaction with T cells [54]. M-MDSC produce high amounts of NO, Arg1 and immune suppressive cytokines. These molecules have much longer half-life than ROS and require cellular proximity, but not close contact between MDSC and T cells. Because of that, M-MDSC potently suppress non-specific T-cell responses and on a per cell basis, M-MDSC have higher suppressive activity than PMN-MDSC [55-57].
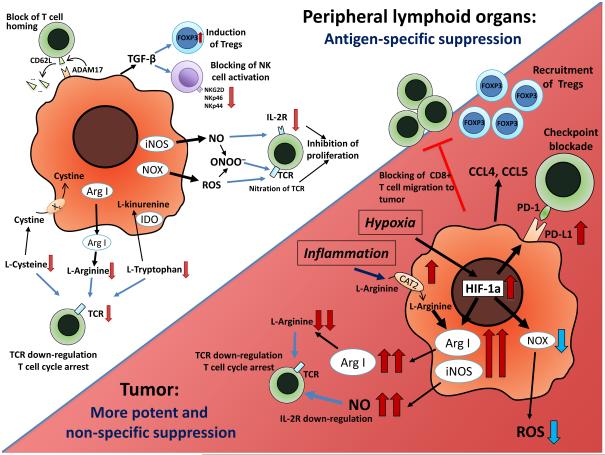
Figure 1. MDSC function in tumor site and peripheral lymphoid organs.
In peripheral lymphoid organs, immune suppression by MDSC is mainly antigen-specific, contact-dependent and utilizes several major pathways, including the production of reactive nitrogen and oxygen species (NO, ROS and PNT), elimination of key nutrition factors for T cells from the microenvironment (L-arginine, L-tryptophan and L-cysteine), disruption of homing of T cells (through the expression of ADAM17), production of immunosuppressive cytokines (TGF-β, IL-10), and induction of T regulatory (Treg) cells. After migration to the tumor, MDSC are exposed to inflammatory and hypoxic tumor microenvironment. This results in significant HIF-1α-mediated elevation of Arg1 and iNOS and downregulation of ROS production, upregulation of inhibitory PD-L1 on MDSC surface, and production of CCL4 and CCL5 chemokines attracting Tregs to the tumor. Overall, these alterations result in more potent non-specific immunosuppressive activity of MDSCs inside the tumor.
A direct comparison of MDSC from spleens and tumors demonstrated that tumor MDSC have a more potent suppressive activity [58-61]. These differences could be explained by changes in the proportion of PMN-MDSC and M-MDSC between tumors and spleens or by changes in functional activity of MDSC. Indeed, whereas PMN-MDSC represent more than 70% of the total population of MDSC in peripheral lymphoid organs, in cells obtained from the tumor, the proportion of M-MDSC is substantially higher [58, 60, 62, 63]. Since, as we discussed above, M-MDSC are more potent suppressors on per cell basis, those difference could result in more potent suppressive activity of the total population of tumor MDSC. At this time, it is not clear whether the differences in the PMN-MDSC/M-MDSC ratio between tumors and spleens are the reflection of preferential migration of M-MDSC to the tumor, their enhanced survival, or selective loss of PMN-MDSC during isolation.
Recent studies analyzing the individual subsets of MDSC indicate that the tumor microenvironment (TME) directly augments MDSC function by changing the properties of these cells. MDSC in RM-1 prostate tumors [60] and 3LL lung tumors [58] had a greater expression of arg1 and nos2 than MDSC in spleens of the same mice, which was associated with significantly stronger suppressive activity of tumor MDSC. Tumor MDSC, in contrast to spleen MDSC, were able to inhibit antigen non-specific CD3/CD28 mediated T-cell proliferation. Elevated suppressive activity of tumor MDSC was associated with a significant increase in the expression of genes associated with fatty acid oxidation (FAO) and was abrogated with FAO inhibitors [58] (Fig. 1). Splenic and tumor-infiltrating MDSC used different mechanisms for immunosuppression in several tumor models (EL4 thymoma, B16.F10 melanoma, and CC10 spontaneous transgenic lung cancer). Splenic MDSC suppressed antigen-specific immune response of T cells in ROS-dependent manner, while tumor-infiltrating MDSC from the same mice demonstrated more potent antigen-specific suppressive activity and also inhibited anti-CD3/28-stimulated response mainly via NO production and secretion of Arg1 [61]. These results were confirmed in MDSC from patients with head and neck cancer. Consistent with these studies, Schlecker et al. demonstrated that arg1 and nos2 were upregulated in tumor M-MDSC, and that ROS levels were decreased in tumor PMN-MDSC [64]. Using the RMA-S lymphoma model, the authors performed genome-wide expression profiling of PMN-MDSC and M-MDSC from blood and tumor of tumor-bearing mice and found increased expression of CCL3 and CCL4 in tumor M-MDSC. Similar results were obtained from the B16 melanoma model with the strongest upregulation observed for CCL3, CCL4 and CCL5. CCL4 and CCL5 were able to attract Tregs in CCR5-dependent manner [64]. Bozkus et al. described the mechanisms regulating MDSC immunosuppressive activity via Arg1 and NO. They demonstrated that the transporter of L-arginine, CAT2 (cationic acid amino transporter 2), could be responsible for the differences in suppressive activity between tumor and spleen MDSC [63]. In the RM1 model of prostate cancer, tumor-infiltrating M-MDSC and PMN-MDSC had more potent suppressive activity than spleen MDSC. This activity was associated with an upregulation of CAT2 and Arg1 in M-MDSC and PMN-MDSC, as well as nos2 in M-MDSC. CAT2−/− tumor M-MDSC and PMN-MDSC had significantly reduced suppressive activity than their wild-type counterparts [63]. Consistent with these observations, Mairhofer et al. reported that tumor PMN-MDSC accumulated in the model of spontaneous melanoma in transgenic (Grm1)EPv mice suppressed melanoma antigen-specific T cells via Arg1, NO, and TGF-β production [62]. The study by Raber et al. confirmed different mechanisms of action for tumor-infiltrating M-MDSC and PMN-MDSC. M-MDSC expressed a high level of inos and used NO as a suppressive molecule. PMN-MDSC expressed nox2 and endothelial NO synthase (nos3) and produced PNT as the main immunosuppressive agent [44] (Fig. 1).
There is now evidence supporting the involvement of ligands for inhibitory checkpoint receptors in immune suppression mediated by tumor MDSC. Tumor-associated hypoxia via hypoxia-inducible factor 1-α (HIF1α), increased expression of PD-L1 on the surface of tumor-infiltrating MDSC, which resulted in more potent suppressive activity of tumor MDSC than splenic MDSC [65]. Expression of PD-L1 in MDSC could be also increased after transfer of tumor-derived exosomes from tumor cells to MDSC in glioma and LLC tumor models. This was associated with increased expression of arg1 and production of TGF-β by MDSC and more potent immune suppressive activity of these cells [66]. The link of arg1 expression with increased expression of inhibitory checkpoint receptors and their ligands was reported in a different study. MDSC from ascites and spleens of ovarian carcinoma-bearing mice expressed PD-1, CTLA-4, PD-L1 and CD80 molecules. The blockade and silencing of either the receptors (PD-1, CTLA-4) or their ligands (PD-L1, CD80) decreased the expression of arg1 as well as the suppressive activity of MDSC [67].
Thus, it appears that there is a consensus in the literature that PMN-MDSC and M-MDSC in the TME are more suppressive than in peripheral lymphoid organs and peripheral blood. This effect is mediated largely via increased expression of various immune suppressive molecules that already exist in MDSC prior to their migration to tumors rather than the emergence of novel mechanisms. As a result, the nature of MDSC mediated immune suppression is changed. In tumors, MDSC acquire the ability to inhibit antigen non-specific T-cell responses. This creates a highly suppressive environment in tumors and prevents rejection of tumors via immune mediated mechanisms. The existence of such non-specific suppression systemically would be highly detrimental to the host, since it would compromise the immune system of the host at early stages of tumor development and would threaten the survival of the host and, therefore, the tumor itself. The mechanisms by which the TME regulate MDSC are discussed below.
The fate of MDSC in tumors and peripheral lymphoid organs
The observations made in the early years of MDSC research concluded that these cells have a decreased ability to differentiate to DCs and MΦ (reviewed in [68]). Those studies were performed with cells isolated from the BM or spleens of tumor-bearing mice or peripheral blood of cancer patients. More recently, it became apparent that those characteristics are associated with M-MDSC, whereas PMN-MDSC are non-proliferating cells that have a short half-life [51]. In recent years, more evidence points out a different pattern of MDSC differentiation in tumors. Under steady state conditions, tissue resident MΦ mainly originate from the yolk sac or fetal liver progenitors and proliferate locally [69, 70]. However, a recent study suggested that classical hematopoietic stem cells were major contributors to tissue MΦ [71]. In cancer, TAM originate primarily from BM-derived blood monocytes/M-MDSC recruited to tumors. By using MMTV-PyMT mice, which express diphtheria toxin receptor (DTR) under control of the Ccr2 locus, Franklin et al. have shown that Ly6C+CCR2+ inflammatory monocytes are required for TAM maintenance [72]. Consistent with these findings, Movahedi et al. have demonstrated that Ly6ChiCX3CR1low monocytes are the main precursors of all subsets of TAM in mammary adenocarcinoma[73]. A distinct subset of monocytes, angiopoietin receptor Tie-2+ monocytes, was shown to migrate to the tumor and differentiate into TAM in mammary carcinoma [74, 75]. Recently, the spleen has been reported to be the reservoir of Ly6Chi monocytes that can migrate to tumors and differentiate into TAM [76]. Interestingly, a significantly higher proportion of splenic precursor cells were found in patients with invasive cancer and upon adoptive transfer into NOD/SCID mice bearing non-small cell lung carcinoma xenograft, they differentiated into CD11b+CD68−CD11c− monocytic-like cells in the spleen and CD68+ macrophages in the tumor [76].
Several factors have been reported to induce monocytes/M-MDSC differentiation to MΦ. Resiquimod, a TLR7/8 agonist, induced differentiation of splenic MDSC into MΦ in 4T-1 mammary carcinoma model [77]. In an ovarian cancer model, thrombin stimulation led to the induction of monocyte differentiation to CD163hiIL-10hiCCL18hiIL-8hi TAM in peritoneum [78]. It was reported that ovarian cancer ascites contained high concentration of Leukemia Inhibitory Factors (LIF) and IL-6, which were involved in monocyte differentiation into TAM [79].
Thus, it appears that in the TME, M-MDSC rapidly differentiate to TAM (Fig. 2). This notion was directly tested in experiments with MDSC differentiation in tumors and spleens of the same mice. MDSC were transferred either directly to the tumor or spleens (via intravenous injection) of EL-4 tumor-bearing mice. In contrast to spleens where MDSC differentiated slowly to MΦ and DCs, in tumors, these cells rapidly differentiated to TAM [61]. A recent study has confirmed this phenomenon and linked it with a different profile of myeloid cells in tumors and spleens of tumor-bearing mice. In spleens of mice bearing various tumors, MDSC were the prevalent population of myeloid cells whereas in tumors of the same mice, TAM were predominant [5]. Thus, it appears that TME not only affects the function of PMN-MDSC and M-MDSC, but it also changes the pattern of M-MDSC differentiation by promoting rapid differentiation into TAM. How important is that process for regulating the immune response in cancer? Would inhibition of M-MDSC differentiation be an attractive therapeutic strategy? Currently there are no data that directly address these questions. The answers may come from experiments that selectively inhibit M-MDSC differentiation and from comparative analyses of the functional activity of M-MDSC and TAM.
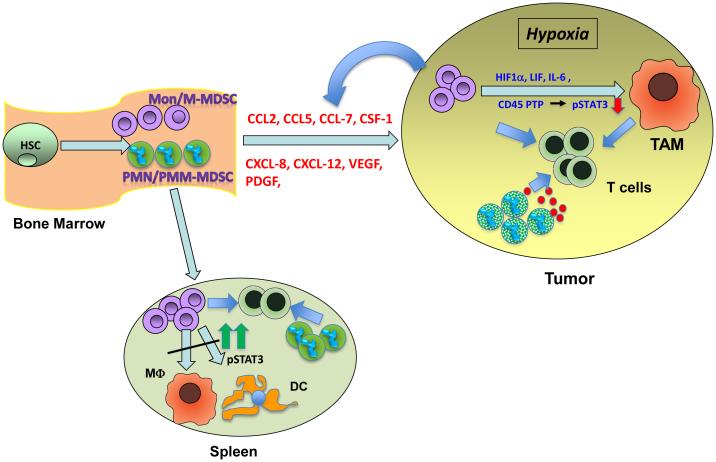
Figure 2. Mechanism of M-MDSC differentiation to tumors and peripheral lymphoid organs.
Mon/M-MDSC are produced in the BM from hematopoietic progenitor cells and recruited to the tumor by several chemokines (described in more details in Table 1). Hypoxic conditions, including HIF1α, prevalent in the TME induces the downregulation of pSTAT3, which results in M-MDSC differentiation to TAM. Other factors known to induce TAM differentiation are activation of CD45 phosphatase, LIF, IL-6, and thrombin. In the spleen, Mon/M-MDSC have a high level of pSTAT3 due to the lack of hypoxic conditions. High STAT3 activity prevents differentiation of Mon/M-MDSC to TAM in the spleen.
The mechanisms regulating the myeloid cell function and differentiation in tumors
What could affect the function and differentiation of myeloid cells in tumors? Recent years have provided evidence in support of the critical role of hypoxia, and specifically HIF1α, in these effects. Exposure of splenic MDSC to hypoxia or chemical stabilization of HIF1α, which prevents its natural degradation, made these cells able to suppress antigen non-specific T cell activation, thus reproducing the effect of the TME [61]. HIF1α also diverted MDSC differentiation towards immune suppressive TAM. MDSC lacking HIF1α did not differentiate into TAM within the TME, but instead acquired markers of DC [61]. The role of HIF1α in regulating the differentiation of MDSC to MΦ was also demonstrated by Liu et al. [80]. Hypoxic conditions inside the tumor upregulated PD-L1 expression on tumor-infiltrating MDSC and resulted in more potent suppressive activity as compared with splenic MDSC. This effect was mediated by HIF1α, but not HIF2α. HIF1α was found to directly bind to a transcriptionally active hypoxia response element in the PD-L1 promoter. Increased PD-L1 expression was not limited only to MDSC, but was also observed in TAM, DC, and tumor cells [81]. A recent study suggested a novel role of hypoxia in the regulation of M-MDSC differentiation to TAM. Hypoxia caused an increase in CD45 tyrosine phosphatase activity in M-MDSC which resulted in the selective decrease of STAT3 activity in myeloid cells in the tumor. The upregulation of CD45 phosphatase activity was mediated by the disruption of CD45 protein dimerization caused by increased sialylation of CD45. Treatment with sialidase abrogated the effect of hypoxia on STAT3 activity in MDSC and differentiation of MDSC to TAM [5].
Changes in oxidative phosphorylation and glycolysis in tumors also affect the function of MDSC. It is now established that to support their rapid proliferation, cancer cells undergo a metabolic shift relying on aerobic glycolysis as the primary means of energy production (Warburg effect). Similarly, activation of myeloid cells leads to a switch towards Warburg metabolism [82]. The metabolism of immune cells in cancer is covered in depth in a recent review [91], but here we focus specifically on the metabolic changes myeloid cells undergo in the TME. The main parameters of such a switch were established for macrophages. Macrophage activation in vitro is typically characterized by two polarized states: M1 and M2 [83]. M1 and M2 macrophages differ in the way they metabolize arginine. M2 macrophages upregulated Arg1 to convert L-arginine to L-ornithine and other polyamines, whereas M1 macrophages upregulated iNos to generate NO [84]. However, in vivo MΦ usually function somewhere in between these two polar conditions. TAM are phenotypically closer to the M2 end of the functional spectrum, although there is evidence indicating that they can display features of M1-like cells [72, 85, 86]. The polarization and function of TAM is dependent on the heterogeneity of the tumor microenvironment in different cancers, and has even been proposed to switch phenotypes through the progression of cancer [75]. TAM, which are exposed to a hypoxic environment and upregulate HIF1α, undergo a glycolytic shift [75]. HIF1α also induces production of NO by TAM, which at the onset of inflammation-induced cancers, can promote tumor development by inducing genetic instability and promoting malignant transformation [87]. Lactic acid, which accumulates in the TME as a result of the Warburg effect, leads to the HIF1α–mediated M2-like polarization of TAM, and contributes to a tumor-promoting phenotype by increasing the expression of VEGFA and Arg1 [88]. However, lactic acid can also upregulate the production of proinflammatory cytokine IL-23 in murine TAM from B16 melanoma [89]. The differential effects of lactic acid on TAM polarization could be due to differences in lactic acid distribution within the tumor, or differences in lactic acid accumulation in different tumors [83]. Because IL-4 promotes FAO and mitochondrial biogenesis in MΦ [90] and Th2 cell-derived IL-4 polarized TAM to an M2 phenotype in mammary carcinoma [91], it is possible that TAM may primarily utilize oxidative phosphorylation over glycolysis. Biswas et al. proposed a model in which the metabolism of TAM shifts with tumor progression: at cancer onset, due to the hypoxic nature of the tumor, TAM are primarily glycolytic, however as the tumor progresses, IL-4 production by Th2 cells and other infiltrates shift the metabolism of TAM towards oxidative phosphorylation and an M2-like phenotype [87]. This hypothesis would need experimental support. Recently, Jha et al. have shown that M2 MΦ are dependent upon glutamine metabolism for UDP-GlcNAc biosynthesis and N-glycosylation, although the role that TME plays in influencing this relationship is not yet known [92]. There are also studies indicating that TAM have differential lipid metabolism leading to either pro- or anti-tumorigenic effects, though further studies are needed to connect lipid metabolism to functional outcome [87] (Fig. 3). Similar to MΦ, under resting conditions, DC rely primarily on oxidative phosphorylation to produce energy, but upon activation, shift to aerobic glycolysis [82]. The increased lactic acid concentration in the TME shifts DC production into a tolerogenic state in response to TLR activation [93]. Moreover, Arg1 and IDO expression in tumor-associated DC (TADC) resulted in an impaired CD8+ T-cell response [94]. As part of lipid metabolism, PGE2 released from tumor cells inhibited DC maturation in an IL-10-dependent manner [95]. TADC upregulate the expression of Msr1, a lipid scavenging receptor, that leads to lipid accumulation and dysfunction in presenting tumor-associated antigens and stimulation of allogeneic T cells [96]. In support of these findings, TADC increased lipid accumulation in an XBP1-dependent manner, leading to inhibition of anti-tumor T cell-response and ultimately promoting ovarian cancer progression [97].
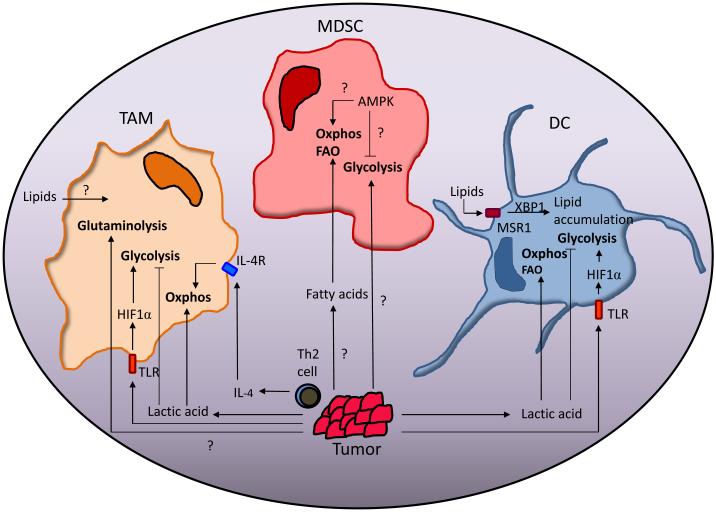
Figure 3. The effect of the tumor microenvironment on the metabolism of myeloid cells.
Lactic acid produced by tumor cells and IL-4 produced by Th2 cells in the TME can drive the metabolism of TAM and TADC towards oxidative phosphorylation (oxphos) while inhibiting glycolysis. Lipids are known to have a role in negative regulation of TADC function. MDSC in peripheral tissue have decreased rates of oxphos and glycolysis compared to tumor-infiltrating MDSC (T-MDSC). Fatty acids derived from the TME drive the metabolism of T-MDSC towards fatty acid oxidation (FAO) and oxphos. Glycolytic rates are also increased in T-MDSC but how the TME influences this process, and the role AMPK plays in this process is unclear.
Since M-MDSC are precursors of MΦ and DC, it is not surprising that changes in oxidative phosphorylation have major impact on their function. Hammami, et al. have shown that in mice, glycolysis increased concurrently with increased Arg1 activity in MDSC. Interestingly, they also note that AMP-activated protein kinase (AMPK) was activated, which normally drives metabolism towards oxidative phosphorylation [98]. In support of this finding, tumor-infiltrating MDSC increased fatty acid uptake and FAO. This was accompanied by an increased mitochondrial mass, upregulation of key FAO enzymes, and increased oxygen consumption rate [58]. Compared to splenic MDSC from a naïve or tumor-bearing mouse, both glycolytic and oxygen consumption rates were increased in tumor MDSC, though based on a comparison of the rates, tumor MDSC primarily used FAO and oxidative phosphorylation as their main metabolic pathway [58]. This is the first evidence that links changes in MDSC function in the tumor with a switch in metabolism in these cells. It appears that this switch is controlled by hypoxia and lactic acid, however, specific mechanisms of this regulation as well as the potential implication for the development of targeted therapeutics remains unclear.
Concluding Remarks
It is now apparent that MDSC play an important role in tumor progression by regulating different aspects of the immune response in cancer. The effect of MDSC depends on their localization. MDSC generated in the BM migrate to peripheral lymphoid organs and tumors. It appears that the migration of M-MDSC and PMN-MDSC to tumors is governed by the same mechanisms as that of neutrophils and monocytes. At this moment, there is no clear evidence that MDSC may have a unique pattern of migration distinct from their control counterparts. However, this issue has not been formally investigated and requires further study (Outstanding Questions). At the systemic level, MDSC contribute largely to tumor antigen-specific T-cell non-responsiveness. Lack of systemic immune suppression in tumor-bearing mice and cancer patients could be a reflection of this phenomenon. It appears that the reason for this could be the prevalence of PMN-MDSC in peripheral lymphoid organs. Due to the nature of immune suppressive mechanisms involved (largely ROS and PNT), these cells require close cell-to-cell contact to inhibit T-cell function. In contrast, in the TME, MDSC contribute largely to non-specific immune suppression. It is well established that MDSC depletion improves T cell immune response and delays tumor progression. However, it is poorly understood whether this is the result of systemic neutralization of MDSC or depends on the depletion of tumor-infiltrating MDSC. Is it enough to block MDSC migration to the tumor or decrease the systemic level of MDSC to achieve an antitumor effect? The answers to these questions are very important for the design of the most effective therapeutics.
The TME has a profound effect on MDSC function and differentiation. Accumulated evidence indicates a substantial enhancement of suppressive activity of both M-MDSC and PMN-MDSC and rapid differentiation of M-MDSC to TAM. Although hypoxia and HIF1α were implicated in this process and some downstream mechanisms were described, the precise molecular mechanism of this phenomenon remains unclear. The TME causes substantial metabolic changes in myeloid cells impacting the function of TAM and DC. Initial studies also demonstrate an important role of this mechanism in regulation of MDSC. Further studies will be required to dissect the metabolic profiles of PMN-MDSC versus M-MDSC, and contributions of glycolysis and oxidative phosphorylation to MDSC function and differentiation in different tumor types.
Outstanding questions.
○ Do MDSC have different migration patterns than neutrophils and monocytes? If this is the case, how do these patterns affect the ability of MDSCs to form pre-metastatic niches and promote tumor metastases? It appears that the migration of M-MDSC and PMN-MDSC to the tumor site is governed by the same mechanisms as neutrophils and monocytes. At this moment, there is no clear evidence that MDSC may have a unique pattern of migration distinct from their control counterparts. However, this issue has not been formally investigated.
○ Do MDSC in peripheral lymphoid organs regulate the immune response in cancer and tumor progression? How do these mechanisms differ from those used by tumor MDSC? MDSC in tumors and peripheral lymphoid organs have different mechanisms of action. It is not clear whether those differences are important for tumor progression.
○ Is it necessary to block MDSC migration to the tumor site in order to have a significant therapeutic effect? Would therapies that decrease the number of MDSCs at a systemic level have a similar effect? There are now various methods to target MDSC in peripheral lymphoid organs and their migration to tumor. However, it is not clear whether either of these methods is sufficient to achieve results in the clinic, or whether combination approaches will be required.
○ What are the molecular mechanisms regulating suppressive activity and differentiation of MDSC in tumors? Although hypoxia and HIF1α were implicated in regulation of MDSC in tumor sites and some downstream mechanisms have been described, a fuller and more precise understanding of the underlying mechanisms is required.
○ How do metabolic changes in tumor MDSC affect their differentiation and function? Initial studies have demonstrated pathways associated with cellular metabolism impact MDSC differentiation and function. However, the mechanisms involved are not well understood, and progress in this regard will be necessary to inform therapeutic manipulation.
Trends Box.
○ In tumor-bearing hosts, MDSC are generated in the bone marrow and migrate to peripheral lymphoid organs and tumor tissues. This process is controlled by a set of defined chemokines, many of which are upregulated in cancer.
○ In peripheral lymphoid organs, M-MDSC differentiate to macrophages and dendritic cells. In contrast, in the tumor site, M-MDSC have an altered differentiation pattern, rapidly differentiating to tumor associated macrophages.
○ The nature of tumor MDSC suppression is different from that of MDSC in peripheral lymphoid organs. As a result, both PMN-MDSC and M-MDSC in the tumor site have more potent suppressive activity than their counterparts in peripheral lymphoid organs. In contrast to MDSC in peripheral lymphoid organs, tumor MDSC suppress T cells in an antigen non-specific manner.
○ Hypoxia appears to play a critical role in the regulation of MDSC differentiation and function in tumors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Condamine T, et al. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ugel S, et al. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125:3365–3376. doi: 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar V, et al. CD45 phosphatase regulates the fate of myeloid cells in tumor microenvironment by inhibiting STAT3 activity Immunity. 2016 in press. [Google Scholar]
- 6.Condamine T, et al. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015 doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porembka MR, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capietto AH, et al. Down-regulation of PLCgamma2-beta-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. J Exp Med. 2013;210:2257–2271. doi: 10.1084/jem.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha P, et al. Proinflammatory s100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youn JI, et al. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corzo CA, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaraj S, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Highfill SL, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arina A, Bronte V. Myeloid-derived suppressor cell impact on endogenous and adoptively transferred T cells. Curr Opin Immunol. 2015;33:120–125. doi: 10.1016/j.coi.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Marigo I, et al. Tumor-Induced Tolerance and Immune Suppression Depend on the C/EBP? Transcription Factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nefedova Y, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 18.Waight JD, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng P, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonda N, et al. miR-142-3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38:1236–1249. doi: 10.1016/j.immuni.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, et al. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueha S, et al. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–788. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Damuzzo V, et al. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry. Part B, Clinical cytometry. 2015;88:77–91. doi: 10.1002/cyto.b.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun E, et al. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell reports. 2015;12:244–257. doi: 10.1016/j.celrep.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raychaudhuri B, et al. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J Neurooncol. 2015;122:293–301. doi: 10.1007/s11060-015-1720-6. [DOI] [PubMed] [Google Scholar]
- 27.Eruslanov E, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–1119. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murdoch C, et al. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 31.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang B, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Letters. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Inamoto S, et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through CCL15-CCR1 Chemokine Axis. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0726. [DOI] [PubMed] [Google Scholar]
- 34.Allavena P, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Sica A, et al. Defective Expression of the Monocyte Chemotactic Protein-1 Receptor CCR2 in Macrophages Associated with Human Ovarian Carcinoma. J Immunol. 2000;164:733–738. doi: 10.4049/jimmunol.164.2.733. [DOI] [PubMed] [Google Scholar]
- 36.Reichel CA, et al. C-C motif chemokine CCL3 and canonical neutrophil attractants promote neutrophil extravasation through common and distinct mechanisms. Blood. 2012;120:880–890. doi: 10.1182/blood-2012-01-402164. [DOI] [PubMed] [Google Scholar]
- 37.Florey O, Haskard DO. Sphingosine 1-phosphate enhances Fc gamma receptor-mediated neutrophil activation and recruitment under flow conditions. J Immunol. 2009;183:2330–2336. doi: 10.4049/jimmunol.0901019. [DOI] [PubMed] [Google Scholar]
- 38.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 39.Seubert B, et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61:238–248. doi: 10.1002/hep.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh SJ, et al. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichikawa M, et al. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quatromoni JG, et al. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J Leukoc Biol. 2015;97:201–209. doi: 10.1189/jlb.5TA0814-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raber PL, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2014;134:2853–2864. doi: 10.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raber P, et al. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava MK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molon B, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan PY, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao Y, et al. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20:4096–4106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]
- 51.Youn JI, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Messmer MN, et al. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64:1–13. doi: 10.1007/s00262-014-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solito S, et al. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann NY Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 54.Nagaraj S, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuenca AG, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dolcetti L, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 57.Haverkamp JM, et al. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity. 2014;41:947–959. doi: 10.1016/j.immuni.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hossain F, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3:1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maenhout SK, et al. Enhanced suppressive capacity of tumor-infiltrating myeloid-derived suppressor cells compared with their peripheral counterparts. Int J Cancer. 2014;134:1077–1090. doi: 10.1002/ijc.28449. [DOI] [PubMed] [Google Scholar]
- 60.Haverkamp JM, et al. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol. 2011;41:749–759. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mairhofer DG, et al. Impaired gp100-Specific CD8(+) T-Cell Responses in the Presence of Myeloid-Derived Suppressor Cells in a Spontaneous Mouse Melanoma Model. J Invest Dermatol. 2015;135:2785–2793. doi: 10.1038/jid.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cimen Bozkus C, et al. Expression of Cationic Amino Acid Transporter 2 Is Required for Myeloid-Derived Suppressor Cell-Mediated Control of T Cell Immunity. J Immunol. 2015;195:5237–5250. doi: 10.4049/jimmunol.1500959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlecker E, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 65.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ridder K, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4:e1008371. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu CY, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 71.Sheng J, et al. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43:382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Franklin RA, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 74.Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Biswas SK, et al. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Seminars in immunopathology. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 76.Cortez-Retamozo V, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trellakis S, et al. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate immunity. 2013;19:328–336. doi: 10.1177/1753425912463618. [DOI] [PubMed] [Google Scholar]
- 78.Mishalian I, et al. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duluc D, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 80.Liu G, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1α-dependent glycolysis. Cancer Res. 2014;74:727–737. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 81.Rigamonti N, et al. Modulators of arginine metabolism do not impact on peripheral T-cell tolerance and disease progression in a model of spontaneous prostate cancer. Clin Cancer Res. 2011;17:1012–1023. doi: 10.1158/1078-0432.CCR-10-2547. [DOI] [PubMed] [Google Scholar]
- 82.Pearce EJ, Everts B. Dendritic cell metabolism. Nature Reviews Immunology. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 84.Modolell M, et al. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow derived macrophages by Th1 and Th2 cytokines. Eur. J. Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 85.Locati M, et al. Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J Immunol. 2002;168:3557–3562. doi: 10.4049/jimmunol.168.7.3557. [DOI] [PubMed] [Google Scholar]
- 86.Qian B-Z, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biswas SK. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity. 2015;43:435–449. doi: 10.1016/j.immuni.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shime H, et al. Tumor-Secreted Lactic Acid Promotes IL-23/IL-17 Proinflammatory Pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 90.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell metabolism. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jha Abhishek K., et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules that Regulate Macrophage Polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Nasi A, et al. Dendritic Cell Reprogramming by Endogenously Produced Lactic Acid. J Immunol. 2013;191:3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 94.Tran Janco JM, et al. Tumor-Infiltrating Dendritic Cells in Cancer Pathogenesis. J Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmadi M, et al. Prevention of Both Direct and Cross-Priming of Antitumor CD8+ T-Cell Responses following Overproduction of Prostaglandin E2 by Tumor Cells In vivo. Cancer Res. 2008;68:7520–7529. doi: 10.1158/0008-5472.CAN-08-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cubillos-Ruiz, Juan R, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hammami I, et al. Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biol. 2012;13:18. doi: 10.1186/1471-2121-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loberg RD, et al. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Passaro C, et al. The oncolytic virus dl 922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. 2015 doi: 10.18632/oncotarget.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Azenshtein E, et al. The CC Chemokine RANTES in Breast Carcinoma Progression: Regulation of Expression and Potential Mechanisms of Promalignant Activity. Cancer Res. 2002;62:1093–1102. [PubMed] [Google Scholar]
- 102.Soria G, et al. Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine. 2008;44:191–200. doi: 10.1016/j.cyto.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kowalczuk O, et al. CXCL5 as a potential novel prognostic factor in early stage non-small cell lung cancer: results of a study of expression levels of 23 genes. Tumour biol. 2014;35:4619–4628. doi: 10.1007/s13277-014-1605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toh B, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS biology. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gijsbers K, et al. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res. 2005;303:331–342. doi: 10.1016/j.yexcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 107.Wang N, et al. Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PloS one. 2014;9:e89991. doi: 10.1371/journal.pone.0089991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trellakis S, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129:2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 109.Eck M, et al. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol. 2003;134:508–515. doi: 10.1111/j.1365-2249.2003.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bellocq A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 111.OuYang L-Y, et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med. 2015;13:47. doi: 10.1186/s12967-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]