Abstract
Background
Proton magnetic resonance spectroscopy (1H-MRS) studies have consistently found abnormal brain concentrations of N-acetylaspartate (NAA) and glutamate in individuals with alcohol use disorders (AUD) relative to light drinkers. However, most such studies have focused on individuals in treatment for severe alcohol dependence and few studies have investigated associations between neurochemical concentrations and recent alcohol consumption. The present study focused on associations between recent drinking and prefrontal neurometabolite concentrations in non-severe, non-treatment seeking individuals with AUD.
Methods
Nineteen treatment naïve alcohol-dependent individuals aged 21–40 completed a 1H-MRS scan. Single-voxel 1H-MRS spectra were acquired in dorsal anterior cingulate (dACC) using a Two-dimensional J-resolved Point Resolved Spectroscopy (2D J-PRESS) sequence. Associations between recent heavy drinking, assessed using the Timeline FollowBack, and dACC metabolite concentrations were estimated via regression controlling for within-voxel tissue composition.
Results
Participants provided a negative breathalyzer reading and reported between 1 and 5 days (M = 2.45, SD = 1.23) since their last drink. Number of heavy drinking days in the 14 days preceding the scan (M = 4.84, SD = 3.32) was significantly inversely associated with both glutamate/water (β = −0.63, t(17) = −3.37, p = 0.004) and NAA/water concentrations (β = −0.59, t(17) = −2.98, p = 0.008).
Conclusions
The present study extends the literature by demonstrating inverse associations between recent heavy drinking and dACC glutamate and NAA concentrations in a sample of non-severe, non-treatment seeking individuals with AD. These findings may support the hypothesis that amount of recent alcohol consumption may account for differences in neuronal metabolism, even in non-severe, non-treatment seeking alcoholics.
Keywords: magnetic resonance spectroscopy, alcohol dependence, non-treatment seeking, glutamate, anterior cingulate
INTRODUCTION
Human postmortem studies have demonstrated that chronic alcohol use disorders are associated with a variety of neuropathologies, including neuronal and/or glial loss in a variety of brain regions (e.g., dorsolateral prefrontal cortex, orbital frontal cortex, hypothalamus, cerebellum, and hippocampus) and white matter loss in primarily frontal brain regions (de la Monte and Kril, 2014). A number of hypothesized mechanisms for these neuropathologies have been explored, including oxidative stress that occurs during alcohol intoxication (Crews and Nixon, 2009) and glutamate-mediated excitotoxicity that occurs during withdrawal from alcohol (Becker, 1998), as well as nutritional deficiencies, deficiencies in brain derived neurotrophic factors, and hepatic disease (Durazzo and Meyerhoff, 2007).
For the past 20 years, researchers have used proton magnetic resonance spectroscopy (1H-MRS), a neuroimaging technique that allows for the in vivo detection of neurochemical abnormalities that may reflect or even precede observed changes in gross morphology, to probe the neurophysiological consequences of chronic alcohol use disorders in humans. Most such studies have focused on individuals in inpatient or outpatient treatment for alcohol dependence (AD) and have traditionally found that such individuals have abnormally low levels of N-acetylaspartate (NAA), a purported marker of neuronal integrity, in frontal lobes and cerebellum; other notable findings include decreased cerebellar choline-containing metabolites and increased thalamic myo-inositol relative to non-AD individuals (Meyerhoff et al., 2013). A number of studies have further demonstrated that many of these neurochemical abnormalities at least partially normalize with prolonged abstinence (Ende et al., 2005).
Recent advances in 1H-MRS have allowed for more accurate measurement of neurometabolites involved in excitatory and inhibitory neurotransmission, including glutamate, glutamine, and ɣ-Aminobutyric acid (GABA). Brain glutamate levels are tightly coupled with both glutamine and GABA concentrations via the glutamate/GABA-glutamine cycle; glutamine, synthesized in astrocytes from glutamate, acts as a precursor for both glutamate and GABA (Bak et al., 2006). 1H-MRS investigations of anterior cingulate glutamate in AD have produced seemingly conflicting results, with reports of increased (Lee et al., 2007), decreased (Thoma et al., 2011), and equivalent glutamate levels among AD relative to non-AD individuals (Bauer et al., 2013, Yeo et al., 2013). However, when independent cross-sectional 1H-MRS investigations of glutamate in AD are sorted by time since participants’ last drink, the literature as a whole supports that brain glutamate levels in individuals with AD are abnormally low during active drinking (e.g., < 24 hours from last alcohol consumption; (Ende et al., 2013)), abnormally high during acute withdrawal (e.g., 48–72 hours from last alcohol consumption; (Hermann et al., 2012)), and abnormally low once again one-week from last alcohol consumption (Mon et al., 2012). Additional research suggests that ACC glutamate levels may normalize approximately 2–5 weeks from last alcohol consumption (Hermann et al., 2012, Mon et al., 2012). 1H-MRS studies of GABA and glutamine in AD have been relatively rare because measurement of GABA and glutamine is more technically challenging relative to glutamate. Nonetheless, while some research has supported decreased GABA (Behar et al., 1999) and increased glutamine (Thoma et al., 2011) in individuals with AD, other studies have failed to replicate these findings (Mason et al., 2006, Mon et al., 2012). These studies most often investigated individuals who were either in treatment or in recovery and as such had relatively severe and chronic alcohol dependence, and often did not control well for recency of drinking, length of abstinence, alcohol withdrawal status or medication treatment.
The present study sought to address previous studies’ shortcomings in several ways. First, whereas most studies have investigated differences in neurometabolite concentrations between individuals with and without AD, the present study focused on associations between recent drinking (i.e., number of heavy drinking days in the past 14 days) and neurometabolite concentrations. Second, unlike most 1H-MRS studies of AD, the present study focused on non-severe, non-treatment-seeking individuals with AD, allowing us to determine whether associations between heavy drinking and frontal neurometabolite concentrations reported in individuals being treated for severe AD generalize to this less severe, untreated population.
METHOD
Participants
Participants were 19 non-treatment-seeking individuals aged 21–40 meeting DSM-IV criteria for current Alcohol Dependence (AD), including criterion 3 and/or 4 “loss of control over drinking” or “the inability to cut-down or stop drinking,” recruited from the community. Exclusion criteria included current DSM-IV Axis I diagnoses except Nicotine Dependence, past-month use of any other psychoactive substance or medication except nicotine and marijuana (by verbal report), past-week use of marijuana (by verbal report), positive urine drug screen for any psychoactive substance (including marijuana), history of severe alcohol withdrawal (seizure, delirium tremens, need for inpatient or outpatient detoxification), and current alcohol withdrawal symptoms (i.e., Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar; (Sullivan et al., 1989)] > 3). Additional MRI-related exclusion criteria included presence of non-MRI safe objects in the body, claustrophobia, history of traumatic brain injury, or pregnancy.
Procedure
Demographic and diagnostic (via the Structured Clinical Interview for DSM-IV; (First, 1998)) information, along with the Alcohol Dependence Scale (Skinner and Horn, 1984), was collected during the baseline assessment of a previous study that participants completed 4–6 weeks prior to their participation in the current study. For the current study, participants were administered a breathalyzer, urine drug screen, pregnancy test (females), and Time-Line Follow-Back interview on the day of the scan.
Following these procedures, participants completed the 1H-MRS scan. Total scan time was approximately 30 minutes in a Siemens 3.0T Trio MR scanner with actively shielded magnet, high-performance gradients (45 mT/m, 200T/m-sec), and a 32-channel head coil. A structural scan was taken for 1H-MRS voxel placement and tissue segmentation (TR/TE=1900/2.26ms; FOV=256mm2; flip angle=9°; spatial resolution=1.0 × 1.0 × 1.0 mm). The 1H-MRS voxel was placed in the dorsal anterior cingulate cortex (dACC) on midsagittal T1-weighted images, posterior and superior to the genu of the corpus callosum, with the ventral edge of the voxel aligned with the dorsal edge of the callosum (Hermann et al., 2012). A voxel size of 2.5 × 2.5 × 3 cm was selected to ensure adequate signal to noise properties. Following placement of six outer-volume suppression bands ≥ 1 cm away from the voxel faces and auto-shimming, single-voxel water-suppressed 1H-MRS spectra were acquired using a Two-dimensional J-resolved Point Resolved Spectroscopy (2D J-PRESS) sequence: TR/TE=2400/31–229ms; ∆TE=2ms; 4 signal averages per TE step with online averaging; 2D spectral width=2000×500 Hz; 2D matrix size=2048×100; total acquisition time=13:28 min (Prescot and Renshaw, 2013); water unsuppressed 2D 1H-MRS data were also acquired from the ACC voxel with 2 signal averages recorded for each TE step (total acquisition time=3:28 min). A 2D J-PRESS sequence was employed because it provides improved quantification of glutamate, glutamine, and GABA by separating all uncoupled and scalar spin-spin J-coupled metabolite resonances across a 2D spectral surface.
Analytic Plan
Skull stripping and whole brain tissue-type segmentation were performed on MP-RAGE images using the FSL BET and FAST tools (Smith et al., 2004). In-house MATLAB functions were used to extract the 3D volume corresponding to the positioned MRS voxel to obtain within-voxel gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content for each subject. Eddy currents and residual water were removed using in-house MATLAB functions. Subsequently, the ProFit algorithm was applied using software-supplied 2D basis sets (which contain 19 metabolites total; as detailed in (Schulte and Boesiger, 2006)). Prior to Fourier transformation, the raw 2D matrix was zero-filled. Cramer-Rao Lower Bound (CRLB) values, which reflect the uncertainty of estimated model parameters, were provided by the ProFit software. Estimated metabolite peak areas were normalized to the unsuppressed water signal. Finally, metabolite/water ratios were corrected for within-voxel CSF fraction (Prescot and Renshaw, 2013).
Metabolite/water ratios for 7 neurometabolites: glutamate, glutamine, GABA, NAA, choline-containing metabolites (“choline”), creatine-containing metabolites (“creatine”), and myo-inositol, were entered as dependent variables in separate regression models (i.e., one metabolite per regression model), with number of heavy drinking days in the 14 days preceding the scan and within-voxel gray matter tissue fraction, expressed as the ratio of gray matter to brain matter (i.e., GM + WM), entered as predictors (the latter to adjust coefficients for individual differences in within-voxel tissue composition). Number of heavy drinking days, where a heavy drinking day is defined as ≥ 4 drinks/day for women and ≥ 5 drinks/day for men, over the preceding 14 days was chosen to represent recent drinking in the present study because it captures both frequency and quantity of alcohol use and most accurately represents the binge-like pattern of alcohol consumption characteristic of our young, non-treatment seeking population of interest (NIAAA, 2006); a relatively brief recall window (i.e., 14 days) was selected to represent recent drinking as research has demonstrated that assessments covering longer intervals (e.g., 30 days) may have reduced accuracy (Hoeppner et al., 2010). An alpha level of p < 0.05 was adopted across regression models.
A thorough confounder analysis was not possible given our small sample size. However, we identified a number of potential confounders based on past research including smoking status, age, gender, days since last drink, and CSF tissue fraction and calculated bivariate correlations between each of these variables and metabolite/water ratios for the 7 estimated neurometabolites. Any variables associated with a given metabolite with p ≤ 0.10 were evaluated as additional predictors in the regression model for that metabolite to test whether inclusion of the potential confounder altered model results.
RESULTS
See Table 1 for demographic and alcohol use characteristics of the sample. Average within-voxel gray matter tissue fraction, expressed as the ratio of gray matter to brain matter (i.e., gray matter + white matter), was 61.06% (SD = 2.05%). CRLBs for metabolite concentration estimates were < 20%, supporting their reliability, with the exception of one participant with a CRLB of 21.58% for glutamine. This participant’s glutamine/water concentration estimate was proximal to the sample mean (i.e., Z = −1.27) and removing this participant from the sample did not alter results; as such they were retained for analysis.
Table 1.
Demographic and alcohol use characteristics of the sample (N=19)
| Variable | No. (%) of participants / Mean |
Standard Deviation |
|---|---|---|
| Gender (male) | 14 (73.7%) | |
| Smoking Status (non-smoker) | 13 (68.4%) | |
| Race (Caucasian) | 17 (89.5%) | |
| Age | 26.68 | 6.40 |
| Alcohol Dependence Scale | 9.79 | 4.72 |
| # Heavy drinking days (past 14) | 4.84 | 3.32 |
| Drinks per drinking day | 7.38 | 3.45 |
| Days since last drink | 2.53 | 1.22 |
See Table 2 for regression model results. Gray matter to brain matter proportion was not significantly associated with metabolite concentrations in any of the regression models (Mean p = 0.70) and was therefore removed from all final models. As detailed in Table 1, number of heavy drinking days in the preceding 14 days was significantly inversely associated with glutamate/water (β = −0.63, t(17) = −3.37, p = 0.004) and NAA/water concentrations (β = −0.59, t(17) = −2.98, p = 0.008), but was not significantly associated with other neurometabolite concentrations. See Figure 1 for bivariate scatterplots between number of heavy drinking days and a) glutamate/water and b) NAA/water. Bivariate associations between metabolite concentrations and potential confounders (i.e., smoking status, age, gender, days since last drink, and CSF tissue fraction) were small (see Table 3), with p’s > 0.10, with the exception of CSF fraction and GABA (r = 0.44, p = 0.06) and smoking status and glutamine (t[17], p = 0.005); specifically, smokers (M = 0.64 × 10−5) had lower glutamine/water concentrations relative to non-smokers (M = 0.85 × 10−5). Including smoking status in the regression model for glutamine did not render number of heavy drinking days a significant predictor of glutamine/water (p = 0.39), but smoking status itself significantly predicted glutamine/water levels (β = −0.63, t(15) = −3.25, p = 0.005). When CSF fraction was added to the regression model for GABA, neither number of heavy drinking days (p = 0.43) nor CSF fraction (β = 0.43, t(16) = 1.92, p = 0.07) significantly predicted GABA/water levels.
Table 2.
Results from regression models with the number of Heavy Drinking Days (# HDD) associated with dorsal anterior cingulate neurometabolite concentrations.
| Metabolite/water | # HDD | R2 | |
|---|---|---|---|
| β | p | ||
| N-acetylaspartate | −0.59 | 0.008 | 0.34 |
| Glutamate | −0.63 | 0.004 | 0.40 |
| GABA | −0.20 | 0.404 | 0.04 |
| Glutamine | 0.11 | 0.666 | 0.01 |
| Choline | −0.33 | 0.166 | 0.11 |
| Creatine | −0.42 | 0.077 | 0.17 |
| Myo-Inositol | −0.24 | 0.317 | 0.06 |
Note: Significant associations are bolded.
Figure 1.
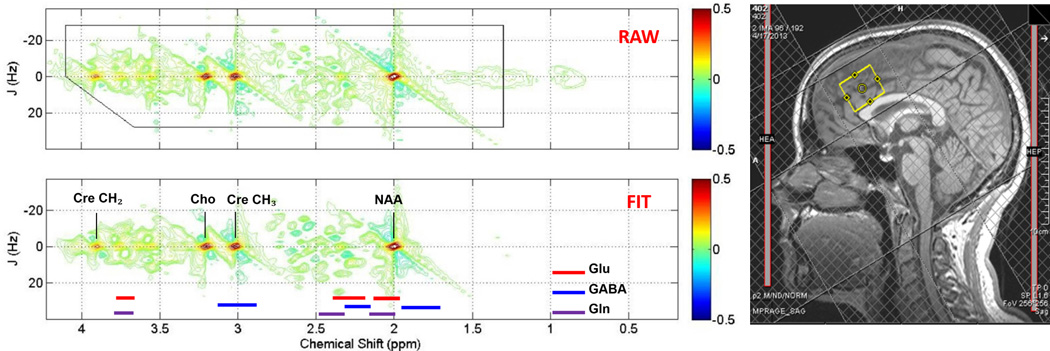
Sample ACC voxel location (right) and raw (top) and fitted (bottom) two-dimensional j-resolved 1H-MRS spectra analyzed using Prior Knowledge Fitting (ProFit). Colored horizontal bars represent approximate primary spectral locations of Glu, GABA, and Gln. Gln=glutamine, Glu=glutamate, GABA=gamma-aminobutyric acid, NAA=n-acetyl aspartate, Cre=creatine, Cho=choline.
Table 3.
Associations between potential confounders and dorsal anterior cingulate neurometabolite concentrations.
| Metabolite/water | Smoking Status | Gender | Age | DSLD | CSF tissue % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | p | t | p | r | p | r | p | r | p | |
| N-acetylaspartate | −0.72 | 0.484 | 0.03 | 0.978 | 0.13 | 0.592 | 0.00 | 0.996 | 0.16 | 0.513 |
| Glutamate | −0.32 | 0.757 | −0.07 | 0.946 | −0.01 | 0.969 | 0.15 | 0.546 | 0.09 | 0.719 |
| GABA | 1.16 | 0.262 | 1.73 | 0.103 | −0.06 | 0.807 | −0.06 | 0.824 | 0.44 | 0.063 |
| Glutamine | 3.20 | 0.005 | 1.59 | 0.130 | 0.06 | 0.815 | −0.30 | 0.214 | 0.21 | 0.395 |
| Choline | 0.35 | 0.732 | 0.22 | 0.828 | 0.10 | 0.969 | 0.33 | 0.173 | 0.02 | 0.945 |
| Creatine | −1.05 | 0.309 | −0.16 | 0.874 | 0.19 | 0.446 | 0.03 | 0.898 | 0.12 | 0.640 |
| Myo-Inositol | −0.62 | 0.545 | −0.41 | 0.688 | 0.10 | 0.968 | 0.10 | 0.682 | −0.05 | 0.844 |
Note: Smoking Status (non-smoker = 0, smoker = 1); Gender (male = 0, female = 1); DSLD = Days Since Last Drink; CSF = Cerebrospinal Fluid. Significant/trend associations are bolded.
DISCUSSION
The present study provided support for inverse associations between recent heavy drinking and dorsal anterior cingulate cortex glutamate and NAA concentrations in non-severe, non-treatment seeking individuals with AD; specifically, more heavy drinking days in the 2 weeks preceding the scan was significantly associated with lower dACC concentrations of both glutamate and NAA. These findings are both consistent with and significantly extend past reported findings using 1H-MRS in AD individuals.
First, over the past 20 years, consistent support has been found for reductions in brain (e.g., frontal, thalamic, cerebellar) NAA in individuals with AD. Although most 1H-MRS studies of alcoholism have focused on treatment-seeking individuals, the few existing studies that have investigated treatment-naïve individuals have also found significant reductions in NAA relative to light drinkers. For example, Meyerhoff and colleagues (2004) found that treatment naïve heavy drinkers (many of whom had AD) demonstrated significantly less NAA in frontal white matter relative to light drinkers (Meyerhoff et al., 2004). They also found that estimated number of lifetime drinks was significantly inversely associated with brainstem NAA. The present study extends this literature by demonstrating a significant negative correlation between dACC NAA and recent heavy drinking in treatment-naïve individuals with AD. This finding may reflect the disrupting influence of recent alcohol consumption on neuronal metabolism in individuals with AD (Baslow et al., 2002).
Second, due in part to advances in MR technology (e.g., increased field strength), a number of more recent 1H-MRS investigations have focused on glutamatergic dysfunction in individuals with AD. As detailed earlier, taken as a whole, this literature suggests that the direction and magnitude of observed glutamate disturbance in AD depends on time since participants’ last drink as well as whether participants are experiencing alcohol withdrawal. Although little research has examined brain glutamate concentrations in active drinking individuals with AD, Ende and colleagues (2013) recently found that glutamate levels in frontal white matter were significantly lower in heavy drinkers reporting a loss of control over their drinking relative to both light and heavy drinkers not experiencing loss of control over drinking (Ende et al., 2013). In contrast, the present study demonstrated a significant inverse association between dACC glutamate and recent heavy drinking in non-treatment-seeking individuals with AD. Although these findings are not completely overlapping, and cannot be directly compared due to a number of methodological differences between the two studies including brain region of interest, both findings are consistent with the adaptations in glutamatergic neurotransmission that occur following active, chronic heavy drinking (Spanagel and Kiefer, 2008).
It is perhaps unsurprising that the same direction of significant association was found between recent heavy drinking and both NAA and glutamate in the present study given that NAA is found in highest concentrations in pyramidal glutamatergic neurons (Urenjak et al., 1993). As noted by Mon and colleagues (2012), who similarly found abnormally low levels of both ACC NAA and glutamate in individuals with AD, relative to controls, after approximately 9 days of abstinence, reductions in both ACC NAA and glutamate may reflect a general, alcohol-related neurometabolic/bioenergetic disturbance in that brain region (Mon et al., 2012). Although intriguing, more research is needed to support or refute this interpretation of the data.
The results of the present study should be interpreted in light of several limitations. First, the study included a relatively small sample of AD participants. Results should therefore be considered tentative until replicated in a larger sample. Second, the recent heavy drinking variable relied entirely on subjective self-report; supplementation of subjective drinking data with objective biomarkers of recent alcohol consumption would strengthen confidence in our findings. Third, the present analytic methods available for our 2D J-PRESS 1H-MRS sequence did not allow for separation of co-edited macromolecules (e.g., proteins) from the observed GABA signal, making the observed lack of significant association between heavy drinking and GABA more difficult to interpret. Fourth, given that we did not acquire data from brain regions other than dACC, we cannot comment on the regional specificity of our findings. Finally, because assessment for the present study was limited and the sample was selected to be homogeneous on a number of key alcohol diagnostic and drinking variables, we were not able to evaluate correlations between metabolite concentrations and additional clinically meaningful variables. For example, although we did not find support for association between number of days since last drink and metabolite concentrations, this lack of association was most likely due to restriction of range on the former variable (i.e., 11 of 19 participants reported drinking 2 days prior to the scan).
These limitations notwithstanding, the present study extends the literature by demonstrating inverse associations between recent heavy drinking and dACC glutamate and NAA concentrations in a sample of relatively young and less severe, non-treatment-seeking individuals with AD. These findings may support the hypothesis that active heavy drinking disrupts neuronal metabolism. Further studies should examine the impact of these findings on other related alcohol issues, like cognitive ability and change, craving, prognosis for further drinking and alcohol use disorder criteria, as well as predicting medication or other treatment interventions.
Figure 2.
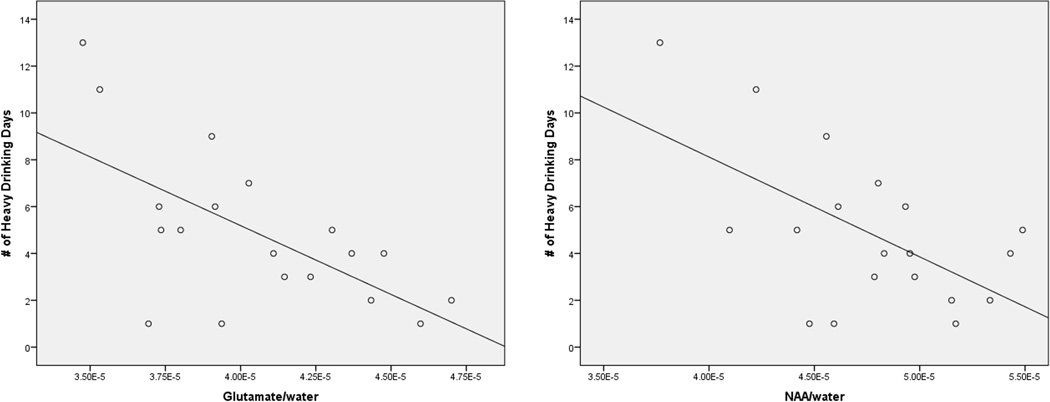
Scatterplots, with linear regression line added, for the associations between number of heavy drinking days and dACC Glutamate/water (left) and NAA/water (right).
Acknowledgments
Funding Sources: This research was supported by NIAA P50 AA010761 (PI: Becker) and K05 AA017435 (PI: Anton). Dr. Prisciandaro is supported by NIAAA K23 AA020842.
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Kitada K, Suckow RF, Hungund BL, Serikawa T. The effects of lithium chloride and other substances on levels of brain N-acetyl-L-aspartic acid in Canavan disease-like rats. Neurochem Res. 2002;27:403–406. doi: 10.1023/a:1015504031229. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38:1401–1408. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, Wichert S, Rabinstein J, Frischknecht U, Mann K, Vollstadt-Klein S. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res. 2013;37:1643–1649. doi: 10.1111/acer.12149. [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- First MB. Structured clinical interview for DSM-IV axis I disorders : patient edition (February 1996 final), SCID-I/P. New York, N.Y.: Biometrics Research Dept., New York State Psychiatric Institute; 1998. [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Hoeppner BB, Stout RL, Jackson KM, Barnett NP. How good is fine-grained Timeline Follow-back data? Comparing 30-day TLFB and repeated 7-day TLFB alcohol consumption reports on the person and daily level. Addict Behav. 2010;35:1138–1143. doi: 10.1016/j.addbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Jang DP, Kim JJ, An SK, Park S, Kim IY, Kim SI, Yoon KJ, Namkoong K. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18:1511–1514. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, De Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr Top Behav Neurosci. 2013;13:511–540. doi: 10.1007/7854_2011_131. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. Focus on Young Adult Drinking. Alcohol Alert. 2006 [Online] [Google Scholar]
- Prescot AP, Renshaw PF. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (profit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. J Magn Reson Imaging. 2013;37:642–651. doi: 10.1002/jmri.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P. profit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Horn JL. The Alcohol Dependence Scale (ADS) User's Guide. Toronto, Canada: Addiction Research Foundation; 1984. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36:1359–1365. doi: 10.1038/npp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Thoma RJ, Gasparovic C, Monnig M, Harlaar N, Calhoun VD, Kalyanam R, Mayer AR, Durazzo TC, Hutchison KE. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2013;211:141–147. doi: 10.1016/j.pscychresns.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


