Abstract
Objectives
To compare the therapeutic effectiveness of corticosteroids (CS) alone versus CS plus D-penicillamine (D-Pen) in severe eosinophilic fasciitis.
Methods
Long term prospective non-randomized trial of D-Pen plus CS vs. CS alone in patients with severe eosinophilic fasciitis, defined as clinically apparent cutaneous fibrotic involvement affecting greater than 15% body surface area (BSA), or greater than 10% BSA with joint flexion contractures.
Results
Sixteen patients with severe eosinophilic fasciitis entered the study. Ten patients received D-Pen plus CS and 6 CS alone. Affected BSA decreased from an average of 29% to 8.9% in the D-Pen plus CS group compared to a decrease in affected BSA from 28% to 22.83% in the CS alone group. The reduction in affected BSA in the D-Pen plus CS group was significantly greater than in the CS alone group (p= 0.038). Clinical improvement occurred in all D-Pen plus CS patients, compared to only 33.3% of CS alone patients (p=0.008). There was no difference in overall adverse events frequency between the groups (p=0.60). The most common adverse event in the D-Pen plus CS group was proteinuria (33.3%). However, proteinuria also occurred in 16.6% in the CS-alone group.
Conclusions
Treatment with CS alone failed to induce clinical improvement in the majority of the severe eosinophilic fasciitis patients. In contrast, D-Pen plus CS resulted in significantly greater clinical improvement. These results suggest that initial treatment of severe eosinophilic fasciitis with CS alone is not sufficient for optimal therapeutic response and that addition of an antifibrotic agent results in an improved outcome.
Keywords: Eosinophilic Fasciitis, Corticosteroids, D-penicillamine
INTRODUCTION
Eosinophilic fasciitis is a rare cutaneous fibrotic disorder characterized by symmetric and often progressive induration of the skin in the absence of clinical manifestations of systemic sclerosis (1,2). Other clinical features include myalgia, weight loss, prominent articular involvement leading to severe joint contractures, and the rare occurrence of aplastic anemia and hematologic malignancies (1–6). Typical laboratory abnormalities include elevated erythrocyte sedimentation rate, hypergammaglobulinemia, and peripheral blood eosinophilia, although the latter is not required for diagnosis. Histopathological examination of full thickness skin biopsies shows marked thickening and fibrosis of the fascia often involving the adjacent muscle and an inflammatory infiltrate composed of lymphocytes, plasma cells and eosinophils (1–3).
The etiology of eosinophilic fasciitis is unknown and its pathogenesis is poorly understood. Furthermore, owing to its rarity epidemiological and demographic data are scarce. There is also no consensus on treatment regimen, duration, or in the definition of treatment effectiveness. Corticosteroids (CS) are generally used as a first line treatment. Other immunosuppressive agents are added when full therapeutic response is not achieved with CS alone, however, the timing and type of second line agents have not been systematically evaluated, and there is no consensus on an optimal second line treatment (5). Numerous second line agents have been utilized including hydroxychloroquine, methotrexate, cyclosporine, ketotifen, infliximab, and D-Penicillamine (D-Pen) (6–13). Other treatment modalities including phototherapy and allogeneic bone marrow transplantation have also been used (14–16). A recent review identified 16 published cases of eosinophilic fasciitis that received treatment with D-Pen and described three additional cases (13). All patients included in this report had a favorable outcome even in CS-refractory cases and, therefore, it was concluded that D-Pen was a highly effective therapy for eosinophilic fasciitis although important side effects occurred in 4 patients (13). However, given the very small number of cases reported, the differences in treatment regimens, and the variable definitions of therapeutic effectiveness, it is difficult to draw conclusions regarding D-Pen effectiveness and safety for eosinophilic fasciitis treatment.
Here, we describe the results of a long-term prospective study conducted at a single institution to compare the therapeutic effectiveness and side effect profiles of either CS alone or D-Pen plus CS in patients with severe eosinophilic fasciitis.
PATIENTS AND METHODS
Study design
A long term (1987–2007) prospective, non-randomized, open label trial of D-Pen plus CS vs CS alone for treating severe eosinophilic fasciitis was conducted at the Scleroderma Center of Thomas Jefferson University, Philadelphia, PA. The requirements for patient’s entry into the study were: 1. The histopathological demonstration of fascial thickening with accumulation of lymphocytes and/or eosinophils in full thickness skin biopsies (including dermis, fascia and subjacent muscle); and 2. The diagnosis of severe eosinophilic fasciitis defined as skin induration affecting more than 15% of total body surface area (BSA) or skin induration affecting between 10% and 15% of BSA, with associated fibrotic lesions causing joint contractures. Patients were excluded from the study if they had a diagnosis of systemic sclerosis, eosinophilia–myalgia syndrome, nephrogenic systemic fibrosis, or localized forms of scleroderma. Patients were also excluded if they had received prior CS therapy or had received any corticosteroid sparing agent.
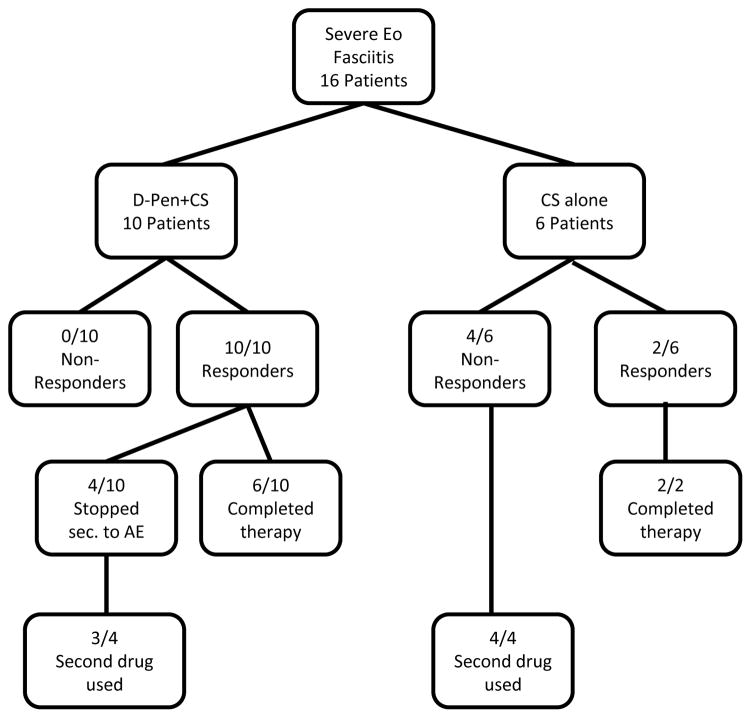
During the period of the study, thirty-two patients with a clinical and histopathological diagnosis of eosinophilic fasciitis were identified and screened (Figure 1). None of the patients included in this study have been described previously. From this cohort, 16 patients were identified with severe eosinophilic fasciitis according with the criteria described above. Following the histopathological confirmation of the diagnosis and the determination that the process was severe, patients were offered therapy with either D-Pen plus CS or with CS alone. The side-effect profile and the fact that the Food and Drug Administration had not approved the use of D-Pen for this condition were explained and discussed in detail with each patient and each patient voluntarily selected to be entered into one of the treatment arms. From the 16 patients fulfilling the criteria for severe eosinophilic fasciitis, 10 patients selected and consented to be treated with D-Pen plus CS whereas 6 chose and consented to treatment with CS alone. Written consent was signed by each patient. The CS treatment protocol for both groups of patients consisted of prednisone (40–50 mg daily) for 3 months followed by a slow taper depending on clinical response. The D-Pen plus CS treatment protocol consisted of prednisone as described above plus D-Pen which was initiated at a dose of 250 mg daily for 2–4 weeks and then increased by 250 mg increments every 4 weeks to reach 750 to 1000 mg daily. All patients were evaluated every 3 to 6 months and laboratory tests which included a complete blood count with differential, comprehensive metabolic panel and complete urinalysis were performed every 2 months. Dose adjustment of D-Pen was allowed if proteinuria of less than 1.5g/24h was developed. Proteinuria greater than 1.5g of protein per 24h was considered clinical significant and was the criterion used for discontinuation of D-Pen therapy. Symptoms suggestive of lupus-like syndrome were evaluated in detail at every clinical visit. All BSA measurements and clinical assessment were performed by a single examiner (SAJ) who was not blinded to the patient treatment protocol. The clinically affected BSA was recorded employing body surface diagrams employed for the visual assessment of the extent of thermal burns. The extent of cutaneous induration/involvement was calculated employing the “rule of nines” (17) and was expressed as a percentage of total BSA. BSA measurements were performed before initiation of treatment and were continued prospectively every 3–6 months. Physical Therapy/Occupational Therapy was recommended for all patients who had any evidence of joint contracture. The response to treatment was assessed according to changes in affected BSA and clinical evaluation by the treating physician. Patients were classified as either non-responder (persistent or progressive physical signs and symptoms) or responder (documentation of softening of the skin and/or reduction of affected BSA). Subjective and objective joint of motion changes were also documented but goniometry measurements were not routinely performed. Data regarding adverse events and relapse rates were collected and analyzed.
Figure 1.
Flow chart of patients entered into the study, treatment modality, and clinical outcomes.
Statistical analysis
Continuous variables were compared using the Mann-Whitney test and categorical variables were assessed using Fisher’s exact test. Tests were two-tailed and a p≤0.05 was considered statistically significant.
RESULTS
Of the 16 patients entered into the study, 10 were male and 6 were female. The average age of the entire cohort was 51 years at presentation (Table 1). In 25% of the patients, recent physical trauma, surgery or strenuous exercise was temporally associated to disease onset. Histopathological examination of full thickness skin biopsies including the fascia showed inflammation of the fascia with lymphocytic and/or eosinophilic infiltrates in all samples and variable levels degrees of fascial thickening/sclerosis. Ten out of 16 patients were treated with D-Pen plus CS and 6/16 patients were treated with CS alone (Figure 1). The average duration of D-Pen treatment in the D-Pen plus CS group was 13.5 ± 10.7 months and the average duration for the CS alone treatment was 19±8.7 months.
Table 1.
Demographic data and frequency/extent of skin and joint involvement at initial evaluation
| CS alone | D-pen plus CS | p values* | Average | |
|---|---|---|---|---|
| Male:Female ratio | 4:2 | 7:3 | 1.00 | 11:5 |
| Interval from first symptoms to diagnosis (months)** | 8.5±7.9 | 15±18.9 | 0.34 | 12.7±15.7 |
| Age (years)** | 39.7±27.7 | 57.8±7.1 | 0.45 | 51±19 |
| Extent of affected Body Surface Area (BSA) | 29.2±17.1% | 28±16% | 0.46 | 27±19% |
| Lesions crossing joints | 100% | 90% | 1.00 | 93.8% |
| Flexion contractures | 100% | 70% | 0.25 | 81.25% |
p: Comparison between CS alone vs. CS + D-Pen.
Values expressed in means± standard deviation.
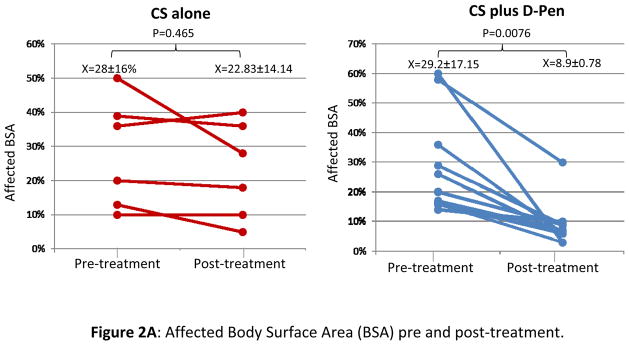
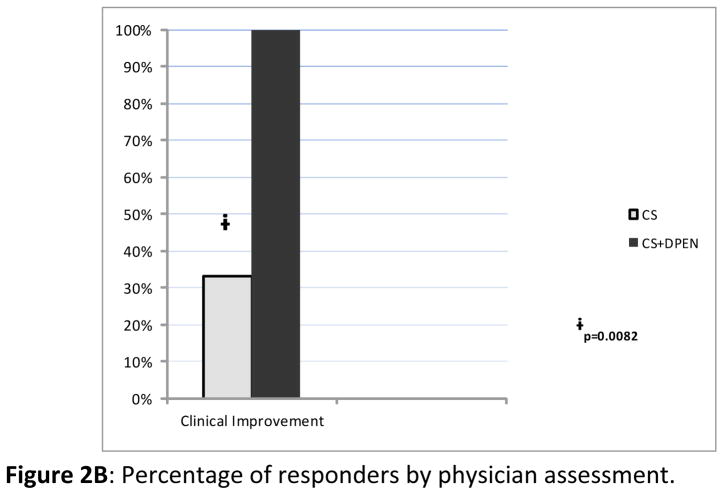
Typical eosinophilic fasciitis lesions involved an average of 29.2±17.1% of BSA in the group receiving CS alone and 28±16.0% of BSA in the D-Pen plus CS therapy group. Flexion contractures were demonstrated in 70% of patients in the D-Pen plus CS group and in 100% of the patients in the CS alone group. Patients treated with D-Pen plus CS showed a decrease in affected BSA from an average of 29.2% prior to treatment to an average of 8.9% following treatment. In contrast, patients treated with CS alone showed only a minor reduction in affected BSA from an average of 28% before treatment to an average of 22.83% after treatment (Figure 2A). The difference in the reduction of the percentage of affected BSA in the CS+ D-Pen group was 20.3±15.34% whereas the difference in the CS group was only 5.17±0.91%. The difference in reduction of the affected BSA was highly significant in favor of the D-Pen + CS group (p= 0.038). By physician assessment, all patients in the D-Pen plus CS group achieved improvement of cutaneous involvement. In contrast, only 33.3% of the patients treated with CS alone had clinically detectable improvement of cutaneous findings. This difference was statistically significant (p=0.008) (Figure 2B). Improvement of joint range of motion was observed in 70% of patients with joint contractures treated with D-Pen plus CS. In contrast, none of the patients in the CS alone group showed improvement in joint range of motion; this difference was also statistically significant (p=0.021).
Figure 2.
A: Affected body surface area (BSA) prior to initiation of treatment (pre-treatment) and at treatment termination (post-treatment) in patients treated with CS alone or with D-Pen plus CS. B: Bar graph showing the averages of clinical response of patients treated with CS alone or with D-Pen plus CS according to treating physician assessment.
Figure 2A. Affected Body Surface Area (BSA) pre and post-treatment.
Figure 2B. Percentage of responders by physician assessment.
Forty percent (4/10) of the patients in the D-Pen plus CS group required discontinuation of D-Pen owing to the occurrence of adverse events and were switched to a different steroid sparing agent. From these 4 patients, 3 required discontinuation of D-Pen owing to proteinuria and 1 owing to bullous penphigoid. In contrast, 66.6% of patients in the CS alone group required the addition of a steroid sparing agent owing to lack of clinical response and/or disease progression (Figure 1).
The adverse events identified in the two treatment groups are listed in Table 2. Adverse events were more frequent in the D-Pen plus CS group (80%) as compared with the CS alone group (66.67%). However, this difference did not reach statistical significance (p=0.60). The most common adverse event in the D-Pen plus CS group was the occurrence of clinically significant proteinuria, (33.3% of patients). Quite unexpectedly, proteinuria also occurred in 16.6% of the patients treated with CS alone. This difference was not statistically significant (p=1.00). There was no obvious explanation for the occurrence of proteinuria in the CS alone group. None of the patients with proteinuria in either group presented any clinical or laboratory test evidence to suggest the occurrence of nephritic syndrome, systemic manifestations of vasculitis, or drug induced lupus.
Table 2.
Adverse events attributed to therapy.
| Adverse Events | CS alone | D-pen plus CS | Total |
|---|---|---|---|
| Proteinuria | 1/6 (16.7%) | 3/10(30.0%) | 4/16(25.0%) |
| Diabetes | 2/6(33.3%) | 1(10.0%) | 3/16(18.8%) |
| Skin rash* | 1/6(16.7%) | 2/10(20%) | 3/16(18.8%) |
| Bone and Musculoskeletal | 1/6(16.7%) | 0/10(0.0%) | 1/16(6.3%) |
| Other | 1/6(16.7%) | 1/10(10.0%) | 2/16(12.5%) |
| Total | 6 | 7 | 13 |
One case of rosacea/acne was reported in the CS alone group. One case of bullous penphigoid and one case of acne were reported in 2 different patients in the CS plus DPen group.
DISCUSSION
A new syndrome of diffuse fasciitis with eosinophilia was first described by Shulman in 1975 (1,2). The term “Eosinophilic Fasciitis” was subsequently used to refer to this rare and highly heterogeneous cutaneous fibrotic disorder (3–6). Eosinophilic fasciitis is often progressive and is frequently associated with severe and incapacitating joint flexion contractures (1–6). Owing to the rarity of the disorder and to the heterogeneity in severity and extent of clinical manifestations, there is no standardized and generally accepted treatment for eosinophilic fasciitis and, currently, this disorder is potentially incapacitating. We describe here the results of a long-term prospective study, comparing the effectiveness of treatment with either CS alone or with D-Pen plus CS for patients with severe eosinophilic fasciitis, which was defined by the presence of either an affected BSA greater than 15% or an affected BSA of 10% to 15% with associated joint flexion contractures. Despite the numerous case reports and small series described in the literature, including a prior series from our institution (18), the remarkable heterogeneity in the therapeutic approach employed precluded a direct comparison with the results reported in the current series. Furthermore, in contrast to prior case reports and series that did not stratify the clinical manifestations in terms of severity, the study described here focused only on patients with severe eosinophilic fasciitis defined as noted above.
Our results indicate that patients with severe eosinophilic fasciitis have a poor response to CS alone. This observation suggests that therapy with CS alone may not be sufficient for patients with severe eosinophilic fasciitis and that simultaneous use of a second agent should be considered in the initial management of these patients. It is important to emphasize that none of the patients reported here were treated with repetitive intravenous pulses of CS, an approach that has been proposed as a possible disease modifying intervention (19,20). Patients treated with both D-Pen and CS had statistically significant greater improvement in the extent of cutaneous involvement measured by BSA and in the assessment of clinical response compared with patients treated with CS alone, although they had greater frequency of adverse events (Table 2). However, the frequency of side effects was not statistically significant between the groups (p=0.60). The most important adverse event was proteinuria, which occurred in 33.3% of the D-Pen plus CS group. The high frequency of proteinuria observed in these patients is substantially greater than that in cohorts of patients with systemic sclerosis treated with similar doses of D-Pen described in the literature in which the incidence of proteinuria ranged from 5.5% to 8.7% (21–23). Interestingly, proteinuria also occurred in 16.6% of patients treated with CS alone. The frequent occurrence of proteinuria in eosinophilic fasciitis has not been described previously. Although the small numbers of patients included in this study does not allow to draw definitive conclusions, the unexpected high occurrence of proteinuria in both treatment groups; suggests that kidney involvement previously described in only two eosinophilic fasciitis patients (24,25), may represent an under-estimated systemic feature of the severe forms of the disease. Alternatively this observation may also suggest that patients with severe eosinophilic fasciitis may be more sensitive to the nephrotoxicity effects of D-Pen owing to an intrinsic predisposition.
The results described here demonstrate that oral CS alone therapy was not effective for the treatment of severe eosinophilic fasciitis as two-thirds of patients in the CS alone group had no improvement of cutaneous and joint involvement and the extent of affected BSA in the group showed only a non-significant reduction. The results, however, conclusively show that administration of the antifibrotic agent D-Pen besides CS, achieved a significantly improved cutaneous and joint contracture outcome. Although various potential mechanisms of action have been proposed to explain the biological effects of D-Pen, ranging from immunomodulation to antifibrotic effects, recent studies (26–28) have provided a novel mechanism for the antifibrotic effects of D-Pen. These studies have shown that increased matrix stiffness mediated by collagen cross-linking and tissue integrin interactions can induce potent up-regulation of pro-fibrotic pathways and further increase the production of collagen and other extracellular matrix proteins with worsening of the fibrotic process (26–28). Ergo, disruption of collagen cross-linking would be expected to exert a potent antifibrotic effect. D-Pen is a potent inhibitor of collagen cross-linking and this property may mediate its antifibrotic effects (29–31). However, it should be emphasized that the occurrence of severe adverse effects will undoubtedly limit its long term use. Additional options including methotrexate and mycophenolate or the novel antifibrotic drugs should be explored by future trials focused on their safety and efficacy in eosinophilic fasciitis.
The nature of the non-randomized open label design and the small size of the cohort are limitations that hamper the ability to draw definitive conclusions from this study. However, on the other hand, the comparison of two treatment approaches in a cohort from a single institution focusing only on patients with severe disease are substantial strengths of this study; providing a new insight on the treatment of this rare and relatively under-studied condition. The results described here suggest that a combination of D-Pen plus CS should be considered as an effective therapeutic regimen for patients with severe eosinophilic fasciitis. However, the high frequency of proteinuria associated with D-Pen treatment should be carefully considered before initiation of this therapeutic regimen.
Key Messages.
Eosinophilic fasciitis often causes disabling cutaneous fibrosis and joint contractures.
Therapy with corticosteroids alone is not sufficient for severe eosinophilic fasciitis.
D-Penicillamine in combination with corticosteroids is effective for treatment of severe eosinophilic fasciitis, although its side effect profile limits its long term use.
Acknowledgments
Supported by NIH grant AR 19616 to SAJ. The expert assistance of Kenneth Brown and Ruth M. Johnson in preparation of the manuscript is gratefully acknowledged. Design of the study by SAJ, manuscript preparation by FAM, RB, AGK and SAJ. Data acquisition by SAJ. Analysis of data by FAM, RB, AGK, and SAJ. Final manuscript revised and approved by FAM and SAJ.
References
- 1.Shulman LE. Diffuse fasciitis with eosinophilia: a new syndrome? Trans Assoc Am Physicians. 1975;88:70–86. [PubMed] [Google Scholar]
- 2.Shulman LE. Diffuse fasciitis with hypergammaglobulinemia and eosinophilia: a new syndrome? J Rheumatol. 1984;11:569–70. [PubMed] [Google Scholar]
- 3.Barnes L, Rodnan GP, Medsger TA, Short D. Eosinophilic fasciitis. A pathologic study of twenty cases. Am J Pathol. 1979;96:493–518. [PMC free article] [PubMed] [Google Scholar]
- 4.Pinal-Fernandez I, Selva-O’Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014;13:379–82. doi: 10.1016/j.autrev.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Antic M, Lautenschlager S, Itin PH. Eosinophilic fasciitis 30 years after - what do we really know? Report of 11 patients and review of the literature. Dermatology. 2006;213:93–101. doi: 10.1159/000093847. [DOI] [PubMed] [Google Scholar]
- 6.Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988;17:221–31. doi: 10.1016/0049-0172(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 7.Pouplin S, Daragon A, Le Loët X. Treatment of eosinophilic fasciitis with methotrexate. J Rheumatol. 1998;25:606–7. [PubMed] [Google Scholar]
- 8.Janzen L, Jeffery JR, Gough J, Chalmers IM. Response to methotrexate in a patient with idiopathic eosinophilic fasciitis, morphea, IgM hypergammaglobulinemia, and renal involvement. J Rheumatol. 1995;22:1967–70. [PubMed] [Google Scholar]
- 9.Bukiej A, Dropiński J, Dyduch G, Szczeklik A. Eosinophilic fasciitis successfully treated with cyclosporine. Clin Rheumatol. 2005;24:634–6. doi: 10.1007/s10067-005-1099-4. [DOI] [PubMed] [Google Scholar]
- 10.Valencia IC, Chang A, Kirsner RS, Kerdel FA. Eosinophilic fasciitis responsive to treatment with pulsed steroids and cyclosporine. Int J Dermatol. 1999;38:369–72. doi: 10.1046/j.1365-4362.1999.00695.x. [DOI] [PubMed] [Google Scholar]
- 11.Ching DW, Leibowitz MR. Ketotifen--a therapeutic agent of eosinophilic fasciitis? J Intern Med. 1992;231:555–9. doi: 10.1111/j.1365-2796.1992.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 12.Khanna D, Agrawal H, Clements PJ. Infliximab may be effective in the treatment of steroid-resistant eosinophilic fasciitis: report of three cases. Rheumatology (Oxford) 2010;49:1184–8. doi: 10.1093/rheumatology/keq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzini CU, Sebastiani M, Giuggioli D, Manfredi A, Colaci M, Cesinaro AM, et al. D-penicillamine in the treatment of eosinophilic fasciitis: case reports and review of the literature. Clin Rheumatol. 2012;31:183–7. doi: 10.1007/s10067-011-1866-3. [DOI] [PubMed] [Google Scholar]
- 14.Romano C, Rubegni P, De Aloe G, Stanghellini E, D’Ascenzo G, Andreassi L, et al. Extracorporeal photochemotherapy in the treatment of eosinophilic fasciitis. J Eur Acad Dermatol Venereol. 2003;17:10–3. doi: 10.1046/j.1468-3083.2003.00587.x. [DOI] [PubMed] [Google Scholar]
- 15.Schiener R, Behrens-Williams SC, Gottlöber P, Pillekamp H, Peter RU, Kerscher M. Eosinophilic fasciitis treated with psoralen-ultraviolet A bath photochemotherapy. Br J Dermatol. 2000;142:804–7. doi: 10.1046/j.1365-2133.2000.03431.x. [DOI] [PubMed] [Google Scholar]
- 16.Cetkovský P, Koza V, Cetkovská P, Svojgrová M. Successful treatment of severe Shulman’s syndrome by allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;21:637–9. doi: 10.1038/sj.bmt.1701137. [DOI] [PubMed] [Google Scholar]
- 17.Knaysi GA, Crikelair GF, Cosman B. The rule of nines: its history and accuracy. Plast Reconstr Surg. 1968;41:560–3. [PubMed] [Google Scholar]
- 18.Derk CT, Bischoff L. Eosinophilic fasciitis: demographics, disease pattern and response to treatment: report of 12 cases and review of the literature. Int J Dermatol. 2008;47:29–35. doi: 10.1111/j.1365-4632.2007.03544.x. [DOI] [PubMed] [Google Scholar]
- 19.Lebeaux D, Francès C, Barete S, Wechsler B, Dubourg O, Renoux J, et al. Eosinophilic fasciitis (Shulman disease): new insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford) 2012;51:557–61. doi: 10.1093/rheumatology/ker366. [DOI] [PubMed] [Google Scholar]
- 20.Lebeaux D, Sène D. Eosinophilic fasciitis (Shulman disease) Best Pract Res Clin Rheumatol. 2012;26:449–58. doi: 10.1016/j.berh.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Steen VD, Medsger TA, Rodnan GP. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med. 1982;97:652–659. doi: 10.7326/0003-4819-97-5-652. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez SA, Sigal SH. A 15-year prospective study of treatment of rapidly progressive systemic sclerosis with d-penicillamine. J Rheumatol. 1991;18:1496–503. [PubMed] [Google Scholar]
- 23.Derk CT, Huaman G, Jimenez SA. A retrospective randomly selected cohort study of d-penicillamine treatment in rapidly progressive diffuse cutaneous systemic sclerosis of recent onset. Br J Dermatol. 2008;158:1063–8. doi: 10.1111/j.1365-2133.2008.08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirschstein M, Helmchen U, Jensen R, Küster RM, Lehmann H. Kidney involvement in a 17-year-old boy with eosinophilic fasciitis. Clin Nephrol. 1999;52:183–7. [PubMed] [Google Scholar]
- 25.Janzen L, Jeffery JR, Gough J, Chalmers IM. Response to methotrexate in a patient with idiopathic eosinophilic fasciitis, morphea, IgM hypergammaglobulinemia, and renal involvement. J Rheumatol. 1995;22:1967–70. [PubMed] [Google Scholar]
- 26.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39:S158–61. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 27.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–54. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;23:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris ED, Jr, Sjoerdsma A. Effect of penicillamine on human collagen and its possible application to treatment of scleroderma. Lancet. 1966;2:996–9. doi: 10.1016/s0140-6736(66)92926-6. [DOI] [PubMed] [Google Scholar]
- 30.Nimni ME. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. I. Metabolic and functional abnormalities in soft tissues. J Biol Chem. 1968;243:1457–66. [PubMed] [Google Scholar]
- 31.Siegel RC. Collagen cross-linking. Effect of D-penicillamine on cross-linking in vitro. J Biol Chem. 1977;252:254–9. [PubMed] [Google Scholar]