Abstract
Background
ACOSOG Z0011 defined clinical node negativity by physical exam alone. Although axillary US with biopsy has a positive predictive value for lymph node (LN) metastases approaching 100%, it may not appropriately identify clinically node-negative women with ≥3 positive LNs (+LNs) who require ALND. We sought to identify the total number of +LNs in women presenting with cT1-2N0 breast carcinoma with a positive preoperative LN biopsy to evaluate the potential for overtreatment when ALND is performed on the basis of a positive needle biopsy in patients who otherwise meet ACOSOG Z0011 eligibility criteria.
Methods
Patients with cT1-2N0 breast cancer by physical exam with a positive preoperative LN biopsy were identified from a prospective institutional database. Clinicopathologic characteristics and axillary imaging results were compared between women with 1-2 total +LNs and ≥3 total +LNs.
Results
Between 5/2006-12/2013, 141 women with cT1-2N0 breast cancer had abnormal axillary imaging and a preoperative +LN biopsy (median patient age: 51yrs, median tumor size:2.4cm,86% ductal histology,79% ER+). 66 (47%) women had 1-2 total +LNs, 75 (53%) had ≥3 total +LNs. Women with ≥3 total +LNs had larger tumors (2.4 vs 2.2cm, p=0.03), fewer tumors with ductal histology (79% vs 94%, p=0.01), more lymphovascular invasion (80% vs 61%,p=0.01) and higher median BMI (29.2 vs 27.1,p=0.04). Having >1 abnormal LN on axillary imaging was significantly associated with having ≥3 total +LNs on final pathology (68% vs 43% p=0.003).
Conclusions
Axillary imaging with preoperative LN biopsy does not accurately discriminate low-versus high-volume nodal disease in clinically node-negative patients.
Keywords: lymph node needle biopsy, ACOSOG Z0011, axillary lymph node dissection, clinically node-negative breast cancer
INTRODUCTION
Axillary management for early-stage breast cancer patients continues to evolve, with the goal of optimal oncologic safety coupled with nominal surgical morbidity. Four trials evaluating axillary management in clinically node-negative breast cancer patients have established the safety of omitting a completion axillary lymph node dissection (ALND) in select pathologically node-positive patients.1-5 The ACOSOG Z0011 trial reported equivalent outcomes for sentinel lymph node biopsy (SLNB) alone compared to completion ALND for women with 1–2 positive sentinel lymph nodes undergoing breast-conserving surgery, whole-breast radiation therapy, and systemic therapy.3,4 In the ACOSOG Z0011 trial, a clinically negative axilla was defined by physical exam alone. However, some studies6,7 suggest that axillary ultrasound (US) and needle biopsy of abnormal-appearing nodes can appropriately allocate node-positive women to an upfront ALND, avoiding a two-step axillary procedure. Although axillary US and needle biopsy has a positive predictive value for the detection of nodal metastases approaching 100%8, it may not appropriately identify clinically node-negative women who require ALND. In the ACOSOG Z0011 era, axillary US and needle biopsy to select patients for ALND has utility only if it appropriately discriminates between women with 1–2 positive SLNs and those with >2 positive SLNs. In this study, we sought to determine if a positive preoperative lymph node needle biopsy in clinically node-negative women with T1–2 tumors identifies a population of women who require ALND and to evaluate the potential for overtreatment when ALND is performed on the basis of a positive needle biopsy in patients who otherwise meet ACOSOG Z0011 eligibility criteria.
MATERIALS AND METHODS
Following institutional review board approval, patients clinically staged as having T1–2N0 invasive breast cancer with a positive preoperative axillary lymph node needle biopsy (fine needle aspiration or core needle biopsy) were identified from a prospective institutional database. Clinical nodal status was confirmed by chart review. Patients undergoing neoadjuvant chemotherapy or managed with a SLNB alone were excluded. Patient and tumor characteristics including age, body mass index, tumor size, tumor histology, nuclear grade, presence of lymphovascular invasion, multifocality, estrogen and progesterone receptor status, HER2neu amplification, and breast surgery were collected. Type of axillary surgery and the total number of positive lymph nodes were determined. Axillary imaging results from mammogram, US, and MRI were abstracted from radiology reports. All outside imaging was reviewed by specialist breast imagers prior to surgery. We do not routinely obtain axillary ultrasound for clinically node-negative patients at our institution. Axillary ultrasounds were performed at the discretion of an outside physician prior to presentation at Memorial Sloan Kettering Cancer Center or were done as additional work-up for abnormal lymph nodes seen on mammogram or MRI. For ultrasound performed at MSKCC, a high-resolution transducer was used by a trained ultrasound technician to perform contiguous radial and anti-radial scanning; a breast radiologist re-scans any questionable findings. For each imaging modality, it was determined whether abnormal axillary lymph nodes were identified, and the number of abnormal lymph nodes were categorized as 1 or >1. Clinicopathologic characteristics and axillary imaging results were compared between women with 1–2 total positive lymph nodes and those with ≥3 total positive lymph nodes.
Clinical characteristics were summarized using frequency and percent for categorical covariates, and median and range for continuous covariates, and were compared between the group with 1–2 positive lymph nodes and the group with >3 positive lymph nodes using chi-squared tests for categorical covariates (Fisher’s exact test in the case of small call frequencies) and t-tests for continuous covariates (Wilcoxon rank-sum test for tumor size). All statistical analysis was performed in SAS 9.2 (SAS Institute, Cary, NC), and p-values <0.05 were considered significant.
RESULTS
Between May 2006 and December 2013, 904 breast cancer patients had a positive preoperative lymph node needle biopsy; 151 of these women were staged as cT1-2N0 by physical exam. 10 women underwent SLNB alone and were excluded, leaving 141 women for the study population. Median patient age for the cohort was 51 years (range 25–91 years), median pathologic tumor size was 2.4cm (range 0.8–9.5cm), 86% of tumors were of ductal histology, and 79% of tumors were estrogen receptor positive. Lumpectomy was performed in 51 (36%) women, while 90 (64%) underwent mastectomy (Table 1). All patients had an ALND.
Table 1.
Clinicopathologic features of patient population based on extent of nodal involvement nodes
| Factor | Total population (n=141) |
1–2 positive LNs (n=66) |
≥3 positive LNs (n=75) |
P-value |
|---|---|---|---|---|
|
| ||||
| Age, years, median (range) |
51 (25–91) | 51.5 (31–80) | 51 (25–91) | 0.80 |
|
| ||||
| BMI, median (range) | 27.7 (17.7–48.6) | 27.1 (17.7–41.4) | 29.2 (18.4– 48.6) |
0.0448 |
|
| ||||
| Tumor size, median (range) |
2.4 (0.8–9.5) | 2.2 (0.8–4.5) | 2.4 (0.9–9.5) | 0.0329 |
|
| ||||
| Tumor histology | 0.0121 | |||
| Ductal | 121 (86%) | 62 (94%) | 59 (79%) | |
| Lobular | 12 (9%) | 1 (2%) | 11 (15%) | |
| Mixed ductal and lobular features |
7 (5%) | 3 (5%) | 4 (5%) | |
| Metaplastic | 1 (1%) | 0 (0%) | 1 (1%) | |
|
| ||||
| Nuclear grade | 0.92 | |||
| Low/intermediate | 43 (38%) | 23 (39%) | 20 (37%) | |
| High | 70 (62%) | 36 (61%) | 34 (63%) | |
| Missing | 28 | 7 | 21 | |
|
| ||||
| LVI present | 100 (71%) | 40 (61%) | 60 (80%) | 0.0114 |
|
| ||||
| Multifocal | 62 (44%) | 26 (39%) | 36 (48%) | 0.30 |
|
| ||||
| ER status | 0.81 | |||
| Positive | 112 (79%) | 53 (80%) | 59 (79%) | |
| Negative | 29 (21%) | 13 (20%) | 16 (21%) | |
|
| ||||
| PR status | 0.64 | |||
| Positive | 102 (72%) | 49 (74%) | 53 (71%) | |
| Negative | 39 (28%) | 17 (26%) | 22 (29%) | |
|
| ||||
| HER2/neu status | 0.41 | |||
| Not amplified | 36 (26%) | 19 (29%) | 17 (23%) | |
| Amplified | 105 (74%) | 47 (71%) | 58 (77%) | |
|
| ||||
| Total positive nodes, median (range) |
3 (1–53) | 1 (1–2) | 6 (3–53) | NA |
|
| ||||
| Total nodes removed, median (range) |
21 (7–60) | 21 (7–53) | 20 (10–60) | 0.83 |
|
| ||||
| Final breast procedure |
0.45 | |||
| Lumpectomy | 51 (36%) | 26 (39%) | 25 (33%) | |
| Mastectomy | 90 (64%) | 40 (61%) | 50 (67%) | |
Sixty-six (47%) women had 1–2 total positive lymph nodes (34 with 1 positive node, 32 with 2 positive lymph nodes), and 75 (53%) women had ≥3 total positive lymph nodes. Table 1 compares clinicopathologic features between these 2 groups. Women with >3 total positive lymph nodes had larger tumor size (2.4cm versus 2.2cm, p=0.033), fewer tumors with ductal histology (79% versus 94%, p=0.012), a higher rate of lymphovascular invasion (80% versus 61%, p=0.011), and a higher median BMI (29.2 versus 27.1, p=0.045).
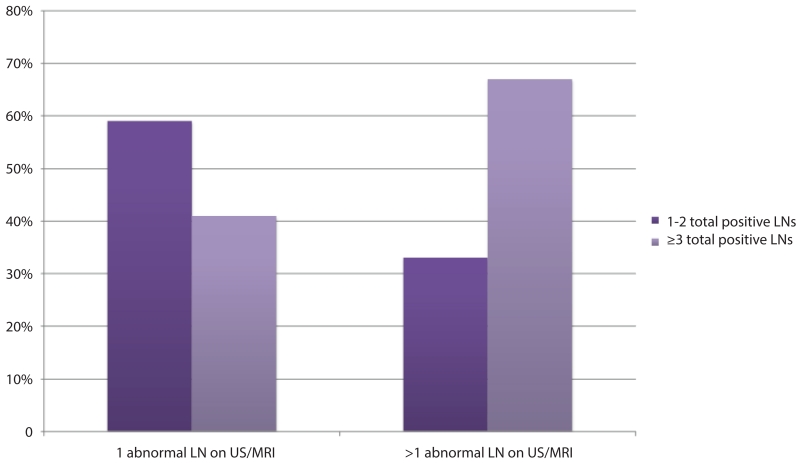
All 141 patients had at least one abnormal axillary lymph node seen on US, and 60 (43%) had abnormal axillary lymph nodes identified on mammogram. 89 (63%) women were also imaged with breast MRI; 16%, 49%, and 35% had 0, 1, or >1 abnormal lymph nodes identified, respectively. Table 2 compares axillary imaging results among women with 1–2 total positive nodes and those with ≥3 total positive nodes. A significantly greater proportion of women with ≥3 total positive nodes had >1 abnormal lymph node identified on preoperative axillary imaging compared to women with only 1–2 total positive lymph nodes (53% versus 29%, p=0.0032). Among women with >1 abnormal lymph nodes identified on preoperative axillary imaging, 68% had ≥3 total positive lymph nodes on final pathology (Fig 1).
Table 2.
Axillary imaging results based on extent of nodal involvement
| Imaging Results | 1–2 Positive LNs (n=66) |
≥3 Positive LNs (n=75) |
P-value |
|---|---|---|---|
|
| |||
| Mammogram with abnormal LNs | 0.75 | ||
| No | 37 (56%) | 44 (59%) | |
| Yes | 29 (44%) | 31 (41%) | |
|
| |||
| Number of abnormal LNs on US | 0.016 | ||
| 1 | 52 (79%) | 45 (60%) | 3 |
| >1 | 14 (21%) | 30 (40%) | |
|
| |||
| MRI performed | 0.0266 | ||
| Yes | 48 (73%) | 41 (55%) | |
| No | 18 (27%) | 34 (45%) | |
|
| |||
| Number of abnormal LNs on MRI (n=88)* | 0.083 | ||
| 0 | 8 (17%) | 6 (15%) | |
| 1 | 28 (58%) | 15 (38%) | |
| >1 | 12 (25%) | 19 (48%) | |
|
| |||
| Number of abnormal LNs on US/MRI axillary imaging** |
0.0032 | ||
| 1 | 47 (71%) | 35 (47%) | |
| >1 | 19 (29%) | 40 (53%) | |
Results unknown for one patient who underwent MRI
This value indicates the greatest number of abnormal lymph nodes seen on either US or MRI for each patient
Fig 1.
Total positive lymph nodes for women with a positive preoperative needle biopsy with 1 or >1 abnormal axillary lymph nodes identified by US or MRI
DISCUSSION
Among a cohort of clinically node-negative breast cancer patients identified as having abnormal axillary imaging with a subsequent positive preoperative lymph node needle biopsy, nearly half (47%) had only 1–2 total positive lymph nodes at the time of axillary surgery. During this time period, almost all patients with a positive lymph node needle biopsy were managed with an upfront ALND, with only 4% of patients having a SLNB followed by completion ALND. Based on final lymph node pathology, our results suggest that at least half of these patients could be safely managed with SLNB alone if treated according to ACOSOG Z0011 criteria. Furthermore, because the number of positive lymph nodes is the total number of positive nodes from an axillary dissection specimen rather than number of positive sentinel lymph nodes, this is likely an underestimate of the number of women who would be spared a completion ALND if managed according to ACOSOG Z0011.
While some suggest that axillary US and needle biopsy are essential in the preoperative work-up of breast cancer patients to help dictate surgical management9-11, the role of preoperative axillary staging is shifting in the ACOSOG Z0011 era. Houssami et al assessed the utility of preoperative axillary US and needle biopsy in discriminating low versus high nodal disease burden based on needle biopsy results in an analysis of 7 studies and report results similar to our findings. In this analysis, high nodal disease burden was defined as >3 positive lymph nodes in 6 studies and ≥2 positive lymph nodes in one study. The pooled odds ratio (OR) for high nodal disease burden with a positive versus negative needle biopsy was 4.38, with the proportion of patients with a positive needle biopsy having a high nodal disease burden estimated to be 59%. Conversely, these results suggest that 41% of patients with a positive preoperative needle biopsy would have only 1–2 total positive lymph nodes and do not require additional axillary surgery.12
Similarly, Schipper et al13 examined the nodal disease burden in women evaluated with preoperative axillary US and needle biopsy. In a cohort of 40 clinically node-negative breast cancer patients with an abnormal axillary US and positive lymph node needle biopsy, 25 (62.5%) were pN1, while 15 (37.5%) had pN2-3 disease on final pathology. In a subset of women with T1-2 tumors undergoing breast-conserving surgery, 12 of 278 cases had abnormal axillary US and needle biopsy. ALND pathology of these 12 patients revealed 1–2 positive lymph nodes in 6 cases, supporting the notion that a positive lymph node needle biopsy does not predict the need for ALND in clinically node-negative patients.
Studies by Verheuvel et al and Caudle et al have also compared node-positive patients identified by US and needle biopsy to women with negative axillary imaging found to have a positive node with a SLNB and concluded that women diagnosed with positive nodes by needle biopsy are higher risk for heavy nodal disease burden and should not be managed according to Z0011 criteria.14,15 Women with a positive needle biopsy had higher risk tumor characteristics, with larger tumor size, and more high-grade tumors with lymphovascular invasion and hormone receptor negativity. Although women identified as being node positive by US and needle biopsy were at higher risk for heavy nodal disease burden, 37%-52% had only 1-2 total positive lymph nodes. Furthermore, while survival was expectedly worse in the needle biopsy cohort reported by Verheuvel el al that presented with more advanced-stage disease, there was no difference in regional recurrence, with only one isolated regional relapse in each group.. A meta-analysis including 6 additional studies also reported a higher percentage of pN2 disease in women with a positive needle biopsy compared to those with negative axillary imaging with a positive sentinel lymph node (46% versus 30%).16 These studies concluded that SLNB may not be appropriate for women diagnosed with nodal disease by US and needle biopsy because this represents a higher-risk population; however, these studies do not represent women meeting Z0011 criteria, and yet 30%–52% of patients had low volume nodal disease and, in the appropriate context, could be spared ALND.
When evaluating nodal disease burden by number of abnormal lymph nodes identified, we found that women with >1 abnormal lymph nodes by US or MRI were more likely to have >3 positive total lymph nodes than women with only 1 abnormal lymph node on axillary imaging (68% versus 43%, p=0.003). Hieken et al17 also compared final nodal pathology for women with 1 versus >1 abnormal lymph node on preoperative axillary imaging and found a greater percentage of pN2 disease in women with >1 abnormal node on US (31% versus 14%, p>0.001). While >1 abnormal lymph nodes is a predictor of higher nodal disease burden, we have previously reported that finding multiple abnormal axillary lymph nodes on preoperative axillary imaging is extremely uncommon in a cohort of clinically node-negative patients meeting ACOSOG Z0011 eligibility criteria. Among 425 women treated with breast conservation found to have a positive sentinel lymph node, >1 abnormal lymph node was identified in 15 (6%) women by US and 20 (12%) women by MRI, with only 10 (2%) women having >2 abnormal lymph nodes by US or MRI (Pilewskie et al, submitted). While finding multiple abnormal lymph nodes is uncommon among women meeting ACOSOG Z0011 criteria, for women with multiple abnormal lymph nodes and a positive preoperative needle biopsy, frozen section could be performed in the operating room to document the number of nodal metastasis to obviate a return to the operating room for a completion ALND in a population of women at high risk for requiring additional axillary surgery.
This study is limited by the retrospective nature of the data collection and a paucity of information regarding indication for axillary imaging. While axillary imaging was not routine for clinically node-negative patients at our institution, many women undergo axillary US prior to presentation, and others have breast MRI at the surgeons discretion. Additionally, while we abstracted axillary lymph node imaging results from mammogram and MRI reports, it is notable that these imaging modalities are not performed to specifically evaluate the axilla. Furthermore, while the presenting clinical stage is consistent with a population of patients meeting ACOSOG Z0011 criteria, many patients were treated with mastectomy and ALND, and therefore this cohort does not represent a population of women managed according to Z0011. However, in a recent study from our institution evaluating the role of axillary imaging in a consecutive cohort of women presenting with cT1-2N0 invasive breast carcinoma and treated according to ACOSOG Z0011 criteria, similar results were reported. Among a small group of women (n=11) with a positive preoperative needle biopsy, 5 of 11 (45%) women required completion ALND while 6 of 11 (55%) had 1–2 positive sentinel lymph nodes and were spared additional axillary surgery (Pilewskie et al, submitted).
While this is a relatively small study, the results strongly suggest that a positive axillary node needle biopsy is insufficient to warrant ALND. Based on the total number of positive axillary lymph nodes identified in this cohort, it appears that approximately half of women presenting with clinically node-negative disease and a positive preoperative lymph node needle biopsy are likely overtreated if managed with upfront ALND, questioning the utility of axillary imaging and needle biopsy in select early-stage breast cancer patients.
Synopsis.
Here we examine whether a positive preoperative lymph node needle biopsy in clinically node-negative women meeting Z0011 criteria identifies patients who require axillary lymph node dissection (ALND). We find that a positive needle biopsy is insufficient to warrant ALND.
REFERENCES
- 1.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–32. doi: 10.1097/SLA.0b013e3181f08f32. discussion 32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sola M, Alberro JA, Fraile M, et al. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20(1):120–7. doi: 10.1245/s10434-012-2569-y. [DOI] [PubMed] [Google Scholar]
- 6.Rao R, Euhus D, Mayo HG, et al. Axillary node interventions in breast cancer: a systematic review. JAMA. 2013;310(13):1385–94. doi: 10.1001/jama.2013.277804. [DOI] [PubMed] [Google Scholar]
- 7.Shah-Khan M, Boughey JC. Evolution of axillary nodal staging in breast cancer: clinical implications of the ACOSOG Z0011 trial. Cancer Control. 2012;19(4):267–76. doi: 10.1177/107327481201900403. [DOI] [PubMed] [Google Scholar]
- 8.Houssami N, Ciatto S, Turner RM, et al. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011;254(2):243–51. doi: 10.1097/SLA.0b013e31821f1564. [DOI] [PubMed] [Google Scholar]
- 9.Fornage BD. Local and regional staging of invasive breast cancer with sonography: 25 years of practice at MD Anderson Cancer Center. Oncologist. 2014;19(1):5–15. doi: 10.1634/theoncologist.2013-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diepstraten SC, Sever AR, Buckens CF, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21(1):51–9. doi: 10.1245/s10434-013-3229-6. [DOI] [PubMed] [Google Scholar]
- 11.Rattay T, Muttalib M, Khalifa E, et al. Clinical utility of routine pre-operative axillary ultrasound and fine needle aspiration cytology in patient selection for sentinel lymph node biopsy. Breast. 2012;21(2):210–4. doi: 10.1016/j.breast.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Houssami N, Diepstraten SC, Cody HS, 3rd, et al. Clinical utility of ultrasound-needle biopsy for preoperative staging of the axilla in invasive breast cancer. Anticancer Res. 2014;34(3):1087–97. [PubMed] [Google Scholar]
- 13.Schipper RJ, van Roozendaal LM, de Vries B, et al. Axillary ultrasound for preoperative nodal staging in breast cancer patients: is it of added value? Breast. 2013;22(6):1108–13. doi: 10.1016/j.breast.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Caudle AS, Kuerer HM, Le-Petross HT, et al. Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol. 2014;21(11):3440–7. doi: 10.1245/s10434-014-3813-4. [DOI] [PubMed] [Google Scholar]
- 15.Verheuvel NC, van den Hoven I, Ooms HW, et al. The Role of Ultrasound-Guided Lymph Node Biopsy in Axillary Staging of Invasive Breast Cancer in the Post-ACOSOG Z0011 Trial Era. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4071-1. [DOI] [PubMed] [Google Scholar]
- 16.van Wely BJ, de Wilt JH, Francissen C, et al. Meta-analysis of ultrasound-guided biopsy of suspicious axillary lymph nodes in the selection of patients with extensive axillary tumour burden in breast cancer. Br J Surg. 2014 doi: 10.1002/bjs.9663. [DOI] [PubMed] [Google Scholar]
- 17.Hieken TJ, Trull BC, Boughey JC, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery. 2013;154(4):831–8. doi: 10.1016/j.surg.2013.07.017. discussion 8-40. [DOI] [PubMed] [Google Scholar]