Abstract
In multiple myeloma (MM) increased numbers of monoclonal plasma cells in the bone marrow induce localized osteolytic lesions that rarely heal, due to increased bone resorption and suppressed bone formation. Numerous studies reported the contributions that different cell types in the MM microenvironment make to MM growth and bone disease, but the role of matrix-embedded osteocytes in MM, which comprise >95% of bone cells and are major regulators of osteoclast and osteoblast activity, is unclear. We report that osteocytes in MM-bearing bones physically interact with MM cells in vivo, undergo caspase3-dependent apoptosis, and express higher RANKL and Sclerostin levels than osteocytes from control mice. Mechanistic studies revealed that osteocyte apoptosis is initiated by activation of Notch signaling in osteocytes through direct contact with MM cells, and is further amplified by MM cell-secreted TNFα. This Notch/TNFα induced osteocyte apoptosis increases osteocytic Rankl expression, the osteocytic Rankl/Opg ratio and the ability of osteocytes to attract osteoclast precursors to induce local bone resorption. Further, osteocytes in contact with MM cells express high levels of Sost/Sclerostin that decrease Wnt signaling in osteoblasts and inhibit osteoblast differentiation. Importantly, direct contact between osteocytes and MM cells reciprocally activates Notch signaling and increases Notch receptor expression in MM cells, in particular Notch3 and 4, and stimulates MM cell growth. These studies reveal a previously unknown role for bidirectional Notch signaling between MM cells and osteocytes that enhances MM growth and bone disease, and suggest the potential of targeting osteocyte-MM cell interactions as a novel MM treatment.
Keywords: Multiple myeloma, osteocytes, Notch signaling, apoptosis, osteoclasts, osteoblasts
Introduction
Multiple myeloma (MM) is characterized by expansion of monoclonal plasma cells in the bone marrow (BM) that induce marked bone destruction in the majority of patients (1). MM patients present with severe bone pain caused by osteolytic lesions that rarely heal (2). The osteolytic lesions result from increased bone resorption and concomitant long-term suppression of bone formation. The bone/BM microenvironment is a major contributor to tumor growth and bone destruction in MM (3). The bone remodeling compartment is disrupted in MM allowing exchange of soluble factors and direct cell-to-cell contact between MM cells and bone cells (4). Previous studies demonstrated that multiple cytokines are secreted or induced by the interaction of MM cells with the different cell types in the bone microenvironment to increase bone destruction, including the receptor of activator nuclear factor-kappa B ligand (RANKL), the chemokine (C-C motif) ligand 3 (MIP1α) and interleukin-3 (IL-3). Further, MM-derived interleukin 7 (IL-7), dickkopf WNT signaling pathway inhibitor 1 (DKK1) and IL-3, as well as direct contact by MM cells with osteoblasts inhibit osteoblast differentiation (1, 5).
Although osteoclasts and osteoblasts remodel bone by resorbing or forming bone respectively, osteocytes, which constitute >95% of all bone cells, are the central regulators of these processes (6, 7). Osteocytes, although buried within bone mineral, extensively communicate with each other and with cells on the bone surface and in the marrow via cytoplasmic projections that run along canaliculi and form the osteocyte network. This network allows cell-to-cell communication and distributes osteocyte-secreted molecules that regulate osteoblast and osteoclast function. Osteocytes are the primary producers of Sclerostin, the product of the Sost gene, a potent inhibitor of bone formation, and are a major source of RANKL, the central osteoclastogenic factor (8, 9). Lack of Sclerostin increases osteoblast number and activity, and deletion of osteocytic RANKL inhibits osteoclast formation and bone resorption, demonstrating that osteocytes are key regulators of osteoblast and osteoclast activity (10–12). Moreover, apoptotic osteocytes, which accumulate with skeletal disuse, glucocorticoid excess or estrogen deficiency, increase local bone resorption by attracting osteoclast precursors to particular areas of bone (6, 13). Although knowledge of the role of osteocytes and the osteocytic network in bone homeostasis and common skeletal diseases has greatly increased, the contribution of osteocytes to the development and progression of cancer involving bone is just beginning to be defined.
In the current study, we determined if reciprocal communication between MM cells and osteocytes occurs and explored the mechanisms involved and the consequences of these interactions for the progression of MM bone disease.
Materials and Methods
Reagents
Reagents used in this study can be found in Supplementary Methods.
Cells and culture conditions
L. Bonewald (University of Missouri at Kansas City, USA) provided the murine MLO-A5 in 1997 and MLO-Y4 in 2001 osteocyte-like cells (14, 15). JJN3, 5TGM1 and MM1.S MM cell lines were provided by N. Giuliani (University of Parma, Italy) in 2006, B. Oyajobi (University of Texas at San Antonio, USA) in 2007, and S. Rosen (Northwestern University, USA) in 2003 (16–18). R. Jilka (University of Arkansas for Medical Sciences, USA) provided the OB-6 osteoblast-like cells in 1997 (19). Non-adherent osteoclast precursors were collected as described before (20). After informed consent, CD138+ cells from MM patients were prepared as previously detailed (16). Studies were approved by the Indiana University School of Medicine Institutional Review Board. Cell lines were authenticated by morphology, gene expression profile, and tumorigenic capacity (MM cells). Co-cultures were established by 1) adding MM cells on top of osteocyte-like cells, 2) adding MM cells in transwell chambers in a 1:5 ratio (osteocytic:MM), or 3) adding 50% conditioned media (CM) from 48h-culture of MM cells to osteocytes. DEVD (50nM), anti-TNFα (0.3μg/mL) or GSIXX (2.5–10μM) were added 1h before addition of MM cells or CM. MLO-A5 cells were treated with 0.01ng/mL TNFα, 5ng/mL TGFβ or 10ng/mL interleukin 6 (IL-6) for 4–24h. For Notch activation, MLO-A5 cells were cultured on DLL1-IgG2 or control IgG2-coated plates for 24h. For OB-6 osteoblast-like cell differentiation, cells were cultured with osteogenic media (OM; 0.2 mM ascorbic acid, 10 mM β-glycerophosphate) or OM containing 50% of CM from MLO-A5 or JJN3 cells cultured alone, or from JJN3 directly co-cultured with MLO-A5 cells.
Ex vivo bone organ cultures
This assays were performed as previously described (21). Detailed description of the assay can be found in Supplementary Methods.
Cell viability and apoptosis
MM cells were separated from adherent osteocyte-like cells using EDTA. Cell death was quantified by trypan blue uptake and apoptosis by chromatin condensation and nuclear fragmentation of cells transfected with nuclear green fluorescent protein (nGFP) (22, 23). At least 100 cells in 5 different fields selected by systematic random sampling were examined for each experimental condition. Representative photomicrographs were taken with an EVOS FLCell Imaging System (Life technologies, Grand Island, NY, USA).
Cell proliferation analysis
Viable cells were enumerated by trypan blue exclusion. Fluorescence emitted by 5TGM1-GFP cells was measured in a SpectraMaxi3 microplate reader (Molecular Devices, Sunnyvale, CA, USA), set at 485 nm/520 nm. 5TGM1-GFP cell numbers linearly correlated with fluorescent units (RFU) (Suppl. Figure 1A).
Transfections
MLO-A5 cells were transiently transfected using Lipofectamine-Plus (Invitrogen) (23, 24).
Gene expression analysis
mRNA expression was quantified as previously described (25). Murine soluble RANKL, OPG or human TNFα were quantified by Enzyme-linked immunosorbent assays (ELISA, R&D systems) of culture supernatants.
Western blot analysis
The assays were performed as described previously (25). Detailed descriptions of antibodies can be found in Supplementary Methods.
Osteoclast precursor migration
48h-CM from JJN3, MLO-A5, or from JJN3 and MLO-A5 co-cultured in direct contact was placed in the bottom of 8 μm pore chambers to attract murine non-adherent BM cells (26). Detailed description of the assay can be found in Supplementary Methods.
Mouse model of human MM
6wk-old female SCID mice B6.CB17-Prkdcscid/SzJ (Jackson laboratories, Bar Harbor, Maine, USA) and NIH-LystbgFoxn1nuBtkxid (Charles River Wilmington, Massachusetts, USA) were injected intratibially with JJN3 cells or saline and sacrificed 4wks later (16). Detailed information regarding imaging and micro-CT analysis can be found in Supplementary Methods. Studies were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. The sample size was calculated based on a previous study (16).
Immunohistochemistry
Antigen detection was performed on paraffin-embedded tibiae as described before (25). Detailed descriptions of antibodies can be found in Supplementary Methods.
Acid etching scanning electron microscopy (SEM)
The assays were performed as described previously (27). Detailed description of the assay can be found in Supplementary Methods.
Statistical analysis
Data were analyzed using SigmaStat (SPSS Science, Chicago, IL). Differences between means were evaluated using unpaired T-test, one-way, or two-way ANOVA, followed by pair-wise multiple comparisons using Student-Newman-Keuls method. Means ± standard deviation (SD) are reported. P values <0.05 were considered significant.
Results
MM cells increase osteocyte apoptosis and osteocytic RANKL and Sclerostin production in an in vivo model of MM bone disease
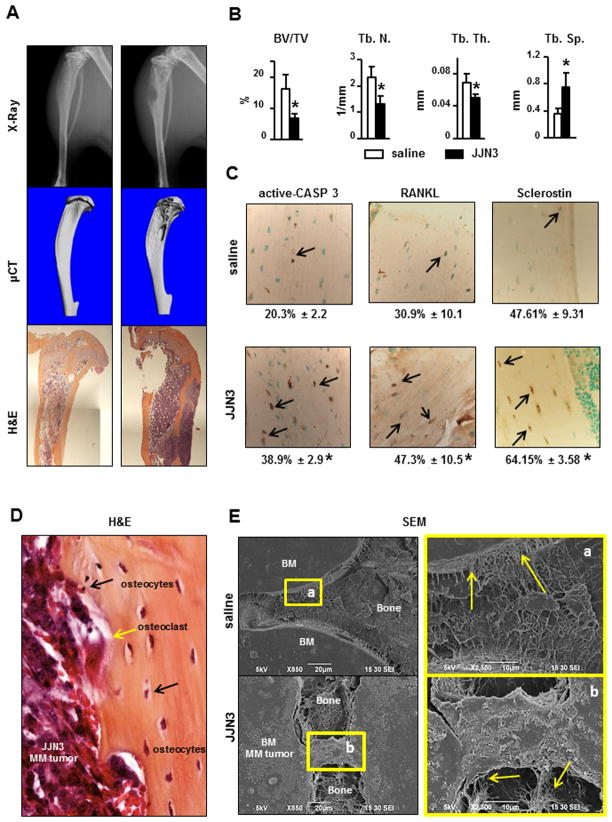
Our murine model of human MM is well-established and reproduces all features of human MM-induced bone disease (16). Osteolytic lesions were first detected radiographically 2wks after JJN3 cell injection (Suppl. Figure 1B) and their size progressively increased up to 4wks (Figure 1A). Micro-CT analysis of JJN3-involved tibia performed at 4wks confirmed the presence of osteolytic lesions and demonstrated decreased trabecular bone volume (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th.) and increased trabecular separation (Tb.Sp.) compared to controls (Figure 1B). The percentage of apoptotic osteocytes, stained with an anti-active CASP3 antibody, was increased 2 fold in bones injected with JJN3 cells compared to controls (Figure 1C). Moreover, the percentage of osteocytes expressing RANKL and Sclerostin was significantly higher in JJN3-injected mice compared to controls. These findings suggest that in addition to survival, osteocyte gene expression is altered in MM-bearing bones. Histological analysis of MM bearing bones showed that MM cells were adjacent to the bone surface, and acid etching-SEM images revealed that osteocytic dendritic processes were in direct contact with MM cells in the marrow (Figures 1D and E). Thus osteocytes are in close contact with MM cells, suggesting that MM-induced changes in osteocytes may result from cell-to-cell contact and/or exchange of soluble factors between osteocytes and MM cells.
Figure 1. Osteocytes interact with MM tumors in the BM to increase apoptosis, RANKL and Sclerostin production in osteocytes in a murine model of human MM.
(A) Osteolytic lesions were detected by radiographs, micro-CT and histology 4wks after injection. Representative images of tibiae are shown. (B) micro-CT analysis of cancellous bone in tumor-bearing tibias (n=5/group). (C) Percentage of cortical osteocytes stained for active CASP3, RANKL, or Sclerostin (n=5–7/group). *p<0.05 vs saline. (D) MM cells adjacent to osteocytes in bone detected by H&E. Black arrows point to osteocytes and the yellow arrow points to an osteoclasts on the bone surface. (E) Osteocytes in direct contact with MM tumors detected by acid etching-SEM. Areas indicated by boxes a and b are magnified on the right. Arrows point to osteocytic cytoplasmic projections in contact with the bone marrow (BM) compartment.
Osteocyte apoptosis is initiated by MM cell-mediated activation of Notch signaling in osteocytes and amplified by MM cell-derived TNFα
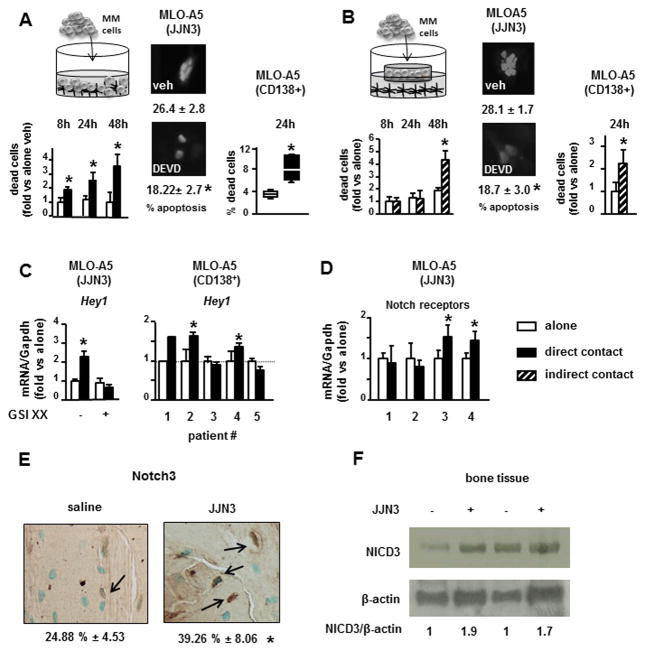
In vitro co-cultures between osteocyte-like MLO-A5 cells and MM cells were performed to determine the mechanisms responsible for the increased osteocyte apoptosis and up-regulation of RANKL and Sclerostin observed in osteocytes in MM-bearing bones. Communication between MM cells and bone cells, including osteocytes, can occur in different manners: 1) via secretion of soluble factors, 2) via direct cell-to-cell contact or 3) a combination of both. First, direct cell-to-cell cultures were established to determine the effect of physical interactions with MM cells on osteocyte apoptosis. Co-culture of MLO-A5 cells in direct contact with human JJN3 MM cells induced a 2–3 fold increase in MLO-A5 cell death (1–4% vs 6–15% in MLO-A5 alone vs. MLO-A5 cells co-cultured with JJN3 cells) (Figure 2A). Cell death was detected at 8h and progressively increased at 24 and 48h. To elucidate the effect of factors released by MM cells on osteocyte apoptosis, the cell types were separated by a porous membrane that prevents physical interactions and only allows exchange of soluble factors. Co-culture of JJN3 and MLO-A5 cells separated by transwell chambers did not increase cell death at 8 or 24h, but it did at 48h (1–3% vs 8–13%) (Figure 2B). These results suggest that different mechanisms mediate the early and late increases in osteocyte cell death induced by MM cells. Co-culture of MLO-A5 cells with JJN3 cells or JJN3-CM increased the percentage of MLO-A5 cells exhibiting chromatin condensation and nuclear fragmentation, sine qua non features of apoptosis (Figure 2A and B). Moreover, osteocyte death was inhibited by the CASP3 inhibitor, DEVD, at all time-points confirming that it was due to apoptosis (Figure 2A, 2B, and Suppl. Figure 1C). Co-culture of MLO-A5 cells with CD138+ cells from 6 different MM patients (Figure 2A) or conditioned media (CM) from CD138+ cells from a MM patient (Figure 2B) also increased MLO-A5 cell apoptosis. Similar results were obtained when MLO-A5 were co-cultured with human MM.1s or murine 5TGM1 MM cells, or when MLO-Y4 cells, another murine osteocytic cell line, were co-cultured with JJN3 cells (Suppl. Figure 1D).
Figure 2. MM-induced osteocyte apoptosis is triggered by activation of Notch signaling in osteocytes and sustained by MM-derived TNFα.
(A) Cell death, measured by trypan blue uptake and apoptosis (24h), measured by nuclear morphology, were quantified in MLO-A5 cells co-cultured in direct contact with JJN3 MM cells or CD138+ cells isolated from 6 MM patients, with or without the caspase inhibitor DEVD for 24h. (B) MLO-A5 cell death or apoptosis (24h) was quantified in co-cultures in indirect contact with JJN3 cells, cultured with 48h conditioned medium (CM) collected from JJN3 cultured alone, or 48h-CM from primary CD138+ cells from a MM patient with or without the caspase inhibitor DEVD. Representative experiments out of 3 (n=3–8) are shown (C and D) Hey1, Notch1, 2, 3 and 4 expression in MLO-A5 cells co-cultured for 4h in direct contact with JJN3 MM cells or CD138+ cells from MM patients. Data from one well per condition are reported for patient #1; 3 wells per condition are reported for the other patients (E) Percentage of cortical osteocytes stained for NOTCH3 (n=3–7/group). *p<0.05 vs saline. (F) Protein levels of NICD3, the activated form of the NOTCH3 receptor, in bone lysates obtained from saline- and JJN3-injected tibias.
Notch signaling is activated by cell-to-cell contact and regulates cell proliferation and apoptosis in multiple cell types (28). Further, Notch signaling is aberrantly activated in MM cells due to the over-expression of Notch receptors and ligands (29, 30), demonstrating that MM cells are potential signalers as well as targets of Notch. We therefore assessed if direct interactions between MM cells and osteocytes activate Notch in these cells and the potential biological consequences. Direct contact of MLO-A5 cells with JJN3 MM cells activated Notch signaling in MLO-A5 cells, as shown by the increased expression of the Notch target gene hairy/enhancer-of-split related with YRPW motif 1 (Hey1) (Figure 2C) and hairy and enhancer of split 1 (Hes1), which persisted up to 48h (Suppl. Figure 1E). JJN3 cell activation of osteocytic Notch signaling was suppressed by the Notch inhibitor GSIXX, confirming the specificity of the effect (Figure 2C). Hey1 expression was also increased in MLO-A5 cells co-cultured with CD138+ cells from 3 of 5 MM patients (Figure 2C). As expected, Notch activation (Hey1 and Hes1) in osteocytes did not occur when MLO-A5 cells were co-cultured with MM cells in transwell chambers (Suppl. Figure 1F). MM1.s or 5TGM1 MM cells also increased the Notch target gene expression in osteocyte-like MLO-A5 or MLO-Y4 cells (Suppl. Figure 1F). In addition, interactions with JJN3 cells rapidly upregulated the expression of receptors Notch3 and Notch4 in MLO-A5 cells, whereas Notch1 or Notch2 receptor expression was unchanged (Figure 2D). Moreover, the levels of NOTCH3 and of NICD3, the active form and downstream effector of the receptor Notch3, were elevated in tumor-bearing bones compared to control bones, demonstrating that Notch signaling activation also occurs in vivo (Figure 2E and F).
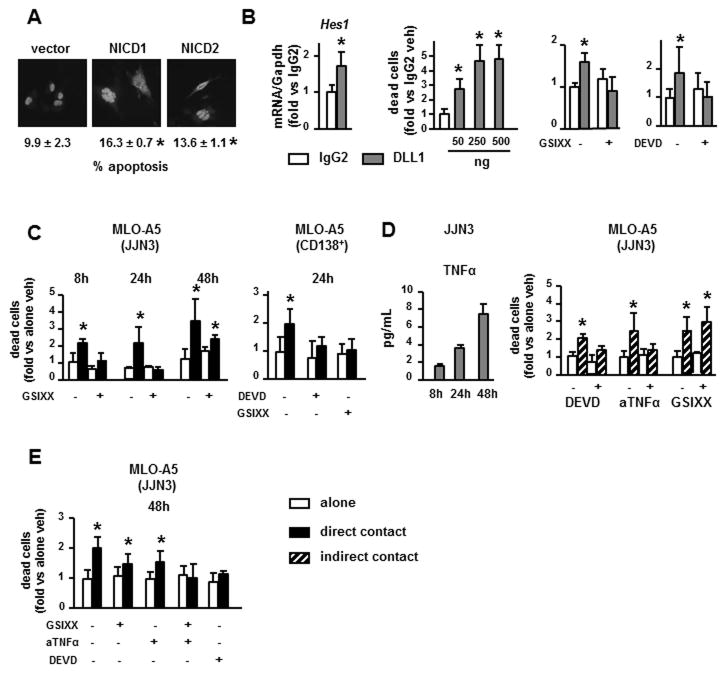
To evaluate the potential impact of gain-of-function of Notch signaling on osteocyte life span, MLO-A5 cells were transfected with nGFP and the Notch intracellular domains (NICD) 1 or 2, which translocate to the nucleus and activate Notch target gene transcription. Cells expressing either NICD1 or NICD2 exhibited increased apoptosis compared to vector controls (Figure 3A). Moreover, MLO-A5 cells cultured on plates coated with the Notch ligand DLL1 expressed higher levels of Hes1, confirming Notch activation, and had increased cell death (2- to 5-fold) compared to control cells cultured on IgG2 (1–2% vs 5–9% in control vs DLL1 treated cells). DLL1-induced death was completely inhibited by the Notch inhibitor GSIXX or by DEVD (Figure 3B), indicating that Notch activation is sufficient to trigger osteocyte apoptosis. Consistent with this observation, GSIXX completely blocked osteocytic cell death induced by direct co-culture with JJN3 cells or CD138+ cells from a MM patient at 8h or 24h (Figure 3C). In contrast, GSIXX only partially prevented MLO-A5 cell death measured at 48h, suggesting that factors secreted by MM cells accumulated during culture contribute to osteocyte apoptosis at later time points. In support of this notion, levels of TNFα, a recognized inducer of osteoblast and osteocyte apoptosis (31), increased 4-fold from 4 to 48h in CM from JJN3 cell cultures (Figure 3D), and addition of TNFα increased MLO-A5 cell death (Suppl. Figure 1G). Consistent with a role for MM-secreted TNFα in MM-induced osteocyte apoptosis, the decrease in osteocyte viability induced by JJN3-CM was blocked by DEVD or by a neutralizing anti-human TNFα antibody, but not by GSIXX (Figure 3D). Moreover, GSIXX or anti-TNFα only partially inhibited cell death, whereas the combination of both completely blocked the increase in osteocytic cell death induced by direct co-culture of MLO-A5 cells with JJN3 cells for 48h (Figure 3E). These findings suggest that MM cells induce osteocyte apoptosis by activating Notch and TNFα signaling pathways in osteocytes.
Figure 3. Activation of Notch signaling induces osteocyte apoptosis.
(A) Apoptosis was measured by nuclear morphology in MLO-A5 cells co-transfected with nGFP and vectors overexpressing NICD1 or NICD2 and quantified after 24h. (B) Cell death measured by trypan blue uptake and Hes1 expression (qPCR) were quantified in MLO-A5 after 24h culture on DLL1-coated plates with or without GSIXX or DEVD. (C) Cell death of MLO-A5 cells co-cultured in direct contact with JJN3 cells or CD138+ from a MM patient with or without the Notch inhibitor GSIXX or the caspase inhibitor DEVD. (D) TNFα protein levels secreted to the media by JJN3 MM cells. MLO-A5 cell death quantified in co-cultures in indirect contact with JJN3 cells, with or without DEVD, anti-TNFα (aTNFα) or GSIXX, as indicated. (E) Cell death of MLO-A5 quantified in co-cultures in direct contact with JJN3 cells, with or without DEVD, GSIXX, anti-TNFα or a combination of GSIXX and anti-TNFα. Representative experiments out of 3 (n=3–8) are shown. *p<0.05 vs MLO-A5 cultured alone (veh), vs vector-transfected cells (A), or vs cells plated on IgG2-coated plates (B).
Osteocyte apoptosis increases the osteoclastogenic potential of osteocytes and stimulates osteoclast precursor recruitment
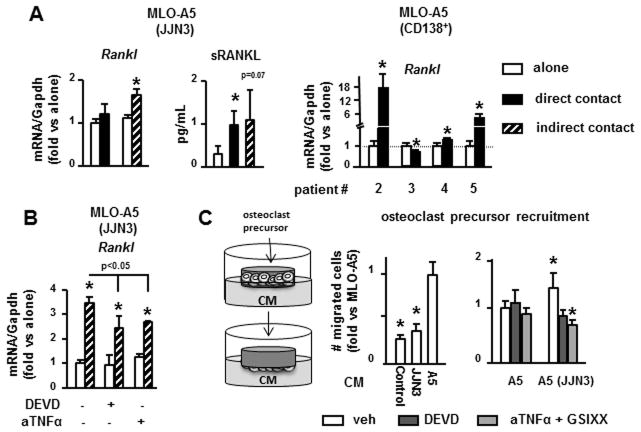
We then investigated the consequences of osteocyte apoptosis on the osteoclastogenic potential of osteocytes. MLO-A5 cells co-cultured with JJN3 MM cells exhibited increased Rankl expression at the mRNA and protein levels (Figure 4A). Further, CD138+ cells from 3 of 4 MM patients also increased Rankl transcripts in MLO-A5 cells. Like apoptosis, Rankl expression in osteocytes was increased by either direct or indirect contact with MM cells, suggesting that soluble factors secreted by MM cells were responsible. Similar results were obtained with other MM and osteocyte-like cell lines (Suppl. Figure 2A). Among several cytokines secreted by MM cells known to upregulate Rankl (32), only TNFα increased Rankl mRNA in MLO-A5 cells after 4h (Suppl. Figure 2B) or 24h. Moreover, anti-TNFα significantly blunted the increased Rankl mRNA levels in MLO-A5 cells treated with CM from JJN3 cells (Figure 4B). Inhibition of osteocyte apoptosis by DEVD similarly reduced Rankl levels to those observed with anti-TNFα.
Figure 4. Osteocyte apoptosis induced by MM increases the osteoclastogenic potential of osteocytes.
(A, B) Rankl gene expression in MLO-A5 cells co-cultured in direct or indirect contact with JJN3 cells (4h) or primary CD138+ cells from MM patients (24h), with or without anti-TNFα or DEVD (24h). (C) Osteoclast precursor migration induced by conditioned media (CM) collected from JJN3 cells cultured alone, MLO-A5 cultured alone or MLO-A5 co-cultured with JJN3 cells in direct contact for 48h, with or without DEVD or a combination of GSIXX and anti-TNFα (aTNFα). Representative experiments out of 2 (n=3–4) are shown. *p<0.05 vs MLO-A5 cells cultured alone (veh), vs osteoclast precursors cultured in the presence of CM from MLO-A5 cells cultured alone (C).
We next determined the effect of osteocyte apoptosis on osteoclast precursor recruitment. CM from MLO-A5 cells cultured alone significantly increased osteoclast precursor migration compared to control media, whereas CM from JJN3 cells did not (Figure 4C). CM from JJN3 and MLO-A5 cells co-cultured in direct contact enhanced osteoclast precursor chemotaxis by 50% compared to CM from MLO-A5 cultured alone. This effect was inhibited by blocking osteocyte apoptosis with DEVD or by the combination of GSIXX and anti-TNFα. Taken together, these results suggest that MM-induced osteocyte apoptosis, even when modest, is sufficient to increase osteocytic Rankl expression and potentiate osteocyte-mediated recruitment of osteoclast precursors.
MM cells upregulate Sost expression in osteocytes, decrease Wnt signaling and inhibit osteoblast differentiation
Consistent with the increased Sclerostin expression in osteocytes in the in vivo MM mouse model, direct co-culture with JJN3 MM cells up-regulated Sost mRNA expression in MLO-A5 cells by 3-fold as early as 4h (Figure 5A), which remained elevated up to 24h (Suppl. Figure 2C). Simultaneously with Sost upregulation, the expression of osteoprotegerin (Opg), a Wnt target gene, was decreased by 50%, both at mRNA (Figure 5A) and protein level (Suppl. Figure 2D). Similarly, the mRNA levels of the other Wnt target genes Axin2 and Smad family member 6 (Smad6) (25, 33, 34) were also decreased. In contrast, Sost/Wnt target gene expression was not altered when MM and MLO-A5 cells were not in direct cell-to-cell contact. Sost upregulation induced by MM cells was also recapitulated in a three dimensional ex vivo bone organ model containing authentic osteocytes (Figure 5B). These findings suggest that interactions between osteocytes and MM cells upregulate the expression of Sost in osteocytes, which in turn decreases Wnt signaling.
Figure 5. MM increases Sost expression in osteocytes, which decreases Wnt signaling and osteoblasts differentiation.
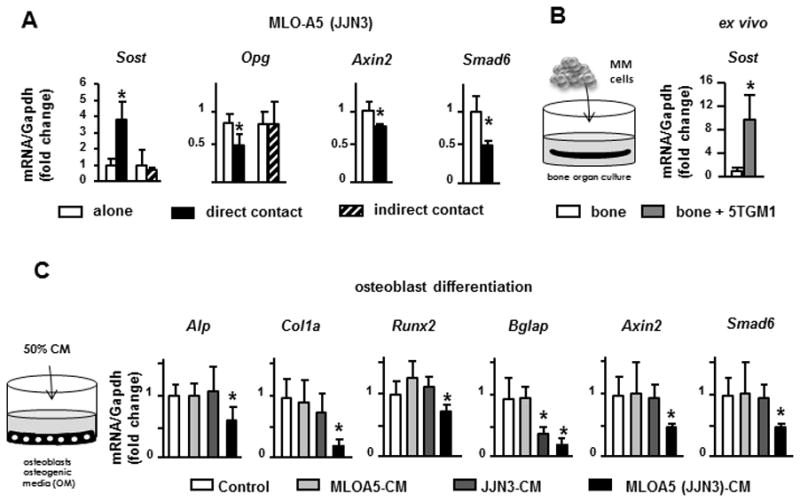
(A) Sost, Opg, and Wnt target genes mRNA levels in MLO-A5 cells co-cultured in direct or indirect contact with JJN3 cells (4h). (B) Sost expression in authentic osteocytes co-cultured with 5TGM1 MM cells in ex vivo bone organ cultures (48h). (C) Osteoblast marker expression (Alp, Col1a, Runx2 and Bglap) and Wnt target genes (Axin2 and Smad6) in osteoblasts cultured in osteogenic media with or without 50% CM from JJN3 cells cultured alone, MLO-A5 cultured alone or MLO-A5 co-cultured with JJN3 cells in direct contact for 48h. Representative experiments out of 2 (n=3–6) are shown. *p<0.05 vs MLO-A5 cells cultured alone (A), vs authentic osteocytes (bone) (B), vs osteoblast cultured with osteogenic media (OM) and without CM (C).
We next examined the consequences of MM-induced upregulation of osteocytic Sost/Sclerostin on osteoblast differentiation. Wnt/β catenin signaling is critical for osteoblast differentiation and this pathway is tightly regulated by pre-receptor antagonists, including the potent osteoblast inhibitor Sclerostin secreted by osteocytes (35). CM from MLO-A5 cells co-cultured with JJN3 MM cells in direct contact markedly decreased the expression of the osteoblast markers alkaline phosphatase (Alp), collagen 1a (Col1a), runt related transcription factor 2 (Runx2) and bone gamma carboxyglutamate protein (Bglap) in OB-6 osteoblastic cells cultured under osteogenic conditions (Figure 5C). Expression of the Wnt target genes Axin2 and Smad6 was also significantly decreased by CM from MLO-A5-JJN3 co-cultures. The expression of these genes was unchanged by treatment with CM from MLO-A5 or JJN3 cells cultured separately.
Taken together, these results demonstrate that direct interactions between osteocytes and MM cells increase expression of Sost/Sclerostin in osteocytes, decrease Wnt signaling/β catenin and inhibit osteoblast differentiation.
Osteocytes activate Notch signaling to increase MM cell proliferation, and alter the Notch receptor repertoire in MM cells
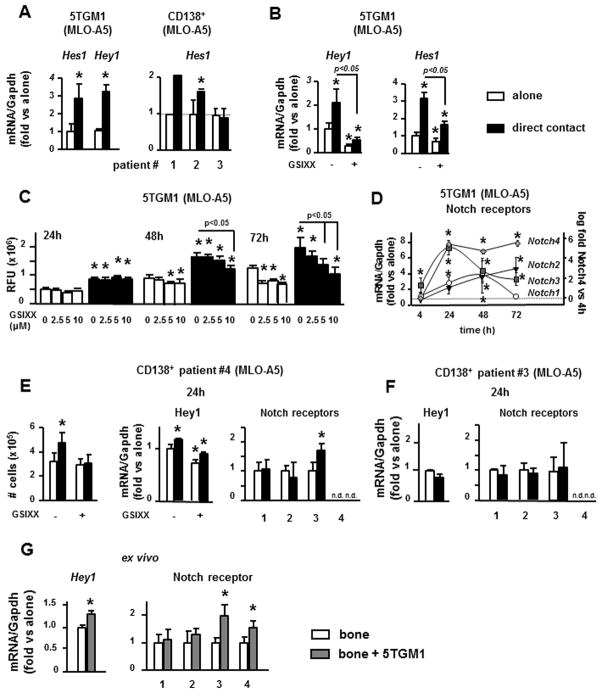
Because Notch signaling can be bidirectional, we next examined if osteocytes modulate Notch signaling in MM cells. Co-culture of 5TGM1 cells in direct contact with MLO-A5 cells for 4h increased Hes1 and Hey1 expression by 2–4 fold in MM cells compared to MM cells cultured alone (Figure 6A). Similar upregulation in Hes1 expression was found in CD138+ cells from 3 of 4 MM patients examined (Figure 6A and E) and in 5TGM1 cells co-cultured in direct contact with MLO-Y4 cells (Suppl. Figure 3A). GSIXX blocked the osteocyte-induced upregulation of Hes1 and Hey1 in 5TGM1 cells (Figure 6B). These results indicate that osteocytes activate Notch signaling in MM cells.
Figure 6. Osteocytes activate Notch signaling, regulate Notch receptor expression, and increase proliferation in MM cells.
(A, B) Hes1 and Hey1 gene expression in 5TGM1 MM cells or CD138+ cells from 3 different MM patients co-cultured in direct contact with MLO-A5 cells, with or without GSIXX. Data from one well per condition are reported for patient #1; 3 wells per condition are reported for the other patients. (C) Cell growth of 5TGM1 cells measured as RFU (relative fluorescence units), cultured alone or co-cultured in direct contact with MLO-A5 cells, with or without GSIXX. (D) Time course of Notch receptors expression in 5TGM1 cells co-cultured with MLO-A5 cells for 4, 24, 48 and 72h. For Notch1, 2 and 3 expression, fold changes were calculated vs 5TGM1 cells cultured alone. For Notch4 expression, fold change was calculated vs Notch4 expression in 5TGM1 cells co-cultured with MLO-A5 for 4h. Cell proliferation (number of viable cells), Hey1 and Notch1, 2, 3 and 4 gene expression in CD138+ cells from a MM patient in which MLO-A5 cells induce (E) or not (F) Notch activation. (G) Hey1 and Notch1, 2, 3 and 4 gene expression in 5TGM1 cells co-cultured with authentic osteocytes in ex vivo bone organ cultures. A representative experiment of 2 (n=4) is shown. *p<0.05 vs MM cells cultured alone (veh), vs MM cultured with MLO-A5 for 4h (D, Notch4).
Co-culture of 5TGM1 cells in direct contact with MLO-A5 cells markedly increased the proliferation of 5TGM1 cells in a time-dependent manner (Figure 6C), whereas no changes were observed when cells were co-cultured without direct contact (Suppl. Figure 3B). Further, inhibition of Notch signaling with GSIXX prevented in a dose-dependent manner the increased proliferation of 5TGM1 cells and CD138+ cell from a MM patient induced by MLO-A5 cells (Figure 6C and E). Higher doses of GSIXX also inhibited proliferation of 5TGM1 cells cultured alone at 48h, and all doses were inhibitory at 72h of culture (Figure 6C).
Osteocyte-like MLO-A5 cells did not activate Notch signaling in all MM cells tested. Thus, MLO-A5 cells did not increase Notch target gene expression or stimulate MM cell proliferation in 1 of 4 CD138+ cell preparations from MM patients (Figure 6F), or in MM1.s or JJN3 MM (Suppl. Figure 3C). We next examined whether differences in proliferative responses of MM cells to osteocytes were due to differences in their Notch receptor repertoire. 5TGM1 cells, which readily respond to osteocytic cell interactions by activating Notch, express higher Notch3 levels (20-fold), and lower Notch1 and 2 compared to JJN3 cells in which osteocytic interactions do not activate Notch (Suppl. Figure 3D). None of the MM cell lines expressed detectable levels of Notch4 when cultured alone. These results show that different MM cells express different levels of Notch receptors, and suggest that osteocytes may activate Notch signaling in MM cells through Notch3, leading to increased MM cell proliferation. Moreover, osteocytic interactions rapidly upregulated Notch3 expression in 5TGM1 cells and induced the expression of Notch4, which is undetectable in MM cells cultured alone (Figure 6D). Further, culture of 5TGM1 MM cells with authentic osteocytes in ex vivo bone organ cultures activated Notch signaling and increased the expression of Notch3 and 4 without altering Notch1 or 2 mRNA levels (Figure 6G). Similarly, CD138+ cells from a MM patient that exhibit Notch activation induced by osteocytes also displayed upregulation of Notch3 (Figure 6E), whereas no changes were found in other Notch receptors. In contrast, no changes in the expression of Notch3 were observed in CD138+ cells from a MM patient that did not show activation of Notch signaling after contact with MLO-A5 cells (Figure 6F). MLO-A5 cells also upregulated Notch1 and 2 in 5TGM1 cells at later time points (Figure 6D). These results demonstrate that osteocytes change the Notch receptor repertoire expressed by MM cells. Thus, the capacity of osteocytes to alter the Notch receptor repertoire on MM cells may regulate osteocyte enhancement of MM cell growth.
Discussion
Osteocytes play a central role in bone homeostasis by regulating osteoclast and osteoblast activity, and premature death of osteocytes leads to targeted bone resorption during skeletal disuse and estrogen loss (6). We report that osteocytes also contribute to a microenvironment favorable for the progression of MM and its associated bone disease. First, we show that osteocytes physically interact with MM tumors in vivo which increases Sclerostin and RANKL production by osteocytes, and reduces osteocyte viability. Second, the reduced viability of osteocytes is due to apoptosis triggered by MM cell-mediated activation of Notch signaling and sustained by MM-derived TNFα. Third, elevated osteocyte apoptosis increases osteocytic Rankl expression and enhances the ability of osteocytes to attract osteoclast precursors. Fourth, the increase in Sost/Sclerostin decreases Wnt signaling and inhibits osteoblast differentiation. Fifth, osteocytes induce reciprocal activation of Notch signaling in MM cells, which in turn enhances MM cell proliferation, and alters the Notch receptor repertoire on MM cells. Importantly, these results were validated in in vivo and in vitro, as well as in a novel ex vivo model, using several MM cell lines and CD138+ cells from MM patients. Taken together, these findings demonstrate that interactions between MM cells and osteocytes within the bone/BM microenvironment generate a permissive niche for MM cell growth and bone destruction (Figure 7). Thus, targeting osteocytes and their derived factors might represent a novel approach to treat MM bone disease.
Figure 7. Interactions between MM cells and osteocytes generate a microenvironment conducive to increased tumor growth and bone destruction.
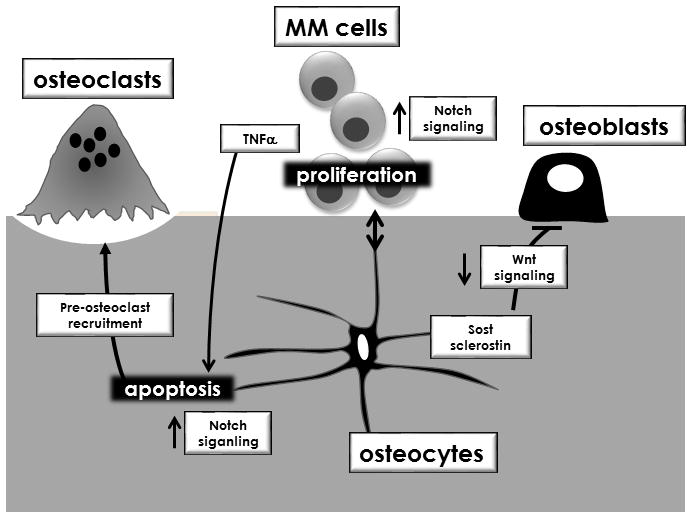
Cell-to-cell contact activates bidirectional Notch signaling in osteocytes and MM cells and triggers osteocyte apoptosis that is maintained by MM-secreted TNFα. Apoptosis enhances the osteoclastogenic potential of osteocytes by increasing osteoclast precursor recruitment and osteocytic RANKL expression. Notch signaling in MM cells increases proliferation, and upregulates Notch1, 2 and 3, in patient MM cells. MM cells increase Sost/Sclerostin expression in osteocytes, which decreases Wnt signaling and inhibits osteoblast differentiation.
Consistent with our findings showing that osteocytes induce MM cell growth by activating Notch signaling, several studies have shown that dysregulation of Notch signaling increases MM growth (36–38), and paracrine Notch activation mediated by BM stromal cells increases MM cell proliferation, through NOTCH1 and 2 signaling (30, 39, 40). However, Notch3 and 4 appear to mediate the osteocytic effects, suggesting that regulation of Notch activation in MM is mediated by complex paracrine interactions with different cell types within the bone/BM microenvironment. Further, the changes in the Notch receptor repertoire on MM cells induced by osteocytic interactions could impact both the homotypic and heterotypic cell-to-cell interactions of MM cells. Although the specific roles of Notch3 and 4 in MM are not fully understood, selective Notch receptor targeting may allow control of MM cell growth and its associated bone disease. Future studies to investigate this possibility and to identify relevant Notch ligands on osteocytes are warranted.
The increased prevalence of osteocyte apoptosis observed in our in vivo model is consistent with a previous report in bone biopsies from MM patients (17), although the mechanism of this phenomenon was not addressed. We show here that osteocyte apoptosis induced by MM cells is triggered by activation of Notch signaling followed by induction of the caspase cascade, and maintained by MM-cell derived TNFα. However, Giuliani et al were unable to block osteocyte apoptosis by neutralizing TNFα (17). This discrepancy, together with their inability to detect changes in osteocytic RANKL, could be explained by the use of cells lines at different stages of differentiation of the osteoblastic lineage. We used the late osteoblast/early osteocyte-like MLO-A5 cell line that expresses the recognized osteocytic genes Sost/Sclerostin and Rankl, whereas the HOB-01 pre-osteocytic cells used in the Giuliani study do not. Moreover, we validated our in vivo observations and mechanistic studies using MLO-Y4 cells, an additional osteocytic cell line widely used as an osteocyte model, and confirmed our findings in authentic osteocytes using ex vivo bone organ cultures. Our results demonstrate that murine and human MM cell lines and CD138+ from MM patients act as Notch signalers in osteocytes that concurrently with MM-secreted TNFα induce osteocyte apoptosis.
Although our current findings demonstrating different outcomes of Notch activation in MM cells (proliferation) versus in osteocytes (apoptosis) may appear counterintuitive, they are consistent with earlier studies showing the cell type- and context-dependent nature of Notch signaling. Thus, whereas activation of Notch signaling protects several cancer cell types from apoptosis (36, 41), it induces apoptosis of neural progenitor cells (42), CD34+ hematopoietic stem/progenitor cells (43), and osteocytes (this report). Similarly, activation of canonical Wnt signaling inhibits apoptosis in different cells of the osteoblastic lineage (31, 44), whereas it increases apoptosis in hematopoietic stem/progenitor cells (45) or in melanoma cells (46). These findings show that the same signaling pathway can elicit different biological responses depending on the ligands/signals present in a particular microenvironment, the receptor repertoire exhibited by the target cell, and the intracellular machinery that integrates extracellular cues and transduces them into biological outcomes.
Osteocyte-derived interleukin 11 has been shown to contribute to osteoclast differentiation in MM (17). We show that apoptotic osteocytes attract osteoclast precursors more potently than MM cells in our in vitro system. Further, although relatively modest, the increased osteocyte apoptosis was sufficient to upregulate osteocyte Rankl. These findings suggest that similar to other pathological conditions, MM-induced osteocyte apoptosis plays a central role in osteoclast recruitment and may initiate and/or sustain bone resorption within the focal lesions observed in MM patients. Further studies are warranted to determine whether osteocyte apoptosis leads to osteoclast differentiation in MM and to identify the molecular mediators.
Sclerostin levels are elevated in the sera of MM patients and correlate with reduced osteoblast function and poor patient survival (47). However, the source of Sclerostin remains unclear. CD138+ cells from MM patients were reported to express Sclerostin (48); but we could not detect Sost mRNA in any patient CD138+ cells studied. In contrast, our in vitro and in vivo models demonstrate that Sost/Sclerostin expression is elevated in osteocytes. Further, Sost/Sclerostin upregulation in osteocytes was associated with decreased Wnt signaling in osteoblasts and reduced osteoblast differentiation. In addition, the expression of Wnt target genes, including OPG, was reduced; thereby further increasing the Rankl/Opg ratio. These results suggest that osteocytes contribute to the generation of a MM microenvironment with high Sclerostin concentrations, affecting both bone formation and bone resorption.
Physical interactions between MM cells and different cells in the bone microenvironment, including stromal cells, osteoclasts and immune cells, were shown to be important for tumor proliferation and survival (49). Our results suggest that osteocytes also contribute to MM cell growth and MM induced bone disease by stimulating osteoclast recruitment and inhibiting bone formation via direct and indirect contact with MM cells. Since osteocytes comprise more than 95% of the bone cells, they should be major contributors to generating a microenvironment favorable for MM progression. However, the relative contribution of osteocytes versus other cell types in the bone/BM microenvironment to MM disease remains to be determined.
Our study identified pathways activated by interactions between MM cells and osteocytes that provide potential new ways to inhibit MM tumor growth and bone disease. Prevention of osteocyte apoptosis might suppress initiation of bone resorption by inhibiting osteoclast recruitment as well as improving the bone fragility syndrome associated with MM and with some of the current anti-tumor drugs (eg. glucocorticoids that also increase osteocyte apoptosis). Pharmacological inhibition of Notch signaling can inhibit MM cell growth (50, 51) and could provide additional benefits by inhibiting osteocyte apoptosis, either alone or in combination with TNFα signaling blockade. Our findings demonstrate that osteocytes regulate the expression and repertoire of Notch receptors on MM cells, supporting development of therapeutic approaches that block specific Notch receptors. This approach might be an effective alternative to overcome the side-effects associated with generalized Notch inhibition (36).
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Institutes of Health Grants (Indiana-CTSI P30, 1R21CA179017-02 and R01AR059679 to GDR; R01AR059357, R01 DK076007, and S10-RR023710 to TB), the Veteran’s Administration (Merit Review to TB and to GDR), and the IBMS Gideon and Sevgi Rodan Fellowship (to JDC) and funds from the CTSI at Indiana University.
We thank Kevin McAndrews, Hannah M. Davis, Keith Condon, Amy Sato, Dan Zhou, Doug Tompkins and Ning Ma for assistance in tissue collection and Caroline Miller (Electron Microscopy Center, Indiana University School of Medicine) for assistance with SEM.
Footnotes
Conflict of interest: Authors have no conflict of interest.
Reference List
- 1.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–41. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg AJ, Rajkumar SV, Therneau TM, Singh PP, Dispenzieri A, Kumar SK. Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia. 2014;28:398–403. doi: 10.1038/leu.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roodman GD. Targeting the bone microenvironment in multiple myeloma. J Bone Miner Metab. 2010;28:244–50. doi: 10.1007/s00774-009-0154-7. [DOI] [PubMed] [Google Scholar]
- 4.Andersen TL, Soe K, Sondergaard TE, Plesner T, Delaisse JM. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br J Haematol. 2010;148:551–61. doi: 10.1111/j.1365-2141.2009.07980.x. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–83. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 6.Bellido T. Osteocyte-Driven Bone Remodeling. Calcif Tissue Int. 2013;94:25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases. Endocr Rev. 2012;33:747–83. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 8.Van Bezooijen RL, Roelen BA, Visser A, Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–14. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonewald LF. The Amazing Osteocyte. J Bone Miner Res. 2011;26:229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–63. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 13.Bellido T. Osteocyte apoptosis induces bone resorption and impairs the skeletal response to weightlessness. BoneKEy-osteovision. 2007;4:252–6. [Google Scholar]
- 14.Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res. 2001;16:1622–33. doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- 15.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–23. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza S, del PD, Jin S, Sun Q, Huston AJ, Kostov FE, et al. Gfi1 expressed in bone marrow stromal cells is a novel osteoblast suppressor in patients with multiple myeloma bone disease. Blood. 2011;118:6871–80. doi: 10.1182/blood-2011-04-346775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla PB, et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–401. doi: 10.1038/leu.2011.381. [DOI] [PubMed] [Google Scholar]
- 18.Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T, et al. Characterization of the MM. 1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol. 2003;31:271–82. doi: 10.1016/s0301-472x(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 19.Lecka-Czernik B, Gubrij I, Moerman EA, Kajkenova O, Lipschitz DA, Manolagas SC, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPAR-gamma 2. J Cell Biochem. 1999;74:357–71. [PubMed] [Google Scholar]
- 20.Pacheco-Costa R, Hassan I, Reginato RD, Davis HM, Bruzzaniti A, Allen MR, et al. High Bone Mass in Mice Lacking Cx37 Due to Defective Osteoclast Differentiation. J Biol Chem. 2014;289:8508–20. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suvannasankha A, Tompkins DR, Edwards DF, Petyaykina KV, Crean CD, Fournier PG, et al. FGF23 is elevated in multiple myeloma and increases heparanase expression by tumor cells. Oncotarget. 2015 doi: 10.18632/oncotarget.3794. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellido T, Plotkin LI. Detection of apoptosis of bone cells in vitro. In: Westendorf JJ, editor. Osteoporosis. Humana Press; 2007. pp. 51–75. [DOI] [PubMed] [Google Scholar]
- 23.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–30. [PubMed] [Google Scholar]
- 24.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with βarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–30. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, et al. Osteocytes mediate the anabolic actions of canonica Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112:E478–E486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Dujaili SA, Lau E, Al-Dujaili H, Tsang K, Guenther A, You L. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J Cell Biochem. 2011;112:2412–23. doi: 10.1002/jcb.23164. [DOI] [PubMed] [Google Scholar]
- 27.Kubek DJ, Gattone VH, Allen MR. Methodological assessment of acid-etching for visualizing the osteocyte lacunar-canalicular networks using scanning electron microscopy. Microsc Res Tech. 2010;73:182–6. doi: 10.1002/jemt.20772. [DOI] [PubMed] [Google Scholar]
- 28.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 29.Houde C, Li Y, Song L, Barton K, Zhang Q, Godwin J, et al. Overexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell lines. Blood. 2004;104:3697–704. doi: 10.1182/blood-2003-12-4114. [DOI] [PubMed] [Google Scholar]
- 30.Jundt F, Probsting KS, Anagnostopoulos I, Muehlinghaus G, Chatterjee M, Mathas S, et al. Jagged1-induced Notch signaling drives proliferation of multiple myeloma cells. Blood. 2004;103:3511–5. doi: 10.1182/blood-2003-07-2254. [DOI] [PubMed] [Google Scholar]
- 31.Jilka RL, Bellido T, Almeida M, Plotkin LI, O’Brien CA, Weinstein RS, et al. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. 3. San Diego, San Francisco, New York, London, Sydney, Tokyo: Academic Press; 2008. pp. 237–61. [Google Scholar]
- 32.Aggarwal R, Ghobrial IM, Roodman GD. Chemokines in multiple myeloma. Exp Hematol. 2006;34:1289–95. doi: 10.1016/j.exphem.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yochum GS, McWeeney S, Rajaraman V, Cleland R, Peters S, Goodman RH. Serial analysis of chromatin occupancy identifies beta-catenin target genes in colorectal carcinoma cells. Proc Natl Acad Sci U S A. 2007;104:3324–9. doi: 10.1073/pnas.0611576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien CA, Plotkin LI, Galli C, Goellner J, Gortazar AR, Allen MR, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 36.Colombo M, Mirandola L, Platonova N, Apicella L, Basile A, Figueroa AJ, et al. Notch-directed microenvironment reprogramming in myeloma: a single path to multiple outcomes. Leukemia. 2013;27:1009–18. doi: 10.1038/leu.2013.6. [DOI] [PubMed] [Google Scholar]
- 37.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–9. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 38.Guo D, Li C, Teng Q, Sun Z, Li Y, Zhang C. Notch1 overexpression promotes cell growth and tumor angiogenesis in myeloma. Neoplasma. 2013;60:33–40. doi: 10.4149/neo_2013_005. [DOI] [PubMed] [Google Scholar]
- 39.Chiron D, Maiga S, Descamps G, Moreau P, Le GS, Marionneau S, et al. Critical role of the NOTCH ligand JAG2 in self-renewal of myeloma cells. Blood Cells Mol Dis. 2012;48:247–53. doi: 10.1016/j.bcmd.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Hu J, Xu S, De BE, Menu E, Van CB, et al. Dll1/Notch activation accelerates multiple myeloma disease development by promoting CD138+ MM-cell proliferation. Leukemia. 2012;26:1402–5. doi: 10.1038/leu.2011.332. [DOI] [PubMed] [Google Scholar]
- 41.Mungamuri SK, Yang X, Thor AD, Somasundaram K. Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Cancer Res. 2006;66:4715–24. doi: 10.1158/0008-5472.CAN-05-3830. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Chadwick N, Nostro MC, Baron M, Mottram R, Brady G, Buckle AM. Notch signaling induces apoptosis in primary human CD34+ hematopoietic progenitor cells. Stem Cells. 2007;25:203–10. doi: 10.1634/stemcells.2005-0303. [DOI] [PubMed] [Google Scholar]
- 44.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–51. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 45.Ming M, Wang S, Wu W, Senyuk V, Le Beau MM, Nucifora G, et al. Activation of Wnt/beta-catenin protein signaling induces mitochondria-mediated apoptosis in hematopoietic progenitor cells. J Biol Chem. 2012;287:22683–90. doi: 10.1074/jbc.M112.342089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerman ZF, Kulikauskas RM, Bomsztyk K, Moon RT, Chien AJ. Activation of Wnt/beta-catenin signaling increases apoptosis in melanoma cells treated with trail. PLoS ONE. 2013;8:e69593. doi: 10.1371/journal.pone.0069593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131:1466–71. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 48.Brunetti G, Oranger A, Mori G, Specchia G, Rinaldi E, Curci P, et al. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann N Y Acad Sci. 2011;1237:19–23. doi: 10.1111/j.1749-6632.2011.06196.x. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125:3049–58. doi: 10.1182/blood-2014-11-568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Chen F, Clifton N, Sullivan DM, Dalton WS, Gabrilovich DI, et al. Combined inhibition of Notch signaling and Bcl-2/Bcl-xL results in synergistic antimyeloma effect. Mol Cancer Ther. 2010;9:3200–9. doi: 10.1158/1535-7163.MCT-10-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarzer R, Nickel N, Godau J, Willie BM, Duda GN, Schwarzer R, et al. Notch pathway inhibition controls myeloma bone disease in the murine MOPC315. BM model Blood Cancer J. 2014;4:e217. doi: 10.1038/bcj.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.