Abstract
Background and Aims
Fat radiodensity, as measured by fat attenuation on computed tomography (CT), has emerged as a potential biomarker of “fat quality.” We sought to characterize the relationship between fat radiodensity and quantity in subcutaneous, visceral, and intermuscular fat depots, and its role in inflammation, insulin resistance, and metabolic syndrome (MetS).
Methods and Results
We studied 1,511 individuals from the Multi-Ethnic Study of Atherosclerosis who underwent CT for measurement of regional fat distribution and radiodensity, along with biomarker assessments and adjudication of incident metabolic syndrome (MetS). Linear, logistic and Cox regression analyses were used to measure association between fat radiodensity and (1) fat quantity, (2) biomarkers of cardiometabolic dysfunction, and (3) both prevalent and incident MetS. In each fat depot, radiodensity was strongly and inversely associated with quantity (e.g., visceral fat radiodensity vs. quantity: ρ=−0.82, P<0.01). After adjustment for age, sex and race, lower visceral fat radiodensity was associated with greater C-reactive protein, leptin and insulin, but lower adiponectin (P<0.01 for all). After full adjustment for cardiovascular disease risk factors, visceral (but not subcutaneous or intermuscular) fat radiodensity was associated with prevalent MetS (OR=0.96, 95% CI=0.93–0.99, P=0.01). Moreover, lower visceral fat radiodensity was associated with incident MetS after the same adjustment (HR=0.95, 95% CI 0.93–0.98, P<0.01). However, this association became non-significant after further adjustment for visceral fat quantity.
Conclusion
Fat radiodensity is strongly correlated with fat quantity and relevant inflammatory biomarkers. Fat radiodensity (especially for visceral fat) may be a complementary, easily assessed marker of cardiometabolic risk.
INTRODUCTION
Human obesity harbors distinct metabolic phenotypes within its traditional definition of a body mass index (BMI) > 30 kg/m2.1–3 Against a backdrop of “metabolically healthy” and “metabolically unhealthy” obese individuals, visceral adiposity is now a well-established cardiometabolic risk factor, which may differentiate human obesity phenotypes3. Although methods to measure adipose tissue (e.g., magnetic resonance imaging or positron emission tomography) have been proposed to examine adipose tissue lipid composition, metabolic activity, and inflammation, their implementation remains complex and relatively inaccessible to a population studies.
Most investigation in the field of ectopic fat has centered on the importance of visceral adipose tissue quantity1, 3. More recently, investigations in the Framingham Heart Study have demonstrated that the radiodensity of subcutaneous and visceral fat by computed tomography (CT; termed “fat quality”) is associated with cardiovascular, metabolic, and clinical risk, independent of fat volume4–6. However, whether fat radiodensity signifies something mechanistically important in adipose tissue remains an open question. As such, understanding the relationship between adipose tissue quantity and radiodensity, and their relative contributions to metabolic syndrome risk, is important to define a role for these imaging parameters in the pathophysiology of obesity.
To address this question, we investigated (1) the interrelationships between fat radiodensity and quantity in each compartment (visceral, subcutaneous, and intermuscular) and (2) the relationship between radiodensity in these compartments with established biomarkers of obesity-related cardiometabolic disease, as well as both prevalent and incident metabolic syndrome, in a wellcharacterized group of community-based, multi-racial individuals enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
Participant population
The overall design of the MESA study has been described previously7. At baseline, the MESA consisted of 6,814 men and women of White, African American, Chinese American, and Hispanic ethnicity enrolled from six sites in the United States who were free of clinical cardiovascular disease (history of myocardial infarction, angina pectoris, prior revascularization, heart failure, atrial fibrillation, stroke, or peripheral arterial disease) at enrollment. The study design, including demographics, medical history collection, medical therapy, and physical examination has been previously described8. The Institutional Review Board at each participating institution approved protocols. All participants provided written informed consent.
Fat Imaging and Measurement
The first MESA examination began in 2000, with subsequent examinations conducted approximately every 2 years. At visits 2 and 3, a random subset of 1,970 MESA participants underwent abdominal computed tomography (CT) scanning, in which regional fat distribution and radiodensity were evaluated (visceral to subcutaneous fat assessment: exam 2, 756 visceral/577 subcutaneous; exam 3, 1,172 visceral/1,114 subcutaneous). In the current study, we evaluated individuals with full data for visceral and subcutaneous fat depots (N=1,687), excluding individuals with missing data for BMI (N=1), history of cirrhosis, cancer, or self-reported renal disease at the time of the baseline CT examination (N=175), leaving 1,511 participants for this analysis. Among this group, 316 subjects (3 for visceral fat, 63 for intermuscular fat and 312 for subcutaneous fat) required use of techniques to account for imaging artifacts such as truncation, which have previously been described in detail3.
Electron-beam CT scanners were used at Northwestern University and University of California, Los Angeles (Imatron C-150), with settings collimation 3 mm, slice thickness 6 mm, reconstruction using 25 6-mm slices with 35-cm field of view and normal kernel. Multi-detector CT scanners were utilized at Columbia University, Wake Forest University, and University of Minnesota field centers (Sensation 64, GE Lightspeed; Siemens S4 Volume Zoom; and Siemens Sensation 16). Image interpretation was blinded to clinical information.
We have previously described the quantification of visceral and subcutaneous fat3. We defined radiodensity by examining attenuation (in Hounsfield units) within each tissue compartment (visceral, subcutaneous and intermuscular). Regions of interest for these compartments were segmented using the MIPAV 4.1.2 software (National Institutes of Health, Bethesda, MD). Within these areas of interest, lean tissue was defined as an attenuation between 0 and 100 HU, while fat tissue was defined as any tissue with attenuation between −190 and −30 Hounsfield units (HU). Notably, a higher fat radiodensity corresponded to a less negative value for attenuation in Hounsfield units (HU; e.g., Hounsfield units closer to zero). Conversely, a lower fat radiodensity corresponded to a more negative value for attenuation in HU. All fat quantity parameters were adjusted for height, as in our prior work3.
Biomarker assessments
Fasting blood samples drawn at the time of CT scan were assayed for the following biomarkers: high-sensitivity C-reactive protein, interleukin-6 (IL-6) and tumor necrosis factor (TNF-α; inflammation); fasting glucose, insulin (insulin resistance); adiponectin, leptin, and resistin (adiposity-associated inflammation). Biomarker assessments were performed as previously described9, 10. Metabolic syndrome (MetS) was determined at each MESA clinic visit and defined by the updated National Cholesterol Education Panel Adult Treatment Panel III guidelines (including waist circumference, serum triglyceride level, high density lipoprotein cholesterol, systolic and diastolic blood pressure, and fasting glucose)11.
Statistical analysis
Covariates are expressed as median and interquartile range, and compared using Wilcoxon rank-sum tests. Visceral, subcutaneous, and intermuscular fat radiodensity was dichotomized by median value, with clinical, biochemical, and imaging variables being compared between these medians. We measured cross-sectional associations between fat radiodensity and selected adipokines and inflammatory biomarkers (log-transformed) via unadjusted and adjusted (for age, race, sex, and regional fat quantity) linear regression models. Then, we used logistic regression to examine the association between prevalent MetS and fat radiodensity, and discrete time Cox proportional hazards models to quantify associations with incident MetS (among those participants without MetS at baseline). Multivariable regression models for prevalent MetS were adjusted for age, sex, race, and time-varying weight, cigarette smoking, total intentional exercise, waist circumference, triglycerides, high-density lipoprotein, systolic and diastolic blood pressure, and fasting glucose and fat quantity?. Multivariable regression models for incident MetS were adjusted for similar covariates (but included those that were time-varying). Lastly, for both incident and prevalent MetS, we adjusted for fat quantity to test the whether association between fat radiodensity and metabolic syndrome was independent of this variable. For the MetS outcome, effect modification was assessed via multiplicative interaction terms with age (as a continuous variable), sex, and race. SAS (version 9.4, SAS Institute, Cary, NC) and R (version 3.2, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/) were used for all analyses. A two-sided p-value less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study population, stratified by radiodensity
The baseline characteristics of our study population, stratified by median radiodensity for each depot are shown in Table 1. In general, and for each fat depot, individuals with higher fat radiodensity had a consistently lower body mass index, waist circumference, insulin, CRP and prevalence of metabolic syndrome, while having higher adiponectin levels. In addition, MESA participants with higher fat radiodensity had lower fat quantity in each adipose tissue depot (e.g., higher visceral fat density was associated with lower visceral fat quantity). This finding led us to investigate the association between adipose tissue radiodensity and quantity in each compartment and with clinical anthropometric markers of obesity. Visceral fat radiodensity was significantly and inversely correlated with BMI (ρ=−0.55) and waist circumference (ρ=−0.58; both P<0.01). Similarly, subcutaneous fat radiodensity was inversely associated with BMI (ρ=−0.46) and waist circumference (ρ=−0.45; both P<0.01). Finally, intermuscular fat radiodensity was inversely associated with BMI (ρ=−0.44) and waist circumference (ρ=−0.51; P<0.01 for both).
Table 1.
Baseline clinical, demographic, and cardiometabolic characteristics of the study population, stratified by median tissue radiodensity. P values represent results of Chi-square (categorical) or Wilcoxon rank-sum (continuous) testing within each tissue compartment (e.g., P value in column 4 represents comparison between low and high visceral fat radiodensity). Median visceral fat attenuation was −90 HU; median subcutaneous fat attenuation was −101 HU; median intermuscular fat attenuation was −62 HU. Maximum number of missing data for biomarkers was N=38 for interleukin-6.
| Characteristic | Lower visceral fat radiodensity (N=755) |
Higher visceral fat radiodensity N=756) |
P value | Lower SQ fat radiodensity (N=755) |
Higher SQ fat radiodensity (N=756) |
P value | Lower MF radiodensity (N=755) |
Higher MF radiodensity (N=756) |
P value |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 63 (57–71) | 64 (56–72) | 0.36 | 63 (56–71) | 65 (57–73) | 0.004 | 64 (57–72) | 63 (56–71) | 0.03 |
| Male sex (%) | 411 (54%) | 344 (44%) | <0.0001 | 270 (36%) | 475 (63%) | <0.0001 | 379 (50%) | 366 (48%) | 0.49 |
| Body mass index (kg/m2) | 28.7 (26.2–31.7) | 24.9 (22.7–27.3) | <0.0001 | 28.2 (25.7–31.6) | 25.3 (23.0–27.8) | <0.0001 | 28.2 (25.6–31.5) | 25.4 (23.2–27.8) | <0.0001 |
| Race (%) | <0.0001 | <0.0001 | <0.0001 | ||||||
| Caucasian | 304 (40%) | 276 (37%) | 306 (40%) | 274 (36%) | 336 (45%) | 244 (32%) | |||
| Chinese | 88 (12%) | 147 (19%) | 89 (12%) | 146 (19%) | 74 (10%) | 161 (21%) | |||
| African | 123 (16%) | 201 (27%) | 143 (19%) | 181 (24%) | 139 (18%) | 185 (25%) | |||
| Hispanic | 240 (32%) | 132 (17%) | 217 (29%) | 155 (21%) | 206 (27%) | 166 (22%) | |||
| Systolic blood pressure (mmHg) |
123 (112–138) | 118 (107–135) | <0.0001 | 121 (110–137) | 120 (108–137) | 0.35 | 121 (110–137) | 120 (108–136) | 0.08 |
| Waist circumference (cm) | 101 (94–108) | 89 (83–96) | <0.0001 | 99 (91–108) | 91 (84–98) | <0.0001 | 100 (92–108) | 91 (84–97) | <0.0001 |
| Fasting glucose (mg/dl) | 94 (87–104) | 88 (83–95) | <0.0001 | 92 (85–99) | 90 (85–99) | 0.07 | 92 (86–101) | 90 (84–98) | 0.001 |
| Triglycerides (mg/dl) | 135 (97–187) | 88 (64–127) | <0.0001 | 124 (86–179) | 97 (67–140) | <0.0001 | 118 (82–169) | 105 (71–149) | <0.0001 |
| High-density lipoprotein (mg/dl) |
45 (38–54) | 55 (46–66) | <0.0001 | 49 (41–59) | 51 (42–63) | 0.003 | 48 (41–59) | 52 (43–62) | <0.0001 |
| Other conditions (%) | |||||||||
| Ever smoker | 384 (51%) | 385 (51%) | 0.98 | 351 (46%) | 418 (55%) | 0.0006 | 412 (55%) | 357 (47%) | 0.004 |
| Diabetes | 120 (16%) | 72 (10%) | 0.0002 | 81 (11%) | 111 (15%) | 0.02 | 92 (12%) | 100 (13%) | 0.54 |
| Hypertension | 376 (50%) | 299 (40%) | <0.0001 | 350 (47%) | 325 (43%) | 0.19 | 359 (48%) | 316 (42%) | 0.02 |
| Metabolic syndrome | 354 (47%) | 137 (18%) | <0.0001 | 321 (43%) | 170 (22%) | <0.0001 | 291 (39%) | 200 (26%) | <0.0001 |
| Tissue quantity (cm2/m) | |||||||||
| Visceral fat | 708.2 (547.6–878.7) | 351.0 (249.5–464.0) | <0.0001 | 468.2 (410.0–789.2) | 435.9 (276.0–629.9) | <0.0001 | 615.0 (437.1–848.5) | 418.8 (277.2–576.9) | <0.0001 |
| Subcutaneous fat | 709.7 (530.4–985.5) | 560.7 (394.2–800.9) | <0.0001 | 816.0 (627.2–1062) | 488.4 (371.6–681.9) | <0.0001 | 731.3 (530.4–1003) | 560.0 (395.8–782.3) | <0.0001 |
| Intermuscular fat | 70.3 (52.1–94.9) | 50.2 (38.8–65.9) | <0.0001 | 68.9 (50.3–93.1) | 51.1 (38.8–67.9) | <0.0001 | 76.0 (60.3–98.8) | 46.2 (36.5–58.6) | <0.0001 |
| Tissue quality (HU) | |||||||||
| Visceral fat | −94 (−97, −92) | −84 (−87, −80) | <0.0001 | −93 (−97, −89) | −86 (−91, −80) | <0.0001 | −93 (−97, −88) | −86 (−91, −81) | <0.0001 |
| Subcutaneous fat | −103 (−106, −100) | −99 (−102, −94) | <0.0001 | −104 (−106, −103) | −97 (−100, −94) | <0.0001 | −103 (−105, −101) | −99 (−102, −94) | <0.0001 |
| Intermuscular fat | −64 (−66, −61) | −60 (−63, −58) | <0.0001 | −64 (−66, −61) | −60 (−63, −58) | <0.0001 | −64 (−66, −63) | −59 (−61, −58) | <0.0001 |
| CRP (mg/dl) | 1.75 (0.88–3.91) | 0.99 (0.55–2.26) | <0.0001 | 1.73 (0.88–3.87) | 1.00 (0.51–2.22) | <0.0001 | 1.56 (0.81–3.56) | 1.13 (0.56–2.49) | <0.0001 |
| Interleukin-6 (pg/ml) | 2.04 (1.39–3.04) | 1.49 (0.99–2.19) | <0.0001 | 1.86 (1.27–2.77) | 1.65 (1.05–2.50) | <0.0001 | 1.93 (1.35–2.90) | 1.56 (1.02–2.35) | <0.0001 |
| Resistin (pg/dl) | 14932.5 (11925.6–18891.0) | 14544.6 (11211.5–18280.0) | 0.03 | 14810.2 (11679.2–18692.9) | 14726.0 (11596.8–18423.6) | 0.45 | 14817.7 (11925.6–18639.3) | 14721.8 (11299.6–18453.1) | 0.22 |
| Leptin (pg/ml) | 16060.7 (7701.1–32636.3) | 8204.9 (3387.2–19951.6) | <0.0001 | 20074.1 (10375.1–36285.7) | 6824.2 (3049.4–14353.8) | <0.0001 | 15567.0 (7173.3–31817.4) | 8683.8 (3672.1–20835.7) | <0.0001 |
| TNFα (pg/ml) | 4.74 (3.61–6.38) | 4.25 (3.17–5.72) | <0.0001 | 4.63 (3.53–6.22) | 4.36 (3.26–6.03) | 0.02 | 4.63 (3.45–6.23) | 4.37 (3.34–6.00) | 0.04 |
| Insulin (pg/ml) | 261.2 (196.0–391.0) | 171.6 (124.7–236.2) | <0.0001 | 248.6 (176.4–373.1) | 189.1 (131.1–258.5) | <0.0001 | 239.1 (167.8–359.9) | 195.3 (137.0–271.0) | <0.0001 |
| Adiponectin (ng/ml) | 14903.4 (10528.1–21759.4) | 21283.9 (14034.3–31789.4) | <0.0001 | 16752.3 (11954.6–24893.5) | 18305.7 (12016.8) | 0.007 | 11954.6 (11954.6–24710.3) | 18754.3 (12091.3–27859.4) | 0.005 |
Abbreviations: BMI, body mass index; HU, Hounsfield unit; CRP, C-reactive protein, TNFα, tumor necrosis factor-α, VF, visceral fat; SQ, subcutaneous fat; MF, intermuscular fat.
There was a significant inverse correlation between fat quantity and radiodensity in each regional tissue compartment, with a lower radiodensity associated with higher fat quantity. More specifically, lower radiodensity of visceral fat was associated with higher visceral fat quantity (ρ=−0.82, P<0.01), with similar results for the subcutaneous (ρ=−0.61, P<0.01) and intermuscular fat depots (ρ=−0.69, P<0.0001; Figure 1A). In addition to correlations within each compartment, we also observed significant interrelationships between visceral and subcutaneous fat density and quantity (displayed graphically in Figure 1B). For example, higher subcutaneous fat radiodensity was associated with lower visceral and intermuscular fat quantities, as well as higher fat radiodensities in those depots.
FIGURE 1.
Associations between height-indexed visceral, subcutaneous, and intermuscular fat area and fat radiodensity. Panel (A) shows scatterplots of relationship between fat quantity (y-axis) and radiodensity (x-axis) across all abdominal tissue compartments. Panel (B) shows cross-correlations between fat radiodensity and quantity across visceral, subcutaneous, and intermuscular fat. All correlations are Spearman coefficients.
Fat radiodensity is associated with inflammatory biomarkers of cardiometabolic risk, independent of clinical risk factors
We then tested whether fat radiodensity in each compartment was associated with circulating biomarkers of cardiometabolic risk, inflammation, and adipocyte biology. Unadjusted and adjusted (age, sex, race, fat quantity) regression coefficients in multivariable regression for selected adipokines are shown in Table 2. After adjustment for age, sex, race/ethnicity, and fat quantity, a higher visceral fat radiodensity was associated with lower CRP, leptin and fasting insulin, but higher adiponectin. Similarly, higher radiodensity in subcutaneous and intermuscular fat depots was associated with lower markers of inflammation and insulin resistance.
Table 2.
Unadjusted and adjusted linear regression coefficients for association of selected circulating adipokines and biomarkers of inflammation and insulin resistance with fat radiodensity. “Adjusted” β indicates adjustment for age, sex, race, and fat quantity in a specific depot (e.g., adjusted column in visceral fat radiodensity represents adjustment for age, sex, race, and visceral fat quantity). Biomarkers were log-transformed for analysis.
| Biomarker | Visceral fat radiodensity (per 10 HU increase) |
Subcutaneous fat radiodensity (per 10 HU increase) |
Intermuscular fat radiodensity (per 10 HU increase) |
|||
|---|---|---|---|---|---|---|
| Unadjusted β | Adjusted β | Unadjusted β | Adjusted β | Unadjusted β | Adjusted β | |
| CRP | −0.36* | −0.22** | −0.41* | −0.17** | −0.53* | −0.14 |
| Interleukin-6 | −0.22* | −0.06 | −0.16* | −0.05 | −0.38* | −0.32* |
| Resistin | −0.03 | −0.02 | −0.02 | 0.002 | −0.04 | 0.03 |
| Leptin | −0.57* | −0.35* | −1.1* | −0.61* | −1.0* | −0.67* |
| TNFα | −0.07** | −0.05 | −0.01 | −0.03 | −0.08 | −0.06 |
| Insulin | −0.36* | −0.14* | −0.29* | −0.19* | −0.40* | −0.21* |
| Adiponectin | 0.31* | 0.28* | 0.16* | 0.27* | 0.16* | 0.34* |
Abbreviations: CRP, C-reactive protein, TNFα, tumor necrosis factor-alpha.
P<0.0001;
P<0.001.
Visceral but not subcutaneous or intermuscular fat radiodensity is associated with increased prevalent metabolic syndrome after adjustment for risk factors
Of the 1,511 MESA participants, 491 (32.5%) had metabolic syndrome (by ATP III criteria) at the CT examination for adiposity assessment. In unadjusted logistic regression analyses, each Hounsfield unit increase in visceral fat radiodensity (higher radiodensity by 1 HU toward zero attenuation) was associated with a 12% reduced odds of MetS (OR=0.88, 95% confidence interval CI=0.86–0.90, P<0.01), which was not significant after adjustment for visceral fat quantity and other clinical covariates (OR=0.99, 95% CI=0.95–1.03, P=0.57). Similarly, each Hounsfield unit increase in subcutaneous fat radiodensity was associated with reduced odds of prevalent MetS (OR=0.90, 95% CI 0.88–0.92, P<0.01), but this did not persist after adjustment (OR = 0.97, 95% CI 0.93–1.01, P=0.13). Finally, each Hounsfield unit increase in intermuscular fat radiodensity was associated with decreased odds of prevalent MetS (OR=0.88, 95% CI 0.86–0.91, P<0.01), which became borderline significant after full adjustment (OR = 0.96, 95% CI 0.90–1.01, P=0.09). There was no evidence of significant interaction between age, race, or gender on the association between radiodensity in any fat depot and prevalent MetS.
Visceral fat radiodensity is associated with incident metabolic syndrome
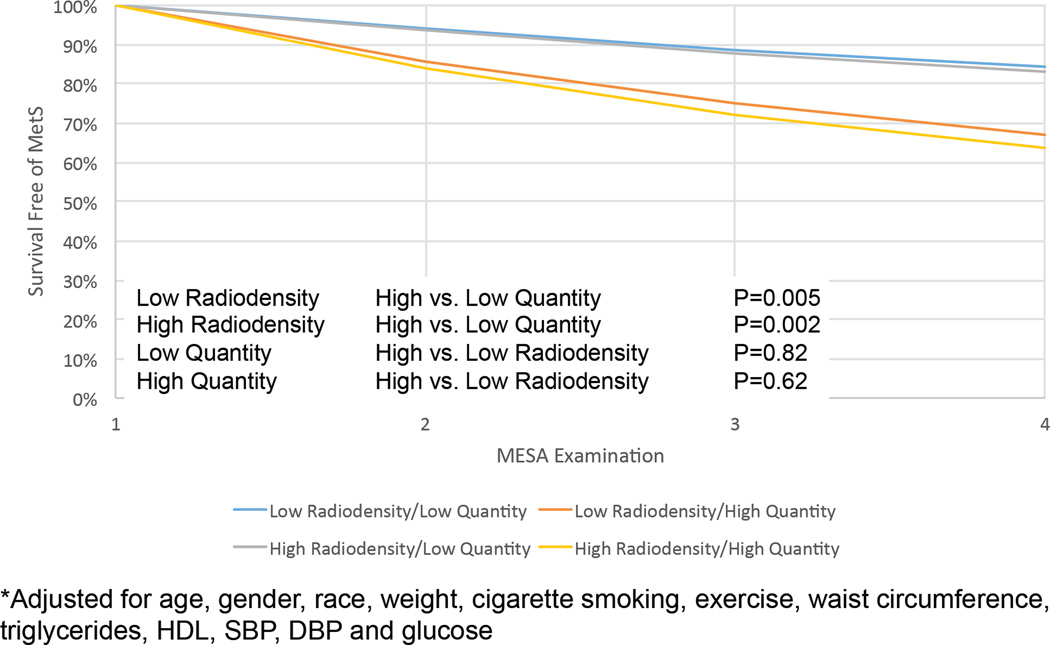
We studied 1,020 MESA participants (of our overall population of 1,511) without MetS at baseline to investigate the relationship between radiodensity and incident MetS. The median follow-up for this group was 6.1 years (IQR 1.9–6.8), and 240 incident MetS events were observed. In unadjusted analyses, higher fat radiodensity (less negative HU values) and lower fat quantity in every compartment (visceral, subcutaneous, and intermuscular) were associated with decreased risk of incident MetS (Table 3). After multivariable adjustment for age, gender, race, and time-varying weight, cigarette smoking, total intentional exercise, waist circumference, triglycerides, high-density lipoprotein, systolic and diastolic blood pressure, and fasting glucose, both higher visceral fat quantity (per 100 cm2/m; HR 1.28, 95% CI 1.17–1.39, P<0.01) and lower visceral fat radiodensity (per HU increase in Hounsfield unit toward zero attenuation; HR 0.95, 95% CI 0.93–0.98, P<0.01) were significantly associated with incident MetS. Conversely, neither subcutaneous or intermuscular fat radiodensity or quantity were associated with MetS with full adjustment. Notably, when both visceral fat radiodensity and quantity were included in the same model, only visceral fat quantity (HR 1.30, 95% CI 1.16–1.46, P<0.01), but not visceral fat radiodensity (HR 1.01, 95% CI 0.97–1.05, P=0.62; Table 3), remained associated with incident MetS,. An adjusted Kaplan-Meier survival curve for visceral fat radiodensity and quantity is presented in Figure 2. There was no effect modification in any model by age, sex, or race.
Table 3.
Cox models for incident metabolic syndrome by regional fat depot quantity and radiodensity. Both Model 1 and Model 2 were adjusted for age, gender, race, weight*, cigarette smoking*, exercise*, waist circumference*, triglycerides*, HDL*, SBP*, DBP*, glucose* (where * signifies time-varying covariates). Model 2 includes both regional fat quantity and radiodensity in the model to test whether radiodensity and quantity were independently associated with incident metabolic syndrome.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variable | Unadjusted HR | P-value | Adjusted HR | P-Value | Adjusted HR | P-Value |
| Visceral Fat Quantity | 1.30 [1.23–1.38] | <0.0001 | 1.28 [1.17–1.39] | <0.0001 | 1.30 [1.16–1.46] | <0.0001 |
| Visceral Fat Radiodensity | 0.91 [0.89–0.93] | <0.0001 | 0.95 [0.93–0.98] | 0.002 | 1.01 [0.97–1.05] | 0.62 |
| Subcutaneous Fat Quantity | 1.16 [1.12–1.20] | <0.0001 | 1.06 [1.00–1.14] | 0.06 | 1.05 [0.99–1.13] | 0.12 |
| Subcutaneous Fat Radiodensity | 0.91 [0.89–0.94] | <0.0001 | 0.97 [0.93–1.00] | 0.06 | 0.97 [0.94–1.01] | 0.12 |
| Intermuscular Fat Quantity | 3.59 [2.39–5.41] | <0.0001 | 1.78 [0.96–3.31] | 0.07 | 1.87 [0.90–3.89] | 0.09 |
| Intermuscular Fat Radiodensity | 0.92 [0.88–0.95] | <0.0001 | 0.98 [0.94–1.03] | 0.43 | 1.01 [0.95–1.07] | 0.81 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
FIGURE 2.
Adjusted Kaplan-Meier survival curve for incident metabolic syndrome (MetS), stratified by median fat density and quantity. MESA examination refers to the time after the initial CT (which was performed at either MESA examination 2 or 3). The model was adjusted for age, gender, race, and time-varying weight, cigarette smoking, total intentional exercise, waist circumference, triglycerides, high-density lipoprotein, systolic and diastolic blood pressure, and fasting glucose. P values for comparison between strata are presented.
DISCUSSION
In a large, community-based, multi-racial, multi-ethnic population, we demonstrated a strong association between fat radiodensity and fat quantity in subcutaneous, visceral, and intermuscular fat depots. Specifically, we found that visceral fat radiodensity was strongly associated with visceral fat quantity, a finding that was similar for subcutaneous and intermuscular fat. In addition, we found that lower fat radiodensity was associated with higher levels of circulating biomarkers of inflammation, insulin resistance and adiponectin, suggesting its role as a marker of dysfunctional adiposity. However, while visceral fat radiodensity was significantly associated with prevalent and incident MetS after adjustment for the CVD risk factors, further adjustment for visceral fat quantity attenuated these associations such that they were no longer significant. Collectively, these findings suggest that fat radiodensity (particularly in the visceral fat compartment) is a clinically important, rapidly assessed imaging biomarker of dysfunctional adipose tissue biochemistry, but may not be independent of total visceral fat quantity in predicting future metabolic syndrome.
Visceral adiposity is a well-established index of metabolically active, “dysfunctional” fat12–15. Alongside these epidemiologic investigations indicating a prognostic role for regional fat in metabolic disease, studies in animal models of obesity and obese humans have suggested that histologic characteristics within adipose tissue identify particularly pathologic fat16–18. A variety of histologic alterations have been described within adipose tissue, namely macrophage infiltration16–18, decreased vascularity19, fibrosis20–22, browning of white adipose tissue23, and adipocyte hypertrophy in response to excessive caloric consumption24. Although these characteristics have been associated with systemic inflammation and insulin resistance16–18—hallmarks of obesity-related cardiometabolic illness—there have been few imaging methods to characterize the “quality” of regional fat compartments. Given observations that not all types of fat equally affect cardiometabolic risk, widely clinically translatable imaging methods to clarify fat quality may provide further risk stratification for cardiometabolic disease and a therapeutic target in studies of metabolic interventions across obesity. While positron emission tomography and magnetic resonance can identify fat inflammation, browning, vascularity, inflammation and are critical to research applications, these modalities are limited for widespread utilization, radiation, and complexity in acquisition that limit enthusiasm for their widespread clinical use.
In this regard, fat attenuation by CT has been proposed as a marker of fat “quality”4–6. In a cross-sectional analysis of 3,198 individuals from Framingham, Rosenquist and colleagues demonstrated that lower attenuation in both visceral and subcutaneous adipose tissue was associated with a higher risk of hypertension, insulin resistance, and metabolic syndrome, independent of fat quantity. Of note, these investigators observed a similar degree of association between fat density and quantity as we observed in the MESA study (e.g., for visceral fat attenuation versus quantity, r=−0.72–0.75, depending on sex). These investigators have also recently identified a quantity-independent association of CT fat attenuation with all-cause and cancer-related mortality6 and with preferential regional expansion of visceral vs. subcutaneous fat (a deleterious fat distribution profile)25.
Nevertheless, studies investigating the histologic basis for changes in fat radiodensity in adipose tissue are limited. In a small study of rats undergoing cold stimulation for brown adipose tissue activation, Baba and colleagues demonstrate a concordant increase in CT attenuation (toward greater radiodensity) with decreased lipid content and activation of brown adipose tissue by positron emission tomography, suggesting that in some adipose tissue subtypes, short-term changes in attenuation may accompany alterations in lipid concentration26. Recent work pre- and post-gastric bypass surgery suggest dynamic changes in CT radiodensity in visceral and subcutaneous fat that parallel improvements in cardiometabolic health and systemic inflammatory biomarkers27. In this report, we extend work in this emerging field by exploring the associations of fat radiodensity with multiple biomarkers of adipocyte function and inflammation, as well as examining fat quantity-independent associations with the metabolic syndrome. Using complementary biomarkers of obesity and cardiometabolic disease, we demonstrate that a more negative fat attenuation (reduced fat radiodensity, or presumed “poorer” fat quality) is associated with greater inflammation (by C-reactive protein), insulin resistance (fasting insulin), dyslipidemia, and a lower adiponectin concentration. In concert with seminal data from the Framingham investigators, these findings provide further evidence that fat radiodensity may provide useful information on metabolic risk.
One important finding in this study was the strong relationship between fat density and quantity. While regional fat quantity is considered a sine qua non of metabolic risk in obesity, the quantitation of visceral and subcutaneous fat generally involves semi-automated contouring, delineation of subcutaneous and visceral fat compartments, and imaging at multiple abdominal levels. In addition, extremely obese individuals may have visceral fat depots that fall outside of the field of view of the CT scan, necessitating methods for imputation3. Our results suggest that an average measure of attenuation within visceral (and subcutaneous or intermuscular) fat depots may be a useful, rapidly assessed surrogate for cardiometabolic disease. While we did not find that radiodensity was independent of quantity in predicting metabolic syndrome risk, given the widespread use of abdominal and thoracic CT imaging in cardiovascular (and general medical) diseases, assessment of fat attenuation may provide a rapid complementary assessment of risk. In addition, while imputation methods may be necessary for fat quantity, fat radiodensity generally does not require this approach, and therefore may be a more robust marker. Further investigation of changes in fat attenuation (preferably with directed metabolic interventions) is warranted.
Our study included a large, well-phenotyped, multi-racial, multi-ethnic population with biomarker studies and careful regional fat phenotyping. However, the study has several important limitations. We did not have histologic confirmation of inflammation, altered lipid content, or adipocyte size in this study. Nevertheless, the association of CT radiodensity with biomarkers of dysfunctional adiposity and inflammation suggests a role for “fat quality” in cardiometabolic disease. In addition, we did not explore the dynamic change of fat radiodensity (using serial CT scans) in this study; this is an area of active research, and large studies with serial changes in fat radiodensity with serial biomarkers and histologic assessments over time in individuals with and without metabolic syndrome are warranted. Finally, metabolic assessments of fat (e.g., using positron emission tomography, as has been reported by other investigators28) would provide interesting comparisons for CT fat radiodensity, and represent areas of active research.
In conclusion, in a large, multi-racial, multi-ethnic population of American adults, we show a strong association between fat radiodensity (as assessed by CT attenuation) and fat quantity in visceral, subcutaneous, and intermuscular fat depots. We further demonstrate that fat radiodensity is associated with mechanistically relevant biomarkers of dysfunctional fat, including inflammation, insulin resistance, and adiponectin. Finally, we show that fat radiodensity in each depot is significantly associated with prevalent and incident metabolic syndrome (though not necessarily independent of visceral fat quantity for the latter). Future studies utilizing metabolic interventions that may fundamentally alter fat radiodensity may assist in understanding the pathobiology of adipose tissue.
Highlights.
Fat radiodensity is strongly linked to total visceral, subcutaneous and intermuscular fat
Fat radiodensity is associated with metabolic syndrome and markers of adipocyte dysfunction
Fat radiodensity is a useful, rapid marker of cardiometabolic risk
Fat radiodensity reflects the adverse biochemical phenotype of obesity
ACKNOWLEDGEMENTS
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Jama. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neeland IJ, Turer AT, de Lemos JA. Measures of adiposity and fat distribution and risk of diabetes--reply. Jama. 2013;309:340. doi: 10.1001/jama.2012.94338. [DOI] [PubMed] [Google Scholar]
- 3.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral Adiposity and the Risk of Metabolic Syndrome Across Body Mass Index: The MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762–771. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvey NJ, Pedley A, Rosenquist KJ, Massaro JM, O'Donnell CJ, Hoffmann U, Fox CS. Association of fat density with subclinical atherosclerosis. Journal of the American Heart Association. 2014;3 doi: 10.1161/JAHA.114.000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, Murabito JM, Hoffmann U, Fox CS. Fat Quality and Incident Cardiovascular Disease, All-Cause Mortality and Cancer Mortality. J Clin Endocrinol Metab. 2014:jc20134296. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multiethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:140–146. doi: 10.1016/j.jacc.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison MA, Bluemke DA, McClelland R, Cushman M, Criqui MH, Polak JF, Lima JA. Relation of leptin to left ventricular hypertrophy (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112:726–730. doi: 10.1016/j.amjcard.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah RV, Abbasi S, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, Kwong R, Bluemke DA, Lima JA, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.01.053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 12.Kramer CK, Zinman B, Retnakaran R. Are Metabolically Healthy Overweight and Obesity Benign Conditions?: A Systematic Review and Meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Kabir M, Stefanovski D, Hsu IR, Iyer M, Woolcott OO, Zheng D, Catalano KJ, Chiu JD, Kim SP, Harrison LN, Ionut V, Lottati M, Bergman RN, Richey JM. Large size cells in the visceral adipose depot predict insulin resistance in the canine model. Obesity (Silver Spring) 2011;19:2121–2129. doi: 10.1038/oby.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I, Hamaguchi M, Nishimura S, Manabe I, Matsuda T, Kimura K, Inoue H, Inagaki Y, Aoe S, Yamasaki S, Ogawa Y. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nature communications. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- 21.Vila IK, Badin PM, Marques MA, Monbrun L, Lefort C, Mir L, Louche K, Bourlier V, Roussel B, Gui P, Grober J, Stich V, Rossmeislova L, Zakaroff-Girard A, Bouloumie A, Viguerie N, Moro C, Tavernier G, Langin D. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell reports. 2014;7:1116–1129. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 22.Chun TH. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte. 2012;1:89–95. doi: 10.4161/adip.19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2014 doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, Rao S, Yusuf S, Gerstein HC, Sharma AM. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE) PLoS One. 2011;6:e22112. doi: 10.1371/journal.pone.0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeoh AJ, Pedley A, Rosenquist KJ, Hoffmann U, Fox CS. The Association between Subcutaneous Fat Density and the Propensity to Store Fat Viscerally. J Clin Endocrinol Metab. 2015:jc20144032. doi: 10.1210/jc.2014-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:246–250. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 27.Torriani M, Oliveira AL, Azevedo DC, Bredella MA, Yu EW. Effects of Roux-en-Y gastric bypass surgery on visceral and subcutaneous fat density by computed tomography. Obesity surgery. 2015;25:381–385. doi: 10.1007/s11695-014-1485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira AL, Azevedo DC, Bredella MA, Stanley TL, Torriani M. Visceral and subcutaneous adipose tissue FDG uptake by PET/CT in metabolically healthy obese subjects. Obesity (Silver Spring) 2015;23:286–289. doi: 10.1002/oby.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]