Abstract
Ornithine decarboxylase (ODC) is a rate-limiting enzyme in the first step of polyamine biosynthesis that is associated with cell growth and tumor formation. Existing catalytic inhibitors of ODC have lacked efficacy in clinical testing or displayed unacceptable toxicity. In this study, we report the identification of an effective and nontoxic allosteric inhibitor of ODC. Using computer docking simulation and an in vitro ODC enzyme assay, we identified herbacetin, a natural compound found in flax and other plants, as a novel ODC inhibitor. Mechanistic investigations defined aspartate 44 in ODC as critical for binding. Herbacetin exhibited potent anticancer activity in colon cancer cell lines expressing high levels of ODC. Intraperitoneal or oral administration of herbacetin effectively suppressed HCT116 xenograft tumor growth and also reduced the number and size of polyps in a mouse model of APC-driven colon cancer (ApcMin/+). Unlike the well established ODC inhibitor DFMO, herbacetin treatment was not associated with hearing loss. Taken together, our findings defined the natural product herbacetin as an allosteric inhibitor of ODC with chemopreventive and antitumor activity in preclinical models of colon cancer, prompting its further investigation in clinical trials.
Keywords: cancer, prevention, ODC, small bowel tumor, colorectal
INTRODUCTION
Polyamines play important roles in normal and cancer cell growth (1), proliferation (2), gene expression (3), and signal transduction from the cell membrane to the nucleus by activating mitogen activated protein (MAP) kinases, including Ras, MEKs and ERKs (4-6). Ornithine decarboxylase (ODC) is a first rate-limiting enzyme in the polyamine biosynthesis pathway in mammals and it is highly expressed in the intestinal mucosa of individuals with familial adenomatous polyposis (FAP), a disease characterized by overexpression of c-myc caused by a deletion mutant adenomatous polyposis coli (APC) gene (7). Ras activation-mediated cell transformation induces ODC transcription, translation and polyamine accumulation (8, 9). In addition, polyamines activate the phosphorylation of ERKs and induce the expression of oncogenes such as myc, jun, and fos (6, 10). Additionally, elevated ornithine decarboxylase (ODC) activity is observed in neoplastic tissues and is highly correlated with tumor growth (11). Furthermore, Myc-induced lymphomagenesis is suppressed by targeting ODC, suggesting that this enzyme is a potential target for cancer prevention or treatment (12).
Previous reports indicated that polyamine inhibitors, including the ornithine decarboxylase (ODC) inhibitor, S-adenosylmethionine decarboxylase (AMD) and N1, N11 or -N14-diethylnorspermine (DENSPM or DEHSPM; polyamine analogues) have been identified (13-16). However, in clinical trials, all of these polyamine inhibitors failed to be effective in treating various cancers (17-19). Difluoromethylornithine (DFMO), an FDA-approved drug, binds to the active site of ODC and acts as an irreversible and specific ODC inhibitor. DFMO significantly inhibited proliferation of adenocarcinoma, squamous and leukemia cells (20) as well as cancers in numerous transgenic animal models (4, 21, 22). DFMO has been evaluated as a prevention agent against several cancers, including bladder, cervical, colorectal, breast, prostate and nonmelanoma skin cancers (11). Intracellular putrescine and spermidine levels were strongly reduced by DFMO; however, in contrast, DFMO can promote the uptake rate of putrescine and spermidine (23). Thus, the initial colon cancer prevention trials with DFMO alone showed a dose limiting cytotoxicity (24). Recently, a combination of low doses of DFMO and non-steroidal anti-inflammatory drugs (NSAIDs) has been studied and shown to have a considerable inhibitory effect on colon cancer (25, 26). Herbacetin is a novel flavonol compound found in natural sources such as herb of ramose scouring rush, flaxseed and Roemeria hybrid (27). Herbacetin is structurally close to quercetin and kaempferol and exerts various pharmacological activities, including antioxidant, anti-inflammatory and anticancer effects (28). Previous studies have shown that herbacetin possesses a strong antioxidant capacity and can also induce oxygen species-mediated apoptosis in hepG2 liver cancer cells (29, 30). Additionally, phosphorylation of c-Met and AKT are strongly inhibited by herbacetin (31). However, this activity is not sufficient to explain herbacetin’s biological activities. The aim of the present study was to identify a novel ODC inhibitor and to investigate the efficacy of the newly discovered ODC inhibitor, herbacetin, in the prevention or treatment of small bowel and colon tumors.
MATERIALS AND METHODS
ODC enzyme assay
ODC activity was measured as the release of CO2 from L-[1-C14] ornithine as previously described (32).
Pull-down assay using CNBr-herbacetin-conjugated beads
A recombinant human ODC protein (200 ng) or total cell lysates (500 μg) were incubated with herbacetin-Sepharose 4B (or Sepharose 4B only as a control) beads (50 μl, 50% slurry). The pull-down assay was performed as described previously (33).
Measurement of polyamine content
Intracellular polyamines were extracted with 0.6 N perchloric acid from herbacetin- or DFMO-treated cell pellets or mouse tissues, then dansylated or benzoylated and content was measured by reverse phase HPLC as described previously (34, 35). Polyamines were detected using a fluorescence detector with an excitation wavelength of 360 nm and an emission cut-off filter of 500 nm and analyzed using chromatography software.
Computer docking and modeling
The structure of ODC (PDB code: 1NJJ) used as the receptor model in our docking program was an X-ray diffraction structure with a resolution of 2.45 Å. Before docking, the ODC protein was prepared for docking following the standard procedure outlined in the Protein Preparation Wizard (36) included in the Schrödinger Suite 2010 (37, 38). The binding pocket was selected by the G418 ligand, which was already bound to the crystal structure chosen. The Traditional Chinese Medicine Database (TCMD), which contains more than 7,500 compound constituents from 352 different herbs, animal products and minerals, was chosen as the ligand database for docking against the ODC protein structure using the Schrödinger docking program Glide (39). One hundred compounds were chosen based on the docking score obtained by high throughput virtual screening. This group was narrowed to 10 compounds based on SP (standard precision) and XP (extra precision) flexible docking.
In vivo studies using the APCMin+ mouse model
Male C57BL/6J(Min/+) mice were obtained from Jackson Laboratory and maintained under “specific pathogen-free” conditions according to the guidelines established by the University of Minnesota Institutional Animal Care and Use Committee. APCMin+ male mice were bred with C57BL/6J APC wildtype female mice. The progeny were genotyped by PCR assay to determine whether they were heterozygous for the min allele or were homozygous wildtype. APCMin+ male or female progeny were randomly assigned to groups after weaning at 3 weeks. Mice (5~6 weeks old) were divided into 3 groups: 1) untreated vehicle group (n = 8); 2) mice treated with 0.4 mg herbacetin/kg of body weight (n = 8); and 3) mice treated with 2 mg herbacetin/kg of body weight (n = 8). Herbacetin or vehicle was injected i.p. 3 times a week for 8 weeks.
In vivo studies using the xenograft mouse model
Athymic nude mice (6 week old nu/nu female mice, Harlan Laboratory, Minneapolis, MN) were inoculated in the right flank with HCT116 cells (2×106 cells/mouse). Mice were maintained under “specific pathogen-free” conditions based on the guidelines established by the University of Minnesota Institutional Animal Care and Use Committee. For treatment by I.P injection, tumors were allowed to grow to an average of ~74.1 ± 56 mm3 and then, based on tumor volume, mice were divided into groups to obtain a similar average tumor volume. Mice were divided into four groups as follows: 1) untreated vehicle group (n = 10); 2) 0.4 mg herbacetin/kg of body weight (n = 10); 3) 2 mg herbacetin/kg of body weight (n = 10); and 4) 200 mg DFMO/kg of body weight (n = 10). Herbacetin, DFMO or vehicle (5% DMSO in 10% tween 20) was injected 3 times per week for 14 days. For treatment by oral administration, tumors were allowed to grow to an average of ~51.3 ± 51.6 mm3 and then mice were divided into 2 groups with a similar average tumor volume as follows: 1) untreated vehicle group (n = 15) and 2) herbacetin at 100 mg/kg (n = 19). Treatment with herbacetin was initiated on Day 17 after inoculation of cells and continued to Day 35 (~3wks) and was administered by oral gavage 5 times a week. Tumor volume was measured 2 times a week and body weight was measured once a week. Herbacetin was prepared in 2.5% DMSO/5% PEG 400/5% Tween-80 in 1× PBS and sonicated for 20 min. Tumor volume was calculated from measurements of 2 diameters of the individual tumor base using the following formula: tumor volume (mm3) = (length × width × height × 0.52). Mice were monitored until tumors reached 1 cm3 total volume, at which time mice were euthanized and tumors extracted.
Cell lines
All cell lines were purchased from American Type Culture Collection (ATCC) and were cytogenetically tested and authenticated before being frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 weeks. Enough frozen vials of each cell line were available to ensure that all cell-based experiments were conducted on cells that had been tested and in culture for 8 weeks or less. HCT116 and HT29 human colon cancer cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and 1% antibiotic-antimycotic. DLD1 human colon cancer cells were cultured in RPMI1640 medium supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) and 1% antibiotic-antimycotic.
Reagents and antibodies
Herbacetin (purity: > 90% by HPLC) was purchased from Indofine Chemical Company (Hillsborough, NJ). CNBr-Sepharose 4B beads were purchased from GE Healthcare (Piscataway, NJ). The recombinant human ODC protein was obtained from Abnova (Walnut, CA). The screening to determine the effect of herbacetin on the activity of 13 kinases was performed by Millipore (Temecula, CA). The antibody to detect Xpress was from Invitrogen (Grand Island, NY). Antibodies to detect total ERKs, phosphorylated ERKs (T202/Y204), total RSK and phosphorylated RSK (T356/S360) were from Cell Signaling Technology (Beverly, MA). Antibodies against ODC and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Lentiviral infection
The lentiviral expression vector, pLKO.1-shODC, and packaging vectors, pMD2.0G and psPAX, were purchased from Addgene Inc. (Cambridge, MA). To prepare ODC viral particles, the viral vector and packaging vectors were transfected using JetPEI into HEK293T cells following the manufacturer’s suggested protocols. The transfection medium was changed at 4 h after transfection and then cells were cultured for 36 h. The viral particles were harvested by filtration using a 0.45 mm sodium acetate syringe filter and then combined with 8 μg/ml of polybrane (Millipore, Billerica, MA) and infected overnight into 60% confluent HCT116 cells. The cell culture medium was replaced with fresh complete growth medium and after 24 h, cells were selected with 1.5 μg/ml of puromycine for 36 h. The selected cells were used for experiments.
Anchorage-independent cell growth
Cells (8 × 103 per well) suspended in complete growth medium (McCoy’s 5A or RPMI1640 supplemented with 10% FBS and 1% antibiotics) were added to 0.3% agar with different doses of each compound in a top layer over a base layer of 0.6% agar with the same doses of each compound as in the top layer. The cultures were maintained at 37°C in a 5% CO2 incubator for 3 weeks and then colonies were counted under a microscope using the Image-Pro Plus software (v.4) program (Media Cybernetics).
Luciferase assay for reporter activity
Transient transfection was conducted using jetPEI (Qbiogene, Carlsbad CA) and assays for the activity of firefly luciferase and Renilla activity were performed according to the manufacturer’s instructions (Promega, Madison, WI). Cells (1 × 104 per well) were seeded the day before transfection into 12-well culture plates. Cells were co-transfected with reporter plasmid (250 ng) and internal control (CMV-Renilla, 50 ng) in 12-well plates and incubated for 24 h. Colon cancer cells were treated with herbacetin for 48 h and harvested in Promega Lysis Buffer. The Luciferase and Renilla activities were measured using substrates in the reporter assay system (Promega). The luciferase activity was normalized to Renilla activity.
Cell proliferation assay
Cells were seeded (1 × 103 cells per well) in 96-well plates and incubated for 24 h and then treated with different doses of each compound. After incubation for 1, 2 or 3 days, 20 μl of CellTiter96 Aqueous One Solution (Promega) were added and then cells were incubated for 1 h at 37°C in a 5% CO2 incubator. Absorbance was measured at 492 nm.
Statistical analysis
All quantitative results are expressed as mean values ± S.D. or ± S.E. as indicated. Significant differences were compared using the Student’s t test or one-way analysis of variance (ANOVA). A p value of < 0.05 was considered to be statistically significant.
RESULTS
Herbacetin is a specific and potent ODC inhibitor
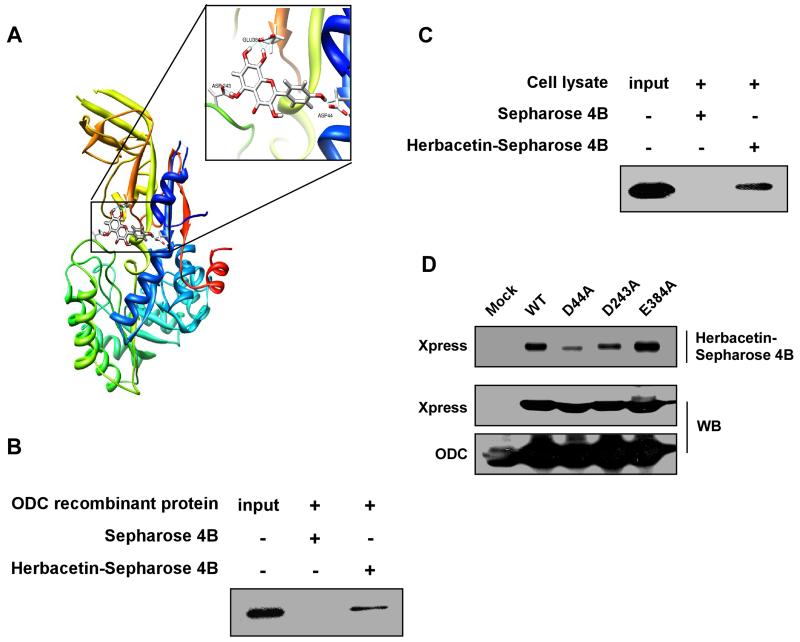
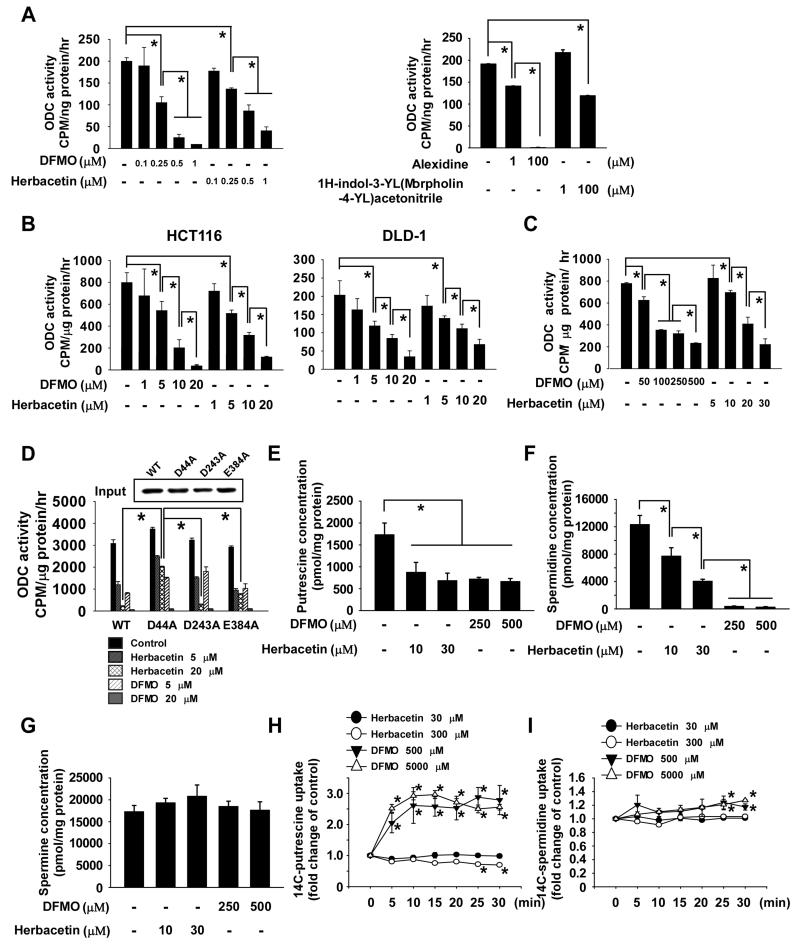
To identify potential active compounds targeting an allosteric site on ODC, we performed docking studies (Supplemental Table 1) using the Traditional Chinese Medicine Database (TCMD). Results indicated that herbacetin was a potential allosteric inhibitory compound that targets ODC (Fig. 1A). To examine the interaction between herbacetin and ODC, we performed in vitro pull-down assays using herbacetin-conjugated Sepharose 4B beads (or Sepharose 4B as a negative control) and a recombinant ODC protein (Fig. 1B) or a HCT116 colon cancer cell lysate (Fig. 1C). Results confirmed that herbacetin directly binds to ODC. Furthermore, computer docking results indicated that Asp44, Asp243, and Glu384 on ODC might be involved in the binding. These sites were mutated to alanine (D44A, D243A, E384A) and ectopically expressed in HCT116 colon cancer cells. Pull-down assays using the wildtype or each mutant and herbacetin-conjugated Sepharose 4B beads revealed that the D44A mutant showed the most reduced binding affinity with herbacetin (Fig. 1D), suggesting that this site is important for binding. Next, we compared the effect of herbacetin and DFMO (Fig. 2A, left panel) and allosteric ODC inhibitors (Fig. 2A, right panel) on ODC activity using a recombinant ODC protein, HCT116 or DLD-1 colon cancer cell lysates (Fig. 2B), or intact HCT116 cells (Fig. 2C). Herbacetin showed inhibitory ODC activity similarly compared to DFMO in vitro (Fig. 2A, B). However, herbacetin was markedly more effective than DFMO in suppressing ODC activity in cell-based assays (Fig. 2C). Furthermore, ODC activity was similar in the wildtype and mutant ODC proteins because the mutated sites originated from the allosteric binding site of ODC rather than the active site. The ODC D44A mutant activity was less susceptible to the effects of herbacetin than the other mutants (D243A, E384A) or the wildtype ODC (Fig. 2D). Additionally, we docked herbacetin in silico to a selected pocket in the 1NJJ (ODC) protein structure, which allowed not only the ligand to be flexible, but also allowed the amino acids forming the protein binding site to achieve a more realistic view of the possible protein-ligand interaction. Results indicated that herbacetin forms numerous favorable interactions and docked nicely within the ODC allosteric site, especially at residue Asp44 (1.64 Å). In contrast, a similar compound, kaempferol, could not form these interactions. In this model, important hydrogen bonds were formed between herbacetin and ODC’s backbone at Asp44, Asp243 and Glu384 (Supplemental Fig. 1A). In contrast, kaempferol formed hydrogen bonds at Glu384, Thr285 and Ser282 (Supplemental Fig. 1B). Kaempferol had little effect on ODC activity compared to herbacetin, suggesting that the binding of herbacetin with Asp44 might be more important for inhibiting ODC activity (Supplemental Fig. 1C). Next, to identify other direct molecular targets of herbacetin, we screened 13 kinases and S-adenosylmethionine decarboxylase (AdoMetDC) enzyme activity against herbacetin using in vitro kinase and enzyme assays. The results showed that none of these kinases were affected by herbacetin, whereas, only high doses of herbacetin or DFMO significantly increased AdoMetDC enzyme activity (Supplemental Fig. 2A-N). Additionally, to determine the effect of herbacetin or DFMO on polyamine content, we measured the level of putrescine, spermidine, and spermine in HCT116 colon cancer cells (Fig. 2E-G). The findings indicated that putrescine and spermidine, but not spermine, levels are significantly inhibited by treatment with herbacetin or DFMO.
Figure 1. Herbacetin binds directly to the ODC protein.
(A) Computer modeling of herbacetin and the ODC protein crystal structure. Several hydrogen bonds are formed between herbacetin and Asp44, Asp243 and Glu384 on the backbone of ODC. (B) Recombinant ODC, (C) an HCT116 colon cancer cell lysate or (D) cells ectopically expressing ODC (WT, mutant D44A, D243A or E384A) were incubated with herbacetin-conjugated Sepharose 4B beads for a pull-down assay and then analyzed by Western blotting. For B-D, similar results were obtained from 3 independent experiments and representative blots are shown.
Figure 2. Herbacetin inhibits ODC activity.
The effect of herbacetin on ODC activity was assessed using a recombinant ODC protein (A) or colon cancer cell lysates (B) incubated for 15 min with reaction buffer and different doses of herbacetin or DFMO and then incubated at 37°C for an additional 1 h. (C) HCT116 cells were treated with herbacetin or DFMO for 48 h and harvested. (D) The effect of herbacetin on ectopically expressed wildtype or mutant ODC (WT, D44A, D243A, E384A) activity was measured as the release of CO2 from L-[1-C14] ornithine. (E-G) The effect of herbacetin or DFMO on polyamine (E, putrescine; F, spermidine; G, spermine) content was analyzed by HPLC in HCT116 colon cancer cells. (H-I) The effect of herbacetin or DFMO on polyamine uptake was measured by using 14C-putrescine (H) or – spermidine (I) in HCT116 colon cancer cells. Cells were treated with herbacetin or DFMO for 24 h and the respective polyamine was or was not added for 30 min. After washing with PBS, the intracellular 14C-putrescine or -spermidine levels were measured. For A-I, all data are represented as means ± S.D. of triplicate values from 3 independent experiments and the asterisk (*) indicates a significant difference (p < 0.05) between herbacetin- or DFMO-treated samples compared to untreated controls.
Furthermore, to identify the effect on polyamine uptake by herbacetin or DFMO, cells were treated with herbacetin or DFMO for 24 h and then 14C-conjugated putrescine or spermidine was added. Results showed that the intracellular 14C-putrescine and -spermidine levels were not changed by herbacetin (Fig. 2H-I). In contrast, DFMO significantly increased intracellular putrescine and spermidine uptake (Fig. 2H-I). Next, to determine the effect on intracellular herbacetin level by polyamines, cells were co-treated with herbacetin and putrescine, spermidine or spermine. Results indicated that the intracellular herbacetin was not significantly changed by polyamine treatment (Supplemental Fig. 3).
Anticancer effects of herbacetin
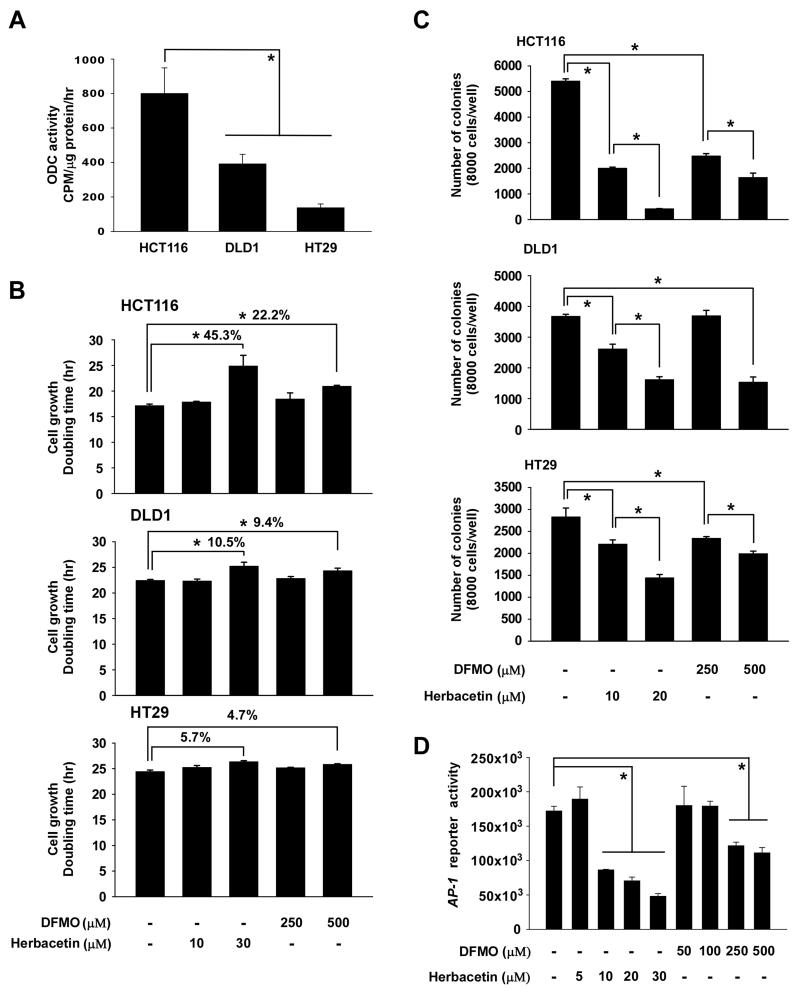
We measured ODC activity in HCT116, DLD1, and HT29 colon cancer cells and determined that, of the three, HCT116 cells had the highest ODC activity (Fig. 3A). We compared the effect of herbacetin and DFMO on the doubling time and cell cycles of each colon cancer cell line (Fig. 3B, Supplemental Fig. 4). The findings indicated that herbacetin had the greatest inhibitory effect on colon cancer cells that expressed higher levels of ODC. We also examined the effect of herbacetin on anchorage-independent colon cancer cell growth. Herbacetin was at least 20-fold more effective than DFMO in suppressing anchorage-independent growth of colon cancer cells (Fig. 3C). Next, we determined whether herbacetin affected the reporter activity of the MAP kinase transcription factor, activator protein-1 (AP-1), in HCT116 cells. HCT116 cells were treated with herbacetin for 48 h and then AP-1 reporter activity was measured (Fig. 3D). Treated cells were also subjected to Western blotting and herbacetin also suppressed phosphorylation of ERK1/2 as well as p90RSK (Supplemental Fig. 5). Overall, these results indicated that herbacetin decreases ODC activity resulting in attenuated AP-1 activation.
Figure 3. Anticancer effects of herbacetin.
(A) The effect of herbacetin on ODC activity in colon cancer cell lines was measured as the release of CO2 from L-[1-C14] ornithine. The asterisk (*) indicates significantly decreased (p < 0.05) ODC activity in DLD1 or HT29 cells compared to HCT116 cells. (B) The effect of herbacetin or DFMO on the doubling time of colon cancer cells and (C) on anchorage-independent colon cancer cell growth. For (C), cells were incubated with respective compound for 3 weeks and then colonies were counted using a microscope and the Image-Pro PLUS (v.6) computer software program. (D) The effect of herbacetin on AP-1 reporter activity in HCT116 colon cancer cells was analyzed using the substrates included in the reporter assay system. All data are represented as means ± S.D. of triplicate values from 3 independent experiments and the asterisk (*) indicates a significant (p < 0.05) effect of herbacetin or DFMO compared to untreated control.
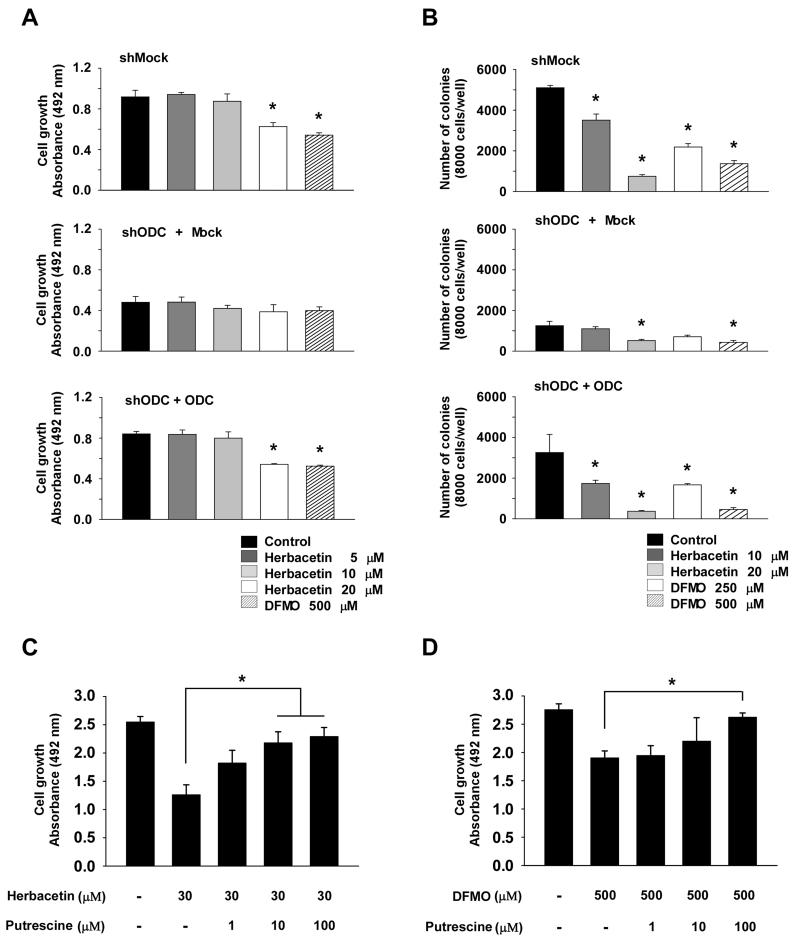
The inhibition of ODC by herbacetin is dependent on the expression of ODC
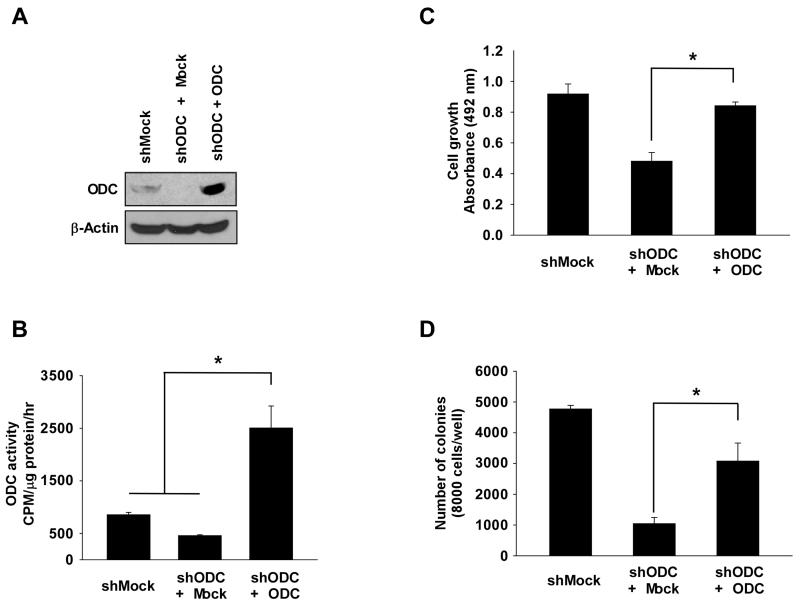
To study the influence of ODC expression on cancer cell growth, we constructed HCT116 cells stably expressing mock (shMock) or knockdown of ODC (shODC) (Supplemental Fig. 6A-E). We also constructed stable shODC HCT116 cells with rescued expression of ODC (shODC + ODC; Fig. 4A) and measured ODC activity (Fig. 4B). Results indicated that colon cancer cell growth is dependent on ODC expression (Fig. 4C-D). We then examined the effect of herbacetin or DFMO on growth of shMock, shODC and shODC + ODC HCT116 cells. Data indicated that cells expressing shODC were resistant to herbacetin’s inhibitory effect on anchorage-dependent and -independent cell growth compared to cells expressing shMock (Fig. 5A, B, middle panels). The shODC cells expressing rescued ODC regained sensitivity to herbacetin (Fig. 5A, B, bottom panels). Furthermore, we investigated the effect of the polyamine, putrescine, plus herbacetin or DFMO on growth. Cells were treated with herbacetin or DFMO for 48 h, and then putrescine was added and cell proliferation and polyamine pools measured after 48 h. Results indicated that cancer cell growth inhibited by herbacetin or DFMO is rescued by putrescine treatment (Fig. 5C-D, Supplemental Fig. 7). Taken together, these findings indicated that the anticancer activity exerted by herbacetin is dependent on ODC and also its anticancer activity against ODC is reversed by putrescine. Next, to determine the influence of polyamine depletion with herbacetin treatment on cancer cell doubling time, we used HCT116 (fast growing) or HT29 (slow growing) cells stably expressing knockdown of ODC or SAT1 (spermidine/spermine N1-acetyltransferase 1). Results showed that cell doubling time was increased by shODC or by overexpressing SAT1 in both cell lines. In contrast, only HCT116 cells expressing SAT1 were more sensitive to herbacetin’s inhibitory effect on cell doubling time (Supplemental Fig. 8A-D).
Figure 4. Effect of ODC expression on anchorage-dependent and -independent colon cancer cell growth.
(A) ODC expression was analyzed by Western blot in HCT116 colon cancer cells expressing shMock, shODC + shMock, or shODC + ODC. (B) ODC activity was assessed as the release of L-[1-C14] ornithine. (C) Anchorage-dependent cell growth was measured by MTS assay. (D) Anchorage-independent colon cancer cell growth was analyzed. Colonies were counted using a microscope and the Image-Pro PLUS (v6) computer software program. Data shown in B, C and D are represented as means ± S.D. of triplicate values from 3 independent experiments and the asterisk (*) indicates a significant (p < 0.05) difference versus shODC + shMock cells.
Figure 5. The anticancer activity exerted by herbacetin is dependent on ODC expression.
(A) The effect of herbacetin on HCT116 colon cancer cell growth was assessed in shMock, shODC and shODC cells with rescued ODC expression. Cells were incubated for 72 h and proliferation was determined by MTS assay. (B) The effect of herbacetin on anchorage-independent HCT116 colon cancer cell growth was assessed in shMock, shODC and shODC cells with rescued ODC expression. Cells were incubated in 0.3% agar for 3 weeks and colonies were counted using a microscope and the Image-Pro PLUS (v.6) computer software program. (C-D) The effect of putrescine and herbacetin or DFMO on cell growth was analyzed. All data are represented as means ± S.D. of triplicate values from 3 independent experiments. The asterisk (*) indicates a significant effect (p < 0.05) of herbacetin or DFMO compared to untreated controls or with putrescine treatment.
Herbacetin as a preventive agent against small bowel and colon tumorigenesis in vivo
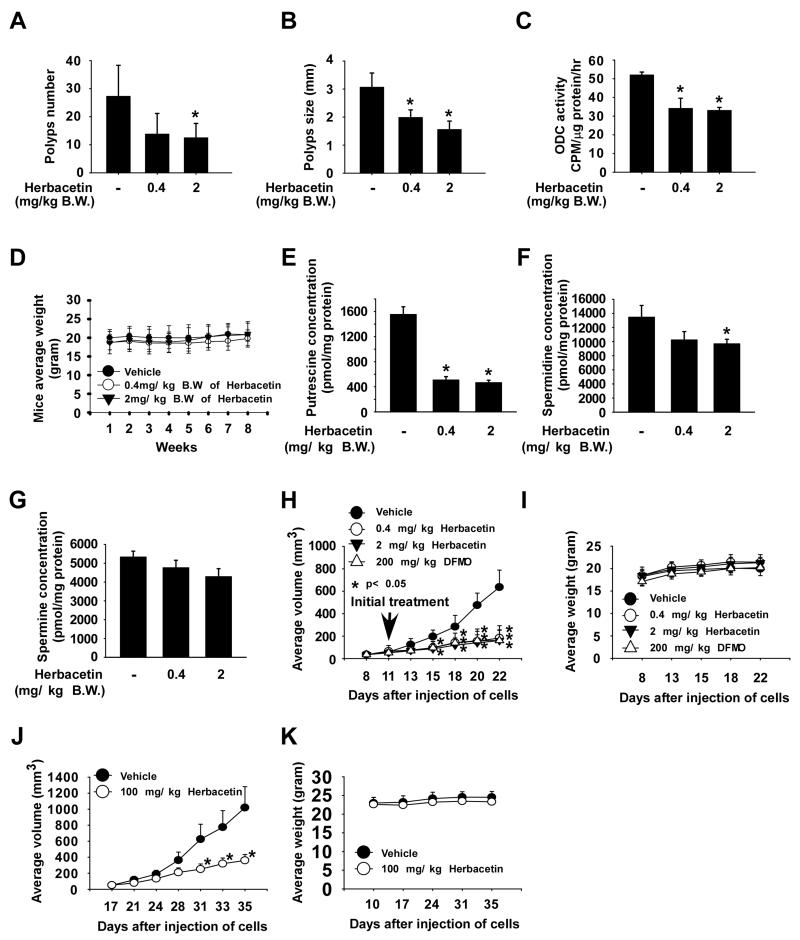
We examined the antitumor activity of herbacetin in colon tumorigenesis using two in vivo mouse models. ODC gene expression is up-regulated in the intestinal tissue of APCMin+ mice, a model that mimics human familial adenomatous polyposis (FAP). APCMin+ mice were administered herbacetin (0.4 or 2 mg/kg body weight) or vehicle 3 times/week for 8 weeks. At the end of 8 weeks, polyp number and size were determined and small intestine samples collected. Treatment of mice with 0.4 or 2 mg/kg of herbacetin significantly suppressed polyp number, size, and ODC activity compared to the vehicle-treated group (Fig. 6A-C; p < 0.05). Mice tolerated treatment with herbacetin without overt signs of toxicity or significant body weight loss (Fig. 6D). Furthermore, the effects of herbacetin on polyamine levels were evaluated by HPLC in the small intestine tissues after 8 weeks of treatment. Results indicated that putrescine and spermidine, but not spermine, content was markedly decreased by treatment with herbacetin (Fig. 6E-G). Additionally, we examined the effect of herbacetin or DFMO on HCT116 colon cancer cell xenograft tumor growth in mice. HCT116 colon cancer cells were injected into the flank of athymic nude mice and mice were injected with herbacetin (i.p. 0.4 or 2 mg/kg body weight), DFMO (i.p. 200 mg/kg body weight) or vehicle 3 times/week for 2 weeks after the average tumor volume grew to about 74 mm3. Treatment of mice with herbacetin or DFMO strongly suppressed HCT116 tumor growth by over 70% relative to the vehicle-treated group (Fig. 6H; p < 0.05). Furthermore, the effects of herbacetin on ODC expression and AP1 signaling were evaluated by Western blotting, immunohistochemistry and H&E staining after 11 days of treatment. The expression of phosphorylated ERKs and RSK was markedly decreased by treatment with herbacetin or DFMO (Supplemental Fig. 9A). However, ODC expression in herbacetin or DFMO treated tissues was similar to the vehicle-treated group (Supplemental Fig. 9A, C). Additionally, mice seemed to tolerate treatment with herbacetin or DFMO without overt signs of toxicity or significant loss of body weight similar to the vehicle-treated group (Fig. 6I). Additionally, we also examined the effect of oral administration of herbacetin (100 mg/kg B.W.) on HCT116 colon cancer cell xenograft tumor growth in mice. HCT116 colon cancer cells were injected into the flank of athymic nude mice and mice were given herbacetin or vehicle by oral gavage 5 times/week for 18 days after the average tumor volume grew to about 51 mm3. Results showed that tumor growth and phosphorylated ERKs and RSK were significantly suppressed in mice fed herbacetin (Fig. 6J, Supplemental Fig. 9B) and body weight was not affected (Fig. 6K). These results indicated that herbacetin is a potent ODC inhibitor and an active anticancer agent against small bowel and colon tumor growth.
Figure 6. Effectiveness of herbacetin as a preventive agent against small bowel and colon tumor growth in vivo.
APCMin+ mice were used as a small bowel tumorigenesis model treated or not treated with herbacetin (i.p 0.4 or 2 mg/kg B.W.) for 8 weeks. (A) Number and (B) size of polyps from APCMin+ mice treated or not treated with herbacetin were calculated following euthanization. (C) ODC activity and (D) body weights from vehicle- and herbacetin-treated groups of mice were measured. (E-G) The effect of herbacetin on polyamine (E, putrescine; F, spermidine; G, spermine) content from APCMin+mice small intestine tissues was analyzed by HPLC. (H) Herbacetin or DFMO suppresses colon tumor growth. Mice were injected with HCT116 colon cancer cells and then treated with herbacetin (i.p 0.4 or 2 mg/kg B.W.), DFMO (i.p 200 mg/kg B.W.) or vehicle 3 times a week for 2 weeks and tumors harvested. (I) Herbacetin or DFMO has no effect on mouse body weight up to 22 days. (J) Oral administration of herbacetin significantly suppresses xenograft HCT116 colon cancer growth. When tumors reached ~51 mm3, mice were administered herbacetin (100 mg/kg B.W) by oral gavage 5 times a week for 18 days. (K) Herbacetin has no effect on mouse body weight up to 35 days. All data are represented as mean values ± S.E. and the asterisk (*) indicates a significant difference (p < 0.05) between herbacetin- or DFMO-treated groups compared to the vehicle-treated group.
Herbacetin is not involved in ototoxicity
Although DFMO is an approved FDA drug as an irreversible inhibitor of ODC, high doses of DFMO in humans can cause hearing loss (19). Therefore, development of new potent, nontoxic ODC inhibitor is important. We demonstrated the anticancer effects of herbacetin as a novel ODC inhibitor against colon cancer and next determined whether herbacetin was associated with ototoxicity (i.e., toxicity to the ear) compared with DFMO. Prepulse inhibition (PPI) of the acoustic startle reflex (ASR) is important to estimate hearing impairment in mice. PPI is the ratio of the startle with a prepulse to the baseline startle response and can be used to assess the behavioral salience of sound. A high percentage PPI value indicates a good PPI. In other words, the subject shows a reduced startle response when a prepulse stimulus is presented compared to the response when the startle stimulus is presented alone and therefore is exhibiting normal hearing. Conversely, a low percentage PPI value indicates a poor PPI or, in other words, the startle responses with and without the prepulse are similar and hearing is therefore defective. Our results indicated that on day 35, oral administration of DFMO (1 g/kg B.W.) resulted in a 20% PPI compared with control group (Supplemental Fig. 10A; data are presented as percent of control with vehicle being 100%) suggesting that DFMO was associated with profound hearing loss in C57BL/6 mice compared to vehicle control. Two additional groups of mice were administered herbacetin intraperitoneally (2 mg/kg B.W.) or orally (100 mg/kg B.W.) to determine whether herbacetin had similar effects on hearing as %PPI. Our results indicated that percentage PPI (i.e., hearing) was unaffected by herbacetin administered orally (Supplemental Fig. 10B) or by intraperitoneal injection (Supplemental Fig. 10C). These results indicate that herbacetin is a potent ODC inhibitor without apparent ototoxicity.
DISCUSSION
Many flavonol compounds have been reported to exert potent anti-proliferative activities through inhibition of multiple targets such as MAPKs, MAPKKs or COX enzymes and have been suggested as agents in the development of molecular target therapy for human cancers (40). Previous reports suggested that alanine mutagenesis in the dimer interface of ODC distant from active site inhibited catalytic activity (41) and G418 (geneticin) induced the disordering of residues in the active site of ODC and allosteric inhibition (42). Therefore, we screened for allosteric ODC inhibitors using computer docking modeling and identified herbacetin as a possible allosteric inhibitor.
Structural and computational technique-based drug discovery, especially the application of molecular modeling, molecular docking, virtual molecular high-throughput and targeted drug screening, has been utilized (43). Recent advances in the development of anticancer drugs involve an emphasis on molecular target-based preventive agents such as erlotinib, tamoxifen and finasteride (44-46). The present study suggests that predicting ODC inhibitors by computer modeling is a useful tool for identifying potential inhibitors. Additionally, computer modeling results of the predicted binding site between herbacetin and ODC showed that herbacetin interacts with Asp44, Asp243, and Glu384 on the ODC backbone and the Asp44 residue appears most important for the inhibitory effect (Fig. 1 and 2). Our in vitro results indicated that the herbacetin interaction with the Asp44 residue appears most important for the inhibition of ODC activity. Furthermore, we determined whether the hydroxyl (OH) residues of herbacetin were involved in its binding to ODC. We docked 4 different flavonols, including herbacetin, luteolin, 7,3’,4’-trihydroxyisoflavone, and kaempferol, in silico to a selected pocket in the 1NJJ (ODC) protein structure and also performed an in vitro ODC activity assay. Herbacetin docked nicely to the ODC allosteric site at residue Asp44 (1.64 Å) as well as strongly inhibiting ODC activity. However, the other compounds could not bind to ODC at Asp44 and only had a weak inhibitory effect on ODC activity (data not shown). Interestingly, herbacetin has little effect on other kinases’ activity suggesting that herbacetin is a specific ODC inhibitor (Supplemental Fig. 2A-M). Therefore, the present findings suggested that herbacetin appears to be relatively specific for ODC rather than other protein targets. Previous studies reported that DFMO inhibited the number of polyps in the middle and distal portions of the small intestine but did not affect polyp size (25). Additionally, oral administration of DFMO (p.o: 500-2000 mg/kg) was shown to strongly inhibit several types of tumor growth in vivo (47-49). However, high doses of DFMO induced cytotoxicity as evidenced by weight loss in several in vivo models (50-52). Therefore, to study potential anticancer effects and examine the possible toxicity of herbacetin, we performed an in vivo study in APCMin+ mice treated with herbacetin (i.p. 0.4, 2, 10 or 20 mg/kg B.W.). Results showed that the number and size of polyps was decreased by herbacetin (Fig. 6A-D) with no overt toxicity. The effects were associated with decreased polyamine content (Fig. 6E-G). In another in vivo study, xenograft tumor growth was also decreased by herbacetin (0.4 or 2 mg/kg B.W.) or DFMO (200 mg/kg B.W.) administered i.p., suggesting that herbacetin is more effective than DFMO. Notably herbacetin administered orally (100 mg/kg B.W.) was equally as effective as twice the dose of DFMO administered i.p. (Fig. 6H,J). Importantly, assessment of PPI as an indicator of auditory function suggested that, unlike DFMO, herbacetin was not associated with ototoxicity.
A combination of DFMO and non-steroidal anti-inflammatory drugs (NSAIDs), sulindac and celecoxib, was shown to exhibit potent inhibitory effects (53, 54). Therefore, further studies are needed to investigate the effectiveness of combining herbacetin with NSAIDs in colon and skin cancers, and to further characterize herbacetin and perform pharmacokinetics and pharmacodynamics studies as well as to elucidate toxicological responses. Overall, the effectiveness of herbacetin as a preventive agent seems to suggest its use as a promising lead compound in the future. The results of this study might be highly significant in that herbacetin is a natural, nontoxic compound that could be combined with (e.g.,) an NSAID, such as sulindac, for an immediate clinical trial to test its effectiveness against colon cancer.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by The Hormel Foundation and National Institutes of Health grants CA120388, R37 CA081064, ES016548 and the Research Funds, M-2011-B0002-00033 and M-2011-B0002-00025 of The Catholic University of Korea.
Footnotes
AUTHOR CONTRIBUTION
D.J.K., M-H.L. and N.O. designed and performed the in vitro and in vivo experiments and prepared the manuscript; E.R., D.Y.L., M.O.K., Y-Y.C., J.W.J., H.E.K. and E.J.C. assisted with the experiments in the in vivo APCmin+ and xenograft mouse model, A.P., H.C. and K.R. performed the computer modeling; J-H.S., J.E.K., and S.Y.L. assisted with the cell based assays; S.C.K., S.P., S-H.K. and Y.I.Y. provided data analysis; A.M.B. supervised the in vivo experimental design and manuscript editing; Z.D. provided the idea, supervised the overall experimental design and data analysis.
Competing Financial Interest Statement: None of the authors have any competing interests.
REFERENCES
- 1.Pegg AE, Feith DJ, Fong LY, Coleman CS, O’Brien TG, Shantz LM. Transgenic mouse models for studies of the role of polyamines in normal, hypertrophic and neoplastic growth. Biochem Soc Trans. 2003;31:356–60. doi: 10.1042/bst0310356. [DOI] [PubMed] [Google Scholar]
- 2.Oredsson SM. Polyamine dependence of normal cell-cycle progression. Biochem Soc Trans. 2003;31:366–70. doi: 10.1042/bst0310366. [DOI] [PubMed] [Google Scholar]
- 3.Childs AC, Mehta DJ, Gerner EW. Polyamine-dependent gene expression. Cell Mol Life Sci. 2003;60:1394–406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feith DJ, Bol DK, Carboni JM, Lynch MJ, Sass-Kuhn S, Shoop PL, et al. Induction of ornithine decarboxylase activity is a necessary step for mitogen-activated protein kinase kinase-induced skin tumorigenesis. Cancer Res. 2005;65:572–8. [PubMed] [Google Scholar]
- 5.Hayes CS, DeFeo K, Lan L, Paul B, Sell C, Gilmour SK. Elevated levels of ornithine decarboxylase cooperate with Raf/ERK activation to convert normal keratinocytes into invasive malignant cells. Oncogene. 2006;25:1543–53. doi: 10.1038/sj.onc.1209198. [DOI] [PubMed] [Google Scholar]
- 6.Tseng CP, Verma AK. Lack of 12-O-tetradecanoylphorbol-13-acetate responsiveness of ornithine decarboxylase introns which have AP-1 consensus sequences. Mol Cell Biochem. 1995;146:7–12. doi: 10.1007/BF00926875. [DOI] [PubMed] [Google Scholar]
- 7.Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, Casero RA., Jr. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57:199–201. [PubMed] [Google Scholar]
- 8.Holtta E, Sistonen L, Alitalo K. The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem. 1988;263:4500–7. [PubMed] [Google Scholar]
- 9.Shantz LM. Transcriptional and translational control of ornithine decarboxylase during Ras transformation. Biochem J. 2004;377:257–64. doi: 10.1042/BJ20030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachrach U, Wang YC, Tabib A. Polyamines: new cues in cellular signal transduction. News Physiol Sci. 2001;16:106–9. doi: 10.1152/physiologyonline.2001.16.3.106. [DOI] [PubMed] [Google Scholar]
- 11.Gerner EW, Meyskens FL., Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–92. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 12.Gerhauser C, Mar W, Lee SK, Suh N, Luo Y, Kosmeder J, et al. Rotenoids mediate potent cancer chemopreventive activity through transcriptional regulation of ornithine decarboxylase. Nat Med. 1995;1:260–6. doi: 10.1038/nm0395-260. [DOI] [PubMed] [Google Scholar]
- 13.Gastaut JA, Tell G, Schechter PJ, Maraninchi D, Mascret B, Carcassonne Y. Treatment of acute myeloid leukemia and blastic phase of chronic myeloid leukemia with combined eflornithine (alpha difluoromethylornithine) and methylglyoxal-bis-guanyl hydrazone (methyl-GAG) Cancer Chemother Pharmacol. 1987;20:344–8. doi: 10.1007/BF00262590. [DOI] [PubMed] [Google Scholar]
- 14.Levin VA, Hess KR, Choucair A, Flynn PJ, Jaeckle KA, Kyritsis AP, et al. Phase III randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–90. [PubMed] [Google Scholar]
- 15.Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, et al. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2003;9:5922–8. [PubMed] [Google Scholar]
- 16.Wilding G, King D, Tutsch K, Pomplun M, Feierabend C, Alberti D, et al. Phase I trial of the polyamine analog N1,N14-diethylhomospermine (DEHSPM) in patients with advanced solid tumors. Invest New Drugs. 2004;22:131–8. doi: 10.1023/B:DRUG.0000011789.79368.ae. [DOI] [PubMed] [Google Scholar]
- 17.Ganju V, Edmonson JH, Buckner JC. Phase I study of combined alpha interferon, alpha difluoromethylornithine (DFMO), and doxorubicin in advanced malignancy. Invest New Drugs. 1994;12:25–7. doi: 10.1007/BF00873231. [DOI] [PubMed] [Google Scholar]
- 18.Horn Y, Schechter PJ, Marton LJ. Phase I-II clinical trial with alpha-difluoromethylornithine--an inhibitor of polyamine biosynthesis. Eur J Cancer Clin Oncol. 1987;23:1103–7. doi: 10.1016/0277-5379(87)90141-6. [DOI] [PubMed] [Google Scholar]
- 19.Lao CD, Backoff P, Shotland LI, McCarty D, Eaton T, Ondrey FG, et al. Irreversible ototoxicity associated with difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 2004;13:1250–2. [PubMed] [Google Scholar]
- 20.Prakash NJ, Schechter PJ, Mamont PS, Grove J, Koch-Weser J, Sjoerdsma A. Inhibition of EMT6 tumor growth by interference with polyamine biosynthesis; effects of alpha-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase. Life Sci. 1980;26:181–94. doi: 10.1016/0024-3205(80)90292-1. [DOI] [PubMed] [Google Scholar]
- 21.Lan L, Trempus C, Gilmour SK. Inhibition of ornithine decarboxylase (ODC) decreases tumor vascularization and reverses spontaneous tumors in ODC/Ras transgenic mice. Cancer Res. 2000;60:5696–703. [PubMed] [Google Scholar]
- 22.Wheeler DL, Ness KJ, Oberley TD, Verma AK. Inhibition of the development of metastatic squamous cell carcinoma in protein kinase C epsilon transgenic mice by alpha-difluoromethylornithine accompanied by marked hair follicle degeneration and hair loss. Cancer Res. 2003;63:3037–42. [PubMed] [Google Scholar]
- 23.Alhonen-Hongisto L, Levin VA, Marton LJ. Modification of uptake and antiproliferative effect of methylglyoxal bis(guanylhydrazone) by treatment with alpha-difluoromethylornithine in rodent cell lines with different sensitivities to methylglyoxal bis(guanylhydrazone) Cancer Res. 1985;45:509–14. [PubMed] [Google Scholar]
- 24.Meyskens FL, Jr., Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–51. [PubMed] [Google Scholar]
- 25.Jacoby RF, Cole CE, Tutsch K, Newton MA, Kelloff G, Hawk ET, et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60:1864–70. [PubMed] [Google Scholar]
- 26.Rao CV, Tokumo K, Rigotty J, Zang E, Kelloff G, Reddy BS. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, alpha-difluoromethylornithine, 16 alpha-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 1991;51:4528–34. [PubMed] [Google Scholar]
- 27.Struijs K, Vincken JP, Doeswijk TG, Voragen AG, Gruppen H. The chain length of lignan macromolecule from flaxseed hulls is determined by the incorporation of coumaric acid glucosides and ferulic acid glucosides. Phytochemistry. 2009;70:262–9. doi: 10.1016/j.phytochem.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer. 2011;11:211–8. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 29.Choe KI, Kwon JH, Park KH, Oh MH, Kim MH, Kim HH, et al. The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. BOR. Molecules. 2012;17:11484–94. doi: 10.3390/molecules171011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao Y, Xiang Q, Yuan L, Xu L, Liu Z, Liu X. Herbacetin induces apoptosis in HepG2 cells: Involvements of ROS and PI3K/Akt pathway. Food Chem Toxicol. 2013;51:426–33. doi: 10.1016/j.fct.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 31.Hyuga S, Hyuga M, Yoshimura M, Amakura Y, Goda Y, Hanawa T. Herbacetin, a constituent of ephedrae herba, suppresses the HGF-induced motility of human breast cancer MDA-MB-231 cells by inhibiting c-Met and Akt phosphorylation. Planta Med. 2013;79:1525–30. doi: 10.1055/s-0033-1350899. [DOI] [PubMed] [Google Scholar]
- 32.Osterman A, Grishin NV, Kinch LN, Phillips MA. Formation of functional cross-species heterodimers of ornithine decarboxylase. Biochemistry. 1994;33:13662–7. doi: 10.1021/bi00250a016. [DOI] [PubMed] [Google Scholar]
- 33.Kim DJ, Reddy K, Kim MO, Li Y, Nadas J, Cho YY, et al. (3-Chloroacetyl)-indole, a novel allosteric AKT inhibitor, suppresses colon cancer growth in vitro and in vivo. Cancer Prev Res (Phila) 2011;4:1842–51. doi: 10.1158/1940-6207.CAPR-11-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marce M, Brown DS, Capell T, Figueras X, Tiburcio AF. Rapid high-performance liquid chromatographic method for the quantitation of polyamines as their dansyl derivatives: application to plant and animal tissues. J Chromatogr B Biomed Appl. 1995;666:329–35. doi: 10.1016/0378-4347(94)00586-t. [DOI] [PubMed] [Google Scholar]
- 35.Morgan DM. Determination of polyamines as their benzoylated derivatives by HPLC. Methods Mol Biol. 1998;79:111–8. doi: 10.1385/0-89603-448-8:111. [DOI] [PubMed] [Google Scholar]
- 36.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, et al. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55:351–67. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 38.Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, Uchimaya M. Epik: a software program for pK( a ) prediction and protonation state generation for drug-like molecules. J Comput Aided Mol Des. 2007;21:681–91. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- 39.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 40.Bode AM, Dong Z. Cancer prevention research - then and now. Nature Reviews: Cancer. 2009;9:508–16. doi: 10.1038/nrc2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers DP, Jackson LK, Ipe VG, Murphy GE, Phillips MA. Long-range interactions in the dimer interface of ornithine decarboxylase are important for enzyme function. Biochemistry. 2001;40:13230–6. doi: 10.1021/bi0155908. [DOI] [PubMed] [Google Scholar]
- 42.Jackson LK, Goldsmith EJ, Phillips MA. X-ray structure determination of Trypanosoma brucei ornithine decarboxylase bound to D-ornithine and to G418: insights into substrate binding and ODC conformational flexibility. J Biol Chem. 2003;278:22037–43. doi: 10.1074/jbc.M300188200. [DOI] [PubMed] [Google Scholar]
- 43.Anderson AC. The process of structure-based drug design. Chem Biol. 2003;10:787–97. doi: 10.1016/j.chembiol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 45.Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA, Jr., Thompson IM. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev Res (Phila) 2008;1:174–81. doi: 10.1158/1940-6207.CAPR-08-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer Metastasis Rev. 2008;27:495–522. doi: 10.1007/s10555-008-9134-y. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto S, Igarashi K, Shrestha RD, Miyazaki M, Okui K. Antitumor effects of two polyamine antimetabolites combined with mitomycin C on human stomach cancer cells xenotransplanted into nude mice. Int J Cancer. 1985;35:821–5. doi: 10.1002/ijc.2910350620. [DOI] [PubMed] [Google Scholar]
- 48.Grossie VB, Jr., Ota DM, Ajani JA, Nishioka K. Amelioration of thrombocytopenia with concomitant ornithine in sarcoma-bearing rats receiving high dose difluoromethylornithine. Invest New Drugs. 1991;9:321–6. doi: 10.1007/BF00183572. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Schut HA, Conran P, Kramer PM, Lubet RA, Steele VE, et al. Prevention by aspirin and its combination with alpha-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis. 1999;20:425–30. doi: 10.1093/carcin/20.3.425. [DOI] [PubMed] [Google Scholar]
- 50.Kingsnorth AN, McCann PP, Diekema KA, Ross JS, Malt RA. Effects of alpha-difluoromethylornithine on the growth of experimental Wilms’ tumor and renal adenocarcinoma. Cancer Res. 1983;43:4031–4. [PubMed] [Google Scholar]
- 51.Kirchner DL, Mercieca MD, Crowell JA, Levine BS. Developmental toxicity studies of 2-(difluoromethyl)-dl-ornithine (DFMO) in rats and rabbits. Toxicol Sci. 1999;50:127–35. doi: 10.1093/toxsci/50.1.127. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Levic S, Gratton MA, Doyle KJ, Yamoah EN, Pegg AE. Spermine synthase deficiency leads to deafness and a profound sensitivity to alpha-difluoromethylornithine. J Biol Chem. 2009;284:930–7. doi: 10.1074/jbc.M807758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyskens FL, Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ignatenko NA, Besselsen DG, Stringer DE, Blohm-Mangone KA, Cui H, Gerner EW. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr Cancer. 2008;60(Suppl 1):30–5. doi: 10.1080/01635580802401317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.