Abstract
Background
Gene expression profiling (GEP) suggests there are three subtypes of muscle-invasive urothelial cancer (UC): basal, which has the worst prognosis; p53-like; and luminal. We hypothesized that GEP of transurethral resection (TUR) and cystectomy specimens would predict subtypes that could benefit from chemotherapy.
Objective
To explore clinical outcomes for patients treated with dose-dense (DD) methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) and bevacizumab (B) and the impact of UC subtype.
Design, setting, and participants
Sixty patients enrolled in a neoadjuvant trial of four cycles of DDMVAC + B between 2007 and 2010. TUR and cystectomy specimens for GEP were available from 38 and 23 patients, respectively, and from an additional confirmation cohort of 49 patients treated with perioperative MVAC.
Outcome measurements and statistical analysis
Relationships with outcomes were analyzed using multivariable Cox regression and log-rank tests.
Results and limitations
Chemotherapy was active, with pT0N0 and ≤pT1N0 downstaging rates of 38% and 53%, respectively, and 5-yr overall survival (OS) of 63%. Bevacizumab had no appreciable impact on outcomes. Basal tumors had improved survival compared to luminal and p53-like tumors (5-yr OS 91%, 73%, and 36%, log-rank p = 0.015), with similar findings on multivariate analysis. Bone metastases within 2 yr were exclusively associated with the p53-like subtype (p53-like 100%, luminal 0%, basal 0%; p ≤ 0.001). Tumors enriched with the p53-like subtype at cystectomy suggested chemoresistance for this subtype. A separate cohort treated with perioperative MVAC confirmed the UC subtype survival benefit (5-yr OS 77% for basal, 56% for luminal, and 56% for p53-like; p = 0.021). Limitations include the small number of pretreatment specimens with sufficient tissue for GEP.
Conclusion
GEP was predictive of clinical UC outcomes. The basal subtype was associated with better survival, and the p53-like subtype was associated with bone metastases and chemoresistant disease.
Patient summary
We can no longer think of urothelial cancer as a single disease. Gene expression profiling identifies subtypes of urothelial cancer that differ in their natural history and sensitivity to chemotherapy.
Keywords: Urothelial cancer; Gene expression profiling; Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin; Neoadjuvant; Bevacizumab; Basal; Luminal; p53; Subtype
1. Introduction
Neoadjuvant chemotherapy remains underutilized for the treatment of muscle-invasive urothelial carcinoma [1,2]. Potential reasons include toxicity, with many elderly patients considered unfit for aggressive cisplatin-based regimens, and a perceived lack of benefit [3,4]. Although neoadjuvant chemotherapy improves survival, more than 40% of patients will ultimately experience relapse and die despite aggressive therapy. Strategies that improve the efficacy or toxicity profile or the selection of patients most likely to benefit from therapy would have a substantial impact in this disease.
In an attempt to improve on the efficacy and toxicity profile of chemotherapy, we added bevacizumab to dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (DDMVAC). Overexpression of the vascular endothelial growth factor receptor (VEGFR) is associated with significantly worse outcomes following neoadjuvant chemotherapy [5,6]. In metastatic disease, DDMVAC improved the complete response rate and toxicity profile compared to traditional MVAC, with double the number of long-term survivors seen in the dose-dense arm [7].
Efforts to improve patient selection have relied on clinical parameters associated with adverse prognosis. Pathologic upstaging has been reported for cases with a three-dimensional mass on examination under anesthesia (EUA), lymphovascular invasion (LVI), or hydronephrosis [8,9], with long-term cause-specific survival exceeding 60% for perioperative cisplatin-based [10] or ifosfamide-based therapy [11]. However, these factors remain difficult to translate into common practice outside a dedicated multidisciplinary setting. Development of a predictive laboratory-based assay could overcome these limitations.
Gene expression profiling (GEP) has recently revealed the presence of distinct intrinsic basal and luminal subtypes of bladder cancer [12–14], with the basal subtype having the worst prognosis in muscle-invasive disease [12,13]. Therefore, we applied this strategy in the context of a homogeneous group of patients treated during a clinical trial.
2. Patients and methods
Between August 2007 and December 2010, 60 patients consented to participate in this institutional review board-approved trial. Transitional cell histology was the predominant component. Micropapillary tumors were allowed, irrespective of the extent of their micropapillary features. Patients had at least one of the following high-risk features: a three-dimensional mass on EUA, direct invasion of the prostatic stroma or vaginal wall, LVI, hydronephrosis, or tumor involving a bladder diverticulum. Upper-tract urothelial cancers (UTUCs) were included if there was high-grade tumor or a measureable mass on imaging. Patients had a Zubrod performance status ≤2, or ≥3 if related to the cancer, an absolute neutrophil count ≥1800/μl, a platelet count ≥150 000/μl, and creatinine clearance, either measured or calculated using the Cockcroft-Gault formula, of ≥50 ml/min. We excluded surgically unresectable disease (cT4b, N+, or M+). Patients with bone pain must have had a negative bone scan. Patients with a history of hypertensive crisis, hypertensive encephalopathy, unstable angina, or myocardial infarction within 6 mo of enrollment or a history of transient ischemic attacks were ineligible.
Treatment consisted of four cycles of neoadjuvant chemotherapy with DDMVAC and bevacizumab every 2 wk with growth factor support. Bevacizumab (10 mg/kg) was given first, followed by methotrexate (30 mg/m2), doxorubicin (30 mg/m2), vinblastine (3 mg/m2), and cisplatin (70mg/m2). Patients received 3 l of post-cisplatin hydration, typically incorporating mannitol. Surgery was performed a minimum of 6 wk after the last dose of bevacizumab.
2.1. GEP
Whole-genome mRNA expression profiling and one nearest-neighbor (1NN) subtype assignments were performed as previously described [12,15]. Total RNA was isolated from macrodissected tumor in formalin-fixed, paraffin-embedded unstained sections using a High Pure miRNA isolation kit (Roche, Indianapolis, IN, USA). GEP was performed using the DASL platform (WG-DASL HT12 V4 chips; Illumina, San Diego, CA, USA). Slides were scanned using a Bead Station 500X system and signal intensities were quantified using GenomeStudio (Illumina). Quantile normalization in the linear models for microarray data (LIMMA) package in the R language environment (R Project for Statistical Computing, Vienna, Austria) was used to normalize the data, and the data were combined with a previously subtyped data set (MD Anderson validation cohort, GSE48276, sample ID GSM1173901-GSM1173957). TUR only and TUR + cystectomy tumors were assigned to subtypes using a 1NN classifier [12]. Analyses were performed using BRB-Array Tools version 4.3.2 by Dr. Richard Simon and Amy Peng Lam. A confirmation cohort was obtained using untreated tissue specimens from a previously published trial of perioperative MVAC [10]. The new GEP data from this study were uploaded to Gene Expression Omnibus with accession numbers GSE69795 (DDMVAC + bevacizumab cohort) and GSE70691 (MVAC confirmation cohort).
2.2. Clinical trial design
The primary endpoint was pathologic downstaging to ≤pT1N0 after neoadjuvant chemotherapy. A response rate of 50% was considered worthy of further investigation; standard therapies achieve a response rate of approximately 35%. With type I and type II error rates of 0.1 and 0.2, respectively, we needed a maximum number of 58 patients. If there were at least eight responses among these first 20 patients, then 38 additional patients would be enrolled. If at least 25 responses were observed from all 58 patients, the treatment would be considered for further investigation. To allow for two unevaluable patients, 60 patients were enrolled.
2.3. Statistics
Summary statistics including frequencies and percentages are reported for categorical variables. Patient risk factors were compared using Fisher’s exact test for categorical variables and a two-sample Wilcoxon rank-sum test for continuous variables. A binary variable was defined for downstaging (pathologic stage below the initial clinical stage and below pT1N0M0). A logistic regression model was used to associate baseline risk factors including tumor subtype with downstaging at surgery. Overall survival (OS), defined as the time from study enrollment to death, and disease-specific survival (DSS), defined as the time to disease-specific death, were estimated using the Kaplan-Meier method. Survival distributions by group were compared using the log-rank statistic. Multivariable Cox proportional hazards models were used to evaluate the effect of subtype before surgery after adjusting for other risk factors. The final multivariable model (both Cox proportional hazards model and logistic regression model) was obtained using a backward selection approach, removing the least significant covariate from the full model one at a time. The Cox proportional hazards assumption was checked for each variable, and no model violation was detected in the final model. All tests were two-sided and p < 0.05 was considered statistically significant. Analyses were conducted using R v.64 (R Project for Statistical Computing) and S+ v.8.0 (TIBCO Software, Somerville, MA, USA).
3. Results
3.1. Patient characteristics
Baseline characteristics are listed in Table 1. Patients with T1 or T2 urothelial cancer had a high-risk factor present to be eligible. All patients with UTUC had high-grade disease.
Table 1.
Patient characteristics
| Parameter | Value |
|---|---|
| Median age at registration, yr (range) | 64 (42–79.6) |
| Gender, n (%) | |
| Female | 20 (33) |
| Male | 40 (67) |
| Race, n (%) | |
| Caucasian | 55 (92) |
| African American | 2 (3) |
| Hispanic | 3 (5) |
| Histology, n (%) | |
| UC/TCC | 48 (80) |
| UC/TCC mixed with variant histology | 12 (20) |
| Micropapillary | 8 (13) |
| Primary site, n (%) | |
| Renal pelvis/ureter a | 16 (27) |
| Bladder/urethra | 44 (73) |
| c Stage (bladder, n = 44), n (%) | |
| T1N0M0 b | 4 (9) |
| T2N0M0 b | 13 (30) |
| T3–4a N0M0 | 27 (61) |
| High-risk feature, n (%) c | |
| Lymphovascular invasion | 23 (38) |
| Hydronephrosis | 20 (33) |
| Diverticula | 1 (2) |
| High-grade upper tract tumor | 16 (27) |
| Three-dimensional mass on examination under anesthesia | 27 (45) |
All patients with upper tract disease had high-grade disease and were N0M0. Since resection to the muscularis is not possible owing to the inherent risk of perforation in upper tract disease, c stage is not available in these patients.
All patients with T1 or T2 disease had a high risk factor to be eligible for the trial. Of the patients with cT1 high-risk disease, two were downstaged to pTisN0 and two were upstaged to pT3 disease.
Patients may have had more than one high-risk feature, so numbers exceed the total number of patients.
3.2. Response and survival
Pathologic downstaging to ≤T1N0 occurred in 53% of patients, with a marginally significant increase in downstaging in UTUC (75% vs 45% in bladder urethral, Fisher’s exact test p = 0.077.) Pathologic T0N0 rates were similar across tumor sites with a 38% pT0N0 rate overall and 39% and 38%, respectively, in bladder/urethral tumors and UTUC.
In univariate analyses, younger patients were more likely to respond to neoadjuvant treatment (median age 61 yr for responders and 66 yr for nonresponders; Wilcoxon test p = 0.02).
Subtype at TUR, LVI, PS, and clinical stage were not significantly associated with the response rate. In the multivariate logistic model, younger patients and those with UTUC were more likely to be downstaged (OR= 0.92 when age increasing 1 yr with 95% CI: 0.85–0.99, p=0.03; OR=3.87, for UTUC against bladder/urethral tumors with 95% CI: 1.0, 15.0, p=0.05).
Survival for all patients was quite good, with 5-yr OS and DSS of 63% and 64%, respectively (median follow-up 49 mo; Fig. 1A). The 5-yr OS and DSS were both 72% for in UTUC, and 60% and 61%, respectively, for bladder/urethra disease (Fig. 1B). Downstaging to ≤pT1N0 significantly impacted survival (OS and DSS of 93% vs 29% at 5 yr; log-rank p ≤ 0.0001, Fig. 1C). Those with pathologic pT4b, N+, or M+ disease had poor survival, with median OS of 17 mo. Patients with micropapillary histology had 5-yr OS and DSS of 50% and 63%, respectively.
Fig. 1.
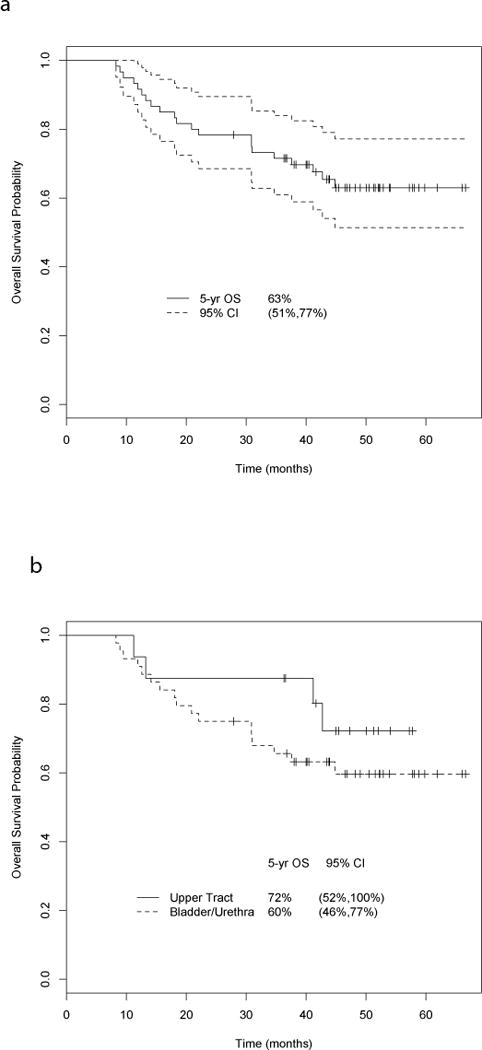
(A) Overall survival (OS) for all patients. The 5-yr OS and disease-specific survival (DSS) were 63% and 64%, respectively, for all patients treated with neoadjuvant chemotherapy with dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (DDMVAC) + bevacizumab (median follow-up 49 mo). (B) OS for patients with urothelial carcinoma of the bladder and upper tract. The 5-yr OS and DSS were both 72% in upper tract disease, and 60% and 61%, respectively, in bladder/urethral cancer. (C) OS by pathologic stage. Downstaging to ≤pT1N0 had a significant impact on 5-yr survival (OS and DSS 93%) compared to those who were not downstaged (OS and DSS 29%; log-rank p ≤ 0.0001). CI = confidence interval.
3.3. Toxicity
All 60 patients completed at least one cycle of neoadjuvant chemotherapy; 51 patients (85%) completed all four cycles before surgery and 56 patients (93%) completed at least three cycles of chemotherapy. In total, 224 cycles of neoadjuvant chemotherapy were administered. Chemotherapy was well tolerated, with 27% of patients experiencing neutropenia/neutropenic fever (Table 2). There were very few episodes of thrombosis and pulmonary embolism associated with this combination (5%). One patient with an extensive cardiac history experienced cardiac ischemia.
Table 2.
Chemotherapy-related toxicity
| Patients, n (%)
|
||
|---|---|---|
| Grade 3 | Grade 4 | |
| Neutropenia/neutropenic fever | 10 (17) | 6 (10) |
| Fatigue | 7 (12) | |
| Hypertension | 4 (7) | |
| Anemia | 4 (7) | |
| Thrombosis/pulmonary embolism | 3 (5) | |
| Nausea/vomiting | 3(5) | |
| Mucositis | 2 (3) | |
| Thrombocytopenia | 2 (3) | |
| Syncope | 2 (3) | |
| Hyponatremia | 1 (2) | |
| Hypotension | 1 (2) | |
| Transaminitis | 1 (2) | |
| Cardiac ischemia | 1 (2) | |
3.4. Surgery
Fifty-nine of the 60 patients had surgery. One patient was diagnosed with a primary lung cancer and did not undergo cystectomy, and is included as a treatment failure. Surgery-related toxicity data are listed in Table 3. One patient experienced an abdominal abscess associated with colitis and was discharged 44 d after surgery.
Table 3.
Surgery-related toxicity for cystectomy, nephrectomy, and nephroureterectomy
| Bladder/urethra (n = 43) | Renal pelvis/ureter (n = 16) | |
|---|---|---|
| Median hospital stay, d (range) | 9 (5–20) | 4 (3–44) |
| Median PRBC units, n (range) | 3 (0–12) | 0 (0–4) |
| Discharge >11 d, n (%) | 10 (23) | 2 (13) |
| Ileus/total parenteral nutrition | 7 (17) | 2 (13) |
| Abdominal abscess | 1 (2) | 1 (6) |
| Jugular vein thrombus | 1 (2) | 0 (0) |
| Incision infection | 1 (2) | 0 (0) |
| Atrial fibrillation a | 1 (2) | 0 (0) |
| Deconditioning | 1 (2) | 0 (0) |
| Abdominal fluid collection | 1 (2) | 0 (0) |
PRBC = packed red blood cell.
Patient had a history of atrial fibrillation with procedures.
3.5. GEP
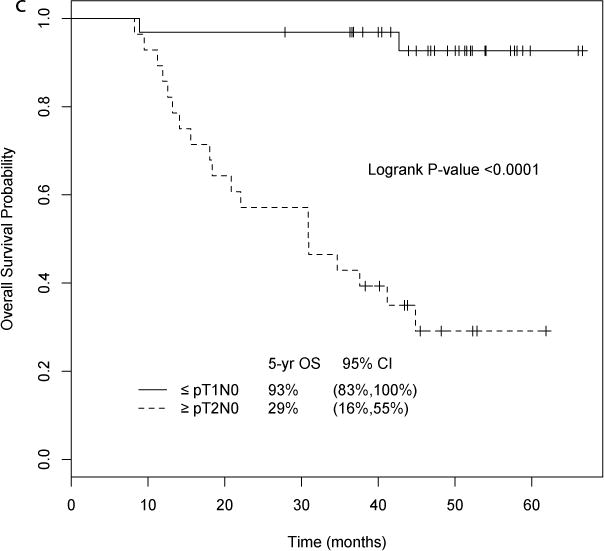
TUR and matched cystectomy specimens were available for GEP in 38 and 23 patients, respectively. Tumor subtype assignments were made using a 1NN classifier on TUR tissue (n = 38) and yielded basal (n = 11), luminal (n = 11), and p53-like proportions (n = 16) that were similar to those described previously [12]. UTUC was more likely to be of the luminal subtype (basal 0, luminal 4, p53 1). Pathologic stage by subtype is shown in Figure 2B. Strikingly, in the neoadjuvant chemotherapy setting, patients with basal tumors had better survival compared to the luminal and p53-like subtypes (5-yr OS 91%, 73%, and 36%, respectively; log-rank p = 0.015, Fig. 2C). In multivariate analysis adjusted for age at registration and LVI, the basal subtype was associated with longer overall survival compared to the p53-like (hazard ratio [HR] 14.6, 95% CI 1.78–120.3; p = 0.013) and luminal subtypes (HR 6.87, 95% CI 0.64–72.9; p = 0.11, Supplementary Table 1). Bone metastases within 2 yr, which are associated with very poor prognosis (median OS 15 mo), only occurred in the p53-like subtype (9/16, 56%), with none in the luminal and basal subtypes (p ≤ 0.001, Fisher’s exact test; Fig. 2D).
Fig. 2.
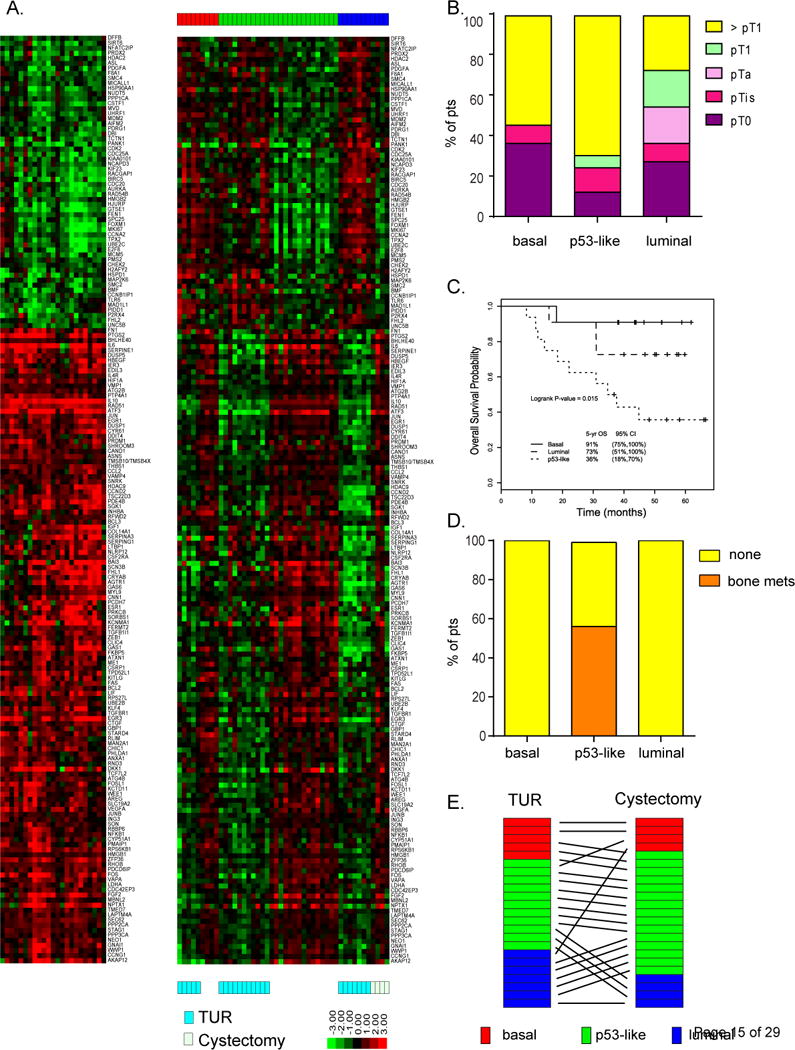
(A) Gene expression profiling in matched transurethral resection (TUR) and cystectomy specimens. Left: Chemotherapy induced the active p53 gene signature (log ratio cystectomy/TUR for matched tumors). Right: relative expression of the p53 gene signature in each pre- and post-treatment tumor arranged according to subtype membership. To visualize gene expression patterns, specific gene expression values adjusted to a median of zero were used. (B) Pathologic stage by subtype. (C) Overall survival (OS) by subtype. The basal subtype was associated with better survival after neoadjuvant chemotherapy compared to the p53-like and luminal subtypes (5-yr OS 91%, 73%, and 36%, respectively; p = 0.015). (D) Bone metastases (mets) by subtype. Bone metastases only occurred in the p53-like subtype (53%), with none in the basal and luminal subtypes. (E) Gene expression profile comparing TUR to cystectomy. Post-treatment tumors were enriched with an active p53 gene expression signature that was distinct from the p-53 like subtype at TUR. Owing to quantile normalization, the subtype membership of a few TUR tumors was changed when we combined TUR and cystectomy. CI = confidence interval.
Tumor subtypes were then compared for combined TUR (n = 38) and cystectomy (n = 23) tissues. Subtype at cystectomy did not correlate with survival outcome. However, there was a shift to the p53-like subtype at cystectomy (basal to p53-like, 60%; luminal to p53-like, 71%; Fig. 2E). All the post-treatment tumors were enriched in an active p53 GEP signature that was distinct from the p53 signature that dictated membership in the p53-like subtype (Fig. 2A, Supplementary Table 2).
3.6. Confirmation cohort
GEP was performed for a separate cohort of 49 patients treated with perioperative MVAC in a previously published clinical trial (Supplementary Table 3) [10]. A survival advantage for basal (5-yr OS 77%, 95% CI 59–100%) compared to luminal (5-yr OS 56%, 95% CI 37–87%) and p53-like tumors (5-yr OS 56%, 95% CI 37–87%) was observed in this separate cohort (p = 0.021, log-rank test; Fig. 3). The HIF1 gene signature was enriched in the basal subtype in our previously described cohort of fresh frozen tissue (p = 4.92 × 10−7, Z-score = 3.606 for ingenuity pathway analysis of upstream regulators) [12], but the enrichment in the current cohort did not reach statistical significance, probably because of the modest sample size (Supplementary Fig. 1).
Fig. 3.
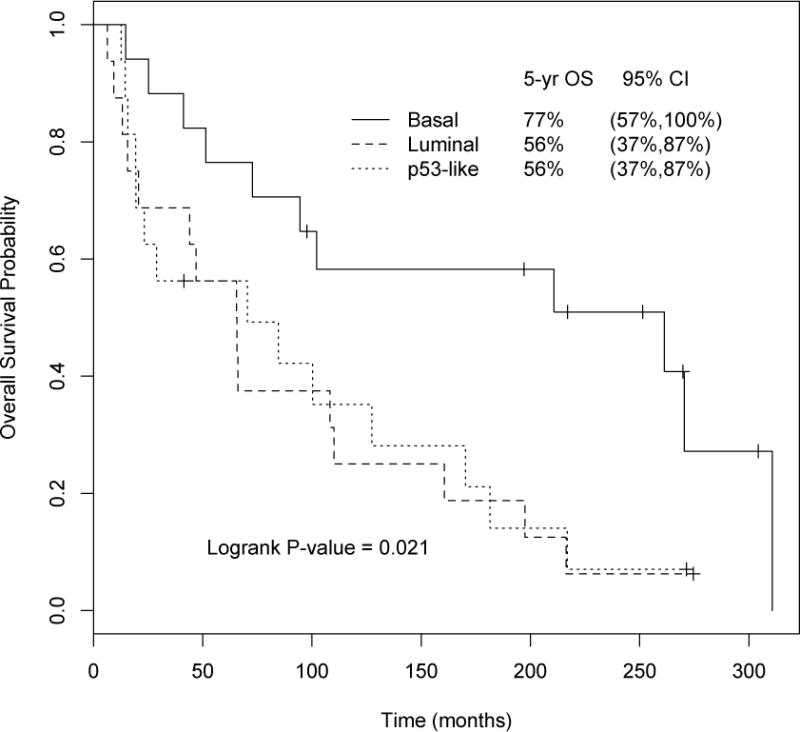
Survival in the confirmatory data set by subtype. Gene expression profiling of pretreatment tissue from patients treated in a clinical trial of perioperative methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) confirmed the survival advantage seen in the basal subtype. After chemotherapy the 5-yr overall survival (95% confidence interval) was 77% (59–100%) for the basal, 56% (37–87%) for the luminal, and 56% (37–87%) for the p53-like subtype (p = 0.021, log-rank test). OS = overall survival; CI = confidence interval.
4. Discussion
We applied a GEP prognostic for survival in muscle-invasive UC to UC patients treated with neoadjuvant chemotherapy. In contrast to the negative prognostic impact of the basal subtype identified previously [12–14,16,17], the basal subtype predicted longer survival and the p53-like subtype shorter survival with a higher frequency of bone metastases. This survival benefit was confirmed in a separate group treated with perioperative MVAC. These findings suggest that the prognostic signature in chemotherapy-naïve UC patients is predictive of outcome after chemotherapy.
Although addition of bevacizumab did meet prespecified clinical criteria for activity in the trial, the downstaging rates are similar to those observed in other recent neoadjuvant trials [11,18,19]. In theory, the basal subtype, which also contains the Hif-1α/angiogenesis signature [12], should be the group most likely to benefit from an angiogenesis inhibitor. Although basal tumors treated with bevacizumab had 5-yr OS of 91% compared to 77% for those treated with perioperative MVAC alone, we were unable to confirm any definitive statistical benefit in this modest sample, which limits the impact of our findings. It would be worth exploring the impact of bevacizumab on the basal subtype in larger clinical trials. We believe that the apparent effects on basal UC reflect the well-described clinical activity of DDMVAC [7,18,19], which altered the natural course of this aggressive subtype. It is likely that these same aggressive features (especially rapid proliferation) make the basal subtype the most susceptible to chemotherapy. The modest sample size may account for our inability to confirm an association between downstaging and the basal subtype. The poor outcomes seen previously for the basal subtype may reflect the heterogeneous sample size, including patients who are not candidates for aggressive chemotherapy [12,13]. Likewise, squamous features, which have been associated with the basal subtype [16], have been linked to benefit from neoadjuvant chemotherapy [20].
Bone metastasis only occurred in the p53-like subtype, which is enriched with extracellular matrix biomarkers and cancer-associated fibroblasts [12]. It is possible that this subtype recapitulates a tumor-stromal environment that is similar to bone marrow, making marrow an attractive landing site for p53-like metastases. The p53-like tumors have lower proliferation and an upregulated p53-associated gene signature, which has been associated with lower response to chemotherapy in patients and UC cell lines [12]. Therefore, it is possible that the bone metastases seen in this subtype could reflect our inability to eradicate chemotherapy-resistant tumor cells in stromal-rich bone marrow.
The molecular characterization of UC may aid in predicting which tumors will benefit from chemotherapy and other targeted agents. The underlying biology of these subtypes may cross tumor types [21,22] and may correlate with pathways associated with UC differentiation [23]. Basal UC shares cytokeratin markers from the basal/stem-cell compartment [12,12,23] with the corresponding transcription factors ΔNp63 [23–25], which is associated with adverse prognosis in muscle-invasive disease [24,25], and Hif-1α, a driver of angiogenesis [12]. Future studies should focus on the impact of antiangiogenic drugs and chemotherapy in basal UC. Luminal UC share cytokeratin markers representative of the umbrella cell layer [12,14]. The presence of FGFR signaling or ERBB2 expression suggest a potential role for targeted agents, while ERBB2 expression may help in selecting for chemosensitive luminal UC [26]. Finally, the low cell-cycle proliferation and poor outcomes for chemotherapy in p53-like UC suggest that patients with these tumors may benefit from initial surgery. The enrichment in stromal markers [12], in addition to the association with bone metastases, suggests a potential role for agents targeting stroma or bone.
5. Conclusions
It is clear that our findings are limited by the modest sample size and require confirmation in larger cohorts. The potential for heterogeneity along the urothelium, including a higher rate of luminal biology in upper tract disease, should be explored. Further work is necessary to confirm these findings and explore the potential benefit of subtype as a predictive biomarker and in developing personalized medicine for UC.
As a result, we can no longer think of UC as a single disease. GEP identifies UC subtypes that differ dramatically in their natural history and sensitivity to chemotherapy in a far more powerful manner than has been possible on the basis of morphology and clinical features. This is a significant development in unraveling the long-appreciated heterogeneity of this disease. GEP should be incorporated into clinical trials to provide a practical starting point for a more refined approach to the development of targeted therapies.
Clinical trials results were previously presented at the 2012 American Society of Oncology (ASCO) Genitourinary Cancer Symposium and the 2012 ASCO Annual Meeting. Gene expression profiling results were presented at a late-breaking minisymposium at the American Association for Cancer Research 2014 Annual Meeting and the 2015 ASCO Genitourinary Cancer Symposium.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: Funding was received from Genentech, M.D. Anderson Bladder SPORE (P50 CA91846), the Baker Foundation, and the Michael and Sherry Sutton Fund. The sponsors played a role in the design and conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Arlene O. Siefker-Radtke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Siefker-Radtke, Millikan, McConkey, Choi.
Acquisition of data: Siefker-Radtke, Millikan, McConkey, Choi, Corn, Dinney, Lee, Porten, Matin, Kamat, Czerniak.
Analysis and interpretation of data: Siefker-Radtke, McConkey, Choi, Shen.
Drafting of the manuscript: Siefker-Radtke, McConkey, Choi.
Critical revision of the manuscript for important intellectual content: Siefker-Radtke, McConkey, Choi, Shen, Lee, Porten, Matin, Kamat, Corn, Millikan, Dinney, Czerniak.
Statistical analysis: Shen, Choi.
Obtaining funding: Siefker-Radtke, McConkey.
Administrative, technical, or material support: Siefker-Radtke, Choi, McConkey, Lee, Porten.
Supervision: Siefker-Radtke, McConkey.
Other: None.
Financial disclosures: Arlene O. Siefker-Radtke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Arlene O. Siefker-Radtke is a scientific advisor for Vertex, Merck, Janssen, Celgene, Threshold, NCCN, Dendreon, and Boehringer Ingelheim, and has received clinical trial funding from Genentech. Arlene O. Siefker-Radtke, Woonyoung Choi, David J. McConkey, and Colin Dinney have a patent pending on Methods of characterizing and treating molecular subsets of muscle-invasive bladder cancer. Ashish M. Kamat has received fees from Photocure, Sanofi, Merck, and FKD. Yu Shen, I.-Ling Lee, Sima Porten, Surena F. Matin, Paul Corn, Randall E. Millikan, and Bogdan Czerniak have nothing to disclose.
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.International Collaboration of Triallists. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternberg CN, Bellmunt J, Sonpavde G, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: chemotherapy for urothelial carcinoma—neoadjuvant and adjuvant settings. Eur Urol. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. 2015;67:165–70. doi: 10.1016/j.eururo.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue K, Slaton JW, Karashima T, et al. The prognostic value of angiogenesis factor expression for predicting recurrence and metastasis of bladder cancer after neoadjuvant chemotherapy and radical cystectomy. Clin Cancer Res. 2000;6:4866–73. [PubMed] [Google Scholar]
- 6.Slaton JW, Millikan R, Inoue K, et al. Correlation of metastasis related gene expression and relapse-free survival in patients with locally advanced bladder cancer treated with cystectomy and chemotherapy. J Urol. 2004;171:570–4. doi: 10.1097/01.ju.0000108845.91485.20. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer protocol no 30924. J Clin Oncol. 2001;19:2638–46. doi: 10.1200/JCO.2001.19.10.2638. [DOI] [PubMed] [Google Scholar]
- 8.Bassi P, Ferrante GD, Piazza N, et al. Prognostic factors of outcome after radical cystectomy for bladder cancer: a retrospective study of a homogeneous patient cohort. J Urol. 1999;161:1494–7. [PubMed] [Google Scholar]
- 9.Wijkstrom H, Norming U, Lagerkvist M, et al. Evaluation of clinical staging before cystectomy in transitional cell bladder carcinoma: a long-term follow-up of 276 consecutive patients. Br J Urol. 1998;81:686–91. doi: 10.1046/j.1464-410x.1998.00637.x. [DOI] [PubMed] [Google Scholar]
- 10.Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19:4005–13. doi: 10.1200/JCO.2001.19.20.4005. [DOI] [PubMed] [Google Scholar]
- 11.Siefker-Radtke AO, Dinney CP, Shen Y, et al. A phase 2 clinical trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine followed by cisplatin, gemcitabine, and ifosfamide in locally advanced urothelial cancer: final results. Cancer. 2013;119:540–7. doi: 10.1002/cncr.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–5. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudoit S, Fridlyand J, Speed TP. Comparison of discrimination methods for the classification of tumors using gene expression data. J Am Stat Assoc. 2002;97:77–87. [Google Scholar]
- 16.Sjodahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–86. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 17.Rebouissou S, Bernard-Pierrot I, de Reynies A, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med. 2014;6:244ra91. doi: 10.1126/scitranslmed.3008970. [DOI] [PubMed] [Google Scholar]
- 18.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–94. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scosyrev E, Ely BW, Messing EM, et al. Do mixed histological features affect survival benefit from neoadjuvant platinum-based combination chemotherapy in patients with locally advanced bladder cancer? A secondary analysis of Southwest Oncology Group-Directed Intergroup Study (S8710) BJU Int. 2011;108:693–9. doi: 10.1111/j.1464-410X.2010.09900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 22.Prat A, Ellis MJ, Perou CM. Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol. 2012;9:48–57. doi: 10.1038/nrclinonc.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol. 2012;9:583–94. doi: 10.1038/nrurol.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi W, Shah JB, Tran M, et al. p63 expression defines a lethal subset of muscle-invasive bladder cancers. PloS One. 2012;7:e30206. doi: 10.1371/journal.pone.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karni-Schmidt O, Castillo-Martin M, Shen TH, et al. Distinct expression profiles of p63 variants during uothelial development and bladder cancer progression. Am J Pathol. 2011;178:1350–60. doi: 10.1016/j.ajpath.2010.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol in press. doi: 10.1016/j.eururo.2015.01.014. http://dx.doi.org/10.1016/j.eururo.2015.01.014. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


