Abstract
OBJECTIVE
Atrial fibrillation (AF) is the most common arrhythmia associated with cardiovascular disease and mortality. Recent studies suggest an association between inflammation, hyperuricemia and AF, but little is known whether gout is associated with AF risk.
METHODS
Using data from a US commercial insurance plan (2004–2013), we conducted a cohort study to evaluate the risk of incident AF in patients with gout versus osteoarthritis. Patients with gout or osteoarthritis were identified with ≥2 diagnoses and ≥1 dispensing for gout or osteoarthritis medications. Incident AF was defined as a new AF diagnosis and a new dispensing for anticoagulants or anti-arrhythmics. The risk of incident AF in gout was also compared to the non-gout group.
RESULTS
We identified 70,015 patients with gout and 210,045 with osteoarthritis, matched on age, sex and index date. The mean age was 57 years and 81% were men. Over the mean 2-year follow-up, the incidence rate of AF per 1,000 person-years was 7.19 in gout and 5.87 in osteoarthritis. The age, sex and index date-matched HR of AF was 1.23 (95%CI 1.14–1.32) in gout versus osteoarthritis. In a multivariable Cox regression adjusting for age, sex, comorbidities, medications and healthcare utilization, the HR of AF in gout was 1.13 (95%CI 1.04–1.23). When compared to non-gout, the multivariable HR of AF in gout was also increased (HR 1.21, 95%CI 1.11–1.33).
CONCLUSIONS
In this large population-based cohort study, gout was associated with a modestly increased risk of incident AF compared with osteoarthritis and non-gout after adjusting for other risk factors.
Keywords: atrial fibrillation, inflammation, uric acid, gout, cohort study
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia affecting over 5 million in the U.S. and associated with substantial morbidity including stroke and congestive heart failure and all-cause and cardiovascular mortality.[1 2] There are increasing data supporting the role of inflammation in the development and maintenance of AF.[3 4] Chronic medical conditions such as diabetes, obesity, and coronary artery disease are associated with systemic inflammation and increased levels of serum inflammatory biomarkers such as reactive oxygen species, transforming growth factor-β, c-reactive protein (CRP), pro-inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-2, and IL-6.[4 5] Increased levels of inflammatory biomarkers, abnormal infiltration of mononuclear lymphocytes in atrial tissue and necrosis of the adjacent myocytes are noted in patients with AF, leading to the hypothesis linking inflammation with AF.[3 4 6–8] Furthermore, several clinical trials show beneficial effects of anti-inflammatory drugs such as statins, colchicine, and steroids in reducing the risk of postoperative AF.[4 9–12]
Gout is a common inflammatory arthritis triggered by the deposition of monosodium urate crystals in joints. It is well-established that urate crystals induce an inflammatory response leading to release of TNF-α, IL-1, IL-6 and IL-8 by activating the NOD-like receptor protein 3 (NLRP3) inflammasome.[13 14] The association between gout, hyperuricemia, and comorbid conditions such as hypertension, chronic kidney disease, and cardiovascular disease is also well-described.[15–18] Although the causal link cannot be confirmed, a number of large prospective cohort studies show significantly increased risks of developing myocardial infarction and stroke in hyperuricemic or gout patients, independent of traditional cardiovascular risk factors.[19–22] Recently, a few cross-sectional or cohort studies report an increased risk of sinus tachycardia, AF and left atrial thrombus in patients with hyperuricemia.[23–25]
To date, it remains unknown whether gout is associated with the risk of AF. The main objective of this study was to assess the rate of incident AF in patients with gout compared to those without gout in a large population-based cohort study.
METHODS
Data Source
We conducted a cohort study using claims data from United HealthCare, a large commercial U.S. health plan, for the period January 1, 2004 to December 31, 2013. This data source has been described in details elsewhere.[26] In brief, the study database contains longitudinal information on medical and pharmacy claims. In addition, on a subset of beneficiaries claims data were linked to laboratory test results provided by two large national laboratory providers. Patient informed consent was not required as the dataset was de-identified to protect subject confidentiality. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Study Cohort
Adult patients aged ≥40 years who had ≥2 visits coded with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD 9-CM) code, 274.0X, 274.8X and 274.9 for gout were eligible for the gout group. We defined the index date for the ‘gout group’ as the date of the first dispensing of a gout-related drug after ≥1 year of continuous health plan enrollment and ≥2 visits for gout. Gout-related drugs were allopurinol, febuxostat, colchicine, probenecid, pegloticase, selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs), and systemic and intra-articular steroids. To ensure that we capture incident cases of AF, we excluded patients who had a diagnosis of arrhythmia or cardiac surgery, or who used anti-arrhythmics or anticoagulants in the 365-day period prior to the index date. Nursing home patients were also excluded as we did not have drug dispensing data from a nursing home stay.
We selected two different non-gout comparison groups aged ≥40 years. The primary comparison group was the ‘osteoarthritis group’ based on ≥2 visits coded with the ICD-9 code, 715.XX for osteoarthritis. The osteoarthritis group was used as the primary comparison group to minimize confounding by obesity, comorbidities and health care utilization intensity as these characteristics are similar to those of the gout group. The index date for the osteoarthritis group was the date of the 1st dispensing of opioids, selective or non-selective NSAIDs after ≥2 physician visits coded for osteoarthritis. The secondary comparison group was the ‘non-gout group’ and included patients who had no diagnosis of gout at baseline and had at least 2 physician visits after ≥12 months of continuous health plan enrollment. The index date for the non-gout patients was the date of the first dispensing of any prescription drugs after ≥2 physician visits. We applied the aforementioned exclusion criteria in both comparisons groups. The osteoarthritis and non-gout groups were not allowed to use allopurinol, febuxostat, colchicine, pegloticase, or probenecid in the 365-day period prior to or on the index date. The osteoarthritis and non-gout group were then matched to the gout group on age, sex and index date (+/− 30 days) with a 3:1 ratio.
Patients in both comparison groups were followed from the index date to the first of any of the following censoring events: development of AF, development of gout (for both osteoarthritis and non-gout groups), insurance disenrollment, admission to nursing home, end of study period, or death.
Outcome Definition
The primary outcome was defined as a combination of an inpatient or outpatient diagnosis of AF (ICD-9: 427.3x) and a new dispensing of anticoagulants or anti-arrhythmics within 30 days after the AF diagnosis date. We also used two secondary more stringent definitions of AF outcomes. One outcome was defined with an inpatient discharge diagnosis code of AF. This algorithm has been previously validated and had both sensitivity and specificity greater than 90%.[27] Another outcome required a combination of an inpatient discharge diagnosis of AF and a new dispensing of anticoagulants or anti-arrhythmics prescription for an anticoagulant within 30 days after the inpatient discharge date.
Covariates
We assessed a number of predefined variables potentially related to gout, health care use or development of AF using data from the 365 days before the index date. These variables were age, sex, comorbidities, use of medications, health care utilization intensity indicators, and physician order of outpatient laboratory tests listed in Table 1. We also calculated a comorbidity score to quantify patients’ comorbidities based on ICD codes.[28] Outpatient laboratory data such as acute phase reactants (i.e., high ESR or CRP versus normal levels) and uric acid levels were also included in a subgroup of all three study groups.
Table 1.
Baseline characteristics of study cohorts in the 365 days before study entry.
| Gout | Osteoarthritis | |
|---|---|---|
| N | 70,015 | 210,045 |
| Percentage or mean ± standard deviation | ||
| Follow-up, year | 2.1 ± 1.8 | 2.0 ± 1.8 |
| Demographic * | ||
| Age, years | 56.8 ± 9.0 | 56.8 ± 9.0 |
| Men | 81.4 | 81.4 |
| Comorbidities | ||
| Hypertension | 69.5 | 50.9 |
| Stroke | 4.0 | 3.7 |
| Cardiovascular disease | 12.4 | 10.5 |
| Valvular heart disease | 4.8 | 4.6 |
| Chronic lung disease | 10.6 | 12.6 |
| Chronic kidney disease | 9.7 | 2.3 |
| Liver disease | 4.3 | 3.2 |
| Hyperlipidemia | 63.9 | 54.2 |
| Obesity | 10.9 | 9.7 |
| Diabetes | 24.1 | 17.8 |
| Smoking | 6.1 | 9.8 |
| Alcoholism | 0.8 | 0.7 |
| Comorbidity score a | 0.1 ± 1.4 | 0.1 ± 1.1 |
| Medications | ||
| Allopurinol or febuxostat | 55.2 | 0 |
| Colchicine | 23.6 | 0 |
| Recent colchicine use b | 10.5 | 0 |
| Lipid-lowering drugs | 43.7 | 38.0 |
| Recent steroid use b | 6.8 | 4.2 |
| 365-day cumulative prednisone-equivalent dose (mg) | 108.4 ± 410.5 | 83.0 ± 512.8 |
| Non-selective NSAIDs | 58.3 | 55.1 |
| Selective cox-2 inhibitors | 4.8 | 11.4 |
| Opioids | 42.3 | 70.1 |
| Beta blockers | 23.8 | 14.2 |
| Calcium channel blockers | 19.6 | 11.7 |
| ACE inhibitors or ARBs | 45.4 | 31.1 |
| Diuretics | 22.6 | 13.2 |
| Bisphosphonates | 1.2 | 2.1 |
| Anti-platelets | 3.7 | 3.5 |
| Health care utilization | ||
| No. of outpatient physician visits | 7.1 ± 5.5 | 8.2 ± 5.6 |
| Emergency room visit | 20.0 | 18.0 |
| Acute hospitalization | 8.1 | 10.0 |
| No. of prescription drugs | 8.6 ± 5.4 | 8.1 ± 5.1 |
| Laboratory test | ||
| CRP ordered | 9.6 | 8.2 |
| ESR ordered | 15.7 | 11.4 |
| CRP or ESR level available | 6.6 | 5.0 |
| Elevated CRP or ESR c | 35.5 | 23.4 |
| Uric acid ordered | 56.2 | 7.1 |
| Uric acid level available | 21.4 | 2.7 |
| Uric acid, mg/dl d (median, IQR) | 7.4 ± 2.0 (7.5, 6.0–8.8) | 5.9 ± 1.4 (5.8, 4.9–6.8) |
ACE: angiotensin-converting enzyme, ARB: angiotensin receptor blocker, NSAID: nonsteroidal anti-inflammatory drug, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, IQR: interquartile range
Gout and osteoarthritis groups are age-, sex- and index date-matched.
The range of the comorbidity score is −2 to 26.
Recent use is defined as any use within 30 days from the index date.
Proportions were calculated among patients with baseline CRP or ESR levels available.
The mean uric acid level was calculated among patients with baseline uric acid levels available.
Statistical Analyses
Baseline characteristics of the gout and osteoarthritis groups were compared. We calculated the incidence rates (IR) and rate ratios of AF with 95% confidence intervals (CI) in both age, sex, and index-date matched groups.[29] Kaplan-Meier curves were plotted for the cumulative incidence of AF in the gout and osteoarthritis groups. We tested for an interaction between gout and sex by including the product term in a multivariable Cox proportional hazards model and did not find a statistically significant interaction (p=0.8). To estimate the hazard ratios (HR) of AF associated gout versus osteoarthritis, we conducted a number of separate partially adjusted Cox models including age, sex (model 1), and age, sex, comorbidity score, and number of unique prescriptions (model 2). The final, fully adjusted Cox model included nearly 40 covariates listed in Table 1.
We conducted multivariable Cox regression analyses stratified by several important risk factors for AF, including obesity, diabetes, and history of cardiovascular disease. In addition, to examine the risk of AF associated with gout versus osteoarthritis adjusting for elevated ESR/CRP levels at baseline, we conducted subgroup analyses in patients with baseline ESR/CRP levels available. We also conducted subgroup analyses adjusting for baseline serum uric acid levels among patients in whom we had a baseline uric acid level available. Lastly, among gout patients in whom we had a baseline uric acid level measured, we assessed the risk of AF in relation to their serum uric acid levels.
All the main and subgroup analyses were repeated for the comparison between the gout and non-gout groups. The proportional hazards assumption was assessed by testing the significance of the interaction term between exposure (i.e., gout or osteoarthritis) and time, and was not violated in any models.[30] All analyses were done using SAS 9.3 Statistical Software (SAS Institute Inc., Cary, NC).
RESULTS
Cohort Selection
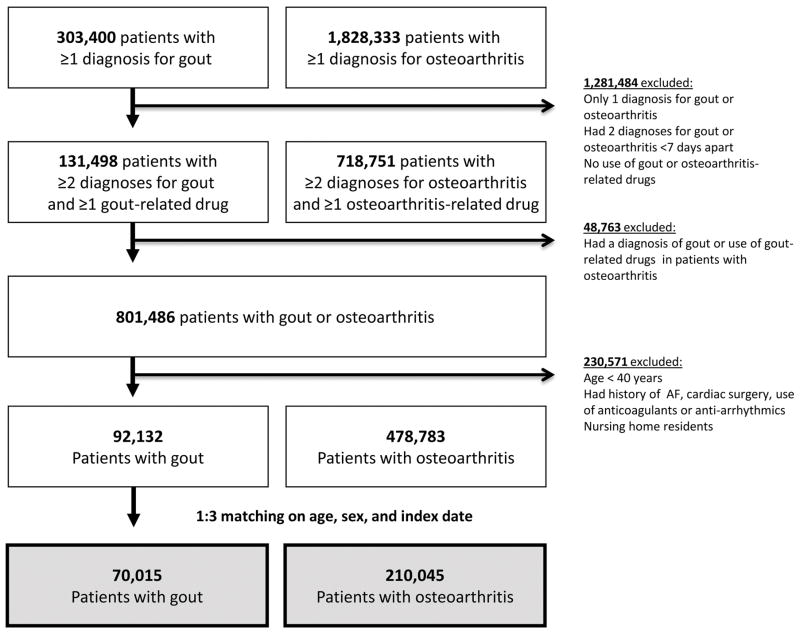
Figure 1 displays the study cohort selection process. There were initially 303,400 patients with at least one gout diagnosis and 1,828,333 with at least one osteoarthritis diagnosis after a 365-day enrollment period. After applying the inclusion and exclusion criteria, we selected 3 osteoarthritis patients matched to each gout patient on age, sex, and the index date. Our final study cohort includes 70,015 gout and 210,045 osteoarthritis patients. The mean (SD) follow-up time was 2.1 (1.8) years for gout and 2.0 (1.8) years for osteoarthritis patients. The most common reason for censoring besides the development of outcome was disenrollment from the plan (63% in gout and 59% in osteoarthritis) followed by the end of database period (33% in gout and 32% in osteoarthritis), nursing home admission (3% in both groups), and death (<1% in both groups). Three percent of osteoarthritis patients were censored because they developed gout during the follow-up time.
Figure 1.
Cohort selection
For the comparison between gout and non-gout groups, there were 91,976 gout patients and 275,928 non-gout patients matched on age, sex, and the index date (Appendix 2). The mean (SD) follow-up time was 2.0 (1.8) years for gout and 2.0 (1.8) years for non-gout patients.
Patient Characteristics
Baseline characteristics of the gout and osteoarthritis groups are presented in Table 1. The mean (SD) age was 56.8 (9.0) years and men comprised 81.4% in both groups. Patients with gout generally had a greater frequency of comorbidities such as hypertension, cardiovascular disease, diabetes, chronic kidney disease, and hyperlipidemia compared to the osteoarthritis group. The proportion of patients with obesity at baseline was 10.9% in the gout group and 9.7% in the osteoarthritis group. Among patients with gout, 55.2% used allopurinol or febuxostat and 23.6% colchicine at baseline. Of those, 10.5% used colchicine within 30 days from the index date. Use of anti-hypertensives, diuretics, lipid-lowering drugs, and steroids were more common in gout than osteoarthritis, while use of bisphosphonates, opioids, and selective NSAIDs were more common in osteoarthritis than gout. Health care utilization intensity was similar between the groups. Among patients with baseline ESR/CRP levels available (n=15,174, 5.4% of the total cohort), 35.5% of gout and 23.4% of osteoarthritis patients had elevated ESR/CRP. Among patients with baseline uric acid levels available (n=20,622, 7.4% of the total cohort), the mean (SD) uric acid level (in milligram/dL) was 7.4 (2.0) in gout and 5.9 (1.4) in osteoarthritis. Baseline characteristics of the gout and non-gout groups are shown in Appendix Table 1.
Risk of Incident AF in Gout
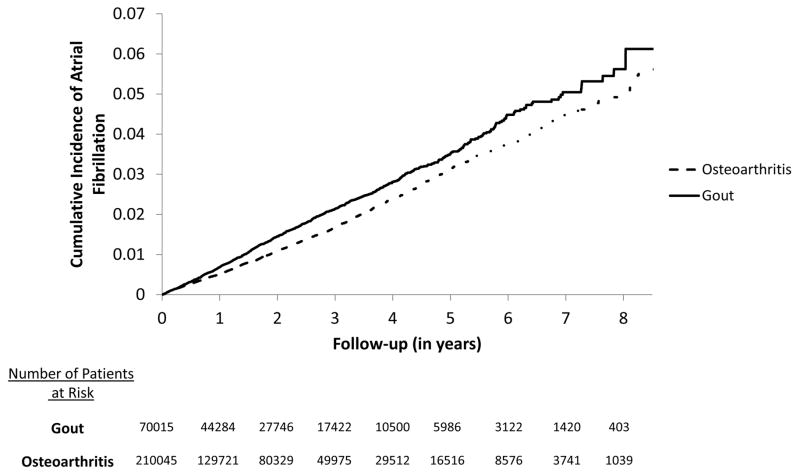
The IR of AF using the primary outcome definition was 7.19 per 1,000 person-years in gout and 5.87 per 1,000 person-years in osteoarthritis patients (Table 2). The age-, sex- and index date-matched rate ratio was 1.22 (95% CI 1.13–1.31) in gout compared to osteoarthritis. Figure 2 illustrates Kaplan-Meier curves for the cumulative incidence of AF in the gout and osteoarthritis groups (log-rank p-value<0.0001). Table 3 summarizes the results from several multivariable Cox regression analyses comparing gout patients to osteoarthritis. After adjusting for age, sex, obesity, comorbidities, medications, and health care utilization intensity listed in Table 1, the HR of AF associated with gout was 1.13 (95% CI 1.04–1.23). Multivariable Cox regression analyses stratified by obesity, diabetes, or history of cardiovascular disease showed no significant heterogeneity with similar HRs between the subgroups (see Table 3).
Table 2.
Incidence rates of atrial fibrillation in patients with gout compared to those with osteoarthritis.
| Gout | Osteoarthritis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atrial Fibrillation | No. of patients | Cases | Person-years | Incidence rate (95% CI) | No. of patients | Cases | Person-years | Incidence rate (95% CI) | Rate ratio* (95% CI) |
| In/outpatient diagnosis + anticoagulants/anti-arrhythmics | 70,015 | 1,029 | 143,185 | 7.19 (6.76–7.64) | 210,045 | 2,438 | 415,578 | 5.87 (5.64–6.11) | 1.22 (1.13–1.31) |
| Inpatient diagnosis | 70,015 | 685 | 143,856 | 4.76 (4.42–5.13) | 210,045 | 1,771 | 416,929 | 4.25 (4.06–4.45) | 1.12 (1.03–1.22) |
| Inpatient diagnosis + anticoagulants/anti-arrhythmics | 70,015 | 451 | 144,116 | 3.13 (2.85–3.43) | 210,045 | 1,186 | 417,659 | 2.84 (2.68–3.01) | 1.10 (0.99–1.23) |
CI: confidence interval, Incidence rate is per 1,000 person-years.
matched on age, sex, and index date
Figure 2. Kaplan-Meier survival curves of atrial fibrillation in gout versus osteoarthritis.
Log-rank P-value <0.0001
Table 3.
Risk of incident atrial fibrillation* in patients with gout compared to those with osteoarthritis.
| Adjustment | Hazard ratio (95% CI) |
|---|---|
| Main analysis | |
|
| |
| Age, sex | 1.23 (1.14–1.32) |
| + comorbidity score and number of prescription drugs | 1.19 (1.11–1.28) |
| Final model a | 1.13 (1.04–1.23) |
|
| |
| Stratified analysis b | |
|
| |
| By obesity | |
| - Yes | 1.26 (1.02–1.56) |
| - No | 1.18 (1.09–1.27) |
| By diabetes | |
| - Yes | 1.11 (0.96–1.27) |
| - No | 1.21 (1.11–1.31) |
| By history of CVD | |
| - Yes | 1.25 (1.01–1.41) |
| - No | 1.15 (1.05–1.26) |
|
| |
| Subgroup analysis | |
|
| |
| Patients with baseline ESR/CRP levels available (n=15,174) | |
| Age, sex | 1.17 (0.78–1.76) |
| + comorbidity score and number of prescription drugs | 1.12 (0.74–1.69) |
| + high ESR/CRP | 1.06 (0.70–1.61) |
| Patients with baseline uric acid levels available (n=20,622) | |
| Age, sex | 1.32 (0.88–1.97) |
| + comorbidity score and number of prescription drugs | 1.33 (0.89–2.00) |
| + serum uric acid level | 1.19 (0.78–1.83) |
CI: confidence interval, CVD: cardiovascular disease, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein
Defined with an inpatient or outpatient diagnosis of AF and a dispensing of anticoagulants/anti-arrhythmics
The final model includes age, sex, comorbidities, medications, and health care utilization factors listed in Table 1.
Stratified analyses were adjusted for age, sex, comorbidity score, and number of prescription drugs.
In the subgroup of patients with baseline ESR/CRP level measured, the HR of AF in gout versus osteoarthritis was 1.12 (95% CI 0.74–1.69) after adjustment for age, sex, comorbidity score, and number of prescription drugs. After further adjusting for elevated ESR/CRP at baseline, the HR of AF was 1.06 (95% CI 0.70–1.61) in gout versus osteoarthritis. Among patients with baseline serum uric acid levels available, the HR of AF was 1.33 (95% CI 0.89–2.00) adjusting for age, sex, comorbidity score, and number of prescription drugs and 1.19 (95% CI 0.78–1.83) further adjusted for serum uric acid levels at baseline. Among gout patients with baseline serum uric acid levels available (n=14,968), the HR of AF associated with a 1 mg/dl increase in the uric acid level was 1.05 (95% CI 0.96–1.16) after adjusting for age, sex, comorbidity score, and number of prescription drugs.
In the secondary comparison between gout and non-gout patients, the IR of AF using the primary outcome definition was 6.44 per 1,000 person-years in gout and 4.11 per 1,000 person-years in non-gout patients (Appendix Table 2). The risk of incident AF associated with gout (HR 1.21, 95% CI 1.11–1.33) was consistently increased in gout compared to non-gout patients after fully adjusting for more than 35 covariates (Appendix Table 3). In the subgroup analyses, similar to the primary comparison, the HR of AF in gout versus non-gout was 1.05 (95% CI 0.64–1.73) adjusting for age, sex, comorbidity score, number of prescription drugs, and elevated ESR/CRP and 1.21 (95% CI 0.70–2.08) adjusting for age, sex, comorbidity score, number of prescription drugs and serum uric acid levels.
DISCUSSION
In this large population-based cohort study, gout was associated with a 13% greater risk of incident AF compared to osteoarthritis and a 21% greater risk compared to non-gout. The risk of AF associated with gout did not differ between men and women. Although the causal relationship between gout and AF is yet to be determined, gout could potentially increase the risk of AF through two distinct but possibly related mechanisms – inflammation and hyperuricemia. We first investigated the association between gout and AF independent of the level of systemic inflammation at baseline in a subgroup analysis adjusting for elevated ESR or CRP at baseline. The risk of AF in gout did not appear to be elevated after further adjusting for elevated ESR/CRP. Second, as prior evidence showed a potential relationship between serum uric acid levels and AF risk,[23 24] we conducted a separate sensitivity analysis restricting to those with baseline uric acid levels. The risk of AF in gout was attenuated toward the null after further adjusting for baseline serum uric acid levels. Lastly, there seems to be a dose-response, albeit not statistically significant due to a smaller subgroup size, between serum uric acid levels and risk of AF relationship (HR 1.05 per 1 mg/dl increase in serum uric acid level, 95% CI 0.96–1.16). While these subgroup analyses were run in only a subgroup of patients with the laboratory data at baseline, these findings support the hypothesis that gout may increase the risk of AF due to complex inter-related mechanisms of systemic inflammation and hyperuricemia.
This study has several strengths. First, the study cohort includes a large number of gout, osteoarthritis and non-gout patients in a population that is representative of the U.S. commercially-insured population. Second, we assessed a wide range of potential confounders at baseline, including comorbidities, medications, and health care use characteristics. Third, we also performed several subgroup analyses to investigate potential mechanism in which gout might increase the risk of AF. Fourth, to minimize surveillance bias and confounding by body mass index and comorbidities, we compared gout patients to those with OA, a chronic non- or minimally inflammatory medical condition requiring frequent medical attention or pharmacologic treatment. Lastly, we also showed an increased risk of AF with a HR of 1.21 in the comparison between gout and non-gout patients, similar to the results from the primary analysis.
There are several limitations in this study. First, there is a possibility of residual confounding due to unmeasured risk factors such as body mass index, abdominal circumference, baseline cardiac function, and severity of comorbidities such as heart failure or chronic obstructive pulmonary disease.[1 31] Second, there is a potential for exposure (i.e. gout versus osteoarthritis) and outcome misclassification as we relied on a combination of diagnosis and procedure codes. However, the algorithm that we used to identify patients with gout has a positive predictive value of >99% in a separate chart review study that we recently conducted using an electronic medical record database linked to claims data (unpublished). For the outcomes, we used three different algorithms to define AF which used previously validated diagnosis codes [27] with and without a dispensing of new anticoagulants or anti-arrhythmics and found similar results across those definitions. Third, because we required gout and osteoarthritis patients to have at least 2 visits and a dispensing for gout- or osteoarthritis-related medications, patients with mild or minimally symptomatic gout or osteoarthritis who did not need to take a drug were not included. Thus, our results may not be generalizable to such patients. Fourth, statistical power of the subgroups analyses was limited due to the relatively small number of patients with baseline ESR/CRP or uric acid levels.
Our findings have important implications for future studies aimed at reducing the risk of AF. While the magnitude of the association between gout and AF is small, it may still have an important impact on the gout population as AF is the most common arrhythmia with substantial consequences such as stroke and heart failure. First, several studies have examined the role of colchicine in reducing AF development or recurrence after cardiac surgery over the past five years.[32 33] In a placebo-controlled clinical trial, a 3-month course of colchicine 0.5 mg twice daily was effective (odds ratio 0.38, 95% confidence interval 0.18–0.80) in preventing recurrent AF in patients with paroxysmal AF who underwent pulmonary vein isolation procedure.[32] The multicenter, double-blind, randomized trial Colchicine for the Prevention of the Post-Pericardiotomy Syndrome (COPPS) trial showed that patients who started colchicine 1.0 mg twice daily 3 days after cardiac surgery and continued a maintenance dose of 0.5 mg twice daily for 1 month had a 45% reduced risk of developing post-operative AF compared to placebo.[33] Since colchicine is commonly used in patients with gout, future research may examine the effect of colchicine on primary or secondary prevention of AF among patients with gout. Second, a previous case-crossover clinical trial of allopurinol was effective in lowering blood pressure in adolescent patients with newly diagnosed hypertension.[34] Future studies may explore the role of urate-lowering therapy in reducing AF risk as hyperuricemia is causally related to gout and associated with cardiovascular disease.
In conclusion, gout was associated with a modestly increased risk of incident AF compared to osteoarthritis and non-gout after adjusting for a number of traditional risk factors. Our results support the hypothesis that gout increases the risk of AF via systemic inflammation combined with hyperuricemia although a dose-response relationship between the level of serum uric acid and the risk of AF needs to be further studied. Future research should confirm this finding in a different setting and may examine the role of gout treatment, controlling systemic inflammation and/or lowering uric acid levels, in the primary or secondary prevention of AF.
Supplementary Material
Acknowledgments
Other Research funding Support: SCK is supported by the NIH grant K23 AR059677. DHS is supported by NIH K24 AR055989.
Abbreviations
- AF
atrial fibrillation
- CI
confidence interval
- CRP
c - reactive protein
- ESR
erythrocytes sedimentation rate
- HR
hazard ratio
- ICD
International Classification of Diseases
- IL
interleukin
- IR
incidence rate
- NSAID
non-steroidal anti-inflammatory drug
- SD
standard deviation
Footnotes
Authors’ contributions: All authors conceived and designed the study, interpreted the data, and critically revised the manuscript for important intellectual content. SCK drafted the paper. SCK has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Potential Conflicts of Interest: Dr. Kim receives salary support from unrelated grants to Brigham and Women’s Hospital from Lilly, Pfizer and AstraZeneca. Dr. Solomon receives salary support from unrelated grants to Brigham and Women’s Hospital from Lilly, Pfizer, AstraZeneca, Amgen and CORRONA. Dr. Liu has nothing to disclose.
Conflict of interest disclosures: No specific funding was given for this study.
SCK receives salary support from unrelated grants to Brigham and Women’s Hospital from Pfizer, AstraZeneca and Lilly. DHS receives salary support from unrelated grants to Brigham and Women’s Hospital from Lilly, Pfizer, Amgen, and Astra Zeneca.
References
- 1.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 2.Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. European heart journal. 2013;34(14):1061–7. doi: 10.1093/eurheartj/ehs469. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26(20):2083–92. doi: 10.1093/eurheartj/ehi350. [DOI] [PubMed] [Google Scholar]
- 4.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nature reviews. Cardiology. 2015 doi: 10.1038/nrcardio.2015.2. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 5.Gungor B, Ekmekci A, Arman A, et al. Assessment of interleukin-1 gene cluster polymorphisms in lone atrial fibrillation: new insight into the role of inflammation in atrial fibrillation. Pacing and clinical electrophysiology : PACE. 2013;36(10):1220–7. doi: 10.1111/pace.12182. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49(15):1642–8. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Lip G, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–70. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 9.Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114(14):1455–61. doi: 10.1161/circulationaha.106.621763. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 10.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124(21):2290–5. doi: 10.1161/circulationaha.111.026153. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 11.Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. Journal of the American College of Cardiology. 2010;56(18):1463–72. doi: 10.1016/j.jacc.2010.04.057. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 12.Cappabianca G, Rotunno C, de Luca Tupputi Schinosa L, Ranieri VM, Paparella D. Protective effects of steroids in cardiac surgery: a meta-analysis of randomized double-blind trials. Journal of cardiothoracic and vascular anesthesia. 2011;25(1):156–65. doi: 10.1053/j.jvca.2010.03.015. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 14.Amaral FA, Costa VV, Tavares LD, et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64(2):474–84. doi: 10.1002/art.33355. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 15.Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67(7):960–6. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SC, Schmidt BM, Franklin JM, Liu J, Solomon DH, Schneeweiss S. Clinical and health care use characteristics of patients newly prescribed allopurinol, febuxostat and colchicine for gout. Arthritis Care Res (Hoboken) 2013 doi: 10.1002/acr.22067. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008;67(9):1310–6. doi: 10.1136/ard.2007.081604. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Pandya B, Choi H. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125(7):679–87. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 19.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010;69(6):1162–4. doi: 10.1136/ard.2009.122770. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Guevara J, Kim K, Choi H, Heitjan D, Albert D. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–92. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Guevara J, Kim K, Choi H, Heitjan D, Albert D. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care & Res. 2010;62(2):170–80. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seminog OO, Goldacre MJ. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology (Oxford, England) 2013;52(12):2251–9. doi: 10.1093/rheumatology/ket293. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 23.Chuang SY, Wu CC, Hsu PF, et al. Hyperuricemia and incident atrial fibrillation in a normotensive elderly population in Taiwan. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2014;24(9):1020–6. doi: 10.1016/j.numecd.2014.03.012. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 24.Cicero AF, Rosticci M, Tocci G, et al. Serum uric acid and other short-term predictors of electrocardiographic alterations in the Brisighella Heart Study cohort. European journal of internal medicine. 2015 doi: 10.1016/j.ejim.2015.02.007. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 25.Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11(7):1102–8. doi: 10.1016/j.hrthm.2014.04.003. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 26.Kim SC, Liu J, Solomon DH. Risk of incident diabetes in patients with gout: a cohort study. Arthritis Rheumatol. 2015;67(1):273–80. doi: 10.1002/art.38918. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazer NL, Dublin S, Smith NL, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167(3):246–52. doi: 10.1001/archinte.167.3.246. [DOI] [PubMed] [Google Scholar]
- 28.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 30.Kleinbaum D, Klein M. Evaluating the Proportional Hazards Assumption. In: Gail M, Krickberg K, Samet J, Tsiatis A, Wong W, editors. Survival Analysis: A Self-Learning Text. 3. Springer; 2012. [Google Scholar]
- 31.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, JBA, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–4. [PubMed] [Google Scholar]
- 32.Deftereos S, Giannopoulos G, Kossyvakis C, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. 2012;60(18):1790–6. doi: 10.1016/j.jacc.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124(21):2290–5. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 34.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA : the journal of the American Medical Association. 2008;300(8):924–32. doi: 10.1001/jama.300.8.924. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.