Abstract
Background
The observation that mismatch negativity (MMN) is consistently impaired in schizophrenia has generated considerable interest in the use of this biomarker as an index of disease risk and progression. Despite such enthusiasm, a number of issues remain unresolved regarding the nature of MMN impairment. The present study expands upon an earlier meta-analysis of MMN impairment in schizophrenia by examining impairment across a range of clinical presentations, as well as across experimental parameters.
Methods
One hundred and one samples of schizophrenia patients were included in the present study, including first-episode (N=13), chronic (N=13), and mixed-stage (N=75) samples. Additionally, MMN was examined in three related conditions: bipolar disorder (N=9), unaffected first-degree relatives (N=8), and clinical high risk (N=16).
Results
We found that MMN impairment (1) likely reflects a vulnerability to disease progression in clinical high risk populations rather than a genetic risk for the condition; (2) is largely unrelated to duration of illness after the first few years of illness, indicating that impairment is not progressive throughout the lifespan; (3) is present in bipolar disorder, albeit to a lesser degree than in schizophrenia; and (4) is not modulated by experimental parameters such as magnitude of change between standard and deviant tones or frequency of deviant tones, but may be modulated by attentional demands.
Conclusions
Such findings lay the foundation for a better understanding of the nature of MMN impairment in schizophrenia, as well as its potential as a clinically useful biomarker.
Keywords: schizophrenia, high risk, prodromal, bipolar disorder, mismatch negativity, meta-analysis
Introduction
The mismatch negativity (MMN) is an event-related potential that has garnered attention in recent years for its promise as a biomarker for schizophrenia. The MMN is an electrophysiological response that is elicited when a sequence of identical auditory stimuli is infrequently interrupted by a stimulus that deviates from the standard stimulus along one or more dimensions, such as pitch, duration, or intensity. This event-related potential therefore appears to represent the automatic change detection process that occurs when an acoustic event violates expectations maintained by the active auditory trace (for a review, see 1). People with schizophrenia exhibit robust and reliable deficits in MMN production (2), a finding that motivates interest in this phenomenon as a putative index of structural impairment in the frontal and temporal cortices (3-5), as a target to validate the clinical and biological relevance of pharmacological compounds (6), and, more recently, as a predictor for conversion to psychosis among high-risk individuals (7, 8).
Given such enthusiasm for this index, recently hailed as a “breakthrough biomarker” for understanding and treating psychosis (9), a next logical step is to use a meta-analytic approach to evaluate what is known about MMN impairment in schizophrenia and provide clarity on issues that have yet to be resolved. One such unresolved issue relates to the progressive nature of MMN impairment. It has been suggested that MMN impairment worsens over the course of the illness (10, 11), an observation that is modestly supported by a trend-level association between MMN impairment and illness duration in an earlier meta-analysis (12). Such findings are consistent with reports of elevated rates of gray matter loss in schizophrenia (13). However, one large study that explicitly tested this hypothesis found that although the MMN tends to decrease in amplitude with age for both groups, the magnitude of group difference was not substantially larger for those individuals at later stages of the illness (14, see also 15). It is therefore unclear whether MMN impairment reflects a stable feature of the illness, or if it follows a progressive course.
A second issue concerns the degree to which MMN impairment is associated with illness state and/or genetic predisposition for developing schizophrenia. Recently, there has been considerable enthusiasm for the use of MMN to predict conversion to psychosis among at-risk individuals. However, it is not yet known whether MMN impairment is associated with the emergence of symptoms specifically, or reflective of genetic risk for developing the disorder. To date, the literature indicates that MMN impairment is greater among high risk samples that later convert to schizophrenia (8, 16, 17), but has also been observed among unaffected first-degree relatives of schizophrenia patients (18). Therefore, it is not yet clear whether MMN impairment represents an index of an emerging illness, or is better conceptualized as an endophenotype marker of genetic vulnerability.
A related issue concerns the specificity of MMN impairment to schizophrenia, as compared with bipolar disorder. To date, the literature is mixed regarding support for MMN impairment in bipolar disorder (for a review, see 19). However, there is increasing evidence that bipolar patients share many of the cognitive deficits found in schizophrenia patients (20), as well as substantial symptom overlap among bipolar patients with psychotic features. Therefore, it is of interest to know whether MMN impairment is diagnosis-specific, or if it is better conceptualized within the RDoC framework as an impairment that is shared across psychotic disorders.
Finally, disagreement remains regarding the role of impaired auditory discrimination on MMN decrements in schizophrenia. For instance, one study reported that group differences in MMN amplitude were minimized when tone pairs were matched to individuals' auditory discrimination thresholds, a phenomenon that may be accounted for by floor effects in MMN amplitude as the standard-deviant difference becomes smaller (21). However, it has also been demonstrated that the most robust between-group differences emerge when the change in stimulus characteristics between standard and deviant stimuli is large, rather than small (22). If impaired auditory discrimination meaningfully impacts the magnitude of the mismatch response in schizophrenia patients, the effect sizes of MMN impairment ought to be largest when the difference between standard and deviant stimuli is the most difficult to detect (see 23, 24). However, the observation that this is not the case suggests that early sensory processing deficits may not be a primary constraint on MMN amplitude in schizophrenia.
The purpose of the present study is to expand upon a previous meta-analysis (12) by exploring the pattern of MMN impairment across multiple levels of risk for psychosis, as well as across the course of the illness. Furthermore, we aimed to better understand the nature of impoverished MMN production among schizophrenia patients by identifying experimental parameters that impact effect size estimates, such as deviant tone properties and attentional demands. Though some of these questions were explored by Umbricht and Krljes (12), this early meta-analysis was conducted using 36 schizophrenia patient samples that were available at the time. Here, we add 65 samples of schizophrenia patients, as well as 33 samples with related conditions including bipolar disorder (BP; N=9 samples), clinical high risk (CHR; N=16 samples), and first-degree relatives (REL; N=8 samples). The schizophrenia patient groups included first episode schizophrenia or first episode psychosis (SZ-F; N=13 samples), chronic schizophrenia patients (SZ-C; N=13 samples), and a broader category of patients that were not separated into illness stage by the experimenters (SZ-All; N=75 samples). Patient samples were assigned to first-episode and chronic groups only if they were identified as such by the authors of the original study. The present study is the first to directly compare MMN integrity across these different groups, and therefore allows for (a) a comparison of MMN impairment across the spectrum of risk and disease progression, and (b) more power to detect relationships between effect size and experimental parameters.
Methods
Literature Search and Study Selection
A literature search was conducted using Web of Science and PubMed (years 1987 to 2014) using combinations of the keywords schizophrenia, schizoaffective, psychosis, prodromal, bipolar disorder, mismatch negativity, and MMN. Furthermore, we examined reference lists from those studies for additional papers not identified in the original search. Only peer-reviewed manuscripts were considered. This initial search strategy identified 216 articles. The following criteria were then used to identify studies for inclusion in the meta-analysis: (a) the MMN amplitude must be reported as a difference wave (deviant minus standard ERP); (b) group differences in MMN amplitude must be reported either in terms of mean and standard deviation, or as a t-test or F-test; (c) the study should include at least one psychiatrically healthy control group, and one comparison group of schizophrenia or bipolar patients that have been diagnosed according to contemporary diagnostic standards (e.g., DSM-III or later, ICD-9 or later), or of individuals who have been identified as high risk for psychosis, prodromal, or first degree relatives of schizophrenia patients; (d) for consistency, only EEG (not MEG) studies of MMN were included in the present analysis; and (e) only studies that presented original data (i.e., no reanalysis of previously published data) were included. Following the initial search, we discovered a small number of studies that examined MMN amplitudes among twin pairs. Given that these samples are likely characterized by dependencies that are not characteristic of the other included studies, the 4 twin studies were excluded.
Using these criteria, 104 unique articles were selected for inclusion in the meta-analysis (see Supplementary Table S1 for list of studies and sample characteristics). Several of these studies included multiple patient comparison groups, yielding a total of 134 comparison samples that consist of 13 SZ-F, 13 SZ-C, 75 SZ-All, 16 CHR, 9 BP, and 8 REL samples. For studies in which drug effects were evaluated, or for which test-retest data were available, only the placebo/baseline condition was included in the present meta-analysis. In a small number of studies, the deviant types were very unusual and therefore not included in the present analysis—for example, Todd and colleagues (25) used intensity-matched duration deviants as one of the experimental conditions. Given the unusual nature of these matched deviant stimuli, only the standard paradigm stimuli from these studies (see also 21) were included. One hundred and twelve of the 216 identified studies were rejected from the present analysis (see Supplementary Table S2).
Effect size calculations
All calculations were performed using Comprehensive Meta-Analysis Software (26). For each study, the effect size of the difference between healthy individuals and the comparison group was calculated as Hedge's g (27) on the basis of either (a) group means and standard deviations, (b) t-tests or F-tests of the group effect, (c) p-value and sample size, or (d) Cohen's d and sample size. Hedge's g is calculated as (M1 – M2)/(SDpooled), where the pooled standard deviation is weighted by the number of participants in each group. Many of the included studies examined MMN amplitude from multiple groups, as well as from multiple deviant types, probabilities, and magnitudes. For the broad group comparisons, the combined F-value for the group effect across all levels of deviant type, magnitude and probability was entered. In the event that a group-level F statistic was unavailable, the means and standard deviations were pooled across stimulus types for each group, separately, to be entered into the meta-analysis. Later, group effects for individual deviant types, magnitude, and probabilities were examined separately where reported data permitted such examination.
Sampling bias
As the “file drawer problem” represents a significant threat to the validity of conclusions drawn from the meta-analysis, three calculations were performed to determine whether sampling bias significantly impacted the estimates of overall effect size by group. These three calculations are: fail-safe N (28), the trim and fill method to correct for nonnormal distribution of effect size (29), and Kendall's tau. All three sampling bias indices were examined separately for each group included in the present meta-analysis. Overall, there was modest evidence of sampling bias for the REL, CHR, and SZ-F samples (see Supplementary Table S3). Following correction for sampling bias, the effect sizes for these three groups was 0.21 (CI = -0.05-0.46), 0.30 (CI = 0.12-0.47), and 0.37 (CI = 0.13 – 0.60), respectively.
Results
Sample Characteristics
The combined demographic information from these studies is presented in Table 1. The average age for healthy, SZ-All, SZ-C, BP, and REL participants was approximately 33 years (range = 31.82–36.22), whereas SZ-F and CHR individuals tended to be younger (average age = 23.57 and 19.71 years, respectively). A larger proportion of participants in the schizophrenia patient groups were male (range = 66.54%–73.95%) compared to healthy controls, CHR, BP, and REL groups (range = 37.43%–59.60%). Finally, duration of illness data was only available for 54 of the patient samples. SZ-C and SZ-All groups had an average illness duration of 14.09 and 12.24 years, respectively; similarly, BP participants had an average illness duration of 9.67 years. As expected, the SZ-F group had the shortest illness duration of 0.62 years.
Table 1.
| Sample Size | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Median | Average | Total | Average Age | % Males | Duration of Illness | |
| Healthy control participants (N= 105 samples) | 22.00 (range = 9-753) | 37.71 ± 79.07 | 3960 | 31.82± 7.91 | 57.97% ± 16.47% | -- |
| All schizophrenia patients (N = 75 samples) | 23.00 (range = 9-966) | 50.63 ± 126.32 | 3797 | 35.74 ± 6.57 | 71.27% ± 16.43% | 12.24 ± 7.04a |
| Chronic schizophrenia patients (N = 13 samples) | 17.00 (range = 10-45) | 20.62 ± 9.64 | 268 | 35.81 ± 7.28 | 73.95% ± 17.82% | 14.09 ± 4.88b |
| First episode schizophrenia patients (N = 13 samples) | 20.00 (range = 11-51) | 23.08 ± 10.84 | 300 | 23.57 ± 2.75 | 66.54% ± 16.22% | 0.62 ± 0.34c |
| Bipolar patients (N = 9 samples) | 25.00 (range = 11-52) | 26.67 ± 12.85 | 240 | 33.53 ± 8.91 | 49.09% ± 21.79% | 9.67 ± 2.91d |
| High-risk individuals (N= 16 samples) | 26.00 (range = 14-67) | 31.56 ± 15.29 | 505 | 19.71 ± 4.21 | 59.60% ± 11.83% | -- |
| First-degree relatives (N = 8 samples) | 21.00 (range = 15-74) | 29.00 ± 19.27 | 232 | 36.22 ± 14.68 | 37.43% ± 10.36% | -- |
Duration of illness data available for 38 samples
Duration of illness data available for 7 samples
Duration of illness data available for 4 samples
Duration of illness data available for 5 samples
MMN effect size by group
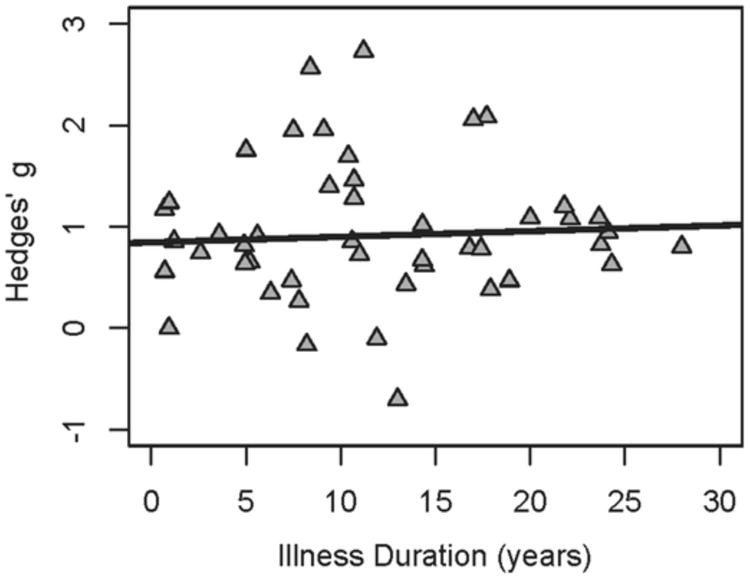
Effect sizes by group are depicted in Figure 1. The SZ-All group exhibited a large effect size of 0.95 (CI = 0.85–1.04), similar to that reported by Umbricht and Krljes (12). When examining the impact of illness duration on estimates of effect size, it was observed that SZ-C exhibited effect sizes comparable to the SZ-All group (effect size = 0.81; CI = 0.59–1.03); however, the SZ-F group had a significantly smaller effect size than any of the other schizophrenia patient groups (effect size = 0.42; CI = 0.19–0.65; p's < 0.05). This observation that first-episode patients have a smaller MMN impairment than do patient groups at later stages of the illness may suggest a progressive nature to MMN impairment in schizophrenia, as proposed by others (e.g., 10). To test this hypothesis, a meta-regression was conducted in which the duration of illness for all of the schizophrenia patient groups was entered as a moderator variable on effect size (47 samples included). The relationship between duration of illness and effect size is shown in Figure 2. Whereas there appeared to be a qualitatively positive relationship between duration of illness and effect size, a linear model was not a significant fit for the data (p = 0.59). Taken together, these observations suggest that any progression in MMN impairment among schizophrenia patients does not follow a linear trajectory.
Figure 1.
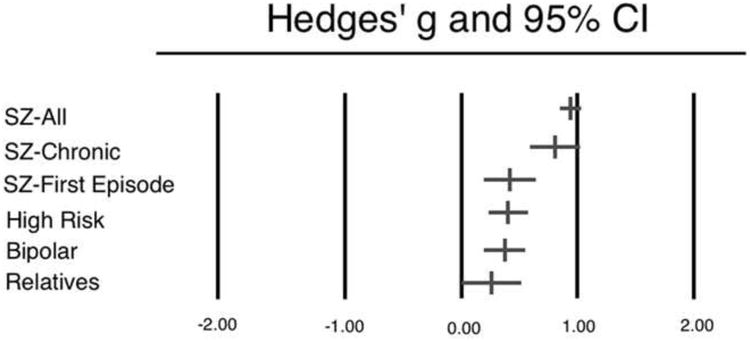
Figure 2.
We next examined MMN amplitude among REL and CHR. REL exhibited an effect size of 0.26 (CI = -0.01–0.52), indicating that the MMN amplitude from this group was qualitatively but not significantly reduced compared to healthy controls (p = 0.053). Furthermore, this effect was significantly lower than effect size estimates from SZ-All and SZ-C (t's > 3.49; p's < 0.05), but not statistically distinguishable from that of the SZ-F group (t = 0.99; p = 0.34). This observation suggests that impaired MMN amplitude may be considered a weak marker of genetic risk for schizophrenia; however, this conclusion should be made with caution as the comparatively small number of REL studies produced the largest variance in effect size estimates of all the groups examined in the present analysis.
CHR participants similarly exhibited a modest effect size of 0.40 (CI = 0.23– 0.58), which was both significantly elevated compared to healthy controls (p < 0.001) and significantly lower than effect size estimates from SZ-All and SZ-C (t's > 2.86; p's < 0.05). CHR effect sizes were not significantly lower than effect sizes from the SZ-F group, however (t = 0.10; p = 0.92). To test the hypothesis that reduced MMN is predictive of conversion to psychosis, a subset of CHR samples was examined for which outcome data were available (8, 16, 17, 30, 31). The results from this analysis are presented in Table 2. Overall, there was a significant difference in effect size between those who went on to develop a psychotic episode by follow-up, approximately 2 years later (effect size = 0.79; CI = 0.44–1.15) and those who did not convert to psychosis (effect size = 0.17; CI = -0.06–0.39). Importantly, and taken together with observations of nonsignificant MMN impairment in unaffected relatives, it can be concluded that MMN impairment may be a useful biomarker for the brain abnormalities that are associated with the onset of diagnosable schizophrenia.
Table 2.
| Sample Size (Converters : Non-Converters) | Follow-up period (years) | Effect size (converters) | Effect size (non-converters) | |
|---|---|---|---|---|
| Bodatsch et al, 2011 (14) | 25:37 | 2.67 | 0.48 | 0.02 |
| Higuchi et al, 2014 (28) | 4: 15 | 2.20 | 1.93 | 0.23 |
| Hsieh et al.,2012 (29) | 11 : 19 | 2.00 | 1.02 | 0.33 |
| Shaikh et al, 2011 (15) | 10:31 | 2.00 | 0.74 | 0.27 |
| Perez et al, 2014 (6) | 15: 16 | 1.00 | 0.67 | 0.05 |
|
| ||||
| AVERAGE | 13.0 : 23.6 | 1.97 | 0.79a | 0.17a*** |
Group effect size is weighted by sample size
p< 0.001
Finally, BP participants exhibited an effect size that was comparable to that of CHR participants (effect size = 0.37; CI = 0.19–0.55), which was significantly elevated compared to healthy controls (p < 0.001) and significantly lower than effect size estimates from SZ-All and SZ-C (t's > 3.04; p's < 0.01). BP effect sizes did not differ significantly from those of SZ-F, however (t = 0.28; p = 0.78). As decreased MMN amplitude appears to be related to disease process rather than genetic vulnerability (see above), this modest effect may reflect overlap of clinical features that are shared by these two groups of patients.
Effect size by deviant type, magnitude, and probability
To test the effect of deviant type, magnitude, and probability on effect size estimates, all schizophrenia patient groups were examined (N = 101 samples). Only three deviant types were examined in two or more of the 101 samples: tone duration (N=63 samples), tone frequency (N=54 samples), and tone intensity (N=7 samples). Consistent with observations reported by Umbricht & Krljes (12), duration deviants produced a significantly larger effect size (0.94; CI = 0.85–1.04) compared to effect sizes produced by frequency deviants (0.72; CI = 0.57–0.87; p < 0.05). Intensity deviants yielded an effect size that was significantly smaller than that of duration deviants (effect size = 0.62; CI = 0.40–0.85; p < 0.05), but not frequency deviants (t = 0.71; p = 0.49).
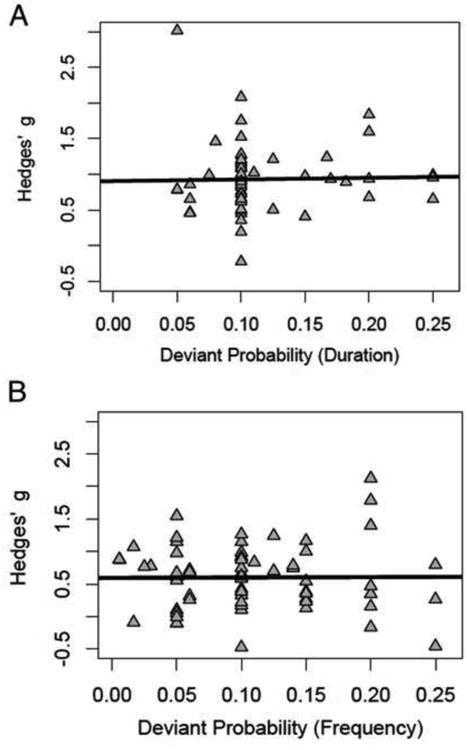
We next examined the relationship between magnitude of deviant change and probability of deviant occurrence separately for duration and frequency deviant types. The results from these meta-regressions are presented in Figures 3 and 4. Broadly, there was no significant effect of these paradigm characteristics on MMN effect size estimates for either duration or frequency deviant types (p's > 0.74). For additional details, see Supplementary Materials.
Figure 3.
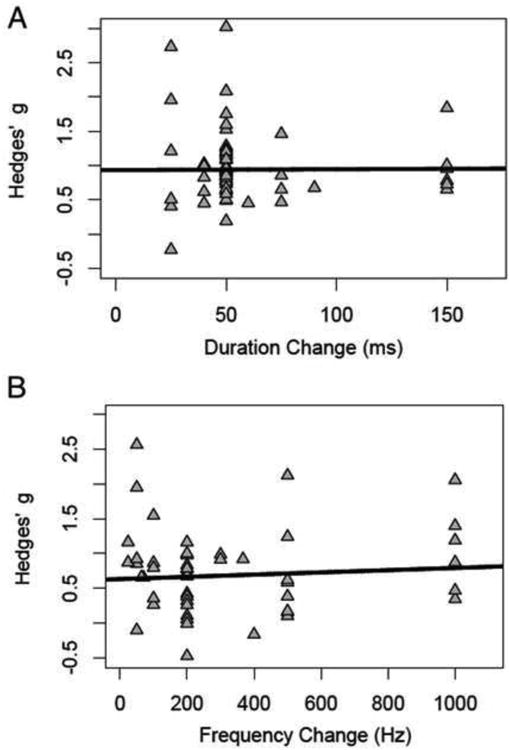
Figure 4.
Effect size by attention
Lastly, we examined effect size as a function of attention. Of the 101 schizophrenia patient samples, 90 completed paradigms in which the auditory stimuli were not attended, and only 6 (32-37) completed a task in which participants were required to make a response to the deviant tones (5 studies contained insufficient information for inclusion). We found that effect sizes were larger when tones were unattended (effect size = 0.87; CI = 0.78–0.97; range = -0.70–2.73) compared to when they were attended (effect size = 0.59; CI = 0.34–0.84; range = 0.35–1.40) at the level of a trend (p = 0.08). Though based on a small number of samples in which tones were attended, these trend-level results suggest that modulation of the overlapping N2b component by directing attention to the stimuli may minimize between-group differences in MMN production.
Discussion
This meta-analysis yields important insights into the nature and course of MMN impairment in schizophrenia. Among the SZ-All participants, the effect size estimated from this meta-analysis (0.95) is quite similar to the group effect size of 0.99 described by Umbricht & Krljes (12), despite the present study reporting findings from an additional 65 patient samples. Extending this work, we found that SZ-C participants have a significantly larger effect size than do SZ-F participants. This observation may suggest that MMN amplitude is associated with disease progression; indeed, this conclusion is modestly supported by Umbricht and Krljes's (12) meta-analysis. However, the nonsignificant relationship between duration of illness and effect size in the meta-regression analysis suggests that any progressive impairment that can be observed in patients is not a linear process. To speculate, one parsimonious explanation for this effect is that MMN impairment worsens within the first 1-2 years of diagnosis, but stabilizes after this critical period has passed. Such a pattern of nonlinear progressive impairment is consistent with reports from Salisbury and colleagues demonstrating a progressive course of MMN impairment over the first 18 months of a psychotic illness (10). Alternatively, these seemingly contradictory findings between the stage of illness group contrasts and the duration of illness regression may be explained by ascertainment biases. That is, the SZ-F group may be comprised of a mix of individuals, some of whom will go on to have a relatively good outcome and may have more intact MMN, and some with robust MMN deficits who go on to have poor outcomes. We suspect that the proportion of poor outcome patients is higher in the SZ-C groups than in the SZ-F groups. In this case, the significant difference between SZ-F and SZ-C MMN production may be better explained by sampling differences, rather than a progressive decline of MMN amplitude over illness course.
We next explored the sensitivity of the MMN to genetic vulnerability and process of conversion among first-degree relatives and patients at clinically elevated risk for developing a psychotic disorder. We found that first-degree relatives exhibit a modest, non-significant effect size of 0.26. Among CHR, we observed that the overall effect size of high-risk participants who converted to psychosis was quite large (0.79), and that high-risk participants who did not convert to psychosis had a non-significant effect size of 0.17, which was statistically indistinguishable from healthy comparison participants. By contrast, CHR participants who converted to psychosis exhibited effect sizes comparable to that of the SZ-C group and quite a bit larger than the first-episode schizophrenia group. Again, this puzzling pattern of findings is not easily accounted for by a linear progression of MMN impairment from prodromal status to early stages of the illness. We offer two speculative explanations for these data. One possibility that has already been identified (see above) again relates to differential composition of patient types within samples. For instance, CHR individuals who have been in need of care for a number of years prior to meeting diagnostic criteria for schizophrenia may represent a relatively homogenous group of patients with poor premorbid functioning. These participants may therefore be expected to have MMN impairment similar to SZ-C individuals, who also have a poor outcome in the long term. Alternatively, it is possible that the effect size estimates for the CHR converters vs. non-converters are unreliable, as yet based on too few small sample studies where influential outliers may impact results. Charting the course of MMN impairment will require longer term follow up studies of cohorts followed from clinical high risk diagnosis to 5 years post schizophrenia diagnosis to resolve these issues definitively. That said, the available data suggest that MMN impairment is best interpreted as a marker for likely progression and conversion to the disorder, rather than as a marker of genetic vulnerability. Whereas its utility as an endophenotype for the illness should be evaluated in futures studies using more targeted methods, the present analysis suggests that MMN impairment is possibly a weak marker for genetic vulnerability in otherwise psychiatrically healthy individuals.
Lastly, BP participants exhibited an effect size that was similar to that of CHR and SZ-F (0.37), and was significantly smaller than SZ-C and SZ-All. Such a finding may reflect shared neurobiology across disorders; indeed, it has been suggested that glutamatergic imbalances that are thought underlie MMN deficits in schizophrenia can also be observed in individuals with bipolar disorders (for a review, see 19). Alternately, this modest group effect that sits halfway between healthy controls and schizophrenia patients may reflect heterogeneity within bipolar disorder. It is possible that bipolar samples with a history of psychosis will exhibit larger MMN deficits than bipolar patients without psychosis (but see 31).
The second aim of the present study was to determine which (if any) stimulus characteristics were associated with robust effect sizes. Similar to Umbricht and Krljes (12), we found that duration deviants produced a significantly larger effect size than frequency deviants (0.94 and 0.72, respectively). Unlike this first meta-analysis, however, we found no evidence of a clear pattern between effect size and deviant probability or deviant magnitude. Umbricht & Krljes (12) reported nonsignificant trends towards larger effect sizes for (1) decreasing deviant probability and (2) decreasing magnitude change between standard and deviant tones, particularly in the duration deviant conditions. To corroborate these trends, within-study designs that explicitly examine the impact of deviant magnitude on MMN production in patients suggest that larger changes between standard and deviant tones are associated with larger between-group differences (22, 38). Similarly, it has been reported that decreased deviant probability yields larger effect sizes in carefully controlled studies (22, 39).
The observation from the present meta-analysis that there appears to be no relationship between these experimental parameters and effect size may indicate that these effects are relatively small, and can only be detected using within-subject designs. These findings have important implications for models of MMN impairment in schizophrenia that emphasize a role for abnormalities in stimulus discrimination or other perceptual impairments (e.g., 21). If MMN impairment were truly a function of poor stimulus discrimination, the most robust group differences ought to emerge at deviant changes that are the most difficult to detect (23, 24). This type of overall impairment regardless of stimulus characteristics appears to be more consistent with a failure in higher order auditory expectations, rather than an early perceptual deficit or otherwise poor stimulus discrimination.
Finally, the trend-level observation that between-group differences are minimized when stimuli are attended suggests that the overlapping N2b component can significantly impact estimates of MMN impairment in schizophrenia. This conclusion should be made with caution, however, as there were a small number of studies in which stimuli were actively attended by participants (N=6). Future studies may therefore be conducted to confirm this hypothesis using a within-sample design.
Taken together, the present results suggest that MMN impairment is a consequence of higher-order auditory expectancy deficits among schizophrenia patients and those who are at elevated clinical risk for converting to psychosis. Furthermore, these deficits may progress in a nonlinear fashion such that may be more closely examined in future studies. Such findings provide important support for the use of this measure in the development of novel diagnostic approaches for early detection and intervention.
Supplementary Material
Acknowledgments
We would like to acknowledge the contributions of Alexandra Gradzka, who assisted with data acquisition.
This work was supported by the National Institute of Mental Health (R01 MH065034 to J.G. and T32 MH067533-10).
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naatanen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118:2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PloS one. 2012;7:e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasser PE, Schall U, Todd J, Michie PT, Ward PB, Johnston P, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi H, Rissling AJ, Pascual-Marqui R, Kirihara K, Pela M, Sprock J, et al. Neural substrates of normal and impaired preattentive sensory discrimination in large cohorts of nonpsychiatric subjects and schizophrenia patients as indexed by MMN and P3a change detection responses. Neuroimage. 2013;66:594–603. doi: 10.1016/j.neuroimage.2012.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rissling AJ, Miyakoshi M, Sugar CA, Braff DL, Makeig S, Light GA. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. NeuroImage Clinical. 2014;6:424–437. doi: 10.1016/j.nicl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 7.Naatanen R, Shiga T, Asano S, Yabe H. Mismatch negativity (MMN) deficiency: A break-through biomarker in predicting psychosis onset. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2015 doi: 10.1016/j.ijpsycho.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–469. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Light GA, Naatanen R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15175–15176. doi: 10.1073/pnas.1313287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 12.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Pol HEH, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophrenia Bulletin. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiang M, Braff DL, Sprock J, Light GA. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:1949–1957. doi: 10.1016/j.clinph.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, et al. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh M, Valmaggia L, Broome MR, Dutt A, Lappin J, Day F, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012;134:42–48. doi: 10.1016/j.schres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Jessen F, Fries T, Kucharski C, Nishimura T, Hoenig K, Maier W, et al. Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neuroscience letters. 2001;309:185–188. doi: 10.1016/s0304-3940(01)02072-9. [DOI] [PubMed] [Google Scholar]
- 19.Chitty KM, Lagopoulos J, Lee RS, Hickie IB, Hermens DF. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23:1348–1363. doi: 10.1016/j.euroneuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Bora E, Pantelis C. Meta-analysis of Cognitive Impairment in First-episode Bipolar Disorder: Comparison with First-episode Schizophrenia and Healthy Controls. Schizophr Bull. 2015 doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. The American journal of psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- 22.Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalography and clinical neurophysiology. 1998;108:143–153. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 23.Todd J, Harms L, Schall U, Michie PT. Mismatch negativity: translating the potential. Frontiers in psychiatry. 2013;4:171–171. doi: 10.3389/fpsyt.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd J, Whitson L, Smith E, Michie PT, Schall U, Ward PB. What's intact and what's not within the mismatch negativity system in schizophrenia. Psychophysiology. 2014;51:337–347. doi: 10.1111/psyp.12181. [DOI] [PubMed] [Google Scholar]
- 25.Todd J, Michie PT, Jablensky AV. Do loudness cues contribute to duration mismatch negativity reduction in schizophrenia? Neuroreport. 2001;12:4069–4073. doi: 10.1097/00001756-200112210-00042. [DOI] [PubMed] [Google Scholar]
- 26.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Engelwood, NJ: Biostat; 2005. [Google Scholar]
- 27.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 28.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychological Bulletin. 1979;85:638–641. [Google Scholar]
- 29.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 30.Higuchi Y, Seo T, Miyanishi T, Kawasaki Y, Suzuki M, Sumiyoshi T. Mismatch negativity and p3a/reorienting complex in subjects with schizophrenia or at-risk mental state. Frontiers in behavioral neuroscience. 2014;8:172. doi: 10.3389/fnbeh.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh MH, Shan JC, Huang WL, Cheng WC, Chiu MJ, Jaw FS, et al. Auditory event-related potential of subjects with suspected pre-psychotic state and first-episode psychosis. Schizophr Res. 2012;140:243–249. doi: 10.1016/j.schres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Alain C, Cortese F, Bernstein LJ, He Y, Zipursky RB. Auditory feature conjunction in patients with schizophrenia. Schizophr Res. 2001;49:179–191. doi: 10.1016/s0920-9964(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 33.Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it's time for a change: failures to track context in schizophrenia. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2010;78:3–13. doi: 10.1016/j.ijpsycho.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasai K, Okazawa K, Nakagome K, Hiramatsu K, Hata A, Fukuda M, et al. Mismatch negativity and N2b attenuation as an indicator for dysfunction of the preattentive and controlled processing for deviance detection in schizophrenia: a topographic event-related potential study. Schizophr Res. 1999;35:141–156. doi: 10.1016/s0920-9964(98)00116-9. [DOI] [PubMed] [Google Scholar]
- 35.Kirino E, Inoue R. The relationship of mismatch negativity to quantitative EEG and morphological findings in schizophrenia. Journal of psychiatric research. 1999;33:445–456. doi: 10.1016/s0022-3956(99)00012-6. [DOI] [PubMed] [Google Scholar]
- 36.Kirino E. Mismatch negativity correlates with delta and theta EEG power in schizophrenia. The International journal of neuroscience. 2007;117:1257–1279. doi: 10.1080/00207450600936635. [DOI] [PubMed] [Google Scholar]
- 37.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 38.Horton J, Millar A, Labelle A, Knott VJ. MMN responsivity to manipulations of frequency and duration deviants in chronic, clozapine-treated schizophrenia patients. Schizophr Res. 2011;126:202–211. doi: 10.1016/j.schres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


