Abstract
Introduction
Muscle cramping is a common symptom in amyotrophic lateral sclerosis (ALS) without efficacious treatment. The natural history of this symptom is unknown, which hampers efforts to design optimal clinical trials.
Methods
We surveyed early stage ALS patients about their experience with cramps each month by phone for up to 21 months.
Results
Cramps developed in 95% of patients over the course of their disease. The number of cramps experienced by an individual varied widely from month-to-month and trended lower after the first year of illness (P = 0.26). Those with limb-onset and age >60 years had more cramps than bulbar-onset (P<0.0001) and younger patients (P<0.0001).
Discussion
The high variability of the number of cramps experienced suggests that clinical trials will need to use crossover designs or large numbers of participants, even when the treatment effect is substantial.
Keywords: amyotrophic lateral sclerosis, cramps, pain, muscle spasms, natural history
INTRODUCTION
Progressive weakness without significant pain or sensory loss is the typical presentation of Amyotrophic Lateral Sclerosis (ALS). Most patients do report painful symptoms at some point during their illness [1], and muscle cramps are a leading source of pain.[2] Cramps have been reported to affect about two-thirds of ALS patients [3,4]; the cause is uncertain, and there is support for both peripheral and central nervous system components in the literature.[5] There is no clearly efficacious treatment for cramps in ALS.[6]
Despite the high prevalence of cramps in the ALS population, only 2 published studies have directly investigated their treatment in the disease [7,8], and practice guidelines reflect the lack of an evidence-based approach.[6] Many of the recommendations are based on anecdotal experience or from studies focused on benign nocturnal cramps.[9] There are many inherent difficulties in studying cramps, because they are variable, subjective, and self-reported. There have been no formal natural history studies of muscle cramps in ALS assessing features such as their prevalence, duration, or intensity. [10] The lack of natural history data makes it difficult to plan outcome measures and sampling intervals for clinical trials.[8] For these reasons, we undertook a prospective, longitudinal study of the prevalence, frequency, distribution, and severity of muscle cramps in a cohort of ALS patients over the course of their disease.
MATERIALS AND METHODS
From July 2010 to December 2012, all ALS patients evaluated at the Wake Forest ALS Center with duration of weakness <18 months were asked to participate in the study. The diagnosis of ALS was made by clinical evaluation with support from electrodiagnostic and imaging studies with a grade of “clinically possible” ALS or higher according to the revised El Escorial Criteria.[11] Limb, bulbar, and respiratory onset patients were included, and symptoms of cramps were not necessary for inclusion in the study. This study was approved by the Wake Forest Health Sciences Institutional Review Board, and participants provided written informed consent.
Longitudinal data regarding cramps were collected during monthly patient interviews for a maximum of 2 years. Interviewers (SC, ES) trained in the definitions of cramps [5] guided participants through a survey about their experience with cramps. Cramps were defined as sudden onset, focal muscle pains with a palpable contraction or feeling of contraction. Symptoms were explored carefully to avoid confusion with similar symptoms, particularly pain related to spasticity or muscle strain, spontaneous clonus, eyelid myokymia, and tremors. The baseline survey was conducted during a routine clinic visit along with collection of demographic data and the revised ALS Functional Rating Scale (ALSFRS) score.[12] Subsequent monthly surveys were administered via telephone or could be conducted face-to face during a routine clinic visit, typically scheduled every 3 months. ALSFRS scores were also collected during these visits. During interviews, caregivers could relay information from the patient but were not allowed to provide information directly. The survey instrument (see supplementary file, available online) was designed for this study and consisted of 10 questions about the number, severity, duration, and location of muscle cramps that could be recalled over the previous month. A cramp index score was calculated by multiplying the number of cramps/month by severity score (1–4) and by duration score (1–3). Daily logs were not kept, and participants were asked to estimate their experience over the preceding month without access to previous data. Use of riluzole, baclofen and gabapentin were recorded each month.
Data were examined using normal probability plots and were appropriate for statistical tests without requiring transformations. Independent 2-sample 2-sided t-tests were conducted to compare the mean number of cramps and the cramp index in bulbar and non-bulbar onset ALS, men and women, and younger and older age groups. Following adjustment of the data to reflect months from onset of ALS, a 1-way ANOVA was used to test for cramp number differences between years 1 through 3. The number of cramps reported by participants taking riluzole was compared with those not taking riluzole at 3-month intervals over the adjusted course of the data collection using simple t-tests. To determine necessary sample size for a hypothetical clinical trial, we assumed post-hoc power analysis with an alpha error of .05 at 80% power.
RESULTS
Forty-one ALS patients were enrolled in the study (Table). The onset location was cervical in 34%, lumbosacral in 46%, and bulbar in 20%. Eight patients provided only baseline data, but the remaining 33 patients were followed for a mean of 8.5 months (range 2–21 months). At baseline, 32 (78%) participants reported cramps, and the mean number of cramps per month was 46 ±103 (range 0–600). Most described the cramp pain as moderate (34%) or severe (24%). Five individuals (12%) experienced extremely frequent (≥100/month) and severe cramps, but most stated that the cramps lasted less than 1 minute and were painless or mildly painful. Frequent cramps (≥2 cramps/day) were reported by 12 participants (29%), and cramps interrupted sleep in 50% of those who reported cramps. Cramps were most commonly reported in the calf and thigh followed by the hand and foot. The mean cramp index at baseline was 225 ± 587 (range 0–3600). Nine individuals did not report cramps at baseline, and nearly half (46%) had fewer than 10 cramps/month. During follow-up of the 33 participants, only 2 never developed cramps; this brings the incidence of cramps over the course of the study to 95%. Over the course of the study, 15 participants (37%) reported ≥60 cramps/month at least once and 10 people (24%) reported ≥100 cramps/month at least once. Of the 17 people who provided follow-up information and experienced infrequent cramps (<10 cramps/month) at baseline, 14 (82%) never developed frequent (≥60) cramps/month.
Table.
Characteristics of participants at baseline
| Mean | Range | |
|---|---|---|
| Age (years) | 64 | 31–84 |
| Number of participants | 41 (23 men) | -- |
| Duration of disease (months) | 11 | 1–18 |
| ALS-FRS | 39 | 27–48 |
| Number with limb onset | 32 | -- |
| Number of cramps per month | 46±103 | 0–600 |
ALS-FRS, Amyotrophic Lateral Sclerosis-Functional Rating Scale
After adjusting data points to the time from onset of weakness, the mean number of cramps trended down and became less variable (first year 46.28 ± 95.73, second year 37.63 ± 62.50, third year 24.14 ± 31.74), but this trend did not reach statistical significance (P = 0.26, Figure). Analyzing all data points throughout the study, the average number of cramps was significantly higher in those with limb-onset ALS (37.48 ± 18.58) compared to those with bulbar-onset (7.12 ± 8.43), P<0.0001. There was no difference in age between the limb-onset (64.27 years) and bulbar-onset (64.25 years) groups. Participants aged 60 or younger reported significantly fewer cramps (mean = 16.08 ± 14.34) than older participants (mean = 45.05 ± 20.61, P<0.0001). There were no differences in cramp number between men and women (P = 0.1347). Similar analysis of the cramp index score mirrored these findings in each case (data not shown). The number of cramps was compared between those who were taking riluzole and those who were not at multiple points in their disease, and no differences were observed (P-values ranged from 0.2370 – 0.9813). To describe the characteristics of likely clinical trial participants, we identified a cohort of 10 participants with at least 2 observations and who complained of 60 or more cramps per month. Over an average of 8.8 observations, the mean numbers of cramps/month was 90.6, SD 67. In this group, standard deviations ranged up to 200% of the mean for an individual.
Figure.
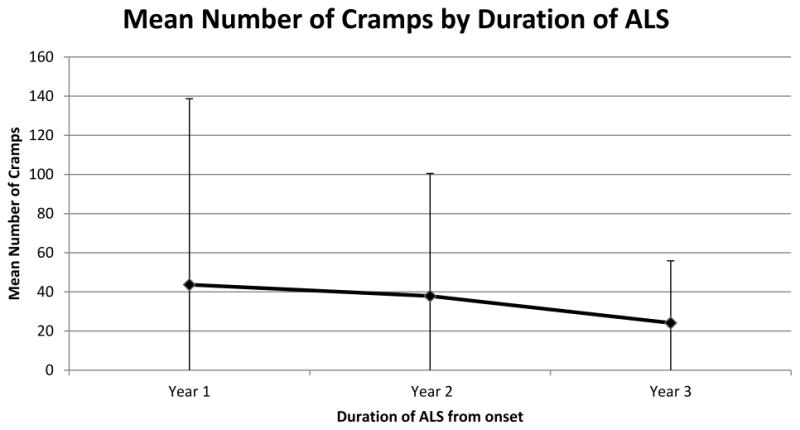
The mean number of cramps per month with standard deviation (whisker lines) reported during progression of ALS. The mean number of cramps declined over time, but this trend did not reach statistical significance (P = 0.26).
DISCUSSION
Muscle cramps were a common symptom of ALS, occurring in 95% of our cohort at some point during their disease course. There was marked variability in the severity and frequency of cramps among these ALS patients. Most never experienced frequent cramps, but nearly a quarter of patients experienced over 100 cramps during a month. In those who experienced numerous cramps, there was also wide variability in the severity from month to month. The majority of those patients who did not have many cramps at the time of diagnosis never experienced frequent cramps.
There is a paucity of literature detailing the ALS patient experience with cramps. A study of 167 ALS patients noted that 72% of patients reported cramps when the mean duration of disease was 2.6 years. [4] This is similar to the prevalence of cramps in our cohort at baseline (78%); the mean disease duration of 11 months was approximately 1.5 years earlier than the previous study, suggesting the prevalence of cramps is remarkably stable from diagnosis until the later stages of ALS. A widely quoted abstract surveyed 84 ALS patients and found a slightly lower prevalence of cramps (62%) [3], but the mean age of the subjects, duration of illness at the time of the survey, and detailed methodology of the survey were not reported. It is possible that differences in the study population or the survey methods explain the lower prevalence of cramps found in this report.
While the prevalence of cramps appears to be stable from the first to the third years of ALS, we found that, the numbers of cramps experienced by patients during the first year of disease trended down over years 2 and 3 but did not reach statistical significance, even between years 1 and 3. A larger sample of patients might have overcome the wide standard deviations and demonstrated a significant decline as the disease progressed. Compared with the healthy baseline state, cramps increase during the months of illness prior to diagnosis, but this cannot be demonstrated without studying a pre-symptomatic cohort as would be possible in familial ALS. In those with at least 2 cramps per day, we found wide month-to-month fluctuation in the cramp number and the cramp index. Previously, there has not been a longitudinal study of cramps in the ALS population except for a few interventional studies and clinical trials where scant details were presented. The day-to-day variability of cramps in the general ALS population has not been studied specifically, but 2 clinical trials provide some indication. In a study of dronabinol for cramps in ALS about 7% of subjects who reported several cramps per day at baseline stopped having cramps during a 2 week lead-in period, indicating significant individual variability.[8] An open-label study of levetiracetam in ALS patients found apparently stable week-to-week measurements of mean cramp severity and frequency, but since daily logs were not kept the results may have been affected by recall bias.[7] Similar to our data collected each month, this study found a large inter-subject standard deviation for cramp frequency (4 cramps/day ± 4.5) but the week-to-week variability for individual subjects was not reported. We found that cramps were more common in patients with limb-onset ALS, likely reflecting the greater degree of lower motor neuron involvement as the disease progressed during the follow-up period. Older patients reported more cramps, perhaps due to the faster progression of disease usually seen in this group and the increasing propensity for cramps with aging suggested by the demographics of benign nocturnal cramp disorder.[13]
The strengths of our study include a prospective design that did not bias towards including those who complained of cramps (about one-quarter did not have these complaints). Our sample is well- balanced for gender and reflects the typical age and onset location distribution for ALS.[14] Vigorous efforts were made to enroll patients immediately after a confirmatory diagnosis of ALS so that cramps in the early phase of the disease could be studied. The mean disease duration in our cohort at baseline was 11 months. This is an indication of considerable success in recruiting participants with early ALS, particularly since the average time from onset to diagnosis is approximately 1 year [15] and is often followed by further delay when they are referred to a multi-disciplinary ALS center. The monthly phone interviews ensured adherence, and since previously collected data were not shared with participants, recall bias was minimized. As our goal was to study cramps over the course of the illness, there was concern that daily logs would be onerous and might lead to incomplete and inaccurate data reporting. Our study also has some limitations, including small sample size, variable length of follow-up, and use of a novel non-validated survey instrument. Also, there was a potential for recall bias representing the days nearest the phone interview.
Our findings have implications for future clinical trial design. The ideal study population, those suffering very frequent and painful cramps, made up only 12% of our cohort, indicating that few participants will be available at any single time at a center. If the inclusion criteria were to be liberalized to include all of those with at least 2 cramps per day, nearly 40% of ALS patients would qualify at some point in their illness, but the high variability in the numbers of cramps creates a challenge. Using a traditional 2-group placebo-controlled study, 70 patients would be needed to show a 50% reduction in cramp number per month, and 276 patients would be needed to detect a 25% reduction in cramps per month. A crossover design would reduce these numbers to 38 patients to detect a 50% drop in the number of cramps, but still a fairly high number of 140 patients to detect a 25% reduction. Others have suggested using a visual analog scale for daily cramps to reduce recall bias and a parallel group design to minimize the effects of high day-to-day variability of cramps.[8,10] Our data indicate that those who did not report cramps at the time of diagnosis are unlikely to later develop significant problems with these symptoms, so they can be effectively excluded from screening. The number of cramps tracked the cramp index very closely suggesting that data about the duration and severity of a cramp may not need to be collected in a clinical trial.
Muscle cramps are a common source of pain in ALS for which there is no effective treatment.[6] Numerous treatments have been suggested to treat cramps in ALS, but few have been rigorously evaluated. Only 2 clinical trials have directly tested medicines for cramps, and a handful of others listed cramps as a secondary outcome measure. A randomized and blinded trial of tetrahydrocannabinol failed to show benefit.[8] Cramps were a secondary outcome measure during a neuroprotection trial of vitamin E in ALS, which did not demonstrate a response treatment.[16] An open-label trial of levetiracetam showed reduced cramp frequency and severity, but no further trials of this agent have been performed.[7] Cramps were recorded as an adverse event during a clinical trial of gabapentin for ALS progression and no difference was found between the gabapentin and placebo groups. [17] A trial of mexiletine for cramps in ALS is underway using a crossover design and number of cramps per day as the primary outcome measure.[18] Our data indicate this is an optimal design, but this will eventually depend on the day-to-day variability of cramps and effect size. As in other studies [19,20], we did not find evidence that riluzole use is associated with fewer cramps, and fewer than 10% of our group were taking other medicines that are sometimes prescribed to reduce cramps (such as gabapentin or baclofen). Our data suggest that medicines prescribed to reduce cramps should be weaned intermittently, as the number of cramps is highly variable and trends downwards.
In conclusion, muscle cramps are a nearly universal occurrence (95%) during the course of ALS and are a major source of pain for about a quarter of patients at some point in their illness. Cramps develop early in the illness and show a trend to decrease over subsequent years. The number of cramps varies widely from month to month in those with frequent cramps. Older patients and those with limb-onset ALS report more cramps than younger and bulbar-onset ALS patients. An improved understanding of the natural history of cramps will help guide future clinical trials.
Supplementary Material
Acknowledgments
This research was supported by the Brian White ALS Research Foundation and NIH K23NS06289223 (MSC). There was no other grant support relevant to this work but Dr. Caress receives current grant support from GlaxoSmithKline and Biogen Idec.
ABBREVIATIONS
- ALS
Amyotrophic Lateral Sclerosis
- ALSFRS
ALS Functional Rating Scale
Footnotes
Parts of this work were presented at the 2013 American Academy of Neurology annual meeting in San Diego, CA.
The remaining authors have nothing to disclose and there are no conflicts of interest.
Contributor Information
James B. Caress, Department of Neurology, Wake Forest School of Medicine, Winston-Salem, NC
Stephanie L. Ciarlone, Department of Molecular Pharmacology and Physiology, Morsani College of Medicine, University of South Florida, Tampa, FL
Elizabeth A. Sullivan, Neurology Associates, Hattiesburg, MS
Leah P. Griffin, Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, NC
Michael S. Cartwright, Department of Neurology, Wake Forest School of Medicine, Winston-Salem, NC
References
- 1.Chio A, Canosa A, et al. Pain in amyotrophic lateral sclerosis: a population-based controlled study. Eur J Neurol. 2012;19:551–55. doi: 10.1111/j.1468-1331.2011.03540.x. [DOI] [PubMed] [Google Scholar]
- 2.Ganzini L, Johnston WS, Hoffman WF. Correlates of suffering in amyotrophic lateral sclerosis. Neurology. 1999;52:1434–1440. doi: 10.1212/wnl.52.7.1434. [DOI] [PubMed] [Google Scholar]
- 3.Heiman-Patterson TD, Rampal N, Brannagan TH, Acosta T, Forshew D, Bromberg MB. The spectrum of patient symptoms in ALS and symptom management. Neurology. 2001;56:A199. [Google Scholar]
- 4.Ringel SP, Murphy JR, Alderson MK, Bryan W, England JD, Miller RG, et al. The natural history of amyotrophic lateral sclerosis. Neurology. 1993;43:1316–1322. doi: 10.1212/wnl.43.7.1316. [DOI] [PubMed] [Google Scholar]
- 5.Caress JB, Paudyal B. Muscle Cramps and Fasciculations. In: Katirji B, et al., editors. Neuromuscular Disorders in Clinical Practice. 2. New York: Springer Science+Business Media; 2014. pp. 1455–1464. [Google Scholar]
- 6.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review) Neurology. 2009;73:1227–1233. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedlack RS, Pastula DM, Hawes J, Hedt D. Open-label pilot trial of levetiracetam for cramps and spasticity in patient with motor neuron disease. Amyotroph Lateral SC. 2009;10:210–215. doi: 10.1080/17482960802430773. [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomized, double-blind crossover trial. J Neurol Neurosur PS. 2010;81:1135–1140. doi: 10.1136/jnnp.2009.200642. [DOI] [PubMed] [Google Scholar]
- 9.Forshew DA, Bromberg MB. A survey of clinicians’ practice in the symptomatic treatment of ALS. Amyotroph Lateral SC. 2003;4:258–263. doi: 10.1080/14660820310017344. [DOI] [PubMed] [Google Scholar]
- 10.Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane DB Syst Rev. 2012;4:1–63. doi: 10.1002/14651858.CD004157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motorneuron disorders: official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 12.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A and the BDNF AKS Study Group (Phase III) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurosci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 13.Haskell SG, Fiebach NH. Clinical epidemiology of nocturnal leg cramps in male veterans. Am J Med Sci. 1997;313:210–14. doi: 10.1097/00000441-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lonergan R, Mitsumoto H, Murray B. In: Neuromuscular Disorders in Clinical Practice. 2. Katirji B, et al., editors. New York: Springer Science+Business Media; 2014. pp. 395–423. [Google Scholar]
- 15.Cellura E, Spataro R, Taiello AC, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosur. 2012;114:550–554. doi: 10.1016/j.clineuro.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Desnuelle C, Dib M, Garrel C, Favier A. A double-blind placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS riluzole-tocopherol Study Group. Amytroph Lateral SC. 2001;2:9–18. doi: 10.1080/146608201300079364. [DOI] [PubMed] [Google Scholar]
- 17.Miller R, Moore D, Young L, et al. Placebo-controlled trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology. 1996;47:1383–1388. doi: 10.1212/wnl.47.6.1383. [DOI] [PubMed] [Google Scholar]
- 18.Oskarsson B. [Accessed 2015 March 19];ClinicalTrials.gov: Mexiletine for the treatment of muscle cramps in ALS. 2014 Available at https://clinicaltrials.gov/ct2/show/NCT01811355.
- 19.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. New Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 20.Bensimon G, Lacomblez L, Delumeau JC, Bejuit R, Truffinet P, Meininger V. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol. 2002;249:609–615. doi: 10.1007/s004150200071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


