Abstract
Alzheimer’s disease (AD) and cerebrovascular diseases share common vascular risk factors that have disastrous effects on cerebrovascular regulation. Endothelial cells, lining inner walls of cerebral blood vessels, form a dynamic interface between the blood and the brain and are critical for the maintenance of neurovascular homeostasis. Accordingly, injury in endothelial cells is regarded as one of the earliest symptoms of impaired vasoregulatory mechanisms. Extracellular buildup of amyloid-β (Aβ) is a central pathogenic factor in AD. Aβ exerts potent detrimental effects on cerebral blood vessels and impairs endothelial structure and function. Recent evidence implicates vascular oxidative stress and activation of the non-selective cationic channel transient receptor potential melastatin (TRPM)-2 on endothelial cells in the mechanisms of Aβ-induced neurovascular dysfunction. Thus, Aβ triggers opening of TRPM2 channels in endothelial cells leading to intracellular Ca2+ overload and vasomotor dysfunction. The cerebrovascular dysfunction may contribute to AD pathogenesis by reducing the cerebral blood supply, leading to increased susceptibility to vascular insufficiency, and by promoting Aβ accumulation. The recent realization that vascular factors contribute to AD pathobiology suggests new targets for the prevention and treatment of this devastating disease.
Keywords: Alzheimer’s disease, β-amyloid, Cerebral blood flow, Cerebral endothelial cells, TRPM2
Introduction
Cerebrovascular endothelial dysfunction is emerging as a major risk factor for brain diseases, and injury to endothelial cells is regarded as one of the earliest symptoms of impaired vasoregulatory mechanisms. Endothelial cells, lining inner walls of cerebral blood vessels, act as an integrator for neurovascular homeostasis in an autocrine and paracrine manner (Fig. 1). Although the impact of endothelial dysfunction in atherosclerosis and cerebrovascular diseases is well appreciated, recent evidence implicates alterations in endothelial function also in Alzheimer’s disease (AD), the major cause of dementia in the elderly (Holtzman et al. 2012; Iadecola 2013). It is now well established that the amyloid-β peptide (Aβ), a key pathogenic factor in AD, has powerful cerebrovascular effects that alter the regulation of the cerebral circulation. Aβ-induced endothelial dysfunction may be responsible for the cerebrovascular dysregulation observed in patients with AD. In this brief review, we will examine the role of endothelial cells in normal cerebrovascular regulation and in the alterations observed in AD. Emphasis will be placed on the role of vascular oxidative-nitrosative stress and the transient receptor potential melastatin 2 (TRPM2) channels in the alterations in endothelial function induced by Aβ.
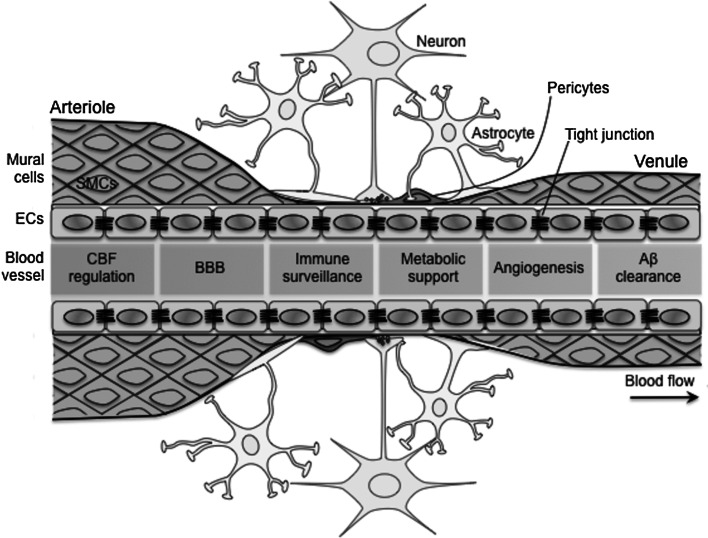
Fig. 1.
The neurovascular unit is formed of endothelial cells, mural cells (smooth muscle cells and pericytes), neurons, astrocytes, and others. The major function of neurovascular unit is to keep the brain in a homeostatic microenvironment. In arteries and capillaries, the astrocytes wrap the abluminal side of blood vessels with their foot processes. In capillaries, mural cells are replaced by pericytes. Endothelial cells line an inner layer of entire blood vessels and play a wide range of critical roles of vascular function in harmony with other neurovascular units and, thus, participate in all aspects of neurovascular homeostasis, including CBF regulation, BBB formation, immune surveillance, metabolic support, angiogenesis, and Aβ clearance. Aβ and cardiovascular risk factors damage the endothelial cells leading to the neurovascular, synaptic, and brain dysfunction. Aβ β-amyloid, BBB blood–brain barrier, ECs endothelial cells, SMCs smooth muscle cells
Role of Endothelial Cells in Neurovascular Regulation
Cerebrovascular cells (endothelial cells, smooth muscle cells, and pericytes), neurons, glia, and perivascular cells, collectively called the neurovascular unit, are tightly interconnected and operate in harmony to provide a homeostatic microenvironment for the brain. Cerebral endothelial cells control blood flow, regulate the exchange across the blood–brain barrier (BBB), contribute to innate immunity, control angiogenesis, and govern clearance of Aβ peptides (Fig. 1).
Cerebral Blood Flow Regulation
The brain is endowed with precise control mechanisms that ensure its energy supply well matched to its energetic needs (Iadecola 2004, 2013; Jackman and Iadecola 2015; Attwell et al. 2010; Lauritzen et al. 2012). Thus, the functional and structural integrity of the brain relies on a continuous and well-regulated blood supply of oxygen and nutrients, and thus, interruption of cerebral blood flow (CBF) results in brain dysfunction and cell death (Iadecola 2004; Lauritzen et al. 2012). Consequently, neural activity evokes a potent increase in CBF, termed functional hyperemia, that is thought to deliver energy substrates and remove metabolic byproducts from the brain (Iadecola 2004, 2013). Cerebral endothelial cells play a critical role in the CBF regulation by producing vasorelaxants, such as NO, bradykinin, prostacyclin, and vasoconstrictors, such as endothelin-1 and thromboxane A2 (Andresen et al. 2006; Faraci 2011; Iadecola 2004, 2013; Katusic and Austin 2014). A recent study demonstrated that a discrete endothelial cell lesion impairs the retrograde vasodilatation induced by neural activity in the somatosensory cortex, highlighting the critical role of these cells in the coordination of responses between intraparenchymal arterioles and pial vessels at the surface of the brain (Chen et al. 2014).
BBB
Cerebral endothelial cells are interconnected and sealed through tight junctions and have reduced transcellular vesicular transport (transcytosis), key features of the BBB. The BBB is highly impermeable so that specialized endothelial transporters control the trafficking of amino acids, ions, macromolecules, neurotransmitters, and other signaling molecules between the blood and the brain, which is a fundamental function of the BBB (Iadecola 2013; Zlokovic 2011). Thus, transporters located on the luminal side of the endothelial cells regulate the entry of nutrients into the brain, while transporters on the abluminal side control disposal of metabolic waste (Neuwelt et al. 2011). The major facilitator superfamily domain containing 2a (Mfsd2a) is selectively expressed in BBB-containing blood vessels in the brain and is a key regulator of BBB function by suppressing transcytosis (Ben-Zvi et al. 2014).
Aβ Clearance
Aβ is a key pathogenic factor involved in the mechanisms of AD, and the BBB plays a key role in regulating the transport of Aβ into and out of the brain (Zlokovic 2008). Therefore, Aβ in peripheral circulation is transported to the brain by receptors for advanced glycation end-products (RAGE) on luminal side of endothelial cells (Zlokovic 2008). Intracerebral Aβ, generally produced by neuronal activity, is removed from the brain via mechanisms involving the lipoprotein receptor-related protein (LRP)-1 and P-glycoprotein, a mechanism regulated by the serum response factor, myocardin, and phosphatidylcholine-binding clathrin assembly protein (PICALM) (Zhao et al. 2015; Zlokovic 2008). Thus, an imbalance between Aβ production and clearance contributes to Aβ deposition in the brain.
Immune Surveillance
Endothelial cells are strategically located and so can detect changes in peripheral and central immune signals. In response to the signals, they express various adhesion molecules, such as intercellular adhesion molecules, platelet-endothelial adhesion molecule-1, selectins, vascular adhesion molecule-1, etc., that recognize cognate molecules on circulating immune cells leading to the adhesion and transmigration of these cells into the brain (Weber et al. 2007). The expression of adhesion molecules, chemokines, and cytokines by endothelium, glia, and perivascular macrophages regulates the trafficking of immune cells across the BBB (Zlokovic 2008; Heppner et al. 2015). Such activity is critical both for immune surveillance in the normal brain and for immune response to injury. Furthermore, the recent discovery of the CNS lymphatic system requires a reassessment of immune surveillance in the brain in health and disease (Louveau et al. 2015).
Metabolic Support
The brain is lack of energy reserve so that metabolic function of endothelial cells is vital for the transport of nutrients to maintain the normal function of neurons and glia (Iadecola 2004, 2013; Iadecola and Nedergaard 2007). Therefore, endothelial cells are equipped with specialized transporter proteins that facilitate the entry of glucose, essential amino acids, vitamins, and other vital nutrients entry into the brain (Neuwelt et al. 2011). Transporters located on the abluminal side of endothelial cells are critical for the removal of potentially noxious byproducts of brain metabolisms. Therefore, endothelial cells are essential for the maintenance of the metabolic homeostasis of the cerebral microenvironment.
Angiogenesis
Endothelial cells, neurons, and glia produce vascular growth factors that provide trophic support for angiogenesis (Zacchigna et al. 2008). Such vasculotrophic support is essential during development when proper guiding cues, such as ephrins, slit ligands, semaphorins, and netrins, are required for correct alignment of both migrating axons and vessels (Carmeliet 2003). Furthermore, after brain injury, angiogenic growth factors released from endothelial cells, neurons, and glia such as vascular endothelial growth factor (VEGF) and angiopoietin-1, activate their receptors on endothelial cells present in preexisting blood vessels and orchestrate their migration and differentiation into new vessels, termed angiogenesis, providing a therapeutic option (Carmeliet and Ruiz de Almodovar 2013). Therefore, the coordinated interaction between endothelial cells, neurons, and glia is critical for vascular and brain repair capacity via angiogenesis
Structural and Functional Alterations in Brain Blood Vessels in AD
There is increasing evidence that the structure and function of the brain vessels are significantly altered in AD (Iadecola 2013; Zlokovic 2008). These alterations markedly disrupt the homeostatic balance and cause neurovascular dysfunction and synaptic dysregulation leading to brain dysfunction.
Structural Alterations
Accumulating evidence suggests that AD is associated with alterations in cerebrovascular structure (Park et al. 2013; Weller et al. 2009; Iadecola 2013; Zlokovic 2008). In large intracranial vessels, atherosclerosis is found in more than 77 % of AD patients (Yarchoan et al. 2012; Beach et al. 2007; Roher et al. 2003; Zhu et al. 2014; Roher et al. 2004). At the microvascular level, arterioles and capillaries show reduction in density, length, and mean diameters in AD (Bouras et al. 2006; Fischer et al. 1990; Kitaguchi et al. 2007; Smith and Greenberg 2009). In AD patients and mouse models, these alterations are often associated with distortion and constriction of capillaries (Smith and Greenberg 2009; Kitaguchi et al. 2007; Meyer et al. 2008; Park et al. 2013). The basement membrane of the vessels also undergoes degenerative changes, and heparin sulfate proteoglycans accumulate in the capillary basement membrane, contributing to the accumulation of Aβ (Morris et al. 2014; van Horssen et al. 2001; Park et al. 2013). In AD and cerebral amyloid angiopathy (CAA), accumulation of Aβ in cortical arterioles and capillaries triggers weakening of the blood vessel wall, a change associated with small cerebral infarcts and hemorrhages (Benedictus et al. 2015; Smith and Greenberg 2009).
Functional Alterations
CBF Dysregulation
CBF is decreased, and functional hyperemia is reduced in AD patients (Bateman et al. 2006; Hirao et al. 2005; Jagust et al. 1998; Johnson and Albert 2000; Luckhaus et al. 2008; Ruitenberg et al. 2005; Schroeter et al. 2007; Yoshiura et al. 2009). In particular, endothelium-dependent vasodilatation is altered in peripheral vessels of AD patients (Dede et al. 2007). In addition, cerebral smooth muscle cells are also converted into a hypercontractile phenotype, which leads to the stronger contractility of cerebral arterioles associated with reduced resting CBF and reactivity (Chow et al. 2007). These vascular effects have been attributed to Aβ, a key pathogenic factor in AD, which potently constricts blood vessels and impairs the fundamental mechanisms controlling the cerebral circulation (Iadecola 2013; Iadecola et al. 1999; Thomas et al. 1996). Endothelial oxidative and nitrosative stress has emerged as key pathogenic factors in cerebrovascular dysfunction (Szabo et al. 2007; Iadecola 2004). Specifically, experimental evidence indicates that free radical species generated from the NADPH oxidase are responsible for the neurovascular alterations induced by vascular risk factors and Aβ (Faraco and Iadecola 2013; Iadecola et al. 1999; Park et al. 2011, 2008). Free radical species can react with NO and form peroxynitrite, which has a powerful effect on CBF regulation by triggering DNA damage, as discussed below.
BBB Impairment
Due to the critical role of the BBB in providing the homeostatic microenvironment for the brain, its alterations have a powerful impact on brain diseases, including cognitive impairment related to aging and AD dementia (Zlokovic 2008). BBB breakdown induced by oxidative–nitrosative stress causes the entry of plasma proteins, an event leading to vascular inflammation, perivascular edema, and further oxidative–nitrosative stress (Pacher and Szabo 2008; Zlokovic 2011). For example, BBB disruption is an early event in the aging humans and mild cognitive impairment patients (Montagne et al. 2015). In addition, Aβ can activate innate immunity receptors and alter cerebrovascular regulation through oxidative stress. For example, the lack of the innate immunity receptor CD36 or RAGE protects endothelium-dependent responses and neurovascular coupling by attenuating vascular oxidative stress in WT mice treated with Aβ and amyloid precursor protein (APP) transgenic mice (Park et al. 2011, 2013). Furthermore, impairments in Aβ clearance through the BBB may also have an impact on the brain amyloid accumulation in AD (Taheri et al. 2011; Zlokovic 2008; Mawuenyega et al. 2010; Roberts et al. 2014). Thus, the reduced expression of the BBB transporters LRP-1, P-glycoprotein, and PICALM or the increased expression of Mfsda2 in the brain endothelium promotes vascular Aβ accumulation and may worsen the vascular dysfunction (Bell et al. 2009; Deane et al. 2003; Zhao et al. 2015; Ben-Zvi et al. 2014). Furthermore, increased circulating levels of Aβ in patients with vascular cognitive impairments and AD can aggravate oxidative stress, inflammation, and endothelial dysfunction and cause cerebrovascular insufficiency associated with accelerated progression of the disease (Iadecola 2013; Zhao et al. 2015; Park et al. 2013).
Impaired Metabolic Support
Glucose is brain’s main energy source and its delivery across the BBB heavily relies on endothelial cells lining cerebral blood vessels. Reduced brain glucose utilization occurs in regions related to learning, memory, overlaps with brain regions that are impacted in persons with AD (Ashe and Zahs 2010). These changes occur early in the course of the disease and are also present in cognitively normal people at genetic risk for AD (Nordberg et al. 2010; Faraco and Iadecola 2013; Iadecola 2013). Using mouse models with a deficit in the gene coding glucose transporter (Glut)-1 (Slc2a1+/−), Winkler et al. demonstrated that vascular Glut-1 deficiency results in marked alterations in vascular, BBB, and neuronal function that are remarkably prominent in the setting of AD pathology (Winkler et al. 2015).
Aberrant Angiogenesis
There is increasing evidence that aberrant angiogenesis is involved in AD pathogenesis (Biron et al. 2011). VEGF, through its binding to cognate receptors, plays a key role in the angiogenesis. Oxidative stress induces marked increase of VEGF expression (Carmeliet and Ruiz de Almodovar 2013; Sanchez et al. 2013). In AD, VEGF immunoreactivity overlaps with Aβ plaques and is found in association with reactive astrocytes, neurons, and blood vessel walls (Biron et al. 2011). Furthermore, in AD patients, the perivascular accumulation of endostatin, a neutrally derived antiangiogenic factor, may also lead to the vascular damage (Deininger et al. 2002). Even though CSF and plasma levels of VEGF are increased in AD patients (Carmeliet and Ruiz de Almodovar 2013; Qin et al. 2015; Tarkowski et al. 2002; Vagnucci and Li 2003), VEGF is sequestered by Aβ plaques (Yang et al. 2004) and its signaling is inhibited by Aβ (Patel et al. 2010), thus reducing its angiogenic property and causing further hypoxia (Sun et al. 2006). In AD mouse model, vascular density is reduced in the cortex, hippocampus, and white matter at 7–9 months, a finding often associated with capillary CAA, vessel occlusion, blood flow disturbances, and BBB leakage (Biron et al. 2011; Lee et al. 2005; Paris et al. 2004; Thal et al. 2009). However, how aberrant angiogenesis occurs remains largely unanswered. One potential mechanism involves TRPM2 channel. Mittal et al. demonstrated that VEGF-induced angiogenesis requires a rise in intracellular Ca2+ via TRPM2 (Mittal et al. 2015). Thus, the underlying mechanism for the aberrant angiogenesis may lie in the extent to which Aβ induces intracellular Ca2+ via TRPM2 activation (see below), but it needs further investigation. These alterations in angiogenesis are likely to have a powerful impact in the cerebrovascular alterations found in AD and vascular dementia as well as in the brain atrophy related to these diseases (Savva et al. 2009; Staekenborg et al. 2009).
Aβ Induces an Oxidative–Nitrosative Stress Associated with Opening of TRPM2 Channels in Cerebral Endothelial Cells (Figs. 2, 3)
Fig. 2.
Aβ1-40 triggers large and sustained increases in intracellular Ca2+ via TRPM2 channels in brain endothelial cell. The Aβ1-40 (Aβ)-induced increases in intracellular Ca2+ are attenuated by the mechanistically distinct TRPM2 inhibitors 2-APB and ACA (a, b) or TRPM2 knockdown using siRNA, but not control siRNA (control si) (c, d). Data are presented as mean ± SEM. *p < 0.05; analysis of variance and Turkey’s test; N = 6–10/group. Modified from Park et al. (2014) with permission
Fig. 3.
Presumed mechanisms through which Aβ1-40 activates endothelial TRPM2 channels. Aβ1-40 (Aβ) activates the innate immunity receptor CD36 leading to production of superoxide via NADPH oxidase. Superoxide reacts with NO, made continuously in endothelial cells, to form peroxynitrite (PN). PN induces DNA damage, which, in turn, activates PARP. ADPR formation by PARG cleavage of PAR activates the Nudix (Nu) domain of TRPM2 leading to massive increases in intracellular Ca2+, which induce endothelial dysfunction. Modified from Park et al. (2014) with permission
Aβ Induces Oxidative Stress in Cerebral Endothelial Cells
There is increasing evidence that ROS are critical mediators of endothelial dysfunction produced by Aβ. Aβ promotes ROS production in cerebral endothelial cells and ROS scavengers counteract the effects of Aβ on endothelial dysfunction and functional hyperemia (Niwa et al. 2000a, b, 2002). In APP mice, overexpression of superoxide dismutase (SOD)-1 rescues cerebral endothelial dysfunction induced by Aβ (Iadecola et al. 1999). Oxidative stress has also been implicated in other vascular effects of Aβ including BBB breakdown and vascular inflammation (Zlokovic 2008).
Aβ Induces Activation of NADPH Oxidase in Cerebral Endothelial Cells
A key mechanism by which Aβ exerts its deleterious vascular effects includes the generation of ROS via the enzyme of NADPH oxidase. Initially identified in phagocytes, NADPH oxidase is also found in vascular cells and is especially abundant in the brain blood vessels (Drummond et al. 2011; Miller et al. 2005). The enzyme consists of membrane-bound (Nox and p22phox) and cytoplasmic (p47phox and p67phox) subunits and some of the enzymes also require the small GTPases, such as Rac1 and Rac2, for its activation (Drummond et al. 2011). Nox subunit is present in 5 isoforms (Nox 1–5) and is a core catalytic subunit of the enzyme (Drummond et al. 2011). Nox 1, 2, and 4 isoforms are present in blood vessels of the brain (Han et al. 2015; Kazama et al. 2004; Miller et al. 2005; Park et al. 2005, 2008). Genetic inactivation of Nox2 counteracts the cerebrovascular oxidative stress and the vascular dysfunction induced by Aβ, pointing to NADPH oxidase as the source of the ROS (Park et al. 2005, 2008). Furthermore, neocortical treatment of a peptide blocking the assembly of NADPH oxidase inhibits the ROS production and endothelial dysfunction induced by Aβ (Park et al. 2005, 2008). More importantly, neurovascular protection provided by Nox2 inactivation is associated with behavioral improvement (Park et al. 2008), providing evidence linking Nox2-containing NADPH oxidase with the neurovascular dysfunction and cognitive decline associated with amyloid pathology.
Aβ Induces Peroxynitrite Formation and DNA Damage in Cerebral Endothelial Cells
The mechanisms by which vascular ROS trigger alterations in cerebrovasculature induced by Aβ have not been elucidated in full. One likely pathway is that ROS inactivate the endogenous vasoactive mediators regulating CBF. For example, ROS formed by Aβ lead to formation of peroxynitrite and attenuate endothelium-dependent vascular responses and functional hyperemia (Park et al. 2004, 2005; Iadecola 2004; Iadecola and Davisson 2008; Tong et al. 2005). Recently, we found that the peroxynitrite inactivators or scavengers prevent the vascular nitration and counteract the cerebrovascular dysfunction triggered by APP overexpression and Aβ in full in vivo (Park et al. 2014). Peroxynitrite is a highly reactive species that causes DNA double-strand breaks. Indeed, a recent study demonstrated DNA damage in cerebral endothelial cells of patients with early AD (Garwood et al. 2014). In agreement with such post-mortem data, we also demonstrated that Aβ increases the immunoreactivity of the DNA damage marker phospho-γH2AX, an effect abolished by ROS scavengers, NADPH oxidase inhibition, NOS inhibition, and peroxynitrite decomposition catalysts (Park et al. 2014). Thus, Aβ induces endothelial DNA damage via oxidative-nitrosative stress.
Aβ Induces Activation of PARP in Cerebral Endothelial Cells
One potential pathway by which Aβ-induced peroxynitrite formation and DNA damage alters endothelial regulation includes activation of PARP-1. PARP-1, the most dominant member of the PARP family, is involved in the repair of oxidative stress-induced DNA damage (Pacher and Szabo 2008), but excessive activation of PARP-1 has deleterious effects on the cell (Pacher and Szabo 2008). Recent evidence suggests that PARP-1 plays a critical role in the cerebrovascular dysfunction induced by Aβ. In APP mice, PARP-1 activity is elevated in penetrating pial arterioles (Park et al. 2004, 2014). Inhibition of PARP-1 activity with the PARP inhibitor PJ-34 prevents the endothelial dysfunction induced by Aβ (Park et al. 2014). Furthermore, Aβ fails to attenuate endothelium-dependent vasodilatation in PARP-1−/− mice, pointing to PARP-1 as the factor responsible for Aβ-induced endothelial dysfunction (Park et al. 2014). Another pathway through which PARP-1 could induce endothelial dysfunction involves the BBB. Inhibition of PARP-1 activity protects BBB in models of neuroinflammation (Rom et al. 2015), raising the possibility that PARP-1 is also involved in the BBB dysfunction induced by Aβ (Fig. 1). However, this hypothesis remains to be tested.
Aβ Induces ADPR Formation Via Activation of PARG in Cerebral Endothelial Cells
Poly(ADP-ribose) glycohydrolase (PARG) is a catalytic enzyme that cleaves ADPR polymers into ADPR (Putt and Hergenrother 2004; Virag and Szabo 2002). PARG inhibition counteracts the cerebrovascular dysfunction both in wild-type (WT) mice treated with Aβ and in APP mice (Park et al. 2014), implicating the activity of PARG pathway in the mechanisms of neurovascular alterations induced by Aβ.
Aβ Triggers Ca2+ Increases in Endothelial Cells Via TRPM2 Channels in Cerebral Endothelial Cells
As described above, ADPR is a potent activator of TRPM2 channels leading to increases of intracellular Ca2+ and other cations (Buelow et al. 2008; Sumoza-Toledo and Penner 2011). TRPM2 channels are expressed in many cells, including neurons and cerebral endothelial cells (Hecquet et al. 2008, 2014; Yamamoto et al. 2008; Kozai et al. 2013), and have been implicated in ischemic injury, traumatic brain injury, and neurodegenerative diseases (Cook et al. 2010; Naziroglu 2011; Nilius and Szallasi 2014; Yue et al. 2015; Zholos et al. 2011; Hermosura et al. 2008). In endothelial cells, Aβ induces inward currents blocked by the TRPM2 antagonists or siRNA knockdown (Park et al. 2014). Aβ-induced TRPM2 currents are attenuated by the PARP-1 inhibitor PJ-34 and by the PARG inhibitor ADP-HPD, pointing to an involvement of PARP and PARG in TRPM2 channel opening (Park et al. 2014). TRPM2 activation by Aβ is associated with massive increases in intracellular Ca2+, an effect attenuated by pretreatment with ACA or 2-APB, and by TRPM2 siRNA (Fig. 2). Thus, Aβ causes opening of TRPM2 channels in cerebral endothelial cells leading to intracellular Ca2+ overload. Accordingly, TRPM2 inhibitors prevent the cerebrovascular effects of Aβ in WT mice and rescue the cerebrovascular dysfunction observed in APP mice (Park et al. 2014). Furthermore, Aβ fails to cause the cerebrovascular dysfunctions in TRPM2 null mice (Park et al. 2014). These data provide pharmacological and non-pharmacological evidence for a crucial involvement of the TRPM2 channels in the endothelial and neurovascular dysfunctions induced by Aβ in vivo.
Concluding Remarks
It is increasingly accepted that AD is a multifaceted disorder associated with multiple pathogenic factors of which vascular factors play a critical role (Holtzman et al. 2011; de la Torre 2012; Iadecola 2013). Aβ alter neurovascular function (Zlokovic 2008; Iadecola 2013), which may play a role in the cognitive deficits by decreasing cerebral perfusion and promoting ischemic brain injury (Iadecola 2013; Zlokovic 2011). In addition, alteration of neurovascular function may accelerate Aβ accumulation in the brain by slowing its perivascular and transvascular clearance (Iadecola 2013; Weller et al. 2009). Considering the enormous public health impact of AD, a disease thus far incurable, it would be important to unravel the mechanisms by which Aβ impairs vascular functions and, based on these mechanisms, develop new therapeutic approaches. Our recent findings that Aβ-induced vascular oxidative–nitrosative stress induces cerebrovascular dysfunction through PARP1 and endothelial TRPM2 channels suggest new therapeutic target to counteract the deleterious cerebrovascular effect of Aβ (Fig. 3). However, additional studies are needed to gain a better understanding of the potential impact of PARP-1 and TRPM2 channels in AD diagnosis and therapy.
Acknowledgments
The authors thank Dr. Costantino Iadecola for critical reading of the manuscript. This work was supported by the National Institutes of Health Grant NS37853, and by the American Heart Association Grant 09SDG2060701. The generous support of the Feil Family Foundation is gratefully acknowledged.
Abbreviations
- ACA
TRPM2 inhibitor
- AD
Alzheimer’s disease
- ADPR
ADP-ribose
- 2-APB
TRPM2 inhibitor
- Aβ
β-amyloid
- APP
Amyloid precursor protein
- BBB
Blood–brain barrier
- CAA
Cerebral amyloid angiopathy
- CBF
Cerebral blood flow
- CD36
Cluster of differentiation 36
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DNA
Deoxyribonucleic acid
- γH2AX
Phosphorylated histone H2AX
- GTP
Guanosine triphosphate
- Mfsd2a
Major facilitator superfamily domain containing 2a
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- Nox
NADPH oxidase
- PARG
Poly(ADP-ribose) glycohydrolase
- PARP
Poly(ADP-ribose) polymerase
- PICALM
Phosphatidylcholine-binding clathrin assembly protein
- PJ34
PARP inhibitor
- phox
Phagocytic oxidase
- Rac
Ras-related C3 botulinum toxin substrate
- RAGE
Receptor for advanced glycogen end-products
- ROS
Reactive oxygen species
- siRNA
Small interfering RNA
- SLC2A1
Solute carrier family 2 (Facilitated Glucose Transporter), Member 1
- SOD
Superoxide dismutase
- TRPM2
Transient receptor potential melastatin 2
- VEGF
Vascular endothelial growth factor
References
- Andresen J, Shafi NI, Bryan RM Jr (2006) Endothelial influences on cerebrovascular tone. J Appl Physiol 100(1):318–327. doi:10.1152/japplphysiol.00937.2005 [DOI] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR (2010) Probing the biology of Alzheimer’s disease in mice. Neuron 66(5):631–645. doi:10.1016/j.neuron.2010.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468(7321):232–243. doi:10.1038/nature09613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC (2006) Quantitative measurement of cerebral haemodynamics in early vascular dementia and Alzheimer’s disease. J Clin Neurosci 13(5):563–568. doi:10.1016/j.jocn.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, Walker DG, Roher AE (2007) Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 113(1):13–21. doi:10.1007/s00401-006-0136-y [DOI] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV (2009) SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol 11(2):143–153. doi:10.1038/ncb1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus MR, Prins ND, Goos JD, Scheltens P, Barkhof F, van der Flier WM (2015) Microbleeds, mortality, and stroke in Alzheimer disease: the MISTRAL study. JAMA Neurol 72(5):539–545. doi:10.1001/jamaneurol.2015.14 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509(7501):507–511. doi:10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron KE, Dickstein DL, Gopaul R, Jefferies WA (2011) Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS One 6(8):e23789. doi:10.1371/journal.pone.0023789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C, Kovari E, Herrmann FR, Rivara CB, Bailey TL, von Gunten A, Hof PR, Giannakopoulos P (2006) Stereologic analysis of microvascular morphology in the elderly: Alzheimer disease pathology and cognitive status. J Neuropathol Exp Neurol 65(3):235–244. doi:10.1097/01.jnen.0000203077.53080.2c [DOI] [PubMed] [Google Scholar]
- Buelow B, Song Y, Scharenberg AM (2008) The Poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem 283(36):24571–24583. doi:10.1074/jbc.M802673200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P (2003) Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet 4(9):710–720. doi:10.1038/nrg1158 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ruiz de Almodovar C (2013) VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci 70(10):1763–1778. doi:10.1007/s00018-013-1283-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM (2014) A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 3(3):e000787. doi:10.1161/JAHA.114.000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV (2007) Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A 104(3):823–828. doi:10.1073/pnas.0608251104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NL, Vink R, Helps SC, Manavis J, van den Heuvel C (2010) Transient receptor potential melastatin 2 expression is increased following experimental traumatic brain injury in rats. J Mol Neurosci 42(2):192–199. doi:10.1007/s12031-010-9347-8 [DOI] [PubMed] [Google Scholar]
- de la Torre JC (2012) Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis 32(3):553–567. doi:10.3233/JAD-2012-120793 [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B (2003) RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9(7):907–913. doi:10.1038/nm890 [DOI] [PubMed] [Google Scholar]
- Dede DS, Yavuz B, Yavuz BB, Cankurtaran M, Halil M, Ulger Z, Cankurtaran ES, Aytemir K, Kabakci G, Ariogul S (2007) Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? J Am Geriatr Soc 55(10):1613–1617. doi:10.1111/j.1532-5415.2007.01378.x [DOI] [PubMed] [Google Scholar]
- Deininger MH, Fimmen BA, Thal DR, Schluesener HJ, Meyermann R (2002) Aberrant neuronal and paracellular deposition of endostatin in brains of patients with Alzheimer’s disease. J Neurosci 22(24):10621–10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG (2011) Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discovery 10(6):453–471. doi:10.1038/nrd3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM (2011) Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol 300(5):H1566–H1582. doi:10.1152/ajpheart.01310.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Iadecola C (2013) Hypertension: a harbinger of stroke and dementia. Hypertension 62(5):810–817. doi:10.1161/HYPERTENSIONAHA.113.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer VW, Siddiqi A, Yusufaly Y (1990) Altered angioarchitecture in selected areas of brains with Alzheimer’s disease. Acta Neuropathol 79(6):672–679 [DOI] [PubMed] [Google Scholar]
- Garwood CJ, Simpson JE, Al Mashhadi S, Axe C, Wilson S, Heath PR, Shaw PJ, Matthews FE, Brayne C, Ince PG, Wharton SB, Function MRCC, Ageing S (2014) DNA damage response and senescence in endothelial cells of human cerebral cortex and relation to Alzheimer’s neuropathology progression: a population-based study in the MRC-CFAS cohort. Neuropathol Appl Neurobiol 40(7):802–814. doi:10.1111/nan.12156 [DOI] [PubMed] [Google Scholar]
- Han BH, Zhou ML, Johnson AW, Singh I, Liao F, Vellimana AK, Nelson JW, Milner E, Cirrito JR, Basak J, Yoo M, Dietrich HH, Holtzman DM, Zipfel GJ (2015) Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc Natl Acad Sci USA 112(8):E881–E890. doi:10.1073/pnas.1414930112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecquet CM, Ahmmed GU, Vogel SM, Malik AB (2008) Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102(3):347–355. doi:10.1161/CIRCRESAHA.107.160176 [DOI] [PubMed] [Google Scholar]
- Hecquet CM, Zhang M, Mittal M, Vogel SM, Di A, Gao X, Bonini MG, Malik AB (2014) Cooperative interaction of trp melastatin channel transient receptor potential (TRPM2) with its splice variant TRPM2 short variant is essential for endothelial cell apoptosis. Circ Res 114(3):469–479. doi:10.1161/CIRCRESAHA.114.302414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16(6):358–372. doi:10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Cui AM, Go RC, Davenport B, Shetler CM, Heizer JW, Schmitz C, Mocz G, Garruto RM, Perraud AL (2008) Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci USA 105(46):18029–18034. doi:10.1073/pnas.0808218105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, Matsuda H, Nemoto K, Imabayashi E, Yamada M, Iwamoto T, Arima K, Asada T (2005) The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. NeuroImage 28(4):1014–1021. doi:10.1016/j.neuroimage.2005.06.066 [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3(77):77sr71. doi:10.1126/scitranslmed.3002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Mandelkow E, Selkoe DJ (2012) Alzheimer disease in 2020. Cold Spring Harb Perspect Med 2(11):a011585. doi:10.1101/cshperspect.a011585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5(5):347–360. doi:10.1038/nrn1387 [DOI] [PubMed] [Google Scholar]
- Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80(4):844–866. doi:10.1016/j.neuron.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL (2008) Hypertension and cerebrovascular dysfunction. Cell Metab 7(6):476–484. doi:10.1016/j.cmet.2008.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10(11):1369–1376. doi:10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA (1999) SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2(2):157–161. doi:10.1038/5715 [DOI] [PubMed] [Google Scholar]
- Jackman K, Iadecola C (2015) Neurovascular Regulation in the Ischemic Brain. Antioxid Redox Signal 22(2):149–160. doi:10.1089/ars.2013.5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Haan MN, Reed BR, Eberling JL (1998) Brain perfusion imaging predicts survival in Alzheimer’s disease. Neurology 51(4):1009–1013. doi:10.1212/WNL.51.4.1009 [DOI] [PubMed] [Google Scholar]
- Johnson KA, Albert MS (2000) Perfusion abnormalities in prodromal AD. Neurobiol Aging 21(2):289–292. doi:10.1016/S0197-4580(00)00137-8 [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Austin SA (2014) Endothelial nitric oxide: protector of a healthy mind. Eur Heart J 35(14):888–894. doi:10.1093/eurheartj/eht544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C (2004) Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95(10):1019–1026. doi:10.1161/01.RES.0000148637.85595.c5 [DOI] [PubMed] [Google Scholar]
- Kitaguchi H, Ihara M, Saiki H, Takahashi R, Tomimoto H (2007) Capillary beds are decreased in Alzheimer’s disease, but not in Binswanger’s disease. Neurosci Lett 417(2):128–131. doi:10.1016/j.neulet.2007.02.021 [DOI] [PubMed] [Google Scholar]
- Kozai D, Ogawa N, Mori Y (2013) Redox regulation of transient receptor potential channels. Antioxid Redox Signal 21(6):971–986. doi:10.1089/ars.2013.5616 [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ (2012) Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. NeuroImage 62(2):1040–1050. doi:10.1016/j.neuroimage.2012.01.040 [DOI] [PubMed] [Google Scholar]
- Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR (2005) Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer’s disease. Brain Res Bull 65(4):317–322. doi:10.1016/j.brainresbull.2004.11.024 [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341. doi:10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaus C, Flub MO, Wittsack HJ, Grass-Kapanke B, Janner M, Khalili-Amiri R, Friedrich W, Supprian T, Gaebel W, Modder U, Cohnen M (2008) Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. NeuroImage 40(2):495–503. doi:10.1016/j.neuroimage.2007.11.053 [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330(6012):1774. doi:10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T (2008) Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc Natl Acad Sci USA 105(9):3587–3592. doi:10.1073/pnas.0709788105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Schmidt HH, Sobey CG (2005) NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res 97(10):1055–1062. doi:10.1161/01.RES.0000189301.10217.87 [DOI] [PubMed] [Google Scholar]
- Mittal M, Urao N, Hecquet CM, Zhang M, Sudhahar V, Gao XP, Komarova Y, Ushio-Fukai M, Malik AB (2015) Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization. Arterioscler Thromb Vasc Biol 35(4):877–887. doi:10.1161/ATVBAHA.114.304802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85(2):296–302. doi:10.1016/j.neuron.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AW, Carare RO, Schreiber S, Hawkes CA (2014) The cerebrovascular basement membrane: role in the clearance of beta-amyloid and cerebral amyloid angiopathy. Front Aging Neurosci 6:251. doi:10.3389/fnagi.2014.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroglu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36(3):355–366. doi:10.1007/s11064-010-0347-4 [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O’Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR (2011) Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12(3):169–182. doi:10.1038/nrn2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Szallasi A (2014) Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66(3):676–814. doi:10.1124/pr.113.008268 [DOI] [PubMed] [Google Scholar]
- Niwa K, Carlson GA, Iadecola C (2000a) Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab 20(12):1659–1668. doi:10.1097/00004647-200012000-00005 [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C (2000b) Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci USA 97(17):9735–9740. doi:10.1073/pnas.97.17.9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C (2002) Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis 9(1):61–68. doi:10.1006/nbdi.2001.0460 [DOI] [PubMed] [Google Scholar]
- Nordberg A, Rinne JO, Kadir A, Langstrom B (2010) The use of PET in Alzheimer disease. Nat Rev Neurol 6(2):78–87. doi:10.1038/nrneurol.2009.217 [DOI] [PubMed] [Google Scholar]
- Pacher P, Szabo C (2008) Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol 173(1):2–13. doi:10.2353/ajpath.2008.080019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M (2004) Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett 366(1):80–85. doi:10.1016/j.neulet.2004.05.017 [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C (2004) Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab 24(3):334–342. doi:10.1097/01.WCB.0000105800.49957.1E [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C (2005) NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci 25(7):1769–1777. doi:10.1523/JNEUROSCI.5207-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C (2008) Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105(4):1347–1352. doi:10.1073/pnas.0711568105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, Van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C (2011) Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci USA 108(12):5063–5068. doi:10.1073/pnas.1015413108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou J, Zhou P, Pistick R, El Jamal S, Younkin L, Pierce J, Arreguin A, Anrather J, Younkin SG, Carlson GA, McEwen BS, Iadecola C (2013) Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci USA 110(8):3089–3094. doi:10.1073/pnas.1300021110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, Iadecola C (2014) The key role of transient receptor potential melastatin-2 channels in amyloid-beta-induced neurovascular dysfunction. Nat Commun 5:5318. doi:10.1038/ncomms6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D (2010) Alzheimer’s beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J Neurochem 112(1):66–76. doi:10.1111/j.1471-4159.2009.06426.x [DOI] [PubMed] [Google Scholar]
- Putt KS, Hergenrother PJ (2004) A nonradiometric, high-throughput assay for poly(ADP-ribose) glycohydrolase (PARG): application to inhibitor identification and evaluation. Anal Biochem 333(2):256–264. doi:10.1016/j.ab.2004.04.032 [DOI] [PubMed] [Google Scholar]
- Qin W, Jia X, Wang F, Zuo X, Wu L, Zhou A, Li D, Min B, Wei C, Tang Y, Xing Y, Dong X, Wang Q, Gao Y, Li Y, Jia J (2015) Elevated plasma angiogenesis factors in Alzheimer’s disease. J Alzheimers Dis 45(1):245–252. doi:10.3233/JAD-142409 [DOI] [PubMed] [Google Scholar]
- Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT 3rd, Derdeyn CP, Bateman RJ (2014) Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol 76(6):837–844. doi:10.1002/ana.24270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG (2003) Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol 23(11):2055–2062. doi:10.1161/01.ATV.0000095973.42032.44 [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG (2004) Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke 35(11 Suppl 1):2623–2627. doi:10.1161/01.STR.0000143317.70478.b3 [DOI] [PubMed] [Google Scholar]
- Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Ramirez SH, Persidsky Y (2015) Poly(ADP-ribose) polymerase-1 inhibition in brain endothelium protects the blood-brain barrier under physiologic and neuroinflammatory conditions. J Cereb Blood Flow Metab 35(1):28–36. doi:10.1038/jcbfm.2014.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57(6):789–794. doi:10.1002/ana.20493 [DOI] [PubMed] [Google Scholar]
- Sanchez A, Tripathy D, Luo J, Yin X, Martinez J, Grammas P (2013) Neurovascular unit and the effects of dosage in VEGF toxicity: role for oxidative stress and thrombin. J Alzheimers Dis 34(1):281–291. doi:10.3233/JAD-121636 [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive Function and Ageing Study (2009) Age neuropathology, and dementia. N Engl J Med 360(22):2302–2309. doi:10.1056/NEJMoa0806142 [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Cutini S, Wahl MM, Scheid R, Yves von Cramon D (2007) Neurovascular coupling is impaired in cerebral microangiopathy—an event-related Stroop study. NeuroImage 34(1):26–34. doi:10.1016/j.neuroimage.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Smith EE, Greenberg SM (2009) Beta-amyloid, blood vessels, and brain function. Stroke 40(7):2601–2606. doi:10.1161/STROKEAHA.108.536839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staekenborg SS, Koedam EL, Henneman WJ, Stokman P, Barkhof F, Scheltens P, van der Flier WM (2009) Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke 40(4):1269–1274. doi:10.1161/STROKEAHA.108.531343 [DOI] [PubMed] [Google Scholar]
- Sumoza-Toledo A, Penner R (2011) TRPM2: a multifunctional ion channel for calcium signalling. J Physiol 589(Pt 7):1515–1525. doi:10.1113/jphysiol.2010.201855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W (2006) Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA 103(49):18727–18732. doi:10.1073/pnas.0606298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discovery 6(8):662–680. doi:10.1038/nrd2222 [DOI] [PubMed] [Google Scholar]
- Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, Rosenberg GA (2011) Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke 42(8):2158–2163. doi:10.1161/STROKEAHA.110.611731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P (2002) Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging 23(2):237–243. doi:10.1016/S0197-4580(01)00285-8 [DOI] [PubMed] [Google Scholar]
- Thal DR, Capetillo-Zarate E, Larionov S, Staufenbiel M, Zurbruegg S, Beckmann N (2009) Capillary cerebral amyloid angiopathy is associated with vessel occlusion and cerebral blood flow disturbances. Neurobiol Aging 30(12):1936–1948. doi:10.1016/j.neurobiolaging.2008.01.017 [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M (1996) beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380(6570):168–171. doi:10.1038/380168a0 [DOI] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Kocharyan A, Hamel E (2005) Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J Neurosci 25(48):11165–11174. doi:10.1523/JNEUROSCI.4031-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnucci AH Jr, Li WW (2003) Alzheimer’s disease and angiogenesis. Lancet 361(9357):605–608. doi:10.1016/S0140-6736(03)12521-4 [DOI] [PubMed] [Google Scholar]
- van Horssen J, Otte-Holler I, David G, Maat-Schieman ML, van den Heuvel LP, Wesseling P, de Waal RM, Verbeek MM (2001) Heparan sulfate proteoglycan expression in cerebrovascular amyloid beta deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol 102(6):604–614 [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54(3):375–429 [DOI] [PubMed] [Google Scholar]
- Weber C, Fraemohs L, Dejana E (2007) The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol 7(6):467–477. doi:10.1038/nri2096 [DOI] [PubMed] [Google Scholar]
- Weller RO, Boche D, Nicoll JA (2009) Microvasculature changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol 118(1):87–102. doi:10.1007/s00401-009-0498-z [DOI] [PubMed] [Google Scholar]
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wenby RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV (2015) GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 18(4):521–530. doi:10.1038/nn.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14(7):738–747. doi:10.1038/nm1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SP, Bae DG, Kang HJ, Gwag BJ, Gho YS, Chae CB (2004) Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol Aging 25(3):283–290. doi:10.1016/S0197-4580(03)00111-8 [DOI] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE (2012) Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 135(Pt 12):3749–3756. doi:10.1093/brain/aws271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T, Hiwatashi A, Yamashita K, Ohyagi Y, Monji A, Takayama Y, Nagao E, Kamano H, Noguchi T, Honda H (2009) Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. AJNR Am J Neuroradiol 30(7):1388–1393. doi:10.3174/ajnr.A1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L (2015) Role of TRP channels in the cardiovascular system. Am J Physiol Heart Circ Physiol 308(3):H157–H182. doi:10.1152/ajpheart.00457.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P (2008) Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci 9(3):169–181. doi:10.1038/nrn2336 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV (2015) Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci 18(7):978–987. doi:10.1038/nn.4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos A, Johnson C, Burdyga T, Melanaphy D (2011) TRPM channels in the vasculature. Adv Exp Med Biol 704:707–729. doi:10.1007/978-94-007-0265-3_37 [DOI] [PubMed] [Google Scholar]
- Zhu J, Wang Y, Li J, Deng J, Zhou H (2014) Intracranial artery stenosis and progression from mild cognitive impairment to Alzheimer disease. Neurology 82(10):842–849. doi:10.1212/WNL.0000000000000185 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723–738. doi:10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]