Abstract
When maintaining postural stability temporally under increased sensory conflict, a more rigid response is used where the available degrees of freedom are essentially frozen. The current study investigated if such a strategy is also utilized during more dynamic situations of postural control as is the case with walking. This study attempted to answer this question by using the Locomotor Sensory Organization Test (LSOT). This apparatus incorporates SOT inspired perturbations of the visual and the somatosensory system. Ten healthy young adults performed the six conditions of the traditional SOT and the corresponding six conditions on the LSOT. The temporal structure of sway variability was evaluated from all conditions. The results showed that in the anterior posterior direction somatosensory input is crucial for postural control for both walking and standing; visual input also had an effect but was not as prominent as the somatosensory input. In the medial lateral direction and with respect to walking, visual input has a much larger effect than somatosensory input. This is possibly due to the added contributions by peripheral vision during walking; in standing such contributions may not be as significant for postural control. In sum, as sensory conflict increases more rigid and regular sway patterns are found during standing confirming the previous results presented in the literature, however the opposite was the case with walking where more exploratory and adaptive movement patterns are present.
Keywords: Biomechanics, Posture, Sway variability, Nonlinear, Sample entropy
INTRODUCTION
Successfully maintaining postural control during standing and walking requires integration of three major sensory systems: visual, vestibular and somatosensory systems.7 It has been suggested that each sensory system monitors postural changes through independent sensorimotor pathways. The central nervous system (CNS) responds by implementing the appropriate corrective muscle synergies based on the integrated input from these three sensory systems.9 If only one sensory system is intact, the CNS determines the response completely based on that particular sensory system; and if two or more sensory systems are intact, the CNS evaluates all signals from the available sensory systems and makes adequate responses.9 Based on this theoretical framework when conditions of reduced perceptual accuracy exist, the CNS recalibrates by reducing inaccurate sensory gains and increasing the functional gain of accurate sensory modalities. During this recalibration process, humans demonstrate difficulties to maintain balance and alter postural control, such as increasing body sway without vision in standing.9 Successful recalibration leads to functional adaptation to the perceived environmental perturbation, as observed for example in the shortening of the stride length on a slippery ground in locomotion.9
In order to quantify the adaptive mechanisms involved in the control of standing posture during sensory conflict, the Sensory Organization Test (SOT) has been widely used in patients with vestibular disorder2,29 concussion,4 stroke,32 and Parkinson’s Disease,23 among others. The design of the SOT is intended to challenge postural control through manipulations of the sensory input. It can manipulate somatosensory and visual inputs individually or in combination to allow assessment of a patient’s ability for maintaining balance. The SOT has allowed scientists to investigate amount of sway variability under these conditions and make inferences about sensory contributions to postural control. In summary these studies found that the amount of sway variability increased as postural control was challenged by manipulating sensory inputs in the SOT.26 These increases have been interpreted as increased noise in the system that could lead to instability.26
To further explore this interpretation, researchers have recently shown interest in the temporal structure of sway variability or in other words how sway variability changes over time while performing the SOT5,6,28,35 This work, which encompasses several different areas including brain function and disease dynamics, has shown that many apparently “noisy” phenomena are the result of nonlinear interactions and have deterministic origins13,37,38 As such, the measured signal, including its “noisy” component, may provide important information regarding the function of the system that produced it. Therefore, new innovative clinical methods that use nonlinear mathematical analysis and investigate the temporal structure of variability have been proposed. These nonlinear methods are being used increasingly to describe complex conditions. For example, nonlinear analysis of the temporal structure of the variability has recently been used to study heart rate irregularities, sudden cardiac death syndrome, blood pressure control, brain ischemia, epileptic seizures, and several other conditions.1,10,12,13,17,41 Such research has allowed for a better understanding of the complexity of these pathologies and eventually led to the development of better prognostic and diagnostic tools in other areas (i.e., cardiology, neurology). Thus, it is fair to assume that nonlinear analysis of the sway variability could allow insight into the complex strategies used to control movement and posture informing clinical practice with respect to movement related disorders.
Such an assumption led investigators to explore the temporal structure of sway variability while performing the SOT.5,6,28,35 Riley et al. used recurrence quantification analysis to investigate the temporal structure of sway variability.28 They found that the temporal structure of postural sway tended to become increasingly regular as the SOT condition increased in difficulty (i.e., as the SOT condition moved from eyes open to eyes closed, to sway-referenced visual surround or support surface, and to sway-reference surface and visual surround). Entropy analysis has also been shown to detect changes in postural control dynamics and results have highlighted the role of such analysis to evaluate postural stability with the SOT condition.4–6,35 Specifically, an overall decrease in entropy values (i.e., more regular sway patterns) with the SOT condition was found even though these studies were not focused on the SOT condition per se but on the effects of vibrating the Achilles tendon.11,35 Similar results were found with entropy values decreasing as the SOT condition increased in difficulty indicating more regular sway patterns.4–6 Therefore, from all the above mentioned studies it can be concluded that sensory manipulation through the SOT condition leads to a more regular and repeatable sway movement pattern.
This strategy could be interpreted as an effort to temporally maintain postural stability under increased sensory conflict. A more rigid (i.e., more regular and repeatable) response has been considered as a freeze of the available degrees of freedom, a phenomenon that is also observed when dealing with novel situations and learning the new skill.21 Will such a strategy be also utilized during more dynamic situations of postural control as in the case with walking? Here this study attempted to answer this question by using an experimental apparatus that combines a treadmill, instrumented with force platform technology, and virtual reality; the Locomotor Sensory Organization Test (LSOT).7 This study hypothesized that a more rigid response will also characterize dynamic postural control during walking on our apparatus that incorporates SOT inspired perturbations of the visual and the somatosensory system.
METHODOLOGY
Subjects
Ten healthy young adults (five males and five females; age 27.20 ± 4.92 years, height 171.30 ± 7.01 cm and weight 64.70 ± 9.90 kg) participated in this study. Subjects were free from any musculoskeletal impairment, had no history of significant lower extremity injuries, which may have affected their posture or gait, and had no visual, somatosensory or vestibular deficits. We excluded individuals without normal or corrected to normal vision, scored above zero on the dizziness handicap inventory for a vestibular deficit18 and with any type of peripheral neuropathy that can affect somatosensory function. Prior to the experiment, each subject signed an informed consent approved by our University’s Medical Center Institutional Review Board.
Protocol
The experiment entailed exposing subjects to sensory perturbations in the SOT and LSOT environments. The SOT was conducted in a quiet room using the Balance Master System 8.4 (NeuroCom International Clackamas, OR, USA). The system contains a moveable visual surround and support surface that rotate in the anterior-posterior (AP) plane. Two 22.9 × 45.7 cm force plates connected by a pin joint are used to collect center of pressure data at 100 Hz. Foot placement is standardized based on subjects’ height according to manufacturer guidelines. While standing in the Balance Master system, subjects wore a vest attached to the safety harness of the system (Fig. 1).
FIGURE 1.

The SMART balance Master (NeuroCom International Clackamas, OR, USA) is used to perform the Sensory Organization Test (SOT). This test contains six conditions: (1) eyes open with fixed surface and fixed visual surrounding; (2) eyes closed with fixed surface; (3) eyes open with fixed surface and sway-referenced visual surroundings; (4) eyes open with sway-referenced surface and fixed visual surroundings; (5) eyes closed with sway-referenced surface; (6) eye open with sway-referenced surface and visual surroundings.
The LSOT consisted of two components: a virtual reality environment, and an instrumented treadmill (Bertec Corp., Columbus, OH, USA) (Fig. 2).7 The LSOT contained six conditions similar to the Sensory Organization Test to manipulate the sensory information during walking (Fig. 3).7 Prior to the data collection, each subject walked for 5 min on the treadmill to determine their preferred walking speed (PWS). After the PWS was determined, all subjects walked on the treadmill with the PWS for 2 min in each of the six conditions of the LSOT test. The LSOT conditions from 1 to 3 required the subject to walk on the treadmill set to the Preferred Walking Speed (PWS). This was done with matching optic flow in condition LSOT 1 (none of the sensory systems was challenged as in SOT 1), vision reduced in condition LSOT 2 (visual blocked as in SOT 2), and eyes open but random optic flow in condition LSOT 3 (visual perturbation matched to SOT 3). The visual perturbation was created by varying the optic flow between 80 and 120% of PWS in randomly assigned time intervals of 1–10 s. The LSOT conditions 4–6 all had random perturbation of the treadmill speed. The random treadmill perturbations was created by varying the treadmill speed between 80 and 120% of PWS in randomly assigned time intervals of 1–10 s. This was done with optic flow matched to PWS in condition LSOT 4 (somatosensory perturbation as in SOT 4), vision reduced in condition LSOT 5 (visual blocked and somatosensory perturbation as in SOT 5) and fi-nally, eyes open with matching random optic flow condition LSOT 6 (simultaneous visual and somatosensory perturbation as in SOT 6). In between conditions, the subjects were allowed to rest for 1 min with closed eyes.
FIGURE 2.
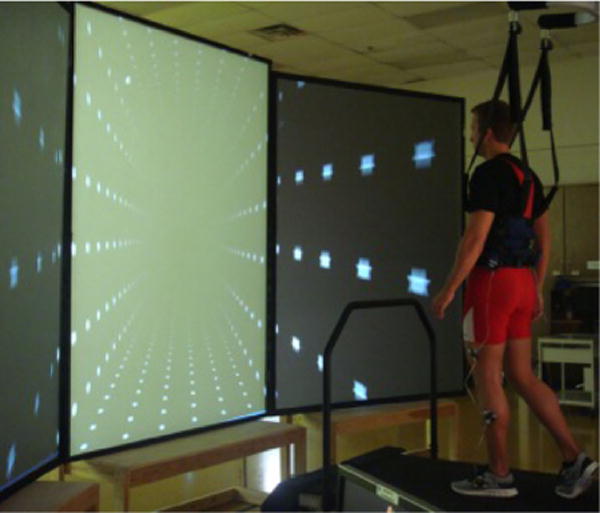
The components of Locomotor Sensory Organization Test (LSOT): virtual reality and the instrumented treadmill.
FIGURE 3.
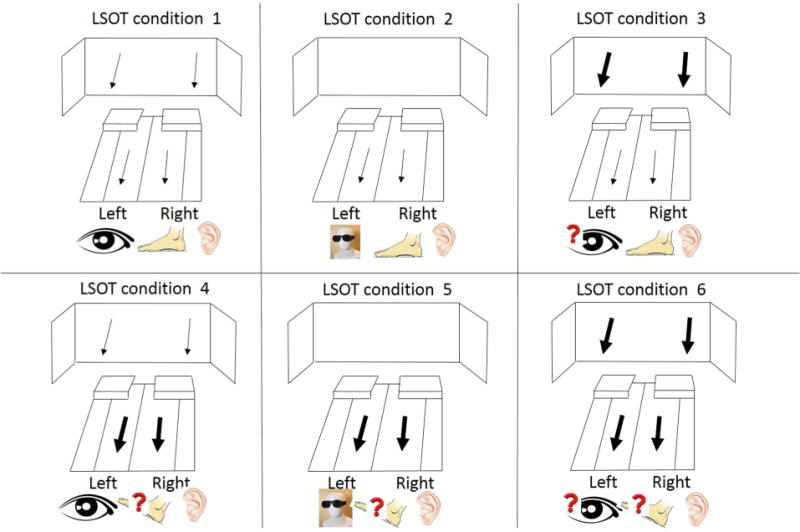
The six conditions of Locomotor Sensory Organization Test (LSOT) that mirrors those of the SOT: (1) normal walking condition; (2) Reduced visual condition by reducing vision capability condition; (3) Perturbed visual condition by manipulating optic flow speed condition; (4) Perturbed somatosensory condition by manipulating treadmill speed condition; (5) Perturbed visual and somatosensory condition by reducing vision capability and manipulating treadmill speed condition and (6) Perturbed visual and somatosensory condition by manipulating optic flow and treadmill speed condition.
Data Reduction
For the SOT, we investigated the temporal structure of sway variability using the COP trajectory in the AP and the medial–lateral (ML) direction. In addition, we only selected the first trial of each SOT condition to reduce the effect of condition adaptation. A similar approach has been used in previous studies.5,6,16,35 For the LSOT, structure of sway variability using the net-COP trajectory in the AP and the ML direction. This measure allows for a direct comparison of the COP measures between standing and locomotion. The net-COP is the point where the total sum of a pressure field acts on a body during walking. The total force vector acting at the netCOP is the value of the integrated vector pressure field.20 The netCOP as is the case with the COP, provides with a net representation of the movement generated by the entire body and all available degrees of freedom.22 Before calculating the temporal structure of the variability present in the COP and netCOP data, the original data was down sampled from 100 to 10 Hz to reduce the irrelevant noise present in the data since there was no physiological signal above 10 Hz in the COP data of both tasks.3
Sample Entropy (SampEn)
For all the COP and netCOP time series, the SampEn values were calculated using a customized script in MatLab R2011a (Mathworks, Natick, MA). The SampEn algorithm is defined as the negative natural logarithm for conditional properties that a series of data points a certain distance apart, m, would repeat itself at m + 1. Sam-pEn takes the logarithm of the sum of conditional probabilities. Given the time series g(n) = g(1), g(2),…, g(N), where N is the total number of data points, a sequence of m-length vectors is formed. Vectors are considered alike if the tail and head of the vector are within the set tolerance level. The sum of the total number of like vectors is divided by m + 1 and defined as A or by N − m + 1 and defined as B. SampEn is then calculated as −ln(A/B). A perfectly repeatable time series has a SampEn value ~0 and a perfectly random time series has a SampEn value converging toward infinity. In the current study, the following parameters were selected and used in the determination of SampEn values in SOT and LSOT: (a) a pattern length (m) of 2, (b) and error tolerance (r) of 0.2.40 The time series length in the SOT trials was 200 data points. The time series length in the LSOT trials was 1200 data points. These data lengths should be sufficient according to the literature.40
Four one-way repeated measure ANOVAs were performed using SPSS (18.0, IBM Corporation, Somers, NY) to determine condition effects of the LSOT and SOT. Specifically, the dependent measures were: the SampEn calculated from the COP data for the SOT in the (1) AP and in the (2) ML direction, and the SampEn calculated from the netCOP data for the LSOT in the (3) AP and in the (4) ML direction. Pairwise comparisons were performed to determine specific differences between conditions using Bonferroni adjustments. The adjusted significance level for the pairwise comparisons was 0.0083.
RESULTS
Anterior-posterior SampEn values in the SOT
The one-way repeated ANOVA revealed a significant condition effect (F = 17.79, p < 0.001) (Table 1; Fig. 4a). The post hoc pairwise comparisons revealed numerous significant differences between conditions. The first three conditions had all significantly larger values than the last three, however, there were no differences between them. The last three conditions had also no differences between them. The largest group mean value was present in condition 1, while the smallest group mean value was present in condition 5 (eyes closed with sway-referenced surface), followed by condition 6 (eyes open with sway-referenced surface and visual surroundings).
TABLE 1.
Group means and standard deviations for all conditions for the dependent measures evaluated.
| Conditions | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| SampEn for SOT in anterior-posterior direction | 0.217 (0.043)d,e,f | 0.185 (0.032)d,e,f | 0.199 (0.058)d,e,f | 0.108 (0.036)a,b,c | 0.094 (0.034)a,b,c | 0.092 (0.045)a,b,c |
| SampEn for LSOT in anterior-posterior direction | 0.259 (0.009)b,c,d,e,f | 0.314 (0.009)a,c,d,e,f | 0.183 (0.016)a,b,d,e,f | 0.346 (0.023)a,b,c,e,f | 0.402 (0.024)a,b,c,d,f | 0.380 (0.018)a,b,c,d,e |
| SampEn for SOT in medial–lateral direction | 0.096 (0.030)d,e,f | 0.082 (0.012)d,e,f | 0.065 (0.012),f | 0.059 (0.010)a,b,f | 0.057 (0.013)a,b | 0.048 (0.012)a,b,c,d |
| SampEn for LSOT in medial–lateral direction | 0.071 (0.006)b,e | 0.105 (0.017)a,c,d | 0.078 (0.012)b | 0.075 (0.009)b | 0.093 (0.016)a | 0.081 (0.016) |
Significant differences between conditions are indicated with superscripts.
Significant difference exhibited when compared to condition 1.
Significant difference exhibited when compared to condition 2.
Significant difference exhibited when compared to condition 3.
Significant difference exhibited when compared to condition 4.
Significant difference exhibited when compared to condition 5.
Significant difference exhibited when compared to condition 6.
FIGURE 4.
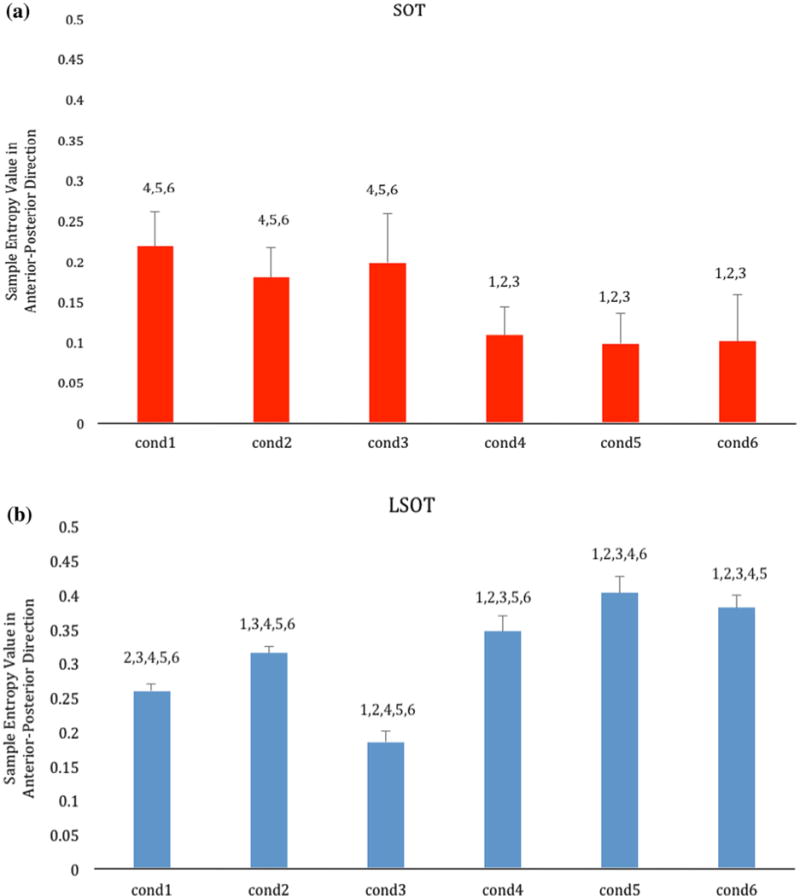
Bar charts showing the group means of the Sample Entropy values of all the subjects for the SOT (red; a) and the LSOT (blue; b) across the six experimental conditions. Error bars are standard deviations. For each condition the post hoc differences are indicated over the bars with the number of the condition found to be significantly different with.
Anterior–Posterior SampEn Values in the LSOT
The one-way repeated ANOVA revealed a significant condition effect (F = 292.96, p < 0.001) (Table 1; Fig. 4b). The post hoc pairwise comparisons revealed that all possible comparisons between conditions were significant. The smallest group mean value was present in condition 3 (variable optic flow), followed by condition 1. The largest group mean value was present in condition 5 (reduced visual information, variable treadmill velocity), followed by condition 6 (variable optic flow and variable treadmill velocity).
Medial–Lateral SampEn Values in the SOT
The one-way repeated ANOVA revealed a significant condition effect (F = 19.49, p < 0.001) (Fig. 5a). The post hoc pairwise comparisons revealed numerous significant differences between conditions. Conditions 1 and 2 had significantly larger values than conditions 4, 5 and 6. Conditions 3 and 4 had also significantly larger values than condition 6. In general the group mean values decreased from condition 1 to condition 6 with the smallest group mean value be present in condition 6 (eyes open with sway-referenced surface and visual surroundings), followed by condition 5 (eyes closed with sway-referenced surface). However, there was no significant difference between conditions 5 and 6.
FIGURE 5.
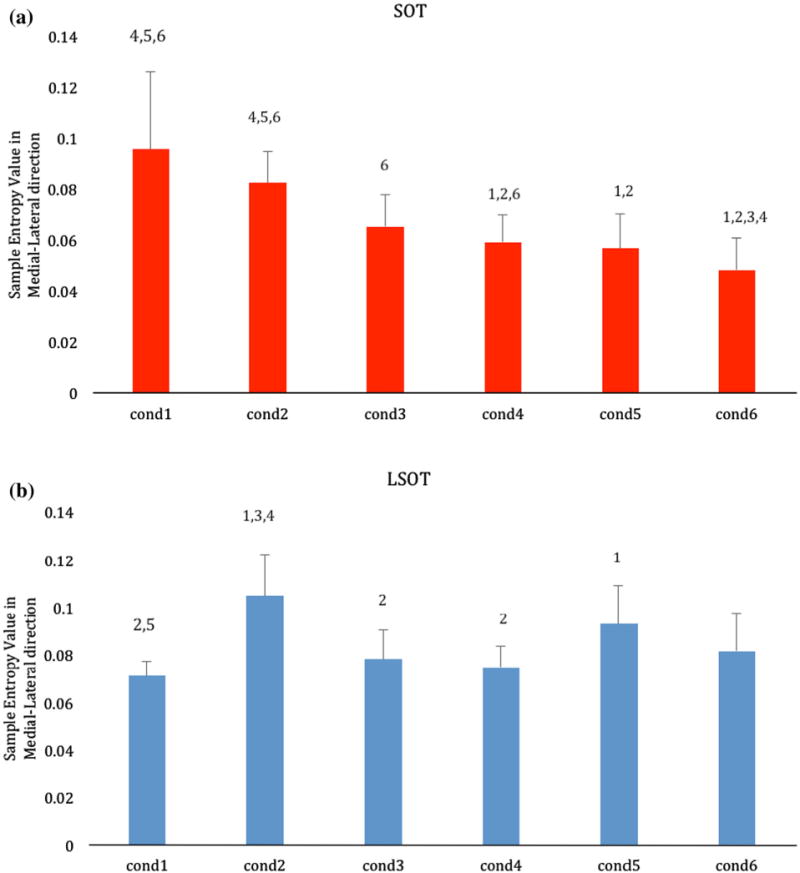
Bar charts showing the group means of the Sample Entropy values of all the subjects for the SOT (red; a) and the LSOT (blue; b) across the six experimental conditions. Error bars are standard deviations. For each condition the post hoc differences are indicated over the bars with the number of the condition found to be significantly different with.
Medial–lateral SampEn Values in the LSOT
The one-way repeated ANOVA revealed a significant condition effect (F = 14.03, p < 0.001) (Fig. 5b). The post hoc pairwise comparisons revealed several significant differences between conditions. The group mean value for condition 1 was significantly smaller than condition 2 (reduced visual information) and condition 5 (reduced visual information, variable treadmill velocity). Condition 2 (reduced visual information) had a significantly larger value than conditions 3 (variable optic flow) and 4 (variable treadmill speed).
DISCUSSION
This current study investigated how increased sensory conflict affects the temporal structure of sway variability during standing and walking. Based on previous studies that have used the SOT and found a more rigid (i.e., more regular and repeatable) response during standing posture in conditions with increased sensory conflict, we hypothesized that a more rigid response will also characterize dynamic postural control during walking in such conditions. To test this hypothesis an apparatus that uses SOT inspired perturbations of the visual and the somatosensory system7 was used. The apparatus combined a treadmill, instrumented with force platform technology, and virtual reality, to create the LSOT.7 The results verified the findings presented in the literature regarding the SOT and revealed a more rigid (i.e., more regular and repeatable) response during standing posture in conditions with increased sensory conflict. They also revealed that the LSOT was successful in producing significant differences between conditions with increased sensory conflict during walking. However, the results did not support our hypothesis as we found a less rigid and more irregular response for dynamic postural control during walking with increased sensory conflict.
As mentioned above, the SOT results were in agreement with the literature. The entropy values decreased as the SOT conditions increased in difficulty indicating more regular sway patterns.5,6,35 One notable difference is that in previous studies that have used the SOT, a different entropy algorithm was utilized, the Approximate Entropy. However, this algorithm has been found to exhibit certain limitations while SampEn was identified as more reliable for short data sets.40 For this reason the SampEn algorithm was used and to the best of our knowledge, our study is the first study to perform such an analysis with SOT derived data sets. The only direct comparison that could possibly attempt to make is with two studies that have used the SampEn algorithm in investigating questions related with postural control.25,27 In these two studies healthy subjects stood on a solid surface with either eyes open or closed. The results of the study by Rigoldi et al. were comparable to the present study (referring to the first two SOT conditions) in terms of the AP direction but not the ML direction where our values were smaller.27 The differences in the ML direction could be due to the fact that the SOT test is performed mainly in the AP direction—both visual surround and sway reference are manipulated in the AP direction. The results of the study by Ramdani et al. were much larger than the present study but these values may be influenced by the fact that we used different m and r parameters (ours were 2 and 0.2, while Ramdani et al. had 3 and 0.3).25 No values on these parameters were reported in the Rigoldi et al. study. In sum, we feel confident about the values of our results at least with respect to the SOT test, since no such comparisons could be made for the LSOT due to lack of related literature.
How is dynamic postural control affected in the AP direction? In our previous work using the LSOT to explore amount of sway variability during locomotion, we found that the contribution of visual input was significantly increased during locomotion compared to standing in similar sensory conflict conditions.7 Thus, it is not surprising that in this study we found that manipulating vision would also alter the temporal structure of sway variability during locomotion. However, the interesting result was that two different kinds of visual manipulation (reduced vision as in condition 2 and perturbed vision as in condition 3) produced completely opposite results. Reduced vision resulted in a significantly more irregular response, while perturbed vision produced a significantly more regular response. It is possible that reduced vision resulted in more uncertainty and larger need to explore the available stepping space leading to more irregular movement patterns. This deduction is supported by Perry et al. who found that when visual information was occluded using special glasses,24 the COM moved closer to the base of support during double support along with more variability in the COM movement, as the subjects were attempting to achieve a final stable position. Further support is provided by our previous findings using the LSOT7 where amount of variability for step length, step width, and netCOP increased significantly when vision was reduced. However in the perturbed vision condition, where we observed a more regular response, the visual input was in conflict with the treadmill moving at PWS resulting in a freeze of the available degrees of freedom as the subjects were learning to walk in a visually unreliable and an unfamiliar condition.21 Additional support is provided by our previous study7 where we found that step length variability decreased in the visual conflict condition and increased in the vision reduced condition. However in the perturbed vision condition, where we observed a more regular response, the visual input was in conflict with the treadmill moving at PWS resulting in a freeze of the available degrees of freedom as the subjects were learning to walk in a visually unreliable and an unfamiliar condition. Such an interpretation is supported by Katsavelis et al. where was found that optic flow manipulation resulted in decreases in measures of the temporal structure of gait variability as compared to normal unperturbed walking.19 Further support is provided by our previous LSOT study7 where we found that step length variability decreased while the increase in netCOP variability was relatively smaller, in comparison with condition 1 of the LSOT.
Beyond the differential effect of visual manipulation on our results, another interesting result from the present study is that altering only the somatosensory input (as in condition 4) produced a larger effect on the temporal structure of sway variability while walking than only reducing the visual input (as in condition 2). This was not expected, as results for the amount of variability in our previous study were different.7 Importantly when perturbed somatosensory input was added to reduced visual input (as in condition 5), an almost linear additive effect was produced on the temporal structure of sway variability. There was also a large effect when perturbed somatosensory input was added to the perturbed visual input (as in condition 6) reversing the decreasing effect observed in condition 3. These results suggest that somatosensory input has a very prominent effect on the temporal structure of sway variability and is even more influential than visual input. It is possible that visual input has a larger effect on amount of variability during locomotion as observed in previous work,7 but somatosensory input may play a bigger role when dealing with the temporal structure of variability in the anterior posterior direction. This interpretation is supported by Clark et al. that found that altered somatosensation can affect prefrontal activity during walking.8 Moreover, investigations of kinesthetic distance perception have shown that perception of distance traveled while blindfolded depends upon the way in which the legs are coordinated.36
The results for dynamic postural control in the AP were not replicated for the ML direction. Interestingly, the only condition that produced significant effects was the reduced visual input (condition 2). Neither perturbed visual (condition 3) nor perturbed somatosensory input (condition 4) had a significant effect and even when these two conditions were combined (as in condition 6), we did not observe any significant results. These results suggest that in the ML direction, control as evaluated through the temporal structure of variability mostly depends on contributions from peripheral vision since it is the reduced visual condition that actually had an effect and not the perturbed vision condition. This interpretation is supported by Graci et al. who found that proprioceptive information as provided by the peripheral visual field is used online to fine tune adaptive gait.14,15 Importantly, these results demonstrate that sensory inputs have directionally dependent contributions.
There are certain interesting observations when comparing SOT and LSOT results. First, in the AP direction during standing, significant differences occurred as soon as the perturbed somatosensory condition was introduced (SOT condition 4). Before this condition and in conditions 2 and 3, there were no effects. Something similar was observed with walking where we found a strong somatosensory effect as described above. In walking we also have a secondary result, which is the differential effect of reduced vision vs. perturbed vision. In the medial lateral direction, we again have a significant effect of the somatosensory input in the SOT results which is similar with the anterior posterior results. However, this is not the case during walking where we found reduced vision to be the most significant sensory condition. Thus, here we have a true difference between the two tasks in terms of sensory systems contributions as observed through analysis of the temporal structure of sway variability. It also might be due to the attentional demands of balance control vary depending on the complexity of the task.39
Another important result is that during standing as sensory conflict increases, in general the values decrease while in walking they increase. These results could suggest that while standing with our feet stationary, we do not have many options or solutions for postural control when we are faced with sensory conflict. Being more rigid and freezing the degrees of freedom is what we always do when we are faced with the unknown especially if we have no options. However, while walking we have more options that allow us further exploration and adaptations in order to compensate for increased sensory conflict conditions.
In conclusion, our results allowed us to identify how increased sensory conflict affects the temporal structure of sway variability during standing and walking. In general we observed that somatosensory input is crucial for the control of the temporal structure of sway variability for both waking and standing in the anterior posterior direction. Visual input also has an effect but is not as prominent as the somatosensory input. It could also have a different effect based on the way it is manipulated. However, in the medial lateral direction reduced visual input has a much larger effect during walking than in standing possibly due to decreased contribution from peripheral visual inputs. Furthermore and regardless of direction, as sensory conflict increases we observe more rigid and regular sway patterns during standing, while the opposite is the case with walking where we observe more exploratory and adaptive movement patterns. This information could enable more comprehensive decision making processes to be made using the LSOT, possibly in parallel with the SOT that is presently readily available in clinics. Such information could allow us to assist patients with sensory and motor disorders by guiding diagnosis and rehabilitation. The present paper provides the foundation for the establishment of the normative data needed for nonlinear measures and further evidence for adaptation of this technology by the biomedical industry.
Acknowledgments
This work was supported by the Center for Research in Human Movement Variability of the University of Nebraska Omaha and the NIH (P20GM109090 and R01AG034995). Additional support was provided by NASA EPSCoR NNX11AM06A. We specially thank for authors from the previous publication (Jung Hung Chien, Diderik-Jan Anthony Eikema, Mukul Mukherjee, and Nicholas Stergiou, Annual of Biomechanics of Engineering, 2014) to allow us to modify several figures.
ABBREVIATIONS
- LSOT
Locomotor Sensory Organization Test
- SOT
Sensory Organization Test
- netCOP
net Center of Pressure
- SampEn
Sample Entropy
Footnotes
Associate Editor Thurmon E. Lockhart oversaw the review of this article.
References
- 1.Abasolo D, James CJ, Hornero R. Non-linear analysis of intracranial electroencephalogram recordings with approximate entropy and Lempel-Ziv complexity for epileptic seizure detection; Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2007; pp. 1953–1956. [DOI] [PubMed] [Google Scholar]
- 2.Black FO, Shupert CL, Horak FB, Nashner LM. Abnormal postural control associated with peripheral vestibular disorders. Prog Brain Res. 1988;76:263–275. doi: 10.1016/s0079-6123(08)64513-6. [DOI] [PubMed] [Google Scholar]
- 3.Borg FG, Laxaback G. Entropy of balance—some recent results. J Neuroeng Rehabil. 2010;7:38. doi: 10.1186/1743-0003-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;9(11):805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanaugh JT, Guskiewicz KM, Giulliani C, Marshall S. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41(3):305–313. [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanaugh JT, Mercer VS, Stergiou N. Approximate entropy detects the effect of secondary cognitive task on postural control in healthy young adults: a methodological report. J Neuroeng Rehabil. 2007;4:42. doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien JH, Eikema DJ, Mukherjee M, Stergiou N. Locomotor sensory organization test: a novel paradigm for the assessment of sensory contributions in gait. Ann Biomed Eng. 2014;42(12):2512–2523. doi: 10.1007/s10439-014-1112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DJ, Christou EA, Ring SA, Williamson JB, Doty L. Enhanced somatosensory feedback reduces prefrontal cortical activity during walking in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(11):1422–1428. doi: 10.1093/gerona/glu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125(Pt 9):2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- 10.Decker LM, Moraiti C, Stergiou N, Georgoulis AD. New insight into anterior cruciate ligament deficiency and reconstruction through the assessment of knee kinematic variability in terms of nonlinear dynamics. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1620–1633. doi: 10.1007/s00167-011-1484-2. [DOI] [PubMed] [Google Scholar]
- 11.Dettmer M, Pourmoghaddam A, O’Connor DP, Layne CS. Interaction of support surface stability and Achilles tendon vibration during a postural adaptation task. Hum Mov Sci. 2013;32(1):214–227. doi: 10.1016/j.humov.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Glenn T, Whybrow PC, Rasgon N, Grof P, Alda M, Baethge C, Bauer M. Approximate entropy of self-reported mood prior to episodes in bipolar disorder. Bipolar Disord. 2006;8(5 Pt 1):424–429. doi: 10.1111/j.1399-5618.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23(1):23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 14.Graci V, Elliott DB, Buckley JG. Peripheral visual cue affect minimum-foot-clearance during overground locomotion. Gait Posture. 2009;30(3):370–374. doi: 10.1016/j.gaitpost.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Graci V, Elliott DB, Buckley JG. Utility of peripheral visual cues in planning and controlling adaptive gait. Optom Vis Sci. 2010;87(1):21–27. doi: 10.1097/OPX.0b013e3181c1d547. [DOI] [PubMed] [Google Scholar]
- 16.Guskiewicz KM, Riemann BL, Perrin DH, Nashner LM. Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exerc. 1997;29(7 Suppl):s213–s221. doi: 10.1097/00005768-199707001-00003. [DOI] [PubMed] [Google Scholar]
- 17.Harbourne RT, Stergiou N. Movement variability and the use of nonlinear tools: principles to guide physical therapist practice. Phys Ther. 2009;89(3):267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolanryngol Head Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 19.Katsavelis D, Mukherjee M, Decker L, Stergiou N. The effect of virtual reality on gait variability. Nonlinear Dyn Psychol life Sci. 2010;14(3):239–256. [PubMed] [Google Scholar]
- 20.Mawase F, Haizler T, Bar-Haim S, Karniel A. Kinetic adaptation during locomotion on a split-belt treadmill. J Neurophysiol. 2013;109:2216–2227. doi: 10.1152/jn.00938.2012. [DOI] [PubMed] [Google Scholar]
- 21.Newell KM. Degrees of freedom and the development of postural center of pressure profiles. In: Newell KM, Molenaar PCM, editors. Applications of Nonlinear Dynamics to Developmental Process Modeling. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 63–84. [Google Scholar]
- 22.Newell KM, Vaillancourt DE. Dimensional change in motor learning. Hum Mov Sci. 2001;20:695–715. doi: 10.1016/s0167-9457(01)00073-2. [DOI] [PubMed] [Google Scholar]
- 23.Nocera J, Horvat M, Ray CT. Effects of home-based exercise on postural control and sensory organization in individuals with Parkinson disease. Parkinsonism Relat Disord. 2009;15(10):742–745. doi: 10.1016/j.parkreldis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry SD, Santos LC, Patla AE. Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res. 2001;913(1):27–34. doi: 10.1016/s0006-8993(01)02748-2. [DOI] [PubMed] [Google Scholar]
- 25.Ramdani S, Seigle B, Lagarde J, Bouchara F, Bernard PL. One the use of sample entropy to analyze human postural sway data. Med Eng Phys. 2009;31(8):1023–1031. doi: 10.1016/j.medengphy.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Riccio GE. Information in movement variability about qualitative dynamics of posture and orientation. In: Newell KM, Corcos D, editors. Variability and Motor Control. Champaign, IL: Human Kinetics; 1993. pp. 317–357. [Google Scholar]
- 27.Rigoldi C, Cimolin V, Camerota F, Celletti C, Albertini G, Mainardi L, Galli M. Measuring regularity of human postural sway using approximate entropy and sample entropy in patients with Ehlers-Danlos syndrome hypermobility type. Res Dev Disabil. 2013;34(2):840–846. doi: 10.1016/j.ridd.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Riley MA, Clark S. Recurrence analysis of human postural sway during the sensory organization test. Neurosci Lett. 2003;342:45–48. doi: 10.1016/s0304-3940(03)00229-5. [DOI] [PubMed] [Google Scholar]
- 29.Rossi-Izquierdo M, Santos-Pérez S, Soto-Varela A. What is the most effective vestibular rehabilitation technique in patients with unilateral peripheral vestibular disorders? Eur Arch Otorhinolaryngol. 2011;268(11):1569–1574. doi: 10.1007/s00405-011-1532-z. [DOI] [PubMed] [Google Scholar]
- 30.Small M, Tse CK. Applying the method of surrogate to cyclic time series. Phys D. 2002;164:187–201. [Google Scholar]
- 31.Small M, Yu D, Harrison RG. Surrogate test for pseudoperiodic time series data. Phys Rev Lett. 2001;87(18):188101–188104. [Google Scholar]
- 32.Smania N, Picelli A, Gandolfi M, Fiaschi A, Tinazzi M. Rehabilitation of sensorimotor integration deficits in balance impairment of patients with stroke hemiparesis: a before/after pilot study. Neurol Sci. 2008;29(5):313–319. doi: 10.1007/s10072-008-0988-0. [DOI] [PubMed] [Google Scholar]
- 33.Stergiou N. Innovative Analyses of Human Motion. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- 34.Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: the method of surrogate data. Phys D. 1992;58(1–4):77–94. [Google Scholar]
- 35.Turnock MJ, Layne CS. Variations in linear and nonlinear postural measurements under achilles tendon vibration and unstable support-surface conditions. J Mot Behav. 2010;42(1):61–69. doi: 10.1080/00222890903397103. [DOI] [PubMed] [Google Scholar]
- 36.Turvey MT, Harrison SJ, Frank TD, Carello C. Human odometry verifies the symmetry perspective on bipedal gaits. J Exp Psychol Hum Percept Perform. 2012;38(2):1014–1025. doi: 10.1037/a0027853. [DOI] [PubMed] [Google Scholar]
- 37.Vaillancourt DE, Newell KM. Complexity in aging and disease: response to commentaries. Neurobiol Aging. 2002;23(1):27–29. doi: 10.1016/s0197-4580(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 38.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23(1):1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 39.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 40.Yentes JM, Hunt N, Schmid KK, Kaipust JP, McGrath D, Stergiou N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann Biomed Eng. 2013;41(2):349–365. doi: 10.1007/s10439-012-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeragani VK, Pohl R, Mallavarapu M, Balon R. Approximate entropy of symptoms of mood: an effective technique to quantify regularity of mood. Bipolar Disord. 2003;5(4):279–286. doi: 10.1034/j.1399-5618.2003.00012.x. [DOI] [PubMed] [Google Scholar]


