Abstract
Adverse intrauterine environments increase vulnerability to chronic diseases across the lifespan. The hypothalamic-pituitary-adrenal (HPA) axis, which integrates multiple neuronal signals and ultimately controls the response to stressors, may provide a final common pathway linking early adversity and adult disease. Both prenatal alcohol exposure (PAE) and prenatal stress (PS) induce a hyperresponsive HPA phenotype in adulthood. As glucocorticoids are pivotal for the normal development of many fetal tissues including the brain, we used animal models of PAE and PS to investigate possible mechanisms underlying fetal programming of glucocorticoid signaling in the placenta and fetal brain at gestation day (GD) 21. We found that both PAE and PS dams had higher corticosterone levels than control dams. However, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) enzyme levels were increased in PAE and unchanged in PS placentae, although there were no differences in 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) levels. Moreover, only PAE fetuses showed decreased body weight and increased placental weight, and hence a lower fetal/placental weight ratio, a marker of placenta efficiency, compared to all other prenatal groups. Importantly, PAE and PS differentially altered corticosteroid receptor levels in placentae and brains. In the PS condition, maternal corticosterone was negatively correlated with both 11β-HSD1 and mineralocorticoid receptor (MR) proteins levels in male and female placentae, whereas in the PAE condition, there were trends for a positive correlation between maternal corticosterone and 11β-HSD1, regardless of sex, and a negative correlation between maternal alcohol intake and MR in male placentae. In fetal brains, sexually dimorphic changes in MR and glucocorticoid receptor (GR) levels, and the MR/GR ratio seen in C fetuses were absent in PAE and PS fetuses. In addition, PS but not PAE female fetuses had higher MR and lower GR expression levels in certain limbic areas compared to C female fetuses. Thus the similar adult HPA hyperresponsive phenotype in PAE and PS animals likely occurs through differential effects on glucocorticoid signaling in the placenta and fetal brain.
Keywords: Prenatal Alcohol Exposure, Hypothalamic-Pituitary-Adrenal Axis, Glucocorticoid Receptor, Mineralocorticoid Receptor, Placenta, 11β-hydroxysteroid dehydrogenase
INTRODUCTION
In the last two decades, a Developmental Origins of Health and Disease (DOHaD) approach has begun to elucidate the links between adverse changes in the intrauterine or early postnatal environment (e.g., alcohol, undernutrition, stress) and the development of chronic diseases or disorders (e.g., cardiovascular disease, type II diabetes, mental health disorders) across the life span (Barker, 1995; Barker, 1998). For example, although the etiology of depression/anxiety disorders is not fully understood, it is generally accepted that the incidence of these mental health problems occurs in greater proportion among vulnerable populations that are exposed to adverse early life experiences, such as prenatal alcohol exposure (PAE) and prenatal stress (PS) (Maccari et al., 2003; Riley and McGee, 2005). This suggests the possibility that different early adverse insults may share some common mechanisms.
Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis, which integrates multiple neuronal signals and ultimately controls the hormonal response to stressors, may provide a final common pathway linking early adversity and adult diseases. The inability to respond appropriately to stress is a crucial determinant in later vulnerability to neuropsychiatric disorders (Nestler et al., 2002). Indeed, epidemiological, clinical, and basic animal studies have shown that both PAE and PS increase the risk of adverse neurodevelopmental outcomes including HPA hyperresponsiveness and vulnerability to mental health disorders (Maccari and Morley-Fletcher, 2007; Hellemans et al., 2010; Weinstock, 2015). These adverse outcomes may be due, at least partly, to increased fetoplacental glucocorticoid (cortisol in human; corticosterone [CORT] in rats) exposure, as studies in animal models have shown that PAE and PS both increase maternal plasma CORT levels (Weinberg and Bezio, 1987; Maccari et al., 2003), which could, in turn, impact the fetus.
As the interface between mother and fetus, the placenta is the key conduit of nutrient, hormone, and oxygen supply to the fetus. The placenta plays an essential role in modulating and filtering signals from the maternal milieu, and thus is critical to fetal development. Although glucocorticoids could potentially cross the fetoplacental barrier freely, levels of fetal glucocorticoid exposure are predominantly regulated by placental 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes. 11β-HSD type 2 (11β-HSD2) catalyzes the conversion of active CORT into inert 11-dehydrocorticosterone (cortisol into cortisone in humans). This enzyme functions as a physiological fetoplacental glucocorticoid barrier by protecting the fetus from overexposure to maternal glucocorticoids. On the other hand, 11β-HSD type 1 (11β-HSD1) catalyzes conversion in the opposite direction, thus regenerating and amplifying glucocorticoid action. In rodents, the expression of 11β-HSD1 increases starting at gestation day (GD) 16.5, which is thought to assist in organ maturation (Thompson et al., 2002). Both 11β-HSD enzymes contribute to the intracellular “gating” of glucocorticoid action (Chapman et al., 2013), and thus play a critical role in glucocorticoid signaling in the placenta and determining the amount and timing of intrauterine glucocorticoid exposure.
Adverse intrauterine environments such as alcohol or stress exposure, and the concomitant increase of maternal glucocorticoids, are important signals for fetuses from the maternal milieu. While gating by the 11β-HSD2 enzymes provides a partial barrier at the fetoplacental interface, as noted, some glucocorticoids will in fact reach the fetus, critically impacting fetal brain development, including the development and activity of the HPA axis, with possible long-term behavioral and physiological effects (Seckl and Holmes, 2007). In the case of maternal alcohol consumption however, effects on the fetus are more complex than those of maternal stress. In addition to the effects of alcohol in activating the maternal HPA axis, and thus, indirectly affecting the fetal HPA axis, alcohol itself can cross the fetoplacental barrier and directly activate the fetal HPA axis, which is functional before birth. Thus, increased plasma and adrenal CORT levels observed in PAE neonates (Taylor et al., 1982; Weinberg, 1989) result from a combination of both direct and indirect effects of alcohol, and indeed, synergistic effects of alcohol and glucocorticoids on the fetus may exist. Of relevance, no studies, to our knowledge, have examined PAE or PS effects on placental 11β-HSD1, and reports on the effects of both PAE and PS on placental 11β-HSD2 mRNA levels have been somewhat inconsistent. Decreased 11β-HSD2 expression levels have been reported in PAE and PS placentae in some studies (Mairesse et al., 2007; Rosenberg et al., 2010; Liang et al., 2011; Pena et al., 2012). However, increased placental 11β-HSD2 expression levels were reported in mouse models of glucocorticoid exposure starting at mid-gestation, rat models of periconceptional alcohol exposure, and in pregnant women at term following inhaled glucocorticoid treatment (Clifton et al., 2006; Cuffe et al., 2012; Gardebjer et al., 2014). Furthermore, in one study that tested effects of PAE on both male and female placentae, 11β-HSD2 mRNA levels were decreased in female, but increased in male placentae (Wilcoxon et al., 2003). Thus it is not clear whether the effects of PAE and PS on the developing HPA axis occur through similar or different mechanisms, at least in terms of effects on 11β-HSD2 expression and subsequent, possibly sexually dimorphic effects, on the fetal brain.
The developing brain is particularly vulnerable to intrauterine adversity. Indeed, neurodevelopmental deficits induced by PAE and PS have been shown in human and animal studies (West et al., 1994; Maccari et al., 2003; Schneider et al., 2004; Riley and McGee, 2005; Seckl, 2008). Importantly, the effects of PAE and PS on neurodevelopmental outcome may be sexually dimorphic. For example, sex differences in HPA responsiveness have been observed in animal models of both PAE and PS (Weinstock, 2007; Weinberg et al., 2008; Brunton and Russell, 2010; Hellemans et al., 2010).
The effects of glucocorticoids in the brain are largely dependent on the site of action and the relative expression levels of mineralocorticoid (MR) and glucocorticoid (GR) receptors, and the balance in functions mediated by MR and GR appears critical for neuronal excitability, stress responsiveness, and behavioral adaptation (De Kloet et al., 1998). The limbic brain areas (medial prefrontal cortex (mPFC), hippocampus and amygdala), which are part of the stress-responsive neurocircuitry, are rich in MR and GR. To date, most of the studies investigating glucocorticoid signaling in the brain have been done in adult PAE and PS offspring. How PAE and PS affect the expression of corticosteroid receptors in key limbic brain areas during the fetal period, and whether these effects are sexually dimorphic, is largely unknown. Understanding the mechanisms underlying fetal programming of the HPA axis and how it is affected by early life adversity such as PAE and PS, may provide insights for both the development of clinical biomarkers during early development and for early intervention.
The present study utilizes animal models of PAE and PS to investigate possible mechanisms underlying fetal programming of glucocorticoid signaling in the placenta and fetal brain at GD 21 and determine whether these mechanisms are similar or different following PAE and PS. In light of the more complex effects of maternal alcohol consumption compared to those of maternal stress on fetal HPA activity, we tested the hypotheses that the similar adult HPA hyperresponsive phenotype in PAE and PS animals will likely occur: 1) through differential effects of these prenatal insults on glucocorticoid signaling in the placenta and fetal brain, and 2) in a sexually dimorphic manner.
EXPERIMENTAL PROCEDURES
Animals, Diets and Feeding
All animal use and care procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the University of British Columbia Animal Care Committee.
Female (265 - 300 g, n = 50) and male (275 - 300 g, n = 18) Sprague-Dawley rats obtained from Charles River Laboratories (St. Constant, PQ, Canada) were pair-housed by sex for a 1 - 2 week adaptation period prior to breeding. Temperature (~21 - 22°C) and lighting (12:12 h light/dark cycle, lights on at 0700 h) were controlled throughout all phases of the experiment.
On GD1, females were singly housed in polycarbonate cages (24 × 16 × 46 cm) with pine-shaving bedding and randomly assigned to one of four treatment groups: 1) Ethanol (PAE), liquid ethanol diet (36 % ethanol-derived calories) and water, ad libitum (n = 8); 2) Pair-fed (PF), liquid control diet, with maltose-dextrin isocalorically substituted for ethanol, and intake matched to the amount consumed by a PAE partner (g/kg body weight/gestation d), and water ad libitum (n = 8); 3) Control (C): pelleted control diet and water, ad libitum (n = 8); and 4) Prenatal stress (PS): pelleted control diet and water, ad libiturm (n = 9). PAE females were gradually introduced to the ethanol diet by providing 1/3 ethanol : 2/3 control diet on GD 1, 2/3 ethanol : 1/3 control diet on GD 2, and full ethanol diet on GD 3. The liquid diets were prepared by Dyets Inc., Bethlehem, PA, USA (Weinberg/Keiver high protein liquid diet – Experimental, #710324; Control, #710109). PAE and PF dams were provided with fresh liquid diet daily within 1.5 h prior to lights off to prevent a shift of the CORT circadian rhythms, which occurs in animals on a restricted feeding schedule, such as those in the PF group (Gallo and Weinberg 1981; Krieger, 1974). The previous night's bottle was measured to determine the amount consumed. Experimental diets were continued through GD 21. The PS model involved a 10-day chronic mild stress paradigm applied to pregnant dams during GD 11-20 in a room separate from the colony room. Animals were exposed to two different stressors per day, one between 0800 h and 1200 h, and the second between 1300 h and 1600 h, with a minimum of two hours between the stressors. The order and type of stressor was randomized, but all animals received the same number of exposures to each stressor over the 10-day period. The stressors were designed to simulate mild, psychological stress and included: (1) space restriction: animals were housed for 12 h (19:00 h to 07:00 h) with food and water in mouse cages with bedding; (2) wet bedding: animals were housed in their home cages with bedding moistened with 700 ml water for 1 h; (3) 30 min restraint in polyvinyl chloride tubes (15 × 6 cm); (4) 1 h exposure to a soiled cage (bedding from a different animal); (5) 2 h cage tilt at a 30° angle; (6) 1 h exposure to a novel cage; and (7) wet cage: animals were put in an empty cage with 1 cm of room-temperature water for 30 min.
All pregnant dams were handled on GD 1, GD 7, and GD 14 for cage changing and weighing, but otherwise left undisturbed. In addition, PS dams were handled everyday during the 10-day chronic mild stress procedure.
Blood Sampling and Tissue Collection
All dams were terminated between 08:00 h and 11:00 h on GD 21. Each pregnant female was carried in her home cage from the colony room to an adjacent testing room where she was weighed and immediately decapitated. A midline abdominal incision was made, the uterine horns were exposed, and all the fetuses and their attached placentae were removed starting from the uterine body to the left uterine horn, followed by the right uterine horn. Fetuses and placentae were lightly wiped and then weighed. The brain and corresponding placenta from the first male fetus and first female fetus sampled were removed and snap frozen in liquid nitrogen and stored at − 80 °C until analysis. Fetal sex was determined by assessing anogenital distance; 4 experienced individuals participating in the tissue collection procedures independently observed and verified the assessment. Maternal serum was collected at the same time and stored at − 20 °C.
Brain Micropunch
Fetal brains and glass slides were acclimated to the Cryostat which was set to −10 °C for at least 2 hours. Sections were cut at 300 μm from the rostral end to the caudal end of the hippocampus (approximately at plate P0 Coronal 30; Ashwell and Paxinos, 2008). Two to three sections were placed on one slide, allowing sections to freeze mount to the slides. Slides were stored on dry ice. Harris Uni-Core disposable micro-punches (1.5mm, Sigma-Aldrich, Oakville, Ontario Canada) were used to dissect out mPFC (pooled infralimbic and prelimbic areas on both sides), hippocampus (both sides), amygdala (both sides), and hypothalamus (both sides). Tissue was deposited into 2 ml sterile tubes and stored at − 80 °C until homogenization.
Radioimmunoassays (RIA)
Blood samples were centrifuged at 2,200g for 10 min at 0 °C. Serum was transferred into 600 μl Eppendorf tubes and stored at − 80 °C until assayed.
Corticosterone
Total serum corticosterone (bound plus free) levels were measured using the ImmuChem Corticosterone Double Antibody RIA kit from (Cat #. 07-120102, MP Biomedicals, Inc., Costa Mesa, CA, USA) with [125I] corticosterone as the tracer. The minimum detectable corticosterone concentration was 0.63 μg/dl and the intra- and inter-assay coefficients of variation were 7.1 % and 7.2 % respectively.
Estradiol
Serum estradiol levels were measured using the ImmuChem Double Antibody 17β-estradiol RIA kit (Cat #. 07- 138102, MP Biomedicals, Inc., Costa Mesa, CA, USA) with [125I] 17β-estradiol as the tracer. The lowest 17β-estradiol standard was 10 pg/ml, and the intra- and inter-assay coefficients of variation were 10.6 % and 11.9 % respectively.
Progesterone
Serum progesterone levels were measured using the ImmuChem Double Antibody progesterone RIA kit (Cat #. 07-170102, MP Biomedicals, Inc., Costa Mesa, CA, USA) with [125I] progesterone as the tracer. The lowest standard was 0.2 ng/ml, and the intra- and inter-assay coefficients of variation were 3.6 % and 6.7 % respectively.
Western Blot
Protein from fetal placentae and fetal brain areas (mPFC, hippocampus, amygdala and hypothalamus) was extracted in RIPA lysis buffer (0.1 % sodium dodecyl sulfate, 1.0 % IGEPAL (v/v), 0.5 % Sarkosyl (w/v), 150 mM NaCl, 50 mM Tris-base, pH = 8.0) containing protease inhibitor (Cat #. 04693116001, Roche, Indianapolis, IN, USA). Protein samples (placentae: 100 μg; brain areas: 20 μg) were electrophoresed on TGX 4-15 % gradient gels (BioRad, Hercules, CA, USA) and wet-transferred onto 0.45-mm nitrocellulose membranes (BioRad, Hercules, CA, USA). After blocking with Odyssey blocking buffer (Li-Cor Biotechnology, distributed by Mandel Scientific Co. Guelph. ON, CA), blotted membranes were incubated with primary antibodies overnight at 4 °C. For placental GR, the primary antibody was a rabbit polyclonal antibody, sc-1004, 1:500 (Santa Cruz Biotechnology, Dallas, TX, USA). For the fetal brain, however, Western Blot utilizing antibody sc-1004 yielded only a faint 95 kDa band, due to the significantly lower GR protein expression in fetal compared to adult hippocampus, which was our positive control. Therefore, GR antibody, sc-8992, at a 1:100 concentration, was utilized for fetal brains. Both antibodies have been shown to react with rat GR protein and are widely used in studies measuring GR levels in rat models. Other primary antibodies included MR (1:2000; sc-11412), 11β-HSD1 (1:200; sc-20175), 11β-HSD2 (1:200; sc-20176), and mouse monoclonal GAPDH antibody from Abcam (ab 9484, Abcam Inc, Toronto, ON, CA). After washing, a 1:10000 dilution in Odyssey buffer of the appropriate secondary antibody was applied to the membranes for 1 hour at room temperature. Secondary antibodies were IRDye 800CW Goat anti-Mouse and IRDye 680RD Goat anti-Rabbit (Li-Cor Biotechnology, distributed by Mandel Scientific Co, Guelph. ON, CA). Membranes were scanned with the Odyssey CLx system (Li-Cor Biotechnology). Densitometric analysis was carried out using Image Studio (Li-Cor Biotechnology) to quantify the expression levels of the targeted protein relative to the internal control protein GAPDH. Each gel contained a total of 8 samples: 1 sample from each prenatal treatment group and sex. A total of 6 gels were run for each area and protein of interest (ie. n = 6/prenatal treatment group/sex).
Statistical Analyses
Maternal hormone and body weight data were analyzed using one-way and repeated measures ANOVAs, respectively. Other data were analyzed using two-way ANOVAs with group (C, PF, PAE, PS) and/or sex (male and female) as between-subjects factors followed by Fisher's LSD post-hoc tests on significant main and interaction effects. To test our a priori hypotheses that PAE and PS would differentially affect glucocorticoid signaling in the placenta and fetal brain, planned comparisons using a Šidák correction for family-wise error (Cardinal and Aitken, 2006) were carried out to examine 1) group differences for PAE vs. PF or C, and PS vs. C overall or within each sex; 2) patterns of sex differences within each prenatal group. Because the Šidák correction accurately controls for family-wise α, an overall F test need not be significant in order to explore a priori differences between factors using planned comparisons (Cardinal and Aitken, 2006; Myers and Well, 2003)
Statistical significance was set at P < 0.05. P values between 0.05 and 0.10 were considered a statistical non-significant trend.
RESULTS
Pregnancy outcome and fetal development
Ethanol intake and blood ethanol levels
Ethanol intake of pregnant females was consistently high throughout gestation, averaging 14.62 ± 0.28, 18.42 ± 0.41, 17.08 ± 0.29 g/kg body weight for weeks one, two and three of gestation, respectively, and resulting in blood ethanol levels of 143.13 ± 2.79 mg/dl measured 2 h after lights off on GD 15.
Maternal body weight
Analysis of % weight gain during gestation (weight relative to GD 1) indicated that while all dams gained weight, overall C dams gained significantly more weight than dams in the other 3 groups (Ps < 0.05; significant main effects of group [F(3,28) = 5.691; P < 0.005] and day [F(3,28) = 778.23; P < 0.001], and a group × day interaction [F(9,84) = 7.97; P < 0.001]; Table 1). PAE dams gained less weight than C dams from GD 7 to GD 21 (Ps < 0.05). PF dams had lower weight gain than C dams on GD 7 and 14 (Ps < 0.05); by GD 21, PF dams were intermediate to and not different in weight from all other groups. PS dams were similar in weight to C dams on GD 7, but weight gain then decreased such that by GD 21, they were similar to PAE dams (PS = PAE < C on GD 21, Ps < 0.05).
Table 1.
Percentage of body weight gain (%) during gestation in C, PF, PAE and PS dams
| C | PF | PAE | PS | |
|---|---|---|---|---|
| GD 1 | 100.0±0.0 | 100.0±0.0 | 100.0±0.0 | 100.0±0.0 |
| GD 7 | 113.0±1.6 | 106.5±0.9a | 106.5±0.5a | 114.9±1.0 |
| GD 14 | 131.3±2.8 | 122.4±1.0b | 121.8±1.0b | 126.9±2.0 |
| GD 21 | 166.3±5.2 | 156.8±1.6 | 150.5±1.9c | 147.5±2.8c |
Abbreviations: C, control; PF, pair-fed; PAE, prenatal alcohol exposure; PS, prenatal stress; GD, gestation day; Ps < 0.05.
PAE = PF < C = PS on GD 7
PAE = PF < C on GD 14
PAE = PS < C on GD 21
Fetal and placental weights
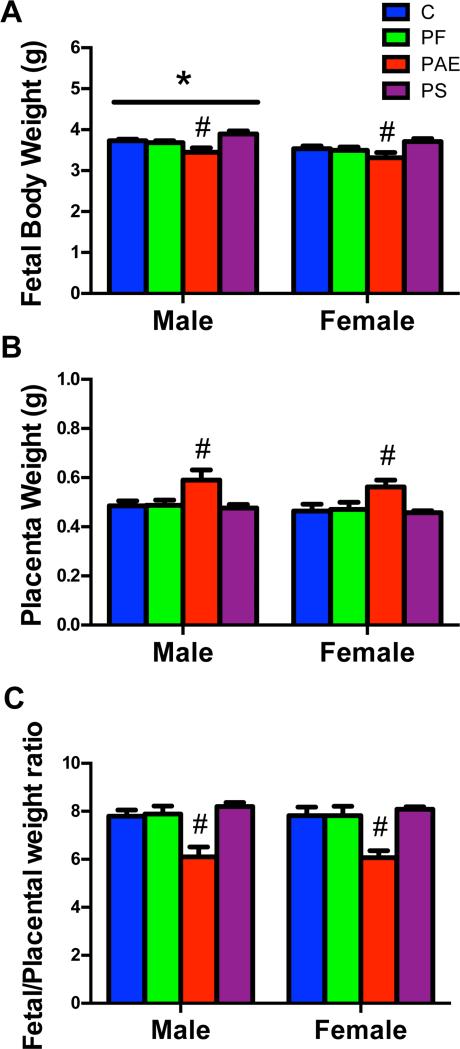
PAE fetuses had the lowest weights compared to fetuses in the other 3 groups (Ps < 0.05), whereas PS fetuses showed a non-significant trend for higher weights than C fetuses (P = 0.067; main effect of group [F(3,42) = 6.84; P < 0.01]; Fig 1A). Not surprisingly, male fetuses weighed more than female fetuses (P < 0.01; main effect of sex [F(1,42) = 7.43; P < 0.01]; Fig 1A).
Fig 1.
Fetal body weight (A), placental weight (B) and fetal/placental weight ratio (C) in the male (left) and female (right) offspring from C, PF, PAE and PS groups on GD 21. (*) Denotes significant differences between sexes, P < 0.01; (#) Denotes significant differences from other prenatal treatment groups, Ps < 0.01.
Placenta weight was higher in PAE fetuses than in fetuses of the other 3 groups (Ps < 0.01; main effect of group [F(3,42) = 6.92; P < 0.01]; Fig 1B). Concomitantly, the fetal/placenta weight ratio, a marker of placental efficiency, was lower in PAE than in fetuses of the other 3 groups (Ps < 0.01; main effects of group [F(3,42) = 14.07; P < 0.01]; Fig 1C).
Maternal hormone levels
Corticosterone (CORT) levels
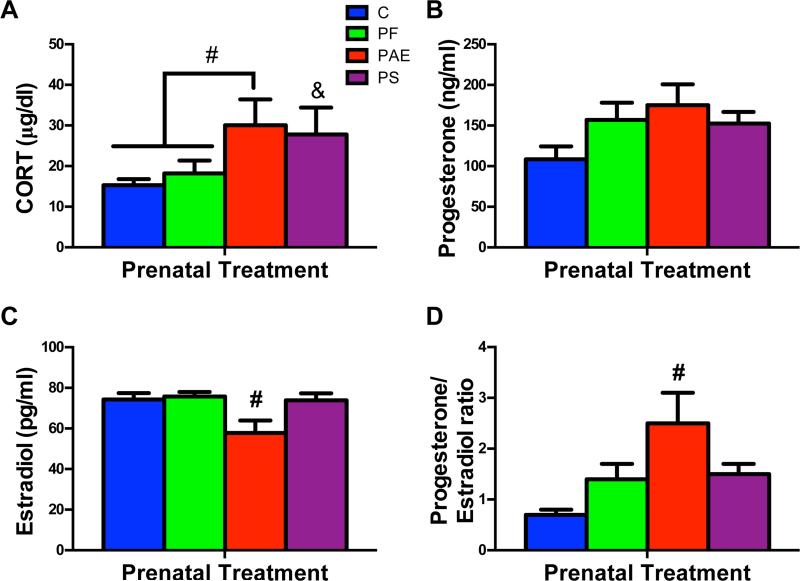
CORT levels were elevated in both PAE and PS dams. PAE dams had higher CORT than both C and PF dams (Ps < 0.05), while PS dams had higher CORT than C (P < 0.05; main effect of group, F(3,24) = 3.62; P < 0.05; Fig 2A).
Fig 2.
Maternal serum CORT (A), progesterone (B), estradiol (C) and progesterone/estradiol ratio (D) on GD 21. (#) Denotes significant differences from other prenatal treatment groups, Ps < 0.05; (&) Denotes significant differences from the C group, P < 0.05.
Progesterone and estradiol levels
For progesterone, There was a non-significant trend for group (F(3,24) = 2.54; P = 0.08; Fig 2B). By contrast, estradiol levels were lower in PAE compared to dams of the other 3 groups (Ps < 0.05; main effect of group, F(3,24) = 4.49; P < 0.05; Fig 2C), and PAE dams thus had a higher progesterone/estradiol ratio than all other dams (Ps < 0.05; main effect of group [F(3,24) = 5.52; P < 0.05]; Fig 2D).
11β-HSD1, 11β-HSD2, MR and GR protein levels and MR/GR ratio in the placenta
11β-HSD1
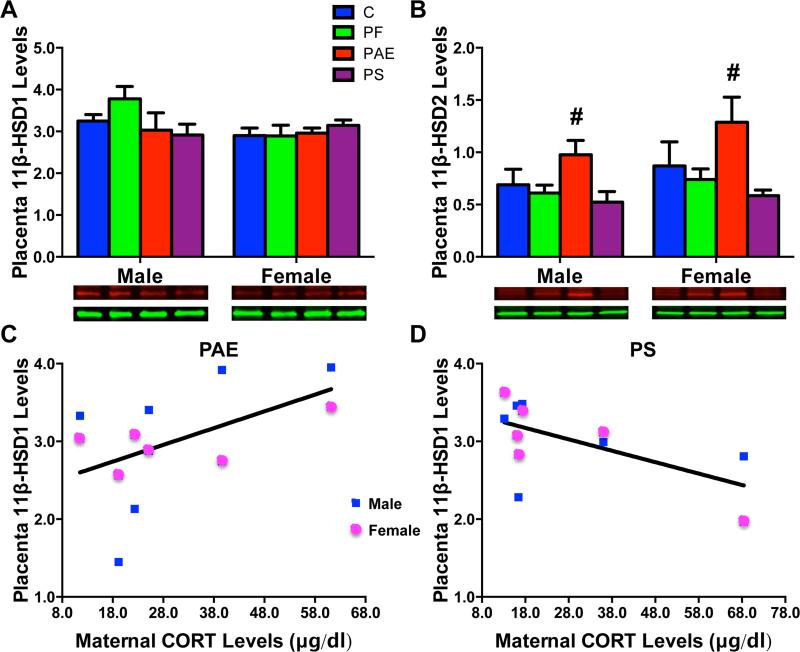
There were no group or sex differences in 11β-HSD1 enzyme levels in the placenta (Fig 3A). However, a significant negative correlation between maternal CORT levels and placental 11β-HSD1 protein levels was found in PS placentae (r = − 0.603, P < 0.05, Fig 3D). Conversely, there was a non-significant trend for a positive correlation between maternal CORT and placental 11β-HSD1 protein levels in the PAE (r = 0.513, P = 0.088) conditions (Fig 3C).
Fig 3.
Placental 11β-HSD1 (A) and 11β-HSD2 (B) protein levels (relative to GAPDH) with representative Western Blots of respective target proteins (11β-HSD1 [37 kDa in red and 11β-HSD 2 [40 kDa in red], and internal control GAPDH [36 kDa in green]) underneath each graph. (#) Denotes significant differences from other prenatal treatment groups, P < 0.05. (C), A non-significant trend for a positive correlation between maternal CORT levels and placenta 11β-HSD1 protein levels in PAE male and female fetuses (P = 0.088). (D), A significant negative correlation between maternal CORT levels and placenta 11β-HSD1 protein levels in PS male and female fetuses (P < 0.05).
11β-HSD2
Overall, 11β-HSD2 protein levels were higher in PAE placentae compared to those of the other 3 groups (Ps < 0.05; significant main effect of group [F(3,42) = 5.37; P < 0.01]; Fig 3B). There were no significant differences between male and female placentae.
MR and GR
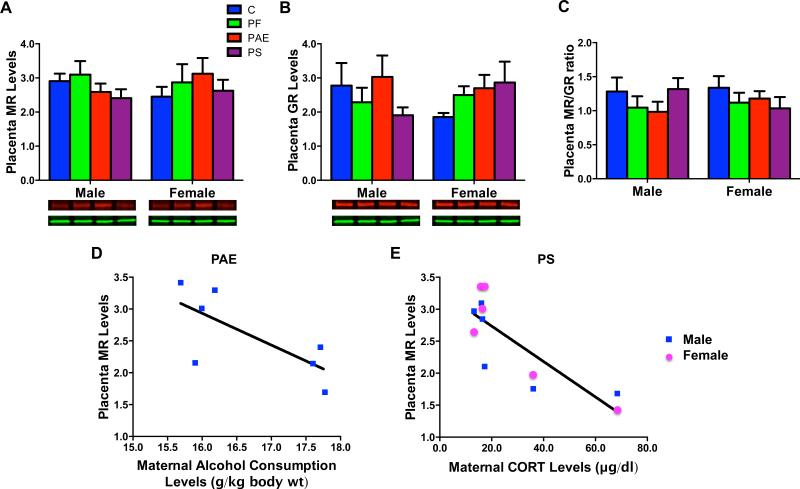
There were no group or sex differences in placental MR (Fig 4A), or GR (Fig 4B) levels or the MR/GR ratio (Fig 4C). However, there was a significant negative correlation (r = − 0.822, P < 0.05) between maternal CORT levels and MR protein levels in PS male and female placentae (Fig 4E). As well, a non-significant trend for a negative correlation (r = − 0.722, P = 0.067) was found between levels of maternal alcohol consumption during gestation week 3 and MR protein levels in PAE male placentae (Fig 4D).
Fig 4.
Placental MR (A), GR (B) protein levels (relative to GAPDH), and MR/GR ratio (C), with representative Western Blots of respective target proteins GR [95 kDa in red], MR [102 kDa in red], and internal control GAPDH [36 kDa in green]) underneath each graph. (D), A negative correlation (P = 0.067) that approached significance between maternal alcohol consumption during gestation week 3 and MR protein levels in male PAE fetuses. (E), A significant negative correlation (P < 0.05) between maternal CORT levels and MR protein levels in PS male and female fetuses.
MR and GR protein levels and MR/GR ratio in the fetal brain
Medial Prefrontal Cortex
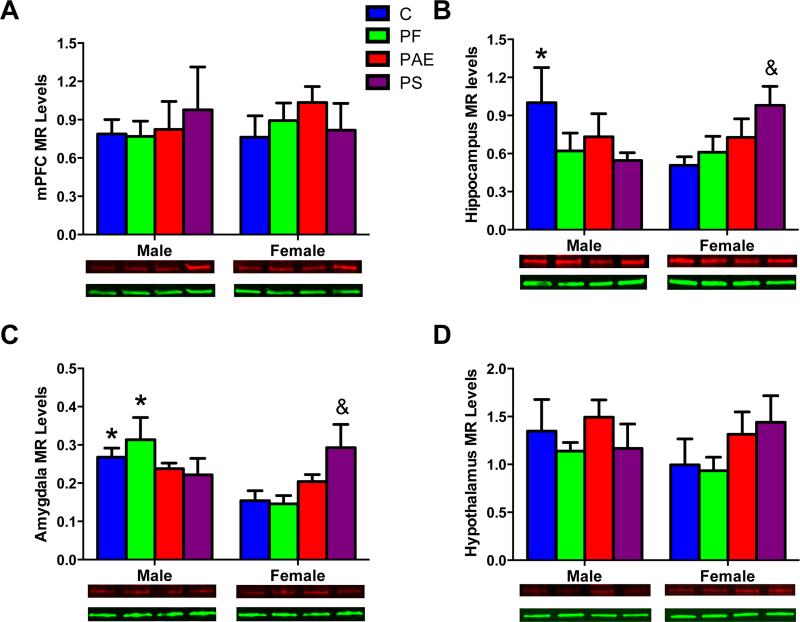
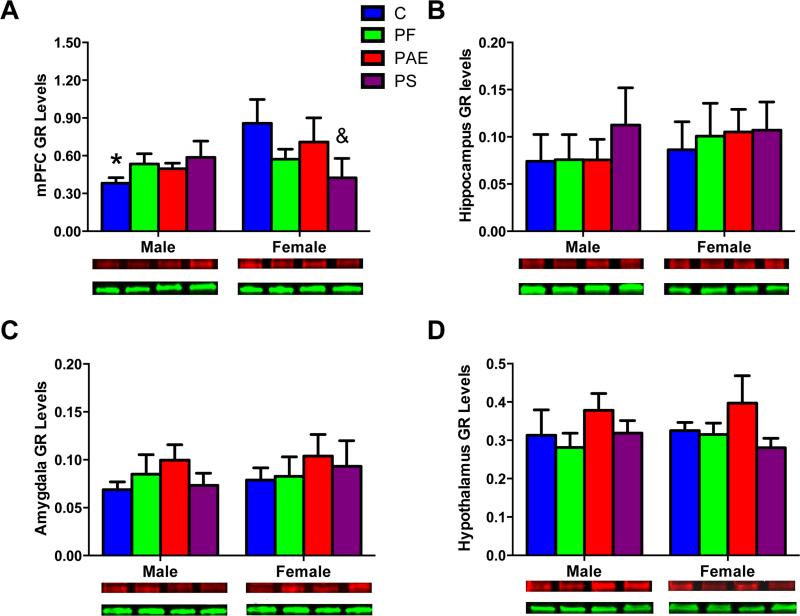
There were no effects of group or sex for MR protein levels (Fig 5A). For GR, non-significant trends for a main effect of sex [F(1, 40) = 3.09; P = 0.087] and a group X sex interaction [F(3, 40) = 2.62; P = 0.064]) were explored by planned comparisons, as per our hypotheses. In the mPFC, only C fetuses showed a sex difference in GR levels (male < female, P < 0.05; Fig 6A). In addition, for female but not male fetuses, GR protein levels were lower in PS than C fetuses (Ps < 0.05; Fig 6A).
Fig 5.
MR protein levels in the fetal brain areas: mPFC (A), hippocampus (B), amygdala (C), and hypothalamus (D) with representative Western Blots of MR (102 kDa in red) and internal control GAPDH (36 kDa in green) images. (*) Denotes significant differences between sexes, P < 0.05; (&) Denotes significant differences from the C group, P < 0.05.
Fig 6.
GR protein levels in the fetal brain areas: mPFC (A), hippocampus (B), amygdala (C), and hypothalamus (D) with representative Western Blots of GR (95 kDa in red) and internal control GAPDH band (36 kDa in green). (*) Denotes significant differences between sexes, P < 0.05; (&) Denotes significant differences from the C group, P < 0.05.
Hippocampus
Sex differences in MR protein levels were observed for the hippocampus in C fetuses (male > female, P < 0.05; significant Group X Sex interaction [F(3,37) = 2.92; P < 0.05]). A non-significant trend for sex differences in MR protein levels in PS occurred in a direction opposite (female > male, P < 0.07) to that in C fetuses (Fig 5B). Furthermore, PS female fetuses had higher MR levels compared to C female fetuses (Ps < 0.05). There were no sex differences in MR expression for PAE fetuses. Analysis of GR showed no significant group or sex effects (Fig 6B). For the MR/GR ratio, however, there was a sex difference for C fetuses only (male > female, P < 0.05; main effect of sex [F(1,35) = 4.16; P < 0.05], data not shown).
Amygdala
Sex differences in MR levels were observed in the amygdala of C and PF but not PAE and PS fetuses (male > female, Ps < 0.05, main effect of Sex [F(1,32) = 5.31; P < 0.05]; Group X Sex interaction [F(3,32) = 3.84; P < 0.05]; Fig 5C). In addition, PS female fetuses had higher amygdala MR protein levels than C female fetuses (Ps < 0.05). There were no group or sex effects for GR protein levels (Fig 6C) or the MR/GR ratio (data not shown) in the amygdala.
Hypothalamus
There were no group or sex differences for MR or GR protein levels or the MR/GR ratio in the hypothalamus (Fig 5D, 6D).
DISCUSSION
In light of the more complex effects of maternal alcohol consumption compared to maternal stress on fetal HPA activity, we tested the hypotheses that the similar adult HPA hyperresponsive phenotype in PAE and PS animals may occur through differential effects on glucocorticoid signaling in the maternal female and the fetus. Consistent with this, we found differential effects of PAE and PS on maternal endocrine function and glucocorticoid signaling in both the placenta and fetal brain. Both PAE and PS dams had higher corticosterone levels than controls. Despite this, 11β-HSD2 levels were increased in PAE and unchanged in PS placentae, suggesting that a possible increase in transplacental passage of active maternal glucocorticoids in PS, but more direct teratogenic effects of alcohol in PAE fetuses, may underlie the impact of these early life insults on the fetus. In addition, only PAE fetuses showed decreased body weight and increased placental weight, and hence a lower fetal/placental weight ratio (a marker of placenta efficiency) compared to all other prenatal treatment groups. Moreover, in the PS condition, maternal CORT was negatively correlated with both 11β-HSD1 and MR protein levels in male and female placentae, whereas in the PAE condition, there were trends for a positive correlation between maternal CORT and 11β-HSD1, regardless of fetal sex, and a negative correlation between maternal alcohol intake and MR in male placentae. In fetal brains, sexually dimorphic changes in MR and GR levels, and the MR/GR ratio seen in C fetuses were absent in PAE and PS fetuses. In addition, PS but not PAE female fetuses had higher MR and lower GR expression levels in certain limbic areas compared to C female fetuses (Table 2). Together, our findings suggest that although there are some similarities in the effects of PAE and PS, it is likely that these early life insults work through different glucocorticoid signaling mechanisms to program HPA activity in offspring.
Table 2.
Summary of changes in PAE and PS compared to Controls
| PAE | PS | |
|---|---|---|
| Fetal/placental wt ratio | ↓ | — |
| Maternal CORT | ↑ | ↑ |
| Maternal Progesterone/Estradiol ratio | ↑ | — |
| Placenta 11-β HSD1 protein levels | Trend for positive correlation with maternal CORT levels in placentae of male and female fetuses | Negative correlation with maternal CORT levels in placentae of male and female fetuses |
| Placenta 11-β HSD2 protein levels | ↑ | — |
| Placenta MR protein levels | Trend for negative correlation with maternal alcohol consumption in placentae of male fetuses | Negative correlation with maternal CORT levels in placentae of male and female fetuses |
| MR protein levels in hippocampus | Absence of sexually dimorphic expression | Absence of sexually dimorphic expression; ↑ in female fetuses |
| MR protein levels in amygdala | Absence of sexually dimorphic expression | Absence of sexually dimorphic expression; ↑ in female fetuses |
| GR protein levels in mPFC | Absence of sexually dimorphic expression | Absence of sexually dimorphic expression; ↓ in female fetuses |
| MR/GR ratio in hippocampus | Absence of sexually dimorphic expression | Absence of sexually dimorphic expression |
The finding that control dams gained more weight than dams in all other groups is not surprising. Alcohol provides “empty calories,” i.e., calories without nutrients, and also affects food intake and nutrient absorption and utilization. Indeed, the food intake of PAE dams was lower than that of controls. Together, these factors can account for the reduced weight gain of PAE dams. PF dams were fed a reduced ration, and thus they also gained less weight than C dams. PS dams experienced chronic mild stress starting at GD 11, which impacted their weight gain from GD 11-21. These findings are consistent with previous work in rodents showing that chronic mild stress reduces weight gain compared to that in their non-stressed counterparts (Paternain et al., 2012; Hellemans et al., 2008). Moreover, administration of CRH suppresses appetite and induces weight loss (Keck et al., 2005). Since both stress and alcohol consumption activate the maternal HPA axis, it is possible that increased hypothalamic corticotropin-releasing hormone (CRH) expression might contribute to the reduced weight gain in PS and PAE females (Hill et al., 2012)
Intrauterine growth retardation was found in PAE but not in PS fetuses. Although low birth weight is often considered an index of prenatal stress in humans, relatively few studies have shown significant reductions of birth weight following PS in rat models. This is likely due to the fact that the placenta of humans is unique in its ability to synthesize CRH, which could mediate some of the effects of maternal stress on fetal growth and neuronal development (Weinstock, 2005). The finding of increased placenta weight in the PAE group is also consistent with previous studies (Weinberg, 1985; Wilcoxon et al., 2003), and has been considered a compensatory response to protect the alcohol-exposed fetus. However, the fetal/placenta weight ratio, which is an index of placenta efficiency, was lowest in the PAE group, suggesting that the efficiency of nutrient and oxygen transport may be decreased, possibly due to direct toxic effect of alcohol on the placenta and/or through secondary effects of altered glucocorticoid signaling (Fowden and Forhead, 2015).
Maternal endocrine function was also affected by alcohol. Progesterone levels are known to be high throughout gestation and gradually decrease before parturition (Grota and Eik-Nes, 1967), whereas estradiol levels are low throughout pregnancy and then increase sharply from approximately GD 18 to parturition (Shaikh, 1971). It has been suggested that changes in the ratio of circulating progesterone and estradiol at the end of pregnancy triggers the onset of parturition and the induction of maternal behavior (Moltz et al., 1970; Rosenblatt et al., 1988). It is possible that the lower estradiol, and hence the higher progesterone/estradiol ratio seen in PAE compared to dams in the other treatment groups may contribute to the delayed parturition that often occurs with prenatal alcohol exposure. Indeed, we (Lan et al., 2006; Weinberg 1985) and others (Bond 1982) have shown that alcohol intake delays parturition in animal models of PAE and in the past, alcohol drip has been used clinically to stop premature labor (Fuchs et al., 1967). Furthermore, in the rat, the first half of pregnancy is established and maintained by hormones released from the anterior pituitary and ovaries, but the placental hormones are the driving factors during the second half of pregnancy (Taya and Greenwald, 1981). Therefore, it is likely that the changes in maternal hormones in PAE dams in the present study reflect primarily an alteration in the placental endocrine function.
Interestingly, both alcohol consumption and stress during pregnancy increased maternal CORT levels. It has been shown that this elevated CORT is unbound and can cross the placenta to influence development of the fetal HPA axis, which is functional from around GD 15 (Aird et al., 1997). For example, maternal adrenalectomy was shown to abolish some of the effects of PAE or PS, such as body weight and HPA alterations (Redei et al., 1993; Tritt et al., 1993; Barbazanges et al., 1996). Furthermore, exogenous glucocorticoid exposure or inhibition of placental 11β-HSD2 mimics some of the effects of PS (Levitt et al., 1996; Welberg et al., 2000). Therefore glucocorticoids may be a candidate for mediating at least some of the effects of PAE and PS on HPA development (Cottrell and Seckl, 2009).
Under normal conditions, levels of 11β-HSD2 increase over the course of gestation to protect the fetus from increasing maternal CORT levels (Harris and Seckl, 2011). However, data from previous studies on the effects of both PAE and PS on placental 11β-HSD2 mRNA levels have been somewhat inconsistent. Both decreased (Mairesse et al., 2007; Rosenberg et al., 2010; Liang et al., 2011; Pena et al., 2012) and increased (Clifton et al., 2006; Cuffe et al., 2012; Gardebjer et al., 2014) 11β-HSD2 expression levels have been reported in PAE and PS placentae and one study reported decreased levels in female, but increased levels in male placentae (Wilcoxon et al., 2003). In the present study, we found that placental 11β-HSD2 protein levels were higher in PAE, and unchanged in PS compared to C dams. Differences in the animal models, timing and levels of alcohol exposure, as well as post-transcriptional events may account for these discrepant results. Our data suggest the possibility that the increased 11β-HSD2 levels in PAE placentae may be a compensatory adaption of placental function in response to increased maternal CORT in PAE dams, whereas the unchanged 11β-HSD2 levels in PS placentae may allow an increased transplacental passage of active maternal glucocorticoids. However, it should be noted that protein levels and catalytic activity of 11β-HSD2 do not always correlate (e.g. Katz et al., 2010). Furthermore, it was reported that placental 11β-HSD2 activity positively correlated with pup birth weight, and negatively correlated with placental weight at term in rats (Benediktsson et al., 1993), suggesting the possibility that 11β-HSD2 catalytic activity may actually be decreased in PAE placentae in the present study. Furthermore, activity of 11β-HSD2 is counterbalanced by the activity of 11β-HSD1, which increases at the end of gestation to facilitate the maturation of organ systems. The two enzymes work together to control the amount and timing of intrauterine glucocorticoid exposure and play a critical role in glucocorticoid signaling in the placenta (Thompson et al., 2002; Chapman et al., 2013). Although we did not find group or sex differences in 11β-HSD1, PAE fetuses showed a marginally positive correlation, whereas PS fetuses showed a significant negative correlation between maternal CORT and placental 11β-HSD1 protein levels. It has been reported that glucocorticoids themselves increase 11β-HSD1 expressions (Chapman et al., 2013). Together, our finding that 11β-HSD1 and 11β-HSD2 respond differentially to the elevated maternal CORT levels in PAE and PS fetuses further supports our hypothesis that these prenatal insults are mediated, at least partly, by different glucocorticoid signaling mechanisms.
Our finding that neither PAE nor PS altered placenta GR protein levels contrasts with previous findings in the literature, where both increased (Cuffe et al., 2012) and decreased (Shukla et al., 2011) GR levels have been reported. Of relevance to our PS model, increased GR mRNA levels were reported in the placentae of fetal male mice exposed to glucocorticoids during the second and third week of gestation (Cuffe et al., 2012), which would result in increased glucocorticoid signaling. While exposure to glucocorticoids could mimic effects of PS, timing and levels of exposure as well as species used differed between our study and that of Cuffe et al. By contrast, Shukla et al. (2011) used rat models very similar to ours, and found decreased protein and mRNA levels of GR in the placentae of male and female PAE fetuses (Shukla et al., 2011). Of note, however, Shukla et al. used Harlan derived rats, and we used Charles River-derived rats, which are known to differ in stress system regulation and inflammatory responses (Turnbull and Rivier, 1999; Pecoraro et al., 2006). Thus vendor-based differences in rats in these two studies may have contributed to the different findings.
Although we did not find group or sex differences in placenta MR levels, we observed a negative correlation between maternal CORT and placenta MR protein levels in PS male and female fetuses, and a marginally negative correlation between maternal alcohol consumption and placenta MR protein levels in PAE male fetuses. Importantly, in addition to inactivating maternal CORT, 11β-HSD2 also protects the specificity of nonselective MR from CORT excess so that circulating mineralocorticoids (ie., aldosterone) can bind to MR in aldosterone target organs. It has been suggested that the placenta has properties closely resembling aldosterone target tissue in terms of its function in electrolyte and water transport (Stulc, 1997). The present data are the first to identify MR protein levels in the rat placenta, although MR mRNA and protein have been found in human placentae (Condon et al., 1998; Hirasawa et al., 2000). Our data suggest the possibility that CORT negatively affects maternal-fetal electrolyte and water transport balance by downregulating placenta MR protein levels, and that alcohol may show a similar trend. For PAE dams, however, the upregulated 11β-HSD2 protein levels found may not only play a role in inactivating CORT, but also may enable more aldosterone to bind to placenta MR, which could partially attenuate at least some of alcohol's adverse effects.
Changes in MR and GR gene expression in the fetal brain can result in long-term changes in the expression of glucocorticoid-regulated genes that participate in the control of HPA activity. The balance between MR and GR determines the sensitivity and response of the brain to glucocorticoid signaling and stress in general (De Kloet et al., 1998). The fetal HPA axis is highly vulnerable to prenatal alcohol or stress, which can alter the MR/GR balance and the set point of HPA regulation (De Kloet et al., 1998, Ong et al, 2013). While we found that PS downregulated GR levels in the female mPFC, our micropunched samples contained a mix of ventral and dorsal mPFC regions, and thus we cannot predict the long-term net stimulatory/inhibitory influences of these mPFC alterations on the hypothalamus of PS animals. Future studies to localize these GR alterations in the mPFC are warranted. Moreover, although we did not measure fetal CORT levels in our study, previous studies have shown that basal CORT levels are lower or unchanged in PS compared to control fetuses on GD 21 (McCabe et al., 2001; Ward and Weisz, 1984; Mairesse et al., 2007). While GR is autologously regulated in adult tissues, such that GR is downregulated when glucocorticoid levels increase (Mueller and Bale, 2008; Brunton and Russell, 2010; Handa and Weiser, 2014), evidence suggests that this autologous regulation may not occur in fetal tissues, where HPA regulation is still developing (McCabe et al., 2001; Holloway et al., 2001; Gupta et al., 2003). Again, further studies on the effects of PAE and PS on HPA development are warranted.
MR plays a role in the tonic modulation of CORT secretion and in stress resilience (ter Heegde, et al, 2015). We found that for PS female fetuses, MR levels were upregulated in the amygdala and hippocampus, which is consistent with previous findings from guinea pig fetuses prenatally exposed to dexamethasone (McCabe et al., 2001). Fetal HPA function was previously shown to be attenuated by PS (Mairesse et al., 2007). The upregulation of MR in the limbic-HPA areas in the present study may be due to decreased feedback of plasma CORT in PS female fetuses at this stage of development, consistent with the attenuated HPA function reported in previous studies..
Interestingly, we also found that both PAE and PS attenuated the sexually dimorphic expression of MR, GR and the MR/GR ratio seen in C fetuses. The perinatal testosterone surge, which is fundamental for masculinization around GD 18-19, is suppressed in PAE and PS fetuses (McGivern et al., 1998; Ward et al., 2003). Fetal testosterone is known to act in a dose dependent manner to masculinize the fetal male brain (McEwen, 1981). The mPFC, hippocampus and amygdala are rich in estrogen and androgen receptors, and therefore, are highly sensitive to gonadal hormones (Shughrue et al., 1997; Williamson and Viau, 2007). It is possible that the organizational effects of androgen were attenuated in PAE, and altered in PS fetuses such that marginally opposite effects were observed. Interestingly, male offspring of rats prenatally exposed to alcohol or stress were reported to display atypical sexual behaviors, such as an increased potential for lordosis, which indicates a failure of masculinization in adulthood (Ward, 1972; Hard et al., 1984; Ward et al., 1994). It is possible that the altered MR and GR expression observed in PAE and PS fetuses sets the stage for further changes in HPA function and altered adrenal-gonadal interactions throughout development and into adulthood.
CONCLUSIONS
Results from the present study build on and expand our knowledge of the link between early life adversity and HPA axis dysregulation. Importantly, the data demonstrate that the effects of PAE and PS on HPA activity begin during gestation and continue to alter and reshape the axis into adulthood. Our data suggest that although PAE and PS induce a similar adult HPA hyperresponsive phenotype and increased vulnerability to mental health problems, glucocorticoid signaling in the placenta and stress-responsive neurocircuitry at GD 21 was differentially altered in PAE and PS, suggesting that programming of the HPA axis in PAE and PS animals likely occurs through different mechanisms. Our data provide an important basis for identifying biomarkers related to glucocorticoid signaling and have clinical implications for early prediction of adult physiological dysregulation and vulnerability to chronic diseases or disorders.
Highlights: Summary of changes in PAE and PS compared to Controls.
➢ Both PAE and PS dams had higher corticosterone levels.
➢ 11β-HSD2 protein levels were increased in PAE and unchanged in PS placentae.
➢ PS female fetuses had higher MR protein levels in hippocampus and amygdala compared to C female fetuses.
➢ PS female fetuses had lower GR protein levels in mPFC compared to C female fetuses.
➢ Both PAE and PS attenuated the sexually dimorphic expression of MR, GR and the MR/GR ratio seen in C fetuses.
ACKNOWLEDGEMENTS
We thank Leyla Innala, Alexandre Lussier, Charlis Raineki, Tamara Bodnar, Vivian Lam, Parker Holman, Wayne Yu, and Wendy Comeau for their expert assistance on the experiments.
Grant/Other Support: NIH/NIAAA R37 AA007789, RO1 AA022460, NeuroDevNet (Canadian Network of Centres of Excellence), and Canadian Foundation on Fetal Alcohol Research to J.W.; National Nature and Science Foundation of China Grant No. 31100793 and IMPART (CIHR) Fellowship to N.L.
Abbreviations
- 11β-HSD1
11β-hydroxysteroid dehydrogenase type 1
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- ANOVA
analysis of variance
- C
control
- CORT
corticosterone
- CRH
corticotropin-releasing hormone
- DOHaD
Developmental Origins of Health and Disease
- GD
gestation day
- GR
glucocorticoid receptor
- h
hour
- HPA
hypothalamic-pituitary-adrenal
- mPFC
medial prefrontal cortex
- MR
mineralocorticoid receptor
- PAE
prenatal alcohol exposure
- PF
pair-fed
- PS
prenatal stress
- RIA
radioimmunoassay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aird F, Halasz I, Redei E. Ontogeny of hypothalamic corticotropin-releasing factor and anterior pituitary pro-opiomelanocortin expression in male and female offspring of alcohol-exposed and adrenalectomized dams. Alcohol Clin Exp Res. 1997;21:1560–1566. [PubMed] [Google Scholar]
- Ashwell KWS, Paxinos G. Atlas of the Developing Rat Nervous System. Academic Press; 360 Park Avenue South, New York, NY 10010-1710, USA.: 2008. [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Bond NW. Prenatal exposure to ethanol: association between increased gestational length and offspring mortality. Neurobehav Toxicol Teratol. 1982;4:501–503. [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MRF. ANOVA for the Behavioural Sciences Researcher. Lawrence Erbaum Associates; London, UK.: 2006. [Google Scholar]
- Chapman K, Holmes M, Seckl J. 11beta-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol Rev. 2013;93:1139–1206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL, Rennie N, Murphy VE. Effect of inhaled glucocorticoid treatment on placental 11beta-hydroxysteroid dehydrogenase type 2 activity and neonatal birthweight in pregnancies complicated by asthma. Aust N Z J Obstet Gynaecol. 2006;46:136–140. doi: 10.1111/j.1479-828X.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Condon J, Gosden C, Gardener D, Nickson P, Hewison M, Howie AJ, Stewart PM. Expression of type 2 11beta-hydroxysteroid dehydrogenase and corticosteroid hormone receptors in early human fetal life. J Clin Endocrinol Metab. 1998;83:4490–4497. doi: 10.1210/jcem.83.12.5302. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffe JS, O'Sullivan L, Simmons DG, Anderson ST, Moritz KM. Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and mRNA expression. Endocrinology. 2012;153:5500–5511. doi: 10.1210/en.2012-1479. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Glucocorticoids as regulatory signals during intrauterine development. Exp Physiol. 2015 doi: 10.1113/EP085212. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Fuchs AR, Poblete VF, Jr, Risk A. Effect of alcohol on threatened premature labor. Am J Obstet Gynecol. 1967;99:627–637. doi: 10.1016/0002-9378(67)90411-5. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Gardebjer EM, Cuffe JS, Pantaleon M, Wlodek ME, Moritz KM. Periconceptional alcohol consumption causes fetal growth restriction and increases glycogen accumulation in the late gestation rat placenta. Placenta. 2014;35:50–57. doi: 10.1016/j.placenta.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Grota LJ, Eik-Nes KB. Plasma progesterone concentrations during pregnancy and lactation in the rat. J Reprod Fertil. 1967;13:83–91. doi: 10.1530/jrf.0.0130083. [DOI] [PubMed] [Google Scholar]
- Gupta S, Alfaidy N, Holloway AC, Whittle WL, Lye SJ, Gibb W, Challis JR. Effects of cortisol and oestradiol on hepatic 11beta-hydroxysteroid dehydrogenase type 1 and glucocorticoid receptor proteins in late-gestation sheep fetus. J Endocrinol. 2003;176:175–184. doi: 10.1677/joe.0.1760175. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard E, Dahlgren IL, Engel J, Larsson K, Liljequist S, Lindh AS, Musi B. Development of sexual behavior in prenatally ethanol-exposed rats. Drug Alcohol Depend. 1984;14:51–61. doi: 10.1016/0376-8716(84)90019-x. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa G, Takeyama J, Sasano H, Fukushima K, Suzuki T, Muramatu Y, Darnel AD, Kaneko C, Hiwatashi N, Toyota T, Nagura H, Krozowski ZS. 11Beta-hydroxysteroid dehydrogenase type II and mineralocorticoid receptor in human placenta. J Clin Endocrinol Metab. 2000;85:1306–1309. doi: 10.1210/jcem.85.3.6429. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Whittle WL, Challis JR. Effects of cortisol and estradiol on pituitary expression of proopiomelanocortin, prohormone convertase-1, prohormone convertase-2, and glucocorticoid receptor mRNA in fetal sheep. Endocrine. 2001;14:343–348. doi: 10.1385/ENDO:14:3:343. [DOI] [PubMed] [Google Scholar]
- Katz A, Oyama RK, Feng N, Chen X, Schlinger BA. 11beta-Hydroxysteroid Dehydrogenase Type 2 in Zebra Finch Brain and Peripheral Tissues. Gen Comp Endocrinol. 2010;166:600–605. doi: 10.1016/j.ygcen.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–889. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–684. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Liang G, Chen M, Pan XL, Zheng J, Wang H. Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp Toxicol Pathol. 2011;63:607–611. doi: 10.1016/j.etp.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology 32 Suppl. 2007;1:S10–5. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–33. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sexual differentiation of the brain. Nature. 1981;291:610. doi: 10.1038/291610a0. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Handa RJ, Raum WJ. Ethanol exposure during the last week of gestation in the rat: inhibition of the prenatal testosterone surge in males without long-term alterations in sex behavior. Neurotoxicol Teratol. 1998;20:483–490. doi: 10.1016/s0892-0362(98)00009-9. [DOI] [PubMed] [Google Scholar]
- Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav. 1970;5:1373–1377. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JL, Well AD. Research Design and Statistical Analysis. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Ong SX, Chng K, Meaney MJ, Buschdorf JP. Decreased hippocampal mineralocorticoid:glucocorticoid receptor ratio is associated with low birth weight in female cynomolgus macaque neonates. J Mol Endocrinol. 2013;51:59–67. doi: 10.1530/JME-12-0218. [DOI] [PubMed] [Google Scholar]
- Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martinez JA, Campion J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology. 2012;96:249–260. doi: 10.1159/000341684. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Ginsberg AB, Warne JP, Gomez F, la Fleur SE, Dallman MF. Diverse basal and stress-related phenotypes of Sprague Dawley rats from three vendors. Physiol Behav. 2006;89:598–610. doi: 10.1016/j.physbeh.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Pena C, Monk C, Champagne F. Epigenetic Effects of Prenatal Stress on 11 beta-Hydroxysteroid Dehydrogenase-2 in the Placenta and Fetal Brain. PLOS ONE. 2012;7 doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E, Halasz I, Li LF, Prystowsky MB, Aird F. Maternal adrenalectomy alters the immune and endocrine functions of fetal alcohol-exposed male offspring. Endocrinology. 1993;133:452–460. doi: 10.1210/endo.133.2.8344191. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rosenberg MJ, Wolff CR, El-Emawy A, Staples MC, Perrone-Bizzozero NI, Savage DD. Effects of moderate drinking during pregnancy on placental gene expression. Alcohol. 2010;44:673–690. doi: 10.1016/j.alcohol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic-hypothalamic-pituitary-adrenocortical axis response to stress in rhesus monkeys. Child Dev. 2004;75:96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental 'programming' and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod. 1971;5:297–307. doi: 10.1093/biolreprod/5.3.297. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Ullmann TM, Redei EE. Candidate placental biomarkers for intrauterine alcohol exposure. Alcohol Clin Exp Res. 2011;35:559–565. doi: 10.1111/j.1530-0277.2010.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulc J. Placental transfer of inorganic ions and water. Physiol Rev. 1997;77:805–836. doi: 10.1152/physrev.1997.77.3.805. [DOI] [PubMed] [Google Scholar]
- Taya K, Greenwald GS. Effect of hypophysectomy on day 12 of pregnancy on ovarian steroidogenesis in the rat. Biol Reprod. 1981;25:692–698. doi: 10.1095/biolreprod25.4.692. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Cooley-Matthews B, Poland RE. Effects of maternal ethanol consumption in rats on basal and rhythmic pituitary-adrenal function in neonatal offspring. Psychoneuroendocrinology. 1982;7:49–58. doi: 10.1016/0306-4530(82)90054-3. [DOI] [PubMed] [Google Scholar]
- ter Heegde F, De Rijk RH, Vinkers CH. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92–110. doi: 10.1016/j.psyneuen.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Thompson A, Han VK, Yang K. Spatial and temporal patterns of expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 messenger RNA and glucocorticoid receptor protein in the murine placenta and uterus during late pregnancy. Biol Reprod. 2002;67:1708–1718. doi: 10.1095/biolreprod.102.005488. [DOI] [PubMed] [Google Scholar]
- Tritt SH, Tio DL, Brammer GL, Taylor AN. Adrenalectomy but not adrenal demedullation during pregnancy prevents the growth-retarding effects of fetal alcohol exposure. Alcohol Clin Exp Res. 1993;17:1281–1289. doi: 10.1111/j.1530-0277.1993.tb05242.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Sprague-Dawley rats obtained from different vendors exhibit distinct adrenocorticotropin responses to inflammatory stimuli. Neuroendocrinology. 1999;70:186–195. doi: 10.1159/000054475. [DOI] [PubMed] [Google Scholar]
- Ward IL. Prenatal stress feminizes and demasculinizes the behavior of males. Science. 1972;175:82–84. doi: 10.1126/science.175.4017.82. [DOI] [PubMed] [Google Scholar]
- Ward IL, Ward OB, Affuso JD, Long WD, 3rd, French JA, Hendricks SE. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm Behav. 2003;43:531–539. doi: 10.1016/s0018-506x(03)00061-8. [DOI] [PubMed] [Google Scholar]
- Ward IL, Ward OB, Winn RJ, Bielawski D. Male and female sexual behavior potential of male rats prenatally exposed to the influence of alcohol, stress, or both factors. Behav Neurosci. 1994;108:1188–1195. doi: 10.1037//0735-7044.108.6.1188. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical development of off spring. Alcohol Clin Exp Res. 1989;13:73–83. doi: 10.1111/j.1530-0277.1989.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcohol Clin Exp Res. 1985;9:49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Bezio S. Alcohol-induced changes in pituitary-adrenal activity during pregnancy. Alcohol Clin Exp Res. 1987;11:274–280. doi: 10.1111/j.1530-0277.1987.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Changes induced by prenatal stress in behavior and brain morphology: can they be prevented or reversed? Adv Neurobiol. 2015;10:3–25. doi: 10.1007/978-1-4939-1372-5_1. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Schwartz J, Aird F, Redei EE. Sexually dimorphic effects of maternal alcohol intake and adrenalectomy on left ventricular hypertrophy in rat offspring. Am J Physiol Endocrinol Metab. 2003;285:E31–9. doi: 10.1152/ajpendo.00552.2002. [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007;503:717–740. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]