Abstract
New achievements in the realm of nanoscience and innovative techniques of nanomedicine have moved micro/nanoparticles (MNPs) to the point of becoming actually useful for practical applications in the near future. Various differences between the extracellular and intracellular environments of cancerous and normal cells and the particular characteristics of tumors such as physicochemical properties, neovasculature, elasticity, surface electrical charge, and pH have motivated the design and fabrication of inventive “smart” MNPs for stimulus-responsive controlled drug release. These novel MNPs can be tailored to be responsive to pH variations, redox potential, enzymatic activation, thermal gradients, magnetic fields, light, and ultrasound (US), or can even be responsive to dual or multi-combinations of different stimuli. This unparalleled capability has increased their importance as site-specific controlled drug delivery systems (DDSs) and has encouraged their rapid development in recent years. An in-depth understanding of the underlying mechanisms of these DDS approaches is expected to further contribute to this groundbreaking field of nanomedicine. Smart nanocarriers in the form of MNPs that can be triggered by internal or external stimulus are summarized and discussed in the present review, including pH-sensitive peptides and polymers, redox-responsive micelles and nanogels, thermo- or magnetic-responsive nanoparticles (NPs), mechanical- or electrical-responsive MNPs, light or ultrasound-sensitive particles, and multi-responsive MNPs including dual stimuli-sensitive nanosheets of graphene. This review highlights the recent advances of smart MNPs categorized according to their activation stimulus (physical, chemical, or biological) and looks forward to future pharmaceutical applications.
1 Introduction
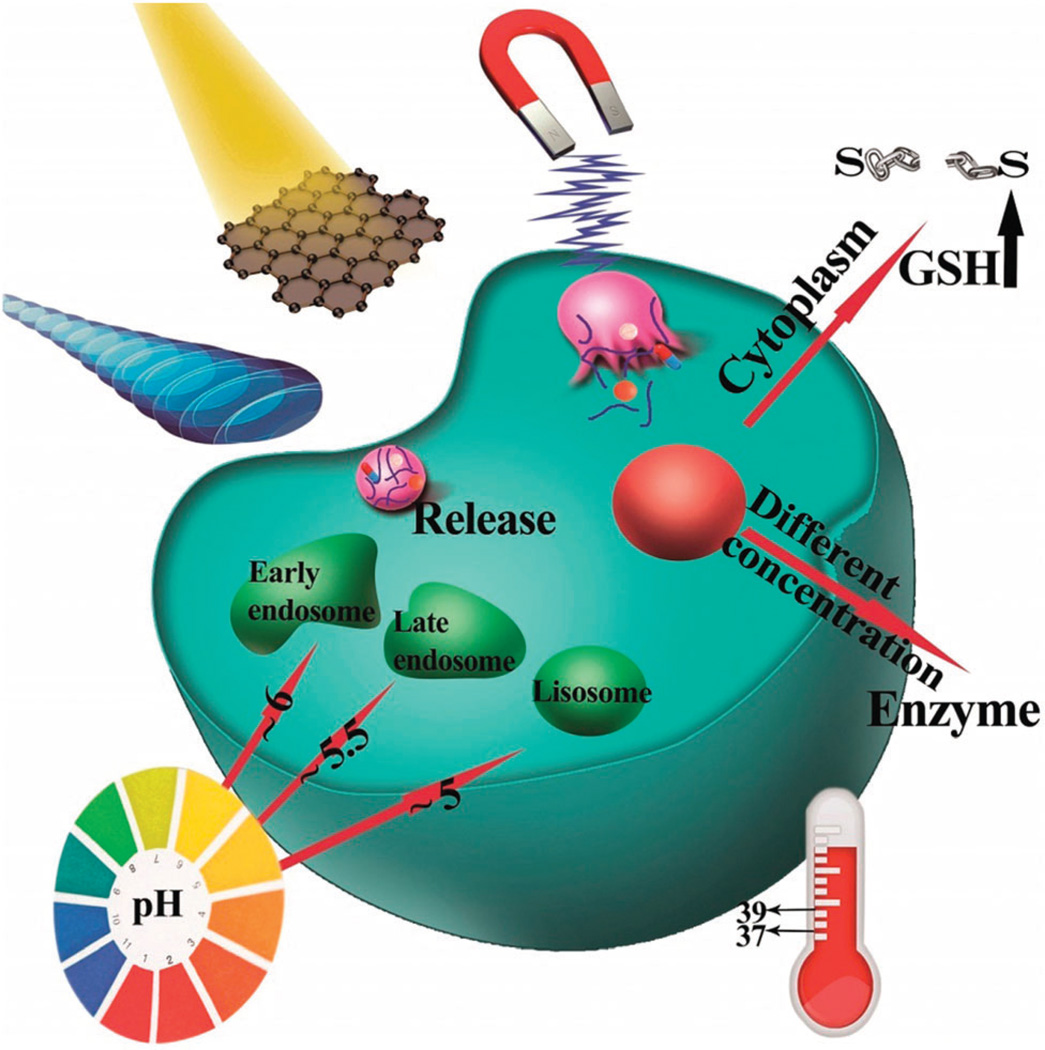
Breakthroughs in nanotechnology have had an important influence on many different industries, especially materials science, biotechnology, and pharmacotherapy. Different nanocarriers such as liposomes, polymers, micelles, and carbon-based nanomaterials are increasingly being used for medical purposes,1–6 such as the delivery of macromolecules (drugs and genes) as therapeutic agents. It is undoubtedly a challenging issue, since the required drug delivery system (DDS) is expected to be both site-specific and time-release controlled. Controlled release of drugs can be triggered by various external or internal stimuli of heat, solvent polarity, ionic strength, action of biomolecules, or the effects of electric/magnetic fields or light, as illustrated in Fig. 1. While temperature can be either an internal or external stimulus, pH, redox, and enzyme activity are known as internal stimuli, and on the other hand, light, magnetic fields, and US are recognized as external stimuli.7–9 Robust and efficient nano-carriers that can respond to changes in their ambient environmental parameters are of particular importance for drug and gene delivery purposes. The term “smart” has been applied to micro/nanoparticles (MNPs) that can react in a predictable and specific way to external/internal stimuli. This property of such carriers to undergo controlled-release of the loaded drugs results in overall mitigation of their side-effects and increases their treatment efficacy. Well-designed smart-MNPs might even be responsive to multiple combinations of different stimuli to further improve their specificity for targeted and controlled drug delivery.10 There exists a similarity between the main action algorithm of multi-responsive smart MNPs and the computer logic gates; an exciting issue to be discussed further.
Fig. 1.
Different physical and chemical stimuli exploited for triggering smart MNPs in controlled release of drugs, namely, magnetic induction, light irradiation, US agitation, temperature difference, enzyme activation, redox potential, or pH difference.
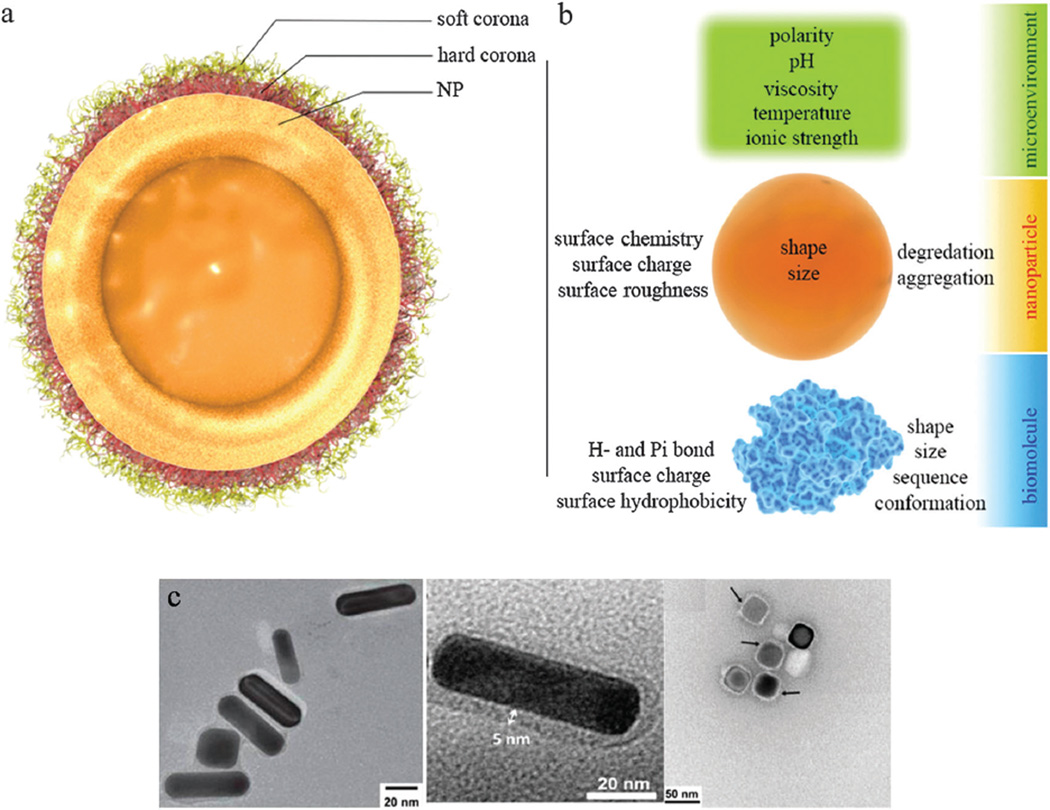
The emergence of novel nanomaterials from the flourishing field of nanoscience has given a boost to research in many areas of technology.11,12 Smart nanostructures have been intensively investigated during the last decade, and their unique and intriguing properties have given hope for the design of more efficient drug delivery vehicles. MNPs can play a crucial role as DDSs as their size (and hence their physical, chemical, and electrical properties) can be tuned and they can be easily functionalized and used in non-toxic concentrations for selective or passive drug delivery. Smart MNPs are considered to be promising agents for targeted delivery of drugs, particularly water-insoluble compounds, and can also be helpful in the field of dual-function cancer diagnosis and treatment systems (theranostics).13 They can escape the reticuloendothelial system and even have the potential to cross the blood–brain barrier (BBB)14 and the blood–testicle barrier (BTB). Their functionalization is a relatively easy process and appropriate functionalization can enable imaging and real-time monitoring of drug delivery to tumors. Although the protein corona-induced screening of MNPs has an important role in mitigation of their cytotoxicity, protein attachment can also reduce the efficiency of targeted MNPs due to covering-up or shielding of active targeting ligands.15 Different organic and inorganic MNPs have been utilized in DDSs. As a particular example, nanosheets of graphene (the first class of two-dimensional crystals16) discovered only a few years ago, have made a tremendous impact on all areas of applied science ranging from nanoelectronics,17 gas sensors,18 and biosensors19 to composites20–23 and somewhat surprisingly, to nanomedicine.24–28 In the field of stimuli-triggered DDSs, intense investigation is being focused on graphene,29 as it is relatively biocompatible30 and can be functionalized easily.31 Thanks to its theoretically-calculated outstanding specific surface area of 2630 m2 g−1,32,33 a single sheet of graphene oxide (GO) can be loaded with drugs to more than two times its own weight. The superior electrical34 and thermal35 conductivity of the graphene nanosheets, the high absorbance of near-infrared (NIR) radiation,36 and most importantly, its nontoxicity at low concentrations37,38 has motivated many investigators to prepare this nanomaterial (micrometer-sized sheets with a thickness of only one nanometer) in a stimuli-responsive form.39,40
The uptake of very small particles by cells is critically dependent on the particle size, which can range from only a few nanometers up to a few microns. For example, although there is no consensus on the optimum size of the particles used for vaccine delivery (according to a review elsewhere41), it is reasonably believed that NPs, particularly, those with diameters less than 100 nm, are better candidates for targeted-delivery purposes due to their good stability in blood circulation, their ability to pass thorough capillaries, their efficiency in permeating biological barriers,42 and even their ability to accumulate in cells without being recognized by efflux pumps such as P-glycoprotein.43 However, due to the fact that the volume of the particles diminishes by three orders of magnitude when the diameter is shortened by a factor of ten, care must be taken that the loading capacity or “cargo space” is large enough to carry the desired drug payload.44
The increasing prevalence of various types of cancer is becoming a serious global health concern as it is the second most common cause of death after cardiovascular diseases. Treatment of cancer is feasible in many cases and can even result in its complete eradication provided it is diagnosed early. However, even though there are innovative attempts in the realm of nanotechnology to achieve detection of cancer at the single-cell level,45 in most cases, due to the low detection limit of the available technologies,46,47 cancer is often not detected until many different parts of the body have been affected. The undesired side-effects of anti-cancer drugs may severely limit their use, since these cytotoxic compounds unfavorably affect the normal proliferating cells at the same time as they kills the cancerous cells, since both have active DNA replication and cell cycle progression. Likewise in radiotherapy, surrounding normal tissue can be damaged by the radiation beam, although improvements are being pursued through emerging approaches of hadron therapy and promising techniques of modern nanomedicine.
Pathological lesions within normal tissue, such as tumors and abnormal cancerous cells, can be discriminated from their normal counterparts due to the unique pathophysiology of tumors, and the peculiarities of malignant and transformed cells.48 Cancerous cells have been reported to differ from normal cells in their morphology, elasticity, and permeability. They often express different glycans compared with their normal counterparts.49 Intracellular glutathione (GSH) levels50 and the concentration of some enzymes51 within tumors can be more than two orders of magnitude higher in comparison with the extracellular levels. Interestingly, it has been reported that the extracellular pH in solid tumors tends to be significantly more acidic (~6.5) than the pH of blood (7.4).52 Controlled drug delivery is not only advantageous in suppressing the side-effects of the toxic drugs by passive or active targeting of the diseased tissue, but also by overcoming the drawbacks of insoluble anti-cancer drugs like the widely-studied doxorubicin (DOX).
The state-of-the-art developments of micro/nanostructures used in smart drug/gene delivery systems are summarized in the present review. A comprehensive perspective is presented for the first time classifying their responsiveness to different stimuli, either physical (temperature, magnetic force, electricity, light irradiation, mechanical agitation, or ultrasound), chemical (pH or redox), or biological factors (specific biomolecules or enzymes). We also discuss the corresponding nanotoxicity of these nanocarriers, their fate and pathology in biological systems, and the cell vision effect resulting from the protein corona formation. Furthermore, a brief background discussion of each category and its corresponding mechanism of action are covered in this compendium and the latest progress and current challenges are highlighted.
2 Different stimuli-responsive MNPs
2.1 Physical stimuli-responsive MNPs
2.1.1 Thermo-responsive MNPs
Temperature is among the most often investigated stimuli to control the release of drugs from stimuli-responsive DDSs with spatiotemporal control. The hyperthermic nature of most inflamed pathological sites and tumours can act as an internal stimulus. Applying external temperature changes can also activate thermo-responsive MNPs to provide an attractive option for stimulus-responsive DDSs as they can rapidly response to temperature changes. Another important advantage of these DDSs is that they can be formulated as injectable fluids so that no surgical operation is needed for implantation in the diseased tissue.53 The ideal smart DDSs should retain their load at inappropriate times and place and release the drug inside with controlled kinetics in the target tissues such as tumors.
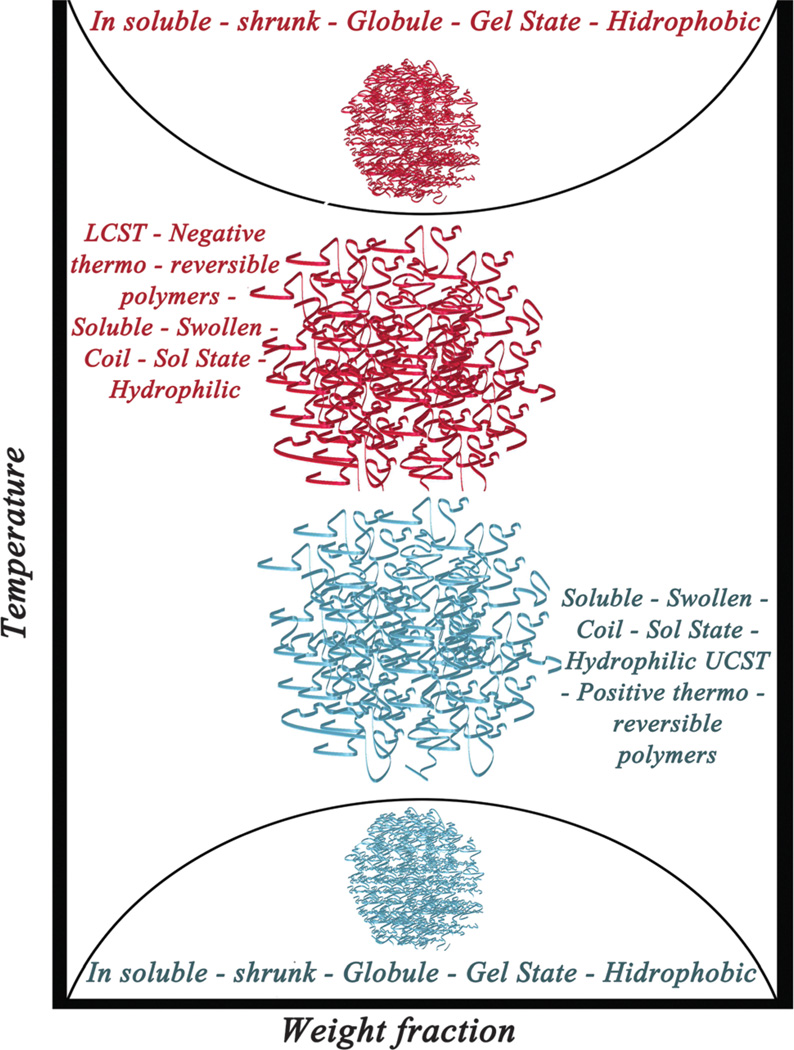
One of the most well-known materials which have been applied in DDSs are temperature-sensitive polymers that can switch their structure from a shrunken form to a swollen form (or vice versa) in response to a change in temperature. In fact, since there is a change in solubility with temperature, the significant properties of these polymers lead to release of the encapsulated drug. They are characterized by an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST). By changing the temperature around the UCST or LCST, a phase transition leading to swelling or shrinking occurs. Fig. 2 illustrates this concept. Drugs can be easily loaded into LCST polymers at room temperature, and then delivered to the target tissue where they are released.54–57
Fig. 2.
Schematic representation of LCST and UCST concepts and their properties.
PNIPAAm and its derivatives have attracted the most attention as thermo-negative hydrogel-based DDSs, since their corresponding LCST is about 32 °C, which is close to the physiological temperature of the human body.58–60 Also, by coupling other polymers, peptides, liposomes and proteins to PNIPAAm, its properties can be optimized in order to get improved targeting and drug release.8,61 For example, in a semi-interpenetrating polymer network with increased LCST and improved swelling ratio synthesized by Fu et al.62 through a free radical polymerization method, the LCST of the modified PNIPAAm increased from 32 °C to 41 °C when AAm content exceeded 5.5% and the hydrophilic nature of AAm could form bonds with PNIPAAm.
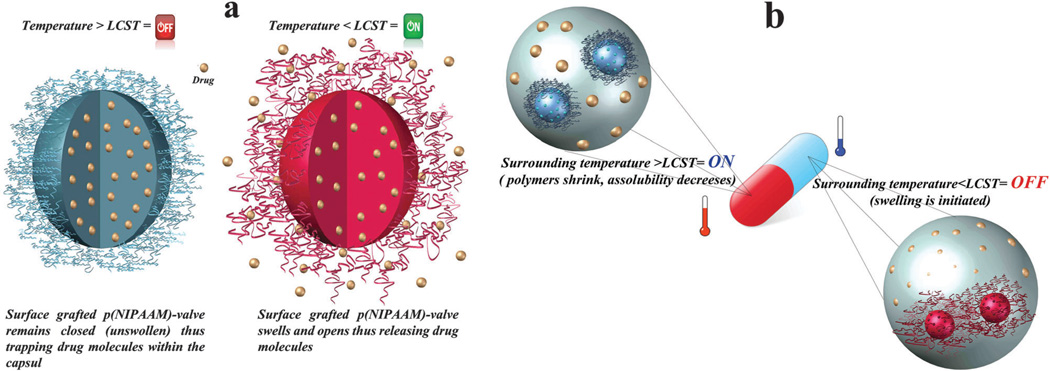
Nanovalves have been used by researchers in order to control drug release and the opening of gates that control the mesopores of mesoporous silica nanoparticles (MSNs) and can be made to be sensitive to light, pH or temperature.63 Thermo-responsive polymers can be used as an “on-off switch”. For example, Okahata et al.64 first used a nylon capsule (membrane) and attached N-isopropylacrylamide (NIPAAm) to the surface of the membrane. They showed that, at temperatures above 35 °C when shrinkage and collapse of NIPAAm occurred, the nanovalve closed and drug release was reduced, whereas at a temperature below the LCST (35 °C), the valve opened and increased the drug release due to swelling of the polymer (Fig. 3a). Lue et al.65,66 used a different technique involving the use of a NIPAAm and acrylic acid (AAc) copolymer as “brush hydrogels” that were grafted on the surface of a porous polycarbonate support, and showed that by increasing the temperature above the LCST, the NIPAAm-co-AAc brush underwent shrinkage resulting in increased release of the drug through the pores, while by decreasing the temperature below the LCST, the drug delivery route was blocked due to swelling of the brushes (Fig. 3b).
Fig. 3.
Schematic illustration of on-off switch/membrane. (a) Drug release in temperature below LCST. (b) Drug release in temperature above LCST.
The insolubility of hydrophobic drugs and anticancer agents in water (e.g., the anticancer drug Deguelin), limits their application in drug delivery.67 To overcome this problem, core–shell thermo-responsive DDSs can be used, which often consist of a hydrophobic core such as polystyrene (PS) acting as a reservoir for the loaded drug, together with a temperature-sensitive shell of a hydrogel such as PNIPAAm,68–70 Yan-Ling Luo et al.71 prepared a four-armed star multiblock copolymer with the hydrophobic HTPB acting as the central block with the thermo-sensitive PNIPAAm arranged as arms. The average hydrodynamic diameter of the micelles was under 150 nm. The micelles showed a temperature-dependent size change, with a LCST of about 33–35 °C. Water insoluble CPT was loaded into the micelles as an anticancer drug which showed strong antitumor properties.71,72
Azobenzene can be attached to temperature-sensitive polymers because of its transition from trans to cis (or cis to trans) by ultraviolet (UV) or visible light irradiation. In this manner, the LCST of temperature-sensitive polymers such as PNIPAAm can be controlled by the amount of azobenzene. For example, reversible alteration of LCST could be achieved through E-to-Z photoisomerization in azobenzene-conjugated PNIPAAm.73 A linear increase in the LCST point (up to 10 °C) upon irradiation was also claimed and attributed to a decrease in the molecular weight of the employed PNIPAAm. In another study, chemotherapy and hyperthermia were concurrently applied by Kim et al.74 activating a core–shell system to improve the effectiveness of gemcitabine for pancreatic cancer. They used super paramagnetic iron oxide NPs with 7 nm diameter which formed a cluster with a core diameter of 60 nm encapsulated in a porous silica shell. The surface of the shell was modified by conjugation to polyvinylpyrrolidone in order to allow grafting of hydroxypropyl cellulose as a thermo-sensitive polymer. By this design gemcitabine release (chemotherapy), magnetic heating and magnetic resonance imaging (MRI) could be accomplished concurrently. They suggested that concurrent chemohyperthermia could improve tumor cell death compared to chemotherapy or hyperthermia alone.
Although PNIPAAm is a well-known candidate for temperature-sensitive DDSs, it is not degradable in the body, so polymers such as polyethylene glycol (PEG) which have better biocompatibility can be an alternative to PNIPAAm.75 Na et al.76 synthesized biodegradable temperature-responsive poly(l-lactic acid)/poly(ethylene glycol) NPs for DOX controlled release. They showed by increasing the temperature, the release of DOX increased and the temperature sensitivity depended on the poly(l-lactide) (PLLA) polymer chain length. Polysaccharides such as alginate, hyaluronic acid (HA), dextran, chitosan, and carrageenan which have no significant toxicity, good biodegradability, biocompatibility, and are natural materials with antibacterial and mucoadhesive properties which can be used in drug delivery, cell encapsulation and injectable matrices for tissue engineering.77–80 For example, Daniel-da-Silva et al.78 synthesized K-carrageenan polysaccharide thermo-sensitive nanogels. Methylene blue was used as a model drug loaded at temperatures between 25–45 °C in phosphate buffered saline (PBS), and it was shown that both encapsulation efficiency and loading capacity doubled when the K-carrageenan increased from 1% to 4%. This behavior was attributed to the interaction between the sulfate group from K-carrageenan and cationic methylene blue. They also reported an increase in methylene blue release when the temperature increased from 25 °C to 37 and 45 °C, as the nanogel swelled. Similarly, poly(methyl vinyl ether), poly(N-vinyl caprolactam, poly(N-ethyloxazoline), poly-(N-vinylalkylamides), PEG, poly(ethylene oxide)–poly(propylene oxide), PAAc and PAAm or their copolymers have been studied and tested in this regard.81–88
Temperature-sensitive liposomes (TSL) can be nanocarriers for DDSs and exhibit a phase transition after a change in temperature. Liposomes formed from lipid bilayers, in addition to reducing drug side-effects and limiting toxicity, can decrease the drug uptake in non-diseased tissues. “ThermoDox” is a liposome-based drug produced by Celsion American Corporation, which received FDA approval to conduct a phase III clinical trial for hepatocellular carcinoma and a phase II clinical trial for breast cancer. ThermoDox gives an increase between 25 and 5 times in drug release in comparison with IV DOX and standard liposomal DOX, respectively, in tumors. In order to provide the external temperature stimulus, heat is often induced by radiofrequency thermal ablation (RFA), microwave hyperthermia and high intensity focused ultrasound (HIFU) for activating TSL. Chen et al.89 encapsulated DOX and incorporated ammonium bicarbonate into the TSL membrane. When the temperature was increased to 40 °C, the ammonium bicarbonate decomposed releasing carbon dioxide that disrupted the liposome bilayers. Then DOX was then delivered to the diseased tissue through the pores generated in the liposomes. TSL nanocarriers have the potential to be tuned and modified by attaching polymers or molecular ligands for specific targeting to receptors. In a study, Ta et al.90 fabricated a TSL using PNIPAAm-co-PAAc in order to release DOX in solid tumors. Here, PNIPAAm acted as a temperature-sensitive component inducing enhanced drug release above 40 °C. Temperature-sensitive nanovalves can be used in liposomes. For example, Al-Ahmady and coworkers91 inserted a leucine peptide zipper into the liposome membrane and when the temperature increased to 43 °C, the zipper opened and DOX was released. However, DOX can cause toxic effects toward healthy tissue in case of its sudden release at 42 °C. To overcome this drawback and in order to render the liposome thermo-sensitive and decrease toxicity, Elk et al.92 conjugated N-(2-hydroxypropyl)methacrylamide mono/dilactate to the liposome surface with different molecular weights and used HIFU as a thermo-generator. They could control DOX release over 10 min at 42 °C using this hyperthermia technique. They also concluded that the temperature required for efficient DOX release had a reverse relation with the polymer molecular weight.
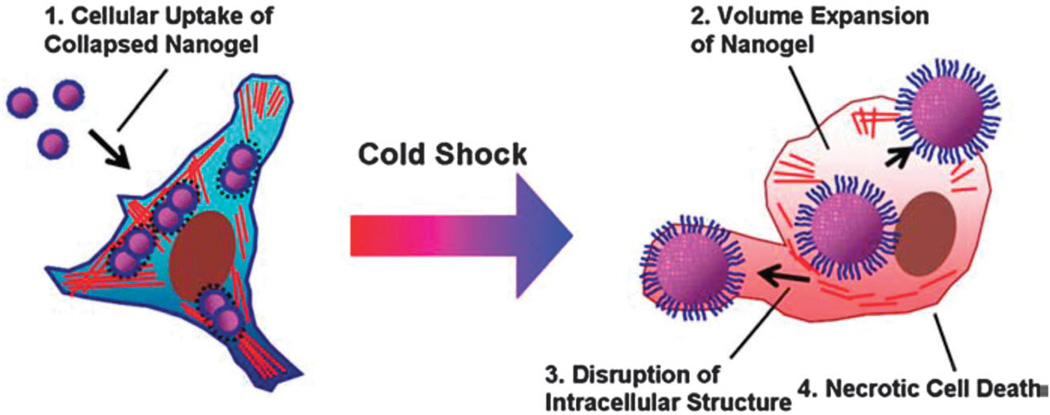
“Smart nanobombs” are another class of temperature-sensitive DDSs showing a reversible phase transition, and which swell from the nanoscale to the microscale when the temperature decreases. This reduction in temperature is similar to “cold shock” or “cryotherapy”. For example, Lee et al.93 fabricated a super-expandable Pluronic/PEI nanogel with 150 nm diameter at 37 °C, which was taken up by diseased cells by an endocytic mechanism. After cell uptake, the temperature was decreased to 20 °C and the nanogel underwent a phase transition with dramatic swelling and its size increased by 800-fold to 1.4 µm. This size increase generated a large internal hydrostatic pressure within the cells and the physical force induced the breakdown of the intracellular endosomal compartments and “blew up” the tumor cells generating necrotic cell death (Fig. 4). Furthermore drugs such as DOX could be loaded in the nanogel and released inside tumor cells.
Fig. 4.
Schematic illustration of intracellular explosion of volume-transition nanogels (Nanobomb) (reproduced from ref. 93. Copyright 2009 with permission from Elsevier).
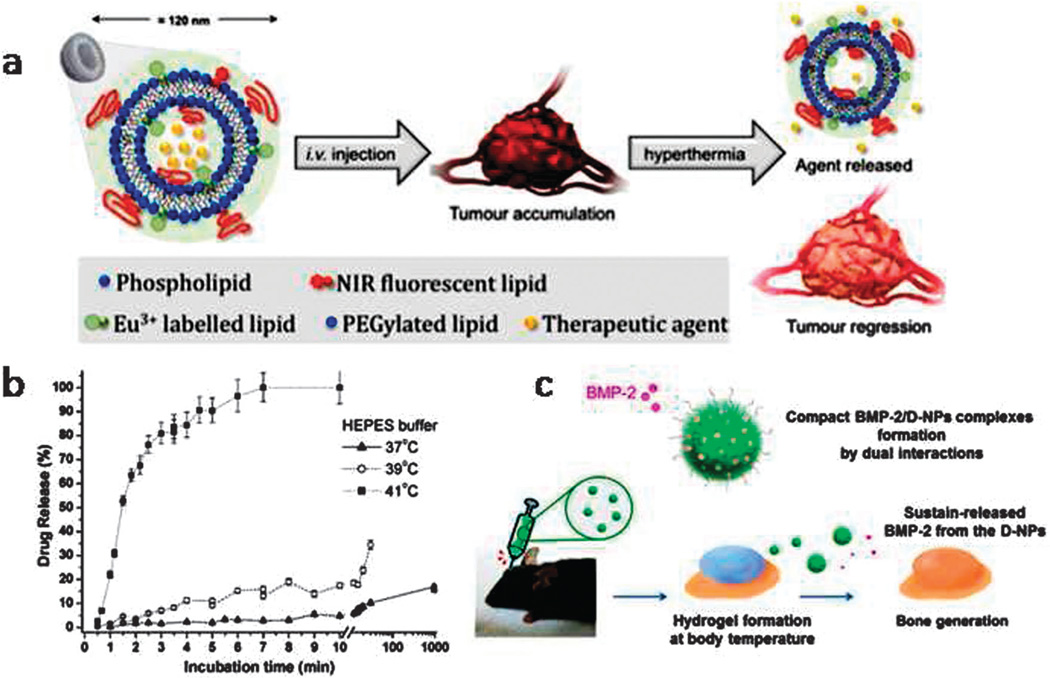
Smart stimuli-responsive nanocarriers with the highest sensitivity to minimal changes of body temperature (such as tumors) are required in the near future. In recent studies thermo-sensitive DDSs have been used for: co-delivery of drugs and genes,94 induced therapeutic angiogenesis in peripheral vascular disease,95 allowed ophthalmic drug delivery with enhanced corneal permeation and retention capacity,96 produced a new intelligent dermatological formulation,97 allowed mucoadhesive-thermogel-based vaginal delivery,98 protein delivery in tissue engineering99 and for bone regeneration.100 Other applications have been reported in cutaneous protein delivery,101 delivery to the uterus for efficacious therapy of uterine diseases (e.g. bovine reproductive disorders),102 therapy of inflammatory bowel disease with enhanced colonic retention of drugs,103 real-time image-guided theranostic drug delivery in cancer chemotherapy,104 efficient mild hyperthermia with increased tumor retention and high specificity toward cancer and angiogenic cells,105 and treatment of hypoxic hepatocellular carcinoma.106 Fig. 5 illustrates studies focused on bone regeneration and image-guided theranostic drug delivery by Seo et al.100 and Rosca et al.,104 respectively.
Fig. 5.
(a) Schematic of image-guided thermo-sensitive liposome (ITSL) injection and their accumulation in the tumor followed by agent release by hyperthermia and consequent tumor regression, (b) topotecan release from ITSL at different incubation temperatures, (c) schematic of dualacting hydrophobic and anionic charged nanocomplex hydrogel carriers for localized release of BMP-2 leading to bone generation (a and b reprinted with permission from ref. 100. Copyright 2015 “American Chemical Society”, and c reproduced from ref. 104. Copyright 2015 “Elsevier”).
Thermo-responsive polymers have also been used for delivery of genes and nucleic acids.107–109 Similar to the case of drug delivery, PNIPAAm has often been used as the temperature-sensitive component in temperature-responsive polymers for nucleic acid delivery.55,110 At a temperature above the LCST, the complexes between the polymer and the DNA become more densely bound, because the PNIPAAm shrinks due to rapid dehydration. As a result, the complexes can better protect the DNA from enzymatic degradation in environments such as the endosomes. By decreasing the temperature below the LCST, hydration occurs and increases the water solubility of PNIPAAm, that in turn makes the complexes less compact and finally results in facilitation of DNA release from polymer/DNA complexes.111,112
2.1.2 Magnetic-responsive MNPs
The force of magnetism is considered as one of the best options for an external stimulus as it scarcely has any physical interaction with the body, in comparison to other stimuli such as light irradiation, US or electrical fields. Also, magnetic-responsive materials can provide a real-time response to a brief triggering impulse.113 Engineered magnetic NPs (MagNPs) can be functionalized and magnetically driven and controlled, and hence magnetic field-responsive particles are being studied as useful vehicles in drug delivery114 and biomedical targeting.115 These engineered MNPs can also be triggered by external stimuli such as alternating magnetic field (AMF) for targeted and controlled drug-release. In 1960, Freeman et al.116 first conceived the idea of using magnetic fields as an external trigger to release drugs. Single domain MNPs with intrinsic superparamagnetic features above their blocking temperature, TB, are promising candidates for biomedical purposes, as compared to ferromagnetic particles, because they display no dipolar attraction in the presence of magnetic fields, have good colloidal stability, and homogenous dispersion within a polymeric matrix.117
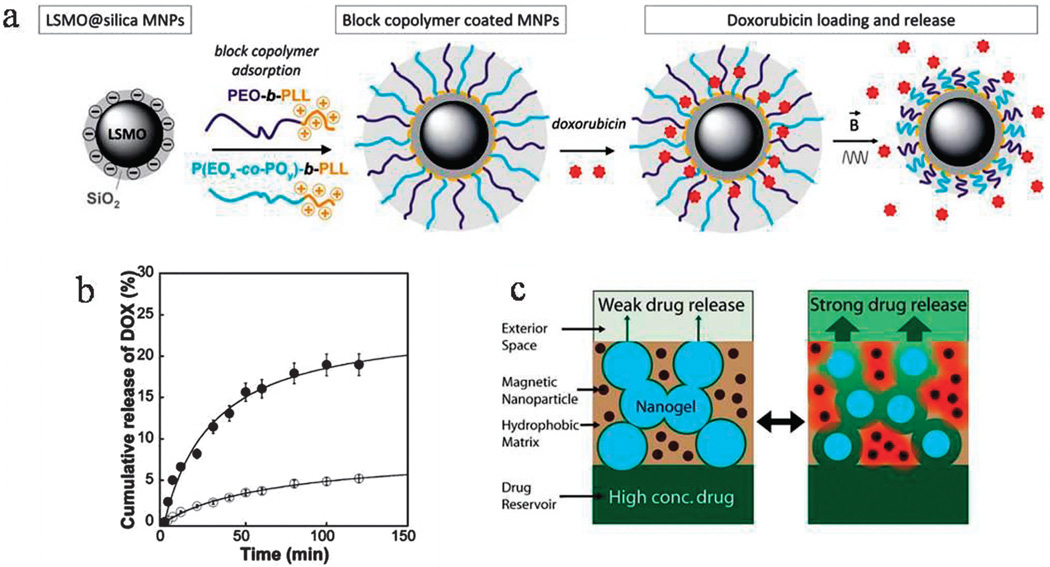
The unique ability of MNPs to generate heat under the influence of external high-frequency AMF is called magnetic hyperthermia. MNPs (with superparamagnetic behavior) exposed to a strong magnetic field can withstand internal stresses caused by the fluctuating distortions induced by magnetic forces (via alternating current (AC) or direct current (DC)). It is considered that the heat generated by MNPs in an AMF is due to two mechanisms of intrinsic rotational motion (Brownian) and extrinsic motion (Neel); i.e. thermal rotation of the particle’s magnetic moment and relaxation via diffusion. This inductive hyperthermia depends on several factors including the saturation magnetization and the hysteresis coercivity, both of which are related to the size of the MNPs. Magnetic fields can be used for applications including controlled/local drug release, guidance, targeting, imaging (MRI and fluorescence) as well as hyperthermal therapy.113 For example, polymeric NPs can undergo thermodynamic phase/conformational transitions depending on their LCST/UCST followed by swelling/shrinkage as described above. This phenomenon can be induced by magnetic heating with potential for drug-release e.g. via structural disruption of the NP or by a “pumping effect” (i.e. squeezing the drugs out from the NPs).113,118,119 It is worth noting that MagNPs can be trapped inside polymer particles e.g. hydrogels with a second polymer chain either as a random copolymer or semi/fully-interpenetrated network, by adsorption of the chains to the surface of MNPs.117 An external magnetic field can also enhance cellular uptake of NPs.119 Louguet et al.118 developed silica-coated MagNPs functionalized with thermo-sensitive block copolymer brushes with an LCST transition. Application of an AC-magnetic field induced heat generation by the magnetic core and enhancement of the triggered release of the drug (DOX) due to conformational changes occurring in the brushes (Fig. 6a and b). Nanoporous membranes prepared as electrically conductive flexible electrodes can act as a magnetically-triggered smart-nanocarrier with tunable drug-release profiles. These can include reversible, burst, stepwise, sustained and slow-release profiles depending on the mode of magnetic triggering. They show zero drug release in the absence of a magnetic field (see Fig. 6c).120 A hybrid nanocarrier composed of iron oxide nanocubes (cubic-IONPs) (with semi-superparamagnetic/semi-ferromagnetic behavior) encapsulated by a thermo-sensitive polymeric shell with a LCST phase transition at 37 °C showed minimal drug release below 37 °C but a consistent on-demand release due to heat generation under an AMF.121
Fig. 6.
(a) Design of a hybrid silica-coated copolymer functionalized magnetic NP followed by the release of DOX via application of an AMF inducing the LCST transition, (b) cumulative release profile of DOX from the nanocarrier, (c) weak and strong drug release (an ON/OFF reversible switch) from a nanoporous membrane comprised of thermo-sensitive nanogels and superparamagnetic MNPs, before and after AMF trigger (a and b reproduced from ref. 118 with permission from the Royal Society of Chemistry; c reprinted with permission from ref. 120. Copyright 2011 American Chemical Society).
Magnetic hyperthermia can also be controlled by adjusting the field strength. Incorporation of micro- and nano-scale metal NPs into the hydrogel matrix has led to the introduction of magnetic hybrid hydrogels as a group of novel materials with unique properties that are of great importance in controlled drug delivery.122 Super paramagnetic iron oxide (SPIO) NPs, functionalized with FA and β-cyclodextrin (β-CD), were reported to generate heat in the presence of an AMF.123 These MNPs showed a specific absorption rate of 132 W g−1 at 230 KHz and 100 Oe where drug release from the CD has been triggered by induced heating. Giani et al.124 cross-linked CoFe2O4 NPs into a carboxymethylcellulose polymer to fabricate hybrid hydrogels. The MagNPs were functionalized with (3-aminopropyl)-trimethoxysilane (APTMS) in order to introduce NH2 groups onto their surface. The preliminary results showed controlled drug-release from the matrix under the influence of AMF. Hyperthermia has been considered to be a promising way to treat cancer for some years, based on the assumption that cancer cells are more affected by heat than the surrounding normal cells.125 Many studies have utilized hyperthermia for clinical use, the results of which have shown tumor regression after application of a magnetic field to solid tumors that had been directly-injected with MNPs.126 In a study by Brulé et al.127 MNPs and DOX were incorporated inside alginate microbeads for dual hyperthermia and drug release. Human breast cancer MCF-7 cells were exposed to 37 °C for 2 hours with or without an AMF (frequency 700 kHz, amplitude 10 mTesla, 45 °C). DOX release was higher at 43 and 50 °C in comparison to temperatures of 20 and 37 °C. No DOX was detected at 4 °C, indicating that drug release was controlled by increased temperature.
Polymersomes have been used for magnetic field-triggered drug delivery.128 In a recent study, it was shown that polymersomes could entrap up to 6% (w/w) of DOX along with 30% (w/w) of ultra-SPION; γ-Fe2O3. When internalized by HeLa cells and subsequently triggered by a high frequency AC magnetic field (14 mT at 750 kHz), an 18% greater cytotoxicity was observed along with increased DOX release. Another important advantage of polymersomes over other delivery systems is the capacity to carry a larger drug load, for instance polymersomes could accommodate up to 12% DOX LC coupled with 50% LC USPIONPs, compared with only 2.4% DOX LC by other systems.129,130 The efficacy of a lipid–polymer hybrid NP system with improved drug release kinetics containing Fe3O4 magnetic beads providing site-specific release of CPT to treat breast cancer of MT2 mouse was evaluated by an external radio frequency (RF) magnetic fields.131
The guidance and triggering abilities of magnetic-responsive materials are superior to those of temperature and pH triggers113 and they are effective over a distance as large as a few centimetres.117 Extracorporeal magnetic guidance can serve to overcome several limitations of DDSs such as natural physiological barriers and lack of specificity to the targeted cells/tissue because spatial/magnetic fields can be spatially focused. For example to overcome high blood flow in arteries or capillaries after an intravenous injection of nanocarriers, powerful magnets can be placed at the desired sites.117 Image-guided drug delivery is an important component of the magnetic responsive DDS approach. Singh et al.132 fabricated magnetic carbon nanotubes (CNTs) ensheathed with surface-layered MSNs (the MNPs were located inside the multiwalled CNTs) for drug delivery accompanied by MR imaging. MSNs were chosen for their enhanced loading of bioactive molecules such as the protein cytochrome C, the drug gentamicin, and the nucleic acid siRNA within the mesopores. Along with high loading-capacity, the cellular uptake was also increased via application of magnetic fields, and negligible cytotoxicity was observed.
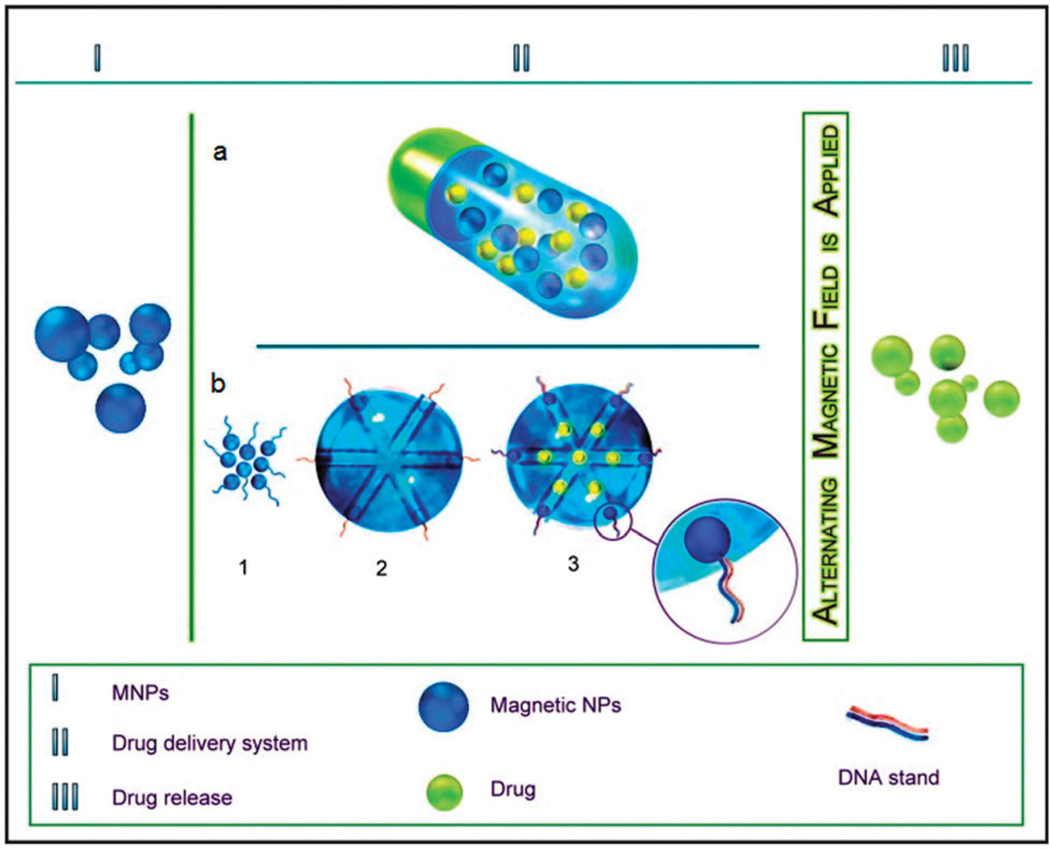
Magnetofection or magnetically-aided transfection is another way for controlled gene delivery. By linking MNPs with viral vectors, Mah et al.133 first reported magnetofection in 2009. A novel magnetite-silica nanocomposite (Fe3O4-SBA-15) was surface-coated with short chain PEI–DNA complexes for the strategic purpose of gene delivery and transfection. Surprisingly, this composite led to 15% higher transfection after applying an external magnetic field.134 Furthermore, MSNs can be utilized to entrap nucleotides in their pores while also being functionalized to form a magnetic field-responsive delivery system as was reported by Lu et al.135 Ruiz-Hernandez et al.136 devised stimulus-responsive MNPs in which ‘caps’ were formed from iron oxide NPs (IONPs) entrapping drug molecules/genes within the porous matrix. The caps were conjugated with one strand of DNA and the complementary strand was conjugated to the mesoporous silica membrane. Hence, the structures could maintain their closed conformation due to the double-helix DNA binding, so under normal conditions no drugs/genes were released. When exposed to an AMF, their temperature was locally raised, the DNA was dehybridized and the entrapped molecules were released from the matrix. This stimuli-responsive delivery mechanism works like an ‘on-off’ switch and aids in controlled drug release (Fig. 7).
Fig. 7.
(a) Alginate-microbead system loaded with MNPs (nanoheaters) and DOX for drug delivery using thermotherapy, (b) magnetically triggered release using reversible magnetic nanogates based on DNA hybridization/dehybridization.
Polymer nanoassemblies made up of biocompatible block copolymers can also be made to be responsive to magnetic fields.137–139 In an innovative approach, where drug molecules (DOX) and IONPs were loaded into block polymer self-assembled nanoassemblies (SNAs) or cross-linked analogues (CNAs) with 100 nm diameter, DOX release was detected at 40–42 °C after the temperature was raised by inductive heating using AMF. It was also observed that CNAs released DOX at a faster rate than SNAs.140 While many research groups have used direct intra-tumoral injection claiming it has low systemic toxicity and permits a higher tumor load of MNPs, the inability of this route to allow MNPs to reach small metastatic cancers, the poor distribution within inhomogeneous tumors, and its relatively invasive approach, make the intravenous approach much more preferable.141 In a study by Huang et al.142 using an intravenous injection, the tumor to non-tumor ratio was >16 where they employed a concentration of 1.9 mg Fe g−1 in a mouse model of subcutaneous squamous cell carcinoma. Their approach was validated as the surrounding normal tissues were completely left intact after a two-minute exposure to AMF (38 kA m−1) at 980 kHz during which the target tumor tissue was heated to 60 °C. In a study by Silva et al.,143 antibiotics were loaded into a nanostructure composed of Fe3O4 NPs with a TiO2-co-pectin shell then were released slowly by application of a remote magnetic field.
2.1.3 Electrical-responsive MNPs
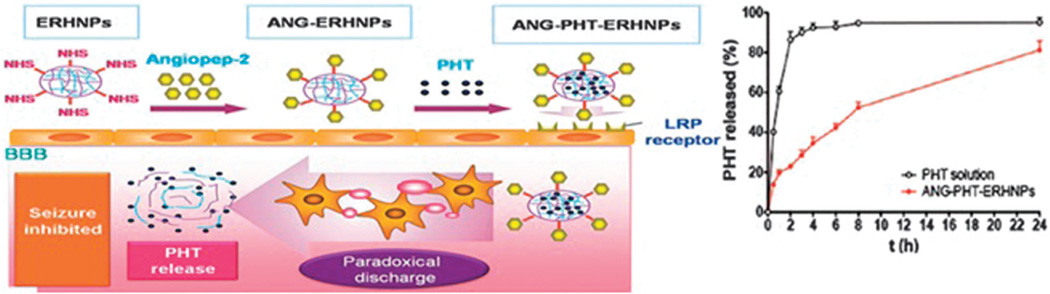
Electrically-triggered DDSs can be utilized for sustained, pulsed, or on-demand drug release via the application of external electric fields. Electro-responsive drug release can be performed with various platforms including electro-responsive nanostructures, electro-responsive compound-loaded nanostructures, and the combination of electroresponsive materials with other stimuli-responsive vehicles such as temperature or magnetic. The introduction of polyelectrolytes containing large numbers of ionizable groups confers responsiveness to an electrical stimulus through shrinking or swelling of the polymers.144,145 For instance, Ying et al. developed electro-responsive hydrogel NPs for targeted delivery of an antiepileptic drug (Phenytoin sodium, Fig. 8). An increased degree of ionization in the structure was achieved under the influence of an electric field due to the presence of the polyelectrolyte poly(sodium 4-vinylbenzene sulfonate) (PSS). The swelling ratio and the particle size could be tuned via the external electric field. In this study in vitro triggering and increased drug release with potential application in epilepsy treatment was shown.146
Fig. 8.
Schematic representation of electro-responsive hydrogel NPs containing PSS, with in vitro favorable sustained release (inset); reproduced from ref. 146. Copyright 2014 with permission from John Wiley & Sons, Inc.
In another example a GO-conducting polymer [poly(pyrrole) (PPy)] nanocomposite film with favorable electrical properties, linear drug release profile, and a good level of temporal control of dexamethasone release in response to electrical magnitudes was developed.147 Electroresponsive scaffolds have also been developed for on-demand and pulsatile drug delivery.148,149 These were composed of a hybrid between graphene and poly-(methylacrylic acid) (PMAA)–(MWNT) hydrogels with electroresponsive properties. The drawbacks of traditional electroresponsive DDSs such as an increase in temperature due to resistive heating after exposure to an electric field, and poor reproducibility of the drug release between the ON/OFF stimuli, were overcome by using the heat-dissipating and good electrical properties of graphene. Other applications of electrically-triggered smart nanostructures include the externally controlled on-demand release of an anti-HIV drug by CoFe2O4@BaTiO3 magneto-electric NPs,150 a polymer–MWCNT network in an electro-sensitive transdermal DDS,151 and PPy-loaded PLGA–PEG–PLGA NPs152 with on-demand drug delivery. These all possess excellent spatio-temporal control of dosage, and have potential clinical applications in multiple diseases. Apart from the above-mentioned advantages of electro-responsive DDSs, there are some limitations such as cell damage and poor tissue penetration, which should be considered in future investigations.
2.1.4 Light-responsive MNPs
Light irradiation can be used to stimulate or trigger drug release. Absorbance of photon energy by materials depends on the corresponding energy band-gap of the electron in the highest occupied molecular orbital. Light absorbing materials have a broad range of practical applications like shielding against UV irradiation in sunlight, generation of electrical power from solar radiation, and acting as optical contrast agents and fluorescent reporter molecules. Among the several stimuli exploited in smart DDSs, light irradiation has attracted a significant amount of attention due to the ease of precisely tuning its intensity, the ability to control the exposure duration and tissue location (via the selection of appropriate beam parameters), and the fact that photo-regulated activation is perceived as non-invasive. Ultraviolet (10–400 nm), visible, or near infrared (IR) regions (650–900 nm) of the light spectrum can be used to trigger drug or gene release from appropriately designed nanocarriers. Although the higher energy per photon of UV light enables it to ionize and cleave covalent bonds with energies of the order of 100 kcal mol−1, the potential hazards of far-UV (wavelengths shorter than 200 nm) to tissues and the possible photodestruction of active molecules makes these wavelengths unsuitable for therapeutic purposes. However the application of continuous wave (CW) long-UV lasers (wavelengths of 200–400 nm) can leave both drugs and tissues intact.153 UV irradiation is much more cytotoxic than the other regions of the light spectrum and its inability to penetrate deeply into the tissue due to its absorption by endogenous chromophores (such as oxy- and deoxy-hemoglobin, lipids, and water) is considered as another disadvantage. Thus, wavelengths below 650 nm are considered to be only suitable to trigger drug release for topical treatment of pathological states affecting the skin and mucosa.153 Supramolecular assemblies designed to use visible light are relatively rare.154
NIR has better transmission through tissue due to its lower absorption and scattering in tissue (penetrating into the body about 10 cm) because hemoglobin, water, and lipids barely absorb it, and causes less damage to cells than visible light, due to its lower energy per photon.155 To solve the problem of low energy of NIR photons, some organic chromophores capable of simultaneously absorbing two photons of low-energy have been studied.156
Diverse MNPs have been exploited for the fabrication of light-sensitive DDSs including carbon dots with strong fluorescent emission,157 polymeric hydrogels exhibiting a hydration/dehydration transition mechanism,158 gold NPs for photogenerated localized heating,159 core-shell particles,160 and MSNs possessing high surface area, good targeting ability and controlled drug-release especially toward tumor cells.135,161
In general, light-responsive NPs have found a wide range of applications. The most commonly studied types are micelles and liposomes.162 Although light-responsive MNPs can be made of different materials, all of them have a chromophore in their architecture to harvest the light. Chromophores are therefore the key component of light-sensitive NPs163 which may rely on photo-isomerization (a conformational change of a double bond that is restricted in rotation usually accompanied by a change in the hydrophilic/hydrophobic balance of the photo-excitable molecules), photo-induced cleavage of bonds, photo-oxidation (generation of singlet oxygen or other reactive oxygen species (ROS)), and reversible photo-cross-linking.164
Photo-cleavage reactions occur in o-nitrobenzyl165,166 and coumarin167,168 containing molecules. In this regard, Dong et al.169 designed an s-(o-nitrobenzyl)-l-cysteine N-carboxyanhydride monomer and synthesized a novel block copolymer derivative for controlled release of DOX via photo-cleavage in the core of the NPs. In another work, Zhao et al.156 synthesized a novel block copolymer containing a coumarin chromophore [7-(diethylamino)coumarin-4-yl]methyl methacrylate that was disrupted by both UVA (365 nm) and by 2-photon NIR (794 nm) absorption. A Ti:sapphire laser, generating 80 fs pulses at 794 nm with a repetition rate of 1 kHz and energy per pulse about 300 mJ was able to photocleave coumarin and release encapsulated nile red from the hydrophobic core of the micelle into a water-based solution.
Photo-induced rearrangements of components of micelles are rare. The most common chromophore exploited in these types of light-sensitive micelles is 2-diazo-1,2-naphthoquinone (DNQ).170 This hydrophobic molecule changes into hydrophilic 3-indene carboxylic acid through a Wolf rearrangement reaction.171 DNQ has been widely used in many nanocarriers in order to deliver different cargos. For example Wang and coworkers synthesized a triple-stimulus responsive copolymer consisting of DNQ as a chromophore which was modulated by pH, light, and temperature.172
Another common type of chromophore employed in light-responsive NPs is azobenzene and its derivatives. These molecules such as azobenzenes, spirooxazines, and spiropyrans are organic photochromic compounds that are used to disrupt NPs due to their ability to undergo reversible isomerization in response to UV.173 Son et al.174 used a synthetic photoresponsive molecule to induce destabilization of polymeric micelles of hyperbranched polyglycerols into individual chains by UV irradiation. In other words, they employed the solubility-switching (from hydrophobic to hydrophilic) characteristic of the molecule to release cargo inside the micelles.
MSNs can contain drugs that are retained in the pores by different capping agents.175 Cargo molecules are released only in the presence of an external stimulus like UV light or visible-light that induces destruction of capping agents. To this end, a supramolecular assembly was used to deliver cargo molecules from a MSN-based structure upon irradiation of visible-light.176 Sulforhodamine 101 was loaded inside the mesopores of mercaptopropyl (MP)-MSN and entrapped by mercaptopropyl-coordinated Ru(bpy)(2)(PPh(3))-moieties.
Photo-isomerization induced by upconversion luminescence (UCL) is another mechanism used for drug release activated via low intensity (e.g. 0.35 W cm−2) light irradiation and with features such as minimal damage to biological sites (considering that the maximum permissible light intensity for skin exposure is 0.726 W cm−2). For example, upconverting NPs (UCNPs) doped with lanthanide ions have been used to convert NIR irradiation to UV/visible light. After the upconversion emission another photosensitive material absorbs the upconverted light producing photoreactions which induce drug-release mechanisms e.g. through degradation of polymeric NPs.177–179
Recently, the assembly/disassembly of NPs through light-triggered reversion of surface charge has been used as an efficient drug release mechanism. For example, colloidosome microcapsules fabricated via the electrostatic assembly of organosilica NPs with oppositely charged surfaces were exposed to light irradiation (365 nm). This induced electrostatic interactions leading to surface charge reversion or “flipping” by which the positive charges turned to negative and thus the repulsive forces between negative charges disassembled the colloidosomes and resulted in drug release.180
An important photo-activated mechanism is two-photon NIR-triggered drug release, in which simultaneous two-photon absorption induces chemical reactions. However the low efficiency of energy absorption by chromophores using this mechanism can reduce its efficiency.181 Furthermore, this mechanism has several advantages such as high three dimensional spatial resolution due to the required tight focus of the laser, low scattering losses, deep penetration in tissues,182 insignificant premature drug release,33 and significant cytotoxic effects by generation of toxic ROC.183,184 In this approach, two-photon fluorophores and photosensitizers are doped or covalently bound to a nanostructure such as MSN based nanovalves. Thereafter, by two-photon NIR irradiation, the fluorophores with high two-photon cross-section and having maximum emission capability through mechanisms e.g. Forster resonance energy transfer (FRET) isomerize the photosensitizers (e.g. azobenzene) located on the fluorophores in the nanostructure. Thus, the drug release process is activated.77,185
The photo-reduction of metal-based prodrugs (e.g. Pt) is another novel technique to induce drug release in reductive milieus such as cancerous sites. Herein, via photo-irradiation, a non-toxic inactive prodrug (e.g. a metal complex containing Pt(IV)) changes to the active cytotoxic form (e.g. a complex with toxic Pt(II)).186,187 Photo-triggers such as UV light,188 visible light189 and NIR irradiation67 have been reported to induce photo-reductive activated prodrug release with advantages such as enhanced specific cytotoxicity against cancer cells, induction of apoptosis, in vivo bioavailability, maximal light penetration, significant inhibition of tumor growth as well as simultaneous imaging capability.
Several efforts have been conducted to develop photolabile protecting groups as a component of nanocarriers which can encapsulate drug molecules and sequester them. Thereafter, these protecting groups can be eliminated by light irradiation, hence the protected drug molecules are uncaged and delivered to a targeted biological site where they can bind to cell receptors.190 Photolabile protecting groups (i.e. photo-uncaging groups) can be employed in different nanostructures such as liposomes, peptides, and lipidic NPs for delivery of biomolecules including antimicrobial peptides, nucleic acids, and anti-tumor agents.190–192
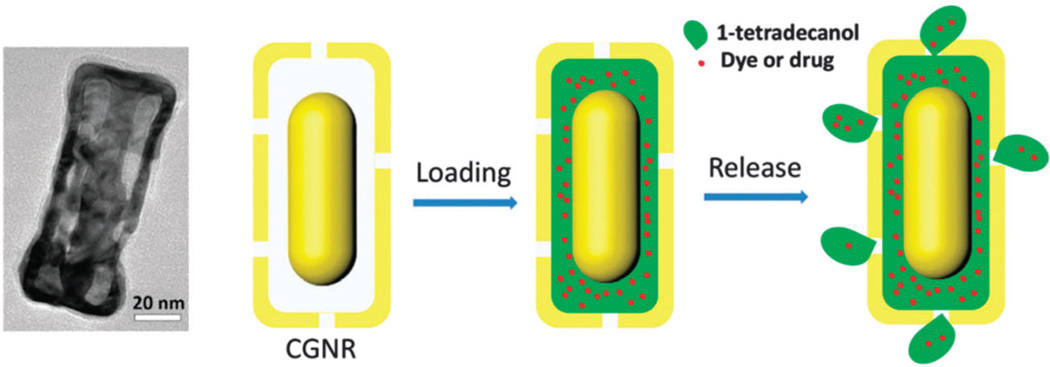
In photothermal therapy by DDSs, the light absorbed by a nanomaterial is converted to localized heat via plasmonic mechanisms (i.e. collective oscillation of free electrons in the conduction band leading to light scattering due to localized surface plasmon resonance (LSPR))193 which can induce enhanced detrimental effects on bacterial cells,194 tumor cells and tissues.195,196 LSPR enhanced heating can be effective although conventional hyperthermia (41–44 °C) has adverse effects such as protein unfolding, and DNA denaturation.197 In photo-triggered thermal activated drug release, low power densities e.g. 0.20 W cm−2 (Ag–Au shell–core),160 0.6 W cm−2 (reduced GO),198 and 1–2 W cm−2 (gold nanocage–CNT hybrid)199 have been reported that do not damage tissue. Photo-triggered thermal systems have also led to a type of photochemotherapy with advantages including on-demand drug release, destruction of tumor cells either in light treated or light-shielded areas (the latter one due to heat-generated diffusing in the tissue), with a light-dosage dependent response.200 Novel hollow plasmonic nanocrystals made from caged gold nanorods (CGNRs) were fabricated by Xiong et al.201 They showed broad-band dipolar surface plasmon resonances to NIR, obtained via tuning their length and thickness. The strong plasmon coupling between the surrounding gold nanocages and the internal gold nanorods could potentially intensify the efficiency of the photothermal effect thus providing a multifunctional theranostic CGNR-based nanoconstruct for cancer treatment (Fig. 9).
Fig. 9.
TEM images of CGNR and the schematic mechanism of 1-tetradecanol and dye/drug loading and release, reproduced from ref. 201 Copyright 2014 with permission from “Royal society of Chemistry”.
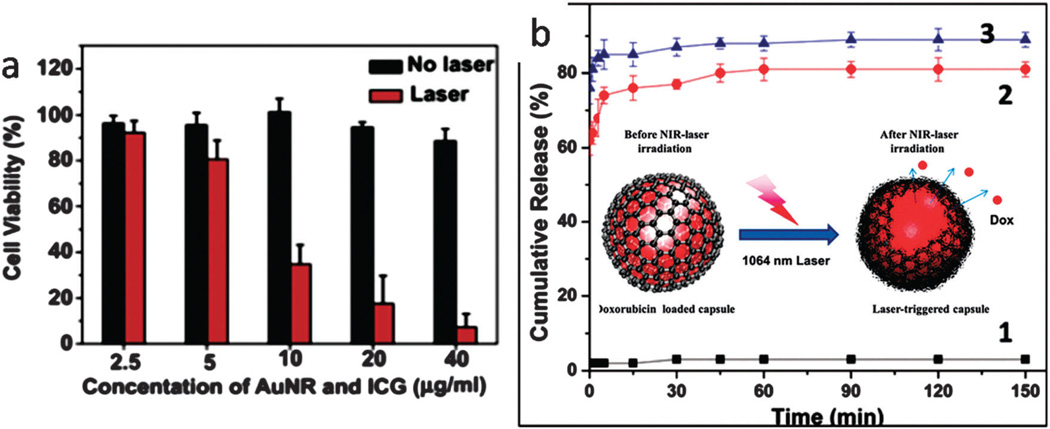
Furthermore, photothermal therapy that is combined with photodynamic therapy (i.e. generation of ROS such as singlet oxygen and free radicals) has shown high cytotoxic efficacies and enhanced damage to tumors45 but can have disadvantageous features such as low ROS production, a tendency toward self destruction, inadequate selectivity, and meager water-solubility.202 A NIR-activated aptamer-silver-gold shell-core nanocarrier showed insignificant destructive effects against normal cells along with highly sensitive detection of various cancer cells at low concentration.160 Another system based on chitosan nanospheres showed a synergistic photothermal effect (due to hyperthermia from gold nanorods) and photodynamic effect (due to ROS production by indocynine green (ICG)) against cancer tissues as well as localized accumulation in these sites. Fig. 10a shows the significantly reduced cell viability after NIR-irradiation.203 Fig. 10b illustrates how a DOX loaded GO–poly(allylamine hydrochloride) (PAH) composite capsule was fabricated. Here, NIR irradiation ruptured the capsules and drugs were released.204 Chang et al. studied drugs encapsulated inside Au-nanorods capped by oligonucleotides. NIR was utilized in their attempt to produce photothermal conversion of Au nanorods and subsequently dehybridization of their light-sensitive gate of duplex DNA.205
Fig. 10.
(a) Cell viability evaluation of cancer cells before and after NIR irradiation using an ICG-Au–chitosan based nanocarrier, reproduced from ref. 203. Copyright 2013, with permission from Elsevier, (b) cumulative release curves before NIR-irradiation (curve 1) and after NIR irradiation (curve 2 and 3) and the schematic of drug release process (inset image), reproduced from ref. 204 with permission from “the Royal Society of Chemistry”.
Another light-sensitive drug release mechanism, known as photochemical internalization (PCI), has recently been used to treat cancer in both animals and patients.206 In PCI, which was first reported in 1999,207 photosensitizing compounds are localized in intracellular endocytic membranes and are then excited by light, producing ROS, whereupon cargo inside the endosomal vesicles is released after disruption of the membranes. This route is often utilized in releasing macromolecules (such as ribosome inactivating proteins or nucleic acid therapeutics) that have been taken up into cells by endocytosis, but need to get out of endosomes to reach the nucleus or the ribosomes to have their best effect.207,208 Oliveira et al.209 utilized this method to silence epidermal growth factor receptors by siRNA. They concluded that endosomal escape was a limiting step for the efficiency of siRNA silencing. In addition they found that lipid-based carriers of nucleic acids had an increased efficacy in PCI. The location of some photosensitizing compounds inside the cytoplasmic membrane is one of the main problems in PCI as photoactivation would be detrimental to the cells.31 It could be suggested that a pH-sensitive linker between the photosensitizing compound and the drug-bearing MNP may be engineered so that the release of drug/gene happens only within either/both lysosomes or/and endosomes. Consequently, only the membrane of these two organelles would be destroyed after light irradiation and release of the loaded material occurring in the cytoplasm.
2.1.5 Mechanical-responsive MNPs
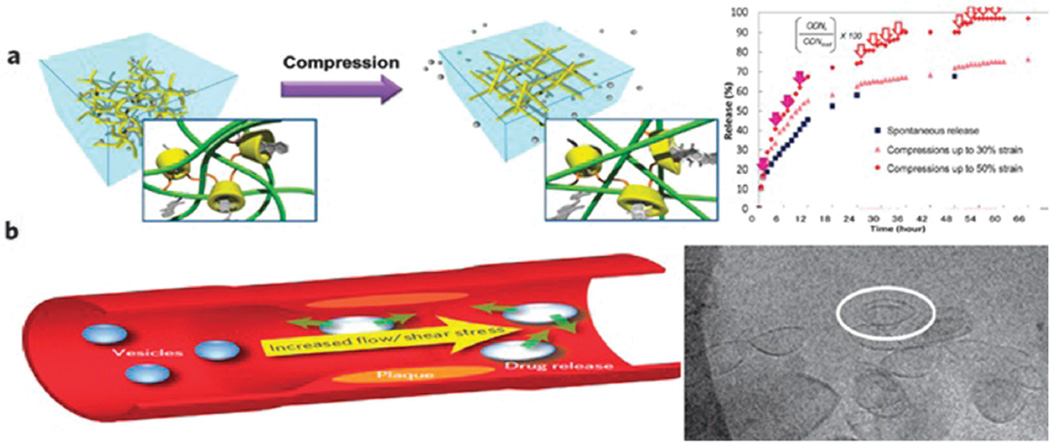
Mechano-responsive nanostructures are another approach to smart DDSs for which only a few studies have so far been reported. Generally this concept is based on the assembly and disassembly of mechano-responsive molecules/structures with applied pressure or other physical stimuli with the aim of changing the overall volume.145 This approach proposes a biomimetic and simple mechanism for controlled DDS compared to other external stimuli-based systems.210 For example cyclodextrin (CyD) inclusion complexes can be affected by pressure, and the destabilization of the inclusion complex in CyD–alginate (AL) hydrogels by mechanical compression was reported for the purpose of patient-controlled release of ondansetron (ODN) (Fig. 11a).211 Accelerated drug release occurred when the inclusion ability of the β-CyD was reduced by applying pressure to an implantable gel thus forming an on-demand strategy for the treatment of severe nausea caused by chemotherapy.
Fig. 11.
(a) Concept of controlled release of ODN from a pressure-sensitive hydrogel. The inset shows accelerated release in response to mechanical compression, (b) endogenous shear stress as a drug releasing stimulus, mechanoresponsive lenticular vesicles 100 nm (inset); (a) reproduced from ref. 211. Copyright 2013 with permission from the Royal Society of Chemistry, (b) reproduced from ref. 213. Copyright 2012 with permission from Nature publishing group.
Shear stress is a less often discussed mechanical stimulus that can be used in the design of smart DDSs. Shear stress is higher in narrowed segments of arteries compared to healthy arterial segments. This difference could be a better physical/mechanical signal to trigger drug release in arteries in comparison with other biological or chemical stimuli.212 Cardiovascular diseases such as stenosis and atherosclerosis could be promising applications of these endogenous mechano-stimuli-responsive systems. For example a study prepared lenticular liposomes that were stable under static conditions but unstable in the elevated shear stress of atherosclerosis.213 The lenticular morphology led to preferential rupture along the equator of the nanostructures followed by increased release of drugs in the areas of high shear stress (Fig. 11b).
Another study developed microscale aggregates of NPs that were broken up into their individual nanoscale components under high local shear stress, for treatment of thrombosis.214 Rapid clot dissolution, lower required doses, restoration of normal flow dynamics, minimized side-effects with maximized drug efficacy were all obtained after in vivo administration.
2.1.6 Ultrasound-responsive MNPs
Ultrasound (US) is one of the external stimuli that can induce mechanical or thermal stimulation. US is of great importance in modern medicine and has a role in many medical applications such as diagnosis (at low frequencies for imaging) and treatment (at high frequencies for the removal of masses such as tumors). In the design of US responsive nano-carriers, three main properties are required; reliable and stable drug encapsulation before delivering the applied US waves to the tissue, efficient drug release owing in response to particular US waves, and the capability of monitoring drug release for imaging and therapeutic purposes. The biomedical range of US waves varies from 0.1 to 50 MHz, although the minimal required frequency is 1 MHz in deep therapy and imaging.4,5,8,215
US not only can trigger the release of drugs from carriers, but can also increase the permeability of biological barriers (cell membranes, blood brain barrier, etc.) by the formation of cavitation bubbles and increased temperature resulting in enhanced drug diffusion.216 Insonated medium can cause unidirectional flow in an aquatic medium, which can affect the tissue structures. Furthermore these waves produce an oscillating pressure between the two layers of biological membranes and create pores in them, thus enhancing the permeability of cell membranes. The effects of US for transdermal drug delivery were widely discussed in a review by Azagury et al.217 The absorption coefficients of US vary among the different body parts and tissues. Some tissues such as bones have much higher coefficients compared to soft tissues, but studies have revealed that the threshold of US pressure and frequency as well as the burst length can influence this tissue disruption and control brain damage when US is applied to the head.218
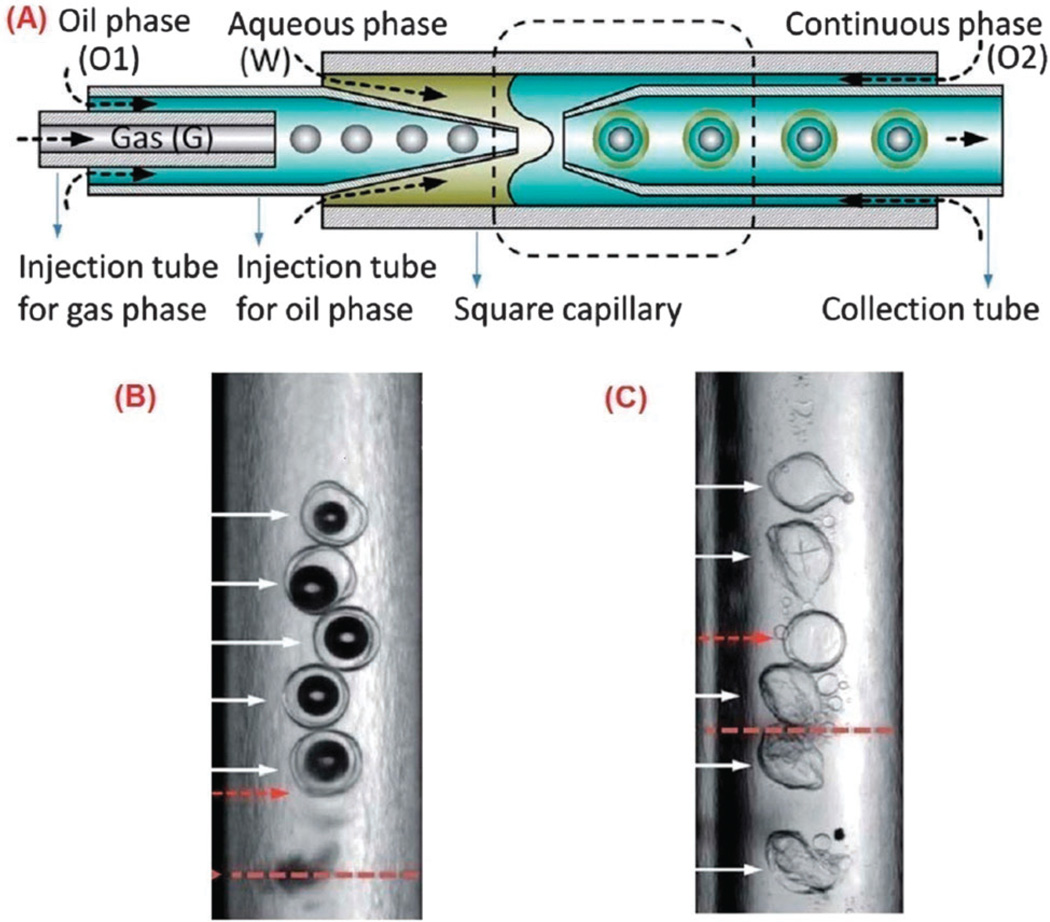
The most suitable structure for US responsive drug-carriers is microbubbles (not nanobubbles) consisting of a gas core such as perfluorocarbons, nitrogen or air, surrounded by a shell of lipids, polymers and proteins such as albumin. The radius of the microbubbles needs to be in a particular range and the number of sites at which microbubbles interact with the vasculature, must be maximized by considering the saturation level. The bubbles are typically on the micron scale (1–10 micrometers) and do not leave the circulation after injection unless they are phagocytosed. Their activation by US also causes physiological changes that enhance drug penetration into the cells.218,219 Optimizing the gas-core is a good strategy to enhance US responsivity of the core–shell nanocarriers, even in more complicated structures in which several liquid and elastic layers surround a gas-core. Each layer of this type of carrier could possess different hydrophobic and hydrophilic properties and could incorporate different types of active cargo molecules. Microfluidic technology provides a good method to produce a gas-core triple emulsion with a gas-in-oil-in-water-in-oil (G/O1/W/O2) structure as shown in Fig. 12. In this study, Chen et al. demonstrated the importance of the gas-core in US responsivity by comparing the effect of US triggering on double oil–water–oil (O/W/O) emulsion structures without the gas-core and the triple G/O/W/O emulsion. The O/W/O emulsions only slightly vibrated without a major response to US waves, whereas the G/O/W/O emulsions disintegrated and their cargo was released220 (Fig. 12B and C). Moreover the materials comprising the core and shell govern the interaction of the immune system with the microbubbles. Soft-shell microbubbles have a thin layer of phospholipid or protein which provides higher sensitivity to the US waves. On the other hand hard-shell microbubbles contain entangled or cross-linked polymers that have higher stability and lower US response.478,480,481
Fig. 12.
Production of US responsive gas-core triple emulsion nanocarrier. (A) Schematic of microfluidic apparatus for the fabrication of the triple emulsion. Dashed lines show the encapsulation of gas in oil and aqueous phases by microcapillary needle. Active cargo might be present in either the oil or the water and then the outer layer of oil surrounds the whole drop. (B) The double emulsion O/W/O drops do not respond to US waves (C) The gas-core triple emulsion disintegrates by US trigger. Arrows show the direction of US waves. Reproduced from ref. 220 with permission from the Royal Society of Chemistry.
The two principal advantages of US, namely energy focusing and good effective depth, make US an attractive route for gene delivery. Many commercial US contrast agents are available such as SonoVue, Optison and Levovist which can be used to monitor the delivery of therapeutic genes by low intensity US. The low intensity of the US prevents the harmful effects on tissues and organs. After the carrier has reached the targeted tissue, high intensity US is applied that causes cavitation in the microbubble gas core and release of the attached DNA to the targeted cells.221–225
He et al.226 used US-mediated collapse of microbubbles to deliver siRNA to enhance therapy against yolk sac carcinoma in vitro. They suggested that owing to the negative charge of DNA, cationic lipid microbubbles would be suitable candidates for gene delivery. Unger et al.216 showed the capability of US to increase gene expression and transfection efficiency using cationic liposomes into cells.227 Lawrie et al.228 reported that US could enhance vascular gene delivery used for a cardiovascular application such as prevention of restenosis. Kuo et al.229 showed that poly-l-lysine (PLL) and polyethylenimine (PEI) both protected nucleic acids from 20 KHz US waves, better than PEG. Hou et al.230 transfected the gene for inducible Smad 7 by US-triggered microbubbles in order to block TGFβ and decrease renal fibrosis in chronic kidney disease. Moreover, nanoscale bubbles also can be designed for targeted drug delivery. For example, Zhang et al.231 combined poly lactic-co-glycolic acid (PLGA) nanobubbles with monoclonal anti-HLA antibody to generate a carrier for targeted delivery to cancer, and enhanced high-intensity focused US ablation of tumors, and Chen et al.232 used US-triggered nano/microbubbles to transfect a model gene (luciferase) into periodontal tissue and observed high gene expression localized in the muscle cells of gingival tissues.
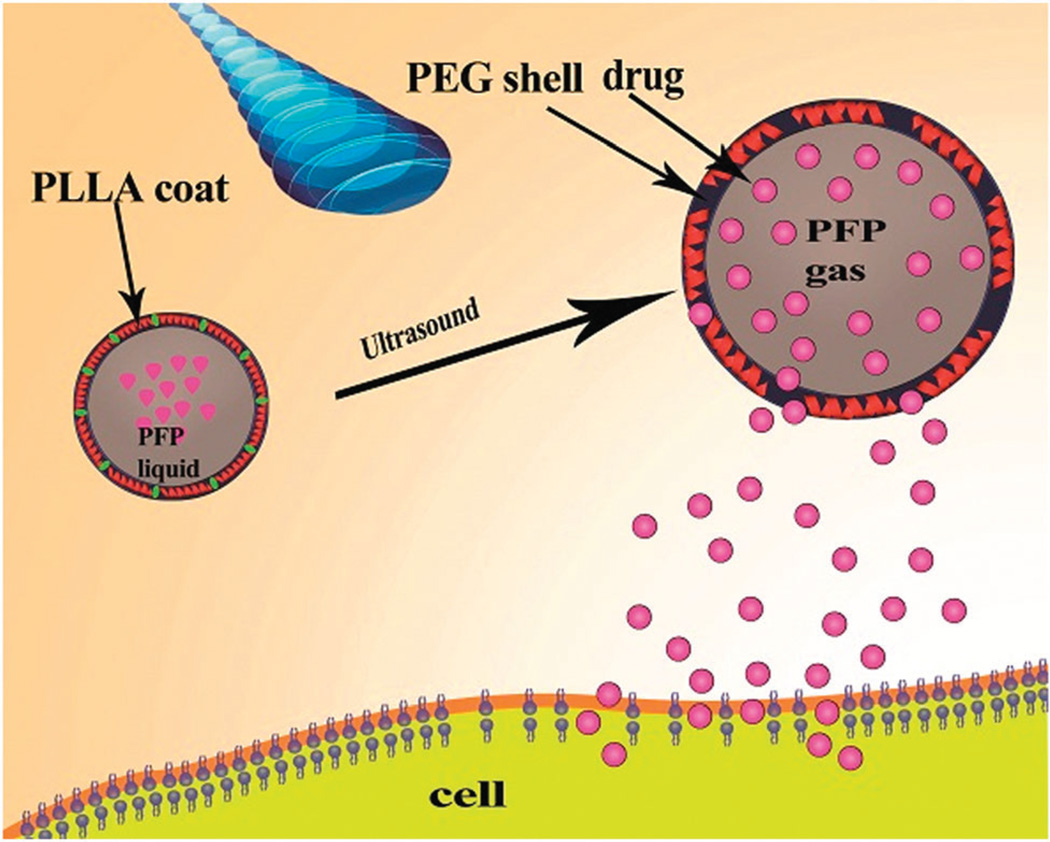
Different structures have been used to deliver drugs and release them by US stimulation. Naked DNA and free drugs can enter into the cell through endocytosis after application of US without using nano/microbubbles and the percentage of Ca2+ has a central role in this phenomenon. Ca2+ increases inside the cell by cell membrane disruption, although the amount of unspecific delivery will also significantly increase which is more important in the case of gene delivery.233 Micelles with 10–100 nm diameter have been used for the delivery of hydrophobic drugs. They self-assemble from amphiphilic polymers in aqueous solution and under the influence of US they release their cargo. The highest drug release occurred at 20 KHz although a low frequency of 20–90 KHz was also effective for drug release from micelles. Rapoport et al.234 generated US-responsive micelles by composing PEG–PLLA poly(ethylene oxide)-co-poly(l-lactide) or PEC-microbubble PCL poly(ethylene oxide-co-polycaprolactone) (PEO-co-PCL). They introduced a core containing 1% PFP (perfluoropentane) that was coated by PEG–PLLA. The resulting microbubbles had a maximum diameter of 500–700 µm and possessed the ability to deliver paclitaxel (PTX) to A2780 ovarian carcinoma or MDA MB 231 breast cancer cells exposed to unfocused 1 MHz US for 1 min. Fig. 13 depicts this mechanism.
Fig. 13.
US-induced phase change particles to facilitate drug release from bubbles.
Husseini et al.235 studied the factors that triggered drug release, and highlighted the role of cavitation as the single most important factor in drug delivery. The size and material properties of bubbles along with the US amplitude directly influenced the type of cavitation (the formation of gas filled cavities such as bubbles when exposed to an oscillating pressure). The mechanical index (MI) of cavitation, in tissue without micro-bubbles, should be less than 1.9, whereas in the presence of micro-bubbles it is reduced to less than 0.8. Increased cell uptake of drugs was proposed as the second reason since the cell membrane can be temporarily permeabilized by the cavitation-induced shock wave. Geer et al.236 designed a liposomal microbubble for increasing cytotoxicity of cancer drugs even at very low doses. Nonlinear reflection of US with low acoustic pressure led to particle destruction in addition to the increased membrane permeability.
The energy of the acoustic waves, when they pass through cells and tissues, is transformed into heat, and therefore applying a focused US beam can generate local tissue heating which can be monitored by different thermometric devices. US also led to the cavitation of the NPs and the destruction of nearby cancer cell membranes, therefore increasing drug uptake. In 2013 Rapoport et al.237 loaded PTX into NPs (nano-emulsions) with 200–300 nm diameter for the treatment of pancreatic cancer activated by FUS (focused US). Here, US delivery (pressure ≥ 1 MPA), 6 h after injection of the NPs, enhanced tumor regression. In addition US can improve the contractile strength of the myocardium. In a study Spivak et al.238 utilized US-mediated sonoporation to increase the uptake of 30 nm AuNPs conjugated with Simdax (a calcium sensitizer) in a rat model of heart failure. The strong anti-oxidant property of AuNPs was observed, and the Simdax was released under US beams.
Rather than the usual advantages of liposomes such as being a stable carrier for lipophilic and hydrophobic compounds, the US beams applied for liposomal drug release should be strong enough to disrupt the liposomal structure and low frequency beams have better efficiency. Furthermore, the combination of US with hyperthermia can also enhance drug release. Smet et al.239 used HIFU mediated drug delivery under MR image guidance. They used temperature responsive liposomes (TSLs) co-encapsulating DOX and an MR contrast agent (250 mM [Gd(HPDO3A)(H2O)]) and showed release of DOX while no leakage of the MRI contrast agent was reported over 1 h at 37 °C. Owing to many simultaneous processes occurring in vivo, identifying the dominant mechanism of action is difficult, US-responsive lipid nano-carriers still have good potential for treatment of cancers.240–242 Xie et al.243 utilized a lipid-encapsulated formulation (MRX 802) with 1 µm diameter that included platelet targeted ligands such as the peptide (cyclo-CRGDWPC)-OH) and targeted glycoprotein 2b/3a receptor. In pigs afflicted with acute left anterior descending thrombotic occlusions, a low-MI (mechanical index) US pulse sequence was utilized to guide the delivery of high-MI (1.9 MI) US and image the myocardium. They observed epicardial recanalization, myocardial blood flow and infarct size were significantly improved by US induced cavitation of MBs.
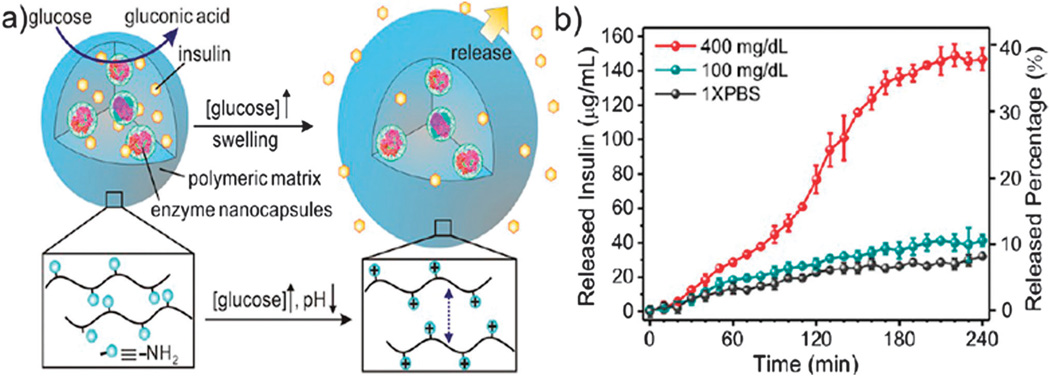
Di et al.244 described a three-dimensional cohesive gel-like nano-network formed from PGLA through electrostatic interaction between positively-charged (chitosan) and negatively-charged (alginate) NPs. This nano-network could be loaded with insulin, subcutaneously injected into diabetic mice, whereupon the insulin was released in a controlled fashion by US-induced shock waves causing cavitation (frequency: 950 kHz, pulse duration: 20 µs, output power: 4.31 W, administration time: 30 s).
Although sometimes the size of microbubbles is too large to reach the targeted tissues outside the vasculature, they can be combined with other NPs such as liposomes and micelles. This strategy can enhance drug loading capacity, increase the systemic targeting capability and increase the vascular permeability of microbubbles. For example, Burke et al.245 showed that the delivery of PLGA NPs containing 5FU was improved by covalently linking them to US-activated microbubbles. After intravenous injection into Rag-1 knockout mice with C6 gliomas, mice were exposed to pulsed 1 MHz US giving a 67% reduction in tumor volume at 7 days. Improved drug delivery to PC3 prostate cancer xenografts and the subsequent controlled release were shown using air-containing microbubbles consisting of poly-(butylcyanoacrylate) (PBCA) loaded with the model drug Nile red after exposure to 300 kHz or 5 MHz US.246
2.2 Chemical stimuli-responsive MNPs
2.2.1 pH-Responsive MNPs
It is well-known that significant pH gradients exist within body (especially the gastrointestinal tract (GI) tract) and also considerable pH differences exist among healthy and pH values vary among the lysosomes (4.5–5), endosomes (5.5–6), Golgi apparatus (6.4), and cytosol (7.4). pH alterations can be induced by the growth of microorganisms (directly or through induction of the release of host enzymes),247 there is an acidic milieu in healing wounds and an alkaline milieu in non-healing wounds.248 Most importantly there is a lower pH profile in tumors than in corresponding normal tissues (i.e. 7.4). This occurs due to the rapid proliferation of cancerous cells outrunning the blood supply, and causing inadequate supplies of oxygen and nutrients, and the thus the formation of lactic acid produced by glycolysis rather than oxidative phosphorylation in normal cells. This phenomenon is known as the Warburg effect. These widely varying pH conditions in diverse biological systems have motivated the design of pH-responsive DDSs.52,249
pH-Responsive nanomaterials have demonstrated a variety of applications including pH-sensors,250 theranostic applications,251,252 controllable switches, controlled-release surfaces, controllable wettability, and cell-recognition devices.,253 Novel pH-responsive nanocarriers of biphasic colloidal particles and “Janus particles”254 have been recently developed with advanced features enabling them to undergo morphological alterations upon pH changes, a new concept of great importance in the design of pH-sensitive DDSs.
There have been abundant advances in smart DDS based on pH-responsiveness of nanosystems in the past few years that have resulted in remarkable breakthroughs in diagnosis and therapy of a range of diseases and disorders such as malignancies and infections. They have shown enhanced antitumor efficacy and reduction of the toxicity of chemotherapy,255 delivery of nucleic acids, proteins, peptides, etc.256 pH-Responsive DDSs can significantly enhance oral bioavailability of anticancer drugs, producing efficient inhibition of tumor growth, reduction of systemic toxicity257 as well as improving selectivity to tumor cells while only giving extremely low cytotoxicity toward normal cells.258
The pH-sensitive nanomaterials that have been used in DDSs can be classified into organic, inorganic and hybrids259 and their methods of synthesis, properties and applications have been recently reviewed.260 The design of pH-responsive nanocarriers requires a good comprehension of their features and mechanisms. For example, the degradation of the spacers conjugating the drug to the polymeric NP at the low environmental pH of tumors or in endosomes/lysosome,261 the protonation of moieties (e.g. carboxylic groups or titratable amines) attached to the surface of the polymeric micellar particles and subsequent structural changes,262 the cleavage of dendrimer particles induced by sensitivity of its hydrophobic groups to acidic conditions,263 and utilizing acid-labile zwitterionic peptide–lipid derivatives in lipidic nanocarriers255 are several examples of pH-dependent mechanisms that can be used for smart drug-delivery. However, the concept of the instability of different pH-responsive nanocarriers in biological media is mostly based on ionizable pH-sensitive groups present in polymeric and peptide NPs, and on acid-labile chemical bonds; both topics are discussed below.
Ionizable chemical groups (e.g. amines, carboxylic acid) including weakly acidic groups (proton donor) or weakly basic groups (proton acceptor) are used in anionic and cationic pH-sensitive polymers, respectively.264,265 These pH-triggered delivery systems show advantageous features such as enhanced cellular uptake, high efficiency of drug or gene (e.g. plasmid DNA (pDNA), and small interfering RNA (siRNA)) delivery, facilitated endosomal membrane rupture induced by the proton sponge effect (i.e. endosomal destabilization by osmotic swelling), surface charge reversion of nanocarriers and deshielding in the low extracellular pH of the tumor environment.266–271 The pH-sensitivity of chitosan derivatives and chitosan-based NPs make them good candidates for pH-responsive oral drug delivery272–274 with properties such as drug release in the intestinal environment (pH 6.8),275 delivery of macromolecules, drugs, and gene (non-viral) delivery,276–278 apoptotic effects produced in tumor cells,279 low cytotoxicity, and increased gene transfection and expression in cancer cells.278 Various chitosan-based nanoassemblies are illustrated in Fig. 14.
Fig. 14.
Chitosan-based nanoassemblies applied in biomedicine. (a) Nano-spheres, (b) vesicles, (c) micelles, and (d) nanogels.
Peptides have been considered as efficient and safe nanocarriers for non-viral delivery of genes to cells,280 as compared to viral gene delivery systems, although the structure has been inspired by these viral vectors. Peptide vectors have advantageous features such as the ability to penetrate the cell membrane, endosomal fusion (in low pH of endosome), and nuclear delivery.281,282 Moreover they avoid the side-effects seen with viral vectors such as inflammation, sustained immune response, and development of cancer and even death.283–285 In addition, multiple repeats of peptide motifs such as glutamic acid–alanine–leucine–alanine (a 30 amino acid) (GALA), shorter version of GALA (shGALA), lysine–alanine–leucine–alanine (KALA) and a 16-amino-acid peptide (RALA) peptides have also been utilized as efficient gene delivery platforms with advantages such as increased interactions with cellular lipid bilayers (especially at lower pH), a long blood circulation time, efficient tumor growth inhibition, improved gene expression, reduced cytotoxicity, increased binding ability to DNA, improved cell-internalization, ability to carry both hydrophilic and hydrophobic molecules with a broad size range (e.g. small molecules, viruses, antibodies, plasmids, proteins), pH-sensitivity to regions of the gastrointestinal tract and good oral delivery.280,286–289 Such gene delivery platforms can be also envisioned for clinical applications.280
Among pH-sensitive moieties, the acid-labile linkers responding to only slight pH changes can provide new classes of highly sensitive pH-responsive MNPs. Acid-labile covalent linkages can be rapidly hydrolyzed in acidic environments such as tumor tissues and several cellular compartments including lysosomes (pH ~ 4.5–5.0), early endosomes (pH ~ 6.0–6.5) and late endosomes (pH 5.0–6.0).290 Polymers containing these cleavable linkers (e.g. acetal/ketal, hydrazine, imine groups etc.) are stable at physiological pH, while drug release occurs due to hydrolysis of the linker bonds in response to the decrease in pH. Cleavable linkages can either be placed in the backbone of the polymer or in the side chains.290,291
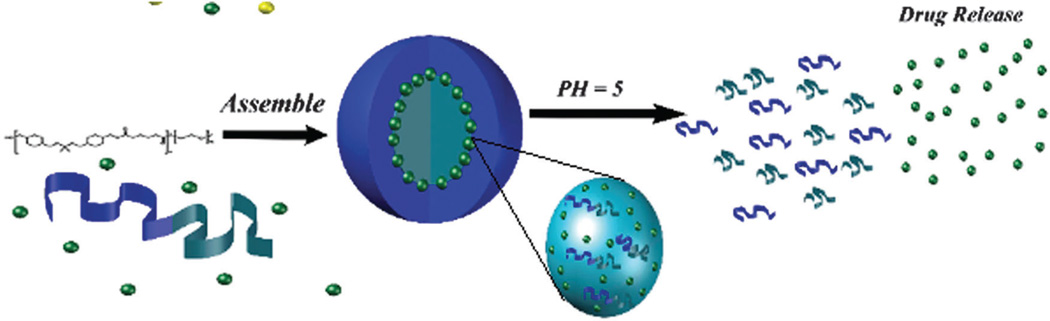
Acid-labile linkages in nanostructures (e.g. polymers) have shown noticeable advantages in gene delivery including biocompatibility, biodegradability, highly efficient delivery, efficient serum-resistant gene transport, low cytotoxicity.292–294 pH-Responsive micelles with an acid-labile ketal linkage in the hydrophobic backbone were dissociated at an acidic pH and thus their anticancer cargo was released. Fig. 15 illustrates the assembly and release of drug from micelles.295
Fig. 15.
Schematic illustration of the assembly and drug release from a micelle encapsulating hydrophobic drugs.
pH-Sensitive micellar structures have other favourable features including multi-functionality, good stability at physiological pH (7.4), partial hydrolysis at the extracellular pH of solid tumors and complete hydrolysis at the lower endosomal pH, a positively charged surface at tumor pH, improved cellular uptake through surface ionization and electrostatic interaction with cell membranes, good endosomal dissociation of micelles, and endosomal membrane disruption leading to enhanced intracellular delivery via the endocytic pathway.296
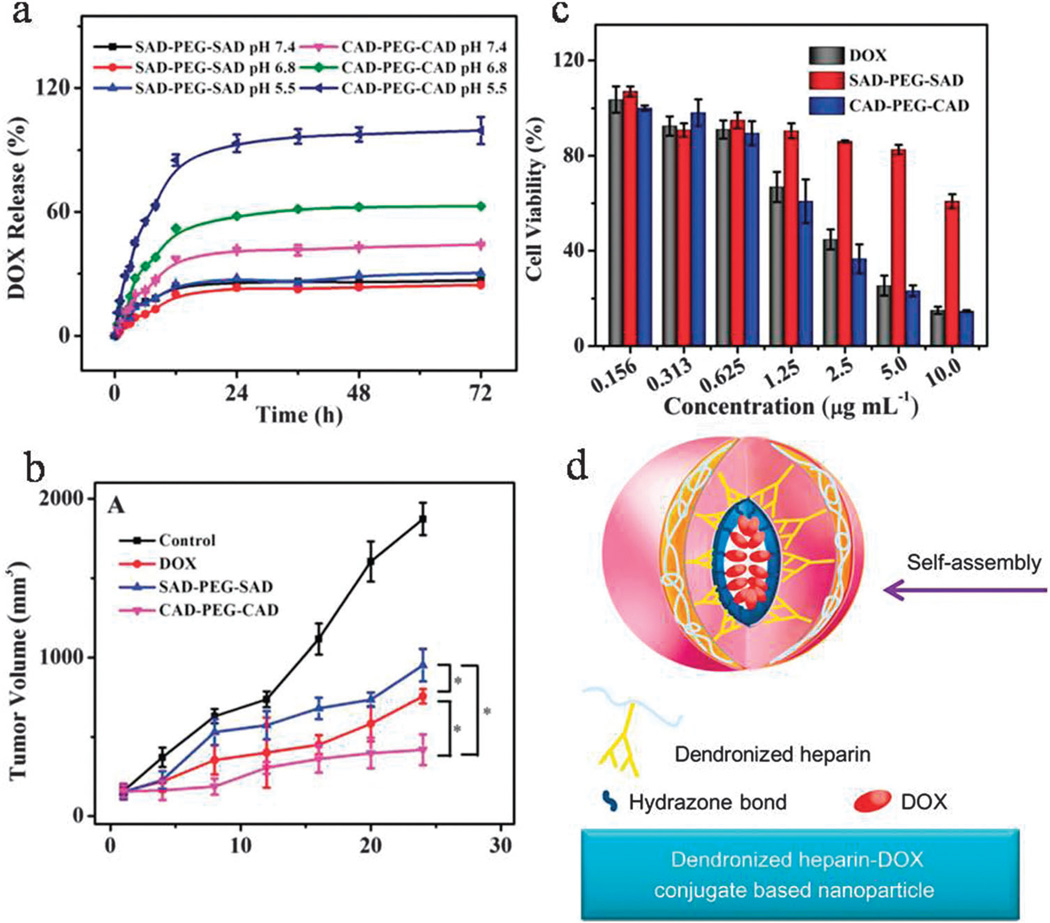
Acid-labile hydrazone bonds can be utilized in pH-responsive DDSs for cancer treatment.297–302 pH-Responsive nanosystems containing hydrazone bonds such as polyurethane (PU) have shown diverse advantages including multi-functionality, biocompatibility, biodegradability of the carriers, anticancer drug release in response to the acidic intracellular environment, ability to be loaded with lipophilic agents in physiological conditions,303 controlled stepwise drug release, and nontoxicity.304 Micelles containing hydrazone bonds have also been developed, especially for cancer therapy305 with advantages such as more rapid drug release at acidic pH,306 and delivery of hydrophobic drugs with no need for conjugation sites.307 Furthermore, the use of nanosystems containing pH-sensitive hydrazone bonds enables co-delivery of drugs such as DOX together with siRNA to tumor cells.298,302 Fig. 16d illustrates a self-assembled dendronized NP containing heparin conjugated to DOX using an acid-labile hydrazone linkage and a negatively charged surface. This NP showed more rapid drug release at pH 5.0 than at pH 7.4 followed by significant killing of 4T1 cancer cells by apoptosis, an antiangiogenesis effect in a breast cancer model with insignificant side-effects.308
Fig. 16.
(a) DOX release from SAD–PEG–SA and CAD–PEG–CAD at pH 5.5, 6.8 and 7.4 at 37 °C, (b) cytotoxic effects and (c) tumor volume evaluation, for free DOX, SAD–PEG–SA and CAD–PEG–CAD, (d) dendronized heparin–DOX based NPs with hydrazone bonds. (a–c reproduced from ref. 313. Copyright 2013 with permission from “John Wiley & Sons, Inc.”, and reproduced from ref. 308. Copyright 2013 with permission from “Elsevier”).
The deep penetration of anticancer drugs (especially those loaded into nanocarriers) into the tumor tissue is a challenging issue. The size and the surface charge of the nanocarriers are the key parameters determining penetration, but the drug release in response to slight pH-changes will lead to better tissue penetration and cellular uptake in cancer cells.309 Smart pH-triggered nanocarriers including micelles with hydrazone bonds310 and liposomes311 have shown good cytotoxic effects and high tumor targeting capability.
Lale et al. reported an acid-sensitive hydrazone-linked nanosystem for breast cancer targeting. It combined the proton sponge-mediated endo-lysosomal escape ability and dual targeting (flate and trastuzumab) had minimal cardiotoxicity, hepatotoxicity, and nephrotoxicity along with its high therapeutic efficacy.312 Sun et al.313 reported two DOX functionalized prodrugs, one that was acid-sensitive (cis-aconitic anhydride conjugated to PEG (CAD–PEG–CAD)) and the other was insensitive to pH changes (succinic anhydride conjugated to PEG (SAD–PEG–SAD)) which both self-assembled into micelles. Both micelles were stable at physiological pH (i.e. pH 7.4), however the CAD–PEG–CAD micelles underwent swelling and drug release at intratumoral and endosomal pH values (pH 6.8 and 5.5, respectively) (Fig. 16a). There was good cellular uptake, remarkable tumor suppression (Fig. 16b) and high cytotoxicity (Fig. 16c) along with reduced side-effects.
Multi/dual pH-responsive DDSs are new concepts with the promise of future refinements in smart drug delivery. Dual pH-responsive delivery nanosystems can be designed to be sensitive to both tumor pH and to endosomal pH. A pH-triggered polymer–DOX drug conjugate smart nanosystem was developed capable of responding to both extracellular and to intracellular pH values. This nanosystem reversed its negative surface charge to positive at extracellular tumor pH (~6.8) leading to facilitated cellular uptake. Afterwards, enhanced intracellular drug release from endocytosed nanocarriers was obtained at the more acidic pH (~5.0) of intracellular endosomal compartments. Increased cytotoxicity and reduction of anticancer drug resistance in cancer cells was achieved.314 Graphene and GO nanocarriers have been designed to be pH-responsive in DDSs.315,316 In anticancer therapy, pH-responsive GO nanocarriers have shown less resistance in cancer cells, accelerated drug release with slow efflux, and synergistic therapeutic effects against tumors.317
Photodynamic therapy (PDT) has been combined with DDSs. Recently fullerenes have shown great potential as photosensitizers. For example, a DOX loaded poly(ethyleneimine)-derivatized fullerene (DOX–PEI-C60) showed a synergistic combination of chemotherapy and PDT, with high cytotoxicity, good tumor targeting, efficacious tumor growth inhibition, and a strong pH dependence with no evident systemic toxic effects against normal cells.318
MSNs containing a pH-sensitive compartment have favorable properties including large surface area, high pore volume and large capacity for cargo delivery,319,320 good pH-sensitivity,321 improved oral bioavailability and long-term preservation of the active drug inside the nanomatrix,322 sensitivity to the acidic endosomal pH,323 and the ability for pH-sensitive gating of controlled drug release.86,324 Very recently Chen et al. fabricated MSNs that were acid-sensitive. These MSNs showed pH-triggered drug release and better cytotoxicity against cancerous cells.325
pH-Responsive DDSs can be potentially used for delivery of protein and peptide drugs such as insulin and can shield them against denaturation by the “crowding effect”.326 A dendritic polyglycerol (dPG) nanogel containing acid-labile benzacetal bonds was prepared. Protein release without loss of activity was achieved at the low pH milieu of acidic cell compartments.326,327 Several pH-sensitive DDSs have been developed for targeted delivery of live bacteria (e.g. Lactobacillus casei) to the small intestine by protecting from the acidic environment of the stomach (i.e. pH 1–3).328 Imbalances in the concentration of metal ions in biological sites can cause several disorders such as Alzheimer’s disease (induced by dysregulation of copper ions that causes the formation of aggregation of amyloid-beta (Aβ) peptides in the brain).329 pH-Responsive delivery systems could be a possibility for treatment330,331 since metal ions could be transported and released at the biological site using pH-triggered delivery systems.332
A carbon dot-coated calcium alginate bead (CA-CD)-based DDS was used to deliver drugs in the gastrointestinal tract at low pH.333 In another study, a nanocarrier comprising a synthetic chitosan-modified gold NP attached to the anionic surface of a liposome (AuChi-liposome) was reported for the delivery of antimicrobial drugs (e.g. doxycycline) to the stomach as a therapy for Helicobacter pylori infection. The nanocarrier was stable at gastric pH = 1.2 but at neutral pH = 7.4 regions (where the bacteria are situated) became destabilized through detachment of the gold NPs followed by the active fusion of the liposomes to the bacterial membrane which induced subsequent drug release (Fig. 17).334
Fig. 17.
(a) Schematic of phospholipid liposomes stabilized by chitosan-modified gold NPs: these are stable at gastric pH 1.2 but become destabilized at pH 7.4 in sites of H. pylori infection by deprotonation and detachment of gold NPs form the liposomes, (b) accumulative doxycycline release profile from AuChi-liposome at pH = 1.2 and 7.4; reprinted with permission from ref. 334. Copyright 2013 “American Chemical Society”.
pH-Sensitive nanocarriers can be used to avoid the premature degradation of drugs in cellular compartments such as lysosomes. These carriers respond to reduced pH by rupturing and destabilizing the endosomal membrane causing drug release into the cytoplasm.7 The drug loading capacity, release rate and dosage are vital factors in this regard. pH-Responsive DDSs can have a high loading capacity for drugs.253,257 In some cases, in order to achieve long-term drug administration, a slower drug release rate can be obtained using pH-Responsive DDSs based on the β-cyclodextrin inclusion complex.333 Furthermore, achieving a sufficient therapeutic dosage of drug after a single oral administration is sometimes important in the case of drugs with limited bioavailability e.g. high isoelectric point therapeutic proteins that need to reach the small intestine and penetrate through the intestinal epithelium. The desired release rate of the therapeutic drug can be tailored to the required dosage.335
Dual cargo release systems with selectively controlled release of two different drugs can be considered an important topic. Wanyuan designed a nanocarrier from which one cargo was released at a lower pH value, while the other one was released at higher pH.336 To overcome cancer multi-drug resistance (MDR), which causes clinical treatment failures, various attempts have been conducted; for example through pH-responsive co-delivery systems.302 In one study nucleic acid (e.g. siRNA) transfection was utilized to induce efficient gene silencing (e.g. luciferase expression) within various carcinoma cell lines accompanied by simultaneous administration of anticancer chemotherapeutics such as PTX.337 Chiang et al.338 designed trastuzumab-conjugated pH-triggered double emulsion nanocapsules (DENCs) for co-delivery of hydrophilic DOX and hydrophobic PTX. Here, PMASH was conjugated to the shell of the DENCs for dual drug release in intracellular acidic compartments (Fig. 18a and b). Enhanced cellular uptake, cytotoxicity and suppressed cancer growth were obtained. Zhang et al.339 showed efficacious accumulation of liposomal nanocarriers in tumors followed by their lysosomal escape (Fig. 18c).
Fig. 18.
(a) Deformation of DENCs due to the shrinkage of acid-labile PMASH at acidic pH leading to drug release, (b) cumulative drug release of PTX-DOX-DENCs containing 1 wt% PMASH at pH 4 and 7, (c) ex vivo images of fT1-bearing BALB/C mice after injecting Cy5-labeled antagomir-10b-loaded liposomes (tumors are indicated by the black arrows); (a and b reproduced from ref. 338). Copyright 2014 with permission from Elsevier, and c reproduced from ref. 339. Copyright 2015 with permission from “Elsevier”.
2.2.2 Redox-responsive MNPs
Redox-responsive DDSs are one of the most efficient systems for stimulus-responsive cancer and gene therapy.334,340 Redox-sensitive degradable nanosystems (RSDNs) provide some benefits over other stimuli such as pH. A good response to the high intracellular levels of GSH, release of the drug directly into the nucleus and the cytosol, and stability in extracellular environment where GSH levels are low are examples of these benefits.341 The redox environment is governed by a linked set of redox couples such as NADP+/NADPH.342
Different redox conditions between the intracellular and extracellular compartments, and also between healthy cells and diseased cells provide the stimulus for drug release. These RSDNs can be designed for different therapeutic goals. The central thiol/disulfide redox couples, GSH/GSSG, cysteine/cystine (CySH/CySSCy), Trx-1 and thioredoxin-2 (Trx-2) govern the redox potential inside cells, and can have a major role in drug delivery. The intracellular concentration of GSH is about 2–10 mM, while the GSH concentration in extracellular fluid in tissue is only about 2–20 µM.343 Furthermore, the endosomal compartment is redox-active due to the gamma-interferon-inducible lysosomal thiol reductase which is a reducing enzyme in the presence of some reducing agents (e.g. cysteine but not GSH). The concentration of GSH in tumor tissues and the cytosol of tumor cells are at least four times higher than that in normal tissues, so tumors can be considered a reducing environment.
The cytoplasm has metabolic oxidases that make an environment for redox signaling which is dependent on NO synthases and NADPH oxidases. Mitochondria contain the most reducing environment and are highly sensitive to oxidation. The rate of electron transfer in mitochondria is higher than other cellular compartments. Nuclei are quite resistant to oxidation but have lower redox potentials. The secretory pathway introduces disulfide bonds into proteins that are exported, by the action of oxidizing systems and enzymes. The redox potentials of cells can be affected by the functional state of the cell such as induction of apoptosis, differentiation, adhesion and proliferation. A general picture showing biologically relevant redox couples and the compartmental organization of cells and their function is given in Fig. 19.344,345
Fig. 19.
Standard reduction potentials of biologically relevant redox couples with subcellular compartmentalization of disulphide/thiol redox potentials in cultured cells. The most validated data (based on limited cell culture studies) is for the cytoplasmic GSH redox potential, ranging from −260 mV (in rapidly proliferating cells) to −220 mV (in non-dividing cells).
The design of reduction-sensitive nanosystems has usually been achieved by degradable micelles using disulphide links in the hydrophobic backbone.346 Amphiphilic copolymers with a single disulphide bond connecting the two polymer blocks346 can self-assemble in to micelles.347,348 Other approaches employ GSH-responsive crosslinking agents that are incorporated either in the core349 or in the shell350 of the micelles. Redox-sensitive systems can also use coated mesoporous silica nanomaterials,351 disulphide crosslinked nanogels,352 liposomes353 or dendrimer-drug conjugates containing thiol-cleavable bonds.354
In polymeric micelles, micellar de-crosslinking and full destabilization/disassembly may be caused by the reduction of disulfide bonds in polymeric assemblies by the action of intracellular GSH.355,356 On the other hand oxidation of redox-active micelles could shift the hydrophobic/hydrophilic balance, leading to micelle fragmentation into water-soluble monomers and release of hydrophobic drugs.357 In redox environments, selective drug release at pathogenic sites can be enhanced by taking advantage of the presence of activated macrophages in inflamed tissues and certain tumors. Different kinds of approaches have been made use to improve the stability of micelles in such biological systems, such as chemical cross-linking of the core358,359 or shell360 of self-assembled micelles. Wang et al.361 showed GSH triggered release of DOX from reduction-sensitive shell-detachable micelles with improved cytotoxicity, and overcoming the MDR in A549 cancer cells pre-treated with GSH monoester (GSH-OEt). This is a laboratory method utilized for artificial elevation of the intracellular GSH level.347
An intracellular GSH responsive RGD containing peptide-capped MSNs including the antitumor drug DOX (DOX@MSN-S-S-RGD) was developed. The RGD containing peptide acted as a gatekeeper and was immobilized onto MSNs using disulfide bonds. Hence, after uptake by tumor cells, DOX was rapidly released by cleaving the disulphide bonds triggered by GSH. This study suggested this nanosystem could have great potential for cancer therapy (Fig. 20).362
Fig. 20.
DOX release from DOX@MSN-S-S-RGD in PBS with different concentrations of GSH for 90 min. Reproduced from ref. 362 with permission from the Royal Society of Chemistry.
Core-cross-linking can increase the stability of micelles with minimal impact on the micelle surface property and can lengthen their half life in the blood circulation.363 In a study, Wang et al.364 developed a promising DDS based on a new class of redox-responsive degradable core-cross-linked (CCL) micelles with conjugated hydrophobic camptothecin (CPT), that were subject to redox-responsive cleavage of the integral disulfide bonds. These CCL micelles showed better stability under physiological conditions compared to non-cross-linked micelles, while they underwent fast dissociation in a reducing environment, leading to burst release of CPT and higher cytotoxicity against human breast cancer cells in vitro.
2.3 Biological stimuli-responsive MNPs
2.3.1 Different biomolecular-responsive MNPs
The existence of specific biomolecules such as glucose, ATP, DNA, and ROS in specific physiological sites or in pathological conditions of living systems has encouraged the development of biomolecule-sensitive smart systems. Glucose-responsive controlled release systems have been the most investigated smart DDS to control insulin therapy for diabetics in response to blood glucose levels. Different mechanisms have been used to fabricate these smart systems, that are usually based on the conversion of glucose to gluconic acid by glucose oxidase (GOx).365 For example human insulin, chitosan and GOx enzyme were incorporated into nanocapsules in the form of monodisperse microgels that swelled in hyperglycemic conditions through enzymatic conversion of glucose, protonation of the pH responsive chitosan matrix and release of insulin as a self-regulating nanovalve system for treatment of type 1 diabetes366 (Fig. 21).
Fig. 21.
(a) Schematic illustration of glucose-responsive insulin encapsulated microgels, (b) insulin in vitro release kinetics in solution with different glucose concentrations at 37 °C reprinted with permission from ref. 366. Copyright 2013 American Chemical Society.
Insulin delivery by glucose-binding proteins such as lectins is another possible strategy. The high affinity interaction between concanavalin A (ConA) lectin and mannose was used to encapsulate insulin cargo within the MSN pores for the construction of a glucose-responsive delivery system.367 Other glucose-responsive micro/nanostructures such as fast responsive implantable microdevices368 and highly selective glucose responsive silica nanocontainers369 have also been reported.
ROS-responsive systems can be designed to operate based on ROS-induced degradation or a ROS-mediated solubility switch.370 ROS species such as hydroxyl radicals (HO•) and hydrogen peroxide (H2O2) are produced in different parts of the cell as a by-product of electron transfer reactions.370 In one study well-defined ROS-sensitive β-CD-based smart DDSs371 were prepared by incorporation of oxidation-responsive boronic ester units into the structure. Cytocompatible micelles containing polypropylene sulfide (PPS) were developed to form a ROS-triggered drug release system taking advantage of the increased hydrophilicity of the PPS core that was disassembled after oxidation by the ROS rich environment.372 Another study took advantage of the EPR effect to deliver DOX nanosystems with controlled drug release after degradation of an aryl boronic ester linker in the presence of ROS.373
ATP is the cellular energy source and is an important cellular metabolite with higher intracellular concentrations compared to the extracellular environment and can be used for the development of smart systems based on natural materials374 or on synthetic polymers.375 In one study high ATP concentrations induced conformational changes of the protein-based nanocarrier formed from multiple units of the barrel-shaped chaperonin protein, followed by the disassembly of the tubular structure powered by ATP hydrolysis, preferential accumulation in tumor tissue and cargo release.374 Another study reported that an ATP/ATP aptamer binding complex could induce dissociation of a two dimensional (2D) DNA–graphene hybrid nanoaggregate for site-specific DOX controlled release in an ATP rich environment376 (Fig. 22).
Fig. 22.
(a) Schematic of an ATP-sensitive DNA–graphene nanosystem for controlled drug delivery; the DNA aptamer bound to ATP was used as a linker. (b) Cumulative DOX in vitro release at different ATP concentrations reproduced from ref. 376. Copyright 2015 with permission from Elsevier.
Other biomolecule-responsive nanostructures have been used in smart DDSs. For example controlled drug release triggered by microRNA-responsive traceable DNA-anchored MSNs operating via competitive hybridization between microRNA and the DNA caps was reported.377 Another study also described protein responsive-amphiphilic polypeptide nanoassemblies that disassembled when a particular protein bound to the nanostructure with potential applications in protein-specific delivery and diagnostics.378
2.3.2 Enzyme-responsive MNPs
As an important component of the bio-nanotechnology toolbox, enzymes play a significant role owing to their exceptional bio-recognition capabilities and outstanding catalytic properties. Their high selectivity and favourable efficiency in enzyme-catalyzed reactions are very beneficial in nanomedicine.379 Since some enzymes are overexpressed in specific tissues, and moreover their concentration may become higher in a diseased state, enzyme-responsive NPs can be an excellent candidate for designing a smart DDS. Moreover, for diagnosis purposes, measuring or detecting an enzyme activity can be very useful as its dysregulation is a particular feature of many diseases.380 A DDS design in this regard can utilize either a physical or a chemical mode of action. In the chemical mode, when the nanomaterials are exposed to the enzyme, it is possible to design the nanomaterials to release their drug load by degrading the different structures ranging from those formed from polymers to those with mesoporous silica cores.381 According to this strategy, transformation or degradation of the nanocarrier by the enzyme can also release the therapeutics and hence multimodal nanomedicines with synergistic effects can be further designed to this end. In the physical mode of action, the enzyme-responsive NPs can be designed so that their macro-scale structure is altered by the enzyme and results in controlled-release. In this approach, the surface of the nanomaterials can be modified by attaching molecules that are affected by enzymatic reactions which causes a change in their physical characteristics. Dysregulation of enzymatic activity has been observed in a number of different pathological situations and this has led to not only new ideas for drug delivery in vivo, but also to fabrication of new ultrasensitive sensors for diagnosing disease.382–384 Nanomaterials which are used in enzyme-responsive DDS are often responsive to hydrolases or to oxidoreductases.385–387
The enzyme-responsive approach to smart drug delivery is based on smart polymers that act as carriers that are able to release the payload only upon the catalytic action of the enzyme. An enzyme-responsive material can be defined as a system that undergoes macroscopic changes in its physical/chemical properties upon the catalytic action of an enzyme. The response mechanism of enzyme responsive materials requires one component to be an enzyme sensitive moiety, that usually is a substrate or a substrate mimic of the enzyme, and a second component that is responsible for changes in the interactions inside the nanomaterial that can lead to macroscopic transitions and drug release. This strategy does not always require modifications of existing drugs, as they need not be chemically attached, but can be physically entrapped.
Hydrolases are often used as effector biomolecules in enzyme-activated DDSs. In a smart DDS, the carrier nanomaterial can be digested by the enzyme, whenever the concentration of both species is high enough.381 This strategy can be particularly useful in some treatment methods such as chemotherapy since it can reduce side effects of some hazardous drugs. Some of the developments of enzyme degradable nanocarriers are reviewed here.
Protease enzymes play powerful roles in many cellular processes such as DNA replication and transcription, cell proliferation, differentiation, angiogenesis, conception, wound repair, stem cell mobilization, hemostasis, inflammation, blood coagulation, immune response, necrosis and apoptosis.388 Proteases are also an ideal enzyme for designing smart DDSs as they are often overexpressed in diseases, such as cancer and inflammation, and protease activation can be used to release drug from carriers at specific locations within cells.389 Under these conditions, the therapeutic effect of cargo release occurs after incorporation of the NPs inside the specific cell by endocytosis. By doing so, the therapeutic effects of the drug will be increased and the toxic effects will be decreased due to lower required doses. For instance, Vicent et al.390 used a specific peptide sequence as a linker to attach DOX and aminoglutethimide (AGM) to N-(2-hydroxypropyl)-methacrylamide HPMA NPs for controlled release.
Proteases are produced either by tumor cells themselves or by other different cells present in the tumor site. However, another approach can use an exogenously administered protease in which a polymeric nanocarrier containing the drug connected by a peptide sequence is administered to the patient. After that, when the NPs have accumulated in the tumor region with leaky blood vessels, another NP that includes the effector protease can be administered so that activation of the first prodrug NP causes localized drug release with a minimum nonspecific toxicity.
Studies show that many proteases are only found in the intracellular compartment. With this in mind, using protease-triggered drug release can enhance the therapeutic effect of the drug and even decrease the toxic side effects of drug release.381 For instance, Imperiale et al.391 innovatively designed a delivery system consisting of pure drug nanocrystals of the potent protease inhibitor indinavir free base (served as a model poorly water-soluble protease inhibitor) fabricated via nanoprecipitation that were encapsulated within mucoadhesive polymeric microparticles. The results supported the outstanding ability of this platform to decrease the dose and the administration frequency of protease inhibitors, a pivotal step to overcome the current problem of patient non-compliance in highly active antiretroviral therapy for HIV infection.
Trypsin is one of the most important digestive proteinases and this enzyme represents the second group of hydrolase-responsive nanomaterials. In the digestive process, trypsin together with the other proteinases lead to breakdown of dietary protein molecules to their constituent peptides and amino acids. It also carries out the digestion process (begun in the stomach) in the small intestine, within which a slightly alkaline milieu elevates its rate of enzymatic activity.392 As the task of trypsin is to degrade dietary protein, it is frequently referred to as a proteolytic enzyme, or proteinase. Also involved in the activation of other digestive enzymes, trypsin plays a key role in controlling functioning of exocrine pancreatic secretion.393
The use of two or more enzymes simultaneously, for enzyme-triggered drug release could increase the accuracy and sensitivity of the method. Radhakrishnan et al.394 investigated the effect of using either hyaluronidase or trypsin enzymes, which are both over-expressed under specific pathological conditions, to degrade capsules produced using protamine and chondroitin sulphate. Recently there was a report of nanocapsules that were degraded in the milieu containing either trypsin or hyaluronidase, which lead to the freeing of encapsulated drugs. When these nanocapsules were at pH 7.4, the cross-linking was maintained, while the drug molecules were rapidly released in the presence of either of the triggering enzymes.395
Hou et al.396 constructed a novel supramolecular NP for controlled drug release at specific sites in the presence of target enzyme. They successfully used two biocompatible materials, namely protamine and sulfato-β-cyclodextrin (SCD), that were non-covalently associated together. The results showed that the disassembly of the nanoparticles was specific to trypsin compared to other enzymes, and efficiently released the encapsulated model drug.
Phospholipases are considered to be therapeutic targets as their expression can be up-regulated in both infectious and inflammatory diseases. Furthermore, they have a high concentration at the invading edge zone of tumors as a part of the host defense mechanism.397,398
Helicobacter pylori infection in the stomach is the cause of several diseases such as peptic ulcers, chronic gastritis, and gastric malignancy.399,400 These bacteria secrete phospholipase A2 (PLA2) that can be used to degrade liposome membrane integrity for triggered cargo release.401 Thamphiwatana et al.402 used a liposomal formulation with a lipid composition responsive to secreted PLA2 and demonstrated that the more bacteria or enzyme that was present at the targeted site, the more drugs was released to treat the infection.
Glycosidase enzymes catalyze the hydrolysis of carbohydrates and can be used for triggering drug release at sites when their concentrations are elevated compared to normal tissue. These conditions include HIV, cancer and its metastases, inflammation and infections. For example researchers have shown that hydrolysis of carbohydrates is increased significantly (85%) in some carcinomas such as breast, lung, pancreas, stomach, uterine, ovarian, osteosarcoma and multiple myeloma. Therefore sugar based NPs could be an appropriate anti-cancer drug carrier.381 Bernardo et al. synthesized a hybrid silica mesoporous (Si-MPs) NPs that showed glycosidase-responsive intracellular controlled release. They found that the delivery of DOX to HeLa and LLC-PK1 cells increased drastically in the presence of β-d-galactosidase.403
Elastase enzymes are another type of proteases (peptidases) that degrades a number of proteins including elastin (present in different organs such as lungs and the extracellular matrix) and collagen. For instance, among elastase enzymes, human neutrophil elastase is over expressed in inflammation.404,405 Elastase based DDSs can be used for triggered release of drugs especially for lung diseases. These therapeutic cargoes include α1-antitrypsin, Eglin C, and peptidyl carbamates which prevent the remodelling of lung tissue and the formation of fibrosis by decreasing the free elastase.
Azoreductase is an enzyme secreted by the microbial flora existing in the human colon, and has received attention as a target for triggered drug release, especially for colonic diseases. In a study by Rao et al. an azoreductase-responsive vehicle was assembled by covalent coupling between azobenzene and an amphiphilic diblock copolymer. In the presence of the enzyme azoreductase and nicotinamide adenine dinucleotide phosphate (NADPH) the vehicle disintegrated releasing its cargo.406,407 Patel et al.408 used enzyme-responsive snap-top covered silica nanocontainers in order to release a payload of small molecules. Porcine liver esterase could trigger the release of rhodamine B, monitored by fluorescence spectroscopy.408
Oxidoreductases have been considered as therapeutic targets owing to their central role in oxidative stress and their involvement in diseases such as Alzheimer’s and cancer.409,410 Recently, many studies have concentrated on oxidoreductase enzymes both for therapeutic drug delivery and diagnostic including bio-imaging probes. The major groups of oxidoreductase enzymes which have been used in DDSs are discussed below.
Hydrogels which have been rendered sensitive to biomolecules could be the next generation of smart biomaterials. Oral administration of proteins and peptides such as insulin is unsuitable due to their sensitivity to chemical and enzymatic hydrolysis in the stomach and poor cellular uptake in the intestine. The release of drugs from hydrogels can be induced by enzyme action that affects the local pH and alters the hydrogel structure. Since glucose-responsive pulsatile insulin release systems are an important goal, glucose oxidase enzymes have been widely studied. For the treatment of diabetes, hydrogels that respond to glucose by swelling have the potential to be a self-regulating insulin release system.411–415
In one study, glucose oxidase was encapsulated in polymeric vesicle nanocarriers.416 In the presence of a moderate amount of glucose, the polymeric shell was degraded and the interaction with glucose oxidase increased. This system that transforms glucose to gluconolactone also produces hydrogen peroxide that can oxidize sulfur containing bonds to sulfones and sulfoxides (Fig. 23). As a result, the nanocarriers can be destabilized and dissolve.
Fig. 23.
Oxidoreductase-responsive vesicles for DDSs; (a) an SEM image of the vesicles that encapsulate glucose oxidase to form a responsive material; (b) enzymatic formation of gluconolactone followed by disruption of the vesicles in order to release the cargo. Reprinted with permission from ref. 417. Copyright 2004 “American Chemical Society”.
Bacterial enzymes have a significant role in combating infectious diseases because they can be potentially used to release antibiotics inside cells as a triggered smart nanosystem. Xiong et al.418 recently treated murine salmonellosis using gentamicin in silica xerogel-based DDSs. They used bacterial enzymes to degrade a polyphosphoester core of the antibiotic-loaded DDSs.
There are many different components that have high potential in smart DDSs. For example, future studies could focus on proteases for targeting the mitochondria in hematological cells of patients, and oxidoreductase for targeting chloroplasts in plants. Owing to recent progress in protein engineering and molecular modeling, different classes of enzymes have been discovered that also play a significant role in disease diagnosis and controlled drug release. Table 1 summarizes different classes of enzymes utilized in different applications.
Table 1.
Examples of enzyme-responsive nanomaterials and their applications
| Enzyme category |
Subgroup | Micro/nano particle |
Application | Benefits | Drawbacks/limitations |
|---|---|---|---|---|---|
| Hydrolases | Trypsin/gelatinase |
|
The diagnosis and treatment of pancreatitis393 Enhancing the hydrolysis of bovine serum albumin419 Quartz crystal microbalance420 |
|
|
| Proteases | Au NPs | Establishing an optical biosensing platform to monitor proteinase activity421 | |||
| Channel-activating proteases (CAPs) | Liposomes | Drug delivery devices389 | |||
| Lipases (PLA2) | Au NPs | High-throughput screening care diagnostics | |||
| Oxidoreductase | Glucose oxidase |
|
Insulin delivery systems366 Biosensor technology for monitoring of blood glucose | ||
| Peroxidase | Au NPs | ELISA422 |
3 Dual and multi-responsive MNPs in DDSs and the role of graphene
The basic principle of dual and multi-responsive drug carriers is based on the action of logic gates. In the logic gate algorithm, two or more factors can stimulate a system and according to the type of the gates, different results will be obtained. The main type of logic gate include AND, OR, and their derivatives such as XOR, NOR, XNOR, and NAND. Table 2 represents these gates’ symbols and their main action. In the OR gates the presence of any one stimuli out of all of them is enough to launch the response, whereas in the case of AND gates, all the stimuli must exist at the same time to trigger the action. NAND gates will not respond to the stimuli when all of them are present and NOR gates will respond only if no single factor can stimulate the gate.
Table 2.
Different types of logic gates: their symbols, response mechanisms, and main action
| Type of the logic gate | Symbol | Algorithm | Main function | ||
|---|---|---|---|---|---|
| AND | x | y | A = x−y | Activated only in the presence of both stimuli. | |
| 0 | 0 | 0 | |||
| 0 | 1 | 0 | |||
| 1 | 0 | 0 | |||
| 1 | 1 | 1 | |||
| NAND | x | y | A= x−y | In the simultaneous presence of all stimuli is inactive, whereas in the presence of none of them or in the presence of some of them is active. | |
| 0 | 0 | 1 | |||
| 0 | 1 | 1 | |||
| 1 | 0 | 1 | |||
| 1 | 1 | 0 | |||
| OR | x | y | A = x + y | Any single stimulus can trigger the response. | |
| 0 | 0 | 0 | |||
| 0 | 1 | 1 | |||
| 1 | 0 | 1 | |||
| 1 | 1 | 1 | |||
| NOR | x | y | A = x + y | Only in the presence of none of the stimuli the gate is active. | |
| 0 | 0 | 1 | |||
| 0 | 1 | 0 | |||
| 1 | 0 | 0 | |||
| 1 | 1 | 0 | |||
| XOR | x | y | A = x + y | In the presence of all stimuli at once and in the presence of none of them the gate is inactive. | |
| 0 | 0 | 0 | |||
| 0 | 1 | 1 | |||
| 1 | 0 | 1 | |||
| 1 | 1 | 0 | |||
| XNOR | x | y | A= x−y | In the presence of all stimuli at once and in the presence of none of them the gate is active. | |
| 0 | 0 | 1 | |||
| 0 | 1 | 0 | |||
| 1 | 0 | 0 | |||
| 1 | 1 | 1 | |||
The human body is a complex collection of different environments. Each cell is surrounded with special receptors and enzymes as well as other molecules to increase its redox potential above that of extracellular body fluid. The combinations of two or more different stimuli described in the preceding sections can be rationally chosen to further increase the versatility and specificity of triggered drug/gene delivery.423,424 Not only may these multi-functional NPs be smarter but they may possess higher loading efficiency and longer sustained release times. Moreover a multi-responsive nano-carrier has better ability to sense very slight changes in the environment such as small variations of pH and temperature.425,426
Combinations of temperature with other factors as a stimulus have some special advantages. The temperature sensitivity can be applied in different ways. First, the sensitivity of the carrier to temperatures at around 37 °C can lead to the release of drug at the normal temperature of the body in the presence of the second stimulus. This strategy would protect the drug out of the body and its life time will be increased. Moreover it is possible to design a gelation system to be active only at body temperature, and as a result the carrier is in the liquid form under laboratory conditions and after injection into the body, it is converted to the gel form. Furthermore if this sensitivity is designed for higher temperature (e.g. 40–42 °C) it would be possible to apply external heating to the local area like a cancer site, leading to the release of the drug in the specific location along with another stimulus such as reduction or pH. These advantages make temperature the most common stimulus in dual-stimuli-responsive carriers.
Among the various dual stimulus-responsive nano-carriers, temperature and pH is the most common combination that has been studied, but the disadvantages of these type of carriers can be premature gelation of the carrier in the body and rapid degradation, while anionic and cationic conditions may influence the pH responsive elements. Nearly all the dual and multi-responsive reported so far carriers have OR or AND logic gates. Shim et al.427,428 generated a dual temperature/pH-responsive hydrogel consisting of three thermo-sensitive polymers. The degradation rate of the pH- and thermo-responsive block copolymers considerably diminished in comparison to the control block copolymer due to the buffering effects of the acidic monomer slowing the rapid degradation. This approach resolved two main disadvantages related to thermo-sensitive block copolymers: rapid degradation and premature gelation. This polymer could provide sustained release of PTX for 2 weeks after a single injection.
In another study, PTX was encapsulated into two types of polymers which self-assembled into supramolecular dendrosomes via a host–guest interaction, and had high sensitivity to pH < 7.4 and temperature > 37 °C. Under these conditions, the dendrosome was destroyed and the polyglycerol released the encapsulated PTX.429 A co-polymer based hydrogel that released human growth hormone (hGH) under both acidic and basic conditions and at body temperature was tested in animal models, and the results displayed both burst and sustained release of hGH.430 In some cases a rise in temperature can stimulate a reaction in the outer part of the nanocarrier, leading to increased proton concentration that stimulates the pH-responsive part of the carrier. In one study, DOX was inserted into the core of a thermo-sensitive micelle having pH-labile histamine residues (due to the presence of imidazole rings) by the heat-shock process. The formation of lactic acid after a temperature increase led to protonation of the histidine imidazole ring and the release of the drug.431
Among logic gates, OR and AND have also been reported. A self-assembled thermo-pH-dual responsive micelle was prepared. These micelles encapsulated methotrexate, and released it at both pH 7 and temperature 37 °C.432 In an interesting study, Du et al. generated an onion-shaped carrier with a pH-responsive inner layer and a thermo-responsive outer layer surrounding a magnetic core of Fe3O4 embedded in SiO2. The thermo-pH responsive action of this polymeric microsphere was triggered by magnetic stimulation.433
The external application of magnetic fields can be combined with another stimulus to make a dual stimuli-responsive carrier many of which are based on Fe3O4·o115 Moreover the presence of a magnetic core allows MRI imaging of drug delivery. The drug release strategy in this system is based on AND gates. Zhu et al.434 reported a multifunctional pH-sensitive SPIO nanosystem for simultaneous tumor MRI and therapy. pH-Sensitive acyl-hydrazone linkages, DOX and PEG were attached to SPIO NPs. The pH-sensitive acyl-hydrazone linkages were cleaved under acidic conditions and DOX was released from the SPIO. High cellular uptake and better antitumor effects were also shown due to the action of externally applied magnetic fields.
Sometimes the presence of a magnetic responsive element in the nanocarrier (such as Fe3O4) can act like a gateway to induce chemical reactions which can be used to follow the carrier distribution. Jing et al.435 loaded DOX onto a polylactic acid polymeric NP and combined it with Mn–porphyrin to create a nano-carrier for imaging as well as therapeutic applications in HeLa cells. A DOX loaded magnetic coated polymeric NP could be useful for imaging and also showed high anticancer activity against prostate tumor cells at 40 °C and pH of 6.436 Moreover external magnetic induced heating can control drug release when it is combined with thermal responsive compounds. For a nanobubble-based dual contrast agent consisting of SPIO encapsulated with perfluoropentane, both the US and MR imaging contrast agent could be optimized by varying the shell thickness and SPIO-concentration. In vivo investigations of SPIO-embedded nano-bubbles in excised tumors under external magnetic fields revealed that both the US and MR signals increased. These dual function nano-bubbles could increase drug delivery by increasing the permeability due to HIFU, and external magnetic fields.437,438 In the case of ischemia, due to acidic conditions caused by low aerobic metabolism, the combination of pH responsive and magnetic responsive elements could simultaneously increase the efficacy of drug release as well as monitoring the location and the extent of ischemia.
Reduction-sensitivity can be combined with pH-responsive carriers for intracellular drug release due to the low pH of intracellular compartment, combined with redox sensitivity. Dual sensitivity to pH and redox is an attractive modification of NPs because the pathogenic tissues almost always contain reducing conditions as well as low pH. Oxygen concentration is also very low in the tumor tissue that can serve as a target for hypoxic-responsive carriers.439 Fig. 24 illustrates the process of drug release based on acid/redox responsivity with an OR logic gate. This carrier consisted of a micelle that has minimum drug release at normal pH and normal redox concentration, but the highest drug release in the presence of 10 mM GSH concentration and an acidic environment (pH 5), although both of them can cause some drug release individually.440
Fig. 24.
Dual-responsive DOX release. The lowest amounts of DOX were released in the normal environment (pH: 7 and 0 mM GSH) (black line). Both GSH concentration and acidic conditions can stimulate drug release based on OR gates (red and dark blue lines). The maximum delivery takes place in the presence of both low pH and 10 mM GSH concentration. Reproduced from ref. 440 with permission from the Royal Society of Chemistry.
Each enzyme possesses its own specific pH, co-enzymes, temperature, and site of action. Therefore an enzyme responsive nano-carrier itself intrinsically possesses multi-responsive modes of action. The presence of particular enzymes at the surface of individual cells can allow the uptake of the drug into that cell whereas the presence of an enzyme inside a cell allows drug release inside that cell. Enzymes can also provide reversibility and compatibility to the carrier according to the acting conditions. Besides, the high levels of ROS in cancer cells suggests that the oxidative environment could be a good target for delivery of anticancer drugs. Self-assembled micelles were prepared by the polymerization of the pro-drug benzoyl oxycinnamaldehyde (BCA) to produce PBCAE that was loaded with zinc protoporphyrin (ZnPP). ZnPP can inhibit heme oxygenaze-1 (HO-1) a pro-tumor enzyme that is over-expressed in tumor cells. ZnPP occurs naturally in the body, but when administered as a targeted drug, ZnPP-PBCAE was able to inhibit human tumor growth in a xenograft mouse model.441 Several dual and multi responsive nano-carriers, their method of preparation, drug type, loading efficiency and size are summarized in Table 3.
Table 3.
Some selected dual and multi responsive nano-carriers, their method of preparation, drug, loading efficiency and size
| Type | Name | pH-Sensitive part |
Method of preparation |
Drug conjugates |
Loading efficiency |
Size (nm) |
Ref. |
|---|---|---|---|---|---|---|---|
| Dual thermo/pH responsive | mPEG–NIPAm–MAAm | MAAm | Crosslinked conjugation | Cisplatin (CDDP) | 95 | 130–250 | 442 |
| Dual redox/pH responsive | Amphiphilic thiolated carboxymethyl chitosan | Chitosan | Self-assembly and ultrasonication in DI water | MTX | 43.4 | 160 | 443 |
| Dual glucose/pH responsive | Concanavalin A (Con A)-glucosyloxyethyl methacrylate-(dimethylamino)ethyl-methacrylamide (Con A-E/GEMA/DMAEMA) | DMAEMA | Transesterification | Insulin | 3.5 | 38 µm | 444 |
| Dual magnetic/pH responsive | DOX–Fe3O4@mSiO2-β-thiopropionate–PEG | β-Thiopropionate | Gatekeeper method | DOX | 2.74 | 65 | 445 |
| Dual enzyme/pH responsive | Hyaluronic acid-diethylaminopropyl (HA-g-DEAP) | Protonation of DEAP | Simple chemical reaction | DOX | 82 | 95 | 446 |
| Dual oxidation/pH responsive | Poly(3-benzoyloxy)phenyl(prop-2-ene-1,1-diyl)bio(oxy)bis(ethane-2,1-diyl)diacrylateco-4,4′(trimethylene dipiperidine). Co poly(ethylene glycol)(PBCAE)–ZnPP | PBCAE copolymer | Conjugated polymerization | Zinc protoporphyrin (ZPP) | 5 | 200 | 441 |
| Dual photo/pH responsive | Azobenzene-containing acrylate units | Acrylic acid | Post-polymerization | Nile red | — | 155–200 | 447 |
| Dual thermomagnetic responsive | Poly-N-isopropylacrylamide-chitosan (PAC) | Chitosan | Hydrothermal and solvent evaporation methods | Curcumin | 86 | 20–35 | 448 |
| Multi pH/redox/photo-responsive | Phenylboronicpluronic chitosan spiropyran poly dimethylaminoethylmethacrylic acid (PC–SPMAc) | Pluronic–chitosan | Cross-linked polymerization | Taxol | — | 226–277 | 449 |
| Multi glucose/enzyme/pH responsive | Glucose oxidase-catalase-chitosan nanogel | Chitosan | Two step enzymatic procedure | Insulin | 45 | 12 | 366 |
| Multi thermo/magnetic/pH | P(NIPAAm-co-MAc) coated magnetic MSNs | Methacrylic acid | Precipitation polymerization | DOX | 91.3 | 210 | 450 |
GO has been under intense investigations as a targeted DDS since it possesses a high surface area454 and can be functionalized with targeting ligands. GO nanosheets have been utilized as dual and even multi-stimuli-responsive drug delivery vehicles.147,451–453 A hybrid hydrogel of GO nano-sheets and PNIPAAm was fabricated by Sun and Wu that showed reversible responsiveness to both temperature and pH.455 Shrinkage occurred through heating from 20 to 50 °C and the sample returned to its initial volume upon rapid (10 s) cooling. The pH-responsiveness of the sample was attributed to the existence of residual carboxyl groups in the hydrogel forming hydrogen bonds that were ionized at high pH leading to swelling of the structure.
There have been a few reports of multi- responsive NPs that respond to three or more different stimuli. In the case of these triple-responsive nanocarriers drug release usually acts based on AND gates, although the recognition processes and the assembly of the carrier might be considered similar to the OR gate systems. For example, Qiao et al. bound OEGA to the thermo-sensitive DMDEA and added bis-(2-acryloyloxyethyl) disulfide to cross-link the polymers to produce a thermo/redox/pH tri-responsive nanocarrier. Subsequently DOX or PTX were encapsulated with high loading efficiency giving drug-release in MCF-7 cancer cells.456 Liu et al.457 used polyvinyl alcohol conjugated with PAA and then loaded with Fe2O3 to form a tri-responsive (magnetic/thermo/pH) carrier with an average size of 7.5 nm and good ability to deliver methylene blue into MEL-5 cells. Using a magnetic hyperthermia/pH approach, daunorubicin hydrochloride (DNR) encapsulated microspheres were fabricated with 98% loading capacity, and good drug release under acidic conditions.458
A self-assembled hydrogel formed from GO nanosheets cross-linked in a Pluronic solution was reported by Sahu et al. to be responsive to the triple stimuli of pH, NIR light, and temperature. Its thermo-sensitive gelation occurred at near body temperature and these nanosheets could also trigger photothermal-assisted gel formation by NIR laser irradiation in less than 30 s.459
Combining the optical properties of graphene, Kurapati et al.204 prepared polymeric microcapsule of GO composite with NIR-laser-controlled release of DOX with a power of only 30 mW that raised the temperature of the capsule suspension from 25 °C to 40 °C. Kim et al. achieved photothermally-controlled delivery of DOX, using a functionalized reduced GO. The loaded drug could be released by GSH from the extensive surface of the nanocarrier that could escape the endosome due to the combination of the proton sponge effect and photothermal-assisted endosomal disruption.460
Dual and multi-responsive smart nano-carriers possess the ability to overcome the general difficulties of specific delivery such as prolonged stability, cellular internalization, cellular uptake and intracellular release of the drug. In this regard different types of stimulations including external stimulation for reproducibility of delivery and internal stimulation for self-regulation can be considered. Their use can provide a real and efficient anti-cancer action with good site-specific targeting, specificity, and controlled-release, while lowering cancer multidrug resistance. Stimulation and response can in principle happen at different times and places separately, but almost always they take place simultaneously. For example, in co-delivery of genes and drugs, the issue of the time-gap between the onset of the function of genes (24–72 h for transcription and translation of genetic materials or for suppression of protein expression) and the onset of drug action must be fully addressed before the co-delivery of combination drug/gene therapy becomes an effective treatment.461 As a suggestion for future work in this regard, the issue may be resolved by the new class of smart MNPs which are capable of the sequential release of genes and drugs at optimized time intervals. For this purpose, the use of internal stimuli (such as pH or enzyme activity) and external stimuli (such as temperature or light462) could act as a release-triggering agent for genes and drugs separately. In these conditions, by internalization of MNPs inside the cells and endosomal compartment, the genes are released by an internal stimulus and within 24–72 h the function can be completed, then by exerting external stimuli the release of drugs in a sequential manner will occur.
Here, it is good to mention that the delivery and releasing systems based on OR logic gates have the lowest specificity although they possess good efficiency. That means each stimuli can trigger the response in carrier whereas if carriers be designed based on AND and NOR logic gates, will provide a restricted system for specific delivery and releasing of the drugs.
4 The protein corona effect and nanotoxicology of NPs in biological systems
4.1 Protein corona
A comprehensive understanding of the interactions between NPs and various biological systems has been the goal of numerous studies and previous literature.463 More importantly, the array of nano-bio interactions have to be considered in the design and improvement of MNP based DDSs especially the stimuli-responsive smart systems covered in the present review. Upon exposure of NPs to a biological environment in the form of a fluid rich in proteins and cells such as plasma and blood, the NPs tend to bind to the surrounding biomolecules (mainly proteins and lipids), and interact with them through various adsorption mechanisms to form a new complex surface layer on the NPs. Therefore, the NP surface is covered by these serum proteins including albumin, apolipoproteins, immunoglobulin G (IgG), complement factors, and fibrinogen. This “natural functionalization”464 is known as a biomolecular corona or a protein corona.15,465–467 Lundqvist et al. studied various proteins detected in different coronas. They compared the corona phenomenon as a “fingerprint” encompassing the history, transport pathways, the biological fate, and behavior of the NPs.468 This fingerprint can be utilized to predict the biological responses to different NPs, NP–cell interactions, specific cell associations, etc.469 Also, a large variety of nanomaterials have been shown to be accompanied by this protein corona in biological environments, whether in vitro or in vivo, including polymers,470,471 metallics and ceramics.469,472
The structure of the protein corona surrounding a NP is divided into two compartments: the hard corona (the inner layer with strong binding, long lifetimes of several hours, and a low exchange rate with the environment) and the soft corona (the outer layer with loose protein binding and higher exchange rates).473–475 The protein corona and its diverse effects on NP–cell interactions strongly depends on various physiochemical characteristics of different NPs, and on the cellular components and biological fluids (e.g. types and concentrations of protein).15,476 The particular characteristics of the NPs such as shape, size and surface chemistry are the most important parameters in determining the protein corona.477 Moreover, the chemical modification of the NP surface can significantly change its physicochemical interactions with biological systems.463,468 Fig. 25 shows the protein corona compartments (Fig. 25a), different factors influencing nanobio interactions (Fig. 25b), and TEM images of protein corona formation around NPs (Fig. 25c).
Fig. 25.
(a) Protein corona compartments including the hard corona (inner side) and soft corona (outer side), (b) various factors influencing the nano-bio interactions related to the features of NPs, microenvironment and biomolecules, (c) TEM images of protein corona surrounding Au NPs (10% FBS), BSA corona-coated Au nano-rods (NRs), protein-coated AgNCs (with an ~3.5 nm thick hard corona after 24 h incubation in RPMI-1640 with 1% FBS); c-(left image) reprinted with permission from ref. 478. Copyright 2013, c-(middle image) reprinted with permission from ref. 479. Copyright 2013, and c-(right image) reprinted with permission from ref. 480. Copyright 2014 “American Chemical Society”.
Biological milieus have an intrinsic ability to sense externally induced changes even at the nanoscale in such components of DDS and nanocarriers.481 So, in such biological milieus, the formation of a protein corona has been suggested to cause significant effects on other phenomena and systems and to have been responsible for ambiguous and vague results.15 It is increasingly understood that the interactions of NPs with cells, as well as their mobility and toxicity is controlled by the protein corona and biological characteristics of the NPs.466,482 The role of the protein corona in the process by which cells recognize nanomaterials has given rise to a new concept called “cell vision”.481 The initial contact point between NPs and the cell surface determines the consequent cell response which is very different between the protein coated NP to the pristine NP. In this respect, the type of the NP and the cell type can significantly affect the cell vision.481 Importantly, the definition of cell vision can be applied in the evaluation of the toxicity of the nanomaterials against cells, and various detoxification strategies that different cells employ when facing various nanomaterials, either in vitro or in vivo.467,481 Notably, the protein corona can lead to changes in the pharmacokinetics and pharmacodynamics of the nanomaterials in biological milieus, and therefore can affect their toxicity.483 Therefore, the protein corona formation can potentially be utilized in decreasing the toxicity of NPs in biological milieus.
The protein corona has been reported to have crucial influences on cellular uptake mechanisms, biodistribution, drug release, localization in subcellular organelles and cellular protein substructures (e.g. cytoskeleton).15,467At the molecular scale, it has been suggested that at the NP–cell membrane interface, the hard corona around the NP interacts with cell receptors.474 For example, in one study, reduced drug release was reported by Behzadi et al.484 for various NPs depending on the protein corona. It has been hypothesized that the protein corona could potentially suppress the mutual interactions between the targeting sites on the cell surface and the functionalized ligands on the surface of NPs.15 Recently, it was shown that the protein corona reduced the specificity of the surface modified NPs used for targeting cells within a biological environment through several different mechanisms including: screening active sites of the functionalized targeting ligands by establishing a protein barrier,15 and shielding the therapeutic agent from binding to the targeting sites (e.g. cell receptors) by interaction and by binding to the therapeutic agent in the biological medium.485 So, it is likely that the destructive effect of the protein corona on the targeting efficiency of nanocarriers is considerable.15 In recent studies, the cellular adhesion and uptake of diverse NPs such as quantum dots486 or carboxylated polystyrene (PS–COOH) NPs487 exposed to a protein corona were shown to be reduced in comparison to bare pristine nanomaterials. In rare circumstances, the uptake of NPs by cells in the presence of a protein corona could be enhanced.488 Protein corona formation can also influence the cell internalization mechanisms of NPs489 and lead to altered transduction of cell signals.490
On the other hand, the protein corona phenomenon can be considered as an opportunity in the design of new DDSs. For example, in one study, Au@protein NPDDS were designed to have pH-sensitive properties and showed reversible agglomeration (due to enzymatic degradation in lysosomes at low pH (4–5)) and disagglomeration and increased stability in biological milieus with higher pH (7.4) e.g. cytosol, extracellular matrix, and blood where proteins are present to form a corona.465 Several copolymers with brush compartments showing conformational changes in response to pH changes can reversibly bind to or repel the charged biomolecules (e.g. proteins, anticancer drugs) at different pH values.8
With regard to drug delivery applications it has been shown that protein corona formation could decrease the release of an anticancer drug from MSN nanocarriers. Furthermore, the hydrophobic drugs could be replaced by the molecules present in the dispersion medium through hydrophobic functionalization of MSN pores.491 Hence, to obtain a controlled and enhanced drug release rate, with smart targeting, inhibition of the protein corona formation can be considered a promising strategy. For instance pH-labile polymer linkers could be explored to control the adsorption and attachment of proteins to the NPs in biological media. It may be possible to engineer the detachment and depletion of adsorbed proteins from the NP surface. For example, the cationic charges occurring near cancer cells induced by the acidic condition around them is a great opportunity to build such a pH-triggered delivery system. It can be suggested that another possibility could be to fabricate nanocarriers with the capability to denature and induce aggregation of protein molecules around the NPs to reduce corona formation. Furthermore, different administration routes for the delivery of smart drug/gene carrier systems can be tested. The different proteins the NPs encounter on their journey to the target is expected to depend on their route of administration.
In one study, high resistance to the adsorption of nonspecific serum proteins to the surface of gold NPs as well as a strong pH-sensitive adsorption to the cell membrane in the biological environment was found.492 Through a computational chemistry simulation, it was shown that protein corona formation on positively charged hydrophobic NPs enhanced their cellular interaction with macrophage cells and reduced the targeting to cancer cells at pH 7.4. However at pH 6.5, the protein corona tended to detach and improved cancer cell internalization was shown for a strongly charged NP.493
4.2 Nanotoxicology of NPs in biological systems
Double-checking the safety of new nanomaterials, and evaluating a range of toxicology issues is a prime priority before utilization of nanotechnology in nanomedicine and clinical trials. Recently, the effect of different features of the nanostructures including material, size and shape, as well as the way the biological systems and the cells interact with well-defined nanostructures, and how this affects the clinical and biomedical applications has been discussed in the literature.494 Besides the biological fate of NPs in various biosystems, the nanotoxicology and related toxicity issues such as cytotoxicty,495–497 ecotoxicity498,499 and genotoxicity500,501 are still of crucial importance in nanotechnology.502 Toxicity issues affecting a wide variety of NPs including oxide NPs, MagNPs, metal NPs, quantum dots (QDs), carbon-based nanomaterials, polymeric NPs, and liposomes have all been extensively reviewed.503–510 Many efforts have been conducted to understand the toxicity mechanisms that apply to nanomaterials511 and to reduce or mitigate them.512,513 Moreover, the way the toxicity of nanomaterials depends on their physicochemical properties (e.g. composition, surface area, surface coating, surface charge, shape, size, etc.) has been studied.514–516 However, in de novo biomedical applications, the cytotoxicity of nanomaterials can also be considered as a therapeutic potential and can be utilized in the killing of cancer cells, and the eradication of bacterial cells, etc.517
Different adverse and toxic effects may be caused by nanomaterials including damage to the cell membrane (e.g. oxidative, surfactant or metal ion-mediated damage), induction of apoptosis, disruption of ATP production and DNA replication, lysosomal degradation/disruption, production of ROS, release of radicals, mitochondrial damage, induction of structural alterations of intracellular proteins (e.g. protein misfolding or oxidation), alternation of gene expression, and blood platelet aggregation.510,515,518–520
New strategies for mitigation of various nanomaterial nanotoxicity have been introduced recently. For example, the protein corona formation on the nanomaterial surface in biological environments (e.g. blood plasma) can cause immunotoxicity by affecting various cells of the immune system.481,521 On the other hand the protein corona has been reported to attenuate cytotoxity of NPs against other cell types.464,522,523 Fig. 26 illustrates the protein corona on the surface of GO nanosheets and its effect on cellular viability and cytotoxicity after exposure to A549 cancer cells. Here, the cell viability was enhanced through an FBS protein corona coating on GOs, but was reduced by increasing the GO concentration and the incubation time.522 Some other approaches have been suggested to mitigate the toxicity of NPs toward healthy tissue and cell. These include utilizing NPs with sizes that can undergo hepatobiliary clearance or can be cleared by the renal system (e.g. after intravenous administration), imparting biodegradability to NPs (e.g. polymeric hydrogels, micelles, etc.) or functionalizing the NP surface with biodegradable groups (e.g. PEG), and using auxiliary devices (e.g. medical catheters, microneedles, etc.) to administer the NPs to the targeted organ. Importantly, biodegradable nanosystems can be employed to design stimuli-responsive DDSs taking advantage of pH changes in different organs, or various routes of administration (e.g. intravaginal administration).524 Slight adjustment of the temperature of the cells, tissues or organs can change the toxicity of nanomaterials.525 This should be considered in the design of temperature-responsive DDSs. Different routes of administration of the NP also seem to influence the immune response.526 Charge-dependent biological interactions of NPs have shown to induce diverse responses for different charged NPs. For example, negatively charged NPs have a lower cytotoxicity than positively charged NPs.527 Cationic NPs induce more damage to plasma membrane integrity, mitochondrial and lysosomal compartments, and a higher extent of autophagosomes compared to anionic NPs.518 Size-dependent investigations have mostly shown that smaller NPs can cause more toxicity than larger ones,528 however contrary results have also been reported.529 For example, 10 nm sized silver (Ag) NPs induced higher cytotoxicity compared to those of larger which was attributed to more release of 10 nm Ag NPs.530 The shape dependency of toxicity of nanomaterials has been shown in several studies. High aspect ratio nanomaterials such as fiber-like nanoparticulates and nanorods/nanowires can induce cytotoxicty, lysosomal damage and pro-inflammatory effects.531 Zhao et al.532 studied the toxicity of hydroxyapatite NPs according to their different shapes including needle, sphere, plate, and rod-shaped NPs and showed that the needle and plate-shaped NPs induced high cytotoxicity and cell-death as well as the least particle–cell association, cellular uptake, and internalization.
Fig. 26.
Cytotoxicity and cell viability evaluation of A549 cells treated with GO nanosheets and FBS-coated GO nanosheets via MTT test: (a) AFM images showing GO (left) and FBS-coated GO nanosheets (right), (b) TEM images showing A549 cells treated with GO nanosheets at a concentration of 100 µg mL−1 (left) and FBS-coated GO nanosheets (right) at 37 C for 2 h, (c) cell viability of A549 cells treated with GO nanosheets obtained at various incubation times and concentrations, and (d) cell viability of the A549 cancer cells incubated with 20 µg mL−1 GO nanosheets or FBS-coated GO nanosheets for 2 h at 4 C and 37 °C. Reprinted with permission from ref. 522. Copyright 2011 “American Chemical Society”.
The crucial dispute of nanotoxicology concerning DDSs must be therefore considered.533 In most cases, the toxicity of nanocarriers must be eliminated or drastically reduced before the DDS could be considered for clinical application. In one study, a hybrid chitosan/carrageenan/tripolyphosphate nanocarrier that was tested for transmucosal delivery showed very low toxicity and negligible inflammatory effects on respiratory cell lines.534 However, the possible toxicity of the nanocarrier should be weighed against other considerations (especially for anticancer therapeutics) such as the lower cytotoxicity of the encapsulated drug compared to the free drug, slower cellular uptake of the encapsulated drug, and limited degradability of the nanocarrier after drug release.327 Through the application of novel stimuli-responsive smart nanocarriers such as pH-responsive DDSs, highly toxic anticancer drugs can be released only where they are required while sparing normal tissues and cells.253,258,535 Regarding the necessity of reducing the toxicity of nanocarriers, highly biocompatible materials such as chitosan are becoming widely accepted in pH-responsive DDSs. Biocompatibility and lower toxicity of chitosan capped MSNs in the pH-responsive anticancer DDSs has been recently confirmed.536
5 From concept to the drugstore shelves
Having comprehensively reviewed the basic concepts and the most important mechanisms of the recently developed stimulus-responsive DDSs, it is in fact worth mentioning that in most of the reported studies, the evaluation tests that have been used have only included simple laboratory testing (in vitro, in vivo, or ex vivo). In reality, only a tiny fraction of these DDSs have any realistic possibility (green light) to advance towards clinical trials. We will examine the current status and the latest achievements in this field, and point out those smart MNPs that have been assessed in preclinical animal testing and even in human clinical trials.
The process from drug discovery towards clinical trials is divided into five phases (Fig. 27) corresponding to five levels of testing, each of which has a specific purpose. The final approval for their clinical utilization as a new drug is only issued after these lengthy stages including clinical trials that progress from Phase 1 to Phase 3.537
Fig. 27.
The five stages of drug development culminating in clinical trials reproduced from ref. 537. Copyright 2013 with permission from Elsevier.
As far as clinical medicine is concerned, the complexity of real human systems sometimes produces unexpected outcomes. The reports on the clinical applications of smart MNPs as controlled-release DDSs indicate that most of the late-stage clinical failures can be caused by less than optimal ADME (absorption, distribution, metabolism, and elimination), lack of efficacy, and toxicity issues. Optimization of these factors not only would reduce their potential adverse effects, but could even reduce drug exposure to off-target sites.538 In addition, the complexity of drug production parameters such as quality control, manufacturing process, and reproducibility, further hamper the translation of stimulus-responsive DDSs from preclinical models to clinical applications.539 Hence, many of these achievements in the initial laboratory studies should be further tested in real biological systems, living animals, and in human beings.
In these consecutive stages of testing culminating in clinical drugs, controlling and understanding the behavior of internal stimulus-responsive MNPs, is much more difficult than the same approach to external stimulus-responsive DDSs. In anticancer stimuli-responsive DDSs for instance, the temperature and pH of the target tumor tissue vary from one model (and one patient) to another. In this regard, control of the delivery depth and focused site-specificity by optimizing the parameters of an externally applied stimulus would be more appropriate to trigger drug release from a nanocarrier. While the investigation by Garcia et al.540 of Doxil® in clinical trials in 1998 showed an acceptable toxicity profile for the fabricated drug; while, the release of the bioavailable drug in this system was very slow compared to the free doxorubicin system. Two years later, favorable clinical results including prolonged clinical responses in gynecologic patients were reported,541 while similar studies on combining Doxil with docetaxel542 and topotecan543 failed at the Phase I clinical trials.
In two separate phase III clinical trials, the treatment effects of pegylated liposomal doxorubicin (PLD) were tested on more than 300 patients with breast cancer.544,545 Although in both studies, the rate of progression-free survival between the arms was comparable and also with the overall survival rate, the results showed that the risk of cardiotoxicity was decreased in case of PLD compared to standard DOX therapy. They also demonstrated that PLD reduced the nausea/vomiting, myelosuppression, and alopecia, which are frequent and troublesome side-effects of DOX therapy.
Recent research on gene delivery systems show that viral and non-viral vectors play a significant role in the final success of gene therapy, so that, the expanded knowledge of in vitro and in vivo behavior of vectors is highly important to reduce the failure rate in clinical trials. As a matter of fact, understanding the mechanisms behind the efficiency of transfection can boost the chance of success in gene therapy applications in an exponential manner.
For instance, Stopeck et al. assayed liposome-based vectors in their clinical trials for cancer treatment. Their product (Allovectin-7®, a plasmid/lipid complex) contained a specific DNA molecule that carries the HLA-B7/β2-microglobulin complex. In their successive studies, they could demonstrate a logical transformation process from the formation of complexes and their intracellular delivery and the eventual achievement of acceptable results in patients with metastatic melanoma in both Phase 1 and Phase 2 clinical trials. Nevertheless, in the phase 3 trial where more than 375 patients from 100 clinical sites were enrolled to test Allovectin-7®, the outcome resulted in its failure to meet its efficacy endpoints. Consequently, the clinical trial failed and the program was terminated.546,547
6 Conclusion and future perspective
The design, construction and testing of smart drug and gene delivery vehicles has undergone a veritable explosion of interest in recent years. In some instances it can be reliably asserted that the era of true molecular engineering has arrived. When polymer chemists can construct analogues of everyday objects at the molecular level, such as “snap-top containers” for instance. There are a number of possible motivations for these efforts, in addition to the obvious one of gratifying the scientific ingenuity of the investigators. The first motivation is to overcome the unfortunate side-effects suffered by patients given otherwise highly effective anti-cancer drugs such as DOX. If the drug can be protected inside a vehicle during its journey to its target and only released at its final destination, then these side-effects could be substantially reduced. But how is the vehicle to know that it has arrived at its destination, without a driver to spot the signposts and pull a handle? One answer is to take advantage of physiological cues that are typical of cancer cells and tumors such as reducing conditions, lower pH and over-expression of various enzymes to trigger release of the drug. The same reasoning can be applied to non-viral gene delivery vehicles, where the need is to protect the cargo from degradation by nucleases that are present in normal tissue, to encourage uptake into target cells, and also to allow escape from endosomes so the nucleic acids can reach the nucleus. Another motivation is to produce sustained release of active molecules, particularly in an auto-regulated manner, so that drug is released from its carrier when some biological signal is detected to show that release is needed. An example of this latter concept is the concept of insulin delivery vehicles that release their cargo in response to high tissue glucose concentrations. A third motivation is to take advantage of some externally applied source of physical energy to trigger the release of cargo, such as heat, magnetic fields, light, or US.
Since the individual stimulus-responsive components of the smart-release toolbox have to some extent been reasonably well validated, it is not surprising that investigators have begun to combine them with each other to form dual-responsive vehicles, tri-responsive vehicles and even to consider more than three response elements. Whether these ever-more complex systems provide sufficiently improved delivery parameters to justify the extra effort involved remains to be seen.
An analogy can be drawn between these smart drug delivery vehicles and the advent of driverless automobiles. A few years ago driverless cars were only dreamt of in science fiction, but now the entire automobile industry is anticipating their imminent arrival with the consequent upheaval it will bring. Will we have the same situation in drug delivery say one decade from now? Only time will tell, but we can be sure that there will be no slow-down in scientific efforts to continuously improve smart nanocarrier technology and new advances in triggered drug and gene release.
Acknowledgments
Michael R. Hamblin was supported by US NIH grant R01AI050875.
Biographies

Mahdi Karimi
Mahdi Karimi received his BSc degree from Iran University of Medical Science (IUMS), MSc in Medical Biotechnology from Tabriz University of Medical Science, and PhD from Tarbiat Modares University. He was a visiting researcher in the laboratory of Professor Michael Hamblin at Wellman Center for Photomedicine at Massachusetts General Hospital, Harvard Medical School in 2012. Currently, he is an Assistant Professor in Department of Medical Nanotechnology at IUMS. His current research interests include smart nanoparticles for drug/gene delivery and microfluidic systems. He has established a scientific collaboration between his lab and the Hamblin lab to design smart nanovehicles in drug/gene delivery systems.

Amir Ghasemi
Amir Ghasemi did his BSc at Sharif University of Technology (SUT). He joined the polymeric materials research group in 2012, and received his MSc in Materials Engineering from SUT. For the MSc project, he worked on Thermoplastic Starch (TPS)/Cellulose Nanofiber (CNF) bio-composites, under the supervision of Professor Bagheri. His research interests lie in the area of mechanical properties of biopolymers and polymer composites. He joined the Advanced Nanobiotechnology & Nanomedicine research Group (ANNRG) in 2013. He works on micro/nano materials, and bio-based polymers as drug carriers under the supervision of Professor Karimi and Professor Hamblin from Harvard Medical School.

Parham Sahandi Zangabad
Parham graduated with a BSc from Sahand University of Technology (SUT), Tabriz, Iran, in 2011. He received his MSc in Nanomaterials and Nanotechnology from Sharif University of Technology (SUT), Tehran, Iran. He became a research assistant at the Research Center for Nanostructured and Advanced Materials (RCNAM), Tehran, Iran. The growth of innovative nanomaterials and nanotechnology interested him in interfacial sciences/technologies especially in nanomedicine, including nanoparticle-based drug delivery systems, and nanobiosensors. He has now joined Professor Karimi’s Advanced Nanobiotechnology & Nanomedicine Research Group (ANNRG) in Iran University of Medical Science, in collaboration with Prof. Hamblin from Harvard Medical School.

Reza Rahighi
Reza Rahighi Yazdi joined professor Alimorad Rashidi at Research Institute of Petroleum Industry (RIPI) in 2014 after graduation from Sharif University of Technology where he obtained MSc and BSc in Physics and established Sharif Ultrahigh Nanotechnologists (SUN) company. Graphene practical applications is his scientific favorite, the newly popular flourishing field in which he has authored several publications.

S. Masoud Moosavi Basri
S. M. Moosavi-Basri did his BSc at Persian Gulf University. He conducted his Masters thesis in Biomimetics and Bioinspiration at Shahid Beheshti University in collaboration with Bioenvironmental Research Center at Sharif University. He founded the Bioinspiration group in BRTeam as the first Iranian group which carries out fundamental research in Bionics. He is developing an approach which will lead to obtaining promising results in Systems Biology. Currently he is pursuing his interests in biological disciplines working as a researcher at ANNRG, and pursuing fundamental studies at the Institute for Research in Fundamental Sciences in Tehran, Iran.

Michael R. Hamblin
Michael R Hamblin PhD is a principal investigator at the Wellman Center for Photomedicine, Massachusetts General Hospital, an associate professor of dermatology, Harvard Medical School and affiliated faculty at Harvard – MIT Division of Health Science and Technology. He directs a laboratory of around 12 scientists who work in photodynamic therapy and photobiomodulation. He has published over 300 peer-reviewed articles, is an associate editor for eight journals and serves on NIH study sections. He has edited ten proceedings volumes, together with ten other major textbooks on PDT and photomedicine. In 2011 Dr Hamblin was honored by election as a Fellow of SPIE.
Notes and references
- 1.Qian W-Y, Sun D-M, Zhu R-R, Du X-L, Liu H, Wang S-L. Int. J. Nanomed. 2012;7:5781. doi: 10.2147/IJN.S34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari M. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 4.Karimi M, Avci P, Mobasseri R, Hamblin MR, Naderi-Manesh H. J. Nanopart. Res. 2013;15:1–14. doi: 10.1007/s11051-013-1651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahromi MAM, Karimi M, Azadmanesh K, Manesh HN, Hassan ZM, Moazzeni SM. Comp. Clin. Pathol. 2013:1–7. [Google Scholar]
- 6.Karimi M, Avci P, Ahi M, Gazori T, Hamblin MR, Naderi-Manesh H. J. Nanopharm. Drug Deliv. 2013;1:266–278. doi: 10.1166/jnd.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torchilin VP. Nat. Rev. Drug Discovery. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley EG, Albert JN, Sullivan MO, Epps TH., III Chem. Soc. Rev. 2013;42:7057–7071. doi: 10.1039/c3cs35512h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Fu X, Fu W, Li Z. Chem. Soc. Rev. 2015;44:612–622. doi: 10.1039/c4cs00271g. [DOI] [PubMed] [Google Scholar]
- 10.Cheng R, Meng F, Deng C, Klok H-A, Zhong Z. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 11.Karimi M, Solati N, Amiri M, Mirshekari H, Mohamed E, Taheri M, Hashemkhani M, Saeidi A, Estiar MA, Kiani P. Expert Opin. Drug Delivery. 2015:1–17. doi: 10.1517/17425247.2015.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi M, Solati N, Ghasemi A, Estiar MA, Hashemkhani M, Kiani P, Mohamed E, Saeidi A, Taheri M, Avci P. Expert Opin. Drug Delivery. 2015:1–17. doi: 10.1517/17425247.2015.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Chen L. Nanomedicine. 2011;7:385–402. doi: 10.1016/j.nano.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Xu G, Mahajan S, Roy I, Yong K-T. Front. Pharmacol. 2013;4:140. doi: 10.3389/fphar.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirshafiee V, Mahmoudi M, Lou K, Cheng J, Kraft ML. Chem. Commun. 2013;49:2557–2559. doi: 10.1039/c3cc37307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dávila M, Xian L, Cahangirov S, Rubio A, Le Lay G. New J. Phys. 2014;16:095002. [Google Scholar]
- 17.Lin Y-M, Dimitrakopoulos C, Jenkins KA, Farmer DB, Chiu H-Y, Grill A, Avouris P. Science. 2010;327:662. doi: 10.1126/science.1184289. [DOI] [PubMed] [Google Scholar]
- 18.Schedin F, Geim A, Morozov S, Hill E, Blake P, Katsnelson M, Novoselov K. Nat. Mater. 2007;6:652–655. doi: 10.1038/nmat1967. [DOI] [PubMed] [Google Scholar]
- 19.Akhavan O, Ghaderi E, Rahighi R. ACS Nano. 2012;6:2904–2916. doi: 10.1021/nn300261t. [DOI] [PubMed] [Google Scholar]
- 20.Justin R, Chen B. J. Mater. Chem. B. 2014;2:3759–3770. doi: 10.1039/c4tb00390j. [DOI] [PubMed] [Google Scholar]
- 21.Nourmohammadi A, Rahighi R, Akhavan O, Moshfegh A. J. Alloys Compd. 2014;612:380–385. [Google Scholar]
- 22.Qiu Y, Yan K, Yang S, Jin L, Deng H, Li W. ACS Nano. 2010;4:6515–6526. doi: 10.1021/nn101603g. [DOI] [PubMed] [Google Scholar]
- 23.Fan W, Lai Q, Zhang Q, Wang Y. J. Phys. Chem. C. 2011;115:10694–10701. [Google Scholar]
- 24.Liu F, Zhang Y, Yu J, Wang S, Ge S, Song X. Biosens. Bioelectron. 2014;51:413–420. doi: 10.1016/j.bios.2013.07.066. [DOI] [PubMed] [Google Scholar]
- 25.Mao HY, Laurent S, Chen W, Akhavan O, Imani M, Ashkarran AA, Mahmoudi M. Chem. Rev. 2013;113:3407–3424. doi: 10.1021/cr300335p. [DOI] [PubMed] [Google Scholar]
- 26.Some S, Gwon A-R, Hwang E, Bahn G-h, Yoon Y, Kim Y, Kim S-H, Bak S, Yang J, Jo D-G. Sci. Rep. 2014;4 doi: 10.1038/srep06314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhavan O, Ghaderi E, Shirazian SA, Rahighi R. Carbon. 2016;97:71–77. [Google Scholar]
- 28.Moradi S, Akhavan O, Tayyebi A, Rahighi R, Mohammadzadeh M, Rad HS. RSC Adv. 2015;5:47529–47537. [Google Scholar]
- 29.Fazaeli Y, Akhavan O, Rahighi R, Aboudzadeh MR, Karimi E, Afarideh H. Mater. Sci. Eng., C. 2014;45:196–204. doi: 10.1016/j.msec.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Akhavan O, Ghaderi E, Shirazian SA. Colloids Surf., B. 2014;126:313–321. doi: 10.1016/j.colsurfb.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Hashemi E, Akhavan O, Shamsara M, Valimehr S, Rahighi R. RSC Adv. 2014;4:60720–60728. [Google Scholar]
- 32.Li D, Müller MB, Gilje S, Kaner RB, Wallace GG. Nat. Nanotechnol. 2008;3:101–105. doi: 10.1038/nnano.2007.451. [DOI] [PubMed] [Google Scholar]
- 33.Jeon I-Y, Choi H-J, Choi M, Seo J-M, Jung S-M, Kim M-J, Zhang S, Zhang L, Xia Z, Dai L. Sci. Rep. 2013;3 doi: 10.1038/srep01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geim AK, Novoselov KS. Nat. Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 35.Koh YK, Bae M-H, Cahill DG, Pop E. Nano Lett. 2010;10:4363–4368. doi: 10.1021/nl101790k. [DOI] [PubMed] [Google Scholar]
- 36.Akhavan O, Ghaderi E. Small. 2013;9:3593–3601. doi: 10.1002/smll.201203106. [DOI] [PubMed] [Google Scholar]
- 37.Akhavan O, Ghaderi E, Akhavan A. Biomaterials. 2012;33:8017–8025. doi: 10.1016/j.biomaterials.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 38.Akhavan O, Hashemi E, Shamsara M, Rahighi R, Esfandiar A, Tayefeh AR. RSC Adv. 2014;4:27213–27223. [Google Scholar]
- 39.Servant A, Leon V, Jasim D, Methven L, Limousin P, Fernandez-Pacheco EV, Prato M, Kostarelos K. Adv. Healthcare Mater. 2014;3:1334–1343. doi: 10.1002/adhm.201400016. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Song L, Zhang Z, Chen N, Qu L. Small. 2013;10:2151–2164. doi: 10.1002/smll.201303080. [DOI] [PubMed] [Google Scholar]
- 41.Oyewumi MO, Kumar A, Cui Z. Expert Rev. Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monfardini C, Veronese FM. Bioconjugate Chem. 1998;9:418–450. doi: 10.1021/bc970184f. [DOI] [PubMed] [Google Scholar]
- 43.Cho K, Wang X, Nie S, Shin DM. Clin. Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 44.Lavan DA, McGuire T, Langer R. Nat. Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 45.Akhavan O, Ghaderi E, Rahighi R, Abdolahad M. Carbon. 2014;79:654–663. [Google Scholar]
- 46.Akhavan O, Ghaderi E, Hashemi E, Rahighi R. Nanoscale. 2014;6:14810–14819. doi: 10.1039/c4nr04589k. [DOI] [PubMed] [Google Scholar]
- 47.Liu Yin J. Best Pract. Res., Clin. Haematol. 2002;15:119–135. doi: 10.1053/beha.2002.0188. [DOI] [PubMed] [Google Scholar]
- 48.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Bies C, Lehr C-M, Woodley JF. Adv. Drug Delivery Rev. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 50.Saito G, Swanson JA, Lee K-D. Adv. Drug Delivery Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 51.Inaji H, Koyama H, Higashiyama M, Noguchi S, Yamamoto H, Ishikawa O, Omichi K, Iwanaga T, Wada A. Virchows Arch. A: Pathol. Anat. Histopathol. 1991;419:29–33. doi: 10.1007/BF01600149. [DOI] [PubMed] [Google Scholar]
- 52.Vaupel P, Kallinowski F, Okunieff P. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 53.Ruel-Gariépy E, Leroux J-C. Eur. J. Pharm. Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Caneba G. Free-Radical Retrograde-Precipitation Polymerization (FRRPP): Novel Concepts, Processes, Materials, and Energy Aspects. Vol. 1. Berlin Heidelberg: Springer-Verlag; 2010. ISBN 978-3-642-03024-6. [Google Scholar]
- 55.Qiu Y, Park K. Adv. Drug Delivery Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 56.Schmaljohann D. Adv. Drug Delivery Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 57.Fitzpatrick SD, Fitzpatrick LE, Thakur A, Mazumder MAJ, Sheardown H. Expert Rev. Med. Devices. 2012;9:339–351. doi: 10.1586/erd.12.24. [DOI] [PubMed] [Google Scholar]
- 58.Coughlan D, Corrigan O. J. Pharm. Sci. 2008;97:318–330. doi: 10.1002/jps.21095. [DOI] [PubMed] [Google Scholar]
- 59.Yan H, Okuzaki H. Compos. Interfaces. 2008;15:661–670. [Google Scholar]
- 60.Beija M, Marty J-D, Destarac M. Chem. Commun. 2011;47:2826–2828. doi: 10.1039/c0cc05184e. [DOI] [PubMed] [Google Scholar]
- 61.Kono K, Henmi A, Yamashita H, Hayashi H, Takagishi T. J. Controlled Release. 1999;59:63–75. doi: 10.1016/s0168-3659(98)00180-1. [DOI] [PubMed] [Google Scholar]
- 62.Fu G, Soboyejo W. Mater. Sci. Eng., C. 2010;30:8–13. [Google Scholar]
- 63.Croissant J, Zink JI. J. Am. Chem. Soc. 2012;134:7628–7631. doi: 10.1021/ja301880x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okahata Y, Noguchi H, Seki T. Macromolecules. 1986;19:493–494. [Google Scholar]
- 65.Lue SJ, Hsu J-J, Wei T-C. J. Membr. Sci. 2008;321:146–154. [Google Scholar]
- 66.Lue SJ, Chen C-H, Shih C-M, Tsai M-C, Kuo C-Y, Lai J-Y. J. Membr. Sci. 2011;379:330–340. [Google Scholar]
- 67.Men K, Liu W, Li L, Duan X, Wang P, Gou M, Wei X, Gao X, Wang B, Du Y. Nanoscale. 2012;4:6425–6433. doi: 10.1039/c2nr31592k. [DOI] [PubMed] [Google Scholar]
- 68.Ballauff M, Lu Y. Polymer. 2007;48:1815–1823. [Google Scholar]
- 69.Crassous JJ, Siebenbürger M, Ballauff M, Drechsler M, Henrich O, Fuchs M. J. Chem. Phys. 2006;125:204906. doi: 10.1063/1.2374886. [DOI] [PubMed] [Google Scholar]
- 70.Picos-Corrales LA, Licea-Claveríe A, Arndt K-F. React. Funct. Polym. 2014;75:31–40. [Google Scholar]
- 71.Luo Y-L, Yang X-L, Xu F, Chen Y-S, Zhang B. Colloids Surf., B. 2014;114:150–157. doi: 10.1016/j.colsurfb.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 72.Reinicke KE, Kuffel MJ, Goetz MP, Ames MM. Cancer Chemother. Pharmacol. 2010;66:575–583. doi: 10.1007/s00280-009-1198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akiyama H, Tamaoki N. Macromolecules. 2007;40:5129–5132. [Google Scholar]
- 74.Kim DH, Guo Y, Zhang Z, Procissi D, Nicolai J, Omary RA, Larson AC. Adv. Healthcare Mater. 2014;3:714–724. doi: 10.1002/adhm.201300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wischerhoff E, Uhlig K, Lankenau A, Börner HG, Laschewsky A, Duschl C, Lutz JF. Angew. Chem., Int. Ed. 2008;47:5666–5668. doi: 10.1002/anie.200801202. [DOI] [PubMed] [Google Scholar]
- 76.Na K, Lee KH, Lee DH, Bae YH. Eur. J. Pharm. Sci. 2006;27:115–122. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Croissant J, Maynadier M, Gallud A, Peindy N’Dongo H, Nyalosaso JL, Derrien G, Charnay C, Durand JO, Raehm L, Serein-Spirau F. Angew. Chem. 2013;125:14058–14062. doi: 10.1002/anie.201308647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daniel-da-Silva AL, Ferreira L, Gil AM, Trindade T. J. Colloid Interface Sci. 2011;355:512–517. doi: 10.1016/j.jcis.2010.12.071. [DOI] [PubMed] [Google Scholar]
- 79.Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Carbohydr. Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 80.Muzzarelli RA, Morganti P, Morganti G, Palombo P, Palombo M, Biagini G, Mattioli Belmonte M, Giantomassi F, Orlandi F, Muzzarelli C. Carbohydr. Polym. 2007;70:274–284. [Google Scholar]
- 81.Moerkerke R, Meeussen F, Koningsveld R, Berghmans H, Mondelaers W, Schacht E, Dušek K, Šolc K. Macromolecules. 1998;31:2223–2229. [Google Scholar]
- 82.Negru I, Teodorescu M, Stanescu PO, Draghici C, Lungu A, Sarbu A. Mater. Plast. 2010;47:35. [Google Scholar]
- 83.Rueda J, Zschoche S, Komber H, Schmaljohann D, Voit B. Macromolecules. 2005;38:7330–7336. [Google Scholar]
- 84.Suwa K, Morishita K, Kishida A, Akashi M. J. Polym. Sci., Part A: Polym. Chem. 1997;35:3087–3094. [Google Scholar]
- 85.Wu J, Wei W, Wang L-Y, Su Z-G, Ma G-H. Biomaterials. 2007;28:2220–2232. doi: 10.1016/j.biomaterials.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 86.Elluru M, Ma H, Hadjiargyrou M, Hsiao BS, Chu B. Polymer. 2013;54:2088–2095. [Google Scholar]
- 87.Xiong W, Wang W, Wang Y, Zhao Y, Chen H, Xu H, Yang X. Colloids Surf., B. 2011;84:447–453. doi: 10.1016/j.colsurfb.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 88.Aoki T, Kawashima M, Katono H, Sanui K, Ogata N, Okano T, Sakurai Y. Macromolecules. 1994;27:947–952. [Google Scholar]
- 89.Chen K-J, Liang H-F, Chen H-L, Wang Y, Cheng P-Y, Liu H-L, Xia Y, Sung H-W. ACS Nano. 2012;7:438–446. doi: 10.1021/nn304474j. [DOI] [PubMed] [Google Scholar]
- 90.Ta T, Bartolak-Suki E, Park E-J, Karrobi K, McDannold NJ, Porter TM. J. Controlled Release. 2014;194:71–81. doi: 10.1016/j.jconrel.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, Kostarelos K. ACS Nano. 2012;6:9335–9346. doi: 10.1021/nn302148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Elk M, Deckers R, Oerlemans C, Shi Y, Storm G, Vermonden T, Hennink WE. Biomacromolecules. 2014;15:1002–1009. doi: 10.1021/bm401904u. [DOI] [PubMed] [Google Scholar]
- 93.Lee Y, Park SY, Kim C, Park TG. J. Controlled Release. 2009;135:89–95. doi: 10.1016/j.jconrel.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 94.Peng Z, Fang E, Wang C, Lu X, Wang G, Tong Q. J. Nanosci. Nanotechnol. 2015;15:3823–3833. doi: 10.1166/jnn.2015.9281. [DOI] [PubMed] [Google Scholar]
- 95.Herron C, O’Neill H, Lopez-Noreiga A, Hastings C, Duffy G, McDonnell C. International Journal of Surgery. 2015;18:240–241. [Google Scholar]
- 96.Liu R, Sun L, Fang S, Wang S, Chen J, Xiao X, Liu C. Pharm. Dev. Technol. 2015:1–7. doi: 10.3109/10837450.2015.1026607. [DOI] [PubMed] [Google Scholar]
- 97.Salmon D, Roussel L, Gilbert E, Kirilov P, Pirot F. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Springer; 2015. pp. 315–328. [Google Scholar]
- 98.Caramella CM, Rossi S, Ferrari F, Bonferoni MC, Sandri G. Adv. Drug Delivery Rev. 2015;92:39–52. doi: 10.1016/j.addr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 99.Sung B, Kim C, Kim M-H. J. Colloid Interface Sci. 2015;450:26–33. doi: 10.1016/j.jcis.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 100.Seo B-B, Choi H, Koh J-T, Song S-C. J. Controlled Release. 2015;209:67–76. doi: 10.1016/j.jconrel.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 101.Witting M, Molina M, Obst K, Plank R, Eckl KM, Hennies HC, Calderón M, Frieß W, Hedtrich S. Nanomedicine. 2015 doi: 10.1016/j.nano.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 102.Lu C, Liu M, Fu H, Zhang W, Peng G, Zhang Y, Cao H, Luo L. Eur. J. Pharm. Sci. 2015;77:24–28. doi: 10.1016/j.ejps.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 103.Sinha SR, Nguyen LP, Inayathullah M, Malkovskiy A, Habte F, Rajadas J, Habtezion A. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosca EV, Wright M, Gonitel R, Gedroyc W, Miller AD, Thanou M. Mol. Pharmaceutics. 2015;12:1335–1346. doi: 10.1021/mp5002679. [DOI] [PubMed] [Google Scholar]
- 105.Dicheva BM, ten Hagen TL, Seynhaeve AL, Amin M, Eggermont AM, Koning GA. Pharm. Res. 2015:1–15. doi: 10.1007/s11095-015-1746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quan S, Wang Y, Zhou A, Kumar P, Narain R. Biomacromolecules. 2015;16:1978–1986. doi: 10.1021/acs.biomac.5b00576. [DOI] [PubMed] [Google Scholar]
- 107.Kamimura K, Suda T, Zhang G, Liu D. Pharm. Med. 2011;25:293–306. doi: 10.2165/11594020-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pişskin E, Dincer S, Türk M. J. Biomater. Sci., Polym. Ed. 2004;15:1181–1202. doi: 10.1163/1568562041753016. [DOI] [PubMed] [Google Scholar]
- 109.Dincer S, Türk M, Pişkin E. Gene Ther. 2005;12:S139–S145. doi: 10.1038/sj.gt.3302628. [DOI] [PubMed] [Google Scholar]
- 110.Stayton PS, Shimoboji T, Long C, Chilkoti A, Ghen G, Harris JM, Hoffman AS. Nature. 1995;378:472–474. doi: 10.1038/378472a0. [DOI] [PubMed] [Google Scholar]
- 111.Kurisawa M, Yokoyama M, Okano T. J. Controlled Release. 2000;69:127–137. doi: 10.1016/s0168-3659(00)00297-2. [DOI] [PubMed] [Google Scholar]
- 112.Kurisawa M, Yokoyama M, Okano T. J. Controlled Release. 2000;68:1–8. doi: 10.1016/s0168-3659(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 113.Chen S-Y, Hu S-H, Liu T-Y. Smart Materials for Drug Delivery. 2013;2:32. [Google Scholar]
- 114.Whitesides GM. Nat. Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 115.Shubayev VI, Pisanic TR, II, Jin S. Adv. Drug Delivery Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Freeman M, Arrott A, Watson J. J. Appl. Phys. 1960;31:S404–S405. [Google Scholar]
- 117.Thévenot J, Oliveira H, Sandre O, Lecommandoux S. Chem. Soc. Rev. 2013;42:7099–7116. doi: 10.1039/c3cs60058k. [DOI] [PubMed] [Google Scholar]
- 118.Louguet S, Rousseau B, Epherre R, Guidolin N, Goglio G, Mornet S, Duguet E, Lecommandoux S, Schatz C. Polym. Chem. 2012;3:1408–1417. [Google Scholar]
- 119.Koppolu B, Bhavsar Z, Wadajkar AS, Nattama S, Rahimi M, Nwariaku F, Nguyen KT. J. Biomed. Nanotechnol. 2012;8:983–990. doi: 10.1166/jbn.2012.1465. [DOI] [PubMed] [Google Scholar]
- 120.Hoare T, Timko BP, Santamaria J, Goya GF, Irusta S, Lau S, Stefanescu CF, Lin D, Langer R, Kohane DS. Nano Lett. 2011;11:1395–1400. doi: 10.1021/nl200494t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kakwere H, Leal MP, Materia ME, Curcio A, Guardia P, Niculaes D, Marotta R, Falqui A, Pellegrino T. ACS Appl. Mater. Interfaces. 2015;7:10132–10145. doi: 10.1021/am5088117. [DOI] [PubMed] [Google Scholar]
- 122.Schexnailder P, Schmidt G. Colloid Polym. Sci. 2009;287:1–11. [Google Scholar]
- 123.Hayashi K, Ono K, Suzuki H, Sawada M, Moriya M, Sakamoto W, Yogo T. ACS Appl. Mater. Interfaces. 2010;2:1903–1911. doi: 10.1021/am100237p. [DOI] [PubMed] [Google Scholar]
- 124.Giani G, Fedi S, Barbucci R. Polymers. 2012;4:1157–1169. [Google Scholar]
- 125.Jordan A, Wust P, Fähling H, John W, Hinz A, Felix R. Int. J. Hyperthermia. 2009;25:499–511. doi: 10.3109/02656730903287790. [DOI] [PubMed] [Google Scholar]
- 126.Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T. J. Biosci. Bioeng. 2003;96:364–369. doi: 10.1016/S1389-1723(03)90138-1. [DOI] [PubMed] [Google Scholar]
- 127.Brulé S, Levy M, Wilhelm C, Letourneur D, Gazeau F, Ménager C, Le Visage C. Adv. Mater. 2011;23:787–790. doi: 10.1002/adma.201003763. [DOI] [PubMed] [Google Scholar]
- 128.Marguet M, Bonduelle C, Lecommandoux S. Chem. Soc. Rev. 2013;42:512–529. doi: 10.1039/c2cs35312a. [DOI] [PubMed] [Google Scholar]
- 129.Sanson C, Diou O, Thevenot J, Ibarboure E, Soum A, Brûlet A, Miraux S, Thiaudière E, Tan S, Brisson A. ACS Nano. 2011;5:1122–1140. doi: 10.1021/nn102762f. [DOI] [PubMed] [Google Scholar]
- 130.Hu SH, Liao BJ, Chiang CS, Chen PJ, Chen IW, Chen SY. Adv. Mater. 2012;24:3627–3632. doi: 10.1002/adma.201201251. [DOI] [PubMed] [Google Scholar]
- 131.Deok Kong S, Sartor M, Jack Hu C-M, Zhang W, Zhang L, Jin S. Acta Biomater. 2013;9:5447–5452. doi: 10.1016/j.actbio.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh RK, Patel KD, Kim J-J, Kim T-H, Kim J-H, Shin US, Lee E-J, Knowles JC, Kim H-W. ACS Appl. Mater. Interfaces. 2014;6:2201–2208. doi: 10.1021/am4056936. [DOI] [PubMed] [Google Scholar]
- 133.Mah C, Fraites TJ, Zolotukhin I, Song S, Flotte TR, Dobson J, Batich C, Byrne BJ. Mol. Ther. 2002;6:106–112. doi: 10.1006/mthe.2001.0636. [DOI] [PubMed] [Google Scholar]
- 134.Yiu HH, McBain SC, Lethbridge ZA, Lees MR, Palona I, Olariu CI, Dobson J. J. Nanosci. Nanotechnol. 2011;11:3586–3591. doi: 10.1166/jnn.2011.3615. [DOI] [PubMed] [Google Scholar]
- 135.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Small. 2010;6:1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruiz-Hernandez E, Baeza A, Vallet-Regí Ma. ACS Nano. 2011;5:1259–1266. doi: 10.1021/nn1029229. [DOI] [PubMed] [Google Scholar]
- 137.Lee HJ, Bae Y. Biomacromolecules. 2011;12:2686–2696. doi: 10.1021/bm200483t. [DOI] [PubMed] [Google Scholar]
- 138.Torchilin V. Adv. Drug Delivery Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 139.Fang J, Nakamura H, Maeda H. Adv. Drug Delivery Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 140.Scott D, Beabout Y, Wydra RJ, Dan M, Yokel R, Hilt JZ, Bae Y. J. Appl. Pharm. Sci. 2013;3:021–028. [Google Scholar]
- 141.van Landeghem FK, Maier-Hauff K, Jordan A, Hoffmann K-T, Gneveckow U, Scholz R, Thiesen B, Brück W, Von Deimling A. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 142.Huang HS, Hainfield JF. Int. J. Nanomed. 2013;8:2521. doi: 10.2147/IJN.S43770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.da Silva EP, Sitta DL, Fragal VH, Cellet TS, Mauricio MR, Garcia FP, Nakamura CV, Guilherme MR, Rubira AF, Kunita MH. Int. J. Biol. Macromol. 2014;67:43–52. doi: 10.1016/j.ijbiomac.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 144.Anal AK. Recent Pat. Endocr., Metab. Immune Drug Discovery. 2007;1:83–90. [Google Scholar]
- 145.Qiu Y, Park K. Adv. Drug Delivery Rev. 2012;64:49–60. [Google Scholar]
- 146.Ying X, Wang Y, Liang J, Yue J, Xu C, Lu L, Xu Z, Gao J, Du Y, Chen Z. Angew. Chem., Int. Ed. 2014;53:12436–12440. doi: 10.1002/anie.201403846. [DOI] [PubMed] [Google Scholar]
- 147.Weaver CL, LaRosa JM, Luo X, Cui XT. ACS Nano. 2014;8:1834–1843. doi: 10.1021/nn406223e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Servant A, Leon V, Jasim D, Methven L, Limousin P, Fernandez-Pacheco EV, Prato M, Kostarelos K. Adv. Healthcare Mater. 2014;3:1334–1343. doi: 10.1002/adhm.201400016. [DOI] [PubMed] [Google Scholar]
- 149.Servant A, Methven L, Williams RP, Kostarelos K. Adv. Healthcare Mater. 2013;2:806–811. doi: 10.1002/adhm.201200193. [DOI] [PubMed] [Google Scholar]
- 150.Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. Nat. Commun. 2013;4:1707. doi: 10.1038/ncomms2717. [DOI] [PubMed] [Google Scholar]
- 151.Im JS, Bai BC, Lee Y-S. Biomaterials. 2010;31:1414–1419. doi: 10.1016/j.biomaterials.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 152.Ge J, Neofytou E, Cahill TJ, III, Beygui RE, Zare RN. ACS Nano. 2011;6:227–233. doi: 10.1021/nn203430m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alatorre-Meda M. Smart Materials for Drug Delivery. 2013;1:304. [Google Scholar]
- 154.Knežević NŽ, Trewyn BG, Lin VS-Y. Chem. Commun. 2011;47:2817–2819. doi: 10.1039/c0cc04424e. [DOI] [PubMed] [Google Scholar]
- 155.Yi Q, Sukhorukov GB. Adv. Colloid Interface Sci. 2013;207:280–289. doi: 10.1016/j.cis.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 156.Babin J, Pelletier M, Lepage M, Allard JF, Morris D, Zhao Y. Angew. Chem., Int. Ed. 2009;48:3329–3332. doi: 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]
- 157.Karthik S, Saha B, Ghosh SK, Singh NP. Chem. Commun. 2013;49:10471–10473. doi: 10.1039/c3cc46078a. [DOI] [PubMed] [Google Scholar]
- 158.Lo C-W, Zhu D, Jiang H. Soft Matter. 2011;7:5604–5609. [Google Scholar]
- 159.Luo Y-L, Shiao Y-S, Huang Y-F. ACS Nano. 2011;5:7796–7804. doi: 10.1021/nn201592s. [DOI] [PubMed] [Google Scholar]
- 160.Wu P, Gao Y, Lu Y, Zhang H, Cai C. Analyst. 2013;138:6501–6510. doi: 10.1039/c3an01375h. [DOI] [PubMed] [Google Scholar]
- 161.Huang X, Teng X, Chen D, Tang F, He J. Biomaterials. 2010;31:438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 162.Moghimi SM, Hunter AC, Murray JC. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 163.Alvarez-Lorenzo C, Deshmukh S, Bromberg L, Hatton TA, Sández-Macho I, Concheiro A. Langmuir. 2007;23:11475–11481. doi: 10.1021/la7019654. [DOI] [PubMed] [Google Scholar]
- 164.Jiang J, Qi B, Lepage M, Zhao Y. Macromolecules. 2007;40:790–792. [Google Scholar]
- 165.Zhao H, Sterner ES, Coughlin EB, Theato P. Macromolecules. 2012;45:1723–1736. [Google Scholar]
- 166.Yan B, Boyer J-C, Branda NR, Zhao Y. J. Am. Chem. Soc. 2011;133:19714–19717. doi: 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- 167.Chung JW, Lee K, Neikirk C, Nelson CM, Priestley RD. Small. 2012;8:1693–1700. doi: 10.1002/smll.201102263. [DOI] [PubMed] [Google Scholar]
- 168.Azagarsamy MA, Anseth KS. Angew. Chem., Int. Ed. 2013;52:13803–13807. doi: 10.1002/anie.201308174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu G, Dong C-M. Biomacromolecules. 2012;13:1573–1583. doi: 10.1021/bm300304t. [DOI] [PubMed] [Google Scholar]
- 170.Liu G-Y, Chen C-J, Li D-D, Wang S-S, Ji J. J. Mater. Chem. 2012;22:16865–16871. [Google Scholar]
- 171.Chen C, Liu G, Liu X, Pang S, Zhu C, Lv L, Ji J. Polym. Chem. 2011;2:1389–1397. [Google Scholar]
- 172.Tian F, Yu Y, Wang C, Yang S. Macromolecules. 2008;41:3385–3388. [Google Scholar]
- 173.Tangso KJ, Fong W-K, Darwish T, Kirby N, Boyd BJ, Hanley TL. J. Phys. Chem. B. 2013;117:10203–10210. doi: 10.1021/jp403840m. [DOI] [PubMed] [Google Scholar]
- 174.Son S, Shin E, Kim B-S. Biomacromolecules. 2014;15:628–634. doi: 10.1021/bm401670t. [DOI] [PubMed] [Google Scholar]
- 175.Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI, Nel AE. ACS Nano. 2011;5:4131–4144. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Knezevic NZ, Trewyn BG, Lin VS. Chem. Commun. 2011;47:2817–2819. doi: 10.1039/c0cc04424e. [DOI] [PubMed] [Google Scholar]
- 177.Liu Q, Sun Y, Yang T, Feng W, Li C, Li F. J. Am. Chem. Soc. 2011;133:17122–17125. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]
- 178.He S, Krippes K, Ritz S, Chen Z, Best A, Butt H-J, Mailänder V, Wu S. Chem. Commun. 2015;51:431–434. doi: 10.1039/c4cc07489k. [DOI] [PubMed] [Google Scholar]
- 179.Viger ML, Grossman M, Fomina N, Almutairi A. Adv. Mater. 2013;25:3733–3738. doi: 10.1002/adma.201300902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li S, Moosa BA, Croissant JG, Khashab NM. Angew. Chem. 2015;127:6908–6912. doi: 10.1002/anie.201501615. [DOI] [PubMed] [Google Scholar]
- 181.Álvarez M, Best A, Pradhan-Kadam S, Koynov K, Jonas U, Kreiter M. Adv. Mater. 2008;20:4563–4567. [Google Scholar]
- 182.Gary-Bobo M, Mir Y, Rouxel C, Brevet D, Basile I, Maynadier M, Vaillant O, Mongin O, Blanchard-Desce M, Morère A. Angew. Chem. 2011;123:11627–11631. doi: 10.1002/anie.201104765. [DOI] [PubMed] [Google Scholar]
- 183.Kim S, Ohulchanskyy TY, Pudavar HE, Pandey RK, Prasad PN. J. Am. Chem. Soc. 2007;129:2669–2675. doi: 10.1021/ja0680257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R. FEMS Microbiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Croissant J, Chaix A, Mongin O, Wang M, Clément S, Raehm L, Durand JO, Hugues V, Blanchard-Desce M, Maynadier M. Small. 2014;10:1752–1755. doi: 10.1002/smll.201400042. [DOI] [PubMed] [Google Scholar]
- 186.Graf N, Lippard SJ. Adv. Drug Delivery Rev. 2012;64:993–1004. doi: 10.1016/j.addr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ruggiero E, Hernández-Gil J, Mareque-Rivas JC, Salassa L. Chem. Commun. 2015;51:2091–2094. doi: 10.1039/c4cc07960d. [DOI] [PubMed] [Google Scholar]
- 188.Xiao H, Noble GT, Stefanick JF, Qi R, Kiziltepe T, Jing X, Bilgicer B. J. Controlled Release. 2014;173:11–17. [PubMed] [Google Scholar]
- 189.Infante I, Azpiroz JM, Blanco NG, Ruggiero E, Ugalde JM, Mareque-Rivas JC, Salassa L. J. Phys. Chem. C. 2014;118:8712–8721. [Google Scholar]
- 190.Fan NC, Cheng FY, Ho JaA, Yeh CS. Angew. Chem., Int. Ed. 2012;51:8806–8810. doi: 10.1002/anie.201203339. [DOI] [PubMed] [Google Scholar]
- 191.Schroeder A, Goldberg MS, Kastrup C, Wang Y, Jiang S, Joseph BJ, Levins CG, Kannan ST, Langer R, Anderson DG. Nano Lett. 2012;12:2685–2689. doi: 10.1021/nl2036047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mizukami S, Hosoda M, Satake T, Okada S, Hori Y, Furuta T, Kikuchi K. J. Am. Chem. Soc. 2010;132:9524–9525. doi: 10.1021/ja102167m. [DOI] [PubMed] [Google Scholar]
- 193.Baffou G, Quidant R. Laser Photonics Rev. 2013;7:171–187. [Google Scholar]
- 194.Fang J, Chen Y-C. Curr. Pharm. Des. 2013;19:6622–6634. doi: 10.2174/1381612811319370006. [DOI] [PubMed] [Google Scholar]
- 195.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Lasers Med. Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 197.Jaque D, Maestro LM, Del Rosal B, Haro-Gonzalez P, Benayas A, Plaza J, Rodríguez EM, Solé JG. Nanoscale. 2014;6:9494–9530. doi: 10.1039/c4nr00708e. [DOI] [PubMed] [Google Scholar]
- 198.Robinson JT, Tabakman SM, Liang Y, Wang H, Sanchez Casalongue H, Vinh D, Dai H. J. Am. Chem. Soc. 2011;133:6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 199.Khan SA, Kanchanapally R, Fan Z, Beqa L, Singh AK, Senapati D, Ray PC. Chem. Commun. 2012;48:6711–6713. doi: 10.1039/c2cc32313c. [DOI] [PubMed] [Google Scholar]
- 200.Hu SH, Fang RH, Chen YW, Liao BJ, Chen IW, Chen SY. Adv. Funct. Mater. 2014;24:4144–4155. [Google Scholar]
- 201.Xiong W, Mazid R, Yap LW, Li X, Cheng W. Nanoscale. 2014;6:14388–14393. doi: 10.1039/c4nr04400b. [DOI] [PubMed] [Google Scholar]
- 202.Lin J, Wang S, Huang P, Wang Z, Chen S, Niu G, Li W, He J, Cui D, Lu G. ACS Nano. 2013;7:5320–5329. doi: 10.1021/nn4011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen R, Wang X, Yao X, Zheng X, Wang J, Jiang X. Biomaterials. 2013;34:8314–8322. doi: 10.1016/j.biomaterials.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 204.Kurapati R, Raichur AM. Chem. Commun. 2013;49:734–736. doi: 10.1039/c2cc38417e. [DOI] [PubMed] [Google Scholar]
- 205.Chang YT, Liao PY, Sheu HS, Tseng YJ, Cheng FY, Yeh CS. Adv. Mater. 2012;24:3309–3314. doi: 10.1002/adma.201200785. [DOI] [PubMed] [Google Scholar]
- 206.Norum OJ, Selbo PK, Weyergang A, Giercksky KE, Berg K. J. Photochem. Photobiol., B. 2009;96:83–92. doi: 10.1016/j.jphotobiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 207.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad Ø, Kjølsrud S, Anholt H. Cancer Res. 1999;59:1180–1183. [PubMed] [Google Scholar]
- 208.Berg K, Berstad M, Prasmickaite L, Weyergang A, Selbo PK, Hedfors I, Hogset A. Top. Curr. Chem. 2010;296:251–281. doi: 10.1007/128_2010_63. [DOI] [PubMed] [Google Scholar]
- 209.Oliveira S, Fretz MM, Høgset A, Storm G, Schiffelers RM. Biochim. Biophys. Acta, Biomembr. 2007;1768:1211–1217. doi: 10.1016/j.bbamem.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 210.Lee KY, Peters M, Mooney D. Adv. Mater. 2001;13:837–839. [Google Scholar]
- 211.Izawa H, Kawakami K, Sumita M, Tateyama Y, Hill JP, Ariga K. J. Mater. Chem. B. 2013;1:2155–2161. doi: 10.1039/c3tb00503h. [DOI] [PubMed] [Google Scholar]
- 212.Saxer T, Zumbuehl A, Müller B. Cardiovasc. Res. 2013:cvt102. doi: 10.1093/cvr/cvt102. [DOI] [PubMed] [Google Scholar]
- 213.Holme MN, Fedotenko IA, Abegg D, Althaus J, Babel L, Favarger F, Reiter R, Tanasescu R, Zaffalon P-L, Ziegler A. Nat. Nanotechnol. 2012;7:536–543. doi: 10.1038/nnano.2012.84. [DOI] [PubMed] [Google Scholar]
- 214.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 215.Sirsi SR, Borden MA. Adv. Drug Delivery Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Unger EC, McCreery TP, Sweitzer RH. Invest. Radiol. 1997;32:723–727. doi: 10.1097/00004424-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 217.Azagury A, Khoury L, Enden G, Kost J. Adv. Drug Delivery Rev. 2014;72:127–143. doi: 10.1016/j.addr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 218.Aryal M, Arvanitis CD, Alexander PM, McDannold N. Adv. Drug Delivery Rev. 2014;72:94–109. doi: 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lindner JR. Nat. Rev. Drug Discovery. 2004;3:527–533. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 220.Chen H, Li J, Wan J, Weitz DA, Stone HA. Soft Matter. 2013;9:38–42. [Google Scholar]
- 221.Miura S-i, Tachibana K, Okamoto T, Saku K. Biochem. Biophys. Res. Commun. 2002;298:587–590. doi: 10.1016/s0006-291x(02)02467-1. [DOI] [PubMed] [Google Scholar]
- 222.Lawrie A, Brisken A, Francis S, Cumberland D, Crossman D, Newman C. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 223.Shohet RV, Chen S, Zhou Y-T, Wang Z, Meidell RS, Unger RH, Grayburn PA. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 224.Miller DL, Pislaru SV, Greenleaf JF. Somatic Cell Mol. Genet. 2002;27:115–134. doi: 10.1023/a:1022983907223. [DOI] [PubMed] [Google Scholar]
- 225.Price RJ, Kaul S. J. Cardiovasc. Pharmacol. Ther. 2002;7:171–180. doi: 10.1177/107424840200700307. [DOI] [PubMed] [Google Scholar]
- 226.He Y, Bi Y, Hua Y, Liu D, Wen S, Wang Q, Li M, Zhu J, Lin T, He D. J. Exp. Clin. Cancer Res. 2011;30:104. doi: 10.1186/1756-9966-30-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Adv. Drug Delivery Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 228.Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, Cumberland DC, Newman CM. Circulation. 1999;99:2617–2620. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 229.Kuo J-hS, Jan M-s, Sung K. Int. J. Pharm. 2003;257:75–84. doi: 10.1016/s0378-5173(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 230.Hou C-C, Wang W, Huang XR, Fu P, Chen T-H, Sheikh-Hamad D, Lan HY. Am. J. Pathol. 2005;166:761–771. doi: 10.1016/s0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang X, Zheng Y, Wang Z, Huang S, Chen Y, Jiang W, Zhang H, Ding M, Li Q, Xiao X. Biomaterials. 2014;35:5148–5161. doi: 10.1016/j.biomaterials.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 232.Chen R, Chiba M, Mori S, Fukumoto M, Kodama T. J. Dent. Res. 2009;88:1008–1013. doi: 10.1177/0022034509346119. [DOI] [PubMed] [Google Scholar]
- 233.Lentacker I, De Cock I, Deckers R, De Smedt S, Moonen C. Adv. Drug Delivery Rev. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 234.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. J. Controlled Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Husseini GA, Pitt WG. Adv. Drug Delivery Rev. 2008;60:1137–1152. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Geers B, Lentacker I, Sanders NN, Demeester J, Meairs S, De Smedt SC. J. Controlled Release. 2011;152:249–256. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 237.Rapoport N, Payne A, Dillon C, Shea J, Scaife C, Gupta R. J. Ther. Ultrasound. 2013;1:11. doi: 10.1186/2050-5736-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Spivak MY, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ulberg ZR. EPMA J. 2013;4:20. doi: 10.1186/1878-5085-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. J. Controlled Release. 2011;150:102–110. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 240.Grüll H, Langereis S. J. Controlled Release. 2012;161:317–327. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 241.Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. J. Controlled Release. 2010;148:13–17. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yudina A, De Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P, Bouchaud V, Voisin P, Grüll H, Moonen C. J. Controlled Release. 2011;155:442–448. doi: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 243.Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Circulation. 2009;119:1378–1385. doi: 10.1161/CIRCULATIONAHA.108.825067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z. Adv. Healthcare Mater. 2013;3:811–816. doi: 10.1002/adhm.201300490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Burke CW, Alexander E, Timbie K, Kilbanov AL, Price RJ. Mol. Ther. 2013;22:321–328. doi: 10.1038/mt.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Eggen S, Fagerland S-M, Mørch Ý, Hansen R, Søvik K, Berg S, Furu H, Bøhn AD, Lilledahl MB, Angelsen A. J. Controlled Release. 2014;187:39–49. doi: 10.1016/j.jconrel.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 247.Alvarez-Lorenzo C, Concheiro A. Chem. Commun. 2014;50:7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 248.Alvarez-Lorenzo C, Concheiro A. Smart materials for drug delivery. 2013;1:1. [Google Scholar]
- 249.Wike-Hooley J, Haveman J, Reinhold H. Radiother. Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 250.Joshi GK, Johnson MA, Sardar R. RSC Adv. 2014;4:15807–15815. [Google Scholar]
- 251.Yu B, Li X, Zheng W, Feng Y, Wong Y-S, Chen T. J. Mater. Chem. B. 2014;2:5409–5418. doi: 10.1039/c4tb00399c. [DOI] [PubMed] [Google Scholar]
- 252.He L, Wang T, An J, Li X, Zhang L, Li L, Li G, Wu X, Su Z, Wang C. Cryst Eng Comm. 2014;16:3259–3263. [Google Scholar]
- 253.Rasouli S, Davaran S, Rasouli F, Mahkam M, Salehi R. Drug Delivery. 2014;21:155–163. doi: 10.3109/10717544.2013.838714. [DOI] [PubMed] [Google Scholar]
- 254.Tu F, Lee D. J. Am. Chem. Soc. 2014;136:9999–10006. doi: 10.1021/ja503189r. [DOI] [PubMed] [Google Scholar]
- 255.Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. AAPS Pharm Sci Tech. 2014;15:694–708. doi: 10.1208/s12249-014-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Grainger SJ, El-Sayed ME. Biol.-Responsive Hybrid Biomater. 2010:171–190. [Google Scholar]
- 257.Wang Y, Chen L, Tan L, Zhao Q, Luo F, Wei Y, Qian Z. Biomaterials. 2014;35:6972–6985. doi: 10.1016/j.biomaterials.2014.04.099. [DOI] [PubMed] [Google Scholar]
- 258.Song S, Chen F, Qi H, Li F, Xin T, Xu J, Ye T, Sheng N, Yang X, Pan W. Pharm. Res. 2014;31:1032–1045. doi: 10.1007/s11095-013-1225-y. [DOI] [PubMed] [Google Scholar]
- 259.Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, Liang X-J. Biotechnol. Adv. 2013;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 260.Dai S, Ravi P, Tam KC. Soft Matter. 2008;4:435–449. doi: 10.1039/b714741d. [DOI] [PubMed] [Google Scholar]
- 261.Kamada H, Tsutsumi Y, Yoshioka Y, Yamamoto Y, Kodaira H, Tsunoda S-i, Okamoto T, Mukai Y, Shibata H, Nakagawa S. Clin. Cancer Res. 2004;10:2545–2550. doi: 10.1158/1078-0432.ccr-03-0544. [DOI] [PubMed] [Google Scholar]
- 262.Ge Y, Li S, Wang S, Moore R. Nanomedicine: Principles and Perspectives. Springer; 2014. [Google Scholar]
- 263.Gillies ER, Jonsson TB, Fréchet JM. J. Am. Chem. Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 264.Foster S, Duvall CL, Crownover EF, Hoffman AS, Stayton PS. Bioconjugate Chem. 2010;21:2205–2212. doi: 10.1021/bc100204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, Liang X-J. Biotechnol. Adv. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 266.Poon G, Gariepy J. Biochem. Soc. Trans. 2007;35:788–793. doi: 10.1042/BST0350788. [DOI] [PubMed] [Google Scholar]
- 267.Pietersz GA, Tang C-K, Apostolopoulos V. Mini-Rev. Med. Chem. 2006;6:1285–1298. doi: 10.2174/138955706778992987. [DOI] [PubMed] [Google Scholar]
- 268.Kitazoe M, Futami J, Nishikawa M, Yamada H, Maeda Y. Biotechnol. J. 2010;5:385–392. doi: 10.1002/biot.200900132. [DOI] [PubMed] [Google Scholar]
- 269.Park S-C, Nam J-P, Kim Y-M, Kim J-H, Nah J-W, Jang M-K. Int. J. Nanomed. 2013;8:3663. doi: 10.2147/IJN.S50911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Oh Y, Suh D, Kim J, Choi H, Shin K, Ko J. Gene Ther. 2002;9:1627–1632. doi: 10.1038/sj.gt.3301735. [DOI] [PubMed] [Google Scholar]
- 271.Meng F, Zhong Y, Cheng R, Deng C, Zhong Z. Nanomedicine. 2014;9:487–499. doi: 10.2217/nnm.13.212. [DOI] [PubMed] [Google Scholar]
- 272.Yang J, Chen J, Pan D, Wan Y, Wang Z. Carbohydr. Polym. 2013;92:719–725. doi: 10.1016/j.carbpol.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 273.Jana S, Maji N, Nayak AK, Sen KK, Basu SK. Carbohydr. Polym. 2013;98:870–876. doi: 10.1016/j.carbpol.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 274.Wu H, Zhu L, Torchilin VP. Biomaterials. 2013;34:1213–1222. doi: 10.1016/j.biomaterials.2012.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Čalija B, Cekić N, Savić S, Daniels R, Marković B, Milić J. Colloids Surf., B. 2013;110:395–402. doi: 10.1016/j.colsurfb.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 276.Chen M-C, Mi F-L, Liao Z-X, Hsiao C-W, Sonaje K, Chung M-F, Hsu L-W, Sung H-W. Adv. Drug Delivery Rev. 2013;65:865–879. doi: 10.1016/j.addr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 277.Yang Y, Wang S, Wang Y, Wang X, Wang Q, Chen M. Biotechnol. Adv. 2014;32:1301–1316. doi: 10.1016/j.biotechadv.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 278.Wang M, Hu H, Sun Y, Qiu L, Zhang J, Guan G, Zhao X, Qiao M, Cheng L, Cheng L. Biomaterials. 2013;34:10120–10132. doi: 10.1016/j.biomaterials.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 279.Nogueira DR, Tavano L, Mitjans M, Pérez L, Infante MR, Vinardell MP. Biomaterials. 2013;34:2758–2772. doi: 10.1016/j.biomaterials.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 280.McCarthy HO, McCaffrey J, McCrudden CM, Zholobenko A, Ali AA, McBride JW, Massey AS, Pentlavalli S, Chen K-H, Cole G. J. Controlled Release. 2014;189:141–149. doi: 10.1016/j.jconrel.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 281.Loughran SP, McCrudden CM, McCarthy HO. Eur. J. Nanomed. 2015;7:85–96. [Google Scholar]
- 282.Akita H, Kogure K, Moriguchi R, Nakamura Y, Higashi T, Nakamura T, Serada S, Fujimoto M, Naka T, Futaki S. J. Controlled Release. 2010;143:311–317. doi: 10.1016/j.jconrel.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 283.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova J-L. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 284.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay J-P, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S. N. Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 285.Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kim A, Szoka FC., Jr Pharm. Res. 1992;9:504–514. doi: 10.1023/a:1015892313856. [DOI] [PubMed] [Google Scholar]
- 287.Sakurai Y, Hatakeyama H, Sato Y, Akita H, Takayama K, Kobayashi S, Futaki S, Harashima H. Biomaterials. 2011;32:5733–5742. doi: 10.1016/j.biomaterials.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 288.Guo XD, Wiradharma N, Liu SQ, Zhang LJ, Khan M, Qian Y, Yang Y-Y. Biomaterials. 2012;33:6284–6291. doi: 10.1016/j.biomaterials.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 289.Watkins KA, Chen R. Int. J. Pharm. 2015;478:496–503. doi: 10.1016/j.ijpharm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 290.Liu Y, Wang W, Yang J, Zhou C, Sun J. Asian J. Pharm. Sci. 2013;8:159–167. [Google Scholar]
- 291.Siepmann J, Siegel RA, Rathbone MJ. Fundamentals and applications of controlled release drug delivery. Springer; 2012. [Google Scholar]
- 292.Shenoi RA, Lai BF, Imran ul-haq M, Brooks DE, Kizhakkedathu JN. Biomaterials. 2013;34:6068–6081. doi: 10.1016/j.biomaterials.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 293.Guk K, Lim H, Kim B, Hong M, Khang G, Lee D. Int. J. Pharm. 2013;453:541–550. doi: 10.1016/j.ijpharm.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 294.Shim MS, Kwon YJ. Polym. Chem. 2012;3:2570–2577. [Google Scholar]
- 295.Lee I, Park M, Kim Y, Hwang O, Khang G, Lee D. Int. J. Pharm. 2013;448:259–266. doi: 10.1016/j.ijpharm.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 296.Ding C, Gu J, Qu X, Yang Z. Bioconjugate Chem. 2009;20:1163–1170. doi: 10.1021/bc800563g. [DOI] [PubMed] [Google Scholar]
- 297.Zhou Z, Li L, Yang Y, Xu X, Huang Y. Biomaterials. 2014;35:6622–6635. doi: 10.1016/j.biomaterials.2014.04.059. [DOI] [PubMed] [Google Scholar]
- 298.Liu C, Liu F, Feng L, Li M, Zhang J, Zhang N. Biomaterials. 2013;34:2547–2564. doi: 10.1016/j.biomaterials.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 299.Nakamura H, Etrych T, Chytil P, Ohkubo M, Fang J, Ulbrich K, Maeda H. J. Controlled Release. 2014;174:81–87. doi: 10.1016/j.jconrel.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 300.Etrych T, Šubr V, Laga R, Říhová B, Ulbrich K. Eur. J. Pharm. Sci. 2014;58:1–12. doi: 10.1016/j.ejps.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 301.Sun T-M, Wang Y-C, Wang F, Du J-Z, Mao C-Q, Sun C-Y, Tang R-Z, Liu Y, Zhu J, Zhu Y-H. Biomaterials. 2014;35:836–845. doi: 10.1016/j.biomaterials.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 302.Dong D-W, Xiang B, Gao W, Yang Z-Z, Li J-Q, Qi X-R. Biomaterials. 2013;34:4849–4859. doi: 10.1016/j.biomaterials.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 303.Ding M, Song N, He X, Li J, Zhou L, Tan H, Fu Q, Gu Q. ACS Nano. 2013;7:1918–1928. doi: 10.1021/nn4002769. [DOI] [PubMed] [Google Scholar]
- 304.Zhou L, Liang D, He X, Li J, Tan H, Li J, Fu Q, Gu Q. Biomaterials. 2012;33:2734–2745. doi: 10.1016/j.biomaterials.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 305.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Adv. Drug Delivery Rev. 2002;54:1015–1039. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 306.Wang X, Wu G, Lu C, Zhao W, Wang Y, Fan Y, Gao H, Ma J. Eur. J. Pharm. Sci. 2012;47:256–264. doi: 10.1016/j.ejps.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 307.Koutroumanis KP, Holdich RG, Georgiadou S. Int. J. Pharm. 2013;455:5–13. doi: 10.1016/j.ijpharm.2013.06.071. [DOI] [PubMed] [Google Scholar]
- 308.She W, Li N, Luo K, Guo C, Wang G, Geng Y, Gu Z. Biomaterials. 2013;34:2252–2264. doi: 10.1016/j.biomaterials.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 309.Raghupathi K, Li L, Ventura J, Jennings M, Thayumanavan S. Polym. Chem. 2014;5:1737–1742. [Google Scholar]
- 310.Kang Y, Zhang X-M, Zhang S, Ding L, Li B-J. Polym. Chem. 2015;6:2098–2107. [Google Scholar]
- 311.Liu Y, Gao F-P, Zhang D, Fan Y-S, Chen X-G, Wang H. J. Controlled Release. 2014;173:140–147. doi: 10.1016/j.jconrel.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 312.Lale SV, Kumar A, Naz F, Bharti AC, Koul V. Polym. Chem. 2015;6:2115–2132. [Google Scholar]
- 313.Sun D, Ding J, Xiao C, Chen J, Zhuang X, Chen X. Adv. Healthcare Mater. 2015;4:844–855. doi: 10.1002/adhm.201400736. [DOI] [PubMed] [Google Scholar]
- 314.Du J-Z, Du X-J, Mao C-Q, Wang J. J. Am. Chem. Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 315.Kavitha T, Abdi SIH, Park S-Y. Phys. Chem. Chem. Phys. 2013;15:5176–5185. doi: 10.1039/c3cp00008g. [DOI] [PubMed] [Google Scholar]
- 316.Ren L, Liu T, Guo J, Guo S, Wang X, Wang W. Nanotechnology. 2010;21:335701. doi: 10.1088/0957-4484/21/33/335701. [DOI] [PubMed] [Google Scholar]
- 317.Feng L, Li K, Shi X, Gao M, Liu J, Liu Z. Adv. Healthcare Mater. 2014;3:1261–1271. doi: 10.1002/adhm.201300549. [DOI] [PubMed] [Google Scholar]
- 318.Shi J, Liu Y, Wang L, Gao J, Zhang J, Yu X, Ma R, Liu R, Zhang Z. Acta Biomater. 2014;10:1280–1291. doi: 10.1016/j.actbio.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 319.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T. Nanomedicine. 2014;11:313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 320.Slowing II, Vivero-Escoto JL, Wu C-W, Lin VS-Y. Adv. Drug Delivery Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 321.Muhammad F, Guo M, Qi W, Sun F, Wang A, Guo Y, Zhu G. J. Am. Chem. Soc. 2011;133:8778–8781. doi: 10.1021/ja200328s. [DOI] [PubMed] [Google Scholar]
- 322.Jia Z, Lin P, Xiang Y, Wang X, Wang J, Zhang X, Zhang Q. Eur. J. Pharm. Biopharm. 2011;79:126–134. doi: 10.1016/j.ejpb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 323.Meng H, Xue M, Xia T, Zhao Y-L, Tamanoi F, Stoddart JF, Zink JI, Nel AE. J. Am. Chem. Soc. 2010;132:12690–12697. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Gan Q, Lu X, Dong W, Yuan Y, Qian J, Li Y, Shi J, Liu C. J. Mater. Chem. 2012;22:15960–15968. [Google Scholar]
- 325.Chen L, Zhang Z, Yao X, Chen X, Chen X. Microporous Mesoporous Mater. 2015;201:169–175. [Google Scholar]
- 326.Choi SR, Jang D-J, Kim S, An S, Lee J, Oh E, Kim J. J. Mater. Chem. B. 2014;2:616–619. doi: 10.1039/c3tb21494j. [DOI] [PubMed] [Google Scholar]
- 327.Nowag S, Haag R. Angew. Chem., Int. Ed. 2014;53:49–51. doi: 10.1002/anie.201308619. [DOI] [PubMed] [Google Scholar]
- 328.Mei L, He F, Zhou R-Q, Wu C-D, Liang R, Xie R, Ju X-J, Wang W, Chu L-Y. ACS Appl. Mater. Interfaces. 2014;6:5962–5970. doi: 10.1021/am501011j. [DOI] [PubMed] [Google Scholar]
- 329.Singh I, Sagare AP, Coma M, Perlmutter D, Gelein R, Bell RD, Deane RJ, Zhong E, Parisi M, Ciszewski J. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14771–14776. doi: 10.1073/pnas.1302212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lindgren J, Segerfeldt P, Sholts SB, Gräslund A, Karlström AE, Wärmländer SK. J. Inorg. Biochem. 2013;120:18–23. doi: 10.1016/j.jinorgbio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 331.Treiber C, Quadir MA, Voigt P, Radowski M, Xu S, Munter L-M, Bayer TA, Schaefer M, Haag R, Multhaup G. Biochemistry. 2009;48:4273–4284. doi: 10.1021/bi900290c. [DOI] [PubMed] [Google Scholar]
- 332.Ejima H, Richardson JJ, Liang K, Best JP, van Koeverden MP, Such GK, Cui J, Caruso F. Science. 2013;341:154–157. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 333.Gogoi N, Chowdhury D. J. Mater. Chem. B. 2014;2:4089–4099. doi: 10.1039/c3tb21835j. [DOI] [PubMed] [Google Scholar]
- 334.Thamphiwatana S, Fu V, Zhu J, Lu D, Gao W, Zhang L. Langmuir. 2013;29:12228–12233. doi: 10.1021/la402695c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Koetting MC, Peppas NA. Int. J. Pharm. 2014;471:83–91. doi: 10.1016/j.ijpharm.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Gui W, Wang W, Jiao X, Chen L, Wen Y, Zhang X. Chem Phys Chem. 2014;16:607–613. doi: 10.1002/cphc.201402732. [DOI] [PubMed] [Google Scholar]
- 337.Yu H, Xu Z, Chen X, Xu L, Yin Q, Zhang Z, Li Y. Macromol. Biosci. 2014;14:100–109. doi: 10.1002/mabi.201300282. [DOI] [PubMed] [Google Scholar]
- 338.Chiang C-S, Hu S-H, Liao B-J, Chang Y-C, Chen S-Y. Nanomedicine. 2014;10:99–107. doi: 10.1016/j.nano.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 339.Zhang Q, Ran R, Zhang L, Liu Y, Mei L, Zhang Z, Gao H, He Q. J. Controlled Release. 2015;197:208–218. doi: 10.1016/j.jconrel.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 340.Rajpoot P, Bali V, Pathak K. Int. J. Pharm. 2012;426:219–230. doi: 10.1016/j.ijpharm.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 341.Meng F, Cheng R, Deng C, Zhong Z. Mater. Today. 2012;15:436–442. [Google Scholar]
- 342.Aon M, Cortassa S, O’Rourke B. Biochim. Biophys. Acta, Bioenerg. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. J. Controlled Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 344.Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Expert Rev. Mol. Med. 2011;13:e39. doi: 10.1017/S1462399411002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Go Y-M, Jones DP. Biochim. Biophys. Acta, Gen. Subj. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sun Y, Yan X, Yuan T, Liang J, Fan Y, Gu Z, Zhang X. Biomaterials. 2010;31:7124–7131. doi: 10.1016/j.biomaterials.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 347.Wang Y-C, Wang F, Sun T-M, Wang J. Bioconjugate Chem. 2011;22:1939–1945. doi: 10.1021/bc200139n. [DOI] [PubMed] [Google Scholar]
- 348.Li J, Huo M, Wang J, Zhou J, Mohammad JM, Zhang Y, Zhu Q, Waddad AY, Zhang Q. Biomaterials. 2012;33:2310–2320. doi: 10.1016/j.biomaterials.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 349.Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, Kato J, Dong TA, Lam KS. Biomaterials. 2011;32:6633–6645. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Koo AN, Lee HJ, Kim SE, Chang JH, Park C, Kim C, Park JH, Lee SC. Chem. Commun. 2008:6570–6572. doi: 10.1039/b815918a. [DOI] [PubMed] [Google Scholar]
- 351.Kim H, Kim S, Park C, Lee H, Park HJ, Kim C. Adv. Mater. 2010;22:4280–4283. doi: 10.1002/adma.201001417. [DOI] [PubMed] [Google Scholar]
- 352.Ryu J-H, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. J. Am. Chem. Soc. 2010;132:17227–17235. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 353.Ong W, Yang Y, Cruciano AC, McCarley RL. J. Am. Chem. Soc. 2008;130:14739–14744. doi: 10.1021/ja8050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R, Kannan RM. Biomaterials. 2009;30:2112–2121. doi: 10.1016/j.biomaterials.2008.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Li Y, Lokitz BS, Armes SP, McCormick CL. Macromolecules. 2006;39:2726–2728. [Google Scholar]
- 356.Kakizawa Y, Harada A, Kataoka K. Biomacromolecules. 2001;2:491–497. doi: 10.1021/bm000142l. [DOI] [PubMed] [Google Scholar]
- 357.Takeoka Y, Aoki T, Sanui K, Ogata N, Yokoyama M, Okano T, Sakurai Y, Watanabe M. J. Controlled Release. 1995;33:79–87. [Google Scholar]
- 358.Crielaard BJ, Rijcken CJ, Quan L, van der Wal S, Altintas I, van der Pot M, Kruijtzer JA, Liskamp RM, Schiffelers RM, van Nostrum CF. Angew. Chem., Int. Ed. 2012;51:7254–7258. doi: 10.1002/anie.201202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wei R, Cheng L, Zheng M, Cheng R, Meng F, Deng C, Zhong Z. Biomacromolecules. 2012;13:2429–2438. doi: 10.1021/bm3006819. [DOI] [PubMed] [Google Scholar]
- 360.Koo AN, Min KH, Lee HJ, Lee S-U, Kim K, Kwon IC, Cho SH, Jeong SY, Lee SC. Biomaterials. 2012;33:1489–1499. doi: 10.1016/j.biomaterials.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 361.Tang L-Y, Wang Y-C, Li Y, Du J-Z, Wang J. Bioconjugate Chem. 2009;20:1095–1099. doi: 10.1021/bc900144m. [DOI] [PubMed] [Google Scholar]
- 362.Li Z-Y, Hu J-J, Xu Q, Chen S, Jia H-Z, Sun Y-X, Zhuo R-X, Zhang X-Z. J. Mater. Chem. B. 2015;3:39–44. doi: 10.1039/c4tb01533a. [DOI] [PubMed] [Google Scholar]
- 363.Kuang H, Wu S, Meng F, Xie Z, Jing X, Huang Y. J. Mater. Chem. 2012;22:24832–24840. [Google Scholar]
- 364.Wang H, Tang L, Tu C, Song Z, Yin Q, Yin L, Zhang Z, Cheng J. Biomacromolecules. 2013;14:3706–3712. doi: 10.1021/bm401086d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lu Y, Sun W, Gu Z. J. Controlled Release. 2014;194:1–19. doi: 10.1016/j.jconrel.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG. ACS Nano. 2013;7:6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 367.Wu S, Huang X, Du X. Angew. Chem. 2013;125:5690–5694. [Google Scholar]
- 368.Chu MK, Chen J, Gordijo CR, Chiang S, Ivovic A, Koulajian K, Giacca A, Wu XY, Sun Y. Lab Chip. 2012;12:2533–2539. doi: 10.1039/c2lc40139h. [DOI] [PubMed] [Google Scholar]
- 369.Chen M, Huang C, He C, Zhu W, Xu Y, Lu Y. Chem. Commun. 2012;48:9522–9524. doi: 10.1039/c2cc34290a. [DOI] [PubMed] [Google Scholar]
- 370.Lee SH, Gupta MK, Bang JB, Bae H, Sung HJ. Adv. Healthcare Mater. 2013;2:908–915. doi: 10.1002/adhm.201200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhang D, Wei Y, Chen K, Zhang X, Xu X, Shi Q, Han S, Chen X, Gong H, Li X. Adv. Healthcare Mater. 2015;4:69–76. doi: 10.1002/adhm.201400299. [DOI] [PubMed] [Google Scholar]
- 372.Gupta MK, Meyer TA, Nelson CE, Duvall CL. J. Controlled Release. 2012;162:591–598. doi: 10.1016/j.jconrel.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Su Z, Chen M, Xiao Y, Sun M, Zong L, Asghar S, Dong M, Li H, Ping Q, Zhang C. J. Controlled Release. 2014;196:370–383. doi: 10.1016/j.jconrel.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 374.Biswas S, Kinbara K, Niwa T, Taguchi H, Ishii N, Watanabe S, Miyata K, Kataoka K, Aida T. Nat. Chem. 2013;5:613–620. doi: 10.1038/nchem.1681. [DOI] [PubMed] [Google Scholar]
- 375.Yan Q, Zhao Y. Chem. Sci. 2015;6:4343–4349. doi: 10.1039/c5sc00965k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Mo R, Jiang T, Sun W, Gu Z. Biomaterials. 2015;50:67–74. doi: 10.1016/j.biomaterials.2015.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhang P, Cheng F, Zhou R, Cao J, Li J, Burda C, Min Q, Zhu JJ. Angew. Chem., Int. Ed. 2014;53:2371–2375. doi: 10.1002/anie.201308920. [DOI] [PubMed] [Google Scholar]
- 378.Molla MR, Prasad P, Thayumanavan S. J. Am. Chem. Soc. 2015;137:7286–7289. doi: 10.1021/jacs.5b04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Hu J, Zhang G, Liu S. Chem. Soc. Rev. 2012;41:5933–5949. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 380.Ghadiali JE, Stevens MM. Adv. Mater. 2008;20:4359–4363. [Google Scholar]
- 381.De La Rica R, Aili D, Stevens MM. Adv. Drug Delivery Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 382.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat. Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 383.Aricò AS, Bruce P, Scrosati B, Tarascon J-M, Van Schalkwijk W. Nat. Mater. 2005;4:366–377. doi: 10.1038/nmat1368. [DOI] [PubMed] [Google Scholar]
- 384.Kawata S, Inouye Y, Verma P. Nat. Photonics. 2009;3:388–394. [Google Scholar]
- 385.Cooper S, Bamford C, Tsuruta T. Allan S. Hoffman. The Netherlands: VSP, Utrecht; 1995. [Google Scholar]
- 386.Matsuda T, Takahashi-Tezuka M, Fukada T, Okuyama Y, Fujitani Y, Tsukada S, Mano H, Hirai H, Witte O, Hirano T. Blood. 1995;85:627–633. [PubMed] [Google Scholar]
- 387.Hoshino K, Taniguchi M, Ueoka H, Ohkuwa M, Chida C, Morohashi S, Sasakura T. J. Ferment. Bioeng. 1996;82:253–258. [Google Scholar]
- 388.López-Otín C, Bond JS. J. Biol. Chem. 2008;283:30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Basel MT, Shrestha TB, Troyer DL, Bossmann SH. ACS Nano. 2011;5:2162–2175. doi: 10.1021/nn103362n. [DOI] [PubMed] [Google Scholar]
- 390.Vicent MJ, Greco F, Nicholson RI, Paul A, Griffiths PC, Duncan R. Angew. Chem. 2005;117:4129–4134. doi: 10.1002/anie.200462960. [DOI] [PubMed] [Google Scholar]
- 391.Imperiale JC, Nejamkin P, del Sole MJ, Lanusse CE, Sosnik A. Biomaterials. 2015;37:383–394. doi: 10.1016/j.biomaterials.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 392.Leiros HKS, Brandsdal BO, Andersen OA, Os V, Leiros I, Helland R, Otlewski J, Willassen NP, Smalås AO. Protein Sci. 2004;13:1056–1070. doi: 10.1110/ps.03498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Xue W, Zhang G, Zhang D. Analyst. 2011;136:3136–3141. doi: 10.1039/c1an15224f. [DOI] [PubMed] [Google Scholar]
- 394.Radhakrishnan K, Tripathy J, Raichur AM. Chem. Commun. 2013;49:5390–5392. doi: 10.1039/c3cc42017e. [DOI] [PubMed] [Google Scholar]
- 395.Radhakrishnan K, Tripathy J, Gnanadhas DP, Chakravortty D, Raichur AM. RSC Adv. 2014;4:45961–45968. [Google Scholar]
- 396.Hou X-F, Chen Y, Liu Y. Soft Matter. 2015;11:2488–2493. doi: 10.1039/c4sm02896a. [DOI] [PubMed] [Google Scholar]
- 397.Wells A, Grandis JR. Clin. Exp. Metastasis. 2003;20:285–290. doi: 10.1023/a:1024088922957. [DOI] [PubMed] [Google Scholar]
- 398.Scott KF, Sajinovic M, Hein J, Nixdorf S, Galettis P, Liauw W, de Souza P, Dong Q, Graham GG, Russell PJ. Biochimie. 2010;92:601–610. doi: 10.1016/j.biochi.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 399.Wu W, Yang Y, Sun G. Gastroenterol. Res. Pract. 2012;2012 doi: 10.1155/2012/723183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Iwańczak F, Iwańczak B. Adv. Clin. Exp. Med. 2012;21:671–680. [PubMed] [Google Scholar]
- 401.Andresen TL, Davidsen J, Begtrup M, Mouritsen OG, Jørgensen K. J. Med. Chem. 2004;47:1694–1703. doi: 10.1021/jm031029r. [DOI] [PubMed] [Google Scholar]
- 402.Thamphiwatana S, Gao W, Pornpattananangkul D, Zhang Q, Fu V, Li J, Li J, Obonyo M, Zhang L. J. Mater. Chem. B. 2014;2:8201–8207. doi: 10.1039/C4TB01110D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Bernardos A, Mondragon L, Aznar E, Marcos MD, Martínez-Máñez Rn, Sancenón Fl, Soto J, Barat JM, Pérez-Payá E, Guillem C. ACS Nano. 2010;4:6353–6368. doi: 10.1021/nn101499d. [DOI] [PubMed] [Google Scholar]
- 404.Semple BD, Trivedi A, Gimlin K, Noble-Haeusslein LJ. Neurobiol. Dis. 2015;74:263–280. doi: 10.1016/j.nbd.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ferreira AVF. 2013 http://hdl.handle.net/1822/28648. [Google Scholar]
- 406.Rao J, Hottinger C, Khan A. J. Am. Chem. Soc. 2014;16:5872–5875. doi: 10.1021/ja501632r. [DOI] [PubMed] [Google Scholar]
- 407.Harnoy AJ, Rosenbaum I, Tirosh E, Ebenstein Y, Shaharabani R, Beck R, Amir RJ. J. Am. Chem. Soc. 2014;136:7531–7534. doi: 10.1021/ja413036q. [DOI] [PubMed] [Google Scholar]
- 408.Patel K, Angelos S, Dichtel WR, Coskun A, Yang Y-W, Zink JI, Stoddart JF. J. Am. Chem. Soc. 2008;130:2382–2383. doi: 10.1021/ja0772086. [DOI] [PubMed] [Google Scholar]
- 409.Kundu JK, Surh Y-J. Pharm. Res. 2010;27:999–1013. doi: 10.1007/s11095-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 410.Butterfield DA, Hardas SS, Lange MLB. J. Alzheimer’s Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Imanishi Y, Ito Y. Pure Appl. Chem. 1995;67:2015–2022. [Google Scholar]
- 412.Kai G, Min Y. J. Controlled Release. 2001;71:221–225. doi: 10.1016/s0168-3659(00)00323-0. [DOI] [PubMed] [Google Scholar]
- 413.Tang M, Zhang R, Bowyer A, Eisenthal R, Hubble J. Biotechnol. Bioeng. 2003;82:47–53. doi: 10.1002/bit.10539. [DOI] [PubMed] [Google Scholar]
- 414.Podual K, Doyle FJ, III, Peppas NA. J. Controlled Release. 2000;67:9–17. doi: 10.1016/s0168-3659(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 415.Miyata T, Uragami T, Nakamae K. Adv. Drug Delivery Rev. 2002;54:79–98. doi: 10.1016/s0169-409x(01)00241-1. [DOI] [PubMed] [Google Scholar]
- 416.Schafer FQ, Buettner GR. Free Radicals Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 417.Napoli A, Boerakker MJ, Tirelli N, Nolte RJ, Sommerdijk NA, Hubbell JA. Langmuir. 2004;20:3487–3491. doi: 10.1021/la0357054. [DOI] [PubMed] [Google Scholar]
- 418.Xiong MH, Li YJ, Bao Y, Yang XZ, Hu B, Wang J. Adv. Mater. 2012;24:6175–6180. doi: 10.1002/adma.201202847. [DOI] [PubMed] [Google Scholar]
- 419.Gogoi D, Barman T, Choudhury B, Khan M, Chaudhari Y, Dehingia M, Pal AR, Bailung H, Chutia J. Mater. Sci. Eng., C. 2014;43:237–242. doi: 10.1016/j.msec.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 420.Stoytcheva M, Zlatev R, Cosnier S, Arredondo M, Valdez B. Biosens. Bioelectron. 2013;41:862–866. doi: 10.1016/j.bios.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 421.Chuang Y-C, Li J-C, Chen S-H, Liu T-Y, Kuo C-H, Huang W-T, Lin C-S. Biomaterials. 2010;31:6087–6095. doi: 10.1016/j.biomaterials.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 422.de la Rica R, Fratila RM, Szarpak A, Huskens J, Velders AH. Angew. Chem. 2011;123:5822–5825. doi: 10.1002/anie.201008189. [DOI] [PubMed] [Google Scholar]
- 423.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Photochem. Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 424.Chan A, Orme RP, Fricker RA, Roach P. Adv. Drug Delivery Rev. 2013;65:497–514. doi: 10.1016/j.addr.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 425.Wu L, Zou Y, Deng C, Cheng R, Meng F, Zhong Z. Biomaterials. 2013;34:5262–5272. doi: 10.1016/j.biomaterials.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 426.Cheng R, Meng F, Ma S, Xu H, Liu H, Jing X, Zhong Z. J. Mater. Chem. 2011;21:19013–19020. [Google Scholar]
- 427.Shim WS, Yoo JS, Bae YH, Lee DS. Biomacromolecules. 2005;6:2930–2934. doi: 10.1021/bm050521k. [DOI] [PubMed] [Google Scholar]
- 428.Singh NK, Lee DS. J. Controlled Release. 2014;193:214–227. doi: 10.1016/j.jconrel.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 429.Adeli M, Fard AK, Abedi F, Chegeni BK, Bani F. Nanomedicine. 2013;9:1203–1213. doi: 10.1016/j.nano.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 430.Huynh CT, Nguyen MK, Lee DS. Chem. Commun. 2012;48:10951–10953. doi: 10.1039/c2cc36134e. [DOI] [PubMed] [Google Scholar]
- 431.Chen Y-C, Liao L-C, Lu P-L, Lo C-L, Tsai H-C, Huang C-Y, Wei K-C, Yen T-C, Hsiue G-H. Biomaterials. 2012;33:4576–4588. doi: 10.1016/j.biomaterials.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 432.Liu Y, Cao X, Luo M, Le Z, Xu W. J. Colloid Interface Sci. 2009;329:244–252. doi: 10.1016/j.jcis.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 433.Du P, Wang T, Liu P. Colloids Surf., B. 2013;102:1–8. doi: 10.1016/j.colsurfb.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 434.Zhu L, Wang D, Wei X, Zhu X, Li J, Tu C, Su Y, Wu J, Zhu B, Yan D. J. Controlled Release. 2013;169:228–238. doi: 10.1016/j.jconrel.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 435.Jing L, Liang X, Li X, Yang Y, Dai Z. Acta Biomater. 2013;9:9434–9441. doi: 10.1016/j.actbio.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 436.Sundaresan V, Menon JU, Rahimi M, Nguyen KT, Wadajkar AS. Int. J. Pharm. 2014;466:1–7. doi: 10.1016/j.ijpharm.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Huang H-Y, Hu S-H, Hung S-Y, Chiang C-S, Liu H-L, Chiu T-L, Lai H-Y, Chen Y-Y, Chen S-Y. J. Controlled Release. 2013;172:118–127. doi: 10.1016/j.jconrel.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 438.Cheng X, Li H, Chen Y, Luo B, Liu X, Liu W, Xu H, Yang X. PLoS One. 2013;8:e85003. doi: 10.1371/journal.pone.0085003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Zhang J, Wu L, Meng F, Wang Z, Deng C, Liu H, Zhong Z. Langmuir. 2011;28:2056–2065. doi: 10.1021/la203843m. [DOI] [PubMed] [Google Scholar]
- 440.Lu Y, Mo R, Tai W, Sun W, Pacardo DB, Qian C, Shen Q, Ligler FS, Gu Z. Chem. Commun. 2014;50:15105–15108. doi: 10.1039/c4cc07004f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Park S, Kwon B, Yang W, Han E, Yoo W, Lee D. J. Controlled Release. 2014;196:19–27. doi: 10.1016/j.jconrel.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 442.Peng J, Qi T, Liao J, Chu B, Yang Q, Li W, Qu Y, Luo F, Qian Z. Biomaterials. 2013;34:8726–8740. doi: 10.1016/j.biomaterials.2013.07.092. [DOI] [PubMed] [Google Scholar]
- 443.Gao C, Liu T, Dang Y, Yu Z, Zhang X, He G, Zheng H, Yin Y, Kong X. Carbohydr. Polym. 2014;111:964–970. doi: 10.1016/j.carbpol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 444.Yin R, Tong Z, Yang D, Nie J. Int. J. Biol. Macromol. 2011;49:1137–1142. doi: 10.1016/j.ijbiomac.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 445.Yang C, Guo W, Cui L, An N, Zhang T, Lin H, Qu F. Langmuir. 2014;30:9819–9827. doi: 10.1021/la501833u. [DOI] [PubMed] [Google Scholar]
- 446.Kim SW, Oh KT, Youn YS, Lee ES. Colloids Surf., B. 2014;116:359–364. doi: 10.1016/j.colsurfb.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 447.Feng N, Han G, Dong J, Wu H, Zheng Y, Wang G. J. Colloid Interface Sci. 2014;421:15–21. doi: 10.1016/j.jcis.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 448.Yadavalli T, Ramasamy S, Chandrasekaran G, Michael I, Therese HA, Chennakesavulu R. J. Magn. Magn. Mater. 2014;380:315–320. [Google Scholar]
- 449.Lee SY, Lee H, In I, Park SY. Eur. Polym. J. 2014;57:1–10. [Google Scholar]
- 450.Chang B, Sha X, Guo J, Jiao Y, Wang C, Yang W. J. Mater. Chem. 2011;21:9239–9247. [Google Scholar]
- 451.Liu X, Liu H-J, Cheng F, Chen Y. Nanoscale. 2014;6:7453–7460. doi: 10.1039/c4nr00739e. [DOI] [PubMed] [Google Scholar]
- 452.Chen Y, Xu P, Shu Z, Wu M, Wang L, Zhang S, Zheng Y, Chen H, Wang J, Li Y. Adv. Funct. Mater. 2014;24:4386–4396. [Google Scholar]
- 453.Yang X, Wang Y, Huang X, Ma Y, Huang Y, Yang R, Duan H, Chen Y. J. Mater. Chem. 2011;21:3448–3454. [Google Scholar]
- 454.Mousavi HZ, Rashidi AM, Ghazaghi M, Shirkhanloo H, Rahighi R. Anal. Chim. Acta. 2015;902:33–42. doi: 10.1016/j.aca.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 455.Sun S, Wu P. J. Mater. Chem. 2011;21:4095–4097. [Google Scholar]
- 456.Qiao Z-Y, Zhang R, Du F-S, Liang D-H, Li Z-C. J. Controlled Release. 2011;152:57–66. doi: 10.1016/j.jconrel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 457.Liu J, Detrembleur C, Debuigne A, De Pauw-Gillet M-C, Mornet S, Vander Elst L, Laurent S, Labrugère C, Duguet E, Jérôme C. Nanoscale. 2013;5:11464–11477. doi: 10.1039/c3nr02861e. [DOI] [PubMed] [Google Scholar]
- 458.Bilalis P, Chatzipavlidis A, Tziveleka L-A, Boukos N, Kordas G. J. Mater. Chem. 2012;22:13451–13454. [Google Scholar]
- 459.Sahu A, Choi WI, Tae G. Chem. Commun. 2012;48:5820–5822. doi: 10.1039/c2cc31862h. [DOI] [PubMed] [Google Scholar]
- 460.Kim H, Lee D, Kim J, Kim T-i, Kim WJ. ACS Nano. 2013;7:6735–6746. doi: 10.1021/nn403096s. [DOI] [PubMed] [Google Scholar]
- 461.Yang Z, Gao D, Cao Z, Zhang C, Cheng D, Liu J, Shuai X. Biomater. Sci. 2015;3:1035–1049. doi: 10.1039/c4bm00369a. [DOI] [PubMed] [Google Scholar]
- 462.Jochum FD, Theato P. Chem. Soc. Rev. 2013;42:7468–7483. doi: 10.1039/c2cs35191a. [DOI] [PubMed] [Google Scholar]
- 463.Mu Q, Jiang G, Chen L, Zhou H, Fourches D, Tropsha A, Yan B. Chem. Rev. 2014;114:7740–7781. doi: 10.1021/cr400295a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C. Nat. Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 465.Chanana M, Rivera_Gil P, Correa-Duarte MA, Liz-Marzán LM, Parak WJ. Angew. Chem., Int. Ed. 2013;52:4179–4183. doi: 10.1002/anie.201208019. [DOI] [PubMed] [Google Scholar]
- 466.Wan S, Kelly PM, Mahon E, Stöckmann H, Caruso F, Dawson KA, Yan Y, Monopoli MP. ACS Nano. 2015;9:2157–2166. doi: 10.1021/nn506060q. [DOI] [PubMed] [Google Scholar]
- 467.Ge C, Tian J, Zhao Y, Chen C, Zhou R, Chai Z. Arch. Toxicol. 2015;89:519–539. doi: 10.1007/s00204-015-1458-0. [DOI] [PubMed] [Google Scholar]
- 468.Lundqvist M, Stigler J, Cedervall T, Berggård T, Flanagan MB, Lynch I, Elia G, Dawson K. ACS Nano. 2011;5:7503–7509. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- 469.Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, Cohen Y, Emili A, Chan WC. ACS Nano. 2014;8:2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 470.Wang C, Dong L. Trends Biotechnol. 2015;33:10–14. doi: 10.1016/j.tibtech.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 471.Ahmad Khanbeigi R, Abelha TF, Woods A, Rastoin O, Harvey RD, Jones M-C, Forbes B, Green MA, Collins HL, Dailey LA. Biomacromolecules. 2015;16:733–742. doi: 10.1021/bm501649y. [DOI] [PubMed] [Google Scholar]
- 472.Casals E, Puntes VF. Nanomedicine. 2012;7:1917–1930. doi: 10.2217/nnm.12.169. [DOI] [PubMed] [Google Scholar]
- 473.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Baldelli Bombelli F, Dawson KA. J. Am. Chem. Soc. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 474.Hu X, Zhou Q. Chem. Rev. 2013;113:3815–3835. doi: 10.1021/cr300045n. [DOI] [PubMed] [Google Scholar]
- 475.Milani S, Baldelli Bombelli F, Pitek AS, Dawson KA, Rädler J. ACS Nano. 2012;6:2532–2541. doi: 10.1021/nn204951s. [DOI] [PubMed] [Google Scholar]
- 476.Yan Y, Gause KT, Kamphuis MM, Ang C-S, O’Brien-Simpson NM, Lenzo JC, Reynolds EC, Nice EC, Caruso F. ACS Nano. 2013;7:10960–10970. doi: 10.1021/nn404481f. [DOI] [PubMed] [Google Scholar]
- 477.Albanese A, Tang PS, Chan WC. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 478.Mahmoudi M, Lohse SE, Murphy CJ, Fathizadeh A, Montazeri A, Suslick KS. Nano Lett. 2013;14:6–12. doi: 10.1021/nl403419e. [DOI] [PubMed] [Google Scholar]
- 479.Wang L, Li J, Pan J, Jiang X, Ji Y, Li Y, Qu Y, Zhao Y, Wu X, Chen C. J. Am. Chem. Soc. 2013;135:17359–17368. doi: 10.1021/ja406924v. [DOI] [PubMed] [Google Scholar]
- 480.Miclaus T, Bochenkov VE, Ogaki R, Howard KA, Sutherland DS. Nano Lett. 2014;14:2086–2093. doi: 10.1021/nl500277c. [DOI] [PubMed] [Google Scholar]
- 481.Mahmoudi M, Saeedi-Eslami SN, Shokrgozar MA, Azadmanesh K, Hassanlou M, Kalhor HR, Burtea C, Rothen-Rutishauser B, Laurent S, Sheibani S. Nanoscale. 2012;4:5461–5468. doi: 10.1039/c2nr31185b. [DOI] [PubMed] [Google Scholar]
- 482.Monopoli MP, Åberg C, Salvati A, Dawson KA. Nat. Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 483.Shannahan JH, Brown JM, Chen R, Ke PC, Lai X, Mitra S, Witzmann FA. Small. 2013;9:2171–2181. doi: 10.1002/smll.201202243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Behzadi S, Serpooshan V, Sakhtianchi R, Müller B, Landfester K, Crespy D, Mahmoudi M. Colloids Surf., B. 2014;123:143–149. doi: 10.1016/j.colsurfb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 485.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA. Nat. Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 486.Treuel L, Brandholt S, Maffre P, Wiegele S, Shang L, Nienhaus GU. ACS Nano. 2014;8:503–513. doi: 10.1021/nn405019v. [DOI] [PubMed] [Google Scholar]
- 487.Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. J. Am. Chem. Soc. 2013;135:1438–1444. doi: 10.1021/ja309812z. [DOI] [PubMed] [Google Scholar]
- 488.Krais A, Wortmann L, Hermanns L, Feliu N, Vahter M, Stucky S, Mathur S, Fadeel B. Nanomedicine. 2014;10:1421–1431. doi: 10.1016/j.nano.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 489.Lunov O, Syrovets T, Loos C, Beil J, Delacher M, Tron K, Nienhaus GU, Musyanovych A, Mailander V, Landfester K. ACS Nano. 2011;5:1657–1669. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- 490.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nat. Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 491.Paula AJ, Araujo Júnior RT, Martinez DSfT, Paredes-Gamero EJ, Nader HB, Durán N, Justo GZ, Alves OL. ACS Appl. Mater. Interfaces. 2013;5:8387–8393. doi: 10.1021/am4014693. [DOI] [PubMed] [Google Scholar]
- 492.Yu M, Zhou C, Liu J, Hankins JD, Zheng J. J. Am. Chem. Soc. 2011;133:11014–11017. doi: 10.1021/ja201930p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Ding H-m, Ma Y-q. Biomaterials. 2014;35:8703–8710. doi: 10.1016/j.biomaterials.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 494.Jiang W, Kim BY, Rutka JT, Chan WC. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 495.Zimmer CC, Liu YX, Morgan JT, Yang G, Wang K-H, Kennedy IM, Barakat AI, Liu G-y. J. Phys. Chem. B. 2014;118:1246–1255. doi: 10.1021/jp410764f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 497.Chen N, He Y, Su Y, Li X, Huang Q, Wang H, Zhang X, Tai R, Fan C. Biomaterials. 2012;33:1238–1244. doi: 10.1016/j.biomaterials.2011.10.070. [DOI] [PubMed] [Google Scholar]
- 498.Tejamaya M, Römer I, Merrifield RC, Lead JR. Environ. Sci. Technol. 2012;46:7011–7017. doi: 10.1021/es2038596. [DOI] [PubMed] [Google Scholar]
- 499.Menard A, Drobne D, Jemec A. Environ. Pollut. 2011;159:677–684. doi: 10.1016/j.envpol.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 500.Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M. Nanotoxicology. 2014;8:233–278. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- 501.Golbamaki N, Rasulev B, Cassano A, Robinson RLM, Benfenati E, Leszczynski J, Cronin MT. Nanoscale. 2015;7:2154–2198. doi: 10.1039/c4nr06670g. [DOI] [PubMed] [Google Scholar]
- 502.Astashkina AI, Jones CF, Thiagarajan G, Kurtzeborn K, Ghandehari H, Brooks BD, Grainger DW. Biomaterials. 2014;35:6323–6331. doi: 10.1016/j.biomaterials.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 503.Lee YK, Choi E-J, Webster TJ, Kim S-H, Khang D. Int. J. Nanomed. 2015;10:97. doi: 10.2147/IJN.S72998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. Chem. Rev. 2011;111:3407–3432. doi: 10.1021/cr1003166. [DOI] [PubMed] [Google Scholar]
- 505.Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Chem. Soc. Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Arora S, Rajwade JM, Paknikar KM. Toxicol. Appl. Pharmacol. 2012;258:151–165. doi: 10.1016/j.taap.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 507.Kumar A, Dhawan A. Arch. Toxicol. 2013;87:1883–1900. doi: 10.1007/s00204-013-1128-z. [DOI] [PubMed] [Google Scholar]
- 508.Soenen SJ, Parak WJ, Rejman J, Manshian B. Chem. Rev. 2015;115:2109–2135. doi: 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- 509.Peynshaert K, Manshian BB, Joris F, Braeckmans K, De Smedt SC, Demeester J, Soenen SJ. Chem. Rev. 2014;114:7581–7609. doi: 10.1021/cr400372p. [DOI] [PubMed] [Google Scholar]
- 510.Elsaesser A, Howard CV. Adv. Drug Delivery Rev. 2012;64:129–137. doi: 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 511.Guarnieri D, Sabella S, Muscetti O, Belli V, Malvindi MA, Fusco S, De Luca E, Pompa PP, Netti PA. Nanoscale. 2014;6:10264–10273. doi: 10.1039/c4nr02008a. [DOI] [PubMed] [Google Scholar]
- 512.Winnik FM, Maysinger D. Acc. Chem. Res. 2012;46:672–680. doi: 10.1021/ar3000585. [DOI] [PubMed] [Google Scholar]
- 513.Luo M, Shen C, Feltis BN, Martin LL, Hughes AE, Wright PF, Turney TW. Nanoscale. 2014;6:5791–5798. doi: 10.1039/c4nr00458b. [DOI] [PubMed] [Google Scholar]
- 514.Hsiao I-L, Huang Y-J. Sci. Total Environ. 2011;409:1219–1228. doi: 10.1016/j.scitotenv.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 515.Sharma VK, Siskova KM, Zboril R, Gardea-Torresdey JL. Adv. Colloid Interface Sci. 2014;204:15–34. doi: 10.1016/j.cis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 516.Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ, van Loveren H, de Jong WH. Biomaterials. 2011;32:9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 517.Jimenez-Cruz CA, Kang Sg, Zhou R. Wiley Interdiscip. Rev.: Syst. Biol. Med. 2014;6:329–343. doi: 10.1002/wsbm.1271. [DOI] [PubMed] [Google Scholar]
- 518.Fröhlich E. Int. J. Nanomed. 2012;7:5577. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Smock KJ, Schmidt RL, Hadlock G, Stoddard G, Grainger DW, Munger MA. Nanotoxicology. 2014;8:328–333. doi: 10.3109/17435390.2013.788749. [DOI] [PubMed] [Google Scholar]
- 520.Kim D, Finkenstaedt-Quinn S, Hurley KR, Buchman JT, Haynes CL. Analyst. 2014;139:906–913. doi: 10.1039/c3an01679j. [DOI] [PubMed] [Google Scholar]
- 521.De Paoli SH, Diduch LL, Tegegn TZ, Orecna M, Strader MB, Karnaukhova E, Bonevich JE, Holada K, Simak J. Biomaterials. 2014;35:6182–6194. doi: 10.1016/j.biomaterials.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 522.Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, Fan C, Huang Q. ACS Nano. 2011;5:3693–3700. doi: 10.1021/nn200021j. [DOI] [PubMed] [Google Scholar]
- 523.Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Elsabahy M, Wooley KL. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Mahmoudi M, Shokrgozar MA, Behzadi S. Nanoscale. 2013;5:3240–3244. doi: 10.1039/c3nr32551b. [DOI] [PubMed] [Google Scholar]
- 526.Wang X, Reece SP, Brown JM. Toxicol. Mech. Methods. 2013;23:168–177. doi: 10.3109/15376516.2012.757686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Hühn D, Kantner K, Geidel C, Brandholt S, De Cock I, Soenen SJ, Rivera_Gil P, Montenegro J-M, Braeckmans K, Mullen K. ACS Nano. 2013;7:3253–3263. doi: 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- 528.Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. J. Biomed. Mater. Res., Part A. 2012;100:1033–1043. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- 529.Gerloff K, Fenoglio I, Carella E, Kolling J, Albrecht C, Boots AW, Förster I, Schins RP. Chem. Res. Toxicol. 2012;25:646–655. doi: 10.1021/tx200334k. [DOI] [PubMed] [Google Scholar]
- 530.Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Part. Fibre Toxicol. 2014;11:1–17. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Ji Z, Wang X, Zhang H, Lin S, Meng H, Sun B, George S, Xia T, Nel AE, Zink JI. ACS Nano. 2012;6:5366–5380. doi: 10.1021/nn3012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Zhao X, Ng S, Heng BC, Guo J, Ma L, Tan TTY, Ng KW, Loo SCJ. Arch. Toxicol. 2013;87:1037–1052. doi: 10.1007/s00204-012-0827-1. [DOI] [PubMed] [Google Scholar]
- 533.Palombo M, Deshmukh M, Myers D, Gao J, Szekely Z, Sinko PJ. Annu. Rev. Pharmacol. Toxicol. 2014;54:581. doi: 10.1146/annurev-pharmtox-010611-134615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Rodrigues S, Cordeiro C, Seijo B, Remuñán-López C, Grenha A. Carbohydr. Polym. 2015;123:369–380. doi: 10.1016/j.carbpol.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 535.Wang J, Liu H, Leng F, Zheng L, Yang J, Wang W, Huang CZ. Microporous Mesoporous Mater. 2014;186:187–193. [Google Scholar]
- 536.Hu X, Wang Y, Peng B. Chem. – Asian J. 2014;9:319–327. doi: 10.1002/asia.201301105. [DOI] [PubMed] [Google Scholar]
- 537.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. Nanomedicine. 2013;9:1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Ho RJ, Chien JY. J. Pharm. Sci. 2009;98:1928–1934. doi: 10.1002/jps.21649. [DOI] [PubMed] [Google Scholar]
- 539.Mura S, Nicolas J, Couvreur P. Nat. Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 540.Garcia A, Kempf R, Rogers M, Muggia F. Ann. Oncol. 1998;9:1131–1133. doi: 10.1023/a:1008439013169. [DOI] [PubMed] [Google Scholar]
- 541.Israel VP, Garcia AA, Roman L, Muderspach L, Burnett A, Jeffers S, Muggia FM. Gynecol. Oncol. 2000;78:143–147. doi: 10.1006/gyno.2000.5819. [DOI] [PubMed] [Google Scholar]
- 542.Iqbal S, Tsao-Wei DD, Quinn DI, Gitlitz BJ, Groshen S, Aparicio A, Lenz HJ, El-Khoueiry A, Pinski J, Garcia AA. Am. J. Clin. Oncol. 2011;34:27–31. doi: 10.1097/COC.0b013e3181cae766. [DOI] [PubMed] [Google Scholar]
- 543.Garcia AA, Roman L, Muderspach L, O’Meara A, Facio G, Edwards S, Burnett A. Cancer Invest. 2005;23:665–670. doi: 10.1080/07357900500359877. [DOI] [PubMed] [Google Scholar]
- 544.Keller AM, Mennel RG, Georgoulias VA, Nabholtz J-M, Erazo A, Lluch A, Vogel CL, Kaufmann M, von Minckwitz G, Henderson IC. J. Clin. Oncol. 2004;22:3893–3901. doi: 10.1200/JCO.2004.08.157. [DOI] [PubMed] [Google Scholar]
- 545.O’brien M, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback D, Tomczak P, Ackland S. Ann. Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 546.Stopeck AT, Hersh EM, Akporiaye ET, Harris DT, Grogan T, Unger E, Warneke J, Schluter SF, Stahl S. J. Clin. Oncol. 1997;15:341–349. doi: 10.1200/JCO.1997.15.1.341. [DOI] [PubMed] [Google Scholar]
- 547.Stopeck AT, Jones A, Hersh EM, Thompson JA, Finucane DM, Gutheil JC, Gonzalez R. Clin. Cancer Res. 2001;7:2285–2291. [PubMed] [Google Scholar]