Abstract
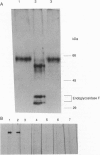
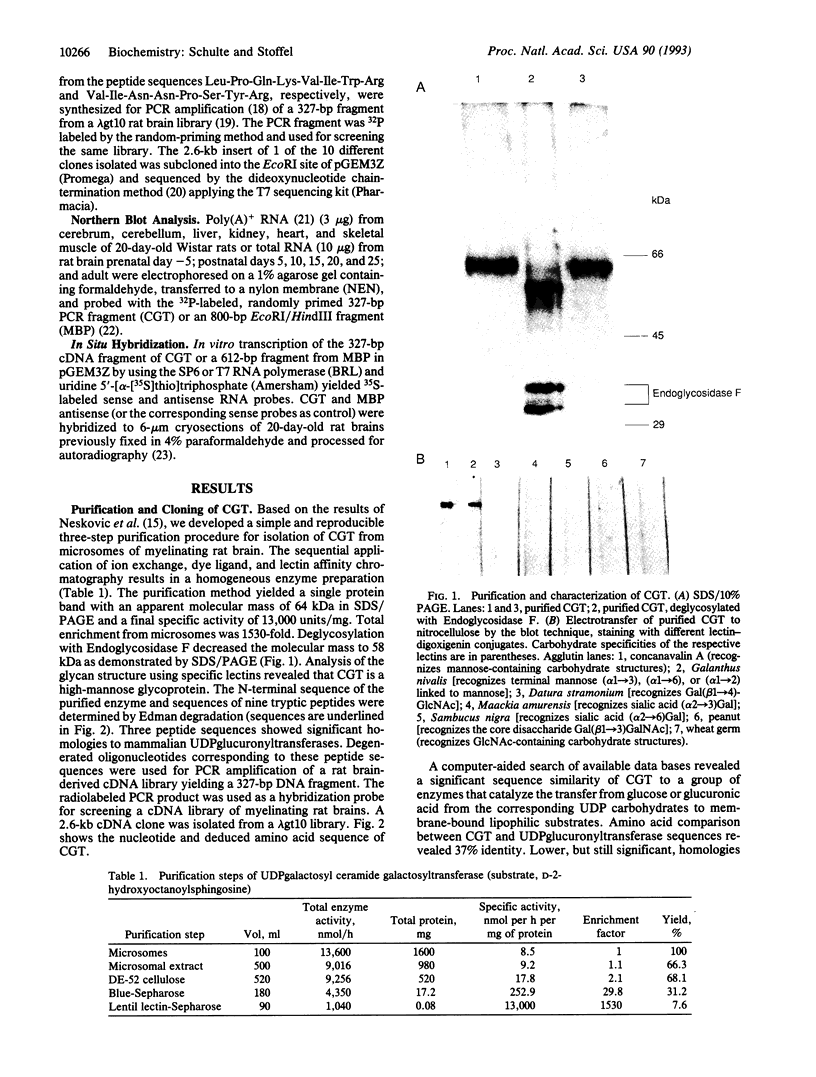
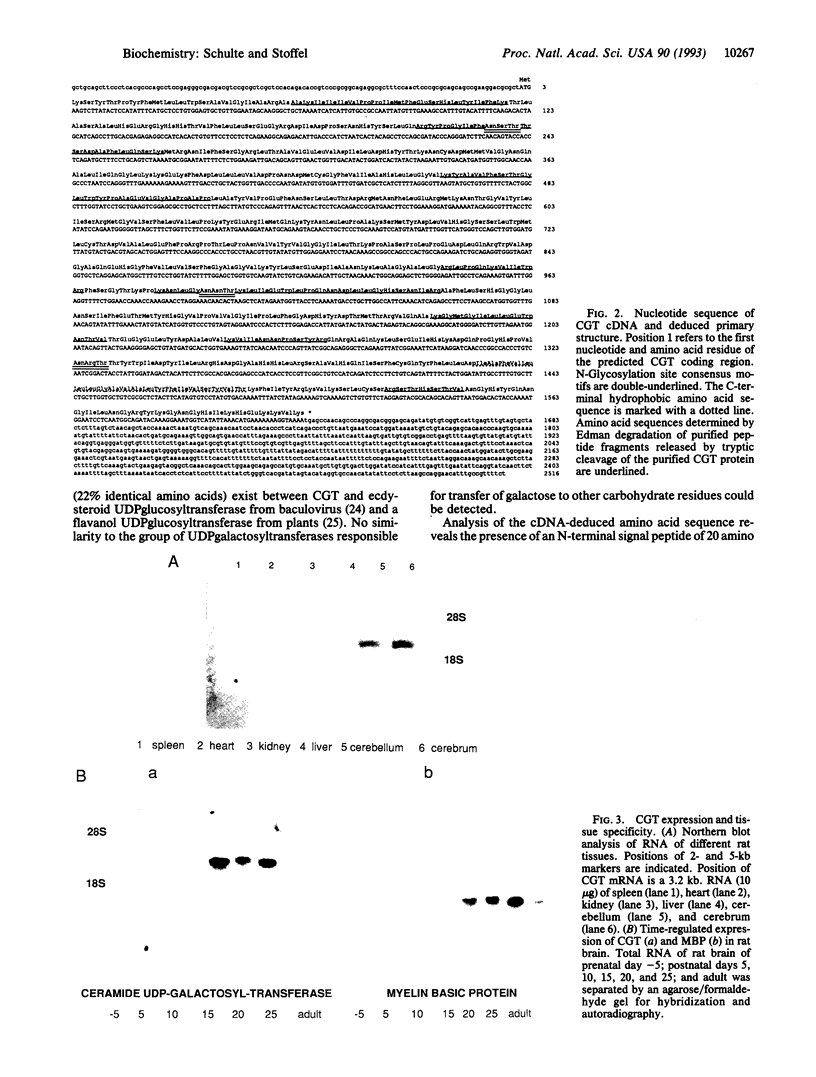
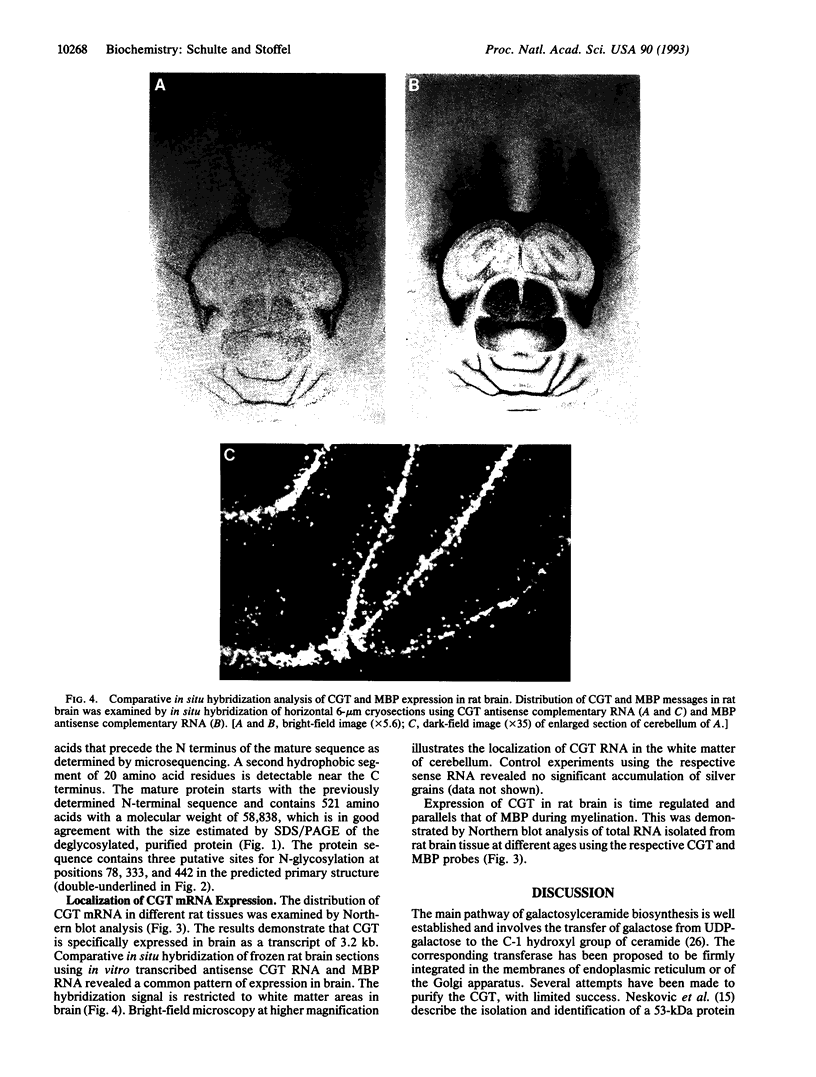
Cerebrosides and sulfatides are major glycosphingolipids of the lipid bilayer of the myelin sheath assembled by oligodendrocytes and Schwann cells during myelination. Cerebrosides are synthesized by ceramide UDPgalactosyltransferase [CGT; 2-hydroxyacylsphinogosine 1-beta-galactosyl-transferase; UDPgalactose:2-(2-hydroxyacyl)sphingosine 1-beta-D-galactosyltransferase; UDPgalactose:2-(2-hydroxyacyl)sphingosine 1-beta-D-galactosyltransferase, EC 2.4.1.45] with UDPgalactose and ceramide as substrates. Here we describe a purification method from microsomes of myelinating rat brains that includes ion exchange, dye ligand, and lectin affinity chromatography. The enzyme was identified as a 64-kDa high-mannose glycoprotein. A CGT-specific cDNA clone was isolated from a rat brain cDNA library using CGT oligonucleotides derived from peptide sequences. The cDNA insert encodes a polypeptide of 541 amino acid residues with a molecular weight of 61,126. The polypeptide has three putative glycosylation sites and one hydrophobic domain at the C terminus. A 20-residue N-terminal signal sequence is lost during cotranslational translocation. Northern blot analysis demonstrates that CGT expression is restricted to brain tissue and is time dependent, correlating with myelin basic protein expression. In situ hybridization reveals that CGT expression is restricted to the oligodendrocyte-containing cell layers of cerebrum and cerebellum, which also express myelin basic protein. The amino acid sequence of CGT shows significant homology to mammalian UDPglucuronyltransferases, which suggests a common evolutionary origin of these enzymes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenkert A., Radin N. S. Synthesis of galactosyl ceramide and glucosyl ceramide by rat brain: assay procedures and changes with age. Brain Res. 1972 Jan 14;36(1):183–193. doi: 10.1016/0006-8993(72)90774-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Costantino-Ceccarini E., Morell P. Biosynthesis of brain sphingolipids and myelin accumulation in the mouse. Lipids. 1972 Oct;7(10):656–659. doi: 10.1007/BF02533072. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B. Synthesis and turnover of cerebroside sulfate of myelin in adult and developing rat brain. J Lipid Res. 1974 Mar;15(2):114–123. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liscum L., Cummings R. D., Anderson R. G., DeMartino G. N., Goldstein J. L., Brown M. S. 3-Hydroxy-3-methylglutaryl-CoA reductase: a transmembrane glycoprotein of the endoplasmic reticulum with N-linked "high-mannose" oligosaccharides. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7165–7169. doi: 10.1073/pnas.80.23.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P., Radin N. S. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry. 1969 Feb;8(2):506–512. doi: 10.1021/bi00830a008. [DOI] [PubMed] [Google Scholar]
- Neskovic N. M., Roussel G., Nussbaum J. L. UDPgalactose:ceramide galactosyltransferase of rat brain: a new method of purification and production of specific antibodies. J Neurochem. 1986 Nov;47(5):1412–1418. doi: 10.1111/j.1471-4159.1986.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Neskovic N. M., Sarlieve L. L., Mandel P. Brain UDPgalactose: ceramide galactosyltransferase Purification of a catalytically active protein obtained after proteolytic digestion. Biochim Biophys Acta. 1976 Apr 8;429(2):342–351. doi: 10.1016/0005-2744(76)90282-5. [DOI] [PubMed] [Google Scholar]
- Neskovic N. M., Sarlieve L. L., Mandel P. Subcellular and submicrosomal distribution of glycolipid-synthesizing transferases in young rat brain. J Neurochem. 1973 May;20(5):1419–1430. doi: 10.1111/j.1471-4159.1973.tb00254.x. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science. 1989 Sep 8;245(4922):1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist H. P., Burkart T., Wiesmann U. N., Herschkowitz N. N., Spycher M. A. Ceramide-galactosyltransferase and cerebroside-sulphotranserase localisation in Golgi membranes isolated by a continuous sucrose gradient of mouse brain microsomes. J Neurochem. 1979 Aug;33(2):497–504. doi: 10.1111/j.1471-4159.1979.tb05180.x. [DOI] [PubMed] [Google Scholar]
- Storck T., Schulte S., Hofmann K., Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]