Abstract
Open retropubic radical prostatectomy (ORP) remains the “gold standard” for surgical treatment of clinically localized prostate cancer (PCa). Robot-assisted radical prostatectomy (RARP) is a robotic surgery used worldwide. The aim of this study is to collect the data available in the literature on RARP and ORP, and further evaluate the overall safety and efficacy of RARP vs. ORP for the treatment of clinically localized PCa. A literature search was performed using electronic databases between January 2009 and October 2013. Clinical data such as operation duration, transfusion rate, positive surgical margins (PSM), nerve sparing, 3- and 12-month urinary continence, and potency were pooled to carry out meta-analysis. Six studies were enrolled for this meta-analysis. The operation duration of RARP group was longer than that of ORP group (weighted mean difference = 64.84). There was no statistically significant difference in the transfusion rate, PSM rate, and between RARP and ORP (transfusion rate, OR = 0.30; PSM rate, OR = 0.94). No significant difference was seen in 3- and 12-month urinary continence recovery (3 months, OR = 1.32; 12 months, OR = 1.30). There was a statistically significant difference in potency between the 3- and 12-month groups (3 months, OR = 2.80; 12 months, OR = 1.70). RARP is a safe and feasible surgical technique for the treatment of clinically localized PCa owing to the advantages of fewer perioperative complications and quicker patency recovery.
Keywords: Robot-assisted radical prostatectomy, Open retropubic radical prostatectomy, Prostate cancer
Introduction
Prostate cancer (PCa) is the most commonly diagnosed male carcinoma, accounting for 28 % (238,590) of incident cases in all male cancers in 2013, and the second leading cause of cancer-related death among men in the USA [1]. With dietary and lifestyle changes in the recent years, the incidence of PCa in China has increased substantially and has become one of the common malignancies in the male urinary system. Prostate-specific antigen (PSA) has routinely been used as a marker for the diagnosis of PCa. According to the American Urological Association (AUA) Guidelines (2013), the Panel strongly recommends that men aged 55–69 years consider PSA routine screening at 2-year intervals [2]. Since the prevalence of PSA screening, clinically localized PCa has been detected increasingly earlier.
Radical prostatectomy (RP) has been shown to be the most efficient treatment for PCa patients [3], and open retropubic radical prostatectomy (ORP) remains the “gold standard” procedure among all surgical treatments [4]. Nonetheless, ORP is associated with more blood loss and transfusion, longer hospital stay, more intraoperative and postoperative complications, and higher in-hospital mortalities as compared with other analogous surgical procedures [5]. These unfavorable factors urge researchers to develop safer and more effective alternative surgical procedures to replace ORP.
Owing to the wide use of minimally invasive radical prostatectomy techniques, robot-assisted radical prostatectomy (RARP) has drawn the attention of the world. Pasticier et al. [6] performed the first robot-assisted laparoscopic prostatectomy (RALP) with the Da Vinci robot (Intuitive Inc., Mountain View, CA, USA) in 2000. In the USA, 52.7 % of the current procedures are robotic assisted laparoscopic surgeries compared to 44.4 % open surgeries in 2008, and now RARP is more prevalent than open surgery in urban hospitals among white patients in high volume [7]. The reasons for the hot trend is that the robotic surgical system has numerous unprecedented advantages in radical prostatectomy, such as 3D vision, precise movements without physical limitations, enhanced magnification, and tremor filtering [8].
Therefore, several studies have been carried out to evaluate the superiority of RARP over ORP. RARP is associated with improving quality of life and intra- and postoperative outcomes as compared with ORP [9, 10]. However, the data in the current literature do not provide convincing evidence to assess the real superiority of this robotic surgical technique over traditional surgery due to the lack of randomized controlled trials. Our main aim is to collect the existing literature on RARP and ORP and further evaluate the overall safety and efficacy of RARP vs. ORP for clinically localized PCa.
Methods
A literature search was performed using PubMed (US National Library of Medicine National, Institutes of Health Search database), Google Scholar, Embase, and Web of Science. We limited our search to English-language articles and time span between January 2009 and October 2013. The following keywords were used: open retropubic radical prostatectomy or ORP, robot-assisted radical prostatectomy or RARP. We included all latest relevant studies comparing RARP and ORP and all included patients of clinically localized PCa, and excluded studies on laparoscopic prostatectomy without comparing robot-assisted, non-comparative studies, patients treated with preoperative radiotherapy or neoadjuvant androgen deprivation therapy, and patients with high-risk PCa.
The Newcastle–Ottawa Scale (NOS) was performed to estimate the methodological quality of these studies. The NOS is known as a “star system” including three broad perspectives: study group selection (four items, four stars), group comparability (two items, two stars), and outcome ascertainment (three items, three stars) [11] .
Two reviewers independently carried out data extraction by searching the full texts of included studies. The extracted data were authors, publication year, treatments, number of patients, operation duration, transfusion rate, positive surgical margins (PSM), nerve sparing, urinary continence, and potency.
A meta-analysis was performed to compare the efficacy and safety of RARP and ORP. Tests for homogeneity were performed by the Cochrane Inconsistency (I2). A value of P >0.10 shows homogeneity of included studies, and I2 <50 % shows acceptable heterogeneity. Dichotomous variables and continuous variables were pooled by odds ratio (OR) and weighted mean difference (WMD), respectively. The fixed-effect model (Mantel–Haenszel method) [12] was applied to calculate pooled estimates for homogeneous studies, and the random-effect model (DerSimonian–Laird method) [13] was used for heterogeneous studies. The pooled effects were measured by means of Z test, and P ≤0.05 was considered statistically significant. Data analysis was carried out with Review Manager (RevMan 5.1, Cochrane Collaboration, Oxford, UK).
Results
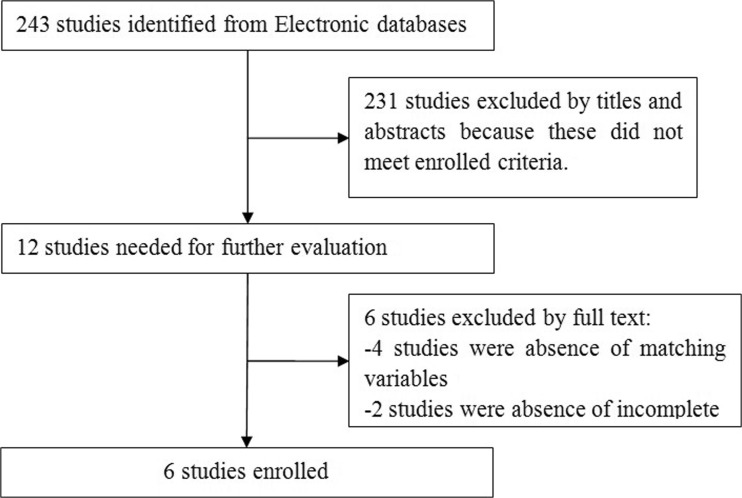
A total of 243 related studies were identified for further evaluation by searching PubMed, Google Scholar, Embase, and Web of Science. Finally, six studies [14–19] were enrolled for this meta-analysis (Fig. 1). Table 1 shows the characteristics of included studies.
Fig. 1.
Flowchart showing filtering studies for the meta-analysis
Table 1.
Characteristics of included studies
| Authors | Year | Treatments | Number of patients | Operation duration (min) | Transfusion (%) | PSM (%) | Urinary continence (%) | Potency (%) | Publication type | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months | 12 months | 3 months | 12 months | ||||||||
| Choo et al. [14] | 2013 | RALP | 77 | 220 | 17 | 40 | 71 | 94 | 17 | 54 | Non RCT |
| ORP | 176 | 151 | 18 | 40 | 80 | 96 | 6 | 40 | |||
| Di Pierro et al. [15] | 2011 | RALP | 75 | 330 | – | 12 | 95 | 89 | 55 | 71 | Non RCT |
| RRP | 75 | 253 | – | 24 | 83 | 80 | 26 | 65 | |||
| Lo et al. [16] | 2010 | RARP | 20 | 306 | 5 | 20 | – | – | – | – | Non RCT |
| ORP | 20 | 289 | 65 | 25 | – | – | – | – | |||
| Doumerc et al. [17] | 2010 | RALP | 212 | 192 | 0.9 | 21.2 | – | – | – | – | Non RCT |
| RRP | 502 | 147 | 2 | 84 | – | – | – | – | |||
| Rocco et al. [18] | 2009 | RARP | 120 | – | – | 22 | 70 | 97 | 31 | 61 | Non RCT |
| RRP | 240 | – | – | 25 | 63 | 88 | 18 | 41 | |||
| Krambeck et al. [19] | 2009 | RARP | 294 | – | – | 15.6 | – | 91.8 | – | 70 | Non RCT |
| RRP | 558 | – | – | 17 | – | 93.7 | – | 62.8 | |||
Data are shown as mean or rate
RALP robot-assisted laparoscopic prostatectomy, RARP robot-assisted radical prostatectomy, ORP open retropubic radical prostatectomy, RRP retropubic radical prostatectomy, PSM positive surgical margins, RCT randomized controlled trials
The results of NOS quality assessment for the enrolled studies are indicated in Table 2. Two high-quality studies won a score of 9. The remaining four studies were scored 8 because of the lack of adequate follow-up data or missing follow-up data.
Table 2.
The Newcastle–Ottawa scale for quality assessment of included studies
| Studies | Selection | Comparability | Outcomes | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest was not present at start of study | Based on the design or analysis | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||
| Choo et al. [14] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Di Pierro et al. [15] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Lo et al. [16] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Doumerc et al. [17] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Rocco et al. [18] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Krambeck et al. [19] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
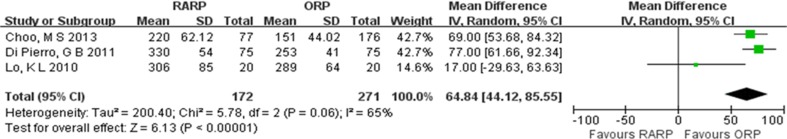
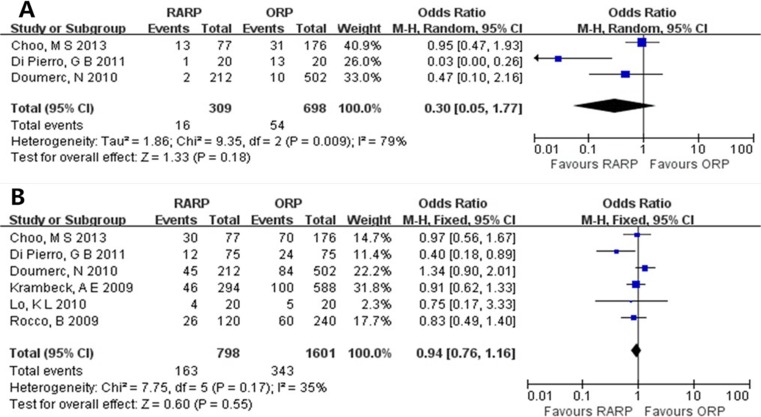
The operation duration of RARP group was longer than that of ORP group [weighted mean difference (WMD) = 64.84, 95 % confidential interval (95 % CI) = 44.12–85.55, P < 0.00001] (Fig. 2). There was no statistically significant difference in the transfusion and PSM rate between RARP and ORP groups (transfusion rate, OR = 0.30, 95 % CI = 0.05–1.77, P = 0.18; PSM rate, OR = 0.94, 95 % CI = 0.76–1.16, P = 0.55) (Fig. 3). There were no statistically significant differences in urinary continence recovery at 3- and 12-month postoperative follow-up between RARP and ORP groups (3 months, OR = 1.32, 95 % CI = 0.58–3.03, P = 0.51; 12 months, OR = 1.30, 95 % CI = 0.55–3.09, P = 0.55) (Fig. 4).
Fig. 2.
Pooled estimates of operation duration
Fig. 3.
Pooled estimates of transfusion rate (a) and PSM rate (b)
Fig. 4.
Pooled estimates of urinary continence recovery at 3 months (a) and 12 months (b) of follow-up
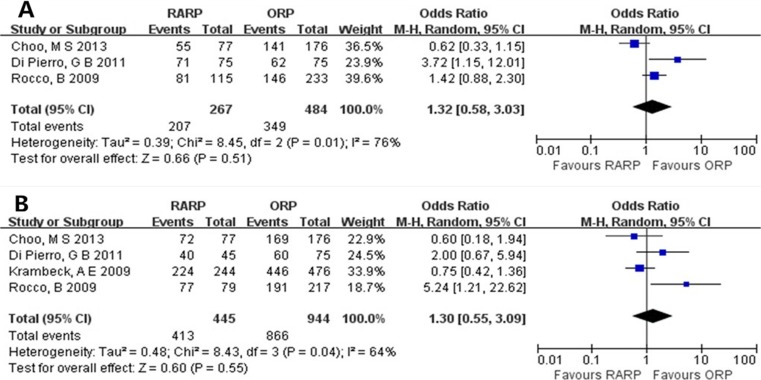
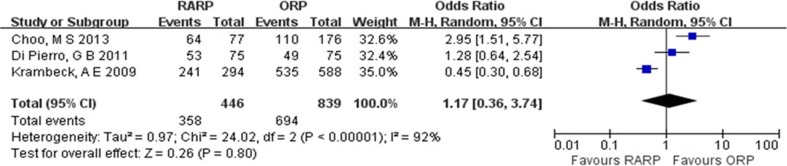
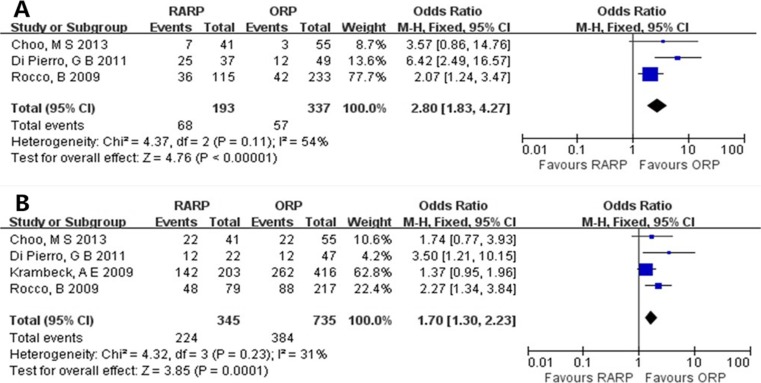
All patients included to assess the potency had satisfactory potency before surgery (defined according to the International Index of Erectile Function–5). Despite similar nerve sparing (bilateral or unilateral) between the 3- and 12-month groups (OR = 1.17, 95% CI = 0.36–3.74, P = 0.80) (Fig. 5), the potency recovery for RARP group was significantly quicker than for ORP group (3 months, OR = 2.80, 95% CI = 1.83–4.27, P < 0.00001; 12 months, OR = 1.70, 95% CI = 1.30–2.23, P = 0.0001) (Fig. 6).
Fig. 5.
Pooled estimates of nerve sparing
Fig. 6.
Pooled estimates of potency recovery at 3 months (a) and 12 months (b) of follow-up
Discussion
Robotic surgery represents a revolutionary progress in the history of urology and has become a hot area among many researchers. A growing body of literature has reported the superiority of robotic surgery as compared with traditional surgery [20–23]. On this foundation, our study would further explain the advantages of RARP for treating clinically localized PCa vs. ORP.
According to a nationwide inpatient sample, blood loss and transfusions of patients treated with RARP were less than ORP [24]. Kordan et al. [25] reported that estimated blood loss of patients undergoing RALP ranged 50–200 ml vs. 300–600 ml in patients undergoing ORP. However, many factors can affect intraoperative blood loss. With the current prevalence of cardiovascular diseases, more PCa patients administer antithrombotic drugs daily, which increases the risk of surgical procedures. A recent study [26] reported that the mean blood loss of patients who administered aspirin 750 ml for ORP and 700 ml for RARP, and the transfusion rate in patients undergoing RARP was significantly lower than that in patients undergoing ORP (8 % vs. 21 %). Another study [27] found that Hct% reduction was more significant in patients who used statins than that in patients without using statins (20.7 % vs. 18.6 %) when they underwent ORP; however, there was no significant change in Hct% in patients undergoing RARP.
Prostate-specific membrane antigen (PSM) is a risk factor contributing to postoperative tumor recurrence. Our meta-analysis showed no statistically significant difference between RARP and ORP. However, a most recent non-randomized observational study reported that RARP had a lower PSM rate than the open approach, and postoperative adjuvant therapies including radiotherapy, chemotherapy, or androgen deprivation therapy seemed less likely to be accepted by patients who received RARP as compared with those who received ORP (OR = 0.59, 95% CI = 0.39–0.88, P = 0.010) [28]. On the contrary, Williams et al. [29] reported that the PSM rate in patients undergoing nerve-sparing RALP and nerve-sparing ORP was 13.5 % and 7.6 %, respectively. For high-risk PCa patients, a recent retrospective study showed no significant difference in oncological outcomes between the two groups [30]. Similarly, Punnen et al. [31] reported no statistically significant difference in the PSM rate in patients with high-risk PCa between RARP and ORP groups.
Urinary incontinence is a common postoperative long-term complication after radical prostatectomy [32]. Therefore, postoperative urinary continence recovery is defined as 0–1 pad per day, which is also a measure of the quality of life for patients treated with RP. In our meta-analysis, the outcome of 3- and 12-month urinary continence recovery in RARP group was similar to that in ORP group. Likewise, Froehner et al. [33] reported no detectable difference in continence recovery between the two groups. However, a study [34] reported that patients undergoing RALP might have a better functional outcome and earlier recovery of urinary incontinence than patients undergoing RP. In a recent single surgeon experience report [35], the 12-month recovery of continence favored RALP as compared with ORP, and factors contributing to this difference were associated with the operation method, patient age, and the membranous urethral length.
Potency after RP is an important indicator for assessing the quality of life. Brandina et al. [10] showed that the potency rate was 79.2–80.4 % during the 1-year follow-up period. Our meta-analysis showed that the 3- and 12-month postoperative potency in RARP group was significantly better than that in ORP group. However, Rocco et al. [14] reported that functional erection of patients undergoing RARP was significantly higher than that of patients undergoing ORP. Additionally, Krambeck et al. [19] reported no statistical difference between the two groups.
Compared with ORP, RARP also showed spectacular advantages in other related complications. Bladder neck contracture (BNC) is a known complication of RP. Breyer et al. [36] reported that the BNC rate was 1.4 % for RALP and 2.6 % for ORP. Webb et al. [37] also reported that RALP was superior to ORP in the incidence of BNC. The incidence rate of inguinal hernia (IH) as a postoperative complication may have a difference due to surgical methods. Stranne et al. [38] reported that the cumulative incidence rate of IH at 48 months was 12.2 % for patients undergoing ORP and 5.8 % for patients undergoing RALP, the difference being statistically significant.
During the past decade, RARP has become the dominant surgical approach in the treatment of PCa due to enormous advantages over ORP. RARP is easily accepted for young surgeons because of the shorter learning curve as compared with other radical prostatectomy procedures. A systematic review [39] recently reported that the learning curve for RALP was 40 procedures as a minimum number compared with the learning curve for ORP ranging from 250 to 1,000 cases. Nonetheless, many factors need to be considered. The cost of treatment for PCa is a concern that most patients have to consider before surgery. The total actual costs associated with RARP were significantly greater than those for ORP [40, 41]. According to the statistics [42], the 1-, 2-, 5-, and 10-year cumulative cost of RARP was $17,824, $18,308, $20,117, and $22,762, respectively, vs. $9,732, $10,360, $12,209, and $15,084 for ORP. The high cost of RARP is attributable to robot purchase, maintenance, and supplies, which was the main factor for limiting its widespread adoption. Another factor is that the Da Vinci robotic surgical system also has inevitable malfunction, although this situation is uncommon. An international survey [43] showed that robotic malfunction could occur at any time during surgery. Therefore, well-trained and experienced surgeons are required to handle these mechanical failures to complete the prostatectomy.
There were some limitations in our study. First, due to the lack of randomized controlled trials in this area, all studies included for meta-analysis were non-randomized controlled studies. Second, several data were unavailable in some studies due to the lack of complete data. For example, blood loss, catheterization, hospital stay, and pelvic lymph node dissection PLND were provided only with median, range, and 25th percentile and 75th percentile in some studies. Third, some included studies lacked adequate follow-up data. Two studies did not show follow-up data and one study did not show early follow-up data.
Conclusions
Despite the absence of randomized controlled trials to provide high-quality evidence, our results demonstrated that RARP has the advantages of fewer perioperative complications and quicker potency recovery as compared with ORP, and therefore it is a safe and feasible surgical technique for the treatment of clinically localized PCa. However, prospective randomized studies are required to further evaluate the role of RARP in the treatment of localized PCa.
Acknowledgments
Disclosure Statement
No competing financial interests exist.
Abbreviations
- PCa
Prostate cancer
- RP
Radical prostatectomy
- ORP
Open retropubic radical prostatectomy
- RRP
Retropubic radical prostatectomy
- RARP
Robot-assisted radical prostatectomy
- RALP
Robot-assisted laparoscopic prostatectomy
- PSA
Prostate-specific antigen
- NOS
Newcastle–Ottawa Scale
- PSM
Positive surgical margins
- OR
Odds ratio
- WMD
Weighted mean difference
- BNC
Bladder neck contracture
- IH
Inguinal hernia
- LC
Learning curve
Footnotes
Xiu-wu Pan and Xin-ming Cui contributed equally to this work.
Contributor Information
Xiu-wu Pan, Phone: +86-21-81885726, Email: panxiuwu@126.com.
Xin-ming Cui, Phone: +86-21-81885726, Email: xinmingcui_2008@163.com.
Jing-fei Teng, Phone: +86-21-81885726, Email: tengjingfei@yahoo.cn.
Dong-xu Zhang, Phone: +86-21-81885726, Email: zdx570786324@126.com.
Zhi-jun Wang, Phone: +86-21-81885726, Email: zhijunwang_08@163.com.
Fa-jun Qu, Phone: +86-21-81885726, Email: fajunqu@163.com.
Yi Gao, Phone: +86-21-81885726, Email: yigao_2009@163.com.
Xin-gang Cui, Phone: +86-21-81885726, Email: cuixingang@163.com.
Dan-feng Xu, Phone: +86-21-81885721, Email: xu-danfeng@hotmail.com.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part I: screening, diagnosis, and treatment of clinically localised disease. Actas Urol Esp. 2011;35:501–514. doi: 10.1016/j.acuro.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein J, Eckersberger E, Sadri H, Taneja SS, Lepor H, Djavan B. Open versus laparoscopic versus robot-assisted laparoscopic prostatectomy: the European and US experience. Rev Urol. 2010;12:35–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Sammon JD, Karakiewicz PI, Sun M, et al. Robot-assisted versus open radical prostatectomy: the differential effect of regionalization, procedure volume and operative approach. J Urol. 2013;189:1289–1294. doi: 10.1016/j.juro.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Pasticier G, Rietbergen JB, Guillonneau B, Fromont G, Menon M, Vallancien G. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. 2001;40:70–74. doi: 10.1159/000049751. [DOI] [PubMed] [Google Scholar]
- 7.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187:1392–1398. doi: 10.1016/j.juro.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 8.Piechaud P. State of the art: urologic surgery. J Visc Surg. 2011;148:e27–e29. doi: 10.1016/j.jviscsurg.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Robertson C, Close A, Fraser C, et al. Relative effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of localised prostate cancer: a systematic review and mixed treatment comparison meta-analysis. BJU Int. 2013;112:798–812. doi: 10.1111/bju.12247. [DOI] [PubMed] [Google Scholar]
- 10.Brandina R, Fau - Berger A, Berger A, Fau-Kimoi K, Fau-Gill IS, Gill IS. Critical appraisal of robotic-assisted radical prostatectomy. Curr Opin Urol. 2009;19:290–296. doi: 10.1097/MOU.0b013e328329a356. [DOI] [PubMed] [Google Scholar]
- 11.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al (2009) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 19 October 2009
- 12.Mantel NHW. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Choo MS, Choi WS, Cho SY, Ku JH, Kim HH, Kwak C. Impact of prostate volume on oncological and functional outcomes after radical prostatectomy: robot-assisted laparoscopic versus open retropubic. Korean J Urol. 2013;54:15–21. doi: 10.4111/kju.2013.54.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Pierro GB, Baumeister P, Stucki P, Beatrice J, Danuser H, Mattei A. A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol. 2011;59:1–6. doi: 10.1016/j.eururo.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Lo KL, Ng CF, Lam CN, Hou SS, To KF, Yip SK. Short-term outcome of patients with robot-assisted versus open radical prostatectomy: for localised carcinoma of prostate. Hong Kong Med J. 2010;16:31–35. [PubMed] [Google Scholar]
- 17.Doumerc N, Yuen C, Savdie R, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int. 2010;106:378–384. doi: 10.1111/j.1464-410X.2009.09158.x. [DOI] [PubMed] [Google Scholar]
- 18.Rocco B, Matei DV, Melegari S, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int. 2009;104:991–995. doi: 10.1111/j.1464-410X.2009.08532.x. [DOI] [PubMed] [Google Scholar]
- 19.Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–453. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 20.Xylinas E, Ploussard G, Durand X, de la Taille A. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: a review of the current literature. Urol Oncol. 2013;31:288–293. doi: 10.1016/j.urolonc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Lim SK, Kim KH, Shin TY, Rha KH. Current status of robot-assisted laparoscopic radical prostatectomy: how does it compare with other surgical approaches? Int J Urol. 2013;20:271–284. doi: 10.1111/j.1442-2042.2012.03193.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang KH, Carter SC, Hu JC. Does robotic prostatectomy meet its promise in the management of prostate cancer? Curr Urol Rep. 2013;14:184–191. doi: 10.1007/s11934-013-0327-8. [DOI] [PubMed] [Google Scholar]
- 23.Duffey B, Varda B, Konety B. Quality of evidence to compare outcomes of open and robot-assisted laparoscopic prostatectomy. Curr Urol Rep. 2011;12:229–236. doi: 10.1007/s11934-011-0180-6. [DOI] [PubMed] [Google Scholar]
- 24.Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol. 2012;61:679–685. doi: 10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Kordan Y, Barocas DA, Altamar HO, et al. Comparison of transfusion requirements between open and robotic-assisted laparoscopic radical prostatectomy. BJU Int. 2010;106:1036–1040. doi: 10.1111/j.1464-410X.2010.09233.x. [DOI] [PubMed] [Google Scholar]
- 26.Leyh-Bannurah SR, Hansen J, Isbarn H, et al. (2013) Open and robotic assisted radical retropubic prostatectomy in men with ongoing low-dose aspirin medication: revisiting old paradigm? BJU Int 76:8 [DOI] [PubMed]
- 27.Truesdale MD, Polland AR, Graversen JA, et al. Impact of HMG-CoA reductase inhibitor (statin) use on blood loss during robot-assisted and open radical prostatectomy. J Endourol. 2011;25:1427–1433. doi: 10.1089/end.2010.0688. [DOI] [PubMed] [Google Scholar]
- 28.Evans SM, Millar JL, Frydenberg M, et al. Positive surgical margins: rate, contributing factors and impact on further treatment: findings from the Prostate Cancer Registry. BJU Int. 2013 doi: 10.1111/bju.12509. [DOI] [PubMed] [Google Scholar]
- 29.Williams SB, Chen MH, D’Amico AV, et al. Radical retropubic prostatectomy and robotic-assisted laparoscopic prostatectomy: likelihood of positive surgical margin(s) Urology. 2010;76:1097–1101. doi: 10.1016/j.urology.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 30.Silberstein JL, Su D, Glickman L, et al. A case-mix-adjusted comparison of early oncological outcomes of open and robotic prostatectomy performed by experienced high volume surgeons. BJU Int. 2013;111:206–212. doi: 10.1111/j.1464-410X.2012.11638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punnen S, Meng MV, Cooperberg MR, Greene KL, Cowan JE, Carroll PR. How does robot-assisted radical prostatectomy (RARP) compare with open surgery in men with high-risk prostate cancer? BJU Int. 2013;112:E314–E320. doi: 10.1111/j.1464-410X.2012.11493.x. [DOI] [PubMed] [Google Scholar]
- 32.Hautmann RE, Sauter TW, Wenderoth UK. Radical retropubic prostatectomy: morbidity and urinary continence in 418 consecutive cases. Urology. 1994;43:47–51. doi: 10.1016/0090-4295(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 33.Froehner M, Koch R, Leike S, Novotny V, Twelker L, Wirth MP. Urinary tract-related quality of life after radical prostatectomy: open retropubic versus robot-assisted laparoscopic approach. Urol Int. 2013;90:36–40. doi: 10.1159/000345320. [DOI] [PubMed] [Google Scholar]
- 34.Iseki R, Ohori M, Hatano T, Tachibana M. Urinary incontinence in early experience with robot-assisted laparoscopic prostatectomy—comparison with radical retropubic prostatectomy. Hinyokika Kiyo. 2012;58:409–414. [PubMed] [Google Scholar]
- 35.Son SJ, Lee SC, Jeong CW, Jeong SJ, Byun SS, Lee SE. Comparison of continence recovery between robot-assisted laparoscopic prostatectomy and open radical retropubic prostatectomy: a single surgeon experience. Korean J Urol. 2013;54:598–602. doi: 10.4111/kju.2013.54.9.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breyer BN, Davis CB, Cowan JE, Kane CJ, Carroll PR. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int. 2010;106:1734–1738. doi: 10.1111/j.1464-410X.2010.09333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb DR, Sethi K, Gee K. An analysis of the causes of bladder neck contracture after open and robot-assisted laparoscopic radical prostatectomy. BJU Int. 2009;103:957–963. doi: 10.1111/j.1464-410X.2008.08278.x. [DOI] [PubMed] [Google Scholar]
- 38.Stranne J, Johansson E, Nilsson A, et al. Inguinal hernia after radical prostatectomy for prostate cancer: results from a randomized setting and a nonrandomized setting. Eur Urol. 2010;58:719–726. doi: 10.1016/j.eururo.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Abboudi H, Khan MS, Guru KA, et al. Learning curves for urological procedures—a systematic review. BJU Int. 2013 doi: 10.1111/bju.12315. [DOI] [PubMed] [Google Scholar]
- 40.Tomaszewski JJ, Matchett JC, Davies BJ, Jackman SV, Hrebinko RL, Nelson JB. Comparative hospital cost-analysis of open and robotic-assisted radical prostatectomy. Urology. 2012;80:126–129. doi: 10.1016/j.urology.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Kim SP, Shah ND, Karnes RJ, et al. Hospitalization costs for radical prostatectomy attributable to robotic surgery. Eur Urol. 2013;64:11–16. doi: 10.1016/j.eururo.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Eldefrawy A, Katkoori D, Abramowitz M, Soloway MS, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol. 2013;31:576–580. doi: 10.1016/j.urolonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kaushik D, High R, Clark CJ, LaGrange CA. Malfunction of the Da Vinci robotic system during robot-assisted laparoscopic prostatectomy: an international survey. J Endourol. 2010;24:571–575. doi: 10.1089/end.2009.0489. [DOI] [PubMed] [Google Scholar]