Abstract
Rectal cancer is one of the common cancers in India. Surgical management is the mainstay of initial treatment for majority of patients. Minimally invasive surgery has gained acceptance for the surgical treatment of rectal cancer because, compared with laparotomy, it is associated with fewer complications, shorter hospitalization, and faster recovery. The aim of this study is to evaluate the safety, feasibility, technique, and outcomes (postoperative, oncological, and functional) of robotic-assisted rectal surgery in comparison with open surgery in the Indian population. A prospective randomized study was undertaken from August 2011 to December 2012. Fifty patients who presented with rectal carcinoma were randomized to either robotic arm (RA) or open arm (OA) group. Both groups were matched for clinical stage and operation type. Technique and feasibility of robotic-assisted surgery in terms of operating time, estimated blood loss, margins status, total number of lymph nodes retrieved, hospital stay, conversion to open procedure, complications, and functional outcomes were analyzed. The mean operative time was significantly longer in the RA than in the OA group (310 vs 246 min, P < 0.001) but was significantly reduced in the latter part of the robotic-assisted patients compared with the initial patients. The mean estimated blood loss was significantly less in the RA compared with the OA group (165.14 vs 406.04 ml, P < 0.001). None of the patients had margin positivity. The mean distal resection margin was significantly longer in the RA than in the OA group (3.6 vs 2.4 cm, P < 0.001). A total of 100 % of patients in the RA group had complete mesorectal excision while two patients in the OA group had incomplete mesorectal excision. The average number of retrieved lymph nodes was adequate for accurate staging. The number of lymph nodes removed by robotic method is slightly higher than the open method (16.88 vs 15.20) but with no statistical significance. Conversion rate was nil. The mean hospital stay was significantly shorter in the RA group (7.52 vs 13.24 days, P < 0.001). Postoperative and functional outcomes were comparable between the two groups. Robotic-assisted surgery is an emerging technique in our country. Robotic-assisted rectal cancer surgery is safe with low conversion rates and acceptable morbidity and is oncologically feasible.
Keywords: Robotic surgery, Rectal cancer, India, Minimally invasive surgery
Patients and Methods
A prospective nonblinded randomized study was undertaken from August 2011 to December 2012 at Manipal Comprehensive Cancer Centre, Manipal-Vattikuti Institute of Robotic Surgery, Bangalore. All patients who presented to us with newly diagnosed rectal cancer were randomized to undergo either robotic or open surgery. Rectal cancer was defined as any tumor within 15 cm of the anal verge. Tumor location was measured using rigid sigmoidoscopy. Fifty patients with rectal cancer were included in the study.
According to our institution protocol, all patients underwent pelvic MRI to assess local infiltration and nodal metastasis. Patients with local invasion (T3–T4) or node involvement were reviewed by the multidisciplinary team and underwent neo-adjuvant chemoradiotherapy. They received 50.4Gy dose of EBRT in 28 fractions over 5.5 weeks, along with systemic 5-flurouracil-based chemotherapy followed by surgery 6–8 weeks later [1].
The study was approved by the institutional review board of our institution. Informed consent was obtained from all the patients.
Although studies have compared robotic with laparoscopic approach for rectal cancer, few prospective studies have compared robotic with open rectal cancer surgery, which is considered the classic gold standard treatment for rectal cancer. The aim of this study is to evaluate the safety, feasibility, technique, and outcomes (postoperative, oncological, and functional) of robotic-assisted rectal surgery in comparison with open surgery in the Indian population.
Patient demographics, basal metabolic index (BMI), location of the tumor, and operative procedure were recorded and depicted in Table 1. Technique and feasibility of robotic-assisted surgery in terms of operating time, estimated blood loss, total number of lymph nodes retrieved, return of bowel function, hospital stay, conversion to open procedure, and intraoperative and postoperative complications were analyzed. Operative time was defined as the time from incision to closure, including docking time. Estimated blood loss was estimated by taking into account all the blood loss in the suction apparatus and weighing of the mops. The quality of mesorectum was scored using three grades (complete, near complete, and incomplete) as defined by the Dutch TME trial [2]. Oncological outcomes were analyzed based on the proximal, distal, and circumferential margins and the number of resected lymph nodes at pathologic analysis.
Table 1.
Patient demographics, basal metabolic index (BMI), location of the tumor, and operative procedure
| Characteristics | Robotic-assisted surgery (n = 25) | Open surgery (n = 25) |
|---|---|---|
| Male:female | 17:8 | 15:10 |
| Age (years) | 56.36 ± 8.21 | 59.56 ± 5.75 |
| Mean BMI (kg/m2) | 31.51 ± 3.02 | 29.84 ± 2.75 |
| Site | ||
| Upper third | 2 | 5 |
| Mid third | 10 | 8 |
| Lower third | 13 | 12 |
| Preoperative CTRT | 7 | 9 |
| Operation type | ||
| Low anterior resections | 22 | 20 |
| Abdominoperineal resections | 3 | 5 |
| Mean blood loss (ml) | 165.14 ± 11.25 | 406.04 ± 111.32 |
| Mean operative time (min) | 310.32 ± 47.62 | 246.32 ± 17.45 |
| Margins | ||
| Proximal/distal | Negative | Negative |
| Circumferential/radial | Negative | Negative |
| Mean total lymph nodes | 16.88 ± 2.18 | 15.20 ± 1.65 |
| Mean hospital stay (days) | 7.52 ± 1.38 | 13.24 ± 2.43 |
| Intraoperative complications | 1 | 0 |
| Postoperative complications | ||
| Minor | 0 | 4 |
| Major | 0 | 1 |
Postoperative recovery included the time for return of bowel function, time requiring parenteral analgesia, and length of hospital stay. Intraoperative complications were defined as bowel, bladder, ureteral, nerve, or vascular injury during surgery. Postoperative complications were categorized into major and minor complications. Major complications included anastomotic dehiscence, pelvic abscess, deep vein thrombosis, pulmonary embolus, myocardial infarction, and sepsis. Minor complications included urinary tract infection, wound infection, and ileus.
Oncological radicality is the primary outcome that determines success in rectal cancer surgery. Secondary outcomes such as postoperative complications, time taken to recover from surgery, urinary continence, and sexual function are all important determinants of quality of life after surgery and were analyzed in the study.
Preoperative Preparation
Patient takes clear liquids with an oral antibiotic (ciprofloxacin + tinidazole) from 2 days prior to surgery. Night before the surgery, proctoclysis enema and two bisacodyl (Dulcolax) tablets are given per oral. We do not administer peglec because we found that it causes dilatation of bowel.
Port Placement and Instrumentation
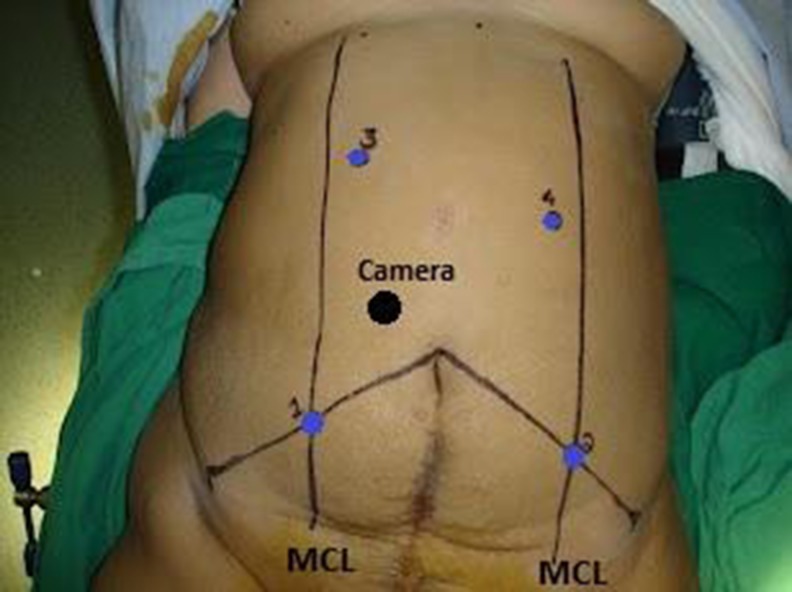
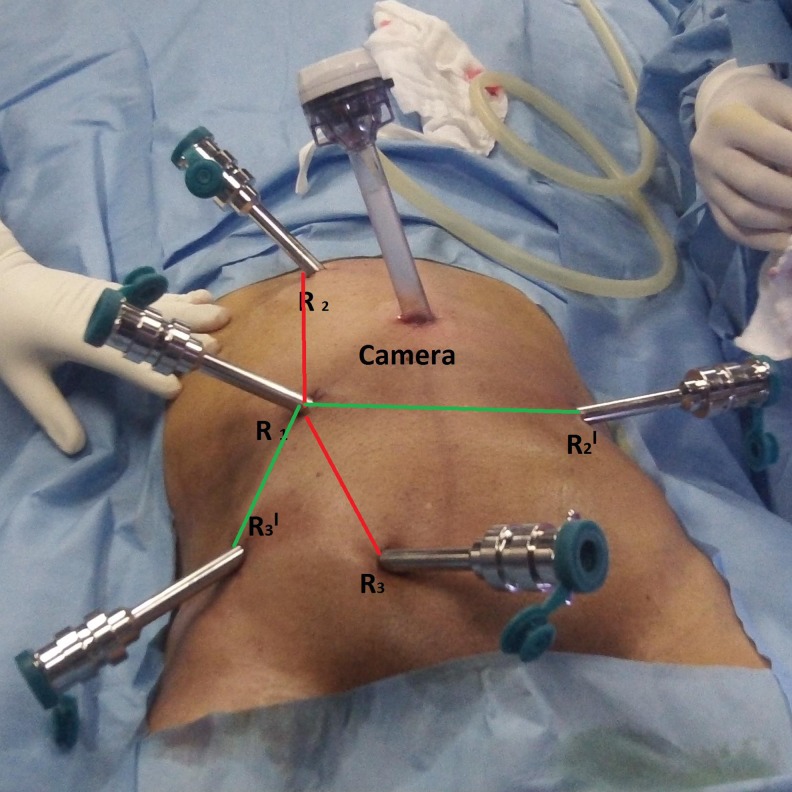
The robotic surgery was performed using the four-arm da Vinci S surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA). Total robotic colorectal mobilization and resection was performed in all. We used two types of port placements in our study. For the initial 10 cases, we used the port placement as depicted in Fig. 1. For the subsequent 15 cases, we prefer the port placement as depicted in Fig. 2. Both types involve dual docking but in fixed position of robotic cart.
Fig. 1.
Port placements used in the initial 10 cases
Fig. 2.
New port placements used in the last 15 cases
Surgical Steps
Patient Positioning
The patient is placed supine on the operating table. After induction of general anesthesia and insertion of an oral gastric tube and Foley catheter, the patient is rotated with the left side up and right side down, to approximately 25 to 30° tilt. The patient is then placed in a Trendelenburg position; this helps gravitational migration of the small bowel away from the operative field.
A 30° down facing camera with three working ports was used. Hot shears is used in R1, Cadiere forceps in R2, and double fenestrated grasper in R3.
Robotic dissection was performed following these steps:
Initial metastatic survey of the abdomen
Identification and isolation of IMA and IMV
Safeguarding autonomic nerves followed by clipping and transection of IMA and IMV
Medial to lateral dissection (identification of ureter and gonadal vessels)
Left colon mobilization up to splenic flexure
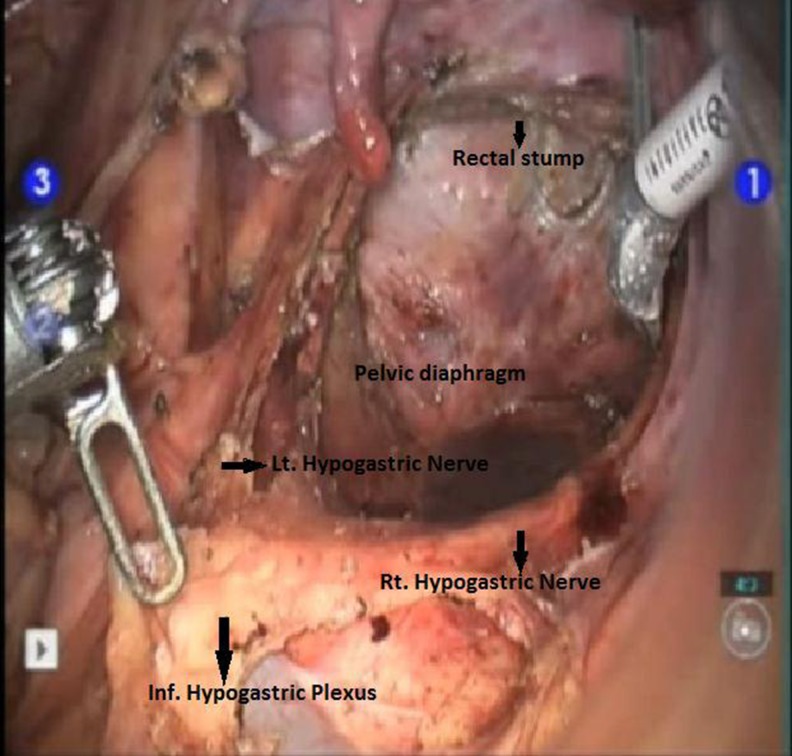
Pelvic dissection and mesorectal excision
Distal transection, exteriorization of specimen
Anastomosis
Statistical analysis
Mean values in each variable are compared, and P value is obtained by using the Student's t test (unpaired) and nonparametric test analysis for comparison between both arms.
Results
Twenty-five patients who underwent robotic-assisted mesorectal excision and autonomic nerve preservation were compared with similar open procedure in 25 patients. The outcomes were compared, and statistical significance was calculated (Table 2).
Table 2.
Postoperative and functional outcomes in the robotic-assisted and open surgeries
| Variable | Robotic-assisted surgery (n = 25) | Open surgery (n = 25) | P value |
|---|---|---|---|
| Age (years) | 56.36 | 59.56 | 0.117 |
| BMI (kg/m2) | 31.19 | 28.84 | 0.105 |
| Operating time (min) | 398 in first 5 cases | 240 | <0.001 |
| 288 in next 20 cases | 247 | <0.001 | |
| Estimated blood loss (ml) | 165.14 | 406.04 | <0.001 |
| Number of lymph nodes removed | 16.88 | 15.20 | 0.055 |
| Hospital stay (days) | 7.52 | 13.24 | <0.001 |
The male sex was more predominant in the study. The patients in the open arm (OA) group were older when compared with the robotic arm (RA) group. The two groups did not differ in terms of gender, physical constitutions, and comorbidities. Totally 16 patients received preoperative concurrent chemoradiation, 7 patients in RA, and 9 patients in OA.
Operative Outcomes
The majority of the patients had the growth in the mid or lower rectum with only 14 % having upper rectal cancer. Thirteen patients in the RA group had lower rectal cancer out of which three patients underwent abdominoperineal resection (APR), while 5 out of 12 patients in the OA group with low rectal cancer required APR. Temporary diverting loop ileostomy was performed in patients who underwent ultra-low anterior resection or in low anterior resection patients who had received preoperative chemoradiotherapy. In our study, 20 patients underwent ileostomies (9 in RA and 11 in OA). The conversion rate was nil.
The mean total operative time was significantly longer in patients who underwent robotic surgery than open surgery (310 vs 246 min, P < 0.001). Estimated blood loss was significantly less with robotic-assisted surgery (165.14 ml) compared with open method (406.04 ml).
Oncologic Outcomes
Circular stapled anastomosis was performed in all patients who underwent LAR, and the donuts were sent for frozen section analysis. The distal and proximal margins were negative for tumor in all the patients. The mean distal resection margin was significantly longer in the RA than in the OA group (3.6 vs 2.4 cm, P < 0.001). The circumferential resection margins (CRM) were inked to assess the completeness and subjected to pathological analysis. The CRM was negative in all patients. Besides the CRM status, the quality of mesorectum was scored by the pathologist using three grades (complete, near complete, and incomplete) as defined by the Dutch TME trial [1]. None of the patients in RA had incomplete mesorectum while two patients in OA had an incomplete mesorectal excision. In the RA group, the nodes (16.88) were slightly higher than those in the open method (15.20) but with no significant difference.
Postoperative Outcome
Length of hospital stay was significantly shorter in the RA group (7.52 vs 13.24 days). One patient in the RA group had bleeding from presacral plexus, which was controlled with pressure. In the open method, three patients had paralytic ileus, and one had wound infection. One patient in the OA group had anastomotic leak and underwent peritoneal toileting with diverting loop ileostomy. There was no operative mortality in either group. The median follow-up of the study is 5 months.
Functionality Assessment
As a part of the trial, all patients underwent urinary catheter removal on the second postoperative day. Five patients in the OA group and two patients in the RA group developed urinary retention and required re-catheterization and had catheter removal after 7 days following catheter clamping exercises. Post-void residual volume was assessed at the end of 1 month in all patients with the help of ultrasound and 3 months in OA, and one patient in RA had significant residual urine. Erectile dysfunction and retrograde ejaculation were assessed in all male patients during follow-up using the European Organization for Research and Treatment of Cancer (EORTC) questionnaire QLQ-C38. A total of 18 % of male patients in the RA group and 26 % in the OA group had sexual dysfunction features.
Discussion
Since the approval of robotic surgery by the FDA, many surgeons have switched over to robotic approach while dealing with colorectal cancers. The experiences of robotic-assisted colorectal cancer surgery have been reported in a number of case series, but only a minority has concentrated on rectal cancer. The concept of robotic total mesorectal excision for rectal cancer was first reported by Pigazzi et al. in 2006 [3].
In India, a few centers have started using this minimally invasive method while treating cancers. The robotic instruments with the “endowrist” technology can move with seven degrees of freedom simulating the movement of the human wrist. The visual quality of the current robotic system is superior due to the stable stereoscopic three-dimensional view that leads to enhanced depth perception with magnification. This improves the surgical precision and facilitates the performance of more complex procedures with a reduced learning curve and at superior comfort to the surgeon. The robotic approach is especially suited for dissection in confined spaces requiring precise movements and fine tissue dissection near critical structures, like in the pelvis (Fig. 1). This is witnessed by the dramatic impact the robot has had in prostate surgery by the large number of robotic radical prostatectomies that are being performed worldwide.
The prospective randomized study performed at a single institution compares consecutive patients treated surgically for rectal cancer from August 2011 to December 2012 with the da Vinci robotic system (Intuitive Surgical) or with conventional open surgery.
The hybrid technique (combination of laparoscopy and robotics) is the most popular method for robotic rectal surgery. We prefer the “totally” robotic rectal cancer resection technique. Proper cart placement with precise port design is of prime importance in robotic surgery. Initially, we used the port placement as depicted in Fig. 2 and later switched to new port placements (Fig. 3). We found with the new port design that there was less arm clashing, better upper abdominal reach allowing colon mobilization up to the splenic flexure, and also easy access to apply the articulating endoscopic linear cutter as distally as possible. In anterior resections, the narrow confines of the pelvis limit the passage and maneuvering of stapling devices leading to suboptimal distal rectal transection from multiple staple firings. In the latter cases, we adopted the technique of passing the umbilical tape circumferentially around the rectosigmoid junction. Adequate rectal retraction is achieved by the operative assistant by this technique in order to provide exposure and tissue tension for dissection and also ensures that adequate distal margin is obtained during the application of linear staple especially in ultra-low anterior resections.
Fig. 3.
The robotic surgery is especially suited for dissection in pelvis which requires precise movements and fine tissue dissection near critical structures
Morbidly obese patients are more suitable for robotic-assisted approach, as chances of arm clash decrease due to adequate spacing. The total operation time for the robotic surgery is the summation of the docking time and operation time. The robot docking time was especially longer in the initial cases during the learning period and decreased significantly with accumulation of operating experience. In our study, the mean operative time was significantly longer for robotic-assisted surgery than for open surgery (310 vs 246 min, P < 0.001). As we gain more experience, we expect the operating time to fall even further and be similar to the open method. The advanced technology in the robotic surgical system shortens the time of actual operative procedure itself, because it mimics intuitively the open procedure as opposed to the laparoscopic procedure in which surgical maneuvering is counterintuitive [4, 5]. Our robotic operating time was comparable with other series. Park et al., Hellan et al., and Luca et al. reported the operation time as 293.8, 285, and 290 min, respectively. Baik et al. reported the shortest operating time of 190 min [6–9].
The estimated blood loss was significantly less in robotic-assisted surgery compared with the open method (165.14 vs 406.04 ml). Magnified vision and improved dexterity helps in better vascular control than the open method. Various studies have noted similar operative blood loss ranging from 60 to 200 ml [10–14].
The return of bowel function was faster, and the need for analgesics was lesser in the RA surgery which was reflected by a shorter hospital stay. In our study, the hospital stay was significantly less in the RA than in the OA group (7.52 ± 1.38 vs 13.24 ± 2.43 days). In the study by Baik et al., the length of hospital stay was 6.9 ± 1.3 days in the robotic arm surgery [9]. No major postoperative complication was noted in the RA surgery while one patient in the OA method had anastomotic leak. Paralytic ileus was seen in three patients in the OA method, and interestingly, all of them had received neo-adjuvant chemoradiotherapy.
In our study, we did not have any conversion to open laparotomy. Baik et al. and Tayfun et al. reported nil conversion rates for robotic low anterior resection. Stavioros A and Sami Al Asari reported 0.4 and 6 % conversion rates [9, 15–17].
None of the patients in our study was CRM positive. A recent report from a multi-institutional study (CLASICC trial) comparing CRM results from laparoscopic colorectal cancer surgery vs open surgery showed a concern of higher positive rate in the laparoscopic arm [17, 18]. One of the most important factors relating to adequacy of rectal excision is the mesorectal grade, which is related to oncological outcome [2]. In the current study, rectal cancer specimen quality following surgery was better with robotic surgery. Incomplete mesorectal excision with distal “coning” was more common in the OA method due to lack of sufficient space in the pelvis. The quality or grade of mesorectum was evaluated in three studies. Baik et al. reported that the mesorectal grade after robotic-assisted low anterior resection was significantly better than after a conventional laparoscopic surgery.
The average number of retrieved lymph nodes was adequate for accurate staging. Number of lymph nodes removed in the RA surgery is slightly higher than in the OA method (16.88 vs15.20) but with no statistical significance. Negative distal and proximal margins were achieved in all the patients. In our study, the length of distal resection margin was found to be significantly longer in the RA patients (3.6vs2.4 cm). The studies by Pigazzi et al. and J. C. Kim et al. noted a similar mean distal margin of 2.9 and 2.7 cm, respectively. [6, 9, 13, 19, 20]
The superior visualization and precise sharp dissection are the core advantages which the surgeon appreciates by using the robotic system especially in locally advanced cancer patients who have received preoperative chemoradiotherapy. The quality of the rectal cancer surgery can be measured by the evaluation of the mesorectal grade or the harvested lymph node number and autonomic nerve preservation. They have been shown to be crucial in reducing local recurrence with improving survival and also better bladder and sexual function [1, 21, 22].
The limitations of the study are the small number of patients and short follow-up. Recurrence and survival outcomes were not evaluated due to the limited follow-up. A blinded study w.r.t the pathologist analyzing the specimen would have been ideal and is also one of the drawbacks of the study.
Recent investigations of laparoscopic colorectal resection for the treatment of cancer have revealed superior short-term operative outcomes and noninferior oncologic outcomes compared with open surgery, the classic standard treatment [18, 23, 24]. Although laparoscopic colon surgery is very popular, there is little doubt that laparoscopic rectal surgery is technically very challenging and the learning curve for this procedure is longer and more demanding than for laparoscopic colectomy. A steep learning curve explains the low penetration rate for laparoscopic rectal resection. The oncologic and short-term operative outcomes may be impaired with inexperienced hands [18, 23]. The steep learning curve is of concern especially, where the quality of mesorectal excision influences long-term outcomes [18]. The MRC CLASICC trial reported a higher rate of CRM positivity in the laparoscopic arm (12.4 vs 6.3 %). This difference in CRM involvement was postulated to reflect the increased technical difficulties in laparoscopic rectal surgery. Higher conversion rate in laparoscopic rectal subgroup (34 %) and higher postoperative morbidity and mortality than in laparoscopic colon subgroup (25 %) highlight the point [18, 24]. Studies have shown that the benefits of the robotic system are best appreciated when accuracy is required within a confined space, such as in the pelvis [25].
The promising advantages offered by robotic colorectal surgery indicate that they may well represent the next major leap in minimally invasive surgery. The robotic surgical system is upgrading rapidly and continuously. The future will also undoubtedly give rise to a far more sophisticated breed of surgical robot that will become smaller and cheaper and possess haptic feedback.
Conclusion
The key to successful rectal cancer resection is to perform complete mesorectal excision. Laparoscopic rectal surgery can be challenging with a long learning curve, especially in the narrow confines of the pelvis. Technical improvements like robotic-assisted surgery, stapling devices, and neo-adjuvant chemoradiation have accelerated sphincter-saving procedures in low rectal cancers. The initial experiences report that the three-dimensional magnified images combined with wristed instrumentation, tremor filtration, motion scaling, ambidextrous capability, and excellent ergonomics allow the surgeon to overcome many of the limitations of laparoscopy with the robotics platform.
In comparison with the open method, it has advantages of decreased blood loss, less postoperative complication, and shorter length of hospital stay. Morbidly obese patients are more suitable for robotic-assisted approach, as chances of arm clash decrease due to adequate spacing. Minor postoperative complications are less with nil conversion rate. The number of lymph nodes retrieved is equal or higher than the open method. None of the patients had margin positivity. The patients who underwent robotic surgery had a superior mesorectal grade and longer distal resection margins. As the surgeons gain experience, operative time will decrease further. We conclude that the robotic-assisted rectal cancer surgery is an oncologically feasible technique in comparison with the open method. However, large study group and long-term follow-up data are required to evaluate the recurrence and survival rates.
Contributor Information
S. P. Somashekhar, FAX: +91-8025266757, Email: somashekhar.sp@manipalhospitals.com, Email: somusp@yahoo.com
K. R. Ashwin, Email: doc.ashwin.kr@gmail.com
Jaka Rajashekhar, Email: rajjaka@yahoo.com.
Shabber Zaveri, Email: drshabber@gmail.com.
References
- 1.Brown G, Daniels IR. Preoperative staging of rectal cancer: the MERCURY research project. Recent Results Cancer Res. 2005;165:58–74. doi: 10.1007/3-540-27449-9_8. [DOI] [PubMed] [Google Scholar]
- 2.Nagtegaal ID, Van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH, Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB. Robotic assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc. 2006;20:1521–1525. doi: 10.1007/s00464-005-0855-5. [DOI] [PubMed] [Google Scholar]
- 4.Rawlings AL, Woodland JH, Crawford DL. Telerobotic surgery for right and sigmoid colectomies: 30 consecutive cases. Surg Endosc. 2006;20:1713–1718. doi: 10.1007/s00464-005-0771-8. [DOI] [PubMed] [Google Scholar]
- 5.D'Annibale A, Morpurgo E, Fiscon V, et al. Robotic and laparoscopic surgery for treatment of colorectal disease. Dis Colon Rectum. 2004;47:2162–2168. doi: 10.1007/s10350-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 6.JS Park, GS Choi, KH Lim, YS Jang (2010) Robotic-Assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes Annals of Surgical Oncology December 2010, Volume 17, Issue 12, pp 3195–3202 [DOI] [PubMed]
- 7.Hellan M, Anderson C, Ellenhorn JDI, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol 14:3168–3173 [DOI] [PubMed]
- 8.Fabrizio Luca, Sabine Cenciarelli, Full robotic left colon and rectal cancer resection: technique and early outcome Annals of Surgical Oncology May 2009, Volume 16, Issue 5, pp 1274–1278 [DOI] [PubMed]
- 9.Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc. 2008;22:1601–1608. doi: 10.1007/s00464-008-9752-z. [DOI] [PubMed] [Google Scholar]
- 10.Mirnezami H, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ. Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Color Dis. 2010;12(11):1084–1093. doi: 10.1111/j.1463-1318.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 11.Vibert E, Denet C, Gayet B. Major digestive surgery using a remote-controlled robot: the next revolution. Arch Surg. 2003;138:1002–1006. doi: 10.1001/archsurg.138.9.1002. [DOI] [PubMed] [Google Scholar]
- 12.Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg. 2010;251(5):882–886. doi: 10.1097/SLA.0b013e3181c79114. [DOI] [PubMed] [Google Scholar]
- 13.Delaney CP, Lynch AC, Senagore AJ, Fazio VW. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum. 2003;46:1633–1639. doi: 10.1007/BF02660768. [DOI] [PubMed] [Google Scholar]
- 14.Woeste G, Bechstein WO, Wullstein C. Does telerobotic assistance improve laparoscopic colorectal surgery? Int J Colorectal Dis. 2005;20:253–257. doi: 10.1007/s00384-004-0671-8. [DOI] [PubMed] [Google Scholar]
- 15.Tayfun K, Inmail H, Bilgi B. Robot surgery for rectal cancer: initial experience from consecutive patients. J Gastrointestinal Surgery. 2012;16:401–407. doi: 10.1007/s11605-011-1737-x. [DOI] [PubMed] [Google Scholar]
- 16.Antoniou SA, Antoniou GA, Koch OO. Robot assisted laparoscopic surgery of the colon and rectum. Surg Endoscop. 2012;26:1–11. doi: 10.1007/s00464-011-1867-y. [DOI] [PubMed] [Google Scholar]
- 17.Sami Al Asari and Byung Soh Min. Robotic colorectal surgery: a systematic review International Scholarly Research Network ISRN Surgery Volume 2012, Article ID 293894 [DOI] [PMC free article] [PubMed]
- 18.Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC trial group. J Clin Oncol. 2007;25(21):3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 19.Alessio P, Fabrizio L, Alberto P, Manuela V, Graziano C, Luciano C, Roberto B, Julio G-A, Jeong-Heum B. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17(6):1614–1620. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim JC, Yang SS, Jang TY, Kwak JY, Yun MJ, Lim SB. Open versus robot-assisted sphincter-saving operations in rectal cancer patients: techniques and comparison of outcomes between groups of 100 matched patients. Int J Med Robot. 2012;8:468–475. doi: 10.1002/rcs.1452. [DOI] [PubMed] [Google Scholar]
- 21.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/S0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 22.Kang Jeonghyun, Yoon Kyu Jong, Min ByungSoh, HurHyuk, BaikSeungHyuk,; Kim Nam Kyu, Lee Kang Young; The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison—open, laparoscopic, and robotic surgery Annals of Surgery: January 2013 - Volume 257 - Issue 1 - p 95–101 [DOI] [PubMed]
- 23.Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 24.PJ Guillou, P Quirke, H Thorpe et al., “Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial,” The Lancet, vol. 365, no. 9472, pp. 1718–1726 [DOI] [PubMed]
- 25.Wexner SD, Bergamaschi R, Lacy A, et al. The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc. 2009;23:438–443. doi: 10.1007/s00464-008-0202-8. [DOI] [PubMed] [Google Scholar]