Abstract
Glomus tumor is a benign vascular tumor derived from the modified smooth muscle cells of the glomus body. The single most common site is the subungual region of the finger, but other common sites include the palm, wrist, forearm, and foot. In this article, we present a rare situation of glomus tumor occurring on the back of the chest over the scapular area in an elderly male patient. The tumor cells exhibited positive expression for CD34 and smooth muscle actin. This paper highlights the fact that a glomus tumor is a benign neoplasm that may occur in multiple locations. Therefore, the significance of a histological and immunohistochemical approach for a correct characterization of this lesion is required.
Keywords: Glomus tumor, Extra digital glomus tumor, Scapular pain, Limberg flap
Introduction
Glomus tumor is a benign vascular tumor derived from the modified smooth muscle cells of the glomus body. The tumor develop as small pinkish red nodules that are usually located in the nail bed and deep dermis or subcutis of the upper or lower extremity. The single most common site is the subungual region of the finger, but other common sites include the palm, wrist, forearm, and foot. Extra digital glomus tumors are occasionally difficult to diagnose, owing to their nonspecific clinical characteristics, including unusual sites and symptoms which vary compared with those of classical glomus tumors. Therefore, it is crucial to include glomus tumors in a differential diagnosis of patients with extra digital lesions, showing the features of small or unrecognized lesion with disproportionate pain and sensitivity to touch and cold.
Case Report
Sixty-seven-year-old male patient presented with excruciating pain over the left scapular area for 6 years. He had been treated with triamcinolone injections, probably suspecting tendinitis, and later, an excision was done by some surgeons but the pain was persistent. He gave a history of exacerbation of pain while taking a bath and when exposed to cold. Clinical examination revealed a vague swelling on the back of scapular area on the left side (Fig. 1). There was wasting of the infraspinatous muscle, trapazius, and latissimus dorsi.
Fig. 1.
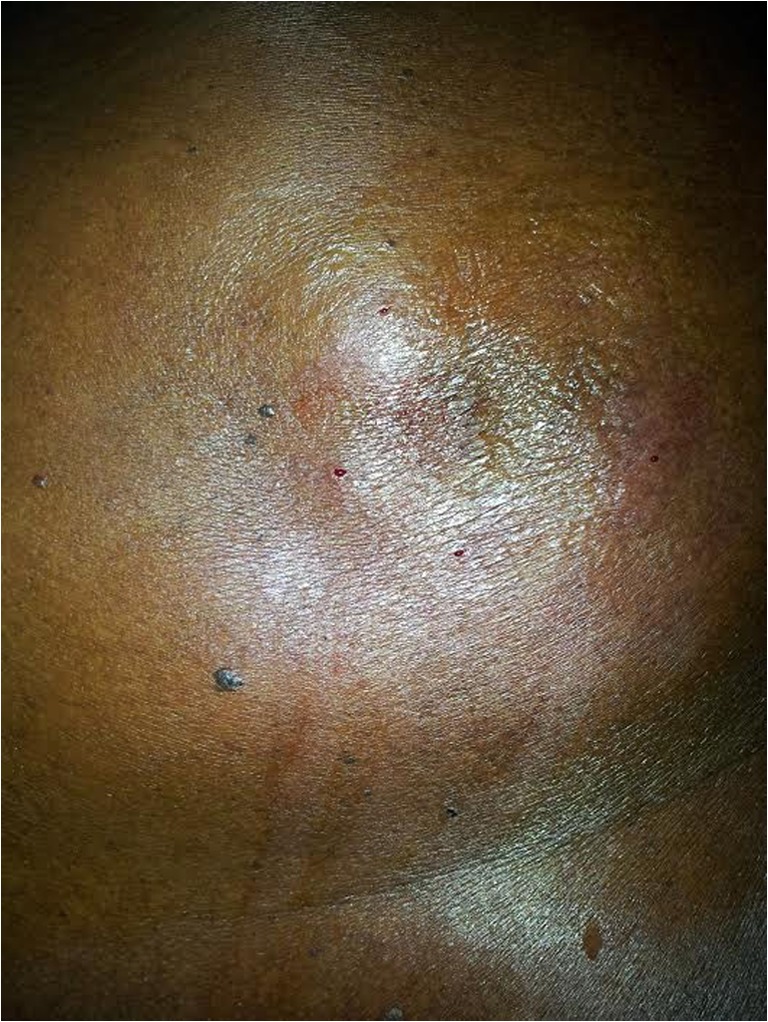
External appearance of the case with flap design
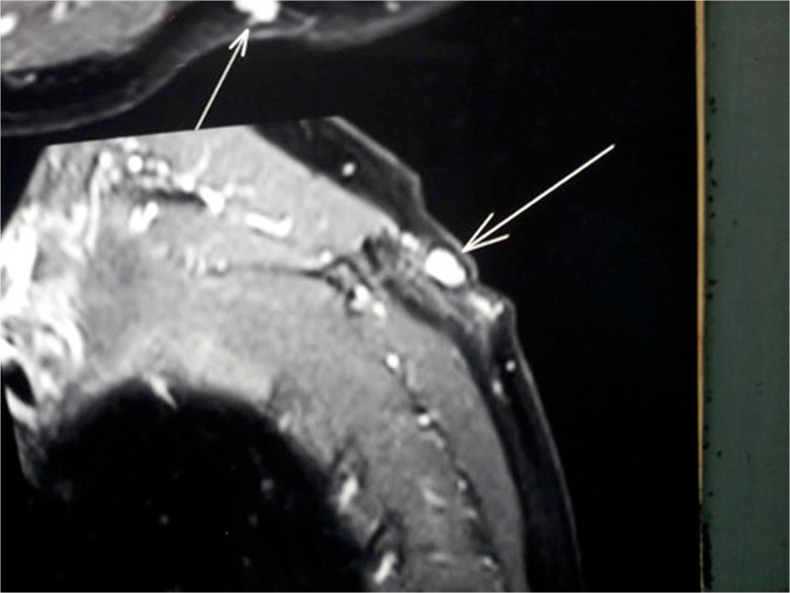
Ultrasound examination revealed a small well-defined hypo-echoic mass. MRI scans showing a hyper-intense lesion on the T2-weighted image (Fig. 2).
Fig. 2.
MRI scans showing the lesion
With clinical diagnosis of glomus tumor with recurrence (one time excision was done by a neurosurgeon who failed to localize and remove the lesion), a wide excision with 6 cm all around was done and the wound was closed with Limberg flap. The defect of 6 cm on the back was covered with thick skin and subcutaneous tissue of the nearby area using this simple flap. A direct closure could have produced a thick painful scar and defect, and a skin graft will produce a depressed painful scar over the pressure-bearing area.
Specimen
The skin excised on the section showed a small pinkish red well-defined pearl like lesion of 2 cm (Fig. 3), and histopathology examination revealed a lesion composed of solid sheets of cells interrupted by vessels of varying size (Fig. 4). The tumor cells exhibited positive expression for CD34 and smooth muscle actin. Immunohistochemically, the tumor cells were reactive for smooth muscle actin (SMA) and vimentin (VMT). Patient was symptom free for the last 6 months.
Fig. 3.
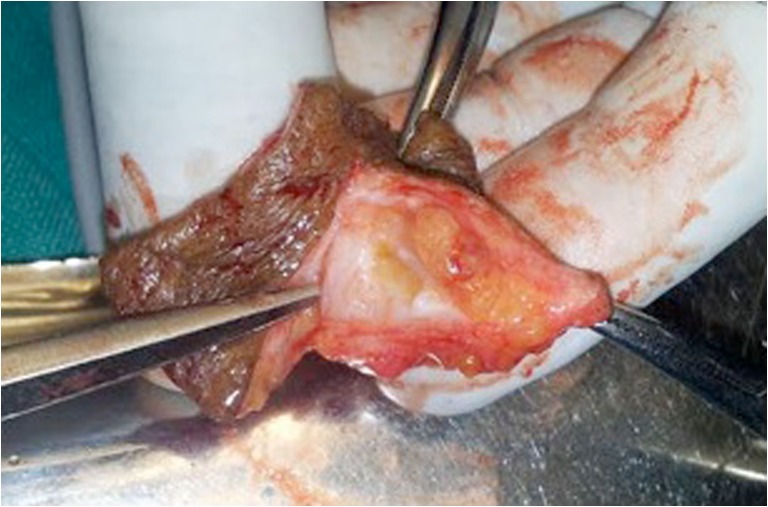
Excised skin with lesion
Fig. 4.
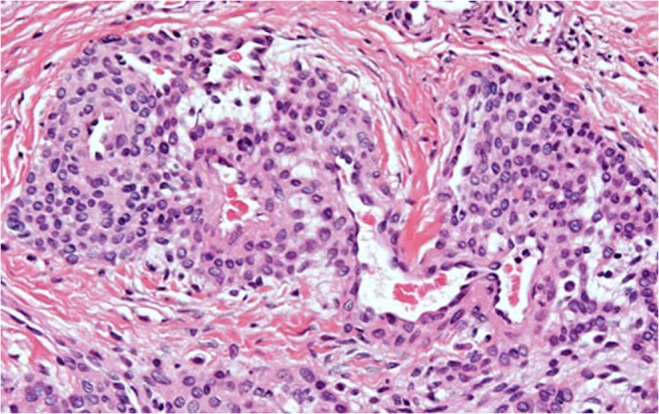
Histological appearance of the tumor
Discussion
Glomus tumors are neoplasm of the glomus body, a neuromyoarterial unit found within the reticular dermis that serves as a specialized arteriovenous anastomosis. The glomus body is made of preglomic arterioles derived from the small arterioles that supply the dermis and is lined by plump cuboidal endothelial cells and surrounded by longitudinal and circular muscle fibers. Scattered throughout the muscle fibers are rounded epitheloid glomus cells. The arterial end of the glomus body or Sucquet-Hoyer canal is surrounded by modified smooth muscle cells called glomus cells that act to regulate blood flow to the skin in response to temperature changes. Histologically, depending on the predominant component, there are three variants of glomus tumor, namely (1) solid, with poor vasculature and scant smooth muscle component; (2) angiomatoid (glomangioma), with a predominant vascular component; and (3) glomangiomyoma, with prominent vascular and smooth muscle components [1]. The tumor cells exhibit positive expression for CD34 and smooth muscle actin. It accounts for 1–6 % of all soft tissue tumors and 1–5 % of hand tumors. Glomus tumors present mostly as solitary masses with a rarer multiple variant. Since the glomus apparatus are more concentrated in the digits, palm, and sole, the lesions are more common in upper extremity. Malignant transformation is extremely rare. Folpe et al. [2] proposed the following classification criteria for malignant glomus tumors: (i) deep location and a size of >2 cm, (ii) presence of atypical mitotic figures, or (iii) combination of moderate to high nuclear grade and mitotic activity (5 MFs/50 HPFs).
Extra digital glomus tumors are occasionally difficult to diagnose, owing to their nonspecific clinical characteristics, including unusual sites and symptoms which vary compared with those of classical glomus tumors. Therefore, it is crucial to include glomus tumors in a differential diagnosis of patients with extra digital lesions, showing the features of small or unrecognized lesion with disproportionate pain and sensitivity to touch and cold. Substance P and TRPV1 were expressed moderately to strongly in glomus cells with no differences in their expression between digital and extra digital glomus tumors, which is responsible for the excruciating pain and sensitivity to cold.
A review of the literature suggests that the extra digital distribution along the upper extremity may be more frequent. The forearm has been noted to be the most common extra digital location, while the shoulder and upper back are the least frequent [2, 3]. The classic triad of symptoms described for glomus tumors consists of pain, localized tenderness, and cold hypersensitivity. Only 20 % of extra digital glomus tumors were diagnosed correctly by the initial physician. The mean size of the tumors was 1.9 cm. The average duration of symptoms was few years. The data indicate that in the subcutaneous location of the extra digital areas, the tumor only becomes visible at a late stage, which correlates with the enlargement of the mass. The absence of objective findings frequently results in a diagnostic delay, a finding that is confirmed by the protracted duration of symptoms observed in the majority of series and case studies. Various diagnostic imaging techniques have been reported to enhance the ability to detect these lesions. There are no specific imaging techniques to aid in the diagnosis. Ultrasonography, despite its low specificity, may aid in locating the lesion. MRI provides more details of the lesion and its association with the adjacent structures [4]. It must be emphasized that a diagnosis relies on a high index of clinical suspicion.
In our case, the lesion was over the scapular area where the skin is very thick and hence it could not be palpated well. One attempt of excision was done by somebody but they could not remove the lesion, and hence the pain was persistent even after the surgery. So, we have done a wider excision and closed it with the Limberg flap since it was a pressure-bearing area. The shoulder and scapular area is a rare site for glomus tumor [5, 6].
Conclusion
Triad of pain, localized tenderness, and cold hypersensitivity of unusual areas has to be investigated for rare glomus tumor. Cases are reported, showing that glomus tumors are not so rare to produce such chronic pain especially in the shoulder area and knee joint area. These cases can be better diagnosed with MRI scan of the area and excision with wide margin in curative.
References
- 1.Enzinger SW, Weiss FM, Editors. Soft Tissue Tumors. 4th edition. Mosby; St Louis: 2001. Perivascular tumors; pp. 985–1001
- 2.Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:58–64. doi: 10.1097/00000478-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lee D-W, Yang J-H, Chang S, Won C-H, Lee M-W, Choi J-H, Moon K-C. Clinical and pathological characteristics of extradigital and digital glomus tumours: a retrospective comparative study. J Eur Acad Dermatol Venereol. 2011;25:1392–1397. doi: 10.1111/j.1468-3083.2011.03979.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg. 2005;30(5):535–540. doi: 10.1016/j.jhsb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Abela M, Cole AS, Hill GA, Carr AJ. Glomus tumor of the scapular region. J Shoulder Elbow Surg. 2000;9(6):532–533. doi: 10.1067/mse.2000.108961. [DOI] [PubMed] [Google Scholar]
- 6.Karakurum G, Tutar E, Pirbudak L, Mizrak A. Glomus tumor of the deltoid muscle. A case report. Acta Orthop Belg. 2009;75:681–683. [PubMed] [Google Scholar]